Abstract
An in vitro pharmacokinetic model was used to simulate the pharmacokinetics of trovafloxacin, ofloxacin, and ciprofloxacin in human serum and to compare their pharmacodynamics against eight Streptococcus pneumoniae strains. The MICs of ofloxacin and ciprofloxacin ranged from 1 to 2 μg/ml. Trovafloxacin was 8- to 32-fold more potent, with MICs of 0.06 to 0.12 μg/ml. Logarithmic-phase cultures were exposed to peak concentrations of trovafloxacin, ofloxacin, or ciprofloxacin achieved in human serum after 200-, 400-, and 750-mg oral doses, respectively. Trovafloxacin was dosed at 0 and 24 h, and ofloxacin and ciprofloxacin were dosed at 0, 12, and 24 h. Human elimination pharmacokinetics were simulated, and viable bacterial counts were measured at 0, 2, 4, 6, 8, 12, 24, and 36 h. Trovafloxacin was rapidly and significantly bactericidal against all eight strains evaluated, with viable bacterial counts decreasing at least 5 logs to undetectable levels. Times to 99.9% killing were only 1 to 3 h. Although the rate of killing with ofloxacin was substantially slower than that with trovafloxacin, ofloxacin was also able to eradicate all eight strains from the model, despite a simulated area under the inhibitory curve/MIC ratio (AUC/MIC) of only 49. In contrast, ciprofloxacin eradicated only five strains (AUC/MIC = 44) from the model. Against the other three strains (AUC/MIC = 22), the antibacterial activity of ciprofloxacin was substantially diminished. These data corroborate clinical data and suggest that trovafloxacin has a pharmacodynamic advantage over ciprofloxacin and ofloxacin against S. pneumoniae in relation to its enhanced antipneumococcal activity.
Streptococcus pneumoniae is a leading cause of pneumonia, bacteremia, otitis media, and sinusitis (24). Since the 1970s, β-lactam resistance and resistance to multiple antibiotics have been steadily increasing worldwide, limiting the therapeutic options for treatment of some infections (19). It is essential, therefore, that the search for more-effective antipneumococcal drugs continue.
The fluoroquinolones are one class of antibiotics currently being developed for improved therapy of pneumococcal infections. In the past, the role of ciprofloxacin and ofloxacin in treatment of pneumococcal infections has been controversial because of their marginal in vitro activity. Ciprofloxacin and ofloxacin MICs at which 90% of the isolates are inhibited against S. pneumoniae are generally two- to fourfold above the susceptible breakpoints (14, 27, 36). Trovafloxacin is a new fluoroquinolone that exhibits enhanced potency against S. pneumoniae. In comparisons with ciprofloxacin and ofloxacin, trovafloxacin is generally 8- to 16-fold more potent, with MICs at which 90% of the isolates are inhibited of 0.12 to 0.25 μg/ml (27, 33, 40). The purpose of this study was to compare the in vitro pharmacodynamics of trovafloxacin with those of ofloxacin and ciprofloxacin against S. pneumoniae and to determine if trovafloxacin’s enhanced potency would translate to enhanced pharmacodynamic killing when serum pharmacokinetics were simulated. With an in vitro pharmacokinetic model (IVPM), oral doses of 200 mg of trovafloxacin, 400 mg of ofloxacin, and 750 mg of ciprofloxacin were simulated and time-kill pharmacodynamic interactions were evaluated over 36 h.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Eight clinical isolates of S. pneumoniae were selected for this study, four of which were resistant to penicillin (MICs of 2 to 4 μg/ml). Logarithmic-phase cultures were prepared by suspending 10 colonies from a 14-h culture on Trypticase soy agar supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) into 6 ml of Todd-Hewitt broth (Unipath/Oxoid, Ogdensburg, N.Y.) supplemented with 0.5% yeast extract (THY) (20). Viable bacterial counts after 10 h of incubation at 37°C in 5% CO2 ranged from 1 × 108 to 5 × 108 CFU/ml.
Antibiotic preparations.
Trovafloxacin powder was supplied by Pfizer Inc., Groton, Conn. Ofloxacin powder was supplied by R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J. Ciprofloxacin powder was supplied by Bayer Corporation, West Haven, Conn. Trovafloxacin and ofloxacin powders were dissolved in 0.2 ml of 0.1 M NaOH, diluted to final volume with distilled water, and sterilized by passage through a 0.20-μm-pore-size Acrodisc syringe filter membrane (Gelman Sciences, Ann Arbor, Mich.). Ciprofloxacin powder was reconstituted with distilled water and filter sterilized as described above.
Antimicrobial susceptibility testing.
Susceptibility tests with trovafloxacin, ofloxacin, and ciprofloxacin were performed by microbroth dilution methodology according to the procedure recommended by the National Committee for Clinical Laboratory Standards (25).
IVPM.
The IVPM used in these studies was a modification of the original model described by Blaser and colleagues (3) and has been described in detail previously (21). The hollow-fiber cartridges (model BR130; Unisyn Fibertech, San Diego, Calif.) used in these studies consisted of 2,250 cellulose acetate hollow fibers contained within a polycarbonate housing, with each fiber having 30,000-molecular-weight pores within its wall. The surface area of exchange between the hollow fibers and the extracapillary space (peripheral compartment) was 1.5 ft2. Medium containing antibiotic was pumped through the lumen of the fibers at a flow rate of 20 ml/min with Masterflex computerized peristaltic pumps (model 7550-90; Cole-Parmer Instrument Company, Vernon Hills, Ill.) and Easy-Load pump heads (model 7518-00; Cole-Parmer). In addition, the bacterial culture within the peripheral compartment was continuously circulated with similar peristaltic pumps at a rate of 20 ml/min through a loop of silicone tubing attached to two ports entering and exiting the peripheral compartment. The initial volume of culture circulated through the peripheral compartment and loop of silicone tubing was 35 to 40 ml. When samples were required from the peripheral compartment, 0.5-ml volumes were removed through a four-way sterile stopcock (Medex, Hilliard, Ohio) positioned within the loop of silicone tubing. The volume of THY within the central reservoir varied with each drug depending upon the elimination half-life, such that the rate of dilution and elimination could be set at the minimum 0.7 ml/min allowed by the peristaltic pumps. The elimination half-lives simulated were 11 h for trovafloxacin (38, 39), 6.5 h for ofloxacin (16), and 4 h for ciprofloxacin (16). The corresponding central reservoir volumes were 700 ml for studies with trovafloxacin, 450 ml for ofloxacin, and 250 ml for ciprofloxacin. In drug-free control experiments, the volume of THY in the central reservoir was 500 ml and the flow rates for addition of fresh medium and elimination from the central reservoir were 2 ml/min.
Pharmacokinetics in the IVPM.
Peak concentrations of trovafloxacin, ofloxacin, and ciprofloxacin achieved in human serum after single oral doses of 200 mg of trovafloxacin, 400 mg of ofloxacin, and 750 mg of ciprofloxacin were targeted in these studies (16, 42). To evaluate the pharmacokinetics of trovafloxacin, ofloxacin, and ciprofloxacin in uninfected chambers, peak concentrations were dosed into the central reservoir at 0 h for trovafloxacin and 0 and 12 h for ciprofloxacin and ofloxacin. Samples were removed from the peripheral compartment at 0, 0.5, 1, 2, 4, 8, 12, 12.5, 16, 20, and 24 h, and drug concentrations were measured by disk diffusion bioassay with a susceptible strain of Escherichia coli. Areas under the concentration-time curves over 24 h (AUC0–24) for trovafloxacin, ofloxacin, and ciprofloxacin were calculated by the trapezoidal rule. The AUC/MIC ratios (AUC/MICs) were calculated by dividing the AUC0–24 by the MIC (31).
Pharmacodynamics against S. pneumoniae.
Logarithmic-phase cultures were diluted into fresh 37°C THY for a final inoculum of 106 to 107 CFU/ml; introduced into the peripheral compartment of the IVPM; and exposed to trovafloxacin, ofloxacin, or ciprofloxacin as described above. Pharmacodynamic experiments were performed in ambient air at 37°C. At 0, 2, 4, 6, 8, 12, 24, and 36 h, samples were removed from the peripheral compartment and viable bacterial counts were measured by plating serial 10-fold dilutions of each sample into Todd-Hewitt agar (THA: BBL) and incubating plates overnight at 37°C in 5% CO2. The lowest dilution plated was 0.1 ml of undiluted sample from the peripheral compartment. Since 30 colonies per plate is the lower limit of accurate quantitation by pour plate methodology, the lowest number of bacteria that could be accurately counted was 300 CFU/ml. The lowest level of detection was 10 CFU/ml.
To prevent antibiotic carryover, 2.5 mM ferric chloride was added to the THA and mixed by inversion before plating (35). Preliminary experiments demonstrated that 2.5 mM ferric chloride was the optimum concentration which would allow for the growth of all the S. pneumoniae strains in the presence of peak concentrations of each quinolone (data not shown). In studies with ciprofloxacin and ofloxacin, ferric chloride was added to THA for the undiluted samples only. Because of the enhanced potency of trovafloxacin, ferric chloride was added to the THA used to plate the undiluted sample, as well as the first 10-fold dilution. Ferric chloride was not required for any other dilutions since the fluoroquinolones were diluted to a concentration at least 200-fold below the MIC.
To evaluate for the selection of mutants with decreased susceptibility to quinolones, samples removed from the IVPM at 36 h were also plated into THA containing the antibiotic at a concentration 4× the MIC.
RESULTS
Characterization of test strains and the IVPM.
Ofloxacin and ciprofloxacin MICs ranged from 1 to 2 μg/ml for all eight strains. When a difference in MIC was observed, the MICs of ofloxacin were always twofold higher than those of ciprofloxacin. Trovafloxacin was 8- to 32-fold more potent than ofloxacin and ciprofloxacin, with MICs of 0.06 to 0.12 μg/ml.
The pharmacokinetic profiles of trovafloxacin, ofloxacin, and ciprofloxacin within the peripheral compartment of the IVPM are shown in Fig. 1. Peak concentrations (mean ± standard deviation [SD]) in the peripheral compartment were achieved 0.5 h after dosing into the central reservoir and were 2.9 ± 0.1 μg/ml for trovafloxacin, 6.3 ± 0.3 μg/ml for ofloxacin, and 4.6 ± 0.1 μg/ml for ciprofloxacin. AUC0–24 were 37 μg · h/ml for trovafloxacin, 44 μg · h/ml for ciprofloxacin, and 96 μg · h/ml for ofloxacin. Calculated peak/MIC ratios and AUC/MICs are shown in Table 1.
FIG. 1.
Pharmacokinetics of trovafloxacin, ofloxacin, and ciprofloxacin in the peripheral compartment of the IVPM after dosing into the central reservoir. Drug levels were measured by bioassay. Each datum point represents the mean drug level in the peripheral compartment (in micrograms per milliliter) for three experimental runs. Error bars show SDs.
TABLE 1.
Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against S. pneumoniae
| S. pneumoniae strain no. | Value for drug:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trovafloxacin
|
Ofloxacin
|
Ciprofloxacin
|
||||||||||
| Peak/ MICa | AUC/ MICb | Time (h)c to 99.9% killing | Time (h)d to eradication | Peak/ MIC | AUC/ MIC | Time (h) to 99.9% killing | Time (h) to eradication | Peak/ MIC | AUC/ MIC | Time (h) to 99.9% killing | Time (h) to eradication | |
| 212 | 48 | 616 | 1.5 | 6 | 3 | 49 | 4.0 | 8 | 5 | 44 | 3.5 | 12 |
| 257 | 48 | 616 | 2.5 | 4 | 3 | 49 | 4.5 | 24 | 2 | 22 | 7.5 | NA |
| 213 | 24 | 313 | 2.0 | 6 | 3 | 49 | 6.0 | 24 | 2 | 22 | NAe | NA |
| 256 | 24 | 313 | 3.0 | 6 | 3 | 49 | 4.5 | 24 | 2 | 22 | NA | NA |
| 3956 | 48 | 616 | 1.5 | 4 | 6 | 98 | 3.5 | 8 | 5 | 44 | 3.5 | 12 |
| 3935 | 24 | 313 | 3.5 | 6 | 3 | 49 | 4.0 | 24 | 5 | 44 | 4.0 | 24 |
| 3938 | 24 | 313 | 1.5 | 4 | 6 | 98 | 3.0 | 24 | 5 | 44 | 6.5 | 24 |
| 3933 | 48 | 616 | 3.5 | 6 | 3 | 49 | 4.5 | 24 | 5 | 44 | 6.0 | 24 |
Ratio of peak antibiotic levels achieved within the peripheral compartment of the IVPM to the MIC of the drug against the strain of S. pneumoniae.
Ratio of the AUC0–24 to the MIC of the drug against the strain of S. pneumoniae.
Time required to achieve 99.9% killing of the inoculum.
Time required to decrease viable counts below the 10-CFU/ml limit of detection.
NA, significant 99.9% killing or eradication of the inoculum was not achieved in these experiments.
Pharmacodynamic studies.
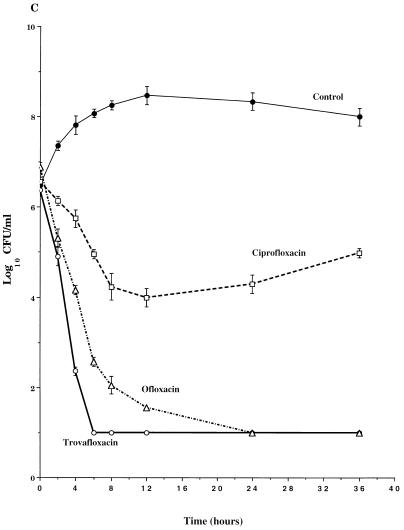
The pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against three representative strains are shown in Fig. 2. Trovafloxacin was rapidly bactericidal against all eight strains of S. pneumoniae, with viable counts falling 5 to 6 logs to undetectable levels (eradication) within 4 to 6 h after the first dose (Fig. 2 and Table 1). Thereafter, no viable bacteria were detected in trovafloxacin-treated cultures through 36 h. The time required for trovafloxacin to achieve a significant 99.9% killing of the S. pneumoniae ranged from 1.5 to 3.5 h (Table 1).
FIG. 2.
Time-kill pharmacodynamics of ofloxacin and ciprofloxacin against representative strains of S. pneumoniae, 3935 (A), 213 (B), and 256 (C). Each datum point represents the mean CFU per milliliter of THY from the peripheral compartment for duplicate experiments. Error bars show SDs.
The times required to achieve 99.9% killing and eradication with ofloxacin were consistently longer than those observed with trovafloxacin (Table 1). In studies with S. pneumoniae 212, S. pneumoniae 257, S. pneumoniae 213, and S. pneumoniae 3956, it took ofloxacin between 2 and 4 h longer than trovafloxacin to achieve a significant 99.9% killing. In studies with the other S. pneumoniae strains, the differences between ofloxacin and trovafloxacin in times to 99.9% killing were ≤1.5 h. Although ofloxacin eventually eradicated all eight S. pneumoniae strains from the IVPM, viable counts of six strains did not decrease below the 10-CFU/ml limit of detection until 12 to 24 h, or after the second dose of ofloxacin had been added to the IVPM.
Similar to ofloxacin, the rates of killing with ciprofloxacin were consistently slower than those observed with trovafloxacin. In studies with six of the eight strains, ciprofloxacin required 2 to 5 h longer to achieve significant 99.9% killing than did trovafloxacin. In contrast to trovafloxacin and ofloxacin, ciprofloxacin failed to eradicate three of the eight strains from the IVPM and failed to even achieve a significant 99.9% killing of S. pneumoniae 213 (Fig. 2B) and S. pneumoniae 256 (Fig. 2C). Furthermore, in studies with S. pneumoniae 213 and S. pneumoniae 256, the antibacterial activities of the second and third doses of ciprofloxacin were substantially diminished such that net bacteriostasis or slight net increases in viable counts were observed over the second and third dose intervals. No mutants with decreased susceptibility to ciprofloxacin were detected on drug selection plates at 36 h.
DISCUSSION
An IVPM was used to simulate maximum oral doses of trovafloxacin, ofloxacin, and ciprofloxacin and to compare their pharmacodynamics against eight clinical isolates of S. pneumoniae. Although all three quinolones bind to serum proteins (70% protein binding for trovafloxacin compared to 20 to 40% protein binding for ciprofloxacin and ofloxacin [23]), these pharmacodynamic studies were performed in the absence of serum proteins. While the degree of protein binding has been shown previously to influence the movement of antibiotics from the bloodstream to extravascular spaces (41), the impact of protein binding on antibacterial activity remains unsettled. The interaction between antibiotics and serum proteins is a dynamic reversible process, and drug molecules are constantly binding and unbinding at a rapid pace (1). In MIC studies, the presence of 40 to 50% serum does not alter the in vitro potency of ciprofloxacin (2, 6) or ofloxacin (4, 18). However, in studies with trovafloxacin, intra- and interspecies variations have been observed, ranging from no effect at all to significant 32-fold reductions in activity (5). Using an IVPM similar to the one in this study, Dudley and colleagues (11a) reported that killing of Staphylococcus aureus with dicloxacillin (∼96% protein bound) was significantly altered over the initial 6 h when 25% serum was present in the medium. How these data relate to this study is uncertain, especially since the impact of protein binding is 8- to 20-fold greater for dicloxacillin than for the quinolones evaluated in this study. Furthermore, the impact of protein binding on β-lactam antibiotics may be much different from that for fluoroquinolones.
Trovafloxacin was rapidly and significantly bactericidal against all eight S. pneumoniae strains and eradicated all strains from the IVPM within 4 to 6 h after the first dose. The significant killing observed with trovafloxacin in this study confirms other pharmacodynamic data in similar pharmacokinetically based models (43, 44). Furthermore the significant bactericidal activity observed with trovafloxacin in this study correlates with the in vivo efficacy of trovafloxacin against S. pneumoniae in clinical trials. Three separate studies have evaluated trovafloxacin for patients with community-acquired pneumococcal pneumonia and have reported rates of clinical efficacy from 90 to 100% at the end of treatment and 86 to 92% at the end of the studies (22, 26, 37). When bacteremia was evaluated, trovafloxacin successfully eradicated S. pneumoniae from the blood of 92 to 96% of patients (22, 26). While similar rates of clinical efficacy and bacterial eradication have been observed with ofloxacin (28, 30) and in some cases with ciprofloxacin (17, 29), the more rapid rates of bacterial killing observed with trovafloxacin in this study suggest that the trovafloxacin possesses a pharmacodynamic advantage over ofloxacin and ciprofloxacin against S. pneumoniae. The increased rate of killing observed with trovafloxacin most likely reflects increased peak/MIC ratios obtained with the 200-mg dose, compared to the peak/MIC ratios obtained with maximum oral doses of ofloxacin and ciprofloxacin. The dose-response relationship associated with the bactericidal activity of fluoroquinolones has been well established previously (11, 34), and in this study, the peak/MIC ratios simulated with trovafloxacin were 4- to 16-fold higher than those simulated for ofloxacin and 5- to 24-fold higher than those simulated for ciprofloxacin. Even if protein binding was not dynamic and the amount of these quinolones bound was no longer available, peak/MIC ratios for trovafloxacin would still be 5- to 10-fold higher than those for ciprofloxacin and 4- to 8-fold higher than those for ofloxacin for the majority of the strains in this study. Although the rates of killing observed for ciprofloxacin and ofloxacin may have been more comparable to those of trovafloxacin if their pharmacokinetics in the IVPM had been adjusted to provide similar peak/MIC ratios, the purpose of this investigation was to determine if the enhanced in vitro potency of trovafloxacin would translate into enhanced pharmacodynamic activity.
Although the rates of killing observed with ofloxacin and ciprofloxacin were slower than that with trovafloxacin, ofloxacin still eradicated all eight strains from the IVPM. These data corroborate clinical experience with ofloxacin in the treatment of community-acquired pneumococcal pneumonia, in which the eradication of S. pneumoniae from the lower respiratory tract is 97% (30). In contrast, ciprofloxacin failed to eradicate three of the eight strains from the IVPM. In studies with two of these strains, gradual net increases in viable counts were observed over the second and third dose intervals. Since no resistant mutants were detected on drug selection plates at 36 h, the decreased antibacterial activity of the second and third doses of ciprofloxacin most likely represents adaptive resistance or the reversible decrease in susceptibility after first exposure to an antibiotic (8, 9). Similar to the variability in killing observed in the IVPM, clinical experience with ciprofloxacin against S. pneumoniae has been variable. In some clinical trials, ciprofloxacin has eradicated 95 to 100% of S. pneumoniae bacteria from the lower respiratory tract, including the eradication of strains exhibiting resistance to ciprofloxacin (17, 29, 32). However, in other clinical studies ciprofloxacin has failed to eradicate 30 to 45% of S. pneumoniae bacteria from the respiratory tract (17, 32), and the development of pneumococcal bacteremia or pneumococcal superinfection has been reported during therapy with ciprofloxacin (7, 15).
The eradication of six strains from the IVPM with ofloxacin and five strains with ciprofloxacin was observed despite simulated AUC/MICs of only 44 to 49. These data suggest that the minimum AUC/MIC required for effective treatment of pneumococcal infections with ofloxacin or ciprofloxacin may be well below the 125 breakpoint suggested by studies with ciprofloxacin against gram-negative bacteria (13). This conclusion is supported by data with oral grepafloxacin in the treatment of acute exacerbations of chronic bronchitis (12). Although the number of patients infected with S. pneumoniae was small, Forrest and colleagues reported an 87.5% (seven of eight) bacteriologic cure when AUC/MICs were in the range of 0 to 92 (12). More systematic studies comparing the antipneumococcal pharmacodynamics of fluoroquinolones over a range of AUC/MICs are required to determine the minimum required for clinical efficacy. This is especially true for those fluoroquinolones which frequently do not provide peak/MIC ratios of 10:1 or greater, since pharmacodynamic data have suggested that the AUC/MIC ratio is most closely linked to treatment outcome when the peak/MIC ratio is less than 10:1 (10).
In summary, the pharmacodynamics of trovafloxacin were enhanced over those of ofloxacin and ciprofloxacin against S. pneumoniae when maximum oral doses were simulated in an IVPM. The higher rates of killing observed with trovafloxacin were most likely the result of the increased potency of trovafloxacin compared to that of the other quinolones, thus providing higher peak/MIC ratios with maximum oral doses. Data from this study and recent clinical data support the use of a once-daily dose of 200 mg of trovafloxacin for the treatment of pneumococcal infections. Furthermore, the consistent eradication of pneumococci from the IVPM with simulated AUC/MICs of 44 to 49 warrants an in-depth investigation into the minimum AUC/MICs required for efficacy of fluoroquinolones against S. pneumoniae.
ACKNOWLEDGMENTS
This work was supported by a grant from Roerig-Pfizer, New York, N.Y.
We thank Stacey Edward for her excellent technical assistance.
REFERENCES
- 1.Barza M, Vine H, Weinstein L. Reversibility of protein binding of penicillins: an in vitro study employing a rapid diafiltration process. Antimicrob Agents Chemother. 1972;1:427–432. doi: 10.1128/aac.1.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser J, Dudley M N, Gilbert D, Zinner S H. Influence of medium and method on the in vitro susceptibility of Pseudomonas aeruginosa and other bacteria to ciprofloxacin and enoxacin. Antimicrob Agents Chemother. 1986;29:927–929. doi: 10.1128/aac.29.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser J, Stone B B, Zinner S H. Two compartment kinetic model with multiple artificial capillary units. J Antimicrob Chemother. 1985;15(A):131–137. doi: 10.1093/jac/15.suppl_a.131. [DOI] [PubMed] [Google Scholar]
- 4.Chantot J F, Bryskier A. Antibacterial activity of ofloxacin and other 4-quinolone derivatives: in vitro and in vivo comparison. J Antimicrob Chemother. 1985;16:475–484. doi: 10.1093/jac/16.4.475. [DOI] [PubMed] [Google Scholar]
- 5.Child J, Andrews J, Boswell F, Brenwald N, Wise R. The in-vitro activity of CP 99,219, a new naphthyridone antimicrobial agent: a comparison with other fluoroquinolone agents. J Antimicrob Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 6.Chin N X, Neu H C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984;25:319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper B, Lawlor M. Pneumococcal bacteremia during ciprofloxacin therapy for pneumococcal pneumonia (brief clinical observation) Am J Med. 1989;87:475. doi: 10.1016/s0002-9343(89)80838-1. [DOI] [PubMed] [Google Scholar]
- 8.Daikos G L, Jackson G G, Lolans V T, Livermore D M. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990;162:414–420. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- 9.Daikos G L, Lolans V T, Jackson G G. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother. 1991;35:117–123. doi: 10.1128/aac.35.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas aeruginosa sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley M N. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med. 1991;91(6A):45S–50S. doi: 10.1016/0002-9343(91)90311-k. [DOI] [PubMed] [Google Scholar]
- 11a.Dudley M N, Blaser J, Gilbert D, Zinner S H. Significance of “extravascular” protein binding for antimicrobial pharmacodynamics in an in vitro capillary model of infection. Antimicrob Agents Chemother. 1990;34:98–101. doi: 10.1128/aac.34.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrest A, Chodosh S, Amantea M A, Collins D A, Schentag J J. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40(Suppl. A):45–57. doi: 10.1093/jac/40.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 13.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu K P, Lafredo S C, Foleno B, Isaacson D M, Barrett J F, Tobia A J, Rosenthale M E. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother. 1992;36:860–866. doi: 10.1128/aac.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon J J, Kauffman C A. Superinfection with Streptococcus pneumoniae during therapy with ciprofloxacin. Am J Med. 1990;89:383–384. doi: 10.1016/0002-9343(90)90355-h. [DOI] [PubMed] [Google Scholar]
- 16.Isreal D, Gillum J G, Turik M, Harvey K, Ford J, Dalton H, Towle M, Echols R, Heller A H, Polk R. Pharmacokinetics and serum bactericidal titers of ciprofloxacin and ofloxacin following multiple oral doses in healthy volunteers. Antimicrob Agents Chemother. 1993;37:2193–2199. doi: 10.1128/aac.37.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan F A. Ciprofloxacin for respiratory tract infections. In: Sanders W E, Sanders C C, editors. Fluoroquinolones in the treatment of infectious diseases. Glenview, Ill: Physicians and Scientists Publishing Co.; 1990. pp. 87–98. [Google Scholar]
- 18.Kumada T, Neu H C. In vitro activity of ofloxacin, a quinolone carboxylic acid compared to other quinolones and other antimicrobial agents. J Antimicrob Chemother. 1985;16:563–574. doi: 10.1093/jac/16.5.563. [DOI] [PubMed] [Google Scholar]
- 19.Lister P D. Multiply-resistant pneumococcus: therapeutic problems in the management of serious infections. Eur J Clin Microbiol Infect Dis. 1995;14:18–25. [PubMed] [Google Scholar]
- 20.Lister P D, Pong A, Chartrand S A, Sanders C C. Rationale behind high-dose amoxicillin therapy for acute otitis media due to penicillin-nonsusceptible pneumococci: support from in vitro pharmacodynamic studies. Antimicrob Agents Chemother. 1997;41:1926–1932. doi: 10.1128/aac.41.9.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lister P D, Sanders W E, Jr, Sanders C C. Cefepime-aztreonam: a unique double β-lactam combination for Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1610–1619. doi: 10.1128/aac.42.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandell L, Williams-Hopkins D, Hopkins S. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Efficacy of trovafloxacin in patients with community acquired pneumonia due to penicillin susceptible and resistant S. pneumoniae, abstr. LM-71; p. 377. [Google Scholar]
- 23.Medical Economics Data. Physicians desk reference. Montvale, N.J: Medical Economics Data; 1998. [Google Scholar]
- 24.Musher D M. Streptococcus pneumoniae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practices of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1811–1826. [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 26.Niederman M, Traub S, Ellison W T, Hopkins D W the Trovan Pneumonia Study Group. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A double blind, randomized, multicenter, global study in hospitalized community acquired pneumonia (CAP) comparing trovafloxacin with ceftriaxone + erythromycin, abstr. LM-72; p. 377. [Google Scholar]
- 27.Pankuch G A, Jacobs M R, Appelbaum P C. Activity of CP-99,219 compared with DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 28.Plouffe J F, Herbert M T, File T M, Jr, Baird I, Parsons J N, Kahn J B, Rielly-Gauvin K T the Pneumonia Study Group. Ofloxacin versus standard therapy in treatment of community-acquired pneumonia requiring hospitalization. Antimicrob Agents Chemother. 1996;40:1175–1179. doi: 10.1128/aac.40.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders W E., Jr Efficacy, safety, and potential economic benefits of oral ciprofloxacin in the treatment of infections. Rev Infect Dis. 1988;10:528–543. doi: 10.1093/clinids/10.3.528. [DOI] [PubMed] [Google Scholar]
- 30.Sanders W E., Jr Oral ofloxacin: a critical review of the new drug application. Clin Infect Dis. 1992;14:539–554. doi: 10.1093/clinids/14.2.539. [DOI] [PubMed] [Google Scholar]
- 31.Schentag J J, Nix D E, Adelman M H. Mathematical examination of dual individualization principles (I): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP Ann Pharmacother. 1991;25:1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 32.Scully B E. Therapy of respiratory tract infections with quinolone antimicrobial agents. In: Wolfson J W, Hooper D C, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1989. pp. 143–165. [Google Scholar]
- 33.Sefton A M, Maskell J P, Seymour A C, Minassian M, Williams J D. Comparative in-vitro activity of CP-99219, a new quinolone, against respiratory pathogens. J Antimicrob Chemother. 1996;37:803–808. doi: 10.1093/jac/37.4.803. [DOI] [PubMed] [Google Scholar]
- 34.Smith J T. Awakening the slumbering potential of the 4-quinolone antibacterials. Pharm J. 1984;233:299–305. [Google Scholar]
- 35.Smith J T. Effects of physiological cation concentration on 4-quinolone absorption and potency. In: Crumplin G C, editor. The 4-quinolones: antibacterial agents in vitro. London, United Kingdom: Springer-Verlag; 1990. pp. 15–21. [Google Scholar]
- 36.Spangler S K, Jacobs M R, Pankuch G A, Appelbaum P C. Susceptibility of 170 penicillin-susceptible and penicillin-resistant pneumococci to six oral cephalosporins, four quinolones, desacetylcefotaxime, Ro 23-9424 and RP 67829. J Antimicrob Chemother. 1993;31:273–280. doi: 10.1093/jac/31.2.273. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan J, Gezon J, Williams Hopkins D the Trovan Community Pneumonia Study Group. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A double blind, randomized, multicenter study in ambulatory community acquired pneumonia (CAP) comparing trovafloxacin with clarithromycin, abstr. LM-73; p. 378. [Google Scholar]
- 38.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 39.Teng R, Liston T E, Harris S C. Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J Antimicrob Chemother. 1996;37:955–963. doi: 10.1093/jac/37.5.955. [DOI] [PubMed] [Google Scholar]
- 40.Thomson K S, Chartrand S A, Sanders C C, Block S L. Trovafloxacin, a new fluoroquinolone with potent activity against Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:478–480. doi: 10.1128/aac.41.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise R, Gillet A P, Cadge B. Influence of protein binding on the tissue levels of 6 β-lactams. J Infect Dis. 1980;142:77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- 42.Wise R, Mortiboy D, Child J, Andrews J M. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:47–49. doi: 10.1128/aac.40.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright D H, Hovde L B, Peterson M L, Hoang A D, Rotschafer J C. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. In vitro evaluation of pharmacodynamic (PD) outcome parameters for three fluoroquinolones (FQ) against Streptococcus pneumoniae, abstr. A-86; p. 53. [Google Scholar]
- 44.Zinner S, Gilbert D, Dickman J, Dudley M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Trovafloxacin killing of penicillin-resistant and susceptible pneumococci in an in vitro pharmacokinetic/dynamic model, abstr. A60; p. 12. [Google Scholar]