Abstract
Cannabis sativa is known among many cultures for its medicinal potential. Its complexity contributes to the historical application of various parts of the plant in ethno-medicines and pharmacotherapy. C. sativa has been used for the treatment of rheumatism, epilepsy, asthma, skin burns, pain, the management of sexually transmitted diseases, difficulties during child labor, postpartum hemorrhage, and gastrointestinal activity. However, the use of C. sativa is still limited, and it is illegal in most countries. Thus, this review aims to highlight the biological potential of the plant parts, as well as the techniques for the extraction, isolation, and characterization of C. sativa compounds. The plant produces a unique class of terpenophenolic compounds, called cannabinoids, as well as non-cannabinoid compounds. The exhaustive profiling of bioactive compounds and the chemical characterization and analysis of C. sativa compounds, which modern research has not yet fully achieved, is needed for the consistency, standardization, and the justified application of Cannabis sativa products for therapeutic purposes. Studies on the clinical relevance and applications of cannabinoids and non-cannabinoid phenols in the prevention and treatment of life-threatening diseases is indeed significant. Furthermore, psychoactive cannabinoids, when chemically standardized and administered under medical supervision, can be the legal answer to the use of C. sativa.
Keywords: medicinal plant, Cannabis sativa, phytochemicals, bioactivity, extraction methods, characterization
1. Introduction
The applications of plants as medicines predates human history. A medicinal plant refers to any plant which contains substances of therapeutic potential in one or more of its parts for the synthesis of plant-based drugs [1]. Active medicinal plant ingredients are referred to as bioactive phytochemicals. [2]. These bioactive compounds are believed to increase the ability of plants to survive or adapt to their surroundings [3] and are used as medicines, flavorings, and recreational drugs in humans. One notable medicinal plant that has continued to garner attention over the years, and in recent times, is Cannabis sativa.
Cannabis sativa L. is known for its medicinal uses since ancient times, because of its rich supply of phytochemicals [4], hence the quest for harnessing its pharmacological potential by scientists. The term “Cannabis” is used to define the products (drugs and essential oils) that are prepared or obtained from the annual herb C. sativa and its variants, which are of the family Cannabaceae [5]. The utilization of this multipurpose plant has been restrained for a long time because of the psychoactive effects of a specific cannabinoid (Δ9-tetrahydrocannabinol; C12H30O2) [6]. It was strongly prohibited in the twentieth century, and was removed from the British pharmacopeia. The plant was demonized due to its high abuse liability and supposedly insufficient health benefits [7]. Furthermore, due to the inability to prepare standardized preparations, and the diffusion of the recreational use of cannabis below therapeutic concentrations from the end of the 19th to the first half of the 20th century, the medical use of cannabis began to decline [7]. In 1937, the “Marihuana Tax Act”, a federal legislation in the United States, functionally ended all medical uses of cannabis and was removed from the “National Formulary and Pharmacopoeia” in 1941 [7]. In 1961, cannabis resin, extracts, and tinctures were listed in the Schedule I of the single Convention on Narcotic Drugs, which prohibits the use, possession, production, manufacture, export, import, and trade of cannabis, except for medical and scientific purposes [7].
However, in multiple countries today, its cultivation and usage are regulated by laws [6,8]. Recent decriminalization policies and new scientific evidence have increased the interest in the medicinal potential of cannabis and have paved the way for the release of marketing authorizations for cannabis-based products [7]. In 1985, the United States Food and Drugs Administration (US FDA) reconsidered the medical use of cannabinoids, and approved Marinol (dronabinol) and Cesamet (nabilone), two synthetic analogues of tetrahydrocannabinol (THC), for the management of nausea and vomiting associated with cancer chemotherapy [7]. The Office of Medical Cannabis Research (OMC), a Dutch government agency in Europe, became the first organization to obtain the exclusive right to supply medical cannabis to research institutes and pharmacies, and, under the Single Convention on Narcotic Drugs of 1961, to import and export cannabis extracts and resin for medical purposes. Several medical cannabis products, all of which are dried female flowering tops, except Bediol (which is ground into small pieces for its easy manipulation by patients with spasticity), are exported by the OMC, with the proper licenses, to other member states of the European Union [7]. In Italy, the Military Pharmaceutical Chemical Works of Florence became the official national settlement for cultivating and manufacturing medical cannabis with a standard cannabinoid content [7,9]. The Italian Ministry of Health in November 2015, in a Ministerial Degree, authorized the indoor cultivation of cannabis flowering tops at a fixed temperature and at fixed light-dark cycles, leading to a standardized composition of different cannabinoids [9]. Two Italian products (FM1 and FM2) are currently available for consumers, and their use was approved for the treatment of chronic pain, neurological disorders, and other diseases resistant to standard therapies [7,9,10]. Non-psychoactive compounds found in C. sativa are associated with fewer side effects and can be used for several industrial applications [6]. The hemp stem supplies both cellulosic and woody fibers. The woody fibers are used for animal beddings, while the cellulosic fibers (bast fibers) are used as a substitute for fiberglass, and to produce bioplastics [4]. Its use as an anti-bacterial finishing agent and in functionalized textiles have also been reported [4]. The inflorescence was used, traditionally, for acute pain, insomnia, coughing, and wounds. The leaves were used for malaria, panting, roundworm, scorpion stings, hair loss, and the greying of hair. The stem bark was used for physical injury and strangury. Vaginal discharge, difficult births, strangury, the retention of the placenta, and physical injuries were treated using the roots [11]. In addition, Cannabis sativa contains essential oils of a high value, which can also improve the effectiveness of cannabinoids in pharmaceutical formulations [6].
Despite the influx of chemical-based medicines for treatments, the relevance of medicinal plants in drug development cannot be overemphasized. In recent years, commercial medicinal cannabis products with several variations in the phytocannabinoid content have been licensed and produced in Canada [7,8] and in several other countries. Several synthetic and standardized products are currently available on the market; however, patients’ preferences lean towards herbal preparations, because they are easy to handle and self-administer [7]. Thus, this review intends to highlight the phytochemicals present in the different plant parts, which potentiates their pharmacological activities, as well as the techniques for the extraction, isolation, and characterization of C. sativa compounds.
2. Methods
Literature on the published works of Cannabis sativa was obtained using electronic search engines, such as Google Scholar, the WSU online database (PubChem), and Science Direct. The keywords included, namely, Cannabis sativa, medicinal plants, Cannabis phytochemicals, ethnopharmacology bioactivity, and medicinal potentials, were used to source for data. An extensive review of the literature from 2011 through to 2021 (the last ten years) on Cannabis sativa L. was used to summarize its medicinal potential. Conversely, an emphasis will be placed on the isolation and characterization techniques from 1970 to 2021 to have a broadened view of the advancements in analytical techniques over the past years. Overall, twenty-nine (29) papers relating to the areas of our focus were chosen and were reviewed by all authors. The results from the search were carefully sorted, based on a general understanding, the review questions, and the related objectives.
3. Origin and Botanical Description of C. sativa
The genus name Cannabis means “cane-like” while sativa means “sown”, which signifies that the plant is propagated from the seed and not from the roots [12]. It is believed to have originated in Asia and occurs widely in Africa [12,13]. Central and south-east Asia are the potential natural origins for the domestication of the Cannabis genus [14] and it is known by different common names in different languages (hemp, marihuana, kannabis sativa, ganja, bhang, and al-bhango) [15]. In South Africa, it is colloquially known, in Afrikaans, as “dagga”; in IsiXhoxa as “umfincafincane”; and in Isizulu as “umunyane” [16,17]. Taxonomically, Carl Linnaeus, a Swedish botanist, was the first to coin the name Cannabis sativa [18]. Other botanists stated that different types of Cannabis existed based on their size, shape, and resin content (breeding and selection). This review discusses, in particular, C. sativa.
The Cannabis phenotype (its observable traits or characteristics, such as its leaf shape and flower color) is based on two main factors: its genetic code (genotype) and the external environmental factors [19].
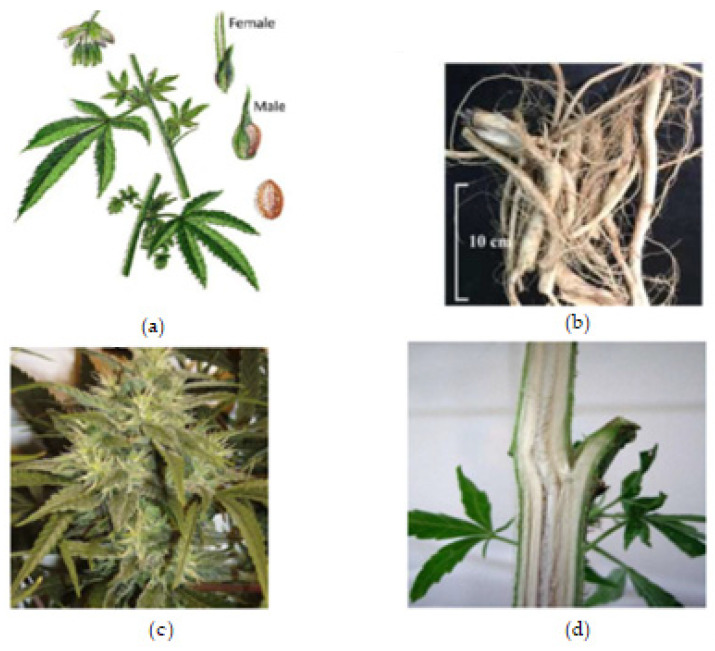
The roots are branched and are about 30–60 cm deep (Farag and Kayser, 2017) [12]. Cannabis inflorescence is made up of several flower heads found on long leafy stems from each leaf axil. A single brownish fruit, about 2-5mm long, is produced per flower, and it contains a single seed tightly covered with a hard shell [12]. The fruit is propagated by bird and the seed germinates after 8–12 days [18]. The leaves, bracts, and stems of the plant are rich in trichrome, which are a diverse set of structures containing the secondary metabolites (phytocannabinoids and terpenoids) responsible for the defense, plant interactions, and typical smell [18]. Figure 1, below, shows the plant parts of C. sativa.
Figure 1.
Cannabis plant parts. (a) Male and female Cannabis flowering parts with fresh leaf and seed. (b) Fresh Cannabis root. (c) Fresh Cannabis inflorescence (flower). (d) Fresh Cannabis stem bark.
4. Phytochemistry of C. sativa
4.1. Chemical Profile of C. sativa
Cannabis, as a herbal medicine, is a complex mixture of compounds, including cannabinoid phenols, non-cannabinoid phenols (stilbenoids, lignans, spiro-indans, and dihydrophenanthrenes), flavonoids, terpenoids, alcohols, aldehydes, n-alkanes, wax esters, steroids, and alkaloids [6,8,11]. Over 500 chemical compounds have been isolated from the cannabis plant and have been reported [13]. The several classes of secondary metabolites are present in different parts of the plant with a wide range of applications (nutraceuticals, cosmetics, aromatherapy, and pharmacotherapy) that are beneficial for humans. However, previous studies have focused mainly on the cannabinoids, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) in particular; hence, the female flower top is only harvested, while other parts of the plant are discarded [11].
Cannabinoids are a class of terpenophenolic compounds obtained by the alkylation of an alkyl-resorcinol with a monoterpene unit [20,21]. They feature alkyl resorcinol and monoterpene moieties in their molecules [20,22]. This specific chemical class in Cannabis is present in the glandular trichomes, which are abundant in the female flower as phytocannabinoid acids, and in the vegetable matrix as neutral phytocannabinoids [6,13]. They are biosynthesized by the alkylation of olivetolic acid with geranyl-pyrophosphate by a prenyltransferase to produce cannabigerolic acid (CBGA). Decarboxylation, a chemical reaction, converts the acidic forms (Δ9 THCA, CBDA, CBCA, and CBGA) into their neutral forms, which are more active and efficient in terms of pharmacological activity [8,23]. To date, 125 cannabinoids have been identified and reported, in addition to five new cannabinoids reported in the past two years, 42 non-cannabinoid phenolics, 34 flavonoids, 120 terpenoids, 3 sterols, and 2 alkaloids [8,11,13]. Terpenoids are the second largest class of cannabis compounds and are responsible for their characteristic aroma [13]. Table 1 below summarizes the classes of compounds isolated from Cannabis sativa and the different plant parts in which they are present.
Table 1.
Chemical compounds Isolated from Cannabis sativa.
| S/N * | Class of Compounds | Plant Part(s) | Isolated Compounds | References |
|---|---|---|---|---|
| 1 | Cannabinoids: -Cannabinoid acids -Neutral cannabinoids -Cannabinoid derivatives -Cannabinoid acid esters |
Leaves, flowers, resin, stembarks, and roots | Δ9-tetrahydrocannabivarin, α/β-fenchyl Δ9-tetrahydrocannabinolate, Δ9-tetrahydrocannabinol, α-terpenyl (−)-Δ9-trans-tetrahydrocannabinolate, γ-eudesmyl (−)Δ9-trans-tetrahydrocannabinolate, 8α-hydroxy-(−)-Δ9-trans-tetrahydrocannabinol, 8b-hydroxy-(−)- Δ9-trans-tetrahydro cannabinol, 8-oxo-(−)-Δ9-trans-tetrahydrocannabinol, Cannabisol, (−)-Δ9-trans-tetrahydrocannabiphorol, (−)-Δ9-trans-tetrahydrocannabihexol, (−)-Δ8-trans-tetrahydrocannabinol, Δ8- trans-tetrahydrocannabinolic acid, Cannabigerol, 6,7-trans/cis-epoxycannabigerolic acid, Sesquicannabigerol, Cannabigerolic acid, Cannnabigerovarin, Cannabidiol, C4-Cannabidiol, Cannabidivarin, C4-tetrahydrocannabinol, Cannabichromene, Cannabichromevarin, Cannabichromanone, -D4-acetoxycannabichromene, Cannabicitran, Cannabiripsol, Cannabicoumaronone, Cannabifuran, Cannabielsoin, Cannabielsoic acid, Cannabicyclol, Cannabinodiol, bornyl/epi-bornyl-Δ9-tetrahydrocannabinlate Cannabinol, Cannabitriol, Cannabimovone, and Cannabioxepane |
[8,11,13,24,25,26,27,28,29,30,31] |
| Non-Cannabinoid Constituents | ||||
| 2 | Non-cannabinoid phenol: -Stilbenoids -Spiro-indans -Phenanthrenes -Lignans, lignanamides, and phenolic amides |
Leaves, flowers, stem, hemp pectin, resin, fruit, seed, and root | Dihydrostilbenes, Dihydrophenathrenes, Cannabistilbene, Canniprene, Cannithrene, Denbinobin, Phloroglucinol β-D-glucoside, Cannabispiran, Cannabispirone, Cannabispirenone, Cannabispirol, Cannabispirketal, a-cannabispiranol-4′-O-β-glucopyranose, prenylspirodienone, 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene, Cannabisin A–O 4,7-dimethoxy-1,2,5-trihydroxyphenathrene, 5-methyl-4-pentyl-2,6,2-trihydroxybiphenyl, 5-methyl-4-pentylbiphenyl-2,2,6-triol, N-trans-coumaroyltyramine, N-trans-feruloyltyramine, Ntrans-caffeoyltyramine, 3,3′-demethylheliotropamide, and Grossamide. |
[8,13,28,32,33,34] |
| 3 | Terpenoids (Terpenes): -Monoterpenes -Sesquiterpenes -Diterpenes -Triterpene, |
Essential oils of fresh and dried leaves, flowers, stembarks, and roots | α-pinene, β-pinene, linalool, linalool oxide, myrcene, limonene, camphene, α-terpinene, γ-terpinene, α-terpinolene, α-terpineol, terpinene-4-ol, sabinene, sabinene hydrate, cis-sabinene hydrate, α-phellandrene, β-phellandrene, 2-methyl-2-heptene-6-one, borneol, piperitenone, geraniol, carvacrol, carvone, cis-carveol, citronellol, bornyl acetate, ipsdienol, germacrene-B, clovandiol, α-bisabolol, β-eudesmol, γ-eudesmol, α-caryophyllene, β-caryophyllene oxide, α-Humulene, Phytol, neophytadiene, friedelin (friedelan-3-one), epifriedelanol, β-amyrin, Vomifoliol, dihydrovomifoliol, and dihydroactinidiolide β-ionone |
[8,11,13,28,29,35,36] |
| 4 | Flavonoids: -Methylated -Glycosylated (C or O glycosides) -Prenylated -Geranylated |
Leaves, flowers, seed, and fruit | Orientin, Vitexin, Isovitexin, Apigenin, Luteolin, Kaempferol, Quercetin, Cytisoside, Cytisoside glucoside, Canniflavone (Cannflavin), Naringenin, and Naringin | [8,11,13,28,29,35,37,38,39] |
| 5 | Sterols | Stembarks, roots, and leaves | Campsterol, Stigmasterol, and β- Sitosterol | [11,40] |
| 7 | Alkaloids | Roots, leaves, stembark | Cannabisativine and Anhydrocannabisativine | [13,40] |
| 8 | Fatty acids: -Saturated and unsaturated fatty acids and their esters |
Seeds | Roughanic acid, Stearidonic acid, α-linolenic acid, and oxylipins. | [8,28] |
| 9 | Hydrocarbons (n-alkane) |
- | Δ9-Tetrahydrocannabiorcolic acid | [8] |
* S/N = Serial Number.
Figure 2, Figure 3 and Figure 4 below show the structures of the different classes of bioactive compounds isolated from Cannabis sativa [13,29,41].
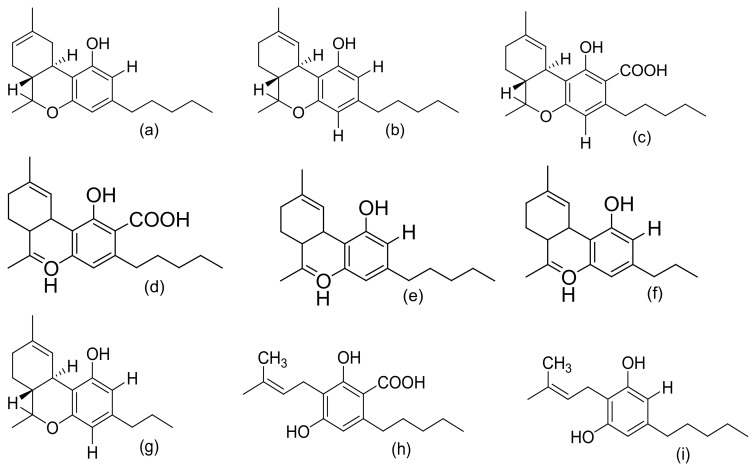
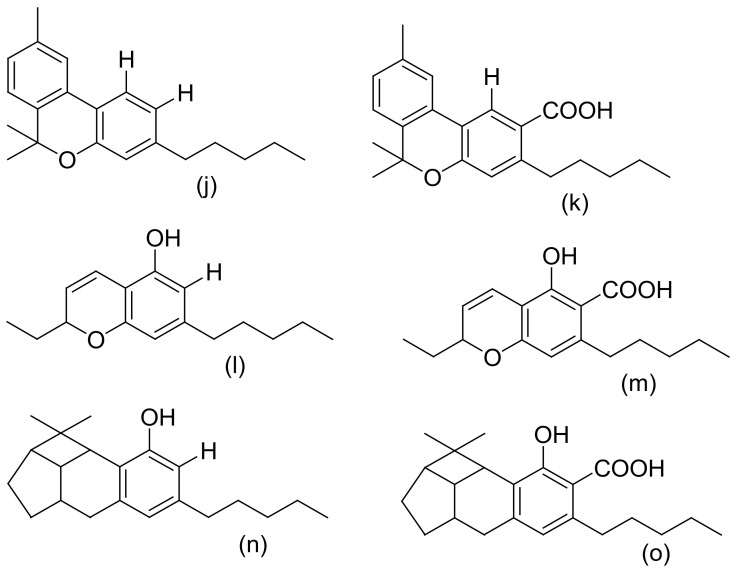
Figure 2.
Chemical structures of major cannabinoids; Δ8 THC, tetrahydrocannabinol (a); Δ9-THC, tetrahydrocannabinol (b); THCA, tetrahydrocannabinolic acid (c); CBDA, cannabidiolic acid (d); CBD, cannabidiol (e); CBDV, cannabidivarin (f); THCV, tetrahydrocannabivarin (g); CBGA, cannabigerolic acid (h); CBG, cannabigerol (i); CBN, cannabinol (j); CBNA, cannabinolic acid (k); CBC, cannabichromene (l); CBCA, cannabichromenic acid (m); CBL, cannabicyclol (n); CBLA, cannabicyclolic acid (o). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
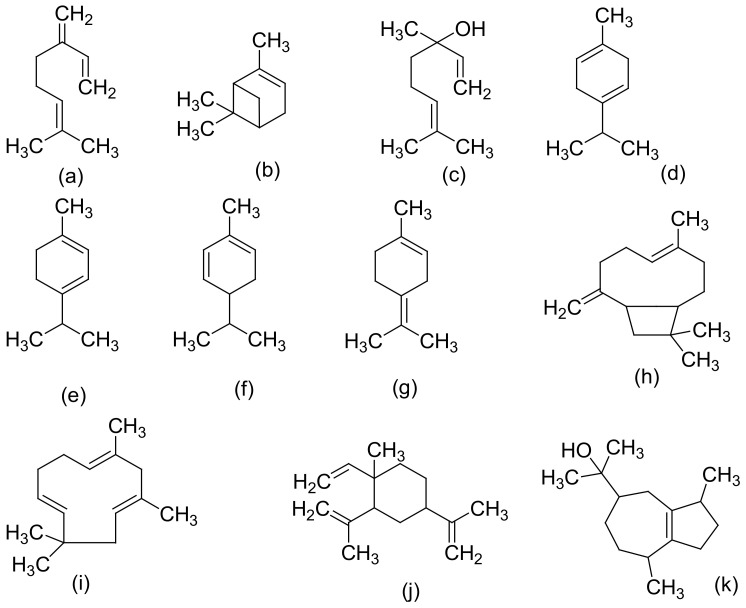
Figure 3.
Chemical structures of some Cannabis sativa terpenes (Monoterpenes, Sesquiterpenes, and Triterpenoids); Mycene (a), α-Pinene (b), D-Linalool (c), Limonene (d), α-Terpinene (e), α-Phellandrene (f), α-Terpinolene (g), β-Caryophyllene (h), α-Caryophyllene (i), β-Elemene (j), Guaiol (k), Friedelin (l), and Epifriedelanol (m). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
Figure 4.
Chemical structures of some non-cannabinoid phenols (Spirans, phenanthrenes, flavonoids, alkaloids); Cannabispiran (a), Cannabispirone (b), Canniprene (c), Cannithrene I (d), Cannithrene II (e), Debinobin (f), Canniflavin A (g), Canniflavin B (h), Cannabisativine (i), and Anhydrocannabisativine (j). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
4.2. Extraction, Isolation, and Chemical Characterization of C. sativa
Many methods have been reported for the extraction of Cannabis in the literature. These include direct maceration (DM), soxhlex extraction, ultrasound-assisted extraction (UAE), supercritical fluid extraction, and microwave-assisted extraction (MAE) [41]. However, two methods of extracting Cannabis are differentiated in the literature [41]. The first is the maceration of the plant material in an organic solvent (direct maceration) and the subsequent removal of the solvent by the concentration of the extract under reduced pressure [41]. The second is the innovative supercritical fluid extraction (SFE) method, which involves the use of pressurized solvents [41]. It is necessary for cannabinoid compounds to be extracted with organic solvents instead of water, because the active compounds are less soluble in polar solvents [41]. The most commonly used solvents are ethanol, ether, chloroform, and methanol [42]. When used for extraction, various compounds, including some undesired substances, dissolve together with the cannabinoids [42]. The high solvent power of ethanol for cannabinoid compounds is the reason why it is frequently used in home-made extracts of Cannabis [41]. However, non-desired compounds (chlorophyll, lipids, and waxy materials) are also extracted which, therefore, requires further steps to remove the co-extracted impurities for a high-purity medicinal product to be obtained [41]. A patent on the method for the isolation of herbal and cannabinoid medicinal extracts stated that the solubility of non-therapeutic substances (chlorophyll and waxy materials) is reduced when the solvent is selected from a group that includes acetonitrile, benzene, dichloromethane, diethyl ether, acetone, butanol, ethanol, chloroform, ethyl acetate, hexane, pentane, propanol, tetrahydrofuran, toluene, xylene, and various combinations of these solvents [41]. The International Conference on Harmonization (ICH) recommends the use of less toxic solvents in the manufacture of drug substances and dosage forms, and sets pharmaceutical limits for residual solvents in drug products [43]. Residual solvents pose risks to human health and are classified into three classes. Class 1 solvents (including carbon tetrachloride, benzene, and methyl chloroform) are regarded as human carcinogens and are environmentally hazardous [41]. Class 2 solvents include methanol and hexane, which are generally said to be limited, and they are possible causative agents of irreversible toxicity, such as neurotoxicity or teratogenicity [41]. Class 3 solvents (ethanol and ethyl acetate) are generally regarded as having a low toxic potential to humans [41]). Above all, ethanol is generally recognized as a safe (GRAS) solvent [41]. In a study by Brighenti et al., they compared the following four extraction techniques to obtain a high yield of medicinal cannabinoids: ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), and direct maceration (DM). They concluded that DM, with ethanol as the extraction solvent at room temperature for an overall time of 45 min, is the best extraction technique (in terms of a high yield) for non-psychoactive cannabinoids from hemp [44].
Over the last decade, compounds in Cannabis have been identified, isolated, and determined by various chromatographic techniques with different spectroscopic detection methods. C. sativa samples are analyzed for both legal and medicinal purposes [41]. Nevertheless, the knowledge of their exact composition remains very significant. In 2009, recommended methods for the identification and analysis of cannabis products were released by the United Nations Office on Drugs and Crime [45]. One notable technique that has been employed in identifying the diverse composition of the compounds found is high-performance liquid chromatography (HPLC) [41]. Spectroscopic approaches or methods are based on the variable absorbance or redirection of electromagnetic (EM) radiation by chemical bonds, resulting in the radiation or transition of the sample’s atoms to a higher energy state [46]. Some advantages are attributed to these spectroscopic methods, such as permitting spatial measurements of metabolites and offering a global metabolic fingerprint of a sample with rapid spectral acquisition [46]. Some of these approaches/methods include Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), HPLC, gas chromatography–mass spectrometry (GC–MS), and liquid chromatography–mass spectrometry (LS–MS) [41,46]. Taking into account the recommended methods and the mandatory requirement of the Ministerial decree to use only chromatographic techniques coupled with mass spectrometric detection, cannabinoid concentrations and its stability in cannabis tea and cannabis oil, prepared from standardized flowering tops obtained from the Military Pharmaceutical Chemical Works of Florence, were studied by Pacifici et al. using easy and fast ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) [9,10]. Table 2 is a summary of the reported extraction solvents, as well as the identification, isolation, and characterization methods of Cannabis.
Table 2.
Reported methods of identification, isolation, and characterization of C. sativa.
| Extraction Solvent(s) | Matrix and Species | Identification, Isolation, and Purification Methods | Elucidation/Analytical Techniques | Analytes | References |
|---|---|---|---|---|---|
| Phenolic Cannabinoids | |||||
| Methanol/chloroform mixture. | C. sativa inflorescence | Qualitative, quantitative, and comparative derivatization study of cannabinoids | Fast GC–MS | CBDA, CBGA, CBG, CBD, THC, Δ8-THC, CBC, THCA, THC | [47] |
| Supercritical fluid extraction | Plant biomass and medicinal Cannabis resin | Quantitative and qualitative analysis of cannabinoids | UHPLC–DAD and statistical analysis | CBDA, THCA, CBD, CBN, CBC, THC | [48] |
| Methanol/chloroform solvent mixture | Cannabis flower samples | Qualitative and quantitative measurement of cannabinoids | HPLC–DAD | Δ9-THC, CBD, CBDA, THCA, CBN, CBG, CBGA, Δ8-THC | [49] |
| Ethanol/ethanolic extracts | (i) Lebanese C. sativa (ii) Cannabis (iii) Decarbo-xylated hemp leaves |
(i) Purified by counter-current distribution and silica gel chromatography (ii) Florisil and silica gel column chromatography |
GCMS, IR, and 1H NMR comparison with an authentic sample | Cannabielsoin, (+)-trans-CBT and (−)-trans -CBT-OEt-C5(Cannibitriol), Cannabicitran, and Monomethylether of CBD | [13,50,51,52] |
| Sequential extraction (hexanes, CH2Cl2, EtOAc, EtOH, EtOH/H2O, and H2O) | (i) Bud and leaves of high-potency variety of C. sativa | (i) Silica gel VLC, C18-solid phase extraction (SPE), and HPLC (ii) VLC chromatography of hexane extract, TLC, flash silica gel, Sephadex LH-20 chromatography, and semipreparative reversed-phase (RP) and chiral HPLC |
HRESIMS, 1D and 2D NMR, GC–MS | epi-bornyl Δ9-tetrahydrocannabinolate, α-terpenyl Δ9-tetrahydrocannabinolate, 4-terpenyl Δ9-tetrahydro-cannabinolate, α-cadinyl Δ9-tetrahydrocannabinolate, γ-eudesmyl Δ9-tetrahydro-cannabinolate, γ-eudesmyl cannabigerolate, 4-terpenyl cannabinolate, bornyl Δ9-tetrahydrocannabinolate, α-fenchyl Δ9-tetrahydro-cannabinolate, α-cadinyl cannabigerolate, Δ9-tetrahydro-cannabinol (Δ9-THC), Δ9-tetrahydrocannabinolic acid A (Δ9-THCA), Cannabinolic acid A (CBNA), and Cannabigerolic acid (CBGA), (±)-6,7-trans-epoxycannabigerolic acid, (+)-6,7-cis-epoxycannabigerolic acid, (±)-6,7-cis-epoxycannabigerol, 5′-Methoxy-cannabigerolic acid, 5′-methyl-4-pentylbiphenyl-2, 2′, 6-triol,7-methoxy-cannabispirone, (±)-6,7- trans-epoxycannabigerol, 8α-hydroxy-(−)-Δ9-trans-tetrahydrocannabinol, 8b-hydroxy-(−)- Δ9-trans-tetrahydro cannabinol, 8-oxo-(−)-Δ9-trans-tetrahydrocannabinol, 10α-hydroxy-Δ8-tetra-hydrocannabinol, 10β-hydroxy- Δ8-tetra-hydrocannabinol, 10a-α-hydroxy-10-oxo-Δ8-tetrahydrocannabinol, (±)-4-acetoxycannabichromene, (±)-3″-hydroxy-Δ(4″, 5″)-cannabichromene,(−)-7-hydroxycannabichromane,(−)-7 R-cannabicoumarononic acid A, 5-acetyl-4-hydroxycannabigerol, 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol, 8-hydroxycannabinol, 8-hydroxycannabinolic acid A, and 2-geranyl-5-hydroxy-3-n-pentyl-1, 4-benzoquinone, (±)-4-acetoxycannabichromene, (±)-3”-hydroxy- Δ4”-cannabichromene, (–)-7-hydroxycannabichromane, 8-hydroxycannabinol, 8-hydroxy cannabinolic acid, 10S-hydroxy-cannabinol, 9b,10b-epoxyhexahydrocannabinol, 9a-hydroxyhexahydrocannabinol, 10a-hydroxyhexahydrocannabinol, and 10aRhydroxyhexahydrocannabinol | [31,53,54,55] |
| Hexane extract | (i) C. sativa (air-dried and powdered buds) (ii) high-potency variety of C. sativa (iii) Illicit Cannabis samples (iv) C. sativa inflorescence (strain CINRO) (v) Lebanese C. sativa (vi) Hemp (vii) Nepalese and Brazilian C. Sativa. |
(i) Column chromatography using silica or alumina, TLC, then fractional distillation and preparative C18 HPLC (ii) VLC (vacuum liquid chromatography) silica gel column chromatography, C18 HPLC and chiral HPLC (iii) Flash silica gel chromatography (iv) Florisil column chromatography |
1H NMR, 13C NMR (2D NMR) HRESIMS, circular dichroism (CD), UV, LC-HRMS, MS/MS, GC–MS, and confirmation by phytochemical transformations. | Δ9-THC, Δ9-THC aldehyde, Cannabinoid esters, Cannabisol, Δ9-trans-tetrahydrocannabiphorol, Δ9-trans-tetrahydrocannabihexol, Cannabidiorcol, Cannabidihexol (CBDH) and Cannabidiphorol (CBDP), Cannabitwinol, Cannabinodivirin and cannabinodiol (CBND), Cannabichromene (CBC), 9a-hydroxyhexahydrocannabinol, 7-oxo-9a-hydroxyhexa-hydrocannabinol, 10a-hydroxyhexahydrocannabinol, and 10a-R-hydroxyhexahydrocannabinol | [53,56,57,58,59,60] |
| Ethyl acetate extracts | Cannabis resins, tinctures, and leaves | - | GC–MS and GC–FID analysis | Δ9-THC and Δ9-THCA | [61] |
| Petroleum ether | (i) Cannabis tincture of Pakistani origin (ii) Brazilian C. sativa (iii) Cannabis leaves and flowers (Maryland and Czechoslovakian origin) (iv) Congo C. sativa (v) Hashish and Cannabis sativa |
(i) Silicic acid column chromatography (ii) Silica gel and Florisil chromatography, preparative TLC |
IR, NMR, MS, GC–MS confirmed by synthesis | Δ9-THCV, Δ9-THCO or Δ9-THC, Δ8-THC (Δ8-THCA), Cannabielsoin acid A (CBEAA,), Cannabielsoin acid B, and Cannabicyclovarin (CBLV) | [61,62,63,64,65,66,67] |
| Benzene | (i) Fresh C. sativa leaves from Thailand (ii) Fresh tops and leaves of C. sativa (iii) Hemp |
Polyamide and silica gel column chromatography | IR, UV, NMR, and comparing UV spectrum with that of derivatives | Δ9-THCVA, CBDV, THCV, CBCV, Cannabigerovarin CBGV, cannabigerovarinic acid (CBGVA), CBDA, cannabidivarinic acid (CBDVA), Cannabicyclolic acid (CBLA), and Cannabichromenic acid (CBCA) | [49,68,69] |
| Acetone extract | (i) Leaves of C. sativa (Mexican strain) (ii) Wax of decarboxylated aerial parts of C. sativa (Carma strain) (iii) Cannabis variety (carmagnola) |
(i) Silica gel column chromatography (ii) Silica and alumina column chromatography, followed by normal phase (NP)-HPLC. (iii). Flash chromatography, over reverse-phased C18 silica gel followed by normal-phase HPLC |
FAB–MS, 1H-NMR, 13C-NMR), and ESI–MS semisyn-thesis. | Cannabigerolic acid (CBGA), dihydroxycannabigerol derivative (camagerol), Sesquicannabi-gerol, Cannabimovone, and Cannabioxepane | [70,71,72,73] |
| Essential/volatile oils | |||||
| Methanol dilutions | Cannabis sativa oil samples | Separation/quantitation of cannabinoids | Fast-GC–FID | CBD, CBN, and THC | [74] |
| Essential oil | Fresh C. sativa L. from India | Fractional distillation and chromatography over alumina. | GC–MS and physico–chemical analyses | α-terpinene, β-phellandrene, γ-terpinene, α-terpinolene, α-pinene, β-pinene, camphene, linalool, α-terpineol, terpinene-4-ol, linalool oxide, and sabinene hydrate | [13] |
| Volatile/essential oils | (i) Cannabis (Dutch and Turkish) (ii) Fresh leaves of Cannabis sativa and Cannabis indica |
(i) Hydrodistillation or through nitrogen extraction (ii) Hydrodistillation, steam distillation, and supercritical fluid extraction |
Capillary gas chromato-graphy, GC–MS analysis |
cis-β-ocimene, trans-β-ocimene, α-phellandrene, D3-carene, Δ4-carene, sabinene and α-thujene, caryophyllene, humulene, trans-β-bergamotene, cis-β–farnesene, δ–limonene, carophyllene oxide, linalool, trans-α- bergamotene, cis- β -farnesene, menthol, eucalyptol, and Carvone. | [13,75] |
| Essential oil | Cannabis (marijuana fresh and dried buds) | Steamdistillation | GC–MS and GC–FID | Ipsdienol, cis-carveol, and cis-sabinene hydrate | [76] |
| Essential oil | C. sativa resin | Minor terpenic component analysis | GC–MS and GC retention time | α-gurjunene, α-bisabolol, α-cedrene, α-cubebene, δ-cadinene, epi-β-santalene, farnesol, γ-cadinene, γ-elemene, γ-eudesmol, guaiol, (E,E)-α-farnesene, (Z)-β-farnesene, and farnesyl acetone | [77] |
| Essential oil | Cannabis | Steam distillation and silica gel chromatography | GC, GC–MS | eugenol, methyleugenol, iso-eugenol, trans-anethol, and cis-anethol (simple phenols) | [78] |
| Essential oil | C. sativa | Column chromatography of the essential oil | GC and GC–MS analyses | Iso-caryophyllene, β-selinene, selina-3,7(11)-diene, and selina-4(14),7(11)-diene | [13,79] |
| Non-cannabinoid phenols | |||||
| Ethanol/ethanolic extract | (i) South African Cannabis variant (ii) Saudi Arabia hashish (iii) Leaves of C. sativa (iv) High-potency Cannabis variety grown in Mississippi (v) Hemp pectin (vi). Cannabis roots (vii) Roots, stem, and leaves of a Mexican variant of Cannabis sativa |
(i) Partitioning and chromatography on silica and polyamide columns (ii) Normal and reversed phase chromatographic techniques (iii) Purification by macro reticular resin, silica gel column chromatography, and Sephadex-LH-20 (iv) TLC, chromatography over alumina, and recrystallization (v) Partitioning and TLC eluted with chloroform:acetone:ammonia (1:1:1) (vi) Series of acid–base extractions and silica-gel chromatography followed by crystallization of the alkaloid from acetone |
IR, GCMS, UV, 1D NMR (1H NMR, 13C NMR) and 2DNMR (COSY, HSQC, HMBC, and ROESY), ESI–MS, comparison with authentic samples, X-ray crystallography, and semi-synthesis | β-cannabispiranol, b-cannabispirol, 5-hydroxy-7-methoxyindan-1-spiro-cyclohexane, 7-hydroxy-5-methoxyindan-1-spiro-cyclohexane, and 5,7-dihydroxyindan-1-spiro-cyclohexane, Cannabispirketal and the glycoside, a-cannabispiranol-4′-O-β-glucopyranose, 3,4′,5-trihydroxy-dihydrostilbene, 4,5-dihydroxy-2,3,7-trimethoxy-9,10-dihydrophenanthrene, 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene and 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene, Rutin, friedelin (friedelan-3-one) and epifriedelanol, Anhydrocannabisativine and cannabisativine, α,α′-dihydro-3′,4,5′-trihydroxy-4′-methoxy-3-isopentenylstilbene, α,α′-dihydro-3,4′,5-trihydroxy-4-methoxy-2,6-diisopentenylstilbene, α,α′-dihydro-3′,4,5′-trihydroxy-4′-methoxy-2′,3-diisopentenylstilbene, α,α′-dihydro-3,4′,5-trihydroxy-4,5′-diisopentenylstilbene, and combretastatin B-2 | [32,33,55,80,81,82,83,84] |
| Benzene | Dried leaves of Japanese cannabis | Chromatographed on a polyamide column followed by silica gel chromatography | IR, 1H NMR, MS, UV. | Cannabispirol and acetyl Cannabispirol | [85] |
| Acetone | C. sativa (CARMA) | Gravity column chromatography on silica gel and purified by crystallization from ether and HPLC | Identified according to its physical and spectroscopic properties and synthesis | Debinoben (1,4-phenanthrenequinone) |
[86] |
| Sequential extraction (Hexane, EtOAc, CH2Cl2, EtOH, EtOH/H2O, and H2O | High-potency variety of C. sativa (Mississippi) | VLC, silica gel column chromatography, and RP–HPLC | 1D and 2D NMR, IR analysis | acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone, 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene, 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene, 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene, Cannflavin C and β-sitosteryl-3-O-β-d-glucopyranoside-2′-O-palmitate, α-cannabispiranol, Chrysoeriol, 6-prenylapigenin, and Cannflavin A and β-acetyl cannabispiranol | [55] |
| Hexane extract | Leaves of Cannabis sativa | Isolation by normal-phase chromatography followed by C18-HPLC | NMR and ESI–MS analysis | Prenylspirodinone and 7-O-methyl-cannabispirone | [55,87] |
| Dichloro-methane extract | (i) Decarboxy-lated C. sativa hemp (ii). Thai Cannabis sativa leaves (iii) Panamanian variety of cannabis |
C18 flash chromatography, followed by silica gel gravity column chromatography and HPLC | HR–ESIMS and NMR (1H, 13C, HSQC, and HMBC) data, X-ray crystallography, and confirmation by hydrogenation | Isocannabispiradienone and a-Cannabispiranol, Cannabispira-dienone, and Cannabidihydro-phenanthrene (Cannithrene1 and Cannithrene2) |
[88,89] |
| Methanol/methanolic extract | (i) Branches and leaves of hemp (ii) Pollen grains of Mexican variety of C. sativa (iii) Dried leaves of South African and Indian cannabis sativa (iv) Panamanian variant of C. sativa (v) Leaves and branches of C. sativa |
(i) TLC, silica gel column chromatography, normal-phase preparative HPLC, and Sephadex LH-20 column chromatography (ii) Partitioning, silica gel chromatography, Sephadex LH-20 chromatography, semi preparative LC |
MS, 1D and 2D NMR, UV experiments, IR, X-ray crystallography and confirmation by total synthesis | Rutin, Quercetin-3-O-α-L-rhamnoside, kaempferol-3-O-α-L-rhamnoside, apigenin-7-O-α-L-rhamnoside, apigenin-7-O-β-D-glucopyranoside, luteolin-7-O-β-D-glucopyranoside, 1,3,6,7-tetrahydroxyl-2-C-β-D-glucopyranosyl xanthone, vitexin, orientin, apigenin-6,8-di-C-β-D-glucopyranoside, vitexin-2″-O-α-L-rhamnoside, orientin-2″-O-β-D-glucopyranoside, quebrachitol, inositol and uracil, kaempferol-3-O-sophoroside (196) and quercetin-3-O-sophoroside, cannabispirone; cannabispirenone, Cannabispiran, Isocannabispiran Canniprene, Cannabistilbene I and Cannabistibene II, and 2,3,5,6-tetramethoxy 9,10-dihydrophenanthrenedione | [90,91,92,93,94] |
| Mixture of hydro-alcoholic and organic solvents | C. sativa inflorescence (Ferimon, Uso-31, Felina 32, and Fedora 17 cultivars) | Metabolic and chemical profiling to identify and quantify compounds of different classes | NMR, GC–MS, UHPLC, and HPLC–PDA | Sugars, organic acids, amino acids, cannabinoids, terpenoids, phenols, tannins, flavonoids (Quercetin, Naringenin, and Naringin) and biogenic amines | [35,37] |
| Diethyl ether | Stem exudate (greenhouse-grown C. sativa) | TLC and acid hydrolysis of the exudate | 1H NMR and GC–MS | Phloroglucinol β-D-glucoside | [95] |
4.3. Biological Evaluation/Potentials of C. sativa
From the biological point of view, the psychoactive cannabinoids reported include Δ9 THC, cannabinol (CBN), and cannabinodiol (CBND), while cannabidiol (CBD) and other cannabinoids are non-psychoactive [8,11]. THC is the major psychoactive component and the toxicity of this metabolite of Cannabis is the most studied [11,28]. Its psychoactive component decreases in the order of inflorescence (the flower), leaves, stem, roots, and seeds, respectively [8]. The interest in the potential medical use of cannabis and cannabinoids rose significantly in the 1990s, following the discovery of the endocannabinoid (eCB) system in mammals [7]. The physiological effects of cannabinoids are exerted through various receptors, such as the cannabinoid receptors (CB1 and CB2), adrenergic receptors, and the recently discovered GPCRs (GPR55, GPR3 and GPR5) [8]. Historically, each part of the Cannabis plant is indicated mostly for pain killing, inflammation, and for mental illnesses. For example, the Cannabis root has been recommended for treating fever, inflammation, gout, arthritis, and joint pain, as well as skin burns, hard tumors, postpartum hemorrhage, difficult child labor, sexually transmitted diseases, gastrointestinal activity, and infections [40]. Cannabis has also been used to treat asthma, epilepsy, fatigue, glaucoma, insomnia, nausea, pain, and rheumatism, as well as being used as appetite stimulant and a digestive aid [7,11,13]. Since concentrations above 0.05% are pharmacologically interesting, Cannabis inflorescence and leaf material may contain sufficient cannabinoids, mono- and sesquiterpenoids, and flavonoids for therapeutic applications [11]. Cannabis terpenoids and flavonoids, mainly myrcene, limonene, pinene, β-caryophyllene, and cannflavin A, act in synergy with cannabinoids to induce pharmacological effects [7]. It was proven that these compounds, which are synthetized in the aerial parts of the plant, enhance CBD’s anti-inflammatory effects and antagonize THC dysphoric action [96]. Cannabidiol (CBD) and Cannabidavarin (CBDV) (neutral cannabinoids) have been reported to have the therapeutic potential for the treatment of epilepsy (focal seizures), as well as treating nausea and vomiting [97,98]. Conversely, THC and CBN have been found to be active in lowering intraocular pressure, and can be applied in all cases of glaucoma that are resistant to other therapies [9]. Cannflavin A and B are also notable flavonoids (prenylflavonoids) with medicinal potentials, such as their anti-inflammatory, anti-neoplastic, antioxidant, neuro-protective, anti-parasitic, and anti-viral effects [99]. Table 3, below, shows a summary of the reported bioactivities (biological potentials) of the bioactive compounds present in Cannabis sativa.
Table 3.
Summary of reported bioactivities associated with isolated compounds and essential oils from Cannabis sativa.
| Isolated Bioactive Compound | Bioactivity/Uses | References |
|---|---|---|
| Tetrahydrocannabinol THC | Antioxidant, anti-pruritic, and anti-inflammatory effects | [29,100] |
| Cannabidiol CBD | Anti-convulsive, anti-inflammatory, immunosuppressive properties, antioxidant, and anti-psychotic effects | [101,102,103] |
| Cannabigerol CBG | Anti-fungal effects, anti-cancer, anti-depressant, mild anti-hypertensive agent, analgesic, and anti-erythemic effects | [29,104] |
| Cannabichromene CBC | Anti-inflammatory and analgesic | [29] |
| Cannabinol CBN | Sedative, anti-convulsant, anti-inflammatory, antibiotic, and anti-MRSA activity | [29] |
| Tetrahydrocannabivarin THCV | Anti-convulsant | [29] |
| Tetrahydrocannabinolic acid THCA | Immunomodulatory, anti-inflammatory, neuroprotective, anti-neoplastic activity, and antiemetic effects | [29,105] |
| Cannabidavarin CBDV | Anti-convulsant (anti-epileptic) properties and anti-emetic properties | [106,107] |
| Cannabidiolic acid CBDA | Anti-emetic effects | [104,108,109,110] |
| β-Myrcene | Anti-inflammatory and analgesic sedative agent | [7,29,111] |
| D-Limonene | Strongly anxiolytic, anti-depressant, antibiotic, and anti-cancer agent | [7,29] |
| β-Ocimene | Anti-convulsant activity, anti-fungal activity, anti-tumor activity, and pest resistance | [112,113] |
| γ-Terpinene |
Anti-inflammatory activity, antioxidant, and anti-proliferative activity | [114,115] |
| α-Terpinene | Antioxidant | [29] |
| α-Pinene | Anti-inflammatory, bronchodilator, anti-microbial, and anxiolytic effects |
[7,11,29,116] |
| Linalool | Analgesic and anticonvulsant, anxiolytic, anti-depressant, anti-glutamatergic, anti-leishmanial activity, anticancer agent, anti-nociceptive, and anti-depressant effects | [111,117,118,119] |
| α-Phellandrene | Anti-nociceptive, anti-depressant, anti-arthritic and allergic, and anti-hyperalgesic effects | [120,121,122] |
| Terpinolene | Anti-fungal and larvicidal, anti-nociceptive, anti-inflammatory antioxidant, and anti-cancer effects | [123,124] |
| β-Caryophyllene | Cardio-protective, hepato-protective, gastro-protective, neuro-protective, nephro-protective, antioxidant, anti-inflammatory, anti-microbial, anti-pruritic, and immunomodulatory activities | [7,125,126,127] |
| Caryophyllene Oxide | Anti-fungal, insecticidal/anti-feedant, and anti-platelet effects | [29] |
| β-Elemene | Anti-cancer and anti-tumor | [128] |
| Guaiol | Anti-inflammatory, antioxidant, anti-cancer anti-rheumatic, antiseptic, diaphoretic, diuretic, and laxative effects |
[29,129] |
| Friedelin | Anti-inflammatory, anti-pyretic, and anti-tuberculosis agent | [130,131] |
| Epifriedelanol | Antioxidant | [132] |
| Cannflavin A and B | Anti-inflammatory, neoplastic, antioxidant, neuroprotective, anti-parasitic, and anti-viral agent | [4,7,29,99] |
| Apigenin | Anxiolytic and estrogenic properties, anti-tumor, antioxidant, anti-inflammatory, anti-osteoporosis, and immune regulation effects | [4,133] |
| Vitexin and Isovitexin | Antioxidant, anti-cancer, anti-inflammatory, anti-diabetic, anti-microbial, anti-viral, anti-hyperalgesic, and neuroprotective effects | [134] |
| Quercetin | Anti-cancer/anti-proliferator, antioxidative/anti-aging, anti-viral, anti-inflammatory, cardio-protective, skin-protective, anti-coagulant, and anti-platelet effects | [135] |
| Luteolin | Neuroprotective effects, anti-inflammatory, and antioxidant effects | [136] |
| Lignans | Antioxidant, anti-viral, anti-diabetic, anti-tumorigenic, and anti-obesity activities | [4] |
Cannabis female flowering tops can be simply administered through commercially available vaporizers (e.g., Micro Vape, G Pen Herbal Vaporizer, and Volcano), buccal sprays (e.g., Sativex), oral capsules (e.g., Cannador), decoctions, or oils [7]. Only cannabis use through oral or inhalatory administration is allowed. Smoking reduces the bioavailability of cannabis ingredients by 40%, and its complete combustion can cause lung diseases and airway obstructions [7]. Homemade decoctions and pharmacy oils are currently the most widespread cannabis formulations in Europe, making the standardization of preparation difficult [7]. Cannabis pharmacological action is dose-dependent and can induce many adverse effects (AEs), principally related to THC, due to unintentional overdosing [7]. The typical symptoms of cannabis acute intoxication that have been reported are dizziness, confusion, tachycardia, postural hypotension, dysphoria, panic depression, hallucinations, allergic reactions, vomiting, and diarrhea [7,137,138]. Furthermore, withdrawal symptoms, such as irritability, aggression, anxiety, insomnia, decreased appetite, tremors, sweating, and headaches may appear after the abrupt cessation of the long-term administration of high doses of cannabis [7]. According to the ICH efficacy and safety guidelines, it is recommended to start with low doses and increase quantities after a satisfactory period of clinic evaluation, depending on the pharmacological effects and the possible adverse effects [139].
In the current COVID-19 pandemic, scientists are repurposing medicines (identifying new therapeutic use(s) of existing drugs) known for their biological potential (anti-viral or anti-inflammatory properties) to tackle the global issue and similar future viruses [140]. They have hypothesized that CBD could be used as an anti-viral agent [141] or anti-inflammatory [142,143] tool, or to inhibit pulmonary fibrosis in COVID-19 patients [144]. In addition, the known growing evidence of the anxiolytic effects of CBD have also been hypothesized to be used as a therapeutic option to treat long-lasting COVID-19-related anxiety and PTSD [145], which is likely to be a significant issue of the pandemic.
5. Conclusions
With the recent evaluation, acceptance, and legalization of Cannabis products for therapeutic purposes, researchers, particularly in the field of natural products, are challenged to improve and standardize the extraction and characterization of the bioactive compounds from Cannabis sativa. Despite various reports of its economic and therapeutic values, it is legal in a handful of jurisdictions (Uruguay, Canada, some US states, and parts of Africa). Presently, Cannabis remains illegal in several countries. This review summarized the biological potential and the techniques for the extraction, isolation, and characterization of Cannabis sativa compounds, and it describes the effectiveness of the various parts of the herb in pharmacotherapy. The usage of C. sativa roots and stem barks in present-day medical research, and the development of new Cannabis-based medicines or products, in contrast to the flowering part only, is highly recommended because they can be exploited for medicine and other uses. In addition, Cannabis-based pharmaceutical products must undergo long purification processes to eliminate unwanted components such as chlorophyll and residual organic solvents. The use of standardized reagents is also very crucial in the analytical studies of C. sativa. Furthermore, future research should seek to clarify the factors responsible for the complexity of C. sativa extracts in terms of their chemical compositions, the physical properties of their active ingredients, and their liability to photochemical oxidation.
Acknowledgments
The authors acknowledge the Directorate of Research and Innovation, Walter Sisulu University (WSU), South Africa and Govan Mbeki Research and Development Center, University of Fort Hare, South Africa.
Author Contributions
Conceptualization, A.E.O., A.O.O., G.U.O., O.O.O., M.G. and Y.S.H.; methodology, A.E.O., G.U.O., O.O.O., M.G., Y.S.H. and A.O.O.; validation, A.E.O., M.G., Y.S.H. and A.O.O.; data curation, A.E.O.; writing—original draft preparation, AEO; writing—review and editing, A.E.O., A.O.O., O.O.O., G.U.O., M.G. and Y.S.H.; supervision, A.O.O., G.U.O., O.O.O., M.G. and Y.S.H.; project administration, A.O.O.; funding acquisition, A.E.O. and A.O.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation (NRF), grant numbers 130205 and 137963.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013;10:210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhary N., Siddiqui M., Bi S., Khatoon S. Variation in preliminary phytochemicals screening of Cannabis sativa L. leaf, stem and root. Int. J. Pharmacogn. 2014;1:516–519. [Google Scholar]
- 3.Bandar H., Hijazi A., Rammal H., Hachem A., Saad Z., Badran B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am. J. Phytomed. Clin. Ther. 2013;1:507–513. [Google Scholar]
- 4.Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsohly M.A., Radwan M.M., Gul W., Chandra S., Galal A. Phytochemistry of Cannabis sativa L. Phytocannabinoids. 2017;103:1–36. doi: 10.1007/978-3-319-45541-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Baldino L., Scognamiglio M., Reverchon E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Pharm. Fluids. 2020;165:104960. doi: 10.1016/j.supflu.2020.104960. [DOI] [Google Scholar]
- 7.Brunetti P., Pichini S., Pacifici R., Busardò F.P., del Rio A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina. 2020;56:237. doi: 10.3390/medicina56050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis M.M., Yang Y., Wasilewski E., Clarke H.A., Kotra L.P. Chemical profiling of medical Cannabis extracts. ACS Omega. 2017;2:6091–6103. doi: 10.1021/acsomega.7b00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacifici R., Marchei E., Salvatore F., Guandalini L., Busardò F.P., Pichini S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017;55:1555–1563. doi: 10.1515/cclm-2016-1060. [DOI] [PubMed] [Google Scholar]
- 10.Pacifici R., Marchei E., Salvatore F., Guandalini L., Busardò F.P., Pichini S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018;56:94–96. doi: 10.1515/cclm-2017-0758. [DOI] [PubMed] [Google Scholar]
- 11.Jin D., Dai K., Xie Z., Chen J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-60172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farag S., Kayser O. Handbook of Cannabis and Related Pathologies. Academic Press; Cambridge, MA, USA: 2017. The Cannabis plant: Botanical aspects; pp. 3–12. [Google Scholar]
- 13.Radwan M.M., Chandra S., Gul S., Elsohly M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules. 2021;26:2774. doi: 10.3390/molecules26092774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens C.J., Murphy C., Roberts R., Lucas L., Silva F., Fuller D.Q. Between China and South Asia: A Middle Asian corridor of crop dispersal and agricultural innovation in the bronze age. Holocene. 2016;26:1541–1555. doi: 10.1177/0959683616650268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra S., Lata H., Khan I.A., Elsohly M.A. Cannabis sativa L.—Botany and Biotechnology. Springer; Berlin/Heidelberg, Germany: 2017. Cannabis sativa L.: Botany and horticulture; pp. 79–100. [Google Scholar]
- 16.Duvall C.S. A brief agricultural history of Cannabis in Africa, from prehistory to canna-colony. Echogéo. 2019;48:1–25. doi: 10.4000/echogeo.17599. [DOI] [Google Scholar]
- 17.Nsuala B.N., Enslin G., Viljoen A. “Wild Cannabis”: A review of the traditional use and phytochemistry of Leonotis leonurus. J. Ethnopharmacol. 2015;174:520–539. doi: 10.1016/j.jep.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Bonini S.A., Premoli M., Tambaro S., Kumar A., Maccarinelli G., Memo M., Mastinu A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018;227:300–315. doi: 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Dutch Passion. Understanding Cannabis Phenotypes, Genotypes and Chemotypes. 2020. [(accessed on 19 February 2022)]. Available online: https://dutch-passion.com/en/blog/understanding-cannabis-phenotypes-genotypes-and-chemotypes-n980.
- 20.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016;33:1357–1392. doi: 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- 21.Appendino G., Chianese G., Taglialatela-Scafati O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011;18:1085–1099. doi: 10.2174/092986711794940888. [DOI] [PubMed] [Google Scholar]
- 22.Hill A.J., Williams C.M., Whalley B.J., Stephens G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012;133:79–97. doi: 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Lewis-Bakker M.M., Yang Y., Vyawahare R., Kotra L.P. Extractions of medical Cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019;4:183–194. doi: 10.1089/can.2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsohly M., Gul W. Constituents of Cannabis sativa. Handb. Cannabis. 2014;3:1093. [Google Scholar]
- 25.Elzinga S., Fischedick J., Podkolinski R., Raber J.C. Cannabinoids and terpenes as chemotaxonomic markers in Cannabis. Nat. Prod. Chem. Res. 2015;3:1–9. [Google Scholar]
- 26.Hazekamp A., Tejkalová K., Papadimitriou S. Cannabis: From cultivar to chemovar II—A metabolomics approach to cannabis classification. Cannabis Cannabinoid Res. 2016;1:202–215. [Google Scholar]
- 27.Lynch R.C., Vergara D., Tittes S., White K., Schwartz C., Gibbs M.J., Ruthenburg T.C., Decesare K., Land D.P., Kane N.C. Genomic and chemical diversity in Cannabis. Crit. Rev. Plant Sci. 2016;35:349–363. doi: 10.1080/07352689.2016.1265363. [DOI] [Google Scholar]
- 28.Pollastro F., Minassi A., Fresu L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018;25:1160–1185. doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- 29.Russo E.B., Marcu J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017;80:67–134. doi: 10.1016/bs.apha.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Upton R., Elsohly M., editors. Cannabis Inflorescence: Cannabis Spp.; Standards of Identity, Analysis, and Quality Control. American Herbal Pharmacopoeia; Scotts Valley, CA, USA: 2014. [Google Scholar]
- 31.Radwan M.M., Elsohly M.A., El-Alfy A.T., Ahmed S.A., Slade D., Husni A.S., Manly S.P., Wilson L., Seale S., Cutler S.J., et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2015;78:1271–1276. doi: 10.1021/acs.jnatprod.5b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo T., Liu Q., Hou P., Li F., Guo S., Song W., Zhang H., Liu X., Zhang S., Zhang J., et al. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018;9:6608–6617. doi: 10.1039/C8FO01896K. [DOI] [PubMed] [Google Scholar]
- 33.Guo T.T., Zhang J.C., Zhang H., Liu Q.C., Zhao Y., Hou Y.F., Bai L., Zhang L., Liu X.Q., Liu X.Y., et al. Bioactive spirans and other constituents from the leaves of Cannabis sativa f. sativa. J. Asian Nat. Prod. Res. 2017;19:793–802. doi: 10.1080/10286020.2016.1248947. [DOI] [PubMed] [Google Scholar]
- 34.Yan X., Tang J., dos Santos Passos C., Nurisso A., Simoes-Pires C.A., Ji M., Lou H., Fan P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015;63:10611–10619. doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- 35.Ingallina C., Sobolev A.P., Circi S., Spano M., Fraschetti C., Filippi A., di Sotto A., di Giacomo S., Mazzoccanti G., Gasparrini F., et al. Cannabis sativa L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules. 2020;25:1908. doi: 10.3390/molecules25081908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menghini L., Ferrante C., Carradori S., D’antonio M., Orlando G., Cairone F., Cesa S., Filippi A., Fraschetti C., Zengin G., et al. Chemical and bioinformatics analyses of the anti-leishmanial and anti-oxidant activities of hemp essential oil. Biomolecules. 2021;11:272. doi: 10.3390/biom11020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Giacomo V., Recinella L., Chiavaroli A., Orlando G., Cataldi A., Rapino M., di Valerio V., Politi M., Antolini M.D., Acquaviva A., et al. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants. 2021;10:44. doi: 10.3390/antiox10010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weston-Green K. Recent Advances in Cannabinoid Research. IntechOpen; London, UK: 2018. The united chemicals of Cannabis: Beneficial effects of cannabis phytochemicals on the brain and cognition. [Google Scholar]
- 39.Smeriglio A., Galati E.M., Monforte M.T., Lanuzza F., D’angelo V., Circosta C. Polyphenolic Compounds and antioxidant activity of cold-pressed seed oil from finola cultivar of Cannabis sativa L. Phytother. Res. 2016;30:1298–1307. doi: 10.1002/ptr.5623. [DOI] [PubMed] [Google Scholar]
- 40.Ryz N.R., Remillard D.J., Russo E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017;2:210–216. doi: 10.1089/can.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez C.L., Fanovich M.A., Churio M.S. Cannabinoids: Extraction methods, analysis, and physicochemical characterization. Stud. Nat. Prod. Chem. 2019;66:143–173. [Google Scholar]
- 42.Płotka-Wasylka J., Rutkowska M., Owczarek K., Tobiszewski M., Namieśnik J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017;91:12–25. doi: 10.1016/j.trac.2017.03.006. [DOI] [Google Scholar]
- 43.International Conference on Harmonisation (ICH) International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline. Impurities: Guideline for Residual solventsq3c(R6) 2016. [(accessed on 20 January 2022)]. Available online: https://database.ich.org/sites/default/files/Q3C-R6_Guideline_ErrorCorrection_2019_0410_0.pdf.
- 44.Brighenti V., Pellati F., Steinbach M., Maran D., Benvenuti S. Development of a new extraction technique and hplc method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp) J. Pharma. Biomed. Anal. 2017;143:228–236. doi: 10.1016/j.jpba.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 45.Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products. 2009. [(accessed on 1 November 2021)]. Available online: http://www.unodc.org/documents/scientific/ST-NAR-40-Ebook.pdf.
- 46.Allwood J.W., Ellis D.I., Goodacre R. Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiol. Plant. 2008;132:117–135. doi: 10.1111/j.1399-3054.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 47.Cardenia V., Toschi T.G., Scappini S., Rubino R.C., Rodriguez-Estrada M.T. Development and validation of a fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018;26:1283–1292. doi: 10.1016/j.jfda.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rochfort S., Isbel A., Ezernieks V., Elkins A., Vincent D., Deseo M.A., Spangenberg G.C. Utilisation of design of experiments approach to optimise supercritical fluid extraction of medicinal Cannabis. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-66119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel B., Wene D., Fan Z.T. Qualitative and quantitative measurement of cannabinoids in Cannabis using modified hplc/dad method. J. Pharma Biomed. Anal. 2017;146:15–23. doi: 10.1016/j.jpba.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Bercht C., Lousberg R., Küppers F., Salemink C., Vree T., van Rossum J. Cannabis: VII. Identification of cannabinol methyl ether from hashish. J. Chromatogr. Anal. 1973;81:163–166. doi: 10.1016/s0021-9673(01)82332-3. [DOI] [PubMed] [Google Scholar]
- 51.Elsohly M., El-Feraly F., Turner C. Isolation and characterization of (+) cannabitriol and () 10 ethoxy 9 hydroxy delta 6a tetrahydrocannabinol: Two new cannabinoids from Cannabis sativa L. extract. Lloydia. 1977;40:275–280. [PubMed] [Google Scholar]
- 52.Shoyama Y., Kuboe K., Nishioka I., Yamauchi T. Cannabidiol monomethyl ether. A new neutral cannabinoid. Chem. Pharm. Bull. 1972;20:2072. doi: 10.1248/cpb.20.2072. [DOI] [Google Scholar]
- 53.Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Khan I.A., Elsohly M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry. 2015;117:194–199. doi: 10.1016/j.phytochem.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Zulfiqar F., Elsohly M.A. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008;71:536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radwan M.M., Elsohly M.A., Slade D., Ahmed S.A., Wilson L., El-Alfy A.T., Khan I.A., Ross S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry. 2008;69:2627–2633. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chianese G., Lopatriello A., Schiano-Moriello A., Caprioglio D., Mattoteia D., Benetti E., Ciceri D., Arnoldi L., de Combarieu E., Vitale R.M., et al. Cannabitwinol, a dimeric phytocannabinoid from hemp, Cannabis sativa L.; is a selective thermo-TRP modulator. J. Nat. Prod. 2020;83:2727–2736. doi: 10.1021/acs.jnatprod.0c00668. [DOI] [PubMed] [Google Scholar]
- 57.Citti C., Linciano P., Russo F., Luongo L., Iannotta M., Maione S., Laganà A., Capriotti A.L., Forni F., Vandelli M.A., et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-: Δ9-tetrahydrocannabiphorol. Sci. Rep. 2019;9:20335. doi: 10.1038/s41598-019-56785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elsohly M.A., Ross S.A. Method of Preparing Delta-9-Tetrahydrocannabinol. US6365416B1. U.S. Patent. 2002 March 4;
- 59.Linciano P., Citti C., Russo F., Tolomeo F., Laganà A., Capriotti A.L., Luongo L., Iannotta M., Belardo C., Maione S., et al. Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: Cannabidihexol. Sci. Rep. 2020;10:22019. doi: 10.1038/s41598-020-79042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zulfiqar F., Ross S.A., Slade D., Ahmed S.A., Radwan M.M., Ali Z., Khan I.A., Elsohly M.A. Cannabisol, a novel Δ9-THC dimer possessing a unique methylene bridge, isolated from Cannabis sativa. Tetrahedron Lett. 2012;53:3560–3562. doi: 10.1016/j.tetlet.2012.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvey D. Characterization of the butyl homologues of D-1-tetrahydrocannabinol, cannabinol and cannabidiol in samples of cannabis by combined gas chromotography and mass spectrometry. J. Pharm. Pharmacol. 1976;28:280–285. doi: 10.1111/j.2042-7158.1976.tb04153.x. [DOI] [PubMed] [Google Scholar]
- 62.Archer R.A., Boyd D.B., Demarco P.V., Tyminski I.J., Allinger N. Structural studies of cannabinoids. Theoretical and proton magnetic resonance analysis. J. Am. Chem. Soc. 1970;92:5200–5206. doi: 10.1021/ja00720a033. [DOI] [PubMed] [Google Scholar]
- 63.Elsohly M.A., Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Gill E.W. Propyl homologue of tetrahydrocannabinol: Its isolation from Cannabis, properties, and synthesis. J. Chem. Soc. C. 1971;3:579–582. doi: 10.1039/j39710000579. [DOI] [Google Scholar]
- 65.Krejcí Z., Šantavý F. Isolation of two new cannabinoid acids from Cannabis sativa L. of Czechoslovak origin. Acta Univ. Olomuc. Fac. Med. 1975;74:161–166. [Google Scholar]
- 66.Shani A., Mechoulam R. Cannabielsoic acids: Isolation and synthesis by a novel oxidative cyclization. Tetrahedron. 1974;30:2437–2446. doi: 10.1016/S0040-4020(01)97114-5. [DOI] [Google Scholar]
- 67.Vree T., Breimer D., van Ginneken C.A.M., van Rossum J.M. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharmacol. 1972;24:7–12. doi: 10.1111/j.2042-7158.1972.tb08857.x. [DOI] [PubMed] [Google Scholar]
- 68.Shoyama Y., Hirano H., Makino H., Umekita N., Nishioka I. Cannabis. X. The isolation and structures of four new propyl cannabinoid acids, tetrahydrocannabivarinic acid, cannabidivarinic acid, cannabichromevarinic acid and cannabigerovarinic acid, from Thai Cannabis,’meao variant’. Chem. Pharma. Bull. 1977;25:2306–2311. doi: 10.1248/cpb.25.2306. [DOI] [Google Scholar]
- 69.Shoyama Y., Hirano H., Oda M., Somehara T., Nishioka I. Cannabichromevarin and cannabigerovarin, two new propyl homologues of cannabichromene and cannabigerol. Chem. Pharm. Bull. 1975;23:1894–1895. doi: 10.1248/cpb.23.1894. [DOI] [Google Scholar]
- 70.Appendino G., Giana A., Gibbons S., Maffei M., Gnavi G., Grassi G., Sterner O. A polar cannabinoid from Cannabis sativa Var. Carma. Nat. Prod. Commun. 2008;3:1934578X0800301207. doi: 10.1177/1934578X0800301207. [DOI] [Google Scholar]
- 71.Pagani A., Scala F., Chianese G., Grassi G., Appendino G., Taglialatela-Scafati O. Cannabioxepane, a novel tetracyclic cannabinoid from hemp, Cannabis sativa L. Tetrahedron. 2011;67:3369–3373. doi: 10.1016/j.tet.2011.03.062. [DOI] [Google Scholar]
- 72.Taglialatela-Scafati O., Pagani A., Scala F., de Petrocellis L., di Marzo V., Grassi G., Appendino G. Cannabimovone, a cannabinoid with a rearranged terpenoid skeleton from hemp. Eur. J. Org. Chem. 2010;2010:2023. doi: 10.1002/ejoc.201090025. [DOI] [Google Scholar]
- 73.Taura F., Morimoto S., Shoyama Y. Cannabinerolic acid, a cannabinoid from Cannabis sativa. Phytochemistry. 1995;39:457–458. doi: 10.1016/0031-9422(94)00887-Y. [DOI] [Google Scholar]
- 74.Borges G.R., Birk L., Scheid C., Morés L., Carasek E., Kitamura R.O.S., Roveri F.L., Eller S., de Oliveira Merib J., de Oliveira T.F. Simple and straightforward analysis of cannabinoids in medicinal products by fast-GC–FID. Forensic Toxicol. 2020;38:531–535. doi: 10.1007/s11419-020-00522-1. [DOI] [Google Scholar]
- 75.Naz S., Hanif M.A., Bhatti H.N., Ansari T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants. 2017;20:175–184. doi: 10.1080/0972060X.2017.1281766. [DOI] [Google Scholar]
- 76.Ross S.A., Elsohly M.A. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J. Nat. Prod. 1996;59:49–51. doi: 10.1021/np960004a. [DOI] [PubMed] [Google Scholar]
- 77.Strömberg L. Minor Components of Cannabis Resin: IV. Mass spectrometric data and gas chromatographic retention times of terpenic components with retention times shorter than that of cannabidiol. J. Chromatogr. A. 1974;96:99–114. doi: 10.1016/S0021-9673(01)81222-X. [DOI] [PubMed] [Google Scholar]
- 78.Turner C.E., Elsohly M.A., Boeren E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 79.Hendriks H., Malingré T.M., Batterman S., Bos R. Alkanes of the essential oil of Cannabis sativa. Phytochemistry. 1977;16:719–721. doi: 10.1016/S0031-9422(00)89239-0. [DOI] [PubMed] [Google Scholar]
- 80.El-Feraly F.S., El-Sherei M.M., Al-Muhtadi F.J. Spiro-indans from Cannabis sativa. Phytochemistry. 1986;25:1992–1994. doi: 10.1016/S0031-9422(00)81194-2. [DOI] [Google Scholar]
- 81.Chen B., Cai G., Yuan Y., Li T., He Q., He J.F. Chemical constituents in hemp pectin I. Chin. J. Exp. Tradit. Med. Form. 2012;18:98–100. [Google Scholar]
- 82.Boeren E., Elsohly M., Turner C., Salemink C. ß-Cannabispiranol: A new non-cannabinoid phenol from Cannabis sativa L. Experiential. 1977;33:848. doi: 10.1007/BF01951236. [DOI] [PubMed] [Google Scholar]
- 83.Slatkin D.J., Doorenbos N.J., Harris L.S., Masoud A.N., Quimby M.W., Schiff P.L. Chemical constituents of Cannabis sativa L. root. J. Pharm. Sci. 1971;60:1891–1892. doi: 10.1002/jps.2600601232. [DOI] [PubMed] [Google Scholar]
- 84.Latter H.L., Abraham D.J., Turner C.E., Knapp J.E., Schiff P.L., Jr., Slatkin D.J. Cannabisativine, a new alkaloid from Cannabis sativa L. root. Tetrahedron Lett. 1975;16:2815–2818. doi: 10.1016/S0040-4039(00)75003-9. [DOI] [Google Scholar]
- 85.Shoyama Y., Nishioka I. Cannabis. XIII. Two new spiro-compounds, cannabispirol and acetyl cannabispirol. Chem. Pharm. Bull. 1978;26:3641–3646. doi: 10.1248/cpb.26.3641. [DOI] [Google Scholar]
- 86.Sánchez-Duffhues G., Calzado M.A., de Vinuesa A.G., Caballero F.J., Ech-Chahad A., Appendino G., Krohn K., Fiebich B.L., Muñoz E. Denbinobin, a naturally occurring 1, 4-phenanthrenequinone, inhibits HIV-1 replication through an NF-κB-dependent pathway. Biochem. Pharmacol. 2008;76:1240–1250. doi: 10.1016/j.bcp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Nalli Y., Arora P., Riyaz-Ul-Hassan S., Ali A. Chemical investigation of Cannabis sativa leading to the discovery of a prenylspirodinone with anti-microbial potential. Tetrahedron Lett. 2018;59:2470–2472. doi: 10.1016/j.tetlet.2018.05.051. [DOI] [Google Scholar]
- 88.Crombie L., Crombie W.M.L. Natural products of Thailand high Δ 1-THC-strain Cannabis. the bibenzyl-spiran-dihydrophenanthrene group: Relations with cannabinoids and canniflavones. J. Chem. Soc. Perkin Trans. 1982;1:1455–1466. doi: 10.1039/P19820001455. [DOI] [Google Scholar]
- 89.Ross S., Elsohly M.A. Constituents of Cannabis sativa L. XXVIII. A review of the natural constituents. Zagazig J. Pharm. Sci. 1995;4:1–10. doi: 10.21608/zjps.1995.169714. [DOI] [Google Scholar]
- 90.Elsohly H.N., Ma G.E., Turner C.E., Elsohly M.A. Constituents of Cannabis sativa, XXV. Isolation of two new dihydrostilbenes from a panamanian variant. J. Nat. Prod. 1984;47:445–452. doi: 10.1021/np50033a008. [DOI] [PubMed] [Google Scholar]
- 91.Ottersen T., Aasen A., El-Feraly F.S., Turner C.E. X-ray structure of cannabispiran: A novel Cannabis constituent. Chem. Commun. 1976;15:580–581. doi: 10.1039/c39760000580. [DOI] [Google Scholar]
- 92.Ross S.A., Elsohly M.A., Sultana G.N., Mehmedic Z., Hossain C.F., Chandra S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005;16:45–48. doi: 10.1002/pca.809. [DOI] [PubMed] [Google Scholar]
- 93.Cheng L., Kong D., Hu G., Li H. A new 9, 10-dihydrophenanthrenedione from Cannabis sativa. Chem. Nat. Compd. 2010;46:710–712. doi: 10.1007/s10600-010-9721-3. [DOI] [Google Scholar]
- 94.Bercht C., van Dongen J., Heerma W., Lousberg R.C., Küppers F. Cannabispirone and cannabispirenone, two naturally occurring spiro-compounds. Tetrahedron. 1976;32:2939–2943. doi: 10.1016/0040-4020(76)80149-4. [DOI] [Google Scholar]
- 95.Hammond C.T., Mahlberg P.G. Phloroglucinol glucoside as a natural constituent of Cannabis sativa. Phytochemistry. 1994;37:755–756. doi: 10.1016/S0031-9422(00)90352-2. [DOI] [Google Scholar]
- 96.Namdar D., Voet H., Ajjampura V., Nadarajan S., Mayzlish-Gati E., Mazuz M., Shalev N., Koltai H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for ellcytotoxic activity. Molecules. 2019;24:3031. doi: 10.3390/molecules24173031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whalley B., Stephens G., Williams C., Guy G., Wright S., Kikuchi T., GW Pharma Ltd. Otsuka Pharmaceutical Co Ltd. Use of One or a Combination of Phyto-Cannabinoids in the Treatment of Epilepsy. 9,066,920. U.S Patent. 2015 June 30;
- 98.Parker L., Rock E., Sticht M., Wills K., Limebeer C.L. Cannabinoids suppress acute and anticipatory nausea in preclinical rat models of conditioned gaping. Pharm. Ther. 2015;97:559–561. doi: 10.1002/cpt.98. [DOI] [PubMed] [Google Scholar]
- 99.Erridge S., Mangal N., Salazar O., Pacchetti B., Sodergren M.H. Cannflavins—From plant to patient: A scoping review. Fitoterapia. 2020;146:104712. doi: 10.1016/j.fitote.2020.104712. [DOI] [PubMed] [Google Scholar]
- 100.Pacher P., Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mcguire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S.J. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 102.Parker L.A., Rock E.M., Limebeer C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011;163:1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeshurun M., Shpilberg O., Herscovici C., Shargian L., Dreyer J., Peck A., Israeli M., Levy-Assaraf M., Gruenewald T., Mechoulam R., et al. Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: Results of a phase II study. Biol. Blood Marrow Transpl. 2015;21:1770–1775. doi: 10.1016/j.bbmt.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 104.Brierley D.I., Samuels J., Duncan M., Whalley B.J., Williams C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology. 2016;233:3603–3613. doi: 10.1007/s00213-016-4397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rock E., Kopstick R.L., Limebeer C.L., Parker L.A. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in S uncus murinus. Br. J. Pharmacol. 2013;170:641–648. doi: 10.1111/bph.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rock E.M., Sticht M.A., Parker L.A. Effect of Phytocannabinoids on Nausea and Vomiting. Oxford University Press; Oxford, UK: 2014. [Google Scholar]
- 107.Tsien R., Whalley B.J., Devinsky O. Cannabinoids and Epilepsy. Neurotherapeutics. 2015;12:747–768. doi: 10.1007/s13311-015-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moreno-Sanz G. Can you pass the acid test? critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016;1:124–130. doi: 10.1089/can.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rock E., Parker L.A. Effect of low doses of cannabidiolic acid and ondansetron on licl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br. J. Pharmacol. 2013;169:685–692. doi: 10.1111/bph.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rock E.M., Connolly C., Limebeer C.L., Parker L.A. Effect of combined oral doses of Δ 9-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea in rat models. Psychopharmacology. 2016;233:3353–3360. doi: 10.1007/s00213-016-4378-7. [DOI] [PubMed] [Google Scholar]
- 111.Russo E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cascone P., Iodice L., Maffei M.E., Bossi S., Arimura G.I., Guerrieri E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015;173:28–32. doi: 10.1016/j.jplph.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 113.Bomfim L.M., Menezes L.R., Rodrigues A.C., Dias R.B., Gurgel Rocha C.A., Soares M.B., Neto A.F., Nascimento M.P., Campos A.F., Silva L.C., et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. 2016;118:208–213. doi: 10.1111/bcpt.12488. [DOI] [PubMed] [Google Scholar]
- 114.De Oliveira Ramalho T.R., de Oliveira M.T., de Araujo Lima A.L., Bezerra-Santos C.R., Piuvezam M.R. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015;81:1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- 115.Fitsiou E., Anestopoulos I., Chlichlia K., Galanis A., Kourkoutas I., Panayiotidis M.I., Pappa A. Antioxidant and antiproliferative properties of the essential oils of Satureja thymbra and Satureja parnassica and their major constituents. Anticancer Res. 2016;36:5757–5763. doi: 10.21873/anticanres.11159. [DOI] [PubMed] [Google Scholar]
- 116.Kasuya H., Okada N., Kubohara M., Satou T., Masuo Y., Koike K. Expression of BDNF and TH mRNA in the Brain Following Inhaled Administration of A-Pinene. Phytother. Res. 2015;29:43–47. doi: 10.1002/ptr.5224. [DOI] [PubMed] [Google Scholar]
- 117.Han H.D., Cho Y.J., Cho S.K., Byeon Y., Jeon H.N., Kim H.S., Kim B.G., Bae D.S., Lopez-Berestein G., Sood A.K., et al. Linalool-incorporated nanoparticles as a novel anticancer agent for epithelial ovarian carcinoma. Mol. Cancer Ther. 2016;15:618–627. doi: 10.1158/1535-7163.MCT-15-0733-T. [DOI] [PubMed] [Google Scholar]
- 118.Russo E.B. Cannabis and epilepsy: An ancient treatment returns to the fore. Epilepsy Behav. 2017;70:292–297. doi: 10.1016/j.yebeh.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 119.Sulak D., Saneto R., Goldstein B. The current status of artisanal Cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017;70:328–333. doi: 10.1016/j.yebeh.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 120.Piccinelli A.C., Santos J.A., Konkiewitz E.C., Oesterreich S.A., Formagio A.S., Croda J., Ziff E.B., Kassuya C. Antihyperalgesic and antidepressive actions of (R)-(+)-Limonene, A-phellandrene, and essential oil from schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015;18:24. doi: 10.1179/1476830514Y.0000000119. [DOI] [PubMed] [Google Scholar]
- 121.Siqueira H.D., Neto B.S., Sousa D.P., Gomes B.S., da Silva F.V., Cunha F.V., Wanderley C.W., Pinheiro G., Cândido A.G., Wong D.V., et al. A-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016;160:27–33. doi: 10.1016/j.lfs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Lima D.F., Brandão M.S., Moura J.B., Leitão J.M., Carvalho F.A., Miúra L.M., Leite J.R., Sousa D.P., Almeida F.R. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012;64:283–292. doi: 10.1111/j.2042-7158.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 123.Aydin E., Türkez H., Taşdemir Ş. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arch. Ind. Hyg. Toxicol. 2013;64:415–424. doi: 10.2478/10004-1254-64-2013-2365. [DOI] [PubMed] [Google Scholar]
- 124.Turkez H., Aydın E., Geyikoglu F., Cetin D. Genotoxic and oxidative damage potentials in human lymphocytes after exposure to terpinolene in vitro. Cytotechnology. 2015;67:409–418. doi: 10.1007/s10616-014-9698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paula-Freire L.I., Andersen M., Gama V., Molska G., Carlini E.L. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine. 2014;21:356–362. doi: 10.1016/j.phymed.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 126.Varga Z.V., Matyas C., Erdelyi K., Cinar R., Nieri D., Chicca A., Nemeth B.T., Paloczi J., Lajtos T., Corey L., et al. Research Paper Themed Issue Β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2017;175:320–334. doi: 10.1111/bph.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katsuyama S., Mizoguchi H., Kuwahata H., Komatsu T., Nagaoka K., Nakamura H., Bagetta G., Sakurada T., Sakurada S. Involvement of peripheral cannabinoid and opioid receptors in β-caryophyllene-induced antinociception. Eur. J. Pain. 2013;17:664–675. doi: 10.1002/j.1532-2149.2012.00242.x. [DOI] [PubMed] [Google Scholar]
- 128.Xu H.B., Zheng L.P., Li L., Xu L.Z., Fu J. Elemene, one ingredient of a chinese herb, against malignant tumors: A literature-based meta-analysis. Cancer Invest. 2013;31:156–166. doi: 10.3109/07357907.2012.756108. [DOI] [PubMed] [Google Scholar]
- 129.Yang Q., Wu J., Luo Y., Huang N., Zhen N., Zhou Y., Sun F., Li Z., Pan Q., Li Y. (−)-Guaiol Regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget. 2016;7:62585. doi: 10.18632/oncotarget.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chinsembu K.C. Tuberculosis and nature’s pharmacy of putative anti-tuberculosis agents. Acta Tropica. 2016;153:46–56. doi: 10.1016/j.actatropica.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Semenya S., Potgieter M., Tshisikhawe M., Shava S., Maroyi A. Medicinal utilization of exotic plants by bapedi traditional healers to treat human ailments in Limpopo province, South Africa. J. Ethnopharmacol. 2012;144:646–655. doi: 10.1016/j.jep.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Yang H.H., Son J.K., Jung B., Zheng M., Kim J.R. Epifriedelanol from the root bark of Ulmus davidiana inhibits cellular senescence in human primary cells. Planta Medica. 2011;77:441–449. doi: 10.1055/s-0030-1250458. [DOI] [PubMed] [Google Scholar]
- 133.Zhou X., Wang F., Zhou R., Song X., Xie M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017;41:e12376. doi: 10.1111/jfbc.12376. [DOI] [Google Scholar]
- 134.He M., Min J.W., Kong W.L., He X.H., Li J.X., Peng B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi: 10.1016/j.fitote.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 135.Sharma A., Kashyap D., Sak K., Tuli H.S., Sharma A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018;7:15–32. doi: 10.4155/ppa-2017-0030. [DOI] [PubMed] [Google Scholar]
- 136.Ashaari Z., Hassanzadeh G., Alizamir T., Yousefi B., Keshavarzi Z., Mokhtari T. The flavone luteolin improves central nervous system disorders by different mechanisms: A review. J. Mol. Neurosci. 2018;65:491–506. doi: 10.1007/s12031-018-1094-2. [DOI] [PubMed] [Google Scholar]
- 137.Rudroff T., Sosnoff J.J. Cannabidiol to improve mobility in people with multiple sclerosis. Front. Neurol. 2018;9:183. doi: 10.3389/fneur.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong K.U., Baum C.R. Acute Cannabis Toxicity. Pediatr. Emerg. Care. 2019;35:799–806. doi: 10.1097/PEC.0000000000001970. [DOI] [PubMed] [Google Scholar]
- 139.International Conference on Harmonisation (ICH) All Guidelines. [(accessed on 19 April 2020)]. Available online: https://www.ich.org/page/ich-guidelines.
- 140.Schlag A.K., O’sullivan S.E., Zafar R.R., Nutt D.J. Current controversies in medical cannabis: Recent developments in human clinical applications and potential therapeutics. Neuropharmacology. 2021;191:108586. doi: 10.1016/j.neuropharm.2021.108586. [DOI] [PubMed] [Google Scholar]
- 141.Hill K.P. Cannabinoids and the coronavirus. Cannabis Cannabinoid Res. 2020;5:118–120. doi: 10.1089/can.2020.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Costiniuk C.T., Jenabian M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020;53:63. doi: 10.1016/j.cytogfr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Byrareddy S.N., Mohan M. SARS-CoV2 induced respiratory distress: Can cannabinoids be added to anti-viral therapies to reduce lung inflammation? Brain Behav. Immun. 2020;87:120. doi: 10.1016/j.bbi.2020.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Esposito G., Pesce M., Seguella L., Sanseverino W., Lu J., Corpetti C., Sarnelli G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020;177:4967–4970. doi: 10.1111/bph.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’sullivan S.E., Stevenson C.W., Laviolette S.R. Could cannabidiol be a treatment for coronavirus disease-19-related anxiety disorders? Cannabis Cannabinoid Res. 2021;6:7–18. doi: 10.1089/can.2020.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.