Abstract
The present study aimed to identify the composition of the aerial parts of Rubia cordifolia L. A chemical investigation on the EtOAc extracts from the aerial parts of Rubia cordifolia resulted in the isolation of four new anthraquinones, namely Cordifoquinone A–D (1–4), along with 16 known anthraquinones. Their structures were elucidated on the basis of NMR and HR-ESIMS data. All isolates were assessed for their inhibitory effects on NO production in LPS-stimulated RAW 264.7 macrophage cells. Compounds 1, 3 and 10 exhibited significant inhibitory activities with IC50 values of 14.05, 23.48 and 29.23 μmol·L−1, respectively. Their antibacterial activities of four bacteria, Escherichia coli (ATCC 25922), Staphylococcus aureus subsp. aureus (ATCC 29213), Salmonella enterica subsp. enterica (ATCC 14028) and Pseudomonas aeruginosa (ATCC 27853), were also evaluated. Our results indicated that the antibacterial activity of these compounds is inactive.
Keywords: Rubia cordifolia, anthraquinones, NO inhibitory activity, antibacterial
1. Introduction
Infectious diarrhea (ID) is a kind of diarrhea caused by multiple pathogens and factors [1]. In 2012, a systematic analysis published in The Lancet showed that infectious diarrhea was the second leading cause of death in children under five years of age worldwide [2]. According to the pathogenesis, it can be divided into inflammatory diarrhea and secretory diarrhea, the former mainly caused by bacterial infection [3]. When the bacteria infect the human body, the pathogens further invade the intestinal mucosa and cause an inflammatory response that can lead to diarrhea. Nitric oxide (NO) is a bioactive molecule with extensive and important biological regulatory functions, which plays an important role in inflammation, tumors, and the cardiovascular system [4,5]. NO has been shown to be synthesized through the L-arginine/iNOS/NO pathway. In this pathway, NO and the amino acid L-citrulline are synthesized from the amino acid L-arginine by inducible nitric oxide synthase (iNOS) [4]. The excessive production of NO plays an important role in inflammatory response. Wink et al. found that when inflammation occurs, immune cells produce large amounts of inducible iNOS, which further produces NO for the immune response [6,7]. Therefore, inhibition of NO production is a direct indicator to verify the anti-inflammatory activity of compounds.
Worldwide, it is common for people to use medicinal plants to treat diarrhea [8,9,10]. Researchers have been working to find natural products with antidiarrheal properties from medicinal plants [11,12,13]. Rubia cordifolia Linn. is a grassy climbing vine herb that belongs to the genus Rubia and is found in China, India, Korea, Japan and the Far East of Russia, growing on sparse forests, forest margins and grasslands [14]. It is a well-known medicinal plant in Traditional Chinese Medicine (TCM). Rubiae Radix et Rhizoma, the dried root and rhizome of R. cordifolia, was first recorded in “Shennong Bencaojing” and used for hemorrhage syndrome and arthralgia. The aerial parts of R. cordifolia used for hemorrhage syndrome and diarrhea were recorded in “Lüchanyan Materia Medica”, another classic book on medicinal plants. In modern studies, R. cordifolia has been found to have a wide range of pharmacological activities. In 1983, Itokawa et al. found that a methanol extract of R. cordifolia had obvious anticancer activity [15]. In 2017, Gong et al. found that the water extract of R. cordifolia could treat senna leaf-induced diarrhea in mice [16]. In 2020, Wang et al. found that the ethanol extract of R. cordifolia could improve arachidonic acid metabolism by inhibiting COX-2 and cPLA2 [17]. Because of its excellent efficacy, R. cordifolia has been listed in the “Chinese Pharmacopeia” and is to this day [18]. Previous chemical studies revealed that the plant contains anthraquinones [19,20], naphthoquinones [20], terpenoids [21], cyclic hexapeptides [22] and lignans [23], some of which have antibacterial [24], anti-inflammatory [25] and antitumor activities [26].
At present, the research on R. cordifolia is mainly focused on the roots and rhizomes, and less on the aerial parts. Therefore, in order to find natural products with antibacterial or anti-inflammatory activities from this plant, we systematically studied the aerial parts of R. cordifolia. Our previous investigation identified eleven lignans from the aerial parts of R. cordifolia for the first time [23]. As a continuing investigation to find more undescribed and bioactive constituents from R. cordifolia, four new anthraquinones (1–4) along with 16 known anthraquinones (5–20) were isolated from the aerial parts of R. cordifolia. Compounds 1–5, 9, 10, 13, 15–17 and 20 were isolated from this plant for the first time. This study describes the isolation, structure elucidation, NO inhibitory and antibacterial assay of these anthraquinones.
2. Results and Discussion
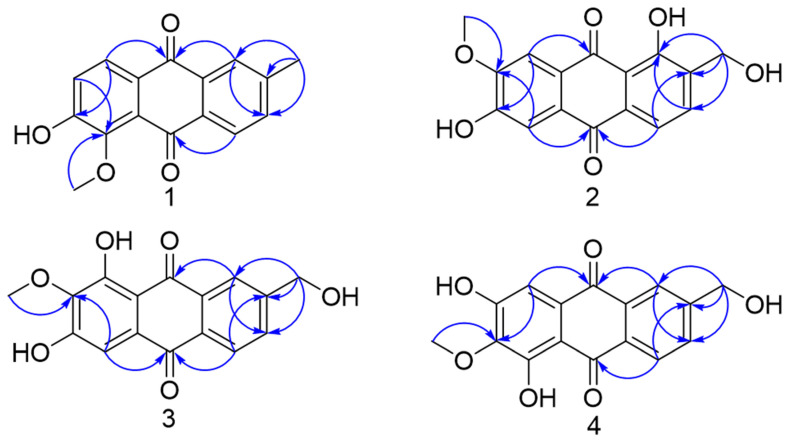
Compound 1 was obtained as a yellow powder, and the molecular formula was determined as C16H12O4, on the basis of HR-ESIMS (m/z 267.0669 [M − H]−, calcd. for C16H11O4, 267.0663). The UV spectrum showed maximum absorptions at 288 nm, suggesting a quinone structure. The 1H NMR data (Table 1) exhibited resonances which attributed to a 1, 3, 4-substituted benzene ring (δH 8.13 (1H, d, J = 7.9 Hz), 8.01 (1H, d, J = 1.8 Hz) and 7.71 (1H, dd, J = 7.9, 1.8 Hz)). The other benzene ring showed two orthodox-coupled aromatic proton resonances at (δH 8.04 (1H, d, J = 8.5 Hz) and 7.38 (1H, d, J = 8.5 Hz)). Moreover, aromatic methyl group δH 2.55 (3H, s), aromatic methoxy group δH 3.95 (3H, s) and phenolic hydroxyl group δH 9.40 (1H, s) protons were also observed. The 13C NMR exhibited 16 carbon signals, which consisted of 14 carbons of anthraquinone nucleus, a methoxy and a methyl. The HMBC (Figure 1) correlations of H-7 with C-5, 5-OMe with C-5 and H-8 with C-6/C-9 revealed that the methoxy group and hydroxyl group were connected at C-5 and C-6, respectively. In addition, the methyl group was deduced to be located at C-2 based on the HMBC correlations of 2-Me with C-1/C-2/C-3 and H-1 with C-3/C-9. Thus, the structure of compound 1 was established as 2-methyl-5-methoxy-6-hydroxyanthraquinone and named Cordifoquinone A, as shown in Figure 1.
Table 1.
1H and 13C NMR spectroscopic data (δ in ppm, J in Hz) for compounds 1–4.
| No | 1 a | 2 a | 3 b | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| δ H | δ C | δ H | δ C | δ H | δ C | δ H | δ C | |
| 1 | 8.01, d (1.8) | 127.2 | 158.6 | 8.12, d (1.7) | 123.5 | 8.06, d (1.7) | 124.0 | |
| 2 | 145.5 | 138.3 | 149.8 | 149.5 | ||||
| 3 | 7.71, dd (7.9, 1.8) | 135.4 | 7.63, d (7.8) | 133.1 | 7.76, dd (7.9, 1.7) | 131.6 | 7.78, dd (7.9, 1.7) | 131.7 |
| 4 | 8.13, d (7.9) | 127.8 | 7.79, d (7.8) | 118.8 | 8.06, d (7.9) | 126.8 | 8.13, d (7.9) | 126.3 |
| 4 a | 133.5 | 131.8 | 131.7 | 132.1 | ||||
| 5 | 148.4 | 7.54, s | 113.4 | 7.19, s | 109.9 | 157.0 | ||
| 6 | 158.1 | 155.1 | 157.0 | 139.8 | ||||
| 7 | 7.38, d (8.5) | 121.6 | 153.5 | 139.6 | 160.3 | |||
| 8 | 8.04, d (8.5) | 125.8 | 7.44, s | 108.8 | 159.2 | 7.16, s | 110.5 | |
| 8 a | 127.9 | 125.2 | 109.6 | 129.3 | ||||
| 9 | 182.6 | 187.8 | 186.1 | 181.9 | ||||
| 9 a | 133.7 | 115.2 | 133.2 | 132.9 | ||||
| 10 | 182.7 | 181.8 | 181.4 | 185.5 | ||||
| 10 a | 127.5 | 129.2 | 129.1 | 109.0 | ||||
| 2-Me | 2.53, s | 21.6 | ||||||
| 2-CH2OH | 4.62, s | 57.9 | 4.67, s | 62.2 | 4.66, s | 62.2 | ||
| 5-OMe | 3.95, s | 61.7 | ||||||
| 6-OMe | 3.82, s | 59.8 | ||||||
| 7-OMe | 3.95, s | 56.3 | 3.83, s | 59.9 | ||||
a 1H at 400 MHz and 13C at 150 MHz in Acetone-d6. b 1H at 400 MHz and 13C at 150 MHz in DMSO-d6.
Figure 1.
Key HMBC of compounds 1–4.
Compound 2 was obtained as a yellow powder, and the molecular formula was determined to be C16H12O6, on the basis of HR-ESIMS (m/z 299.0561 [M − H]−, calcd. for C16H11O6, 299.0560). The 1H NMR data (Table 1) exhibited two meta-coupled aromatic proton resonances at δH 7.79 (1H, d, J = 7.8 Hz) and 7.63 (1H, d, J = 7.8 Hz), two aromatic proton singlets at δH 7.54 and 7.44 (each 1H, s), an aromatic methine proton signals at δH 4.62 (2H, s), an aromatic methoxy group δH 3.95 (3H, s) and a phenolic hydroxyl group δH 13.03 (1H, s). The HMBC (Figure 1) correlations from the aromatic methine protons to C-1/C-2/C-3 and from H-4 to C-2/C-10, together with the 1H NMR data, implied the presence of a phenolic hydroxyl group at the C-1 and a hydroxymethyl group at the C-2 position. Moreover, the methoxy group was deduced to be located at C-7, and a phenolic hydroxyl group was deduced to be located at C-6, based on the HMBC correlations of the 7-OMe with C-7, H-5 with C-7/C-10 and H-8 with C-6/C-9. Therefore, the structure of compound 2 was confirmed as 1,6-dihydroxy-2-(hydroxymethyl)-7-methoxyanthraquinone and named Cordifoquinone B.
Compound 3 was obtained as a yellow powder and had the same molecular formula of C16H12O6 as compound 2, based on HR-ESIMS (m/z 299.0561 [M − H]−, calcd. for C16H11O6, 299.0560). According to the NMR data (Table 1), 3 and 2 were found to have similar structures, except that the phenolic hydroxyl group on the hydroxymethyl side was moved to the methoxy side. The 1H NMR data exhibited resonances attributed to a 1, 3, 4-substituted benzene ring (δH 8.12 (1H, d, J = 1.7 Hz), 8.06 (1H, d, J = 7.9 Hz) and 7.76 (1H, dd, J = 7.9, 1.7 Hz)), an aromatic proton singlet at δH 7.19 (1H, s), an aromatic methine proton signals at δH 4.67 (2H, s), an aromatic methoxy group δH 3.83 (3H, s) and an aromatic hydroxyl group δH 13.03 (1H, s). The methoxy group was deduced to be located at C-7 based on the HMBC (Figure 1) correlations of 7-OMe with C-7 and H-5 with C-7/C-10. Moreover, the HMBC correlations from the aromatic methine protons to C-1/C-2/C-3, from H-4 to C-2/C-10 and from H-1 to C-9 suggested that the hydroxymethyl group was directly connected to C-2. Based on the data mentioned above, the structure of compound 3 was defined to be 6,8-dihydroxy-2-(hydroxymethyl)-7-methoxyanthraquinone and named Cordifoquinone C.
Compound 4 was obtained as a yellow powder and had the same molecular formula of C16H12O6 as compound 3, based on HR-ESIMS (m/z 299.0561 [M − H]−, calcd for C16H11O6, 299.0560). Its 1H and 13C NMR data (Table 1) were very similar to those of compound 3. The difference between them was that two phenolic hydroxyl groups and the methoxy group position of compound 4 were connected to C-5/C-7 and C-6, respectively, which were supported by the HMBC (Figure 1) correlations from the aromatic methine protons to C-1/C-2/C-3, H-1 to C-9/C-3, 6-OMe to C-6 and H-8 to C-6/C-9. Accordingly, the whole structure of compound 4 was finally determined as 5,7-dihydroxy-2-(hydroxymethyl)-6-methoxyanthraquinone and named Cordifoquinone D.
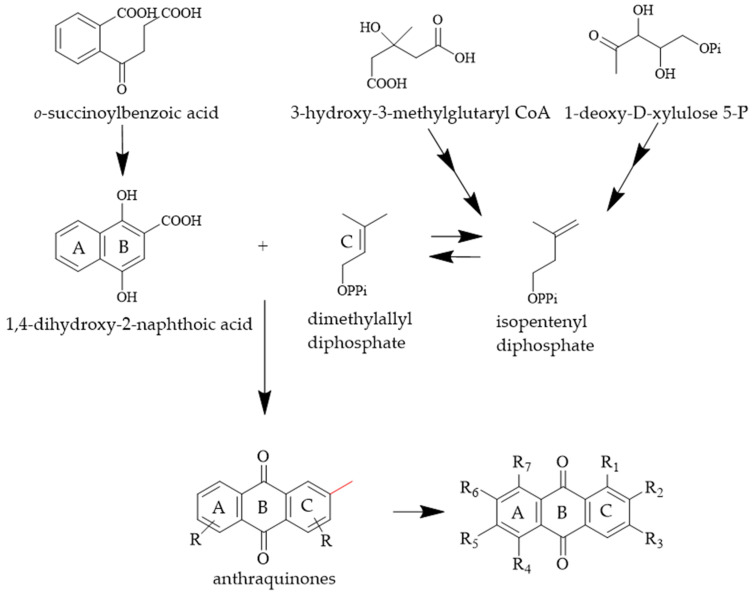
Additionally, 16 known anthraquinones, pustuline [27], 5,7-dihydroxy-6-methoxy-2-methylanthraquinone [28], soranjidiol [29], 1,6-dihydroxy-5-methoxy-2-methylanthraquinone [29], 1,5-dihydroxy-6-methoxy-2-methylanthraquinone [29], copareolatin 6-methylether [30], rubiadin [29], 2-methyl-1,3,6-trihydroxyanthraquinone [19], 7-hydroxy-2-(hydroxymethyl)-6-methoxyanthraquinone [31], 1-hydroxy-2-(hydroxymethyl)anthraquinone [32], 1,6-dihydroxy-2-(hydroxymethyl)-5-methoxyanthraquinone [33], 1,6-dihydroxy-2-(hydroxymethyl)-5,7-dimethoxyanthraquinone [34], 1,3-dihydroxy-2-hydroxymethyl-6-methoxyanthraquinone [35], 1,3-dihydroxy-2-(hydroxymethyl)-5,6-dimethoxyanthraquinone [36], 3,6-dihydroxy-2-(hydroxymethyl)-1-methoxyanthraquinone [37] and alizarin 1-methyl ether [38] were isolated from the aerial parts of R. cordifolia, as shown in Table 2. Their structures were elucidated by comparison of the NMR and ESI data with those reported in the literature. It is worth mentioning that anthraquinones in R. cordifolia are considered to be of the Rubia type; rings A and B are derived from horismite and α-ketoglutarate [39,40], whereas ring C is formed from isopentenyl diphosphate (IPP) [41]. Anthraquinone biosynthesized by this pathway has a methylation site in the C ring [42]. The aryl methyl group at site 2 can be further oxidized to hydroxymethyl. So, we hypothesized that compounds 1–20 might be synthesized by this pathway, as shown in Scheme 1.
Table 2.
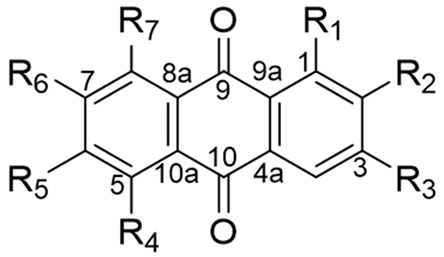
Chemical structures of isolated compounds 1–20 from Rubia cordifolia.
| |||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |
| 1 | H | Me | H | OMe | OH | H | H |
| 2 | OH | CH2OH | H | H | OH | OMe | H |
| 3 | H | CH2OH | H | H | OH | OMe | OH |
| 4 | H | CH2OH | H | OH | OMe | OH | H |
| 5 | H | Me | H | H | OMe | OH | H |
| 6 | H | Me | H | OH | OMe | OH | H |
| 7 | OH | Me | H | H | OH | H | H |
| 8 | OH | Me | H | OMe | OH | H | H |
| 9 | OH | Me | H | OH | OMe | H | H |
| 10 | OH | Me | H | OH | OMe | OH | H |
| 11 | OH | OMe | OH | H | H | H | H |
| 12 | OH | OMe | OH | H | OH | H | H |
| 13 | H | CH2OH | H | H | OMe | OH | H |
| 14 | OH | CH2OH | H | H | H | H | H |
| 15 | OH | CH2OH | H | OMe | OH | H | H |
| 16 | OH | CH2OH | H | OMe | OH | OMe | H |
| 17 | OH | CH2OH | OH | H | OMe | H | H |
| 18 | OH | CH2OH | OH | OMe | OMe | H | H |
| 19 | OMe | CH2OH | OH | H | OH | H | H |
| 20 | OMe | OH | H | H | H | H | H |
Scheme 1.
Plausible biosynthetic pathway for compounds 1–20.
All isolated compounds 1–20 were evaluated for their inhibitory effects on NO production in LPS-activated RAW264.7 macrophage cells. Cell viability was first examined by MTT assay to exclude false positive results caused by the potential cytotoxicity of the tested compounds. NG-Methyl-L-arginine acetate salt, an inhibitor of NO synthase, was used as a positive control (IC50 42.36 ± 2.47 μmol·L−1). As a result, compounds 1, 3 and 10 showed significant inhibitory effects against NO production, with IC50 values of 14.05 ± 0.48, 23.48 ± 1.05 and 29.23 ± 0.34 μmol·L−1 (Table 3), respectively, which were more potent than the positive control. By comparing the structure and activity of compounds 1, 3, 10, we found that the orthoposition of methoxyl and phenolic hydroxyl groups in the A ring of anthraquinone was essential for the NO inhibitory activity, and the hydroxyl group at position C-6 or C-7 provided a greater contribution. It is known that the synthesis of NO requires the addition of superoxide anions [43]. So, we hypothesized that the hydroxyl group in anthraquinone may capture superoxide anions, which are necessary for the synthesis of NO. Compound 1 showed stronger NO inhibitory activity than compounds 3 and 10, indicating that the activity was not necessarily related to the number of hydroxyl groups but may be related to the position of hydroxyl groups. We hypothesized that it may be due to the formation of intramolecular hydrogen bonds between hydroxyl group at position C-5 or C-8 and ketone carbonyl group at position C-9 or C-10, which makes the compound structure more stable and reduces its ability to capture superoxide anions. Through this speculation, it is reasonable to explain why compound 3 showed slightly higher NO inhibitory activity than compound 10. In addition, all compounds were evaluated for their antibacterial activities against four bacteria: Escherichia coli (ATCC 25922), Staphylococcus aureus subsp. aureus (ATCC 29213), Salmonella enterica subsp. enterica (ATCC 14028), and Pseudomonas aeruginosa (ATCC27853). However, no antibacterial activity was observed for these compounds at concentrations below 128 μg·mL−1 in this bioassay.
Table 3.
NO inhibitory activities of compounds 1, 3 and 10.
| Compounds | NO Inhibitory Effects (IC50/μmol·L−1) |
RAW 264.7 Cell Viability a (%) |
|---|---|---|
| 1 | 14.05 ± 0.48 | 105.69 ± 0.25 |
| 3 | 23.48 ± 1.05 | 97.67 ± 1.21 |
| 10 | 29.23 ±0.34 | 101.80 ± 1.10 |
| L-NMMA b | 42.36 ± 2.47 | 98.72 ± 0.94 |
a RAW 264.7 cells treated with samples at 50 μmol·L−1. b Positive control.
3. Experimental
3.1. General Experimental Procedures
UV spectra were taken on a Shimadzu UV-2401 spectrophotometer (Shimadzu, Kyoto, Japan). IR (KBr) spectra were recorded on a Bruker Vertex 70 (Bruker, Bremerhaven, Germany). The 1D and 2D NMR experiments were carried out using Avance III-400 and III-600 spectrometers (Bruker, Bremerhaven, Germany) with TMS as an internal standard. HR-ESIMS data were recorded on an Agilent 6540 Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Silica gel (200–300 mesh, Linyi Haixiang Co. Ltd., Linyi, China) and Sephadex LH-20 gel (Amersham Biosciences, Uppsala, Sweden) were employed for column chromatography. Thin-layer chromatography (TLC) analyses were performed on silica gel GF254 plates (Haohong, Chemical Co. Ltd., Yantai, China). HPLC purification was achieved on an Agilent 1100 instrument (Agilent Technologies, Santa Clara, CA, USA) with an Agilent ZORBAX SB-C18 column (5 μm, 9.4 × 250 mm, Agilent Technologies, Santa Clara, CA, USA).
3.2. Plant Materials
The aerial parts of Rubia cordifolia Linn. Were collected in June 2020 from Huaiyuan County, Anhui Province, China, and authenticated by Prof. Shou-Jin Liu from Anhui University of Traditional Chinese Medicine (Anhui, China). A voucher specimen (No. 20200601QC) was deposited at the Chinese Medicine Resource Center, Anhui University of Traditional Chinese Medicine (ACM).
3.3. Extraction and Isolation
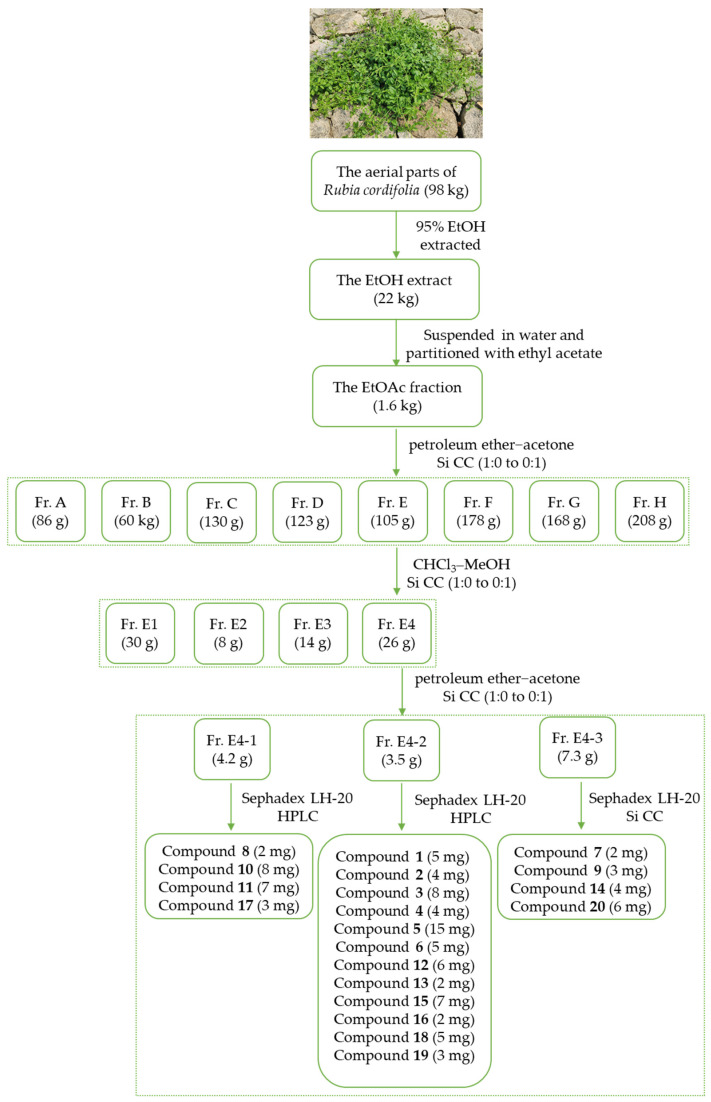
The air-dried and powdered aerial parts of R. cordifolia (22 kg) were extracted with 95% EtOH at room temperature. After removal of the solvent under reduced pressure, the EtOH extract (22 kg) was suspended in water and partitioned with EtOAc. The EtOAc fraction (1.6 kg) was dissolved in EtOAc and chromatographed on silica gel column chromatography (Si CC), eluted with petroleum ether−acetone (1:0 to 0:1) to yield eight fractions, Fr. A–H (86 g, 60 g, 130 g, 123 g, 105 g, 178 g, 168 g, and 208 g). Fr. E (105 g) was dissolved in acetone and subjected to Si CC eluted with a gradient of CHCl3–MeOH (1:0 to 0:1) to obtain four major fractions, Fr. E1 to Fr. E4 (30 g, 8 g, 14 g, and 26 g). The extraction and separation process is shown in Figure 2.
Figure 2.
The extraction and separation process of the aerial parts of Rubia cordifolia L.
Fr. E4 (26 g) was dissolved in acetone and further chromatographed over Si CC using petroleum ether−acetone (1:0 to 0:1) to give three subfractions, Fr. E4-1 to Fr. E4-3 (4.2 g, 3.5 g, 7.3 g). Compounds 8 (2 mg), 10 (8 mg), 11 (7 mg) and 17 (3 mg) were purified from Fr. E 4-1 (4.2 g) by Sephadex LH-20 (MeOH-CHCl3, 1:1) and semipreparative HPLC (H2O-MeOH, 75:25, 2.0 mL/min). Fr. E 4-2 (3.5 g) was applied to Sephadex LH-20 (CHCl3-MeOH, 1:1) to yield four subfractions (Fr. E4-2-1 to Fr. E4-2-4). Fr. E4-2-2 (127 mg) was separated by semipreparative HPLC (H2O-MeOH, 70:30, 2.0 mL·min−1) to obtain 12 (6 mg) and 18 (5 mg). Fr. E4-2-3 (238 mg) was separated by semipreparative HPLC (H2O-MeOH, 60:40, 2.0 mL·min−1) to afford 1 (5 mg), 2 (4 mg), 3 (8 mg), 5 (15 mg) and 15 (7 mg). Fr. E4-2-4 (182 mg) was separated by semipreparative HPLC (H2O-MeOH, 60:40, 2.0 mL·min−1) to afford 4 (4 mg), 6 (5 mg), 13 (2 mg), 16 (2 mg) and 19 (3 mg). Fr. E4-3 (7.3 g) gave 7 (2 mg), 9 (3 mg), 14 (4 mg) and 20 (6 mg) after repeated chromatography over Sephadex LH-20 (CHCl3-MeOH, 1:1) and Si CC gradient of CHCl3-MeOH (1:0 to 0:1).
Cordifoquinone A (1): Yellow powder; UV (MeOH) λmax (log ε) 251 (4.01), 264 (3.97) nm; IR (KBr) νmax 3423, 2955, 1666, 1629, 1581, 1428, 1363, 1302, 1256, 1024, 799 cm−1; 1H and 13C NMR data, see Table 1 and Figure S1; HR-ESIMS (m/z 267.0669 [M − H]−, calcd. for C16H11O4, 267.0663).
Cordifoquinone B (2): Yellow powder; UV (MeOH) λmax (log ε) 288 (3.89), 249 (3.53) nm; IR (KBr) νmax 3433, 2936, 1632, 1591, 1518, 1424, 1384, 1308, 1221, 1116, 1064, 754 cm−1; 1H and 13C NMR data, see Table 1 and Figure S2; HR-ESIMS (m/z 299.0561 [M − H]−, calcd. for C16H11O4, 299.0560).
Cordifoquinone C (3): Yellow powder; UV (MeOH) λmax (log ε) 248 (3.74), 285 (3.73) nm; IR (KBr) νmax 3443, 2952, 1662, 1632, 1596, 1452, 1385, 1340, 1279, 1219, 1134, 988 cm−1; 1H and 13C NMR data, see Table 1 and Figure S3; HR-ESIMS (m/z 299.0561 [M − H]−, calcd. for C16H11O6, 299.0560).
Cordifoquinone D (4): Yellow powder; UV (MeOH) λmax (log ε) 248 (3.47), 285 (3.41) nm; IR (KBr) νmax 3439, 2926, 1633, 1601, 1577, 1384, 1282, 1162, 975, 851 cm−1; 1H and 13C NMR data, see Table 1 and Figure S4; HR-ESIMS (m/z 299.0561 [M − H]−, calcd. for C16H11O6, 299.0560).
3.4. NO Inhibitory Assay
Murine macrophage cell line RAW264.7 was obtained from Shanghai Cell Bank of Chinese Academy of Sciences. RAW264.7 cells were seeded in 96-well cell culture plates (1.5 × 105 cells/well), stimulated with 1 μg·mL−1 LPS (Sigma, St. Louis, MO, USA) for 18 h, and treated with serial dilutions of the compounds with a maximum concentration of 50 μM in triplicate during this period. NG-Methyl-L-arginine acetate salt (L-NMMA, Sigma), a well-known nitric oxide synthase (NOS) inhibitor, was used as a positive control [44]. NO production in the supernatant was assessed by Griess reagents (Reagent A and Reagent B, respectively, Sigma). The absorbance at 570 nm was measured with a microplate reader (Thermo, Waltham, MA, USA). The viability of RAW264.7 cells was evaluated by the MTS assay simultaneously to exclude the interference of the cytotoxicity of the test compounds. All tests were performed in triplicate, and the results were expressed as IC50 values.
3.5. Antibacterial Assay
Escherichia coli (ATCC 25922), Staphylococcus aureus subsp. aureus (ATCC 29213), Sal-monella enterica subsp. enterica (ATCC 14028), and Pseudomonas aeruginosa (ATCC 27853) were obtained from China General Microbiological Culture Collection Center. The inoculated strains and suspension were cultured for 24 h and adjusted to 0.5 McFarlane standard turbidity. A 96-well plate was prepared by injecting 100 µL of Muller Hinton broth/Luria-Bertani into each well. A volume of 100 µL of stock solution from the initial prepared samples was added to the first well. Then, 100 µL from their two-fold serial dilutions were transferred into consecutive wells, and 100 µL of the inoculums was added to achieve a final inoculum concentration of 5 × 105 CFU/mL. The final volume in each well was 200 µL. Ceftazidime (Yuanye Co. Ltd., Shanghai, China) and sodium penicillin G (Labgic Co. Ltd., Beijing, China) were used as positive controls. After incubation at 37 °C for 24 h, growth was monitored by a Microplate Reader at 625 nm (PowerWave XS, BioTek, Winooski, VT, USA) [45].
4. Conclusions
Four new anthraquinones (1–4) and 16 known anthraquinones (5–20) were isolated from a 95% EtOH extract of aerial prat from R. cordifolia and elucidated by UV, IR, HR-ESIMS and NMR spectroscopic data. Among the isolated compounds, compounds 1, 3 and 10 displayed NO inhibitory activity with IC50 values of 14.05, 23.48 and 29.23 μmol·L−1. The structure and activity relationship analysis revealed that the orthoposition of methoxyl and phenolic hydroxyl groups in the A ring of anthra-quinone was essential for the NO inhibitory activity. The hydroxyl group at position C-6 or C-7 may have promoted the NO inhibitory activity, whereas the hydroxyl group at position C-5 or C-8 had the opposite effect. In addition, this series of anthraquinones showed no activity in tests of antibacterial activity. This study proved the reasonableness of using the aerial part of R. cordifolia as a medicine to treat diarrhea in Traditional Chinese medicine. It also provides scientific evidence and a foundation for the understanding of the anti-inflammatory effects and further utilization of R. cordifolia. In future research, both the extract and the natural products of R. cordifolia can be considered a drug or a health supplement to treat diarrhea.
Abbreviations
| ID | infectious diarrhea |
| NO | nitric oxide |
| iNOS | inducible nitric oxide synthase |
| TCM | Traditional Chinese Medicine |
| HR-ESIMS | high resolution electrospray ionization mass spectroscopy |
| UV | ultraviolet-visible |
| NMR | nuclear magnetic resonance |
| HMBC | heteronuclear multiple bond correlation |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromid |
| IR | infrared ra |
| Q-TOF | quadrupole-time of flight |
| HPLC | high performance liquid chromatography |
| TLC | thin-layer chromatography |
| Si CC | silica gel column chromatography |
| EtOH | ethyl alcohol |
| EtOAc | ethyl acetate |
| MeOH | Methanol |
| USA | The United States of America |
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: 1H, 13C and HMBC NMR spectra of compound 1; Figure S2: 1H, 13C and HMBC NMR spectra of compound 2; Figure S3: 1H, 13C and HMBC NMR spectra of compound 3; Figure S4: 1H, 13C and HMBC NMR spectra of compound 4.
Author Contributions
Conceptualization, S.-J.L. and J.-M.H.; methodology, H.L. and F.-C.R.; software, H.L.; validation, W.Q., H.Z. and W.-T.F.; investigation, H.L., Q.-H.K., and J.-M.Z.; re-sources, C.-W.F.; writing—original draft preparation, L.H; writing—review and editing, H.L. and F.-C.R.; visualization, L.Y.; supervision, J.-M.H.; project administration, S.-J.L.; funding acquisition, S.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by the Special Subsidies for TCM public health services, grant number RH2100000743. The APC was funded by National Administration of Traditional Chinese Medicine (NATCM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds compound 1 to 20 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Snyder J.D., Merson M. The magnitude of the global problem of acute diarrhoeal disease: A review of active surveillance data. Bull. WHO. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E., Rudan I., Campbell H., Cibulskis R., Li M., et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Stephen J. Pathogenesis of infectious diarrhea. Can. J. Gastroenterol. 2001;15:669–683. doi: 10.1155/2001/264096. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;2:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wink D.A., Mitchell J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–456. doi: 10.1016/S0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 7.Grisham M.B., Jourd’Heuil D., Wink D.A. Nitric oxide—I. Physiological chemistry of nitric oxide and its metabolites: Implications in inflammation. Am. J. Physiol. 1999;276:315–321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 8.Umer S., Tekewe A., Kebede N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complementary Altern. Med. 2013;13:21. doi: 10.1186/1472-6882-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orsi P.R., Bonamin F., Severi J.A., Santos R.C., Vilegas W., Hiruma-Lima C.A., Di Stasi L.C. Hymenaea stigonocarpa Mart. ex Hayne: A Brazilian medicinal plant with gastric and duodenal anti-ulcer and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012;143:81–90. doi: 10.1016/j.jep.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Ali N., Alam H., Khan A., Ahmed G., Shah W.A., Nabi M., Junaid M. Antispasmodic and antidiarrhoeal activity of the fruit of Rosa moschata. BMC Complementary Altern. Med. 2014;14:485. doi: 10.1186/1472-6882-14-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atta A.H., Mouneir S.M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J. Ethnopharmacol. 2004;92:303–309. doi: 10.1016/j.jep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Mbagwu H.O.C., Adeyemi O.O. Anti-diarrhoeal activity of the aqueous extract of Mezoneuron benthamianum Baill (Caesalpiniaceae) J. Ethnopharmacol. 2008;116:16–20. doi: 10.1016/j.jep.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Praxedes de Sales I.R., Frota Machado F.D., Marinho A.F., Suassuna Carneiro Lucio A.S., Barbosa Filho J.M., Batista L.M. Cissampelos sympodialis Eichl. (Menispermaceae), a medicinal plant, presents antimotility and antidiarrheal activity in vivo. BMC Complement. Altern. Med. 2015;15:253. doi: 10.1186/s12906-015-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinese Academy of Sciences Flora of China Commission . Flora of China. Volume 71. Science Press; Beijing, China: 1999. pp. 287–315. [Google Scholar]
- 15.Itokawa H., Takeya K., Mihara K., Mori N., Hamanaka T., Sonobe T., Iitaka Y. Studies on the antitumor cyclic hexapeptides obtained from Rubiae radix. Chem. Pharm. Bull. 1983;31:1424–1427. doi: 10.1248/cpb.31.1424. [DOI] [PubMed] [Google Scholar]
- 16.Gong X.P., Sun Y.Y., Chen W., Guo X., Guan J.K., Li D.Y., Du G. Anti-diarrheal and anti-inflammatory activities of aqueous extract of the aerial part of Rubia cordifolia. BMC Complementary Altern. Med. 2017;17:20. doi: 10.1186/s12906-016-1527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K., Gao L., Zhang Q., Zhang Y., Yao W.F., Zhang M., Tang Y.P., Ding A.W., Zhang L. Revealing the mechanisms and the material basis of Rubia cordifolia L. on abnormal uterine bleeding with uniting simultaneous determination of four components and systematic pharmacology approach-experimental validation. J. Pharm. Biomed. Anal. 2020;189:113475. doi: 10.1016/j.jpba.2020.113475. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. China Medical Science Press; Beijing, China: 2020. p. 245. [Google Scholar]
- 19.Itokawa H., Mihara K., Takeya K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem. Pharm. Bull. 1983;31:2353–2358. doi: 10.1248/cpb.31.2353. [DOI] [Google Scholar]
- 20.Hu Y.Y., Zhang X.J., Zhang Z.H., Wang Z., Tan N.H. Qualitative and quantitative analyses of quinones in multi-origin Rubia species by ultra-performance liquid chromatography-tandem mass spectrometry combined with chemometrics. J. Pharm. Biomed. Anal. 2020;189:113471. doi: 10.1016/j.jpba.2020.113471. [DOI] [PubMed] [Google Scholar]
- 21.Arisawa M., Ueno H., Nimura M., Hayashi T., Morita N. Rubiatriol, a new triterpenoid from the Chinese drug Qian Cao Gen, Rubia cordifolia. J. Nat. Prod. 1986;49:1114–1116. doi: 10.1021/np50048a027. [DOI] [Google Scholar]
- 22.Chen X.Q., Zhao S.M., Wang Z., Zeng G.Z., Huang M.B., Tan N.H. Rubicordins A-C, new cyclopeptides from Rubia cordifolia with cytotoxicity and inhibiting NF-kappa B signaling pathway. Tetrahedron. 2015;71:9673–9678. doi: 10.1016/j.tet.2015.10.044. [DOI] [Google Scholar]
- 23.Zhang M.T., Yang L., Hu J.M., Zhang H., Shi X.Q., Liu S.J. Study on lignan constituents from aerial part of Rubia cordifolia. Chin. Tradit. Herb. Drugs. 2017;48:4856–4859. [Google Scholar]
- 24.Qiao Y.F., Wang S.K., Wu L.J., Li X., Zhu T.R. Antibacterial constituents from the roots of Rubia cordifolia L. Acta. Pharm. Sin. 1990;25:834–839. [PubMed] [Google Scholar]
- 25.Tao J., Morikawa T., Ando S., Matsuda H., Yoshikawa M. Bioactive constituents from chinese natural medicines. XI. Inhibitors on NO production and degranulation in RBL-2H3 from Rubia yunnanensis: Structures of rubianosides II, III, and IV, rubianol-g, and rubianthraquinone. Chem. Pharm. Bull. 2003;51:654–662. doi: 10.1248/cpb.51.654. [DOI] [PubMed] [Google Scholar]
- 26.Son J.K., Jung S.J., Jung J.H., Fang Z., Lee C.S., Seo C.S., Moon D.C., Min B.S., Kim M.R., Woo M.H. Anticancer constituents from the roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008;56:213–216. doi: 10.1248/cpb.56.213. [DOI] [PubMed] [Google Scholar]
- 27.Nunez Montoya S.C., Agnese A.M., Cabrera J.L. Anthraquinone Derivatives from Heterophyllaea pustulata. J. Nat. Prod. 2006;69:801–803. doi: 10.1021/np050181o. [DOI] [PubMed] [Google Scholar]
- 28.El-Lakany A.M., Aboul-Ela M.A., Abdel-Kader M.S., Badr J.M., Sabri N.N., Goher Y. Anthraquinones with antibacterial activities from Crucianella maritima L. growing in Egypt. Nat. Prod. Sci. 2004;10:63–68. [Google Scholar]
- 29.Ismail N.H., Ali A.M., Aimi N., Kitajima M., Takayama H., Lajis N.H. Anthraquinones from Morinda elliptica. Phytochemistry. 1997;45:1723–1725. doi: 10.1016/S0031-9422(97)00252-5. [DOI] [Google Scholar]
- 30.Koyama J., Ogura T., Tagahara K. Anthraquinones of Galium spurium. Phytochemistry. 1993;33:1540–1542. doi: 10.1016/0031-9422(93)85131-A. [DOI] [Google Scholar]
- 31.Kang X.D., Li X., Zhao C.C., Mao Y. Two new anthraquinones from Hedyotis diffusa W. J. Asian. Nat. Prod. Res. 2008;10:193–197. doi: 10.1080/10286020701394464. [DOI] [PubMed] [Google Scholar]
- 32.Itokawa H., Ibraheim Z.Z., Qiao Y.F., Takeya K. Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 1993;41:1869–1872. doi: 10.1248/cpb.41.1869. [DOI] [PubMed] [Google Scholar]
- 33.Longue Ekon J.P., Zra T., Lobe Songue J., Wondja Ngoko M.L., Ngassoum M.B., Talla E., Kamdem Waffo A.F., Sewald N., Wansi J.D. New anthraquinone derivatives from the stem barks of Morinda lucida Benth. Phytochem. Lett. 2020;39:94–98. doi: 10.1016/j.phytol.2020.06.010. [DOI] [Google Scholar]
- 34.El-Gamal A.A., Takeya K., Itokawa H., Halim A.F., Amer M.M., Saad H.E.A., Awad S.A. Anthraquinones from the polar fractions of Galium sinaicum. Phytochemistry. 1996;42:1149–1155. doi: 10.1016/0031-9422(96)00080-5. [DOI] [PubMed] [Google Scholar]
- 35.Banthorpe D.V., White J.J. Novel anthraquinones from undifferentiated cell cultures of Galium verum. Phytochemistry. 1995;38:107–111. doi: 10.1016/0031-9422(94)00579-I. [DOI] [Google Scholar]
- 36.Wisetsai A., Lekphrom R., Schevenels F.T. New anthracene and anthraquinone metabolites from Prismatomeris filamentosa and their antibacterial activities. Nat. Prod. Res. 2021;35:1582–1589. doi: 10.1080/14786419.2019.1627352. [DOI] [PubMed] [Google Scholar]
- 37.Ling S.K., Komorita A., Tanaka T., Fujioka T., Mihashi K., Kouno I. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae) Chem. Pharm. Bull. 2002;50:1035–1040. doi: 10.1248/cpb.50.1035. [DOI] [PubMed] [Google Scholar]
- 38.Halim A.F., Abd El-Fattah H., El-Gamal A.A., Thomson R.H. Anthraquinones from Galium sinaicum. Phytochemistry. 1991;31:355–356. doi: 10.1016/0031-9422(91)83077-X. [DOI] [Google Scholar]
- 39.Leistner E. Second pathway leading to anthraquinones in higher plants. Phytochemistry. 1971;10:3015–3020. doi: 10.1016/S0031-9422(00)97345-X. [DOI] [Google Scholar]
- 40.Sieweke H.J., Leistner E. o-Succinylbenzoate:coenzyme A ligase from anthraquinone producing cell suspension cultures of Galium mollugo. Phytochemistry. 1992;31:2329–2335. doi: 10.1016/0031-9422(92)83275-4. [DOI] [Google Scholar]
- 41.Lv X., Wang F., Zhou P., Ye L., Xie W., Xu H., Yu H. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat. Commun. 2016;7:12851. doi: 10.1038/ncomms12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y.S., van der Heijden R., Lefeber A.W.M., Erkelens C., Verpoorte R. Biosynthesis of anthraquinones in cell cultures of Cinchona ‘Robusta’ proceeds via the methylerythritol 4-phosphate pathway. Phytochemistry. 2002;59:45–55. doi: 10.1016/S0031-9422(01)00296-5. [DOI] [PubMed] [Google Scholar]
- 43.Ignarro L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 44.Reif D.W., McCreedy S.A. N-Nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch. Biochem. Biophys. 1995;320:170–176. doi: 10.1006/abbi.1995.1356. [DOI] [PubMed] [Google Scholar]
- 45.Walker R.D. Standards for antimicrobial susceptibility testing. Am. J. Vet. Res. 1999;60:64–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.