Abstract
Although tuft cells were discovered over 60 years ago, their functions have long been enigmatic, especially in human health. Nonetheless, tuft cells have recently emerged as key orchestrators of the host response to diverse microbial infections in the gut and airway. While tuft cells are epithelial in origin, they exhibit functions akin to immune cells and mediate important interkingdom interactions between the host and helminths, protists, viruses, and bacteria. With broad intra- and intertissue heterogeneity, tuft cells sense and respond to microbes with exquisite specificity. Tuft cells can recognize helminth and protist infection, driving a type 2 immune response to promote parasite expulsion. Tuft cells also serve as the primary physiologic target of persistent murine norovirus (MNV) and promote immune evasion. Recently, tuft cells were also shown to be infected by rotavirus. Other viral infections, such as influenza A virus, can induce tuft cell–dependent tissue repair. In the context of coinfection, tuft cells promote neurotropic flavivirus replication by dampening antiviral adaptive immune responses. Commensal and pathogenic bacteria can regulate tuft cell abundance and function and, in turn, tuft cells are implicated in modulating bacterial infiltration and mucosal barrier integrity. However, the contribution of tuft cells to microbial sensing in humans and their resulting effector responses are poorly characterized. Herein, we aim to provide a comprehensive overview of microbial activation of tuft cells with an emphasis on tuft cell heterogeneity and differences between mouse and human tuft cell biology as it pertains to human health and disease.
I. Introduction
Tuft cells are rare, chemosensory epithelial cells named for their characteristic tufted apical microvilli that project into the lumen of hollow organs [1]. Despite their rarity, tuft cells have been found in the respiratory tract, gastrointestinal tract, urogenital tract, and thymus at varying levels of abundance [2–13]. In the respiratory tract, tuft cells compose 1% to 10% of the upper airway mucosal epithelium but are absent in the lower airway until tissue damage [3,14]. In the intestinal tract, tuft cells constitute approximately 0.4% to 2% of the epithelium [15,16]. Tuft cell numbers are elevated in biliary and pancreatic tracts, comprising 20% to 30% of the epithelium in these sites [10,17]. Based on their morphology in these locations, tuft cells have amassed many names, including brush and solitary chemosensory cells (airway), caveolated cells (stomach), and multi- or fibrillovesicular cells (olfactory epithelium) [3,18–20]. The evolutionary origin of tuft cells is unknown, but they are widespread across vertebrate species, including placental mammals, snakes, and bullfrogs [3,11,21]. Developmentally, tuft cells require the transcription factor POU Class 2 Homeobox 3 (POU2F3) for differentiation in diverse mucosal surfaces and the thymus [15,22–25]. In the gastrointestinal tract where tuft cells are best characterized, tuft cells are terminally differentiated cells that arise from Leucine Rich Repeat Containing G Protein–Coupled Receptor 5 (Lgr5+) intestinal stem cells in the crypt [15,26]. While the exact environmental cues and transcriptional regulation that drive this process are not completely clear, it has been shown that immune cell–derived cytokines such as interleukin (IL)-13 act on Lgr5+ stem cells to drive polarization toward the tuft cell lineage [27–30]. Other proteins, such as the Taste 1 Receptor Member 3 (TAS1R3) and mechanistic target of rapamycin (mTORC1), also regulate homeostatic tuft cell differentiation and abundance [31,32]. Whether tuft cells emerge from a secretory (Atonal BHLH Transcription Factor 1 (ATOH1-dependent) or nonsecretory (ATOH1-independent) lineage progenitor may vary by anatomic location [15,33–35].
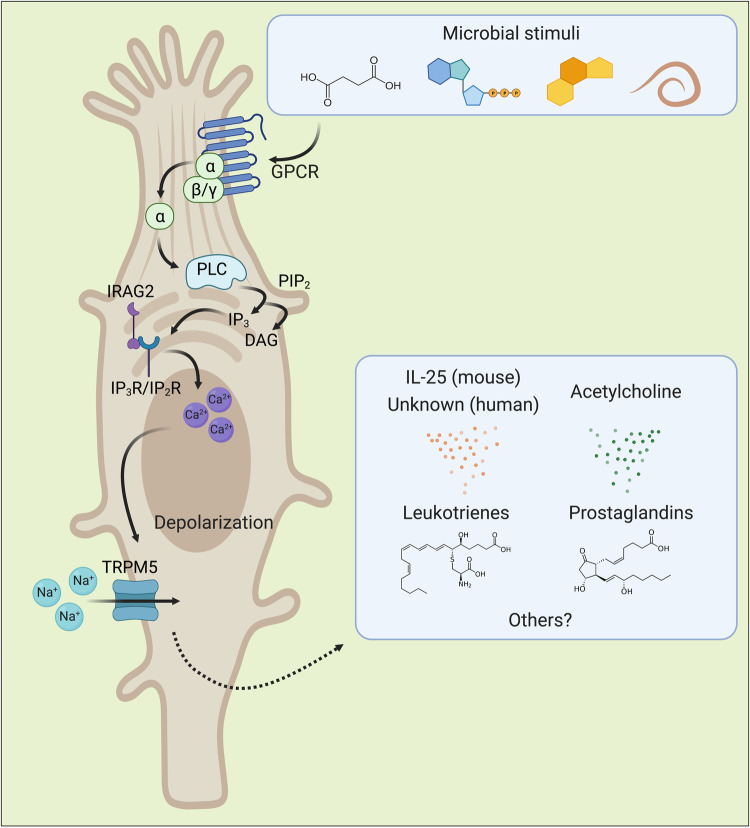
Since their original discovery in the rat trachea and mouse stomach in 1956, the functional roles of tuft cells have remained elusive for over 60 years, even after their identification in humans [2,4,36]. The general signal transduction pathways and effector biosynthetic pathways within tuft cells have been extensively reviewed elsewhere and are outlined generally in Fig 1 [1,37–39]. Despite different sensor and effector functions, tuft cells share many signal transduction pathways with type II taste cells [37]. Briefly, ligands bind to G protein–coupled receptors (GPCRs) on the surface of tuft cells. Intracellular G protein alpha subunits are subsequently activated and promote cleavage of the membranous lipid phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C beta 2 (PLCβ2) or another PLC family member, producing diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 then binds its cognate receptor IP2R in the small intestine or IP3R in the airway and causes calcium efflux from the endoplasmic reticulum. Calcium efflux also appears to require interactions between Inositol 1,4,5-Triphosphate Receptor Associated 2 (IRAG2) with IP3 receptors [40]. Intracellular calcium flux activates transient receptor potential cation channel subfamily M member 5 (TRPM5), which allows sodium influx and subsequent cellular depolarization. This calcium flux and depolarization are canonical measures of tuft cell activation. Downstream of TRPM5, undefined pathways trigger the release of the tuft cell effectors IL-25, acetylcholine (ACh), leukotrienes (LTs), and prostaglandins (PGs) (Fig 1) [29,41–46]. The secretory systems that control effector release are not well understood, and their regulation requires further research. It is known that release of IL-25 can be blocked with the vesicular transport inhibitor Brefeldin A, suggesting that it may be stored in vesicles until triggered for vesicular release [47]. ACh, in contrast, may be secreted in a noncanonical mechanism, as tuft cells do not detectably express the machinery required for ACh to undergo synaptic release [48].
Fig 1. Canonical tuft cell ligands, downstream signal transduction, and effector molecules as understood to date in mouse and human tuft cells.
Molecules derived or produced by microbes and damage-associated by-products of microbial infection can drive tuft cell activation through GPCRs. Most commonly, the GPCR alpha subunit GNAT3 activates PLCβ2 and promotes this signaling cascade after receptor binding, but other alpha subunits and PLC family members have also been implicated. It is not known whether all responses share downstream intracellular signaling pathways, but evidence suggests there may be pathogen-specific and location-specific differences. The transcriptional regulation driving expression of tuft cell signaling components is poorly characterized, but it is known that p53 regulates expression of the transmembrane protein coding gene Irag2, which is required for calcium flux. Additional molecules that activate tuft cells likely exist that have not been discovered [23,29,31,40,43,47,49–55]. GNAT3, G Protein Subunit Alpha Transducin 3; GPCR, G protein–coupled receptor; IRAG2, Inositol 1,4,5-Triphosphate Receptor Associated 2; PLCβ2, phospholipase C beta 2.
Based on their known signaling pathways, tuft cells have been linked to a wide variety of bodily functions, such as the establishment of T cell tolerance, cross talk with the nervous system, epithelial repair and remodeling, cell division, and luminal sensing of microbes [9,24,56–60]. Given these roles, it is unsurprising that tuft cells have been implicated in allergy, inflammatory bowel disease (IBD), and cancer [25,44,45,60–71]. Tuft cells are suggested to have protective functions in the 2 manifestations of IBD: Crohn disease and ulcerative colitis [60,62–64]. The specific mechanisms by which tuft cells mitigate IBD have not been elucidated, but this may be microbiome dependent. Perhaps tuft cells regulate microbiota populations and support protective mucosal barrier function, ameliorating the dysbiosis and bowel dysfunction observed in mouse models and IBD patients. As in IBD, tuft cells may possess protective roles against cancer, such as by impeding Kras-mediated pancreatic tumorigenesis via the production of prostaglandin D2 (PGD2) [45]. In a seemingly juxtaposed role, a number of cancers (colon, pancreatic, thymic, gastric, and small cell lung cancer) have been evidenced to arise from tuft cell origin or to be mediated at least in part by tuft cells [25,65–73]. Further investigation on these topics is warranted, as our understanding of the specific mechanisms by which tuft cells modulate cellular behavior to prevent or promote disease is incomplete.
The best described function of tuft cells emerged after their recent implication in immune sensing of helminths and protists. Through use of the canonical signal transduction pathways delineated in Fig 1, tuft cells can sense the luminal environment. In responding to these microbes at steady state and during insult, tuft cells boast diverse functions ranging from regulating immunity, driving epithelial repair, and maintaining homeostasis. In the mouse small intestine, parasites can trigger tuft cells to release IL-25, stimulating type 2 innate lymphoid cells (ILC2s) to produce IL-5/9/13. These cytokines then drive a type 2 immune response—an inflammatory adaptive immune response that is classically associated with allergy and parasitic infection—which then skews epithelial differentiation [23,29,43,47,49–52,64]. Interestingly, the upstream signaling molecules that drive this pathway differ depending on the pathogen, suggesting unique specificity to tuft cell–mediated immune responses. Tuft cells in the mouse upper airway and lung similarly mediate this type 2 immune pathway, reacting to aeroallergens and transitory helminth infection, respectively [44,55,74,75]. Tuft cell involvement in type 2 immunity has also been reported in the context of coinfection, where tuft cell–IL-4 circuits can impair virus-specific CD8+ T cells during concurrent helminth and viral infection [76,77]. Unlike the small intestine, tuft cells in the colon do not perform parasite-driven tuft cell–ILC2 circuits and instead respond to bacteria. Bacterial microflora can regulate colonic tuft cell numbers and induce tuft cell expansion, and colonic tuft cells have been reported to reduce bacterial infiltration and facilitate epithelial repair [16,60,64,78]. In both the small and large intestine, tuft cells serve as the primary physiologic target of persistent murine norovirus (MNV) viral infection and may promote immune evasion [78–80]. Ultimately, tuft cells respond to both commensal and pathogenic microorganisms, and they perform heterogeneous immunomodulatory functions that vary by anatomic location. This review aims to provide a comprehensive overview of microbial activation of tuft cells with an emphasis on tuft cell heterogeneity and differences between mouse and human tuft cell biology as they relate to human health and disease.
II. Tuft cell heterogeneity
Tuft cells express several characteristic markers that distinguish them from other cell types (Table 1). Tuft cells exhibit broad inter- and intracellular diversity with variation in their expression profiles, phenotypic behavior, and development. While tuft cell receptor and effector gene expression may differ by anatomic location, only a small fraction of these transcriptional differences have been functionally mapped. For example, in the thymus, in addition to their canonical markers, tuft cells also uniquely express antigen presentation hallmarks such as L1cam and genes that encode for major histocompatibility complex II proteins (H2-Aa, H2-Ab, and CD74) that support their function in thymocyte development and immune tolerance [9,52]. Despite their conservation among mammals, tuft cells also exhibit distinct differences in both marker profiles and phenotypic behavior between mice and humans (Table 1).
Table 1. Expression of canonical tuft cell markers across anatomical locations and tuft cell subsets in Mus musculus and Homo sapiens.
| Marker | Name | M. musculus | H. sapiens | Expression patterns and exceptions | Source(s) |
|---|---|---|---|---|---|
| DCLK1 | Doublecortin-like Kinase 1 | ✓ | Most tuft cells (>95%) | [1,64,81] | |
| POU2F3 | POU Class 2 Homeobox 3 | ✓ | ✓ | All tuft cells | [23–25] |
| GFI1B | Growth Factor Independent 1B Transcriptional Repressor | ✓ | All tuft cells | [22,34] | |
| AVIL | Advillin | ✓ | ✓ | Intestinal tuft cells | [82,83] |
| ALOX5AP | Arachidonate 5-Lipoxygenase Activating Protein | ✓ | ✓ | All tuft cells | [82,83] |
| ALOX5 | Arachidonate 5-Lipoxygenase | ✓ | ✓ | All tuft cells | [43,82] |
| PTGS1 (COX-1) | Prostaglandin-Endoperoxide Synthase 1 (Cyclo-oxygenase-1) | ✓ | ✓ | All tuft cells | [82,83] |
| PTGS2 (COX-2) | Prostaglandin-Endoperoxide Synthase 2 (Cyclo-oxygenase-2) | ✓ | ✓ | All tuft cells | [16,82] |
| HPGDS | Hematopoietic Prostaglandin D Synthase | ✓ | ✓ | Small intestinal tuft cells | [15,83] |
| IL-25 | Interleukin-25 | ✓ | * | All tuft cells* | [22,29,84–86] |
| PLCβ2 | Phospholipase C Beta 2 | ✓ | Most tuft cells; skewed toward tuft-1 populations | [87,88] | |
| ChAT | Choline O-Acetyltransferase | ✓ | ✓ | Most tuft cells; not in type II taste bud cells | [46,83,89,90] |
| SIGLECF | Sialic acid-binding Immunoglobulin-like Lectin F | ✓ | Intestinal and pancreatic tuft cells | [22,23] | |
| pEGFR | Epidermal Growth Factor Receptor | ✓ | ✓ | All tuft cells | [16] |
| GNAT3 | G Protein Subunit Alpha Transducin 3 | ✓ | Most tuft cells, skewed toward tuft-1 populations | [22,91] | |
| TAS2Rs and TAS1Rs | Taste 2 Receptors and Taste 1 Receptors | ✓ | ✓ | Lowly/undetectably expressed in the intestinal tract; specific receptor expression and combinations may vary by tissue; skewed toward tuft-1 populations | [31,47,52,88] |
| TRPM5 | Transient Receptor Potential Cation Channel Subfamily M Member 5 | ✓ | ✓ | All tuft cells | [82,92] |
Transcriptomic analyses reveal distinct tuft cell subsets
Single-cell RNA sequencing (scRNA-seq) analyses have uncovered distinct tuft cell subsets. Tuft cells cluster separately by expression profile both within and between anatomic locations [52]. Some studies have found 2 distinct mature tuft cell populations within a given site deemed “tuft-1” and “tuft-2” [93,94]. In the small intestine, tuft-1 and tuft-2 populations share consensus tuft cell signatures and express Dclk1, CD24, Pou2f3, Trpm5, and IL25 [93]. In the airway, canonical consensus tuft cell markers are also shared but some skew more toward tuft-1 (Pou2f3 and Gnat3) or tuft-2 (Gfi1b, Alox5ap, Sox9, Dclk1, and CD24) populations [52,94]. In general, tuft-1 populations express tuft cell–specific genes related mostly to taste transduction, such as Tas2r and Tas1r gene families, Gnat3, and Plcb2, although taste receptor expression is limited in intestinal tuft cells [52,93,94]. Tuft-2 populations overall surprisingly express immune-related genes, including Ly6e, TSLP, and even the hematopoietic lineage marker Ptprc (CD45) [93,94]. However, not all single-cell sequencing analyses have unveiled tuft-1 and tuft-2 populations and instead identify tuft cells as a single cluster [22,35,52,64]. Within their cluster, some of these tuft cells are still heterogeneous in their expression of specific effector pathway components. For instance, only a subset of tuft cells expresses detectable Fcgr2a (the Fc receptor activated by IgG) or the vesicular ACh transporter required for ACh trafficking and secretion [22,48]. In response to cholinergic blockade, some tuft cells can expand and adopt an enteroendocrine transcriptional profile, releasing more ACh to restore cholinergic homeostasis [95].
Long- and short-lived tuft cells
Various studies have demonstrated that mature tuft cells transit upward along the crypt-villus axis as terminally differentiated cells [15,96,97]. With a half-life of approximately 3 to 4 days, tuft cells are generally described as short-lived, postmitotic cells that continually undergo turnover in the intestinal epithelium. A small subset of tuft cells may exhibit long-lived, quiescent stem cell-like behavior with self-renewal properties. This long-lived subpopulation may constitute 5% of the overall tuft cell population and is characterized by the expression of pluripotency factors (Oct4, Nanog, Klf4, and Sox2), survival factors (Survivin, Akt, and p53), and markedly low turnover rates [66,98]. Although DCLK1 is expressed at the +4 position of the crypt, a known site of quiescent intestinal stem cells, long-lived tuft cells express other tuft cell–specific markers, like COX2, and likely do not function as reserve stem cells [66,98–102]. Instead, their expression profiles poise them for injury repair and uncontrolled proliferation. Upon inflammatory insult, tuft cells can serve as tumor stem cells in colorectal cancer and some colorectal tumors can originate from long-lived tuft cells [66,97]. Whether long-lived tuft cells are present in all mice and in extraintestinal sites is unknown. Further, the presence of long-lived tuft cells in humans has yet to be confirmed, although their existence may have important ramifications in human cancers. In mice, long-lived tuft cells have been traced using Cre recombinases driven by expression of the mouse-specific tuft cell marker Dclk1 [35,66,98,103]. Therefore, probing for a comparable tuft cell subset in humans will likely require an alternative cognate tuft cell marker.
Murine versus human tuft cells
Murine and human tuft cells share similar structure and anatomical distribution, although tuft-1 versus tuft-2 populations and the presence of tuft cells in the cecum have yet to be described in humans [1,56,83]. Some human tuft cell functions that mirror those of mice have also been identified, including their role in thymocyte development and potentiating type 2 immune circuits [75,85,104]. However, IL-25 production of human tuft cells has only been described in the nasal epithelium [84–86]. IL-25 transcripts are not robustly detected in tuft cells from intestinal tissue in nonhuman primates or humans [22,42]. Whether human tuft cells produce an alternative effector molecule instead of IL-25 and under what circumstances human tuft cells promote type 2 immunity is an active area of investigation. In addition to IL-25, human and mouse tuft cells exhibit differential expression of some canonical tuft cell markers, most notably DCLK1, but how these differences affect tuft cell functionality is unknown (Table 1) [64,103]. With a heavy reliance on mouse models, our understanding of human tuft cell biology remains impeded by the rarity of tuft cells in human scRNA-seq analyses, limited genetic modeling tools, and the fact that many human samples are derived from diseased patients. Nonetheless, translating these key findings to humans is an important future direction.
III. Tuft cells in host–microbe interactions
Located in the mucosal epithelium, tuft cells are subject to continual microbial exposure and insult. It is thus unsurprising that tuft cells have evolved to perform critical functions in modulating host–microbe interactions. Tuft cells are best characterized as type 2 immune mediators, specifically during parasitic infection, and the role of tuft cells in other microbial interactions remains an active area of investigation. Tuft cells function as luminal sentinels that can sense and respond to a variety of microbial stimuli beyond parasitic helminths and protozoa, including bacteria, viruses, and fungi. In each anatomic location, tuft cells have adaptively tuned their responses to each class of microbe with exquisite specificity, potentially in part due to their heterogeneity. Still, it is poorly understood how many of these interactions are regulated and how specific tuft cell subpopulations differentially contribute to microbial sensing. Importantly, it is even less understood how many these interactions translate to human health, as the majority of tuft cell functions associated with luminal perturbations have been studied only in small animal models.
Parasites (protists, protozoa, and helminths)
It was long appreciated that IL-25 is one of the earliest cytokines induced in response to helminth infection, but the cells that sense helminths and produce IL-25 were unknown until the simultaneous discovery that tuft cells were the elusive source [23,29,49]. Infection with the transitory helminth Nippostrongylus brasiliensis induces a strong type 2 immune response driven by IL-25 and IL-13 that facilitates worm clearance within 7 to 10 days [23,49]. By employing an IL-25 reporter mouse known as Flare25 (flox and reporter of IL25; IL25F25/F25), tuft cells were found to constitutively express IL-25, implicating them as possible mediators of type 2 immunity [29]. At resting state, tuft cell–derived IL-25 acts in a paracrine manner on the IL-25 receptor IL17RB on ILC2s to support homeostatic production of IL-13 [29]. IL-13 in turn acts on IL4RA/IL13RA dimers on epithelial progenitors in the crypt, regulating intestinal homeostasis and supporting tuft cell and goblet cell differentiation [23,29]. During N. brasiliensis infection, this feed-forward loop becomes more pronounced, inducing a 10- to 15-fold increase in tuft cell abundance and small intestine lengthening [23,29,49–51]. Tuft cell activation by N. brasiliensis requires expression of TRPM5, ILC2 production of IL-13, downstream signaling through IL4RA, and Signal Transducer and Activation of Transcription 6 (STAT6) [23,29,49,50,52]. Despite its role in signaling, GNAT3 is not required for tuft cell sensing of N. brasiliensis [52]. While recombinant IL-4 and IL-13 can drive this circuit, endogenous IL-4 is unaffected by loss of tuft cells and is dispensable for in vivo N. brasiliensis infection [23]. This same positive feedback loop has been identified during infection with the protozoan Tritrichomonas muris and the intestinal helminths Trichinella spiralis, Heligmosomoides polygyrus, and Hymenolepis microstoma, although the degree of tuft cell hyperplasia may vary [29,49,53,93]. In addition to IL-25, helminths trigger small intestinal tuft cells to secrete cysteinyl leukotrienes (CysLTs) [43]. The CysLTs LTC4 and LTD4 bind CysLTR1 on the surface of ILC2s, likely activating ILC2s via induction of Nuclear Factor of Activated T-cell (NFAT) signaling [43,74]. CysLTs constitute a critical and nonredundant component of the anti-helminth immune response, as they operate synergistically with IL-25 signaling and worm clearance is significantly delayed in their absence [43]. Surprisingly, the type 2 immune response against intestinal protists does not require CysLTs, indicating that tuft cell effectors can differ between microbial taxonomies within the same tissue [43]. Much like CysLTs, ACh is indispensable for driving optimal tuft cell–ILC2 immune responses [105]. N. brasiliensis and T. muris can induce ChAT expression and ACh production by ILC2s, and IL-25 up-regulates ACh receptors on ILC2s [105]. In the absence of ILC2-derived ACh acting in an autocrine loop, helminth expulsion is significantly dampened [105]. Whether tuft cell–derived ACh acts directly on ILC2s is not clear. Tuft cells also up-regulate Hpgds2, Cox1, and Cox2 and produce PGD2 during late N. brasiliensis infection [106]. Tuft cell–derived PGD2, unlike CysLTs and ACh, acts as a negative regulator of tuft cell–ILC2 immunity by down-regulating Il13ra and limiting intestinal stem cell differentiation programs driven by type 2 immune cytokines [106]. Ultimately, PGD2 seems to aid in restoring intestinal cell populations to homeostatic baseline after infection [106]. Whether this mechanism is true for all intestinal parasites is not known and requires further investigation.
The tuft cell–ILC2 type 2 circuit is present but restrained in the absence of infection, requiring an activating trigger from parasitic infection. For T. muris and other related Tritrichomonad species, the activating signal has been identified as the fermentative end product and tricarboxylic acid (TCA) cycle intermediate succinate [51,52]. Succinate activates tuft cells directly by binding to the GPCR succinate receptor 1 (SUCNR1/GPR91) and initiating a GNAT3-dependent signal cascade that drives IL-25 release in a TRPM5-dependent manner [51,52]. The tuft cell receptor TAS1R3 also facilitates a type 2 immune response against T. muris colonization. Although TAS1R3 is not required for tuft cells to sense succinate, genetic ablation of Tas1r3 dampens tuft cell hyperplasia. Increased duration of succinate treatment can overcome a TAS1R3 deficiency, suggesting that while TAS1R3 is not required for tuft cells to sense succinate, this receptor may potentiate the response or improve tuft cell response kinetics [31].
Unlike T. muris, tuft cell activation by the helminths N. brasiliensis, T. spiralis, and H. polygyrus is SUCNR1- and TAS1R3 independent [47,50,52]. These helminths preferentially colonize the proximal small intestine, where Sucnr1 expression is relatively low. In contrast, T. muris colonizes the SUCNR1-rich distal small intestine [43,51,52]. Tuft cells in the proximal small intestine are less responsive to succinate as a result, and tuft cells have adapted alternative strategies to mount immune responses against these parasites [43,51,52]. In the case of T. spiralis, excretory–secretory (E–S) products and worm extracts activate tuft cells by acting on the bitter taste receptor TAS2R143 [47]. The specific molecule(s) from T. spiralis that trigger tuft cell activation and whether additional TAS2R family members are involved in initial T. spiralis sensing are undetermined [47]. To date, the identity and nature of the activating signal(s) in N. brasiliensis and H. polygyrus colonization remain unknown. Whether N. brasiliensis activates this circuit by first directly activating tuft cells or indirectly by triggering ILC2s is not clear. In either case, it is likely that N. brasiliensis activates tuft cells by introducing an activating ligand or removing an inhibitory signal that propels this circuit forward. Direct positive and negative regulators of tuft cell function remain obscured, but multiple negative regulators of ILC2-mediated type 2 immune responses have been described. For example, the E3 ubiquitin ligase A20 acts as a negative regulator of IL17RB on ILC2s and that A20 deficiency can spontaneously trigger this type 2 immune loop [51]. A20 is notably down-regulated during N. brasiliensis infection [51]. Cytokine-inducible SH2-containing protein (CISH) also negatively regulates ILC2s at homeostasis and during N. brasiliensis infection, as CISH restricts IL-25–dependent ILC2 activation [107]. Global or ILC2-specific CISH knockout also expedites N. brasiliensis expulsion and increases tuft cell numbers at early infection time points [107]. Perhaps N. brasiliensis interferes with A20, CISH, or other negative regulators of ILC2s to release the brakes on this type 2 immune loop.
Whatever the activating signal, tuft cell activation is characterized by a series of intracellular signaling cascades that proceed as outlined in Fig 1. This pathway has been best defined during T. spiralis infection, where TAS2R activation drives tuft cell trimeric G proteins Gα-Gustducin/Gβ1γ13 and/or Gαo/Gβ1γ13 to dissociate and activate PLCβ2 to cleave IP3, which drives calcium release from the endoplasmic reticulum via binding at IP3R2 [47]. Calcium release then induces TRPM5 to open, causing cellular depolarization that stimulates IL-25 release—pushing the classical tuft cell–ILC2 loop into action [47]. Downstream mucosal type 2 immune responses aid in mounting a classical “weep and sweep” response and gasdermin C–mediated activation that drive helminth expulsion [23,108].
Parasitic infections by N. brasiliensis, T. spiralis, and H. polygyrus are typically self-limiting with rapid clearance mediated by the tuft cell–ILC2 circuit. However, unlike N. brasiliensis and T. spiralis, tuft cell hyperplasia is markedly lower (approximately 5- to 10-fold) during H. polygyrus infection and downstream immune-mediated clearance takes months [23,109]. Recently, it was shown that H. polygyrus E–S products suppress tuft cell differentiation, and this likely has critical consequences for tuft cell–mediated control of chronic helminth infection [109]. With the prevalence of endemic helminth infection in human populations, the potential role of human tuft cells in intestinal type 2 immunity and chronic/long-term or secondary helminth infection are of vital interest. How the tuft cell–ILC2 circuit translates to repeated helminth infection or endemic helminth infection in the small intestine is currently unexplored.
Bacteria
During pathogenic bacterial infection with Salmonella enterica, tuft cells in the small intestine do not undergo significant transcriptional changes, suggesting that they may not respond to bacteria [93]. A similar effect has been observed with commensal microbes in the small intestine, where tuft cells are generally resistant to transcriptional perturbation by antibiotic-mediated depletion of the gut microbiome [64,78]. However, evidence thus far suggests that tuft cells in the small intestine can sense some bacteria such as members of the Bifidobacterium genus in a succinate-dependent manner [64]. In response to bacterial succinate, ATOH1-independent ileal tuft cells initiate the same type 2 immune circuit driven by Tritrichomonads via SUCNR1 and up-regulate a variety of TCA genes to modulate inflammation in the small intestine [50,64]. The precise mechanisms by which succinate alters tuft cell gene expression and the consequences of small intestinal tuft cell hyperplasia on the microbiome are unclear.
In contrast to the small intestine, ATOH1-dependent colonic tuft cells are highly sensitive to intestinal bacteria and respond independently of SUCNR1 or type 2 immune circuits. After exposure to antibiotics, tuft cells in the large intestine are significantly depleted [78]. Moreover, the microbiome can causes changes in colonic tuft cell gene expression and tuft cell expansion, as evidenced by helminth-free fecal gavage in germ-free mice [16]. Tuft cell expansion under these conditions is mitigated after 8 weeks, and tuft cell numbers then return to baseline [16]. Furthermore, when the mucosal barrier is breached by bacteria, tuft cell differentiation is stifled, and tuft cell numbers decrease [110]. Previous work has shown that tuft cells are critical for promoting epithelial repair and limiting this bacterial infiltration, especially in bacterial-induced colitis [60]. Together, these findings imply balanced bidirectional regulation, whereby the intestinal microbiome maintains homeostatic colonic tuft cell populations and tuft cells prevent bacterial infiltration and dysbiosis via unidentified direct or indirect mechanism(s). As tuft cells have been linked to IBD in humans, the interactions of tuft cells with the bacterial microbiome may have implications for human health [62].
A similar phenomenon has been described in the airway of mice and humans, where formylated peptides, gram-negative quorum sensing molecules (QSMs, e.g., acyl homoserine lactones), and some D-amino acids can activate tuft cells [41,57,89,111–113]. While the receptors for formylated peptides and QSMs have not been identified, D-amino acids appear to trigger tuft cells using the sweet taste receptor TAS1R2/3 [41,57,89,111–113]. The functional consequence of these interactions is poorly characterized, but they may alter immune responses against pathogenic bacteria. It was previously shown that calcium flux originating from upper airway tuft cells can propagate through gap junctions and trigger antimicrobial peptide (AMP) secretion from neighboring epithelial cells [114]. Importantly, Staphylococcus-derived D-amino acids can impair antibacterial innate immune responses by reducing AMP production or release [113]. In contrast, QSMs can incite tuft cells to produce ACh, which may facilitate mucociliary clearance of virulent bacteria [41,57].
Viruses
Tuft cells can mediate virus pathogenesis both directly by serving as a target cell for infection and indirectly by coinfection driven immune-mediated effects. Both MNV and murine rotavirus can directly infect and replicate within tuft cells, whereas helminth promotion of West Nile virus (WNV) pathogenesis requires the immunomodulatory functions of tuft cells. Tuft cells express the MNV receptor CD300lf and are the primary physiologic target for the persistent strain MNVCR6 [78]. Inducing tuft cell hyperplasia in the small intestine with type 2 cytokines promotes MNVCR6 infection, while reducing colonic tuft cells with broad-spectrum antibiotics is associated with resistance to MNVCR6 infection [78,79]. Tuft cell tropism also enables MNVCR6 evasion of the adaptive immune system. While CD8+ T cells activated by MNVCR6 appear functional, they fail to eliminate infected tuft cells. Similarly, MNVCR6 elicits a neutralizing antibody response, but this is not sufficient to clear infection from tuft cells [115]. This suggests tuft cells may serve as an immune-privileged niche that promotes norovirus immune escape [78,80]. Furthermore, MNVCR6 infection of tuft cells in germ-free mice restores disrupted barrier integrity, regulates immune cell populations, and supports intestinal homeostasis [116]. Taken together, these findings demonstrate that tuft cells are critical for intestinal epithelial homeostasis and immunity. Whether tuft cells mediate human norovirus infection and pathogenesis remains unclear. The receptor for human norovirus remains unknown, but human CD300lf does not appear to act as a receptor [117]. Intestinal epithelial cells including enteroendocrine cells support human norovirus infection and B cells support replication of the human norovirus strain GII.4-Sydney (genogroup II, genotype 4, Sydney isolate) [118–120].
Very recently, it was demonstrated that murine rotavirus also infects tuft cells [121]. While rotavirus primarily infects mature enterocytes near the apical villi of the small intestine, infected tuft cells were identified by scRNA-seq and immunofluorescence [121]. More research will be required to clarify whether rotavirus productively infects tuft cells or whether tuft cells employ any postentry barriers that restrict rotavirus replication. In the rotavirus-infected epithelium, tuft cells up-regulate interferon stimulated genes and a number of viral defense pathways, suggesting they can effectively sense enteric viral infection [121]. During rotavirus infection, tuft cell responses are categorically different than those seen during parasitic infection. In the rotavirus-infected epithelium, tuft cells down-regulate Il25, Alox5, Alox5ap, and Ltc4s and up-regulate Trpm5, Plcb2, and Plcg2 [121]. The functional consequence of up-regulating intermediate signaling genes while simultaneously down-regulating downstream effector genes is unclear, but this finding suggests that tuft cells may perform divergent chemosensory pathways in the presence of enteric viral infection. Maybe other chemosensory effectors are secreted in response to rotavirus, as TRPM5 and PLC family proteins are essential for tuft cell activation. How tuft cells contribute to rotavirus pathogenesis and how tuft cells offer an immune privileged niche for some viruses but not others remain open questions.
In the context of coinfection, tuft cells also potentiate viral infection. During coinfection with the helminth T. spiralis and MNVCR6, tuft cells populations expand and virus-specific CD8+ T cell populations trend modestly downward, which is associated with increases in MNVCR6 viral load during persistent infection [76,78]. A similar phenotype has been described during coinfection with helminth H. polygyrus bakeri during infection with the flaviviruses WNV, Zika virus, and Powassan virus [77]. During flavivirus and helminth coinfection, this tuft cell–IL-4 circuit acts directly on the intestinal epithelium to impair virus-specific CD8+ T cell survival, enabling higher viral replication in multiple segments of the gastrointestinal tract and central nervous system [77]. Taken together, these findings suggest that tuft cells may modulate viral persistence and the CD8+ adaptive immune response. Despite high rates of norovirus and flavivirus infection in countries with endemic helminth burden and previous reports of coinfection, the role of tuft cells in helminth–virus coinfection in humans is unclear [122–124].
Viral infection can also prompt de novo tuft cells development. At steady state, tuft cells are not present in the alveolar epithelium of the lower airway [14]. After infection with the influenza A subtype H1N1, the lung undergoes dramatic dysplastic remodeling and tuft cells appear de novo at approximately 25 to 51 days postinfection [14]. The functional role for these tuft cells is unknown, but it has been suggested that they may facilitate injured lung tissue to rapidly respond to damage signals [14]. Much like in tuft cell type 2 immune feed-forward circuits, tuft cells that appear after lung damage appear promote and reinforce their own expansion [14]. The proposed the role of tuft cells in lung inflammation and repair is poorly explored across other respiratory viruses, although a modest 3-fold increase in tuft-like cells in the upper airway and ectopic development of tuft-like cells in the lung has been recently described in human patients with Coronavirus Disease 2019 (COVID-19) [125]. The physiological relevance and activities of these tuft cells merit further investigation in virus-induced lung injury, but they may contribute to pathophysiology or tissue repair.
Other
Tuft cells in the upper airway have been implicated in type 2 immune responses, especially after stimulation with aeroallergens. Exposure to fungal chitin, dust mites, or Alternaria mold can elicit the release of ATP, a typical damage-associated molecular pattern (DAMP). ATP release triggers the purinergic P2Y2 receptor on tuft cells and stimulates tuft cells to release IL-25 and CysLTs like LTE4 [44]. LTE4 subsequently binds the high affinity receptor CysLT3R on tuft cells while other CysLTs bind their cognate receptors on ILC2s, driving a feed-forward type 2 immune circuit similar to that of small intestine [44,55]. Although other LTs can activate ILC2s directly, LTE4 may indirectly activate ILC2s via tuft cell release of IL-25 in vivo, as LTE4 poorly binds other CysLTRs and ILC2s lack CysLT3R expression [55,74]. While this pathway is IL-25–dependent in the upper airway, it likely operates in a STAT6-independent manner, unlike in the intestinal tract [55]. In humans, Aspergillus fungus and Alternaria can cause IL-25 release and tuft cell expansion in rhinosinusitis patients, but whether this pathway translates to healthy individuals or involves LTs is unclear [75].
IV. Concluding remarks
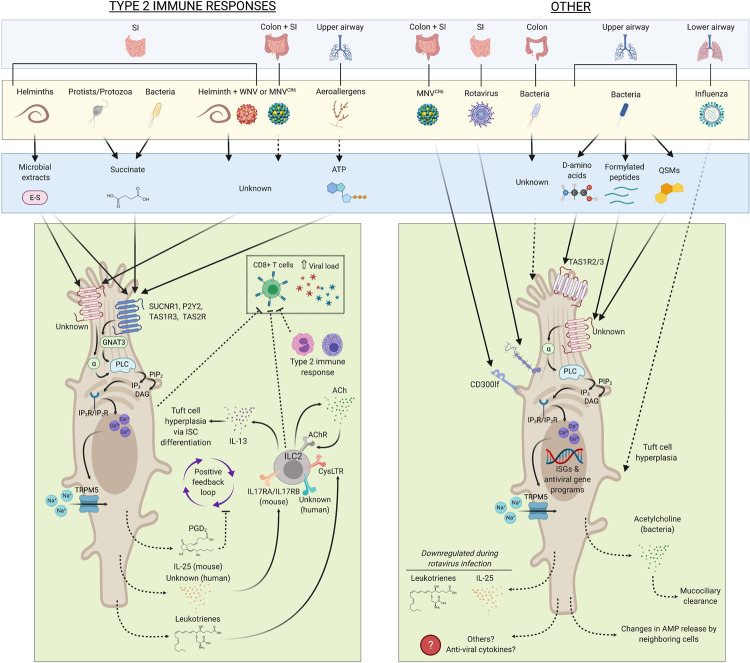
Tuft cells modulate a variety of host-pathogen interactions and act as key mediators of mucosal immunity to diverse microbes (Fig 2). Tuft cells can sense metabolic by-products, specific microbial components, and DAMPs using noncanonical pattern recognition receptors, including TAS1R3, SUCNR1 and P2Y2. In this sense, tuft cells function as immune sensory cells. Tuft cells can also maintain epithelial integrity by regulating commensal bacteria in the large intestine. With only a handful of ligands known to activate tuft cells and limited knowledge of downstream tuft cell effectors, there are likely others that have not been discovered. Overall, the role of tuft cells in orchestrating host microbial responses is highly context and location dependent, with unique activators and effectors depending on the luminal biome and tissue type. This likely has important implications between species, particularly in relating mouse tuft cell biology to that of humans. Given that luminal biomes, metabolites, and nutrient contents can vastly differ between mice and humans, we speculate that tuft cell function and behaviors in humans may be distinct. Perhaps human tuft cells sense different suites of luminal microbes or their by-products, resulting in unique downstream responses. To fully probe these differences, a scalable in vitro culture system for tuft cells will be needed to characterize the molecular mechanisms of tuft cell chemosensation and their downstream effector functions.
Fig 2. A current model of the sensor–effector pathways mediated by tuft cells in response to microbial stimuli.
Dotted lines indicate intermediate or downstream pathways and indirect mechanisms (e.g., secretion mechanisms, CD8+ T cell ablation, or de novo tuft cell hyperplasia). ILC2 negative regulators (e.g., A20 and CISH) are not depicted for simplicity. Evidently, our understanding of tuft cell–microbial interactions and tuft cell responses are poorly characterized in the large intestine or outside of the context of type 2 immunity, especially in humans. ACh, acetylcholine; AMP, antimicrobial peptide; DAG, diacylglycerol; E–S, excretory–secretory; IL, interleukin; ILC2, type 2 innate lymphoid cell; MNV, murine norovirus; PGD, prostaglandin D; PLC, phospholipase C; QSM, quorum sensing molecule; TAS1R3, Taste 1 Receptor Member 3; TAS2R, Taste 2 Receptor; TRPM5, transient receptor potential cation channel subfamily M member 5; WNV, West Nile virus.
Tuft cells have known involvement in human cancers, but their specific roles and how tuft cells behave in other human diseases are relatively unexplored territories. With the recent discovery of thymic tuft cells in humans, the role of tuft cells in human tolerance and autoimmunity will likely bring critical insights into the human immune system [104]. As reduced helminth burdens and dysregulated microbiomes in human populations are correlated with increases in autoimmunity and allergy, it will be compelling to see whether tuft cells support anti-helminth immune responses or bacterial microflora in humans and how this relates to allergy and autoimmunity [123,124]. Finally, how tuft cells interact with human enteric viruses remains to be seen. Whether human tuft cells support viral infection in humans is unclear, but the prospect that tuft cells could offer an immune-privileged niche may have important consequences for chronic viral infections. Ultimately, tuft cells are a rare chemosensory cell type that facilitate striking interkingdom interactions between microbes and their hosts by integrating viral, bacterial, and parasitic pathogenesis and immunity.
Funding Statement
This research was funded by NIH K08 A128043 and R01AI148467, Burroughs Wellcome Fund, the Robert Leet and Clara Guthrie Patterson Trust (CBW) and the National Science Foundation (fellowship DGE1752134 to M.S.S). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ting H-A, von Moltke J. The Immune Function of Tuft Cells at Gut Mucosal Surfaces and Beyond. 2019. doi: 10.4049/jimmunol.1801069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Zeitschrift fur Zellforschung und mikroskopische Anatomie (Vienna, Austria: 1948). 1956;44(4). doi: 10.1007/BF00345847 . [DOI] [PubMed] [Google Scholar]
- 3.Meyrick B, Reid L. The alveolar brush cell in rat lung—a third pneumonocyte. J Ultrastruct Res. 1968;23(1). doi: 10.1016/s0022-5320(68)80032-2 . [DOI] [PubMed] [Google Scholar]
- 4.Järvi O, Keyriläinen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand Suppl. 1956;39(Suppl 111). . [PubMed] [Google Scholar]
- 5.Sato A, Miyoshi S. Fine structure of tuft cells of the main excretory duct epithelium in the rat submandibular gland. Anat Rec. 1997;248(3). doi: [DOI] [PubMed] [Google Scholar]
- 6.Isomäki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta pathologica et microbiologica Scandinavica Section A, Pathology. 1973. . [PubMed] [Google Scholar]
- 7.Okamoto K, Hanazaki K, Akimori T, Okabayashi T, Okada T, Kobayashi M, et al. Immunohistochemical and electron microscopic characterization of brush cells of the rat cecum. Med Mol Morphol. 2008;41(3). doi: 10.1007/s00795-008-0412-0 . [DOI] [PubMed] [Google Scholar]
- 8.Luciano L, Castellucci M, Reale E. The brush cells of the common bile duct of the rat. Cell Tissue Res. 2021;218 (2):403–20. doi: 10.1007/BF00210353 [DOI] [PubMed] [Google Scholar]
- 9.Miller C, Proekt I, von Moltke J, Wells K, Rajpurkar A, Wang H, et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature. 2018;559(7715). doi: 10.1038/s41586-018-0345-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110(3). doi: 10.1007/s004180050292 . [DOI] [PubMed] [Google Scholar]
- 11.Deckmann K, Krasteva-Christ G, Rafiq A, Herden C, Wichmann J, Knauf S, et al. Cholinergic urethral brush cells are widespread throughout placental mammals. Int Immunopharmacol. 2015;29(1). doi: 10.1016/j.intimp.2015.05.038 . [DOI] [PubMed] [Google Scholar]
- 12.Moran DT, Rowley JC, Jafek BW. Electron microscopy of human olfactory epithelium reveals a new cell type: the microvillar cell. Brain Res. 1982;253(1–2). doi: 10.1016/0006-8993(82)90671-0 . [DOI] [PubMed] [Google Scholar]
- 13.Finger TE, Böttger B, Hansen A, Anderson K, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100(15). doi: 10.1073/pnas.1531172100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rane CK, Jackson SR, Pastore CF, Zhao G, Weiner AI, Patel NN, et al. Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am J Physiol Lung Cell Mol Physiol. 2019;316(6). doi: 10.1152/ajplung.00032.2019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerbe F, Es JHv, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192(5). doi: 10.1083/jcb.201010127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinley ET, Sui Y, Al-Kofahi Y, Millis BA, Tyska MJ, Roland JT, et al. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI insight. 2017;2(11). doi: 10.1172/jci.insight.93487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iseki S. Postnatal development of the brush cells in the common bile duct of the rat. Cell Tissue Res. 1991;266(3). doi: 10.1007/BF00318592 . [DOI] [PubMed] [Google Scholar]
- 18.Sbarbati A, Crescimanno C, Benati D, Osculati F. Solitary chemosensory cells in the developing chemoreceptorial epithelium of the vallate papilla. J Neurocytol. 1998;27 (9):631–5. doi: 10.1023/a:1006933528084 [DOI] [PubMed] [Google Scholar]
- 19.Nabeyama A, Leblond CP. "Caveolated cells" characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat. 1974;140(2). doi: 10.1002/aja.1001400203 . [DOI] [PubMed] [Google Scholar]
- 20.Hammond JB, LaDeur L. Fibrillovesicular cedlls in the fundic glands of the canine stomach: evidence for a new cell type. Anat Rec. 1968;161(4). doi: 10.1002/ar.1091610401 . [DOI] [PubMed] [Google Scholar]
- 21.Toshiaki G, Akihiko K, Hiromassa T, Takeshi H, Kazuyoshi H, Shozo S. Electron microscopic observations of the alveolar brush cell of the bullfrog. Zoolog Sci. 1987;4 (4):613–20. [Google Scholar]
- 22.Elmentaite R, Kumasaka N, Roberts K, Fleming A, Dann E, King H, et al. Cells of the human intestinal tract mapped across space and time. Nature. 2021;597 (7875):250–5. doi: 10.1038/s41586-021-03852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585). doi: 10.1038/nature16527 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14(6). doi: 10.1038/nn.2820 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YH, Klingbeil O, He XY, Wu XS, Arun G, Lu B, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32(13–14). doi: 10.1101/gad.314815.118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116(1). doi: 10.1016/s0016-5085(99)70222-2 . [DOI] [PubMed] [Google Scholar]
- 27.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell. 2018;175(5). doi: 10.1016/j.cell.2018.10.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Z, Dean JW, Xiong L, Dougherty MW, Oliff KN, Chen ZE, et al. Mitochondrial transcription factor A in RORγt+ lymphocytes regulate small intestine homeostasis and metabolism. Nat Commun. 2021;12 (1):1–16. doi: 10.1038/s41467-020-20314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585). doi: 10.1038/nature16161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher MA, Hsieh JJ, Liu CY, Appel KL, Waddell A, Almohazey D, et al. Sprouty2 limits intestinal tuft and goblet cell numbers through GSK3β-mediated restriction of epithelial IL-33. Nat Commun. 2021;12 (1):1–16. doi: 10.1038/s41467-020-20314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howitt MR, Cao YG, Gologorsky MB, Li JA, Haber AL, Biton M, et al. The Taste Receptor TAS1R3 Regulates Small Intestinal Tuft Cell Homeostasis. ImmunoHorizons. 2020;4(1). doi: 10.4049/immunohorizons.1900099 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aladegbami B, Barron L, Bao J, Colasanti J, Erwin CR, Warner BW, et al. Epithelial cell specific Raptor is required for initiation of type 2 mucosal immunity in small intestine. Sci Rep. 2017;7(1). doi: 10.1038/s41598-017-06070-w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gracz AD, Samsa LA, Fordham MJ, Trotier DC, Zwarycz B, Lo YH, et al. Sox4 Promotes Atoh1-Independent Intestinal Secretory Differentiation Toward Tuft and Enteroendocrine Fates. Gastroenterology. 2018;155(5). doi: 10.1053/j.gastro.2018.07.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjerknes M, Khandanpour C, Möröy T, Fujiyama T, Hoshino M, Klisch TJ, et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. 2012;362(2). doi: 10.1016/j.ydbio.2011.12.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herring CA, Banerjee A, McKinley ET, Simmons AJ, Ping J, Roland JT, et al. Unsupervised Trajectory Analysis of Single-Cell RNA-Seq and Imaging Data Reveals Alternative Tuft Cell Origins in the Gut. Cell systems. 2018;6(1). doi: 10.1016/j.cels.2017.10.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodin J. LXVII Ultrastructure of the Tracheal Ciliated Mucosa in Rat and Man:. Annals of Otology. Rhinology & Laryngology. 1959;68 (4):964–74. doi: 10.1177/000348945906800402 [DOI] [Google Scholar]
- 37.Schneider C, O’Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol. 2019;19 (9):584–93. doi: 10.1038/s41577-019-0176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billipp TE, Nadjsombati MS, von Moltke J. Tuning tuft cells: new ligands and effector functions reveal tissue-specific function. Curr Opin Immunol. 2021;68. doi: 10.1016/j.coi.2021.10.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevo S, Kadouri N, Abramson J. Tuft cells: From the mucosa to the thymus. Immunol Lett. 2019;210. doi: 10.1016/j.imlet.2019.02.003 . [DOI] [PubMed] [Google Scholar]
- 40.Chang CY, Wang J, Zhao Y, Liu J, Yang X, Yue X, et al. Tumor suppressor p53 regulates intestinal type 2 immunity. Nat Commun. 2021;12(1). doi: 10.1038/s41467-021-23587-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perniss A, Liu S, Boonen B, Keshavarz M, Ruppert AL, Timm T, et al. Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides. Immunity. 2020;52(4). doi: 10.1016/j.immuni.2020.03.005 . [DOI] [PubMed] [Google Scholar]
- 42.Inaba A, Arinaga A, Tanaka K, Endo T, Hayatsu N, Okazaki Y, et al. Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate. Int J Mol Sci. 2021;22(15). doi: 10.3390/ijms22157921 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGinty JW, Ting H-A, Billipp TE, Nadjsombati MS, Khan DM, Barrett NA, et al. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity. Immunity. 2020;52(3). doi: 10.1016/j.immuni.2020.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ualiyeva S, Hallen N, Kanaoka Y, Ledderose C, Matsumoto I, Junger WG, et al. Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Science immunology. 2020;5(43). doi: 10.1126/sciimmunol.aax7224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DelGiorno KE, Chung CY, Vavinskaya V, Maurer HC, Novak SW, Lytle NK, et al. Tuft Cells Inhibit Pancreatic Tumorigenesis in Mice by Producing Prostaglandin D 2. Gastroenterology. 2020;159(5). doi: 10.1053/j.gastro.2020.07.037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31(1). doi: 10.1016/j.ccell.2016.11.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo XC, Chen ZH, Xue JB, Zhao DX, Lu C, Li YH, et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci U S A. 2019;116(12). doi: 10.1073/pnas.1812901116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schütz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6. doi: 10.3389/fphys.2015.00087 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science (New York, NY). 2016;351(6279). doi: 10.1126/science.aaf1648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei W, Ren W, Ohmoto M, Urban JF, Matsumoto I, Margolskee RF, et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A. 2018;115(21). doi: 10.1073/pnas.1720758115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider C, O’Leary CE, von Moltke J, Liang HE, Ang QY, Turnbaugh PJ, et al. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell. 2018;174(2). doi: 10.1016/j.cell.2018.05.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity. 2018;49(1). doi: 10.1016/j.immuni.2018.06.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, et al. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J Exp Med. 2019;216(12). doi: 10.1084/jem.20180610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. 2014. doi: 10.1073/pnas.1402251111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bankova LG, Dwyer DF, Yoshimoto E, Ualiyeva S, McGinty JW, Raff H, et al. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25-producing airway brush cells leading to type 2 inflammation. Science immunology. 2018;3(28). doi: 10.1126/sciimmunol.aat9453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morroni M, Cangiotti AM, Cinti S. Brush cells in the human duodenojejunal junction: an ultrastructural study. J Anat. 2007;211(1). doi: 10.1111/j.1469-7580.2007.00738.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107(7). doi: 10.1073/pnas.0911934107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng X, Voss U, Ekblad E. Tuft cells: Distribution and connections with nerves and endocrine cells in mouse intestine. Exp Cell Res. 2018;369(1). doi: 10.1016/j.yexcr.2018.05.035 . [DOI] [PubMed] [Google Scholar]
- 59.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, et al. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells (Dayton, Ohio). 2014;32(3). doi: 10.1002/stem.1566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi J, Bergstrom K, Fu J, Shan X, McDaniel JM, McGee S, et al. Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis. Cell Death Differ. 2019;26(9). doi: 10.1038/s41418-018-0237-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leyva-Castillo JM, Galand C, Kam C, Burton O, Gurish M, Musser MA, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity. 2019;50(5). doi: 10.1016/j.immuni.2019.03.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kjærgaard S, Jensen TSR, Feddersen UR, Bindslev N, Grunddal KV, Poulsen SS, et al. Decreased number of colonic tuft cells in quiescent ulcerative colitis patients. Eur J Gastroenterol Hepatol. 2021;33(6). doi: 10.1097/MEG.0000000000001959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu D, Weygant N, May R, Chandrakesan P, Madhoun M, Ali N, et al. Ablation of Doublecortin-Like Kinase 1 in the Colonic Epithelium Exacerbates Dextran Sulfate Sodium-Induced Colitis. PLoS ONE. 2015;10(8). doi: 10.1371/journal.pone.0134212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banerjee A, Herring C, Chen B, Kim H, Simmons AJ, Southard-Smith AN, et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology. 2020;159(6). doi: 10.1053/j.gastro.2020.08.029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goto N, Fukuda A, Yamaga Y, Yoshikawa T, Maruno T, Maekawa H, et al. Lineage tracing and targeting of IL17RB + tuft cell-like human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2019;116(26). doi: 10.1073/pnas.1900251116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124(3). doi: 10.1172/JCI73434 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Qu D, Weygant N, Peng J, Houchen CW. Cancer Stem Cell Marker DCLK1 Correlates with Tumorigenic Immune Infiltrates in the Colon and Gastric Adenocarcinoma Microenvironments. Cancer. 2020;12(2). doi: 10.3390/cancers12020274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71(6). doi: 10.1158/0008-5472.CAN-10-2299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao T, Wang M, Xu L, Wen T, Liu J, An G. DCLK1 is up-regulated and associated with metastasis and prognosis in colorectal cancer. J Cancer Res Clin Oncol. 2016;142(10). doi: 10.1007/s00432-016-2218-0 . [DOI] [PubMed] [Google Scholar]
- 70.Li J, Wang Y, Ge J, Li W, Yin L, Zhao Z, et al. Doublecortin-Like Kinase 1 (DCLK1) Regulates B Cell-Specific Moloney Murine Leukemia Virus Insertion Site 1 (Bmi-1) and is Associated with Metastasis and Prognosis in Pancreatic Cancer. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, Pharmacol Ther. 2018;51(1). doi: 10.1159/000495228 . [DOI] [PubMed] [Google Scholar]
- 71.Yamada Y, Simon-Keller K, Belharazem-Vitacolonnna D, Bohnenberger H, Kriegsmann M, Kriegsmann K, et al. A Tuft Cell-Like Signature Is Highly Prevalent in Thymic Squamous Cell Carcinoma and Delineates New Molecular Subsets Among the Major Lung Cancer Histotypes. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2021;16(6). doi: 10.1016/j.jtho.2021.02.008 . [DOI] [PubMed] [Google Scholar]
- 72.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137(2). doi: 10.1053/j.gastro.2009.05.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weygant N, Qu D, May R, Tierney RM, Berry WL, Zhao L, et al. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget. 2015;6(4). doi: 10.18632/oncotarget.3059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Moltke J O ’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med. 2017;214(1). doi: 10.1084/jem.20161274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel NN, Triantafillou V, Maina IW, Workman AD, Tong CCL, Kuan EC, et al. Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis. International forum of allergy & rhinology. 2019;9(7). doi: 10.1002/alr.22334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science (New York, NY). 2014;345(6196). doi: 10.1126/science.1256942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desai P, Janova H, White JP, Reynoso GV, Hickman HD, Baldridge MT, et al. Enteric helminth coinfection enhances host susceptibility to neurotropic flaviviruses via a tuft cell-IL-4 receptor signaling axis. Cell. 2021;184(5). doi: 10.1016/j.cell.2021.01.051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL, et al. Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science (New York, NY). 2018;360(6385). doi: 10.1126/science.aar3799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science (New York, NY). 2015;347(6219). doi: 10.1126/science.1258025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomov VT, Palko O, Lau CW, Pattekar A, Sun Y, Tacheva R, et al. Differentiation and Protective Capacity of Virus-Specific CD8 + T Cells Suggest Murine Norovirus Persistence in an Immune-Privileged Enteric Niche. Immunity. 2017;47(4). doi: 10.1016/j.immuni.2017.09.017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137(6). doi: 10.1053/j.gastro.2009.06.072 . [DOI] [PubMed] [Google Scholar]
- 82.Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, le Coutre J, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509(5). doi: 10.1002/cne.21768 . [DOI] [PubMed] [Google Scholar]
- 83.Schütz B, Ruppert AL, Strobel O, Lazarus M, Urade Y, Büchler MW, et al. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep. 2019;9(1). doi: 10.1038/s41598-018-36956-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;142(2). doi: 10.1016/j.jaci.2018.03.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel NN, Kohanski MA, Maina IW, Triantafillou V, Workman AD, Tong CCL, et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. International forum of allergy & rhinology. 2018. doi: 10.1002/alr.22142 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sell EA, Ortiz-Carpena JF, Herbert DR, Cohen NA. Tuft cells in the pathogenesis of chronic rhinosinusitis with nasal polyps and asthma. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2021;126(2). doi: 10.1016/j.anai.2020.10.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rössler P, Kroner C, Freitag J, Noè J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77(3). doi: 10.1016/s0171-9335(98)80114-3 . [DOI] [PubMed] [Google Scholar]
- 88.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32(1). doi: 10.1093/chemse/bjl034 . [DOI] [PubMed] [Google Scholar]
- 89.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91(21–22). doi: 10.1016/j.lfs.2012.06.014 . [DOI] [PubMed] [Google Scholar]
- 90.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A. 2011;108(23). doi: 10.1073/pnas.1019418108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93(13). doi: 10.1073/pnas.93.13.6631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, et al. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8 (1):1–12. doi: 10.1186/1471-2202-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551 (7680):333–9. doi: 10.1038/nature24489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718). doi: 10.1038/s41586-018-0393-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Middelhoff M, Nienhüser H, Valenti G, Maurer HC, Hayakawa Y, Takahashi R, et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat Commun. 2020;11(1). doi: 10.1038/s41467-019-13850-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsubouchi S, Leblond CP. Migration and turnover of entero-endocrine and caveolated cells in the epithelium of the descending colon, as shown by radioautography after continuous infusion of 3H-thymidine into mice. Am J Anat. 1979;156(4). doi: 10.1002/aja.1001560403 . [DOI] [PubMed] [Google Scholar]
- 97.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45(1). doi: 10.1038/ng.2481 . [DOI] [PubMed] [Google Scholar]
- 98.Chandrakesan P, May R, Qu D, Weygant N, V VET, Li JD, et al. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget. 2015;6(31). doi: 10.18632/oncotarget.5129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269(5628). doi: 10.1038/269518a0 . [DOI] [PubMed] [Google Scholar]
- 100.Umar S. Intestinal stem cells. Curr Gastroenterol Rep. 2010;12(5). doi: 10.1007/s11894-010-0130-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells (Dayton, Ohio). 2008;26(3). doi: 10.1634/stemcells.2007-0621 . [DOI] [PubMed] [Google Scholar]
- 102.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells (Dayton, Ohio). 2009;27(10). doi: 10.1002/stem.193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, et al. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217(2). doi: 10.1084/jem.20191130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bautista JL, Cramer NT, Miller CN, Chavez J, Berrios DI, Byrnes LE, et al. Single-cell transcriptional profiling of human thymic stroma uncovers novel cellular heterogeneity in the thymic medulla. Nat Commun. 2021;12 (1):1–15. doi: 10.1038/s41467-020-20314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chu C, Parkhurst CN, Zhang W, Zhou L, Yano H, Arifuzzaman M, et al. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Science immunology. 2021;6(57). doi: 10.1126/sciimmunol.abe3218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oyesola OO, Shanahan MT, Kanke M, Mooney BM, Webb LM, Smita S, et al. PGD2 and CRTH2 counteract Type 2 cytokine-elicited intestinal epithelial responses during helminth infection. J Exp Med. 2021;218(9). doi: 10.1084/jem.20202178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotas ME, Mroz NM, Koga S, Liang HE, Schroeder AW, Ricardo-Gonzalez RR, et al. CISH constrains the tuft-ILC2 circuit to set epithelial and immune tone. Mucosal Immunol. 2021;14(6). doi: 10.1038/s41385-021-00430-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xi R, Montague J, Lin X, Chanyi L, Lei W, Tanaka K, et al. Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity. 2021. doi: 10.1073/pnas.2026307118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drurey C, Lindholm HT, Coakley G, Poveda MC, Löser S, Doolan R, et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J Exp Med. 2022;219(1). doi: 10.1084/jem.20211140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyata N, Morris LL, Chen Q, Thorne C, Singla A, Zhu W, et al. Microbial Sensing by Intestinal Myeloid Cells Controls Carcinogenesis and Epithelial Differentiation. Cell Rep. 2018;24(9). doi: 10.1016/j.celrep.2018.07.066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sbarbati A, Tizzano M, Merigo F, Benati D, Nicolato E, Boschi F, et al. Acyl homoserine lactones induce early response in the airway. Anatomical record (Hoboken, NJ: 2007). 2009;292(3). doi: 10.1002/ar.20866 . [DOI] [PubMed] [Google Scholar]
- 112.Hollenhorst MI, Jurastow I, Nandigama R, Appenzeller S, Li L, Vogel J, et al. Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34(1). doi: 10.1096/fj.201901314RR . [DOI] [PubMed] [Google Scholar]
- 113.Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, et al. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal. 2017;10(495). doi: 10.1126/scisignal.aam7703 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee RJ, Kofonow JM, Rosen RL, Siebert AP, Chen B, Doghramji L, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3). doi: 10.1172/JCI72094 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee S, Liu H, Wilen CB, Sychev ZE, Desai C, Hykes BL, et al. A Secreted Viral Nonstructural Protein Determines Intestinal Norovirus Pathogenesis. Cell Host Microbe. 2019;25(6). doi: 10.1016/j.chom.2019.04.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516(7529). doi: 10.1038/nature13960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graziano VR, Walker FC, Kennedy EA, Wei J, Ettayebi K, Strine MS, et al. CD300lf is the primary physiologic receptor of murine norovirus but not human norovirus. PLoS Pathog. 2020;16(4). doi: 10.1371/journal.ppat.1008242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Green KY, Kaufman SS, Nagata BM, Chaimongkol N, Kim DY, Levenson EA, et al. Human norovirus targets enteroendocrine epithelial cells in the small intestine. Nat Commun. 2020;11 (1):1–10. doi: 10.1038/s41467-019-13993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karandikar UC, Crawford SE, Ajami NJ, Murakami K, Kou B, Ettayebi K, et al. Detection of human norovirus in intestinal biopsies from immunocompromised transplant patients. J Gen Virol. 2016;97(9). doi: 10.1099/jgv.0.000545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. Replication of human noroviruses in stem cell–derived human enteroids. 2016. doi: aaf5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bomidi C, Robertson M, Coarfa C, Estes MK, Blutt SE. Single-cell sequencing of rotavirus-infected intestinal epithelium reveals cell-type specific epithelial repair and tuft cell infection. Proc Natl Acad Sci U S A. 2021;118(45). doi: 10.1073/pnas.2112814118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ballard SB, Saito M, Mirelman AJ, Bern C, Gilman RH. Tropical and travel-associated norovirus: current concepts. Curr Opin Infect Dis. 2015;28(5). doi: 10.1097/QCO.0000000000000197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14(11). doi: 10.1038/ni.2736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Desai P, Diamond MS, Thackray LB. Helminth-virus interactions: determinants of coinfection outcomes. Gut Microbes. 2021;13(1). doi: 10.1080/19490976.2021.1961202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595(7865). doi: 10.1038/s41586-021-03569-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]