Abstract
Background and Objectives:
Separate lines of research indicate sleep quality may impact recognition of facial expressions in anxious or depressed individuals. This study examined facial emotion recognition ability in the context of self-perceived sleep quality and anxiety and depression symptom levels in individuals with and without internalizing psychopathologies.
Methods:
Seventy anxious and/or depressed patients and 24 demographically matched healthy controls completed the Pittsburgh Sleep Quality Index (PSQI), standard measures of anxiety and depression, and an Emotion Recognition Task comprising negative and positive facial expressions.
Results:
Analyses of variance results revealed patients reported worse sleep quality than controls. Linear mixed-effects models indicated that all participants demonstrated better emotion recognition abilities in identifying positive versus negative emotions. For reaction time, but not accuracy, regression results revealed significant individual differences, with worse sleep quality predicting slower reaction times for positive faces, specifically for happiness.
Limitations:
The use of a subjective measure of sleep quality and a specific behavioral paradigm for emotion recognition may impact the generalizability of the findings.
Conclusions:
Associations between task performance and emotional valence of facial expression implies that poor sleep quality, beyond internalizing symptom severity, may disrupt emotion processing.
Keywords: sleep habits, facial recognition, affect, behavior, anxiety disorders, mood disorders
1. Introduction
The ability to recognize facial expressions accurately and efficiently plays a critical role in everyday social behavior and interactions. For instance, emotion recognition is related to social adjustment, with lower accuracy corresponding with decreased social competency and peer interactions (Leppänen and Hietanen, 2001). The speed in which individuals recognize and process emotions has also been linked to proficiency in social interpretations and reciprocity (Sonneville et al., 2002). Therefore, emotion recognition difficulties may result in adverse interpersonal consequences, which could impact psychological well-being (Carton et al., 1999; Persad and Polivy, 1993). A factor that is expected to play a role in emotion recognition is sleep. For example, sleep deprivation studies involving healthy individuals or convenience samples (Killgore et al., 2017; Maccari et al., 2014; Sack et al., 2019; van der Helm et al., 2010) show insufficient sleep impairs emotion recognition ability and increases the time to identify the expression of a face in general (Cote et al., 2014; Pallesen et al., 2004). Also, studies of insomnia disorder in individuals free from psychiatric illness show links between symptoms of insomnia and reduced emotion recognition ability (e.g., lower accuracy, slower response time, compromised ratings of emotion intensity) (de Almondes et al., 2020; Kyle et al., 2014; Zhang et al., 2019).
Yet, despite interactions between sleep and emotion recognition, findings have been inconsistent with regard to the type of emotion conveyed by a facial expression. For example, sleep deprivation was shown to reduce accuracy in healthy participants when identifying subtle displays of happiness and sadness but not surprise, fear, disgust, or anger (Killgore et al., 2017). In another study where healthy participants rated the emotional intensity of faces, sleep loss blunted recognition for anger and happiness but not sadness (van der Helm et al., 2010). In a study of insomnia disorder, patients took longer to identify displays of happiness, fear, anger, and sadness but only exhibited less accuracy for fear compared to healthy controls (de Almondes et al., 2020). Comparatively, another study found no difference in task performance between groups though patients with insomnia rated facial expressions of sadness and fear as emotionally less intense than controls (Kyle et al., 2014). Lastly, studies have also reported a lack of associations between emotion recognition ability and sleep deprivation (Holding et al., 2017) or insomnia (Brand et al., 2019). Despite mixed findings, the majority of findings suggest sleep loss has the potential to impair emotion recognition, and insufficient sleep may interact with the valence of facial expressions (i.e., positive or negative).
Another important factor that contributes to aberrant emotion recognition facility is psychopathology. Meta-analysis studies (irrespective of sleep considerations) have found both anxiety and depressive disorders to be linked to reduced accuracy in emotion recognition performance (Demenescu et al., 2010). Additionally, the socio-emotional signal of expressions may interact with psychopathology. For example, negative facial expressions are associated with shorter reaction times in anxiety and depression, indicative of a ‘negativity’ bias (Bar-Haim et al., 2007; Peckham et al., 2010). Even in remission, response bias is evident as individuals with a history of depression had higher accuracy for negative faces (i.e., sadness, anger, fear) relative to healthy controls (Anderson et al., 2011). In line with negativity bias, a mismatch between the emotional content of expressions and primary psychiatric illness may also impact task performance. For example, in a study that manipulated the intensity of emotions presented, depressed individuals required happiness to be of greater intensity to accurately identify than healthy controls (Joormann and Gotlib, 2006). Findings highlight the impact of psychopathology on emotion recognition. However, negativity bias in anxiety or depression is of a moderate effect size (Bar-Haim et al., 2007; Peckham et al., 2010) suggesting other factors may contribute to bias. In light of reports of sleep-emotion recognition interactions, it stands to reason sleep quality may factor into aberrant emotion recognition in individuals with psychopathology.
Though problematic sleep is ubiquitous and often studied in individuals without psychopathology, less is known about sleep-emotion relationships in individuals with psychopathology. In general, more than one-third of the U.S. population reported sleeping less than the recommended 7 hours in a 24-hour period (Liu et al., 2016), inclusive of individuals who are unable or choose not to sleep for a longer duration. In internalizing psychopathologies, problematic sleep is also highly prevalent (Almeida and Pfaff, 2005; Mellman, 2006; Raffray et al., 2011). Findings have been mixed in studies of sleep deprivation, showing a reduction of clinical symptoms in some but the worsening of symptoms in others (Babson et al., 2010; Roy-Byrne et al., 1986). Moreover, research beyond lab-based sleep interventions is needed to evaluate the effects of naturalistic sleep on emotion processing across anxious and depressed patients, given evidence that self-reported poor sleep exacerbates symptom severity in internalizing conditions (Cox and Olatunji, 2016; Liu et al., 2007; Zalta et al., 2013). Poor sleep quality may have an additive impact on emotion recognition performance in anxiety or depression in light of negative bias tendencies.
Therefore, the objective of the present study was to expand on previous findings. In line with the literature, we expected individuals with an internalizing psychopathology to report more problematic sleep in the clinical range compared to healthy controls. Since problematic sleep is transdiagnostic, we hypothesized levels of poor sleep quality would be similar between anxious and depressed individuals (Almeida and Pfaff, 2005; Cox and Olatunji, 2016). We also anticipated that worse sleep would correspond with less accuracy and slower response times during emotion recognition across participants, regardless of anxiety and depression severity. Moreover, we hypothesized worse sleep quality would magnify negativity bias. Therefore, we expected lower accuracy and longer response times for positive faces and higher accuracy and shorter response times for negative faces across participants but that this effect would again be driven by patients. Lastly, we explored diagnostic status as anxiety and depression may have a differential effect on emotion recognition (e.g., Mathews and MacLeod, 2005; Oehlberg et al., 2012).
2. Materials and Methods
2.1. Participants
The study is based on a secondary analysis of data collected from a treatment outcome study that was designed in accordance with the Research Domain Criteria (RDoC) initiative by the National Institute of Mental Health that focused on internalizing psychopathologies. Participants were recruited from referrals in local outpatient clinics and the community through sources including flyers, newspapers, and online advertisements. All participants completed a consent form approved by the local Institutional Review Board. A master’s-level or a PhD/MD clinician performed the Structured Clinical Interview for DSM-5 Disorders (‘SCID-5’; First et al., 2015) and clinician-administered measures with all participants. Participants were enrolled if they were 18 to 65 years old, inclusive, and treatment-seeking patients were required to have at least one DSM-5 anxiety or depressive disorder.
The study emphasized transdiagnostic features of psychopathology as opposed to a categorical approach focused on principal diagnosis. Therefore, participants were required to have a total score of ≥ 23 on the Depression, Anxiety, and Stress Scale (‘DASS-21’; Lovibond & Lovibond, 1995). The DASS-21 is based on a dimensional, as opposed to a categorical conception of psychopathology, thus ratings on each subscale (i.e., depression, anxiety, stress) represent symptoms that range from normal to extremely severe (Lovibond and Lovibond, 1995). Accordingly, the cut-point of ≥ 23 is indicative of at least moderate levels of symptom severity, regardless of primary complaint, that both warrants treatment and captures a range of symptoms associated with internalizing psychopathologies.
Participants were primarily female (n = 60; 63.8%), and the average age was 25.99 ± 7.68 years. With regard to diagnosis, treatment-seeking patients met criteria for generalized anxiety disorder (n = 30), social anxiety disorder (n = 17), panic disorder (n = 4), post-traumatic stress disorder (n = 2), or major depressive disorder (n = 17). Comorbidity was permitted (see Table 1). To explore possible differences regarding diagnostic status, patients were assigned to an ‘anxiety disorder’ (AD) group or ‘major depressive disorder’ (MDD) group. Due to the small number of patients with posttraumatic stress disorder (n = 2), these patients were assigned to the AD group. Additionally, 24 demographically-matched healthy controls (HC) completed the same assessments and tasks at the same time points as patients. Table 2 shows the breakdown by diagnostic groups of participant demographic characteristics.
Table 1.
Distribution of principal diagnosis and comorbidity.
| Comorbidity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Principal Diagnosis | N (%) | GAD (%) | MDD (%) | SAD (%) | PD (%) | PTSD (%) | PDD (%) | Specific Phobia (%) | SUD (%) | OCD (%) | ED (%) |
|
| |||||||||||
| Generalized anxiety disorder | 30 (32%) | 8 (27%) | 20 (67%) | 4 (13%) | 3 (10%) | 5 (17%) | 5 (17%) | 1 (3%) | 1 (3%) | 1 (3%) | |
| Major depressive disorder | 17 (18%) | 8 (47%) | 8 (47%) | 4 (24%) | 4 (24%) | 2 (12%) | 3 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Social anxiety disorder | 17 (18%) | 10 (59%) | 10 (59%) | 6 (35%) | 0 (0%) | 4 (24%) | 6 (35%) | 2 (12%) | 1 (6%) | 0 (0%) | |
| Panic disorder | 4 (4%) | 1 (25%) | 3 (75%) | 1 (25%) | 1 (25%) | 0 (0%) | 1 (25%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Posttraumatic stress disorder | 2 (2%) | 2 (100%) | 1 (50%) | 2 (100%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||||||||
| Total | 21 (30%) | 22 (31%) | 31 (44%) | 15 (21%) | 8 (11%) | 11 (16%) | 15 (21%) | 3 (4%) | 2 (3%) | 1 (1%) | |
GAD=Generalized Anxiety Disorder; MDD=Major Depressive Disorder; SAD=Social Anxiety Disorder; PD=Panic Disorder; PTSD=Posttraumatic Stress Disorder; PDD=Persistent Depressive Disorder; SUD=Substance Use Disorder; OCD=Obsessive Compulsive Disorder; ED=Eating Disorder
Table 2.
Participant demographic and clinical characteristics.
| HC (n = 24) | AD (n = 53) | MDD (n = 17) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | N | % | ||
| Gender | Male | 14 | 58% | 16 | 30% | 4 | 24% |
| Female | 10 | 42% | 37 | 70% | 13 | 76% | |
| Race/Ethnicity | Caucasian | 10 | 42% | 32 | 60% | 11 | 65% |
| African American | 5 | 21% | 7 | 13% | 3 | 18% | |
| Asian | 5 | 21% | 6 | 11% | 3 | 18% | |
| Hispanic/Latino | 5 | 21% | 12 | 23% | 2 | 12% | |
| Native Hawaiian or Other Pacific Islander | 0 | 0% | 0 | 0% | 0 | 0% | |
| American Indian or Alaskan Native | 0 | 0% | 1 | 2% | 0 | 0% | |
| Other or Unknown | 4 | 17% | 6 | 11% | 0 | 0% | |
| More than one race | 0 | 0% | 1 | 2% | 0 | 0% | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 25.63 | 7.72 | 25.57 | 7.62 | 27.82 | 7.99 | |
| PSQI | 3.46 | 2.08 | 10.17 | 4.34 | 11.71 | 3.80 | |
| DASS-21 | 2.63 | 3.62 | 33.38 | 7.86 | 34.06 | 7.57 | |
| HAM-A | 0.71 | 0.81 | 15.06 | 5.88 | 15.35 | 4.47 | |
| HAM-D | 0.38 | 0.65 | 9.02 | 3.44 | 13.12 | 3.28 | |
HC=Healthy Controls; AD=Anxiety Disorders; MDD=Major Depressive Disorder
PSQI=Pittsburgh Sleep Quality Index; DASS-21=Depression, Anxiety, and Stress Scale; HAM-A/D=Hamilton Anxiety/Depression Rating Scale
Healthy participants could not have current or lifetime history of an Axis I disorder. Exclusion criteria for all participants included current treatment (medication or psychotherapy), a major medical or neurological problem, lifetime history of major psychiatric illness (e.g., bipolar disorder, psychotic disorder), active suicidal ideation, cognitive dysfunction (e.g., traumatic brain injury, pervasive developmental disorder), and current substance abuse or dependence (within the past 6 months). All participants were monetarily compensated for their time, and all procedures complied with the Helsinki Declaration.
2.2. Sleep measure
The Pittsburgh Sleep Quality Index (‘PSQI’; Buysse et al., 1989) was used to assess self-perceived sleep. The PSQI is a 19-item self-report instrument which assesses sleep quality over a 1-month time period, with validated internal homogeneity, consistency, and validity (Carpenter and Andrykowski, 1998). It is commonly used as a measure of sleep in clinical and research settings (Mollayeva et al., 2016). PSQI items contribute to 7 component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of these component scores yield a total global score. Global scores range from 0 to 21, with higher scores representing worse sleep. A PSQI global score > 5 denotes clinically problematic sleep (Backhaus et al., 2002; Buysse et al., 1989).
2.3. Emotion recognition task
The Emotion Recognition Task (ERT) was drawn from the Cambridge Neuropsychological Test Automated Battery (‘CANTAB’; Sahakian & Owen, 1992), which consists of a series of computerized neuropsychological tests designed to assess a range of cognitive domains. CANTAB has been shown to have reliable and valid sensitivity to cognitive impairments in psychiatric populations (Lowe and Rabbitt, 1998). For the ERT, participants were presented with 6 emotional expressions – happiness, sadness, anger, disgust, surprise, and fear along a continuum of expression magnitude. Each expression was a computer-morphed image based on 12 different individual males performing the emotion. More specifically, a composite face was generated by delineating each of the 12 original images with 172 facial feature points that allowed both shape and color to be averaged. The composite faces displayed the 6 emotions, and within each emotion there were 15 different levels of intensity (i.e., neutral/ambiguous to full/unambiguous expressions). Each stimulus (i.e., emotional face of a given intensity level) was pseudorandomized and presented only once per block.
Each expression was displayed on the screen, one at a time, for 200 milliseconds followed by a mask to prevent residual processing of the image. Subsequently, participants were presented with a list of emotion labels (i.e., happiness, sadness, anger, disgust, surprise, and fear) and instructed to select which emotion the preceding face displayed as quickly and accurately as possible. A fixation cross appeared on screen between stimuli presentations. There were 180 trials, separated into two blocks of 90 trials each. Within each block, the number of trials was distributed equally across the 6 emotions for 15 trials per emotion. The total duration of the task was approximately 10 minutes.
In the ERT, responses were considered correct if participants matched the emotion with its corresponding label in each trial. Thus, accuracy was assessed as the percentage of correct responses, which was determined as the proportion of the number of correct trials divided by the number of trials displayed for each emotion. Reaction time, in milliseconds (ms), was based on the time it took to accurately identify the target emotion.
2.4. Anxiety and depression measures
Primary clinician-administered measures were the Hamilton Anxiety Rating Scale (‘HAMA’; Hamilton, 1959) and the Hamilton Depression Rating Scale (‘HAMD’; Hamilton, 1960). The HAMA is a 14-item clinician-administered assessment of anxiety, evaluating worries, trouble sleeping, fears, tension, chest pain, shortness of breath, and fidgeting over the period of a week. The HAMD is a 21-item clinician-administered assessment of depression, evaluating feelings of sadness/hopelessness, trouble sleeping, loss of interest, loss of appetite, and irritability over the period of a week. Greater total scores indicate higher levels of anxiety or depression, respectively.
2.5. Analytic approach
Analysis was limited to pre-treatment data. To examine relationships with sleep, as indexed with PSQI global score, in addition to levels of anxiety and depression, sleep-related questions in the HAMA and HAMD were removed. To evaluate overall task performance and determine whether participants exerted adequate effort, we report average accuracy and average reaction time for accurate trials across all emotional facial expressions across all participants.
To test the hypothesis of a significant difference in sleep quality between patients and healthy controls but not between patient groups (AD vs. MDD), a one-way analysis of variance (ANOVA) was performed. Significant results were followed by Tukey’s Honestly Significant Difference (HSD) post-hoc test.
Due to mixed findings in prior studies, we did not have hypotheses for specific emotions. Therefore, to reduce multiple comparisons, we evaluated the emotional valence of facial expressions when examining potential interactions between sleep quality and task performance. Specifically, positive valence comprised average accuracy and reaction time for happiness and surprise, and negative valence, the average accuracy and reaction time for sadness, anger, disgust, and fear. If results were significant, follow-up analysis was performed to determine whether a particular emotion(s) explained the finding.
A linear mixed model was performed to address the mixed-effects sampling scheme arising from participants’ exposure to facial expressions of both emotional valences when performing the task. Our model consisted of emotional valence and sleep quality (i.e., PSQI global score) as fixed effects, while participants were treated as nested random effects. Specifically, to evaluate task performance, accuracy was submitted to a linear mixed model, with participants entered as a random intercept and main predictors of emotional valence, sleep quality, and their interaction term of emotional valence × sleep quality. Diagnostic groups (i.e., AD, MDD, HC), symptom severity (i.e., HAMA, HAMD) and demographic characteristics (i.e., age, gender) were entered as covariates. The same analysis was performed for reaction time (RT) for accurate trials.
Significant results were followed up with simple regression analyses to examine which emotion(s) explained significant results. For example, in one model the dependent variable (DV) could consist of accuracy for a particular negative emotion (e.g., fear). Independent variables (IVs) would comprise the PSQI global score, along with any significant covariates following a backward elimination approach. To determine whether collinearity was acceptable, variance inflation factor (VIF) was required to be < 4 (Allison, 1999).
All statistical analyses, with reported confidence intervals and effect sizes, were two-tailed with an alpha level of 0.05 and performed using R (Version 3.5.2).
3. Results
Across all participants, the average PSQI global score was 8.73 ± 4.90; thus, a portion met criteria for clinically problematic sleep (i.e., PSQI global score > 5). Concerning clinical symptom severity, average anxiety level assessed with the HAMA was 11.45 ± 7.93, and average depression level assessed with the HAMD was 7.55 ± 5.36. Table 2 shows the clinical characteristics by diagnostic groups. Concerning overall ERT performance across participants, accuracy was 71.42 ± 5.78% (i.e., > chance or 16.67%, t(93) = 91.89, p < .001) and RT was 1071.99 ± 168.00 ms across all emotional facial expressions. See Table 3 for detailed task performance statistics.
Table 3.
Accuracy (%) and reaction time (milliseconds) for all and individual emotions across diagnostic groups.
| HC | AD | MDD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Accuracy | Reaction Time | Accuracy | Reaction Time | Accuracy | Reaction Time | |
|
| ||||||
| % | ms | % | ms | % | ms | |
| Overall | 71.83 ± 6.21 | 1070.6 ± 154.38 | 71.02 ± 5.62 | 1045.35 ± 159.11 | 72.08 ± 5.88 | 1157.03 ± 193.6 |
| Happiness | 79.47 ± 17.88 | 834.99 ± 164.37 | 79.61 ± 14.65 | 867.54 ± 209.13 | 83.53 ± 13.12 | 980.48 ± 218.68 |
| Surprise | 67.27 ± 13.63 | 1018.93 ± 234.42 | 67.07 ± 11.7 | 1008.12 ± 225.66 | 68.12 ± 9.41 | 1107.45 ± 261.25 |
| Fear | 68.48 ± 19.22 | 1359.56 ± 390.08 | 68.02 ± 20.41 | 1302.81 ± 350.7 | 63.7 ± 21.75 | 1423.4 ± 412.35 |
| Anger | 87.3 ± 9.72 | 1055.02 ± 256.41 | 85.51 ± 13.02 | 1005 ± 187.4 | 85.34 ± 12.29 | 1040.73 ± 271.22 |
| Sadness | 81.27 ± 15.04 | 1078.65 ± 109.57 | 74.34 ± 15.24 | 1083.97 ± 230.06 | 72.98 ± 16.3 | 1174.26 ± 311.26 |
| Disgust | 72.54 ± 13.64 | 1288.49 ± 353.93 | 72.23 ± 15.39 | 1184.77 ± 292.13 | 73.82 ± 13.57 | 1338.85 ± 268.83 |
HC=Healthy Controls; AD=Anxiety Disorders; MDD=Major Depressive Disorder
3.1. Sleep by diagnostic group
The one-way ANOVA for PSQI global score revealed a main effect of diagnostic group (F(2, 91) = 32.24, p < .001, f = .84, 90% CI [.63, 1.02]). Post-hoc comparisons, using the Tukey HSD test, indicated that the PSQI global score for HC (M = 3.46, SD = 2.08) was significantly different from AD (M = 10.17, SD = 4.34, p < .001, 95% CI [4.49, 8.93], Hedges’ g = 1.75) and MDD patients (M = 11.71, SD =3.80, p < .001, 95% CI [5.38, 11.11], Hedges’ g = 2.78). However, AD and MDD patients were not significantly different (p = .318, 95% CI [−0.98, 4.05], Hedges’ g = .36) (see Table 2).
3.2. Sleep and accuracy
The linear mixed model for accuracy revealed a main effect of emotional valence (B = 15.31, 95% CI [10.93, 19.68], t(92) = 6.97, p < .001, R2 = .21). Participants were more accurate in identifying positive faces (M = 83.02%, SD = 6.13%) than negative faces (M = 65.62%, SD = 8.57%) (see Figure 1). There was no main effect of sleep quality (B = −0.10, 95% CI [−0.49, 0.28], t(86) = −0.54, p = .589, R2 = .002) or emotional valence × sleep quality interaction (B = 0.24, 95% CI [−0.20, 0.68], t(92) = 1.09, p = .278, R2 = .006). As for covariates, anxiety (HAMA) was negatively associated with accuracy regardless of emotional valence (B = −0.36, 95% CI [−0.62, −0.11], t(86) = −2.89, p = .005, R2 = .04). However, results were not significant for diagnostic group (AD p = .312, R2 = .006; MDD p = .468, R2 = .003), depression (HAMD, p = .339, R2 = .004), age (p = .288, R2 = .006), or gender (p = .136, R2 = .01). We did not perform follow-up analysis due to the absence of sleep effects of interest.
Figure 1.
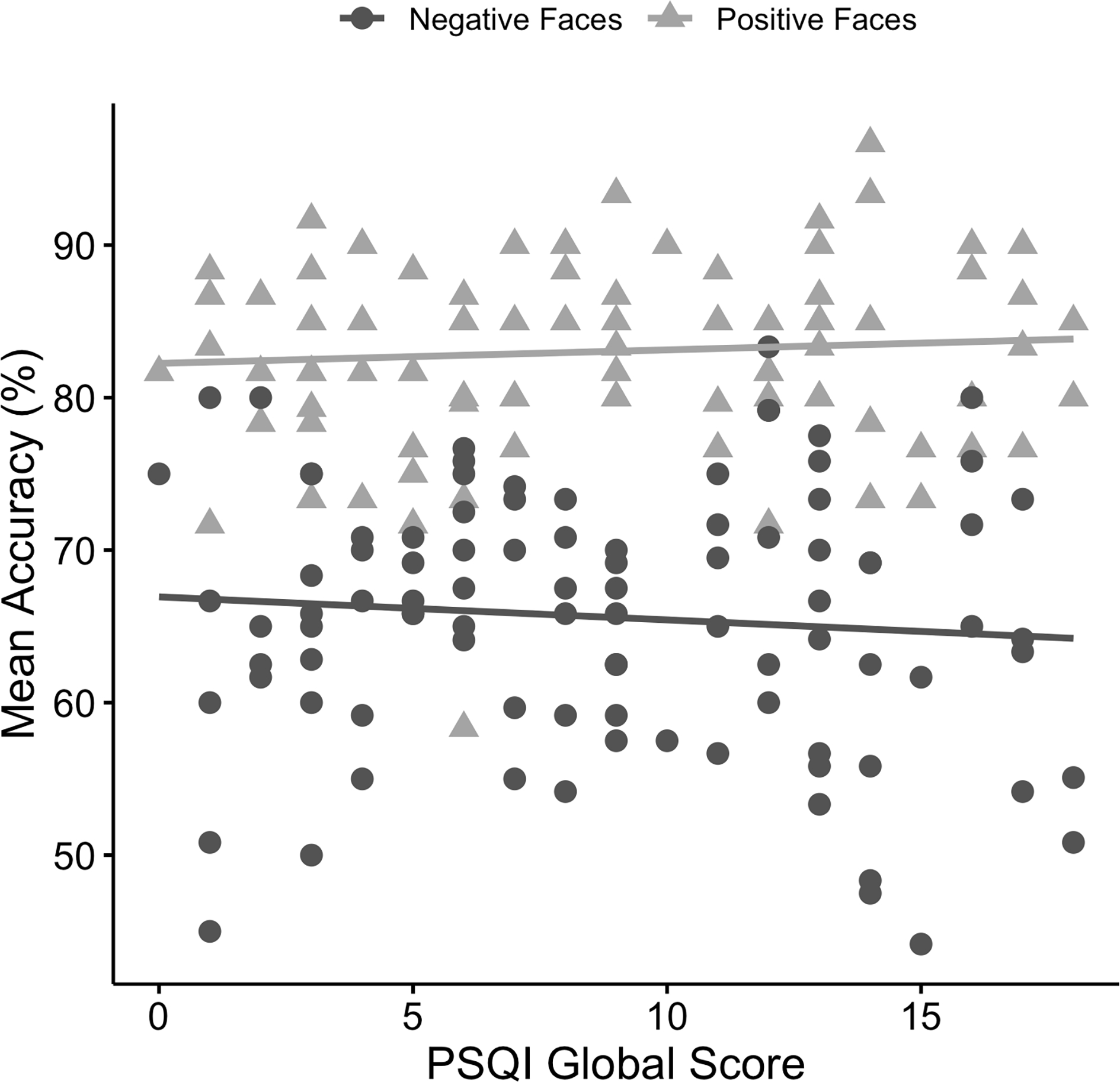
Scatterplot depicting relationships between mean accuracy (%) for positive and negative faces and Pittsburgh Sleep Quality Index (PSQI) global scores.
3.3. Sleep and reaction time
The linear mixed model for RT also revealed a main effect of emotional valence (B = −266.77, 95% CI [−324.91, −208.63], t(92) = −9.11, p < .001, R2 = .16). Participants were faster in identifying positive faces (M = 946.76 ms, SD = 177.62 ms) than negative faces (M = 1154.79 ms, SD = 186.49 ms) (see Figure 2). There was no main effect of sleep quality (B = 1.11, 95% CI [−9.25, 11.74], t(86) = 0.21, p = .836, R2 = .001), but the emotional valence × sleep quality interaction was significant (B = 6.73, 95% CI [0.91, 12.54], t(92) = 2.30, p = .024, R2 = .01). None of the covariates of diagnostic group (AD p = .954, R2 < .001; MDD p = .337, R2 = .01), anxiety (HAMA, p = .233, R2 = .009), depression (HAMD, p = .303, R2 < .001), age (p = .182, R2 = .01), or gender (p = .972, R2 < .001) were significant; thus, covariates were not included in follow-up analyses.
Figure 2.
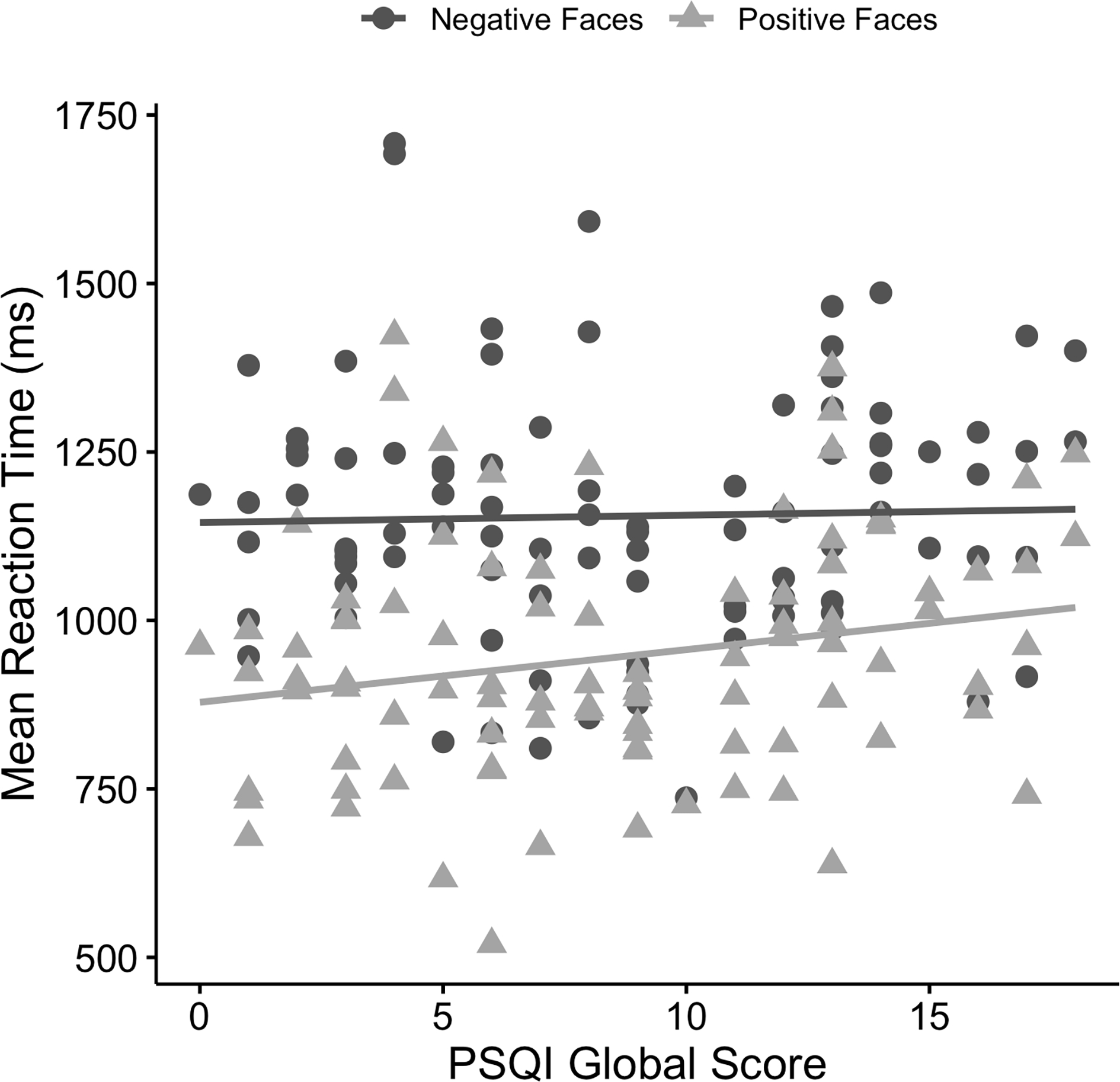
Scatterplot depicting relationships between mean reaction time (ms) for positive and negative faces and Pittsburgh Sleep Quality Index (PSQI) global scores.
To determine which emotion(s) explained the significant emotional valence × sleep quality interaction, we first conducted post-hoc regression analyses where RT to negative faces was the DV and sleep quality (PSQI global score) was the IV; the result was not significant (R2 = .001, F(1, 92) = 0.08, p = .784). However, with RT for positive faces as the DV, the model was significant (R2 = .05, F(1, 92) = 4.49, p = .037) as sleep quality (PSQI global score) significantly predicted RT (B = 7.81, 95% CI [0.49, 15.14], t(92) = 2.12, p = .037).
Follow-up analysis with RT for happiness as the DV was significant (R2 = .06, F(1, 92) = 5.56, p = .021) as sleep quality (PSQI global score) was a significant predictor (B = 9.96, 95% CI [1.57, 18.34], t(92) = 2.36, p = .021) (see Figure 3). The result with RT for surprise was not significant (R2 = .01, F(1, 92) = 1.16, p = .284) as sleep quality (PSQI global score) was not significant (B = 5.35, 95% CI [−4.50, 15.21], t(92) = 1.08, p = .284).
Figure 3.
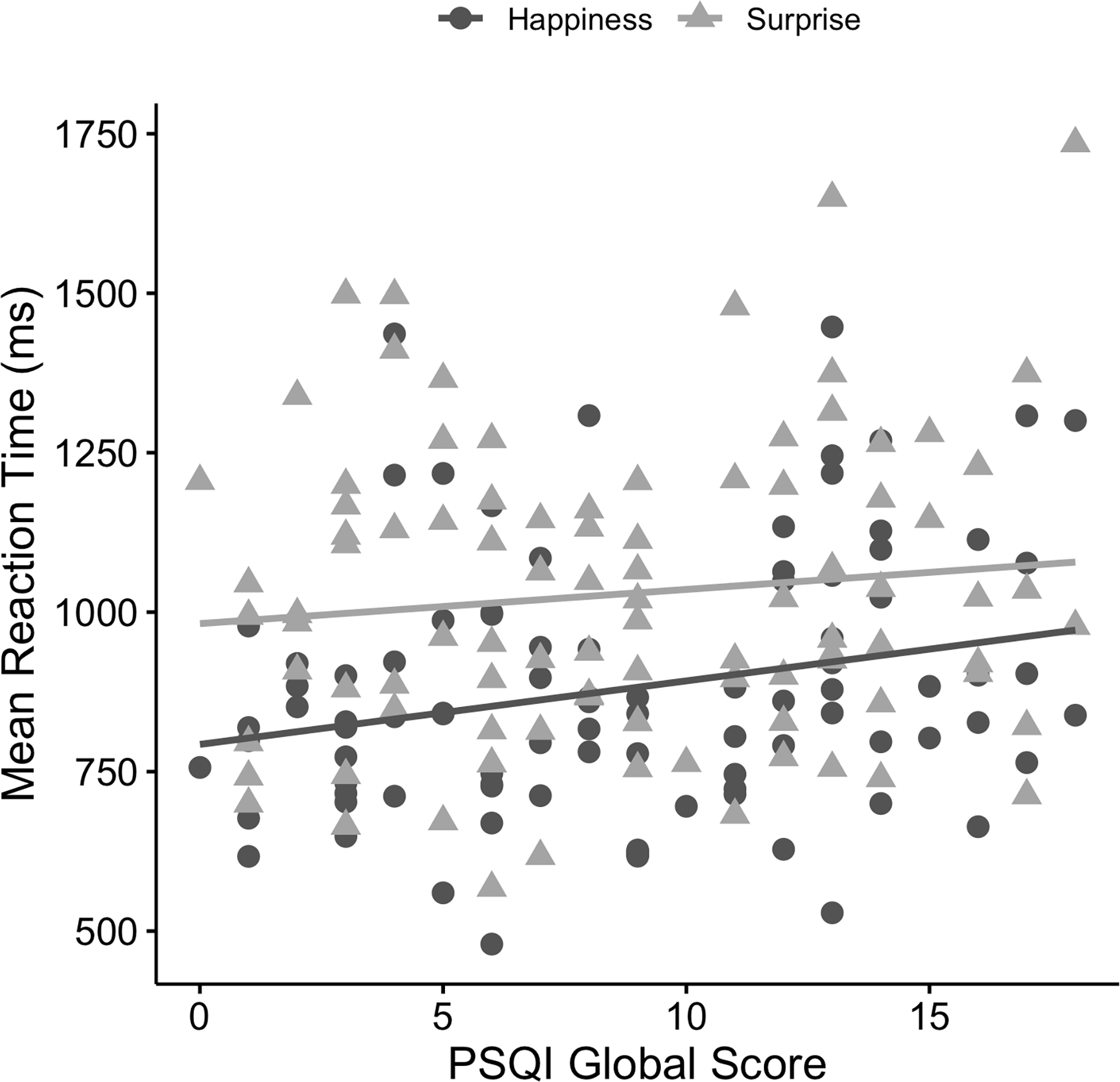
Scatterplot depicting relationships between mean reaction time (ms) for happiness and surprise and Pittsburgh Sleep Quality Index (PSQI) global scores.
3.4. Post-hoc power analyses
For the one-way ANOVA comparing sleep quality between healthy controls and patient groups, the statistical power was revealed to be greater than .99 for detecting the large effect size of f = .84 (Cohen, 1988) at alpha = .05. For the main linear mixed models examining accuracy and reaction time during the ERT, the current study also appeared to have acceptable power (i.e., > .99 at alpha = .05) due to the higher number of observations obtained per participant for the ERT. Each participant completed 180 trials of the task, and this repeated sampling was adequate for the main objective of examining sleep quality on task performance across all participants.
4. Discussion
The primary goal of our study was to examine the impact of sleep quality on emotion recognition in individuals with and without internalizing psychopathologies. Overall performance indicated participants engaged in the task at hand, and as expected, more patients than healthy controls reported worse sleep quality. Results also revealed main effects of emotional valence such that all participants were more accurate and had faster reaction times when identifying positive faces compared to negative faces. Yet, an emotional valence and sleep quality interaction was observed for task performance based on reaction time. Post-hoc analysis showed worse sleep quality specifically predicted slower reaction times for positive faces, particularly for happiness, across participants.
Findings partially support our hypotheses. As expected, more patients than healthy individuals reported poor sleep quality. This is consistent with research demonstrating that the presence of psychiatric symptoms is related to more sleep disturbances or that worse sleep corresponds to higher levels of symptom severity (Alvaro et al., 2013; Chorney et al., 2008; Gregory et al., 2011). However, it is notable that a portion of healthy participants free from a history of psychiatric illness (16%) also reported problematic sleep in the clinical range (Buysse et al., 1989) suggesting poor sleep quality may be dimensional (i.e., cutting across diagnostic groups).
In contrast to expectations, linear mixed model results revealed a main effect of emotional valence but no sleep quality effects. Across participants, accuracy was higher and reaction time faster for positive relative to negative faces. Findings are consistent with prior work, which has shown positive expressions are more easily identified in comparison to negative expressions, potentially due to positive emotions being presented in higher frequencies, being anatomically simpler, and having lower overlap with other emotions (Biehl et al., 1997). Moreover, there was an effect of anxiety levels on accuracy, where higher anxiety symptoms corresponded with less accuracy regardless of valence. Variance in depression symptoms on the other hand did not explain accuracy and may pertain to the sample, which mostly comprised patients with a principal anxiety disorder who had less depression than patients with principal major depressive disorder.
Yet, linear mixed model results revealed a significant emotional valence by sleep quality interaction for reaction time. Specifically, higher PSQI global scores significantly predicted slower reaction time for positive but not negative faces when taking anxiety and depression symptom severity into account. This provides added support to prior studies that demonstrated strong ties between insufficient sleep and longer duration when recognizing facial expressions in general (Cote et al., 2014; de Almondes et al., 2016). Results thus further enhance the overall negative contributions of poor sleep on emotion processing (Killgore et al., 2017).
Post-hoc regression analysis revealed individual differences in the processing of positive emotions. Expected task performance results were partially supported as reaction time effects were observed for positive faces. Specifically, slower reaction time for happiness was predicted by worse sleep quality. Reaction time for all other positive and negative faces were not impacted by sleep quality. Generally, previous work has shown a behavioral advantage in recognizing happy facial expressions more accurately and faster than other expressions (Calvo and Lundqvist, 2008; Tottenham et al., 2009). However, when taking sleep into consideration, our findings suggest this effect may be negatively impacted. Namely, while we also observed a behavioral advantage in recognizing positive facial expressions, worse sleep quality appeared to reduce this effect. Our findings are somewhat consistent with studies that demonstrated sleep deprivation impairs the recognition of happy facial expressions (e.g., reduces accuracy; Killgore et al., 2017; van der Helm et al., 2010). Altogether, our preliminary findings suggest self-reported poor sleep quality may not be sufficient to impair accuracy but may increase the time it takes to accurately identify happiness.
Accuracy and reaction time results do not align with the hypothesized negativity bias, as lower accuracy and slower reaction time was observed for negative faces in comparison to positive faces across individuals with and without internalizing psychopathologies. It is possible that the paradigm was not optimal in detecting negativity bias. In studies of anxiety and depression, negativity bias is commonly evaluated with tasks that probe the attention system such as attentional control (e.g., emotional Stroop), orientation to stimuli, disengagement from stimuli (e.g., dot probe, exogenous cueing task) (Bar-Haim et al., 2007; Peckham et al., 2010). In contrast, the current study focused on emotion recognition. Secondly, faces were presented briefly (i.e., 200 ms) and masked. Though anxiety and depression are associated with negativity bias, it is proposed that relatively long stimulus durations (e.g., 1,000 ms or more) capture attentional bias in depression (Mathews and MacLeod, 2005; Mogg and Bradley, 2005). Even though many participants had elevated levels of anxiety, individuals with major depression and comorbid depression were included, which may have reduced our ability to detect negativity bias.
However, in line with the hypothesis that poor sleep quality may contribute to aberrance in emotion recognition, results showed that worse sleep increased the time it took to accurately identify happiness. Though we are not able to test this in the current study, it is possible that the longer the time it takes to recognize an approach-related expression like happy in real-world situations, the longer it takes to respond, which may impact interpersonal interactions. Findings have other implications as well. For example, sleep may be involved in the regulation of emotional assessment as indicated by a study in which the lack of sleep appeared to bias emotional labeling of stimuli toward negative responses (Fox et al., 2007). This is also in agreement with another study demonstrating that worse sleep quality is associated with cognitive bias for emotionally negative stimuli and decreased sustained attention to non-emotional stimuli (Gobin et al., 2015). Moreover, insufficient sleep may help explain the comparable negative effects of decreased speed in processing emotional content. For instance, studies involving reaction time tasks have concluded that increased reaction times may be a result of impaired attention (e.g., decreased attentional allocation, rapid loss of attention) during sleep deprivation (McCarthy and Waters, 1997). Although this relationship was not directly tied to internalizing symptoms in our sample comprised of mostly patients, findings further highlight the significance of sleep on emotion recognition ability.
This study is not without important limitations. Partial rather than full support of our hypotheses, particularly regarding emotion recognition interactions with anxiety and depression symptom severity, may suggest a lack of sensitivity of this specific behavioral task in capturing the negativity bias often observed in individuals with internalizing psychopathologies in other studies (Bar-Haim et al., 2007; Peckham et al., 2010). Due to the rapid presentation of stimuli during the ERT, participants with poor sleep may have also been subject to compromised attention when performing the task. Study participants were also not taking psychotropic medications, which may reduce generalization of findings. Additionally, sleep was assessed via self-report and studies have reported disagreement between subjective and objective sleep measurements (Girschik et al., 2013; McCall and McCall, 2012; O’Brien et al., 2016; Van Den Berg et al., 2008; Van Ravesteyn et al., 2014). Therefore, findings may not generalize to objectively-defined problematic sleep. Among other self-report measures, the PSQI has also been shown to be more closely correlated with psychiatric symptoms (Buysse et al., 2008), which may have affected endorsement on the questionnaire in our patient sample. Other limitations include the cross-sectional and correlational nature of our study, which does not allow for inferences that worse sleep leads to comprised emotion processing skills. Lastly, there was no direct manipulation of sleep, so outcomes were based on estimates of sleep quality.
4.1. Conclusions
However, despite limitations, findings of the present study have important implications in our understanding of sleep and emotion processing in clinical populations. Results support the notion that poor sleep quality is transdiagnostic and dimensional and that it may negatively impact the processing of positive facial expressions in particular. Since problematic sleep is pervasive and facial expressions important in guiding our behavior (Joormann and Gotlib, 2006), our preliminary findings warrant further investigation in larger samples to increase our understanding of the role sleep plays in emotion recognition in individuals with and without internalizing psychopathologies.
Highlights.
Poor sleep quality and anxiety symptoms impact emotion recognition capability
Emotion recognition task performance was better for positive than negative emotions
Anxiety was negatively associated with accuracy during emotion recognition
Compared to healthy controls, anxious or depressed patients reported worse sleep
Worse sleep was associated with slower reaction times for happy expressions
Acknowledgements
Funding: This work was supported by National Institute of Mental Health of the National Institutes of Health [grant numbers R01MH101497, R01MH112705]; and the Center for Clinical and Translational Research, Chicago, IL [grant number UL1RR029879].
Footnotes
Conflict of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that there are no conflicts of interest.
Data Statement
The data described in the study will be available for access by request, in line with open data practices.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison PD, 1999. Multiple Regression: A Primer. Pine Forge Press. [Google Scholar]
- Almeida OP, Pfaff JJ, 2005. Sleep complaints among older general practice patients: Association with depression. British Journal of General Practice, 55(520), 864–866. [PMC free article] [PubMed] [Google Scholar]
- Alvaro PK, Roberts RM, Harris JK, 2013. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36(7), 1059–1068. 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Shippen C, Juhasz G, Chase D, Thomas E, Downey D, Toth ZG, Lloyd-Williams K, Elliott R, Deakin JFW, 2011. State-dependent alteration in face emotion recognition in depression. The British Journal of Psychiatry, 198(4), 302–308. 10.1192/bjp.bp.110.078139 [DOI] [PubMed] [Google Scholar]
- Babson KA, Trainor CD, Feldner MT, Blumenthal H, 2010. A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: An experimental extension. Journal of Behavior Therapy and Experimental Psychiatry, 41(3), 297–303. 10.1016/j.jbtep.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F, 2002. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53(3), 737–740. 10.1016/S0022-3999(02)00330-6 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH, 2007. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, Ton V, 1997. Matsumoto and Ekman’s Japanese and Caucasian Facial Expressions of Emotion (JACFEE): Reliability data and cross-national differences. Journal of Nonverbal Behavior, 21(1), 3–21. 10.1023/A:1024902500935 [DOI] [Google Scholar]
- Brand S, Schilling R, Ludyga S, Colledge F, Sadeghi Bahmani D, Holsboer-Trachsler E, Pühse U, Gerber M, 2019. Further evidence of the zero-association between symptoms of insomnia and facial emotion recognition—Results from a sample of adults in their late 30s. Frontiers in Psychiatry, 9. 10.3389/fpsyt.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA, 2008. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. Journal of Clinical Sleep Medicine, 4(6), 563–571. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Lundqvist D, 2008. Facial expressions of emotion (KDEF): Identification under different display-duration conditions. Behavior Research Methods, 40(1), 109–115. 10.3758/BRM.40.1.109 [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA, 1998. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research, 45(1), 5–13. 10.1016/S0022-3999(97)00298-5 [DOI] [PubMed] [Google Scholar]
- Carton JS, Kessler EA, Pape CL, 1999. Nonverbal decoding skills and relationship well-being in adults. Journal of Nonverbal Behavior, 23(1), 91–100. 10.1023/A:1021339410262 [DOI] [Google Scholar]
- Chorney DB, Detweiler MF, Morris TL, Kuhn BR, 2008. The interplay of sleep disturbance, anxiety, and depression in children. Journal of Pediatric Psychology, 33(4), 339–348. 10.1093/jpepsy/jsm105 [DOI] [PubMed] [Google Scholar]
- Cote KA, Mondloch CJ, Sergeeva V, Taylor M, Semplonius T, 2014. Impact of total sleep deprivation on behavioural neural processing of emotionally expressive faces. Experimental Brain Research, 232(5), 1429–1442. 10.1007/s00221-013-3780-1 [DOI] [PubMed] [Google Scholar]
- Cox RC, Olatunji BO, 2016. A systematic review of sleep disturbance in anxiety and related disorders. Journal of Anxiety Disorders, 37, 104–129. 10.1016/j.janxdis.2015.12.001 [DOI] [PubMed] [Google Scholar]
- de Almondes KM, Júnior FWNH, Alves NT, 2016. Sleep deprivation and implications for recognition and perception of facial emotions. Sleep and Biological Rhythms, 14(1), 13–22. 10.1007/s41105-015-0029-3 [DOI] [Google Scholar]
- de Almondes KM, Júnior FWNH, Leonardo MEM, Alves NT, 2020. Facial emotion recognition and executive functions in insomnia disorder: An exploratory study. Frontiers in Psychology, 11. 10.3389/fpsyg.2020.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu LR, Kortekaas R, Boer J.A. den, Aleman A, 2010. Impaired attribution of emotion to facial expressions in anxiety and major depression. PloS One, 5(12), e15058. 10.1371/journal.pone.0015058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams J, Karg R, Spitzer RL, 2015. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, 1–94. [Google Scholar]
- Fox E, Mathews A, Calder AJ, Yiend J, 2007. Anxiety and sensitivity to gaze direction in emotionally expressive faces. Emotion, 7(3), 478–486. 10.1037/1528-3542.7.3.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girschik J, Heyworth J, Fritschi L, 2013. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. American Journal of Epidemiology, 177(4), 316–327. 10.1093/aje/kws422 [DOI] [PubMed] [Google Scholar]
- Gobin CM, Banks JB, Fins AI, Tartar JL, 2015. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. Journal of Sleep Research, 24(5), 535–542. 10.1111/jsr.12302 [DOI] [PubMed] [Google Scholar]
- Gregory AM, Buysse DJ, Willis TA, Rijsdijk FV, Maughan B, Rowe R, Cartwright S, Barclay NL, Eley TC, 2011. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. Journal of Psychosomatic Research, 71(4), 250–255. 10.1016/j.jpsychores.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. [DOI] [PubMed] [Google Scholar]
- Holding BC, Laukka P, Fischer H, Bänziger T, Axelsson J, Sundelin T, 2017. Multimodal emotion recognition is resilient to insufficient sleep: Results from cross-sectional and experimental studies. Sleep, 40(11). 10.1093/sleep/zsx145 [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH, 2006. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology, 115(4), 705–714. 10.1037/0021-843X.115.4.705 [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Balkin TJ, Yarnell AM, Capaldi VF, 2017. Sleep deprivation impairs recognition of specific emotions. Neurobiology of Sleep and Circadian Rhythms, 3, 10–16. 10.1016/j.nbscr.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle SD, Beattie L, Spiegelhalder K, Rogers Z, Espie CA, 2014. Altered emotion perception in insomnia disorder. Sleep, 37(4), 775–783. 10.5665/sleep.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Hietanen JK, 2001. Emotion recognition and social adjustment in school–aged girls and boys. Scandinavian Journal of Psychology, 42(5), 429–435. 10.1111/1467-9450.00255 [DOI] [PubMed] [Google Scholar]
- Liu X, Buysse DJ, Gentzler AL, Kiss E, Mayer L, Kapornai K, Vetró Á, Kovacs M, 2007. Insomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depression. Sleep, 30(1), 83–90. 10.1093/sleep/30.1.83 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB, 2016. Prevalence of healthy sleep duration among adults — United States, 2014. Morbidity and Mortality Weekly Report, 65(6), 137–141. 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- Lovibond SH & Lovibond PF, 1995. Manual for the depression, anxiety, stress scales. Sydney: Psychology Foundation, 1–3. [Google Scholar]
- Lowe C, Rabbitt P, 1998. Test\re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. Neuropsychologia, 36(9), 915–923. 10.1016/S0028-3932(98)00036-0 [DOI] [PubMed] [Google Scholar]
- Maccari L, Martella D, Marotta A, Sebastiani M, Banaj N, Fuentes LJ, Casagrande M, 2014. Effects of sleep loss on emotion recognition: A dissociation between face and word stimuli. Experimental Brain Research, 232(10), 3147–3157. 10.1007/s00221-014-3995-9 [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C, 2005. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol, 1, 167–195. 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- McCall C, McCall WV, 2012. Objective vs. subjective measurements of sleep in depressed insomniacs: First night effect or reverse first night effect?. Journal of Clinical Sleep Medicine, 8(1), 59–65. 10.5664/jcsm.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy ME, Waters WF, 1997. Decreased attentional responsivity during sleep deprivation: Orienting response latency, amplitude, and habituation. Sleep, 20(2), 115–123. 10.1093/sleep/20.2.115 [DOI] [PubMed] [Google Scholar]
- Mellman TA, 2006. Sleep and anxiety disorders. Psychiatric Clinics, 29(4), 1047–1058; abstract x. 10.1016/j.psc.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, 2005. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research, 29(1), 29–45. 10.1007/s10608-005-1646-y [DOI] [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A, 2016. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 25, 52–73. 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- O’Brien E, Hart C, Wing RR, 2016. Discrepancies between self-reported usual sleep duration and objective measures of total sleep time in treatment-seeking overweight and obese individuals. Behavioral Sleep Medicine, 14(5), 539–549. 10.1080/15402002.2015.1048447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlberg KA, Revelle W, Mineka S, 2012. Time-course of attention to negative stimuli: Negative affectivity, anxiety, or dysphoria? Emotion, 12(5), 943–959. 10.1037/a0027227 [DOI] [PubMed] [Google Scholar]
- Pallesen S, Johnsen BH, Hansen A, Eid J, Thayer JF, Olsen T, Hugdahl K, 2004. Sleep deprivation and hemispheric asymmetry for facial recognition reaction time and accuracy. Perceptual and Motor Skills, 98(3), 1305–1314. 10.2466/pms.98.3c.1305-1314 [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW, 2010. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety, 27(12), 1135–1142. 10.1002/da.20755 [DOI] [PubMed] [Google Scholar]
- Persad SM, Polivy J, 1993. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. Journal of Abnormal Psychology, 102(3), 358–368. [DOI] [PubMed] [Google Scholar]
- Raffray T, Bond TLY, Pelissolo A, 2011. Correlates of insomnia in patients with social phobia: Role of depression and anxiety. Psychiatry Research, 189(2), 315–317. 10.1016/j.psychres.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW, Post RM, 1986. Effects of one night’s sleep deprivation on mood and behavior in panic disorder: Patients with panic disorder compared with depressed patients and normal controls. Archives of General Psychiatry, 43(9), 895–899. 10.1001/archpsyc.1986.01800090085011 [DOI] [PubMed] [Google Scholar]
- Sack B, Broer K, Anders S, 2019. Sleep deprivation selectively enhances interpersonal emotion recognition from dynamic facial expressions at long viewing times: An observational study. Neuroscience Letters, 694, 225–230. 10.1016/j.neulet.2018.10.035 [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM, 1992. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. Journal of the Royal Society of Medicine, 85(7), 399–402. [PMC free article] [PubMed] [Google Scholar]
- Sonneville LMJD, Verschoor CA, Njiokiktjien C, Veld V.O. het, Toorenaar N, Vranken M, 2002. Facial identity and facial emotions: Speed, accuracy, and processing strategies in children and adults. Journal of Clinical and Experimental Neuropsychology, 24(2), 200–213. 10.1076/jcen.24.2.200.989 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, Nelson C, 2009. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJA, Vos H, Tulen JHM, Hofman A, Miedema HME, Neven AK, Tiemeier H, 2008. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research, 17(3), 295–302. 10.1111/j.1365-2869.2008.00638.x [DOI] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Walker MP, 2010. Sleep deprivation impairs the accurate recognition of human emotions. Sleep, 33(3), 335–342. 10.1093/sleep/33.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ravesteyn LM, Tulen JHM, Kamperman AM, Raats ME, Schneider A.J. (Tom), Birnie E, Steegers EAP, Hoogendijk WJG, Tiemeier HW, Lambregtse–van den Berg MP, 2014. Perceived sleep quality is worse than objective parameters of sleep in pregnant women with a mental disorder. Journal of Clinical Sleep Medicine, 10(10), 1137–1141. 10.5664/jcsm.4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalta AK, Dowd S, Rosenfield D, Smits JAJ, Otto MW, Simon NM, Meuret AE, Marques L, Hofmann SG, Pollack MH, 2013. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Depression and Anxiety, 30(11), 1114–1120. 10.1002/da.22170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chan AB, Lau EYY, Hsiao JH, 2019. Individuals with insomnia misrecognize angry faces as fearful faces while missing the eyes: An eye-tracking study. Sleep, 42(2). 10.1093/sleep/zsy220 [DOI] [PubMed] [Google Scholar]


