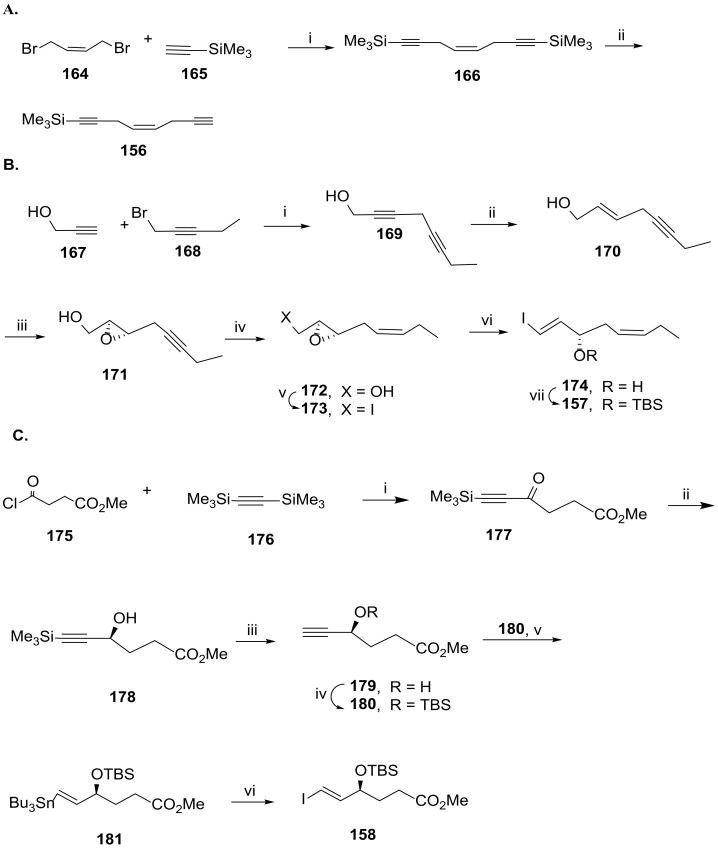
Scheme 22.
Synthesis of Sonogashira coupling partners, as reported by Rodríguez and Spur [94]. (A). i. CuI, Na2SO3, K2CO3, DMF, rt; ii. AgNO3, CH3OH, H2O, NaCN, 0 °C; (B). i. DBU, CuI, HMPA, THF; ii. LiAlH4, ether, 0 °C to rt; iii. l-(+)-DMT, Ti(OiPr)4, TBHP, molecular sieves 4 Å, CH2Cl2; iv. H2, Lindlar cat., Et3N, hexane; v. I2, Ph3P, imidazole, iPr2EtN, CH3CN, ether; vi. NaHMDS, DMF, −60 °C; vii. TBSCl, imidazole, DMF, 67%; (C). i. 10% InBr3, CH2Cl2, 0 °C to rt, 94%; ii. Ru[(S,S)-TsDPEN](p-cymene), iPrOH, rt; iii. KF·2H2O, 18-crown-6, DMF, rt; iv. TBSCl, imidazole, DMF, 0 °C to rt, 63% over the three steps; v. Bu3SnH, AIBN, 130 °C; vi. I2, ether, 0 °C, 93%.