Abstract
Thrombin-induced platelet microbicidal protein-1 (tPMP-1) and human neutrophil defensin-1 (HNP-1) are small, cationic antimicrobial peptides. These peptides exert potent in vitro microbicidal activity against a broad spectrum of human pathogens, including Staphylococcus aureus. Evidence suggests that tPMP-1 and HNP-1 target and disrupt the bacterial membrane. However, it is not yet clear whether membrane disruption itself is sufficient to kill the bacterium or whether subsequent, presumably intracellular, events are also involved in killing. We investigated the staphylocidal activities of tPMP-1 and HNP-1 in the presence or absence of pretreatment with antibiotics that differ in their mechanisms of action. The staphylocidal effects of tPMP-1 and HNP-1 on control cells (no antibiotic pretreatment) were rapid and concentration dependent. Pretreatment of S. aureus with either penicillin or vancomycin (bacterial cell wall synthesis inhibitors) significantly enhanced the anti-S. aureus effects of tPMP-1 compared with the effects against the respective control cells over the entire tPMP-1 concentration range tested (P < 0.05). Similarly, S. aureus cells pretreated with these antibiotics were more susceptible to HNP-1 than control cells, although the difference in the effects against cells that received penicillin pretreatment did not reach statistical significance (P < 0.05 for cells that received vancomycin pretreatment versus effects against control cells). Studies with isogenic pairs of strains with normal or deficient autolytic enzyme activities demonstrated that enhancement of S. aureus killing by cationic peptides and cell wall-active agents could not be ascribed to a predominant role of autolytic enzyme activation. Pretreatment of S. aureus cells with tetracycline, a 30S ribosomal subunit inhibitor, significantly decreased the staphylocidal effect of tPMP-1 over a wide peptide concentration range (0.16 to 1.25 μg/ml) (P < 0.05). Furthermore, pretreatment with novobiocin (an inhibitor of bacterial DNA gyrase subunit B) and with azithromycin, quinupristin, or dalfopristin (50S ribosomal subunit protein synthesis inhibitors) essentially blocked the S. aureus killing resulting from exposure to tPMP-1 or HNP-1 at most concentrations compared with the effects against the respective control cells (P < 0.05 for a tPMP-1 concentration range of 0.31 to 1.25 μg/ml and for an HNP-1 concentration range of 6.25 to 50 μg/ml). These findings suggest that tPMP-1 and HNP-1 exert anti-S. aureus activities through mechanisms involving both the cell membrane and intracellular targets.
Neutrophils represent a key component of innate host defenses against infection by virtue of their opsonophagocytic and oxidative microbicidal mechanisms (5, 6, 11, 24). Platelets share many functional properties with neutrophils, including chemotactic response and generation of oxygen metabolites that have microbicidal action (34, 35). Recent evidence indicates that platelets and neutrophils also provide significant contributions to antimicrobial host defenses via nonoxidative mechanisms through an array of endogenous antimicrobial peptides (5, 6, 34, 35). For example, a group of small, antimicrobial cationic peptides has been isolated from human and rabbit platelets; these have been termed platelet microbicidal proteins (PMPs) (22, 23, 28, 30, 33). Recently, Krijgsveld et al. have confirmed similar peptides in thrombin-stimulated human platelets (9a). The predominant PMP secreted from thrombin-stimulated rabbit platelets, thrombin-induced PMP-1 (tPMP-1), has been the most thoroughly studied PMP to date (8, 9, 26, 28–32, 35). Similarly, rabbit and human neutrophils also contain small, cationic microbicidal peptides, including defensins, which are concentrated within the neutrophil azurophilic granule. In humans, there are four predominant defensins or human neutrophil peptides (HNPs). HNP-1 (HNP-1) is the most extensively studied defensin in terms of structure and function (5, 6, 10, 12, 19, 35). Both tPMP-1 and HNP-1 exert rapid and potent in vitro microbicidal activity against a broad spectrum of common microbial pathogens, including Staphylococcus aureus, Escherichia coli, and Candida albicans (10, 18, 28, 30, 31, 33). However, the mechanisms of the microbicidal activities of tPMP-1 and HNP-1 have not been fully defined.
Evidence from previous studies in our laboratory and other laboratories indicates that the microbial cytoplasmic membrane is a principal target for the microbicidal actions of tPMP-1 and HNP-1. However, the membrane-targeting effects of the two peptides appear to differ (7, 8–10, 12, 26, 35). For example, ultrastructural studies revealed that both peptides induce rapid and extensive damage on the staphylococcal cell membrane, followed by cell death (19, 26, 35). However, flow cytometric data from our laboratory indicate that certain PMPs (e.g., PMP-2), as well as HNP-1, depolarize and permeabilize the staphylococcal membrane in vitro, leading to cell death (9, 35). In contrast, tPMP-1 failed to depolarize but did permeabilize the staphylococcal membrane (9, 35). Additionally, the staphylocidal activity of tPMP-1 has been demonstrated to be influenced by the transmembrane electrical potential (Δψ) in S. aureus (8, 35). In contrast, the microbicidal activity of HNP-1 is independent of Δψ in the range of −100 to −150 mV (34). Thus, S. aureus cell membrane perturbations due to tPMP-1 and HNP-1 likely involve differential mechanisms.
It should be emphasized that the effects on the cytoplasmic membrane induced by tPMP-1 or HNP-1 noted above occur rapidly, within minutes of peptide exposure. Yet, cell death lags behind these membrane effects by 1 to 2 h, suggesting that other, likely intracellular, processes are involved in the microbicidal cascade initiated by tPMP-1 or HNP-1. Our present investigation was designed to further explore the staphylocidal mechanisms of tPMP-1 and HNP-1 in this regard. Thus, our current studies were intended to explore the potential intracellular targets of both microbicidal peptides by pretreatment of S. aureus cells with antibiotics that differ in their mechanisms of action.
(This study was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [27].)
MATERIALS AND METHODS
Organisms.
S. aureus ISP 479C is a well-characterized strain that is the spontaneous, plasmid-cured variant of parental strain ISP 479. S. aureus ISP 479C is highly susceptible to tPMP-1 in vitro, as determined by the microtiter well assay as described previously (28). By using a breakpoint for in vitro resistance to tPMP-1 of ≥40% survival of a 103 CFU/ml inoculum (after 2 h of exposure to 1 μg of tPMP-1 per ml [25]), the mean ± standard deviation (SD) rate of survival of ISP 479C was 8% ± 3.5% (21). Bacillus subtilis ATCC 6633 (obtained from the American Type Culture Collection [ATCC]) is also highly susceptible to tPMP-1 (>99% killing within 30 min of exposure to 1 μg of tPMP-1 per ml) and has been used to quantify and standardize the bioactivity of tPMP-1 (28). Both strains have previously been described in detail (3, 21). In the current studies, both organisms were grown on sheep blood agar plates for 18 h at 37°C. Mid-logarithmic-phase cells were then obtained by inoculating several colonies into brain heart infusion broth (Difco Laboratories, Detroit, Mich.) (optical density at 600 nm [OD600] = 0.05) and incubating at 37°C until an OD600 of 0.6 was achieved. Mid-logarithmic-phase cells were harvested by centrifugation, washed twice in phosphate-buffered saline (PBS; 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 140 mM NaCl, 3.0 mM KCl [pH 7.2]), quantified by spectrophotometry (λ = 600 nm), and cultured on sheep blood agar.
Two additional pairs of S. aureus strains were used in this investigation to define the role of the autolytic enzyme system in the in vitro staphylocidal effects of cationic peptides (see below). Autolysis-deficient strain Lyt−1 is a transposon (Tn917-lacZ)-derived mutant of parental strain ISP 2018 (a derivative of strain 8325-4 [13]). Autolysis-deficient mutant SH 108 was derived from autolysis-deficient mutant SH 105 (in the background of strain RN4220) by transduction of phage 85 into S. aureus 8325-4 (4). These strains were kindly provided by R. K. Jayaswal, Illinois State University, Normal. All strains were stored at −70°C until they were thawed for use.
Antibiotics and peptides.
Penicillin was purchased from Marsam Pharmaceuticals Inc. (Cherry Hill, N.J.), vancomycin was purchased from Eli Lilly & Company (Indianapolis, Ind.), tetracycline was obtained from Aldrich Chemical Company, Inc. (Milwaukee, Wis.), novobiocin was purchased from Sigma Chemical Co. (St. Louis, Mo.), azithromycin was kindly supplied by Pfizer Inc. (New York, N.Y.), and quinupristin and dalfopristin were kindly supplied by Rhone-Poulenc Rorer (Collegeville, Pa.). Antibiotics were prepared from standard powders according to the manufacturer’s recommendations; stock solutions were diluted in the appropriate medium and were used on the same day on which they were diluted. High-performance liquid chromatography (HPLC)-purified HNP-1 was kindly provided by T. Ganz (Los Angeles, Calif.). tPMP-1 was prepared as described previously by thrombin stimulation of washed rabbit platelets (28, 29). Reversed-phase HPLC and acid-urea or sodium dodecyl sulfate-polyacrylamide gel electrophoresis have shown that tPMP-1 accounts for the predominant PMP-mediated antimicrobial activity in this preparation (33).
Determination and standardization of bactericidal activity of tPMP-1.
The bactericidal activity of tPMP-1 was determined as described previously by using Bacillus subtilis ATCC 6633 as a highly tPMP-1-susceptible indicator organism (28). The bioactivity of tPMP-1 (in units per milliliter) was quantified as the reciprocal of the highest dilution that retained >95% killing of an inoculum of 103 CFU of B. subtilis per ml after 30 min of exposure at 37°C. The specific bioactivity of tPMP-1 was then estimated as units per milligram of protein, and the value was then converted to the tPMP-1 concentration, expressed as micrograms per milliliter (5.0 μg/ml ≈ 100 U/ml).
In vitro susceptibility of S. aureus to tPMP-1 or HNP-1.
For tPMP-1 assays, the peptide (prepared in Eagle’s minimal essential medium [MEM; Irvine Scientific, Santa Ana, Calif.]) was added to logarithmic-phase S. aureus suspensions in low-protein-binding microtiter plates to achieve a final tPMP-1 concentration range of 0.04 to 1.25 μg/ml and a final bacterial inoculum of 103 CFU/ml (final volume, 500 μl). For in vitro HNP-1 assays, the peptide (prepared in 0.01% acetic acid) was added to logarithmic-phase S. aureus cell to achieve a final HNP-1 concentration range of 1.56 to 50 μg/ml and a final inoculum of 105 CFU/ml. These bacterial inocula and assay conditions have been used for previous in vitro susceptibility testing studies with tPMP-1 and HNP-1 (8, 28). At 0 and 2 h of incubation at 37°C, 15-μl aliquots were sampled from each microtiter well, briefly sonicated, diluted in PBS containing 0.01% sodium polyanethol sulfonate to inactivate the further bactericidal effects of tPMP-1 and HNP-1, and quantitatively cultured onto sheep blood agar. The staphylocidal effects of tPMP-1 and HNP-1 at each peptide concentration tested were then determined over time as the mean change in the log10 number of CFU per milliliter over the 2-h sampling period (Δlog10 CFU/ml/2 h). All assays were performed a minimum of two times in triplicate, and the mean ± SD Δlog10 CFU/ml/2 h (bactericidal rate) was calculated.
Antibiotic susceptibility testing.
The MICs of penicillin, vancomycin, tetracycline, novobiocin, azithromycin, quinupristin, and dalfopristin for S. aureus ISP 479C were determined in Mueller-Hinton broth (MHB; Difco Laboratories) according to the guidelines of the National Committee for Clinical Laboratory Standards (17). A broth microdilution technique was performed in plastic microtiter plates with a final S. aureus inoculum of either 105 or 107 CFU/ml. These inocula were chosen since 105 CFU/ml is a standard inoculum for antibiotic susceptibility testing and 107 CFU/ml represented the starting inoculum for all antibiotic pretreatment studies (see below). The range of antibiotic concentrations tested was 0.003 to 128 μg/ml for all antibiotics. MICs were read after 18 h of incubation at 37°C and were considered to be the lowest antibiotic concentration that yielded no visible growth. All MICs were redetermined at least twice on separate days and were highly consistent.
Bactericidal effects of tPMP-1 and HNP-1 on S. aureus cells pretreated with antibiotics that differ in their mechanisms of action.
The influence of antibiotic pretreatment upon the subsequent staphylocidal effects of tPMP-1 and HNP-1 was evaluated. Logarithmic-phase S. aureus cells were exposed to one of the following antibiotics, which differed in their mechanisms of action, prior to exposure to tPMP-1 or HNP-1: a cell wall synthesis inhibitor (penicillin or vancomycin), a 30S ribosomal subunit protein synthesis inhibitor (tetracycline), an inhibitor of the B subunit of the bacterial DNA gyrase (novobiocin), a 50S ribosomal subunit protein synthesis inhibitor (azithromycin), and an inhibitor of the 23S RNA component of the 50S ribosomal subunit (quinupristin or dalfopristin). Antibiotic pretreatment was performed with an initial bacterial inoculum of 107 CFU/ml in MHB for 1 h at 37°C with agitation. For all antibiotics tested, antibiotic pretreatments were carried out at 5× the MIC. Pilot studies demonstrated that minimal killing of logarithmic-phase S. aureus cells occurred over a 1-h incubation with the antibiotics at this MIC multiple. After 1 h of incubation in MHB, antibiotic-pretreated S. aureus cells or control cells (without antibiotic pretreatment) were quantitatively cultured. Antibiotic-pretreated or control S. aureus cells were harvested in parallel by centrifugation at 1,000 × g for 10 min, washed twice in PBS (pH 7.2) to remove residual medium and antibiotic, resuspended in PBS (pH 7.2), sonicated, and diluted to the final desired inoculum for assays with tPMP-1 and HNP-1. A final S. aureus inoculum of 103 CFU/ml was then added to tubes containing tPMP-1 (prepared in MEM as described above) to achieve a final concentration range of 0.04 to 1.25 μg/ml or to control tubes containing MEM buffer only (pH 7.4). Likewise, a final S. aureus inoculum of 105 CFU/ml was similarly used to test the anti-S. aureus effects of HNP-1 (concentration range, 1.56 to 50 μg/ml) in the presence or absence of antibiotic pretreatment. These bacterial inocula have been used for previous in vitro susceptibility testing studies with tPMP-1 and HNP-1 (8, 27). All assays were conducted over a 2-h incubation period at 37°C. Aliquots were removed at the end of the incubation period and were diluted in PBS (pH 7.2) containing 0.01% sodium polyanethol sulfonate as described above. The surviving bacterial population was enumerated by quantitative culture on sheep blood agar, and the data were expressed as Δlog10 CFU/ml/2 h (bactericidal rate). All experiments were performed in triplicate on separate days. The final data are means ± SDs.
Role of autolytic enzyme system in staphylocidal activity of tPMP-1.
Several cationic peptides have been shown to activate the autolytic enzyme system in S. aureus (1). To examine the relative contributions of autolytic enzyme system activation on the staphylocidal effects of the cationic peptides used in the present study, we tested well-defined S. aureus mutants with defects in autolytic enzyme activity. In these investigations, we compared the ability of a range of tPMP-1 concentrations to kill the autolytic mutants or their parental strains.
(i) Phenotypic confirmation of autolysin status in S. aureus strains.
To confirm the retention of intact autolytic activity in the parental strain versus the defective autolytic activity in the mutant strains, detergent-induced and penicillin-induced lysis were quantified (modified from Jayaswal et al. [13]). Exponential-phase S. aureus cells (OD580 ≅ 0.7) grown in brain heart infusion broth were collected by centrifugation (1,000 × g for 10 min), washed twice with ice-cold water, and resuspended in Tris-HCl buffer (0.05 M; pH 7.2) containing 0.05% (vol/vol) Triton X-100 (Sigma, St. Louis, Mo.). The cell suspensions were incubated with shaking at 30°C. Cell lysis was measured as a decrease in OD580 over the ensuing 20 h and was expressed as a percentage of the initial (0 h) OD580. Penicillin-induced lysis was also evaluated for these parent-mutant strain pairs. The MICs of penicillin G for the test strains were identical (0.25 μg/ml). For penicillin lysis studies, parental strain ISP 2018 and autolytic mutant Lyt−1 were exposed to penicillin at 4× the MIC (1 μg/ml) during the exponential phase of growth, and cell lysis was monitored over the ensuing 24 h, as described above.
(ii) tPMP-1-induced killing of parental versus autolysis-deficient mutants.
The bactericidal effects of tPMP-1 against the parental and autolysis-deficient strain pairs were assessed over the same peptide concentration range (0 to 1.25 μg/ml) as described above for strain ISP 479C. The data were expressed as the mean ± SD Δlog10 CFU/ml/2 h as described above. All experiments were performed in triplicate on separate days.
Statistical analyses.
The differences in bactericidal rates (Δlog10 CFU/ml/2 h) between S. aureus cells exposed to tPMP-1 or HNP-1 at each concentration in the presence or absence of antibiotic pretreatment were compared by the unpaired Student t test. A probability (P) value of ≤0.05 was considered to represent a significant difference.
RESULTS
Antibiotic susceptibility of S. aureus ISP 479C.
The MICs of antibiotics alone for S. aureus ISP 479C are presented in Table 1. With both inocula tested (105 and 107 CFU/ml), the strain was susceptible to most of the antibiotics tested, with only slight inoculum effects. The MICs determined with an inoculum of 107 CFU/ml were used as the basis for the antibiotic pretreatment strategies (see below).
TABLE 1.
In vitro susceptibility of S. aureus ISP 479C to antibiotics
| Antibiotic | MIC (μg/ml) for the following
inoculum:
|
|
|---|---|---|
| 105 CFU/ml | 107 CFU/ml | |
| Penicillin | 0.03 | 0.125 |
| Vancomycin | 1 | 2 |
| Tetracycline | 2 | 4 |
| Novobiocin | 0.25 | 0.5 |
| Azithromycin | 4 | 8 |
| Quinupristin | 16 | 16 |
| Dalfopristin | 32 | 32 |
Bactericidal effects of antibiotics.
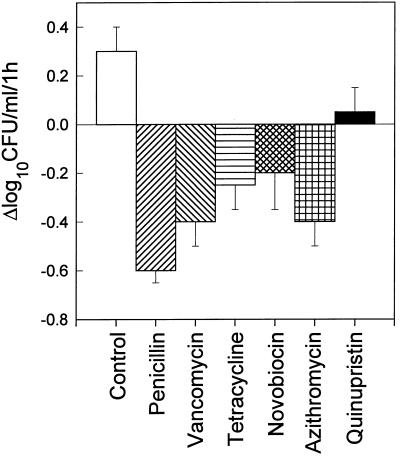
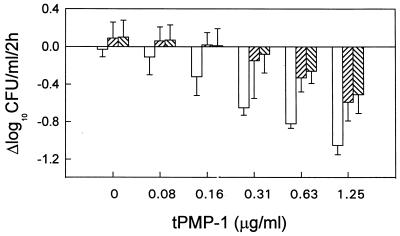
The individual bactericidal effects of antibiotics alone on logarithmic-phase S. aureus ISP 479C cells (107 CFU/ml) over a 1-h period are presented in Fig. 1. Penicillin, vancomycin, tetracycline, novobiocin, or azithromycin (at 5× the MIC) produced only slight bactericidal effects (range, −0.2 to −0.6 Δlog10 CFU/ml). Quinupristin and dalfopristin (data not shown) exhibited no bactericidal activity over the 1-h period.
FIG. 1.
Bactericidal activities of penicillin, vancomycin, tetracycline, novobiocin, azithromycin, and quinupristin against logarithmic-phase S. aureus ISP 479C. All antibiotics tested were used at 5× the MICs. S. aureus cells were exposed to antibiotics at 37°C for 1 h with agitation. Bacterial survival was enumerated on solid medium.
Influence of bacterial cell wall synthesis inhibitors on staphylocidal activity.
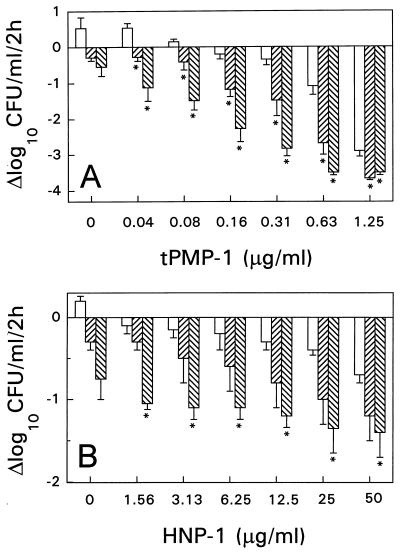
tPMP-1 or HNP-1 alone (without antibiotic pretreatment) each demonstrated concentration-dependent staphylocidal activity. However, exposure of S. aureus cells to either penicillin or vancomycin prior to tPMP-1 or HNP-1 treatment resulted in significantly increased S. aureus killing compared with the killing achieved with either peptide alone (control cells) (Fig. 2). This enhanced effect was most marked following vancomycin pretreatment. For example, tPMP-1 (0.63 μg/ml) alone (without antibiotic pretreatment) yielded a mean bactericidal rate of −1.09 Δlog10 CFU/ml/2 h. However, pretreatment with either penicillin or vancomycin before exposure to the same tPMP-1 concentration significantly increased the staphylocidal effects (−2.68 and −3.5 Δlog10 CFU/ml/2 h, respectively; P < 0.05 for both penicillin- and vancomycin-pretreated cells versus control cells). Significantly greater killing of S. aureus was observed when tPMP-1 exposure followed penicillin or vancomicin pretreatment, regardless of the tPMP-1 concentration (P < 0.05) (Fig. 2A). Similarly, exposure of S. aureus to either penicillin or vancomycin prior to HNP-1 exposure also increased the subsequent staphylocidal effects (Fig. 2B). For example, HNP-1 (25 μg/ml) alone produced a bactericidal effect of −0.4 Δlog10 CFU/ml/2 h. However, vancomycin-pretreated S. aureus cells were substantially more susceptible to the same HNP-1 concentration, with a bactericidal rate of −1.35 Δlog10 CFU/ml/2 h (P < 0.05 for vancomycin-pretreated cells versus control cells). A significant enhancement of staphylocidal effects by vancomycin pretreatment was seen for all HNP-1 concentrations tested (P < 0.05). However, for penicillin pretreatment, these differences did not reach statistical significance.
FIG. 2.
Susceptibility of S. aureus ISP 479C pretreated with a bacterial cell wall synthesis inhibitor (penicillin or vancomycin) to tPMP (A) or HNP-1 (B). S. aureus cells were pretreated with either 5× the MIC of penicillin (▨), 5× the MIC vancomycin (▧), or MHB only (□) at 37°C for 1 h prior to exposure to either tPMP or HNP-1 for 2 h in MEM buffer (pH 7.2). Survivors were enumerated on solid medium. ∗, statistically significant reduction (P < 0.05).
Influence of bacterial 30S ribosomal subunit protein synthesis inhibitor on bactericidal activity.
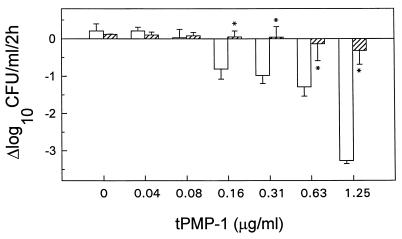
The relationship among tetracycline pretreatment, cationic peptide exposure, and the ensuing staphylocidal peptide activity is shown in Fig. 3. The staphylocidal effect of tPMP-1 was significantly (P < 0.05) reduced by tetracycline pretreatment across the peptide concentration range 0.16 to 1.25 μg/ml.
FIG. 3.
Susceptibility of S. aureus ISP 479C pretreated with a bacterial 30S ribosomal subunit protein synthesis inhibitor (tetracycline) to tPMP. S. aureus cells were pretreated with 5× the MIC of tetracycline (▨) or MHB only (□) at 37°C for 1 h prior to exposure to tPMP for 2 h in MEM buffer (pH 7.2). Survivors were enumerated in solid medium. ∗, statistically significant reduction (P < 0.05).
Influence of inhibition of bacterial DNA gyrase B subunit or 50S ribosomal subunit on bactericidal activity.
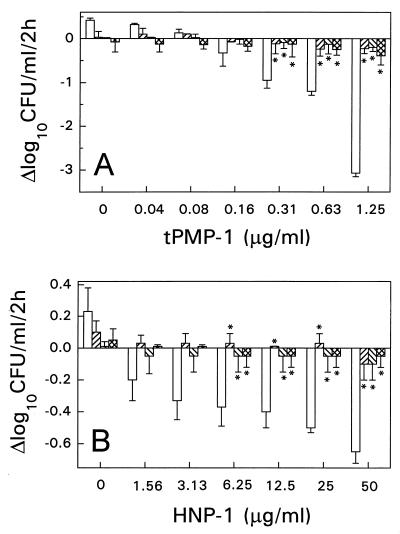
Our results indicate that staphylocidal effects were significantly reduced by pretreatment with novobiocin (a bacterial DNA gyrase B subunit inhibitor), azithromycin, quinupristin, or dalfopristin (bacterial 50S ribosomal subunit protein synthesis inhibitors) prior to tPMP-1 or HNP-1 exposure (Fig. 4). S. aureus cells pretreated with novobiocin were significantly less susceptible than control cells to subsequent microbicidal effects when they were then exposed to tPMP-1 (P < 0.05 for a concentration range of 0.31 to 1.25 μg/ml) (Fig. 4A) or HNP-1 (P < 0.05 for a concentration range of 6.25 to 50 μg/ml) (Fig. 4B). For example, at the highest concentration of tPMP-1 (1.25 μg/ml) and HNP-1 (50 μg/ml) tested, the staphylocidal rates for S. aureus control cells (without antibiotic pretreatment) were −3.1 and −0.7 Δlog10 CFU/ml/2 h, respectively. However, with these same peptide concentrations, tPMP-1 or HNP-1 exposure resulted in only a −0.2 or −0.1 Δlog10 CFU/ml/2 h, respectively, for novobiocin-pretreated cells (P < 0.05). Similarly, pretreatment of S. aureus cells with azithromycin substantially inhibited subsequent bactericidal effects following exposure to tPMP-1 (P < 0.05 for a concentration range of 0.31 to 1.25 μg/ml) (Fig. 4A) or HNP-1 (P < 0.05 for a concentration range of 6.25 to 50 μg/ml) (Fig. 4B) compared to the effects of exposure to tPMP-1 or HNP-1 alone. Likewise, quinupristin and dalfopristin, which are 50S ribosomal subunit protein inhibitors, significantly antagonized the staphylocidal effects subsequent to tPMP-1 exposure (P < 0.05 for a concentration range of 0.31 to 1.25 μg/ml) or HNP-1 exposure (P < 0.05 for a concentration range of 6.25 to 50 μg/ml) (data not shown for dalfopristin).
FIG. 4.
Susceptibility of S. aureus ISP 479C pretreated with the bacterial B subunit of a DNA gyrase inhibitor (novobiocin) or a bacterial 50S ribosomal subunit protein synthesis inhibitor (azithromycin or quinupristin) to tPMP (A) or HNP-1 (B). S. aureus cells were pretreated with 5× the MIC of novobiocin (▨), azithromycin (▧), quinupristin ( ), or MHB only (□) at 37°C for 1 h prior to exposure to either tPMP or HNP-1 for 2 h in MEM buffer (pH 7.2). Survivors were enumerated in solid medium. ∗, statistically significant reduction (P < 0.05).
Influence of autolytic enzyme system on the in vitro staphylocidal activity of tPMP-1.
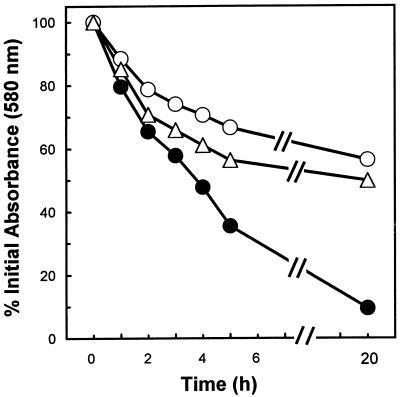
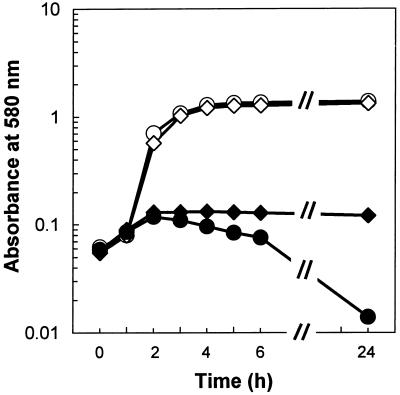
Both autolysis-deficient mutants were shown to be defective in detergent-induced and penicillin-induced lysis, as expected (4, 13). For example, Triton X-100 exposure of parental strain ISP 2018 yielded 90% lysis over a 20-h observation period (Fig. 5). In contrast, autolysis-deficient mutants Lyt−1 and SH 108 exhibited only 50 and 56% lysis in the presence of Triton X-100, respectively, over the same time period. Similarly, exposure of parental strain ISP 2018 to 4× the MIC of penicillin G induced nearly complete cell lysis over the 24-h observation period (Fig. 6). In contrast, neither autolysis-deficient mutant exhibited any cell lysis over this same time period (results for Lyt−1 are presented in Fig. 6; for SH 108, data are not shown). By using tPMP-1 as the cationic peptide in these studies, mutant strains exhibiting the autolysis-deficient phenotype were killed at a moderately reduced rate compared to the rate of killing of the parental strains (Fig. 7). The differences in the tPMP-1-induced mean Δlog10 CFU/ml/2 h in the mutant versus parental strains ranged from ∼−0.13 to −0.57 over the peptide concentration range tested. None of these differences reached statistical significance.
FIG. 5.
Autolysis of whole cells of S. aureus. Mid-exponential-phase cultures were resuspended in 0.05 M Tris-HCl (pH 7.2) containing 0.05% Triton X-100 and were incubated at 30°C. The changes in OD580 were determined as described in Material and Methods. Symbols: ●, S. aureus ISP 2018 (parental strain); ○, Lyt−1 autolytic mutant; ▵, SH 108 autolytic mutant.
FIG. 6.
Effect of penicillin G on the growth of S. aureus. Penicillin (1 μg/ml) was added to exponentially growing cultures, and the OD580 was recorded at various times. Symbols: ○, S. aureus ISP 2018 (parental strain); ◊, Lyt−1 autolytic mutant; ●, S. aureus ISP 2018 plus penicillin; ⧫, Lyt−1 plus penicillin.
FIG. 7.
Susceptibility of S. aureus parental and autolytic mutant strains to tPMP-1. S. aureus 2018 (parental strain) (□), Lyt−1 autolytic mutant (▨), and SH 108 autolytic mutant (▧) cells were exposed to tPMP-1 (0 to 1.25 μg/ml) for 2 h.
DISCUSSION
PMPs (as represented by tPMP-1 in the current study) and neutrophil defensins (as represented by HNP-1 in the current study) are small, endogenous cationic peptides which reside within mammalian blood cell granules. PMPs are believed to originate within platelet α granules (33), while neutrophil defensins are contained within neutrophil azurophilic granules (5). In addition to sharing charge similarities, PMPs (e.g., tPMP-1) and neutrophil defensins (e.g., HNP-1) also share the following features: (i) microbicidal spectra (e.g., staphylococci, E. coli, and Candida [10, 18, 28, 30, 31, 33]), (ii) ultrastructural evidence of disruption of cell membranes of target organisms (8, 19, 26, 35), (iii) electrophysiologic evidence of voltage-dependent membrane permeabilization (8, 35), and (iv) flow cytometric demonstration of functional membrane perturbations (9, 34, 35). Despite these similarities, HNP-1 and tPMP-1 appear to exhibit differences in their microbicidal mechanisms of action. For example, in S. aureus, the intrinsic state of the transcytoplasmic membrane electrical potential (Δψ) substantially influences the in vitro microbicidal effects of tPMP-1. The normal Δψ of logarithmic-phase S. aureus cells is ∼−140 to −150 mV (8, 14). S. aureus strains exhibiting a Δψ of −90 to −100 mV by virtue of electron transport defects (e.g., hemin or menadione auxotrophies [14, 15]) are more resistant to the lethal action of tPMP-1 (8, 9) and show reduced tPMP-1-induced membrane disruption by flow cytometry compared with the susceptibility and membrane disruption found for normal cells. These microbiologic and membrane disruption defects are reversible by nutrient reconstitution of auxotrophic cells to normalize Δψ (9, 35). Furthermore, exposure of tPMP-1-susceptible cells to this peptide during the stationary growth phase or to 4°C (when membrane bioenergetics are substantially reduced) renders such cells relatively more tPMP-1 resistant compared with the greater susceptibility of cells during the logarithmic growth phase or at 37°C, respectively. Finally, protoplasts derived from either tPMP-1-resistant cells or stationary-phase tPMP-1-susceptible cells are each relatively more resistant to tPMP-1-induced disruption (9). In contrast, the microbicidal effects of HNP-1 against S. aureus exhibit little evidence of Δψ dependence. For example, the small-colony-variant S. aureus cells described above are efficiently killed by HNP-1 in a concentration-dependent manner (8). Moreover, the ability of HNP-1 to depolarize and permeabilize the cytoplasmic membranes of S. aureus cells occurs independently of Δψ in the range of −100 to −150 mV (35). These data suggest either that the microbicidal action of HNP-1 is independent of microbial Δψ or that the Δψ threshold for HNP-1 activity is below −100 mV.
Preliminary data presented by van Den Broek et al. (25) suggested that a protein synthesis inhibitor (i.e., azithromycin) could interfere with the antistaphylococcal actions of HNP-1. Moreover, studies by Lehrer et al. (10), who used E. coli as the model organism, provided evidence that protein synthesis and/or nucleic acid synthesis inhibition is involved in the microbicidal mechanism of HNP-1. Our current data provide further evidence that inhibition of protein synthesis and DNA synthesis may well contribute to the overall microbicidal effects of both tPMP-1 and HNP-1.
Several interesting findings emanated from our current studies. Inhibition of DNA synthesis via gyrase B subunit blockade or protein synthesis at either the 30S or the 50S ribosomal subunit level essentially abrogates the subsequent microbicidal effects normally resulting from exposure to tPMP-1 or HNP-1. Importantly, each of the three distinct 50S ribosomal subunit inhibitors tested (azithromycin, quinupristin, and dalfopristin) interfered virtually identically with the ensuing staphylocidal effects of tPMP-1 and HNP-1. Collectively, these data strongly suggest that these peptides both disrupt the cytoplasmic membrane (26, 31, 35) and interfere with intracellular targets to execute their microbicidal actions. This hypothesis is further supported by previous data demonstrating rapid tPMP-1- and HNP-1-induced membrane perturbations (within minutes of exposure) prior to the major microbicidal events (1 to 2 h postexposure) (19, 28, 33, 35).
Our current investigation also demonstrated that penicillin or vancomycin pretreatment, followed by either tPMP-1 or HNP-1 exposure, increased the staphylocidal effects over a wide peptide concentration range. The precise mechanism(s) of this enhanced bactericidal interaction is not known. However, we have previously demonstrated that cell wall-active agents enhance the multiple microbicidal and nonmicrobicidal effects of tPMP-1. For example, simultaneous exposure of S. aureus to tPMP-1 and cell wall-active agents (oxacillin, vancomycin) yielded a synergistic microbicidal effect in vitro (30). Moreover, pretreatment of S. aureus cells with vancomycin substantially lengthened the duration of the postantibiotic effect induced by tPMP-1. Furthermore, pretreatment of S. aureus cells with ampicillin-sulbactam substantially increased the capacity of tPMP-1 to produce platelet antiadherence effects in such cells in vitro (32). Several studies have shown that pretreatment of bacterial cells with penicillin promotes facilitated uptake of the cationic molecule gentamicin by such cells (16). Since both tPMP-1 and HNP-1 carry a cationic charge, it is possible that a similar mechanism accounts for the enhanced staphylocidal activity associated with penicillin or vancomycin pretreatment followed by tPMP-1 or HNP-1 exposure observed in the current study. This notion, however, will require further investigation.
In contrast, it is also conceivable that activation of autolytic enzymes may play a role in the enhanced bactericidal effects of cationic peptides in cells preexposed to cell wall-active agents. Since cationic peptides (e.g., nisin or Pep 5 [1]) and cell wall-active antibiotics (e.g., penicillin and vancomycin [2, 20]) each activate the autolytic enzyme system in S. aureus, it is possible that enhancement of cationic peptide activity in cells pretreated with penicillin or vancomycin is based on the fact that both test agents stimulate the same cellular target. To quantify the relative contribution of the autolytic enzyme system in S. aureus to the magnitude of tPMP-1-induced killing, we used isogenic pairs of strains that were intact or defective in their autolytic enzyme activities. We demonstrated that there is a modest but not statistically significant reduction in the level of tPMP-1-induced killing of autolysis-deficient strains compared to the level of killing of the parental strains. These data suggested that activation of the autolytic enzyme system in S. aureus may well contribute to the direct and penicillin- and vancomycin-enhanced killing by cationic peptides, but it is not likely to be the primary mechanism of action.
In summary, the present results provide evidence that the microbicidal mechanisms of tPMP-1 and HNP-1 are multimodal and involve both early effects upon the structure and function of the cytoplasmic membrane and secondary, delayed effects upon protein and DNA synthesis. These early and late effects result in a microbicidal cascade. Current studies are in progress to further define and characterize both these early and later microbicidal events.
ACKNOWLEDGMENTS
This work was supported in part by research grants from the National Institutes of Health (AI39108 to A.S.B. and AI39001 to M.R.Y.).
We gratefully acknowledge Tomas Ganz (University of California, Los Angeles) for kindly supplying purified HNP-1 and Deborah Kahler for excellent technical assistance.
REFERENCES
- 1.Bierbaum G, Sahl H G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 2.Blumel P, Reinicke B, Lahav M, Giesbrecht P. Cell wall degradation of Staphylococcus aureus by autolysins and lysozyme. The target of penicillin. The murein sacculus of bacteria cell walls. Architecture and growth. In: Hakenbeck R, Holtje J V, Labischinski H, editors. Proceedings of the International FEMS Symposium Berlin (West). Berlin, Germany: Walter de Gruyter; 1983. pp. 323–328. [Google Scholar]
- 3.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster S J. Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus8325/4. J Bacteriol. 1995;177:5723–5725. doi: 10.1128/jb.177.19.5723-5725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T, Selsted M E, Lehrer R I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T, Selsted M E, Szklarek D, Harwig S S L, Daher K, Bainton D F, Lehrer R I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides from voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo S P, Bayer A S, Sahl H G, Proctor R A, Yeaman M R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo S P, Yeaman M R, Bayer A S. Cell membrane is a principal target for the staphylocidal action of platelet microbicidal protein. Infect Immun. 1997;65:4795–4800. doi: 10.1128/iai.65.11.4795-4800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Krijgsveld J, Zaat S A, Kuijpers A J, Engbers G H, Feijen J, Dankert J. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Thrombocidins, bactericidal proteins from human blood platelets, are C-terminal deletion products of cxc-chemokines, abstr. F-179; p. 278. [Google Scholar]
- 10.Lehrer R I, Barton A, Daher K A, Harwig S S, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli: mechanism of bactericidal activities. J Clin Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehrer R I, Ganz T, Selsted M E, Babior B M, Curnutte J T. Neutrophils and host defense. Ann Intern Med. 1988;109:127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein A. Mechanisms of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Invest. 1991;88:93–100. doi: 10.1172/JCI115310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani N, Tobin P, Jayaswal R K. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol. 1993;175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mates S M, Eisenberg E S, Mandel L J, Patel L, Kaback H R, Miller M H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci USA. 1982;79:6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M H, Edberg S C, Mandel L J, Behar C F, Steigbigel N H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980;18:722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moellering R C, Jr, Wennersten C B, Weinberg A N. Synergy of penicillin and gentamicin against enterococci. J Infect Dis. 1971;124(Suppl.):S207–S209. doi: 10.1093/infdis/124.supplement_1.s207. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 2nd ed. Document M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 18.Segal A W, Venge P. The neutrophils in inflammation. In: Venge P, Lindbom A, editors. Inflammation: basic mechanisms, tissue injuring principles and clinical models. Stockholm, Sweden: Almqvist & Wiksell International; 1985. pp. 105–119. [Google Scholar]
- 19.Shimoda M, Ohki K, Shimamoto Y, Kohashi O. Morphology of defensin-treated Staphylococcus aureus. Infect Immun. 1995;63:2886–2891. doi: 10.1128/iai.63.8.2886-2891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullam P M, Bayer A S, Foss W M, Cheung A L. Diminished platelet binding in vitro by Staphylococcus aureusis associated with reduced virulence in a rabbit endocarditis model. Infect Immun. 1996;64:4915–4921. doi: 10.1128/iai.64.12.4915-4921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y Q, Yeaman M R, Selsted M E. Purification, characterization, and antimicrobial properties of peptides released from thrombin-induced human platelets. Blood. 1995;86:910a. (abstr 3626). [Google Scholar]
- 23.Tang Y Q, Yeaman M R, Selsted M E. Microbicidal and synergistic activities of human platelet factor-4 (hpf-4) and connective tissue activating peptide-3 (CTAP-3) Blood. 1995;86:556a. (abstr. 2212). [Google Scholar]
- 24.Thomas E L, Lehrer R I, Rest R F. Human neutrophil antimicrobial activity. Rev Infect Dis. 1988;10(Suppl. 2):S450–S456. doi: 10.1093/cid/10.supplement_2.s450. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Broek P J, Bril-Bazuin C, Mattie H. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Antibacterial activity of defensins against staphylococcus aureus pretreated with benzylpenicillin or azithromycin, abstr. C-88; p. 50. [Google Scholar]
- 26.Wu T M, Yeaman M R, Nast C, Itatani C, Bayer A S. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Ultrastructural evidence that platelet microbial protein (PMP) targets the bacterial cell membrane, abstract A-72; p. 146. [Google Scholar]
- 27.Xiong Y Q, Yeaman M R, Bayer A S. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. In vitro microbicidal activities of thrombin-induced platelet microbicidal protein (tPMP) and human neutrophil defensin (HNP-1) on Staphylococcus aureus are substantially influenced by pretreatment with antibiotics differing in mechanism of action, abstr. F-169; p. 275. [Google Scholar]
- 28.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial purification and staphylocidal activities of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeaman M R, Norman D C, Bayer A S. Staphylococcus aureussusceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992;60:2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeaman M R, Ibrahim A, Filler S G, Bayer A S, Edwards J E, Ghannoum M A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeaman M R, Sullam P M, Dazin P F, Bayer A S. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureusadherence to platelets in vitro. Infect Immun. 1994;62:3416–3423. doi: 10.1128/iai.62.8.3416-3423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeaman M R, Tang Y-Q, Shen A J, Bayer A S, Selsted M E. Purification and antimicrobial activities of rabbit platelet microbicidal proteins. Infect Immun. 1996;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 35.Yeaman M R, Bayer A S, Koo S P, Foss W, Sullam P M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureuscytoplasmic membrane by distinct mechanisms of action. J Clin Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]