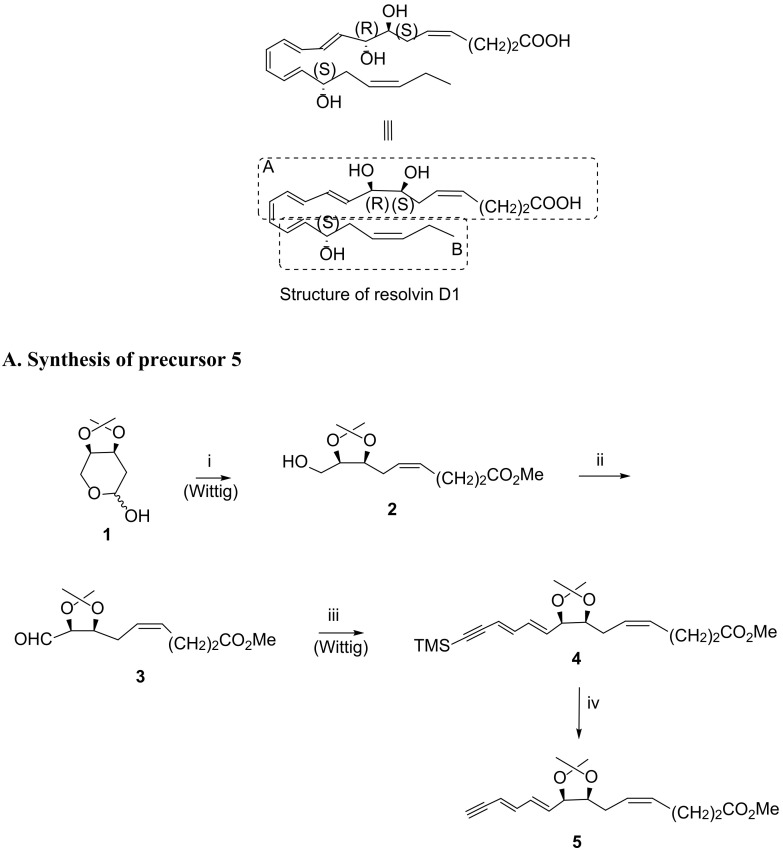
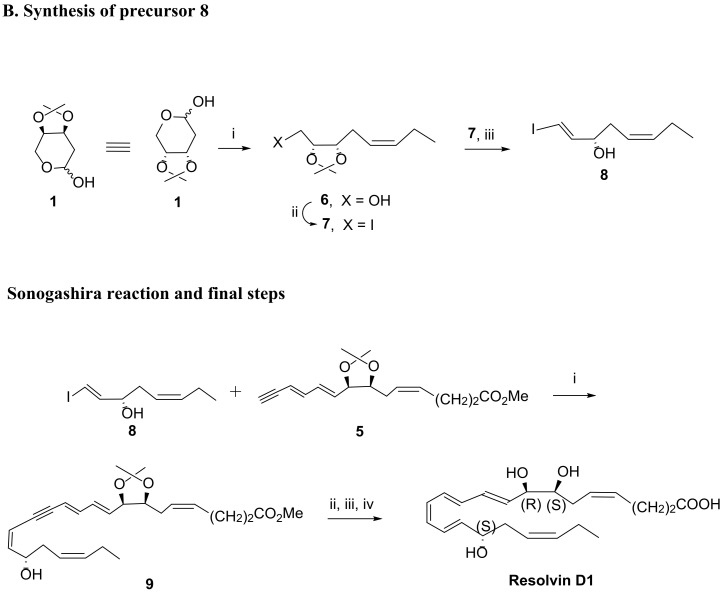
Scheme 4.
A RvD1 total synthesis as reported in 2012 by Rodríguez and Spur [80] (A). Synthesis of compound 5. Reagents and conditions: i. KN(TMS)2, MeO2C-(CH2)2CH2PPh3+ Br−, THF, −70 °C to rt, 43%; ii. Dess–Martin periodinane, CH2Cl2, rt, 84%; iii. 1.TMS-C≡C-CH = CHCH2PPh3 + Br−, nBuLi, THF, −70 °C to 0 °C; 2. I2, benzene, rt, 87%; iv. KF·2H2O, 18-crown-6, DMF, rt, 88%. (B) RvD1 total synthesis as described in 2012 by Rodríguez and Spur [80]. (B). Synthesis of compound 8. Reagents and conditions: i. CH3CH2CH2PPh3+ Br−, NaN(TMS)2, Et2O, −78 °C to rt, 60%; ii. I2, Ph3P, imidazole, toluene, 60 °C, 82%; iii. LDA, THF, −78 °C, 40%; Synthesis of RvD1 via Sonogashira reaction. Reagents and conditions: i. Pd(PPh3)4, CuI, piperidine, benzene, rt, 86%; ii. CH3COCl cat., CH3OH, 0 °C, 90%; iii. Zn(Cu/Ag), CH3OH, H2O, 50 °C, 5 h, 67%; iv. LiOH 1N, CH3OH, H2O, 0 °C, then sat. NaH2PO4, 94%.