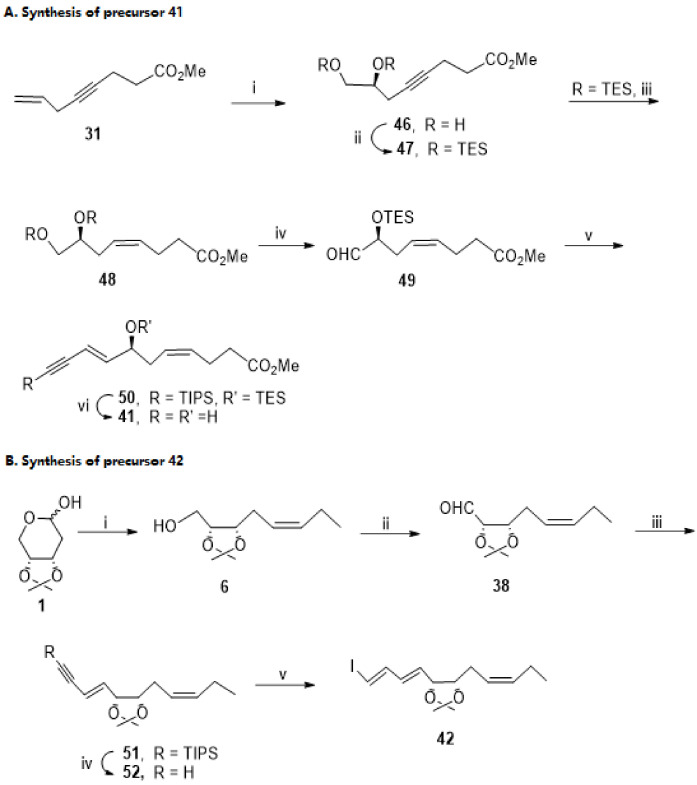
Scheme 9.
Preparation of precursors 41 (A) and 42 (B), as reported by Rizzacasa et al. [86]. Reagents and conditions: (A). i. Admix-α, MeSO2NH2, tBuOH, H2O, 69%; ii. TESCl, imidazole, 93%; iii. Ni (OAc)2.4H2O, NaBH4, EDA, MeOH, 73%; iv. (COCl)2, DMSO, Et3N, CH2Cl2,78%; v. 1. TIPS-≡-CH2PPh3+Br−, LiHMDS, −78 °C, then addition of 38, −40 °C, (3E,8Z)-isomer 49% and (3Z,8Z)-isomer 12%; vi. TBAF. (B). CH3CH2CH2PPh3+ Br−, NaHMDS, −78 °C to 0 °C, 74%; ii. DMP, 93%; iii. TIPS-≡-CH2PPh3+ Br−, nBuLi, THF, −40 °C to 0 °C, (1E,3E,8Z)-isomer 57%, (1Z,3E,8Z)-isomer 26%; iv. TBAF, 73%; v. 1. ZrCp2Cl2, iBu2AlH, THF, 0 °C; 2. I2, −78 °C, 64%.