Abstract
Rifampin is a first-line drug useful in the treatment of tuberculosis. By using biocompatible polymeric excipients of lactide and glycolide copolymers, two microsphere formulations were developed for targeted and sustained delivery of rifampin, with minimal dosing. A small-microsphere formulation, with demonstrated ability to inhibit intracellularly replicating Mycobacterium tuberculosis H37Rv, was tested along with a large-microsphere formulation in an infected mouse model. Results revealed that by using a single treatment of the large-microsphere formulation, it was possible to achieve a significant reduction in M. tuberculosis H37Rv CFUs in the lungs of mice by 26 days postinfection. A combination of small (given as two injections on day 0 and day 7) and large (given as one injection at day 0) rifampin-loaded microsphere formulations resulted in significant reductions in CFUs in the lungs by 26 days, achieving a 1.23 log10 reduction in CFUs. By comparison, oral treatment with 5, 10, or 20 mg of rifampin/kg of body weight, administered every day, resulted in a reduction of 0.42, 1.7, or 1.8 log10 units, respectively. Thus the microsphere formulations, administered in one or two doses, were able to achieve results in mice similar to those obtained with a daily drug regimen within the range of the highest clinically tolerated dosage in humans. These results demonstrate that microsphere formulations of antimycobacterial drugs such as rifampin can be used for therapy of tuberculosis with minimal dosing.
Tuberculosis has historically been a significant life-threatening human disease and is still a worldwide health threat (6), with an increasing incidence of multiple-drug-resistant (MDR) clinical strains of Mycobacterium tuberculosis (3, 4, 11). First-line drugs for therapy of tuberculosis are generally effective when used properly. However, it has been suggested that one of the major reasons for the increased numbers of MDR strains of M. tuberculosis is inefficient therapy, sometimes due to lack of compliance (17). In addition, M. tuberculosis is a facultatively intracellular parasite, capable of growing within host macrophages (31), where the access of antimicrobics is limited. Development of effective therapy for treatment of tuberculosis is important for reducing the incidence of tuberculosis as well as for treating patients who are coinfected with human immunodeficiency virus. Tuberculocidal therapies that reduce dosing intervals should facilitate the elimination of viable bacilli by improving patient compliance. Microencapsulation technology can be used to accomplish sustained release of antibiotics, when they are formulated in larger sizes of >50 μm, or to target drug delivery to specific cells (i.e., macrophages), when antibiotics are formulated in smaller sizes of <10 μm (1). Effective, continuous therapies, especially when used in combination, may help to improve compliance and reduce the emergence of drug-resistant clinical isolates.
Liposomes can also be used to deliver biological agents either entrapped within the internal aqueous compartments, reconstituted in the lipid bilayer, or attached to the outer surface. Liposomes are artificial lipid vesicles composed of concentric lipid bilayers that alternate with aqueous compartments. They have permeability properties similar to those of biological membranes. Liposome administration has been shown to provide delivery of antibiotics in mice infected with Mycobacterium avium (2, 8, 10, 12, 15, 24, 25, 27) or M. tuberculosis (9, 26, 36) with some success.
Microspheres, on the other hand, are discrete particles. As with liposomes, biological agents can either be encapsulated within the microsphere or attached to the surface. The use of polymers such as lactide-co-glycolide polymers allows significant flexibility with respect to the time at which encapsulated material can be released, an option not available with liposomes. In addition, there are stability issues of concern when liposomes are administered. Poly(lactide-co-glycolide) microspheres are degraded by hydrolysis only and are not susceptible to enzymatic degradation. The advantage of microspheres would be to extend the time for drug release from days to months with the small microspheres and for a year or more with large microspheres. These time intervals are much longer than those for most liposome preparations. In addition, the amount of drug that can be administered in large or small preparations of microspheres will exceed the amounts contained in liposome preparations on a weight basis comparison (26, 36).
Microsphere formulations, using polymeric excipients of lactide and glycolide polymers, are known to be biocompatible, as are their metabolic by-products, lactic acid and glycolic acid (33–35). Their chemical composition is based on the formulation for synthetic resorbable sutures (16), which degrade by nonenzymatic reaction (29). Microsphere technology is an established method for sustained delivery of antigens, steroids, peptides, proteins, and antibiotics (7, 13, 14, 18, 19, 28, 30). Larger microspheres release contents by diffusion and by degradation of polymeric excipients (29). Recently, results in our laboratory have demonstrated the effectiveness of smaller microspheres for the delivery of rifampin to host macrophages to significantly reduce levels of intracellularly replicating M. tuberculosis (1). The small microspheres were more efficient at delivering effective doses of rifampin intracellularly than equivalent doses of free drug (1). A combination of small- and large-microsphere formulations would be ideal for use in tuberculosis treatment regimens because a drug could be targeted to host macrophages with the small microspheres and delivered systemically by means of the large microspheres.
Microencapsulation of rifampin, a well-recognized tuberculocidal drug, was performed so as to yield both large and small microspheres. Large- and small-microsphere formulations of rifampin were administered only once and twice, respectively, following initial infection with M. tuberculosis H37Rv. In other experimental groups, oral doses of rifampin were administered daily by gavage, and the reduction in CFUs obtained from lung samples at 26 days was used for comparison. Plasma rifampin levels were quantitated by means of a previously described bioassay (1).
MATERIALS AND METHODS
Preparation of microspheres for extended release of rifampin.
The process used to prepare large microspheres (i.e., 10 to 150 μm in diameter), designed to release rifampin over time, is very similar to the process used to make the smaller microspheres (i.e., 1 to 10 μm) (1). An excipient solution was prepared by dissolving 4.0 g of poly(dl-lactide-co-glycolide) (DL-PLG) in 16.0 g of an organic solvent, in this case, methylene chloride or ethyl acetate. Approximately 1 g of rifampin was introduced into the excipient solution, and again a homogeneous solution was obtained after thorough mixing. The rifampin–DL-PLG solution was introduced into an aqueous process medium consisting of 5.0% (wt/wt) polyvinyl alcohol. An emulsion was formed by using a standard laboratory mixer and again was transferred into 5.0 liters of water. The resulting microspheres were collected over standard American Society for Testing and Materials (ASTM) mesh sieves (Fisher Scientific). The relatively poor solubility of rifampin was helpful in encapsulating the drug but proved a hindrance to achieving its timely release. Thus, we ultimately had to add some low-molecular-weight polymer to the polymer of choice in order to enhance the hydrolysis of the polymer and achieve quicker release of the drug.
Rifampin was used in microencapsulation procedures which consisted of preparing a 25% polymer solution of thermoplastic polyesters of polylactide and copolymers of lactide and glycolide [or poly(dl-lactide) and poly(dl-lactide-co-glycolide)] in ethyl acetate or methylene chloride. Rifampin was introduced into the polymer solution and mixed. The solution was then introduced into an aqueous continuous phase of polyvinyl alcohol. An emulsion was formed by using a Silverson emulsifier and was then transferred into water. The ethyl acetate or methylene chloride was extracted from the emulsion, and the microspheres were collected by centrifugation and then lyophilization of the concentrate. Microspheres were sterilized by gamma irradiation (25 kGy) prior to use in mice. Each lot of microspheres was analyzed for drug content by spectrophotometric and high-pressure liquid chromatographic (HPLC) assays, for size by standard procedures using a Malvern particle size analyzer, and for release characteristics prior to use in mice. Matching placebo formulations were made for each drug-loaded preparation. Rifampin (Sigma Chemical Company, St. Louis, Mo.) was also prepared as a suspension for daily oral gavage in a solution of 10% dimethyl sulfoxide (DMSO; tissue culture grade; Sigma) in sterile 0.9% sodium chloride (for injection; Baxter) solution. A dosing rate of 0.1 ml per 10 g of body weight was administered daily to each mouse, with solutions of rifampin yielding doses of 36, 20, 10, 5, 2.5, 1.25, and 0.42 mg/kg of body weight.
Drug content of rifampin microspheres.
The rifampin content of each lot of microspheres was determined by first extracting the rifampin from a known quantity of microspheres and quantifying the amount of drug spectrophotometrically (1). The concentration of rifampin contained in each sample was determined by measuring the absorbance on a spectrophotometer at a λ of 474 nm. A series of rifampin solutions of known concentrations in ethyl acetate were prepared, and absorbances were measured in order to generate a standard curve. The rifampin concentrations in the microsphere and control samples were then obtained by linear regression, and the total amount of rifampin was calculated as (rifampin concentration in micrograms per milliliter) × (milligrams/1,000 μg) × (sample volume in milliliters)/(microsphere sample weight in milligrams).
In vitro release analysis of rifampin microspheres.
In vitro release of rifampin was determined by a procedure previously described (1). Briefly, multiple samples of each microsphere lot were weighed into 16-by 100-mm glass test tubes equipped with serum separators (Fisher Scientific). To each tube, 3.0 ml of receiving fluid consisting of 0.05 M sodium phosphate solution was added. The test tubes were placed in an incubator (37°C), and the receiving fluid was removed and replaced with new fluid at 2, 6, and 24 h and every 24 to 48 h thereafter. The concentration of rifampin was determined by a standard HPLC assay described in the U.S. Pharmacopeia (32). An absorption maximum of 254 nm was used for rifampin.
Mycobacterial strains.
M. tuberculosis H37Rv (ATCC 27294; SRI no. 1345) was maintained on Middlebrook 7H10 agar slants, containing 0.5% glycerol and 10% oleic acid, albumin, dextrose, and catalase (OADC) (Difco). The MIC of rifampin for this strain is 0.06 to 0.25 μg/ml (37).
Bioassay.
A previously described bioassay, using Staphylococcus aureus (ATCC 29213), was used to determine rifampin concentrations in mouse plasma (1). The bioassay was modified slightly by preparing the standard curve for rifampin in filter-sterilized control mouse plasma instead of culture medium (1). Sterile filter paper disks (13 mm in diameter; Schleicher and Schuell) were aseptically placed into individually coded wells of 12-well tissue culture plates, and 80 μl of either rifampin standard solutions or plasma samples was absorbed onto appropriate disks. A suspension of S. aureus (ATCC 29213) was prepared and adjusted to match a 0.5 McFarland turbidity standard, then swabbed onto a 150-mm Mueller-Hinton agar plate (BBL) for lawn growth (1). The previously loaded disks were aseptically applied to the inoculated plates, which were incubated in a 37°C incubator (no CO2) for 18 to 20 h (1). At the termination of incubation, zones for drug standards were measured and a standard curve was plotted. Zone diameters for test samples were measured, and values were entered into the computer for regression analysis to determine the amount of drug in 80 μl of mouse plasma (1). The value was converted to micrograms of drug per milliliter of plasma.
Mice.
Female CD-1 mice (14 to 16 g) were obtained from Charles River Laboratories and maintained on a diet of Teklad sterilizable laboratory feed (Harlan) and water in a biosafety level III facility throughout the studies. All animal research programs and facilities at Southern Research Institute are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). Animals were euthanized by CO2 asphyxiation, consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Approval for these studies was given by the Institutional Animal Care and Use Committee at Southern Research Institute.
In vivo release characteristics of microspheres.
Release characteristics of rifampin-loaded microspheres were evaluated in mice prior to use in the infected mouse model. Three groups, consisting of three mice each, were evaluated. One group received an intraperitoneal injection of small rifampin-loaded microspheres that have previously been described by us (1). The small formulations are further described in Results, under “Characteristics of microsphere formulations.” The intraperitoneal injection was administered as two 50-mg/mouse doses, one given at day 0 and the other at day 7. A second group of mice received a single 100-mg subcutaneous injection of large rifampin-loaded microspheres (27% [wt/wt]), described in this report as formulation 6 (Table 1). The third group of mice received an oral gavage of rifampin daily (10 mg/kg), from day 0 to day 25. At 7, 14, and 21 days, mice were anesthetized with ketamine-xylazine for exsanguination by cardiac puncture, and plasma was assayed for rifampin by using the bioassay described previously (1).
TABLE 1.
Representative formulations of rifampin-loaded microspheres
| Formulation no. (lot no.) | Excipient | Excipient solvent | Rifampin content (% [wt/wt])
|
Microsphere size (μm)a | In vitro release (%) after 2 days | |
|---|---|---|---|---|---|---|
| Theoretical | Observed | |||||
| 1 | 60:40 DL-PLG | Ethyl acetate | 30 | 1.38 | 101.1 | NDb |
| 2 | 60:40 DL-PLG | Ethyl acetate | 30 | 18 | 106.1 | 2 |
| 3 | 50:50 DL-PLG | Methylene chloride | 30 | 19 | 110.7 | 5 |
| 4 | 65:35 DL-PLG | Ethyl acetate | 30 | 17.6 | 68.4 | 15 |
| 5 | 60:40 DL-PLGc | Ethyl acetate | 30 | 17 | 106.1 | 25 |
| 6 | 60:40/50:50 DL-PLGd | Ethyl acetate | 30 | 27 | 101.1 | 2.5 |
| 7 | 60:40/50:50 DL-PLGd | Ethyl acetate | 30 | 25.7 | 130.2 | 1.8 |
Data reported as 90 volume percentiles.
ND, not done.
Microspheres prepared by incorporating dextrose.
Excipient is a blend of 60:40 DL-PLG and low-molecular-weight 50:50 DL-PLG.
Infection and treatment of mice.
The mouse model described here is a nonlethal, short-term model that was developed from previous studies (5, 21–23) in order to investigate antituberculosis drugs. The inoculum size and time frame were, therefore, selected to ensure that death would not occur due to large inoculum size and also that drugs could be screened with a short turnaround time. Mice were inoculated via the lateral tail vein on day 0 with approximately 105 viable bacilli of M. tuberculosis H37Rv in a volume of 0.1 ml of 0.9% sterile sodium chloride solution. Drug treatments were initiated approximately 2 to 4 h postinoculation. Each treatment group contained 10 mice. Formulations of small microspheres, placebo and rifampin loaded, were injected intraperitoneally on days 0 and 7 in a 50-mg dose suspended in a volume of 0.25 ml of sterile saline by using a sterile tuberculin syringe with a 23-gauge needle. Formulations of large microspheres, placebo and rifampin loaded, were administered subcutaneously in a 100-mg dose suspended in a 0.5-ml volume of a vehicle consisting of 0.5% (wt/wt) carboxymethyl cellulose with 0.1% (wt/wt) Tween 80 and 5.0% (wt/wt) mannitol, on day 0 only, over the dorsal thoracolumbar area by using an 18-gauge needle attached to a sterile tuberculin syringe. Mice were maintained under general anesthesia with ketamine-xylazine (10 and 1.5 mg/100 g of body weight, respectively, given intramuscularly) during the subcutaneous injections. Oral gavage of rifampin was performed daily from day 0 to day 25. Mice were restrained and dosed by gavage with a stainless steel gavage needle attached to a tuberculin syringe. All mice were weighed daily and observed for clinical signs of toxicity. On day 26, mice were anesthetized with ketamine-xylazine for aseptic blood collection using a sterile tuberculin syringe with a heparinized needle to withdraw volumes of 0.5 to 1.0 ml of blood from the heart. Mice were immediately euthanized with CO2 following blood collection. Blood was centrifuged briefly, and plasma was collected and frozen at −70°C until it was used in the bioassay. Organs were frozen individually in sterile Tekmar bags, thawed, hand homogenized with a Bayer roller, diluted with sterile saline containing 0.05% Tween 80, and plated onto OADC-supplemented 7H11 Middlebrook Mycobacterium solid agar. Colonies were enumerated after 14 to 21 days of incubation in a 37°C, 5% CO2 incubator.
RESULTS
Characteristics of microsphere formulations.
The small microsphere formulations have been described previously and were referred to as formulations 5 and 6 (1). In order to avoid confusion in this paper, these formulations will be referred to as S5 and S6. Briefly, S5 and S6 were prepared with a mixture of 60:40/50:50 DL-PLG, using methylene chloride as a solvent. Their rifampin contents were 1.4 and 1.8% (wt/wt), respectively, and their sizes, as 90 volume percentiles, were 7.5 and 8.8 μm (1). This type of small formulation has demonstrated effectiveness for delivery and release of rifampin within macrophages and a proven ability to significantly reduce intracellular replication of M. tuberculosis H37Rv in both murine and human monocytic cell lines (1).
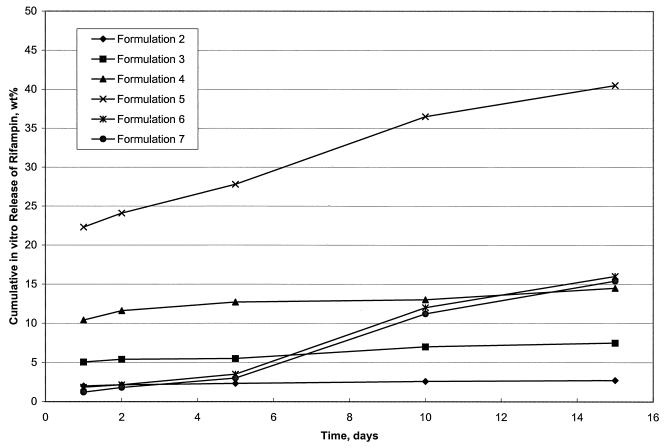
With regard to the large microspheres, numerous formulations were prepared and tested prior to use in treatment of infected mice. Only representative formulations are given here (Table 1). The first level of testing for microsphere preparations involves an examination of various parameters, including observed drug content (percent by weight), morphology, size, and in vitro release characteristics. These characteristics are improved by adjusting ratios and components of polymers and modifying the solvents used during extraction. These parameters are given in Table 1 and Fig. 1. Formulation 1 was not acceptable because the core loading (1.38%) was insufficient (Table 1); it was not tested further. Formulations 2 and 3 had good core loadings (18 and 19% [wt/wt], respectively) and sizes (106.1 and 110.7 μm, respectively) (Table 1) but did not show sustained in vitro release during 15 days (Fig. 1). Formulations 4 and 5 had good core loadings (17.6 and 25% [wt/wt], respectively) and sizes (68.4 and 106.1 μm, respectively) (Table 1), but their release of drug was premature and too fast (Fig. 1). Formulations 6 and 7 proved to be the best because they not only had good core loadings (27 and 25.7% [wt/wt], respectively) and sizes (101.1 and 130.2 μm, respectively) (Table 1) but also showed limited initial release followed by an optimum sustained release throughout the 15-day in vitro test period (Fig. 1).
FIG. 1.
In vitro release characteristics of large rifampin-loaded microsphere formulations over a 15-day period in receiving fluid. Microsphere formulations 2 through 7 are represented.
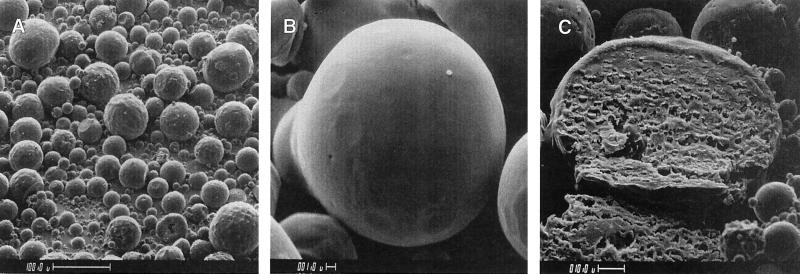
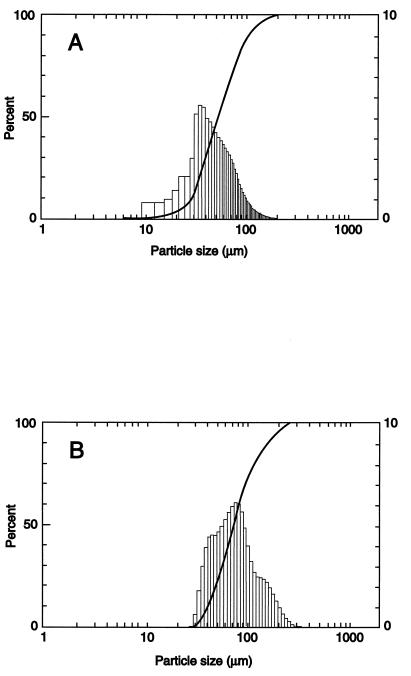
Following the first level of testing, each microsphere formulation is evaluated for surface morphology and size distribution in order to ensure optimum delivery and release parameters. Surface morphology reflects the quality of the polymer film of the microspheres. The more porous the surface, the more likely the microspheres will release the encapsulated drug more rapidly. Size distribution is important from two perspectives. Firstly, we know that microspheres need to be less than 10 μm in diameter to be phagocytized by macrophages. Additionally, we know that the smaller the microspheres are, the more efficiently they are phagocytized. These points were further demonstrated in our previous study (1). Secondly, the size of the microspheres will affect how much drug can be encapsulated as well as how rapidly the drug is released. The typical morphology of the large microspheres is shown in Fig. 2, which also depicts a cross section of a representative large microsphere (Fig. 2C). The morphology of the small microspheres has been presented previously (1). Size distributions for large microspheres, as determined by using a Malvern particle size analyzer, are given for formulations 6 and 7 in Fig. 3. For small microspheres, this information has been given previously (1).
FIG. 2.
Scanning electron micrograph of large, rifampin-loaded microsphere formulation 7. Scope magnifications, ×200 (A), ×3,000 (B), and ×900 (C). Panel C depicts a cross section of a single microsphere. Bars, 100, 1.0, and 10.0 μm in panels A through C, respectively.
FIG. 3.
Size distributions of large, rifampin-loaded microsphere formulations 6 (A) and 7 (B) as determined with a Malvern particle size analyzer. Data are plotted as volume percentile (percent) versus particle diameter (in micrometers).
Effects of microsphere preparations on mice.
Generally, all microsphere preparations were well tolerated by the mice. Some animals that received the small placebo microspheres exhibited a slight ruffled coated appearance on days 1 and 2, but by day 6, they appeared clinically normal. Mice treated with placebo- or rifampin-loaded large microspheres tolerated the formulations very well. All mice exhibited normal behavior, continued to consume feed and water, and did not exhibit abnormal behavior directed at the injection sites (i.e., chewing or biting). Following euthanasia, the postmortem examinations revealed subcutaneous deposits of microspheres in mice that had received the large preparations. Rifampin-loaded preparations were still distinctly orange, while placebo preparations were white. Similarly, intraperitoneal microspheres were observed in mice that had received the small formulations. Again, rifampin-loaded microspheres were still distinctly orange, while placebo preparations were white. Intraperitoneal microspheres were observed to be adherent to various abdominal organs, including the spleen, liver, and intestines. The presence of microspheres did not appear to cause adhesions or other adverse conditions.
Release characteristics in mice.
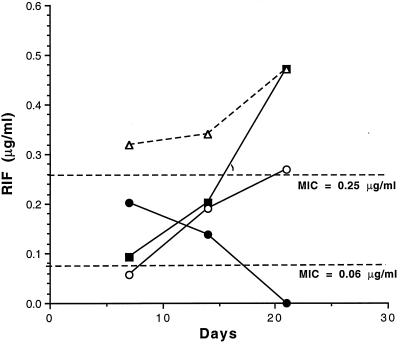
Before experiments involving infected mice were conducted, it was important to verify that rifampin-loaded microspheres could release sufficient quantities of drug to rationalize their use in an infected animal model. This was accomplished by injecting one group of mice with small microspheres on days 0 and 7 and another group with large-microsphere formulations on day 0, and subsequently monitoring plasma levels throughout an experimental period, duplicating our infected animal model of 26 days. Figure 4 depicts the results of that experiment.
FIG. 4.
Release characteristics of small and large rifampin-loaded microspheres in mice. One group of mice received an oral gavage of rifampin daily (10 mg/kg) (○) from day 0 to day 25. Another group of mice was injected intraperitoneally with 50 mg of the small-microsphere formulation S6 (1.4% [wt/wt] rifampin) (●), on day 0 and on day 7. A third group of mice was injected subcutaneously with 100 mg of the large-microsphere formulation 6 (27% [wt/wt] rifampin) (■), on day 0. Additive means for plasma rifampin levels with small and large microspheres (▵) are also given. At 7, 14, and 21 days, plasma was assayed for rifampin by using the bioassay described in Materials and Methods.
At 7 days after injection of rifampin-loaded microspheres, rifampin was detectable in mice receiving small or large formulations; the highest levels were observed in those receiving the small formulation (Fig. 4). By 7 days, levels of rifampin released from small or large formulations were approximately similar (Fig. 4). After 21 days postinjection, rifampin was not detectable in plasma from mice receiving the small formulation (Fig. 4). Rifampin levels in mice receiving the large formulation were substantially greater (Fig. 4). During the 21-day postinjection period, the additive means for both formulations exceeded the MIC for M. tuberculosis H37Rv (MIC of 0.06 to 0.25 μg/ml) (Fig. 4). At 21 days, the large formulation alone was still releasing sufficient quantities of rifampin to exceed the higher MIC (Fig. 4). By comparison, mice receiving an oral dose of 10 mg of rifampin/kg/day had approximately five- and twofold less rifampin in plasma than the combined total for the small and large microspheres at 7, 14, and 21 days postinjection, respectively. These results demonstrated that the use of these formulations would predictably result in sufficient levels of rifampin to affect M. tuberculosis infections in the mice.
Treatment of M. tuberculosis-infected mice with oral and microsphere formulations of rifampin.
Mice were infected with M. tuberculosis H37Rv and treated either with oral doses of rifampin or with microsphere preparations of rifampin. Oral doses of rifampin were given daily for as many as 25 days postinfection at quantities that varied from 0.42 to 36 mg of rifampin/kg/day. For mice receiving rifampin in the form of large microspheres, only one treatment was administered, at the time of infection. For mice receiving rifampin by small-microsphere formulations, two treatments were given. One was given at the time of infection and one was administered 7 days postinfection. No other drug therapy was given to mice receiving microsphere formulations.
With oral administration of rifampin at concentrations of 0.42, 1.25, and 2.5 mg/kg, no significant reductions in viable M. tuberculosis H37Rv levels were observed at 26 days postinfection (Table 2). For mice receiving oral concentrations of 5.0, 10, 20, and 36 mg of rifampin/kg/day, significant reductions in viable M. tuberculosis H37Rv levels were observed at 26 days postinfection (Table 2). It has been reported that a concentration of 10 mg/kg is equivalent to the clinically tolerated dosage for rifamycin derivatives in humans (20).
TABLE 2.
Levels of viable M. tuberculosis H37Rv in lungs of mice receiving oral rifampina
| Value | Log10 CFU in lungs with the following treatment:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | 0.42 mg/kg (n = 9) | 1.25 mg/kg (n = 9) | 2.5 mg/kg (n = 10) | 5.0 mg/kg (n = 10) | 10 mg/kg (n = 9) | 20 mg/kg (n = 10) | 36 mg/kg (n = 10) | |
| Mean | 5.69 | 5.82 | 5.68 | 5.51 | 5.27 | 3.99 | 3.86 | 2.00 |
| SEM | 0.05 | 0.06 | 0.06 | 0.08 | 0.05 | 0.20 | 0.23 | 0.05 |
| P | NA | >0.05 | >0.05 | >0.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Mice were infected with M. tuberculosis H37Rv and treated with rifampin. Rifampin was given orally on a daily basis for 25 days in a solution of 10% DMSO in sterile 0.9% sodium chloride. Lungs were processed, and CFUs were determined and reported as log10 units. Results are reported as the means and standard errors of the means, and probability (P) was determined by the Mann-Whitney U test. Control mice received an oral gavage of the DMSO solution only. n, number of mice in each group. NA, not applicable.
Treatment of M. tuberculosis H37Rv-infected mice with the small-microsphere formulation alone did not result in a significant reduction in levels of viable mycobacteria in the lungs by 26 days postinfection (Table 3). However, treatment with either the large formulation or a combination of small and large formulations did result in a significant reduction in viable M. tuberculosis H37Rv levels in the lungs by 26 days postinfection (Table 3). In addition, it is important to realize that of 10 mice treated with the large formulation, 2 demonstrated no detectable CFUs by day 26 at the lowest dilution plated (10−3) (Table 3). Likewise, of 10 mice treated with the small and large combination, 4 demonstrated no detectable CFUs by day 26 at a 10−3 dilution (Table 3). Three other groups, consisting of 10 infected mice each, received placebo preparations of small, large, or small plus large microspheres. No significant reduction in CFUs was observed in these groups after 26 days postinfection (data not shown).
TABLE 3.
Levels of viable mycobacteria in lungs of mice treated with microsphere-encapsulated rifampina
| Value | Log10 CFU in lungs with the following treatment:
|
|||
|---|---|---|---|---|
| Control (n = 10) | Small microspheres (n = 9) | Large microspheres (n = 9)b | Small + large microspheres (n = 10)c | |
| Mean | 5.61 | 5.50 | 4.61 | 4.38 |
| SEM | 0.14 | 0.07 | 0.32 | 0.41 |
| P | NA | 0.50 | 0.008 | 0.03 |
Mice were infected with M. tuberculosis H37Rv and treated with small microspheres (formulation S6; 1.8% [wt/wt] rifampin; 50 mg; given on day 0 and day 7), large microspheres (formulation 7; 25.7% [wt/wt] rifampin; 100 mg; given on day 0), and both small and large microspheres (formulations S6, and 7). Small-microsphere formulations were suspended in sterile saline prior to intraperitoneal injections. Large-microsphere formulations were administered subcutaneously in a vehicle consisting of 0.5% (wt/wt) carboxymethyl cellulose with 0.1% (wt/wt) Tween 80 and 5.0% (wt/wt) mannitol. Control mice received an equivalent subcutaneous injection of the vehicle solution. Lungs were processed, and CFUs were determined 26 days postinfection and reported as log10 units. Results are reported as means and standard errors of the means, and probability (P) was determined by the Mann-Whitney U test. n, number of mice in each group.
No CFUs detected at plating dilution of 10−3 for two mice.
No CFUs detected at plating dilution of 10−3 for four mice.
When mice were sacrificed, it was observed that aggregates of small microspheres remained within the peritoneal cavity, suggesting that optimum circulation of the microspheres did not occur. This is most likely the reason why significant reduction of levels of mycobacteria in those animals did not take place. This is a problem with the intraperitoneal route of injection; it can most likely be resolved by using alternative injection routes (e.g., the intravenous route). Because intravenous injection of microspheres is not easily performed in mice, larger animal models are being considered for those studies. With regard to the large microspheres, subcutaneous deposits were also observed in mice receiving these formulations. Although degradation of the large microspheres was apparent, deposits still remained at the end of 26 days and still retained the orange color associated with rifampin. This observation, along with the plasma rifampin levels given in Table 4, suggests that the large microspheres were still releasing drug at the end of the experimental period.
TABLE 4.
Plasma rifampin levels in infected mice at 26 days postinfectiona
| Mouse no. | Concn of rifampin in plasma (μg/ml) with:
|
||
|---|---|---|---|
| Small microspheres | Large microspheres | Small + large microspheres | |
| 1 | 0.10 | 0.16 | 0.26 |
| 2 | 0.15 | 1.51 | 0.84 |
| 3 | 0.20 | 0.20 | 0.15 |
| 4 | 0.11 | 2.69 | 2.69 |
| 5 | <0.10 | 0.15 | 1.51 |
| 6 | <0.10 | 0.20 | 1.13 |
| 7 | <0.10 | <0.10 | 0.26 |
| 8 | <0.10 | <0.10 | 0.26 |
| 9 | <0.10 | <0.10 | 0.35 |
| Meanb | 0.14 | 0.82 | 0.83 |
| SEM | ±0.02 | ±0.43 | ±0.28 |
| Range | <0.10–0.20 | <0.10–2.69 | 0.15–2.69 |
Parameters are the same as those given in Table 3. The small and large formulations were S6 (1.8% [wt/wt] rifampin) and 7 (25.7% [wt/wt] rifampin), respectively.
Mean reported for samples with detectable levels of rifampin.
Posttherapy plasma rifampin levels in microsphere-treated mice.
Following the M. tuberculosis H37Rv infection experiment described above, plasma samples were processed from the mice immediately following the termination of the experiment. Plasma levels of rifampin were then quantitated by means of the bioassay procedure described in Materials and Methods. Of nine mice that were treated with the small-microsphere rifampin formulation only, four (44%) had detectable levels of rifampin in their plasma, ranging from 0.10 to 0.20 μg/ml (Table 4). Six of nine mice (67%) treated with the large-microsphere rifampin formulation only demonstrated detectable levels of rifampin in their plasma, ranging from 0.15 to 2.69 μg/ml (Table 4). In the group of mice that received a combination of small and large rifampin-loaded microspheres, nine of nine (100%) had detectable levels of rifampin in their plasma, ranging from 0.15 to 2.69 μg/ml (Table 4).
DISCUSSION
Previously, we reported on the use of microsphere technology as a means of delivery for rifampin to macrophages infected with M. tuberculosis H37Rv (1). By using a small-microsphere formulation (1 to 10 μm in diameter), we were able to show that delivery of rifampin by this method not only reduces toxicity but is able to achieve greater reduction of intracellular replication of M. tuberculosis than an equivalent concentration of rifampin given as free drug (1). As an extension of that study, we report here on the development of a large-microsphere formulation that can effectively deliver rifampin systemically for a prolonged period. This new large formulation was tested along with the previously described small formulation in an M. tuberculosis-infected mouse model. Results demonstrate that these formulations can be used to effectively reduce replication of M. tuberculosis in the lungs of an infected animal.
Reductions in lung CFUs following administration of microencapsulated rifampin was observed, and statistical significance was achieved in some evaluations. Improvements in both drug content and release characteristics of microspheres have been made, allowing for the use of small and large formulations in combination for effective management of infected mice. Although rifampin was released from large and small microspheres, and reductions in numbers of recovered viable bacilli occurred, the postmortem observations suggested residual quantities of rifampin within the microspheres after 26 days in vivo. This assumption was supported by the fact that the bioassay demonstrated substantial quantities of rifampin in the plasma at the termination of the experiment. In addition, the aggregates of small microspheres within the peritoneal cavity indicated that a large percentage of small microspheres was not taken into circulation. This would suggest that the small microspheres were unable to be efficiently distributed to macrophages in lungs or other organs and might explain why optimum reduction of mycobacterial levels was not observed in the animals receiving the small formulation only. This assumption was also supported by the results obtained with the bioassay, which demonstrated a lack of detectable rifampin in five of nine plasma samples from mice receiving the small formulation only.
Although intraperitoneal administration of soluble test compounds is used routinely in mice for efficacy experiments, intravenous administration of microspheres would have been preferable. Attempts to inject small microspheres via the lateral tail vein were made. However, the low diluent volumes, reduced total dose of microspheres, small vein size, small gauge needle size, and tendency for microspheres to aggregate during injection made this route technically difficult in mice. Studies with larger animal models are currently being conducted in order to alleviate these technical delivery problems.
Techniques have been developed for a bioassay for detecting levels of rifampin in plasma from animals treated with microencapsulated rifampin. These evaluations now allow for demonstration of the in vivo release characteristics and documentation of therapeutic levels over time. Use of the bioassay in the development of microsphere studies is critical in order to plan for experimentation in an infected animal model. This methodology proved to be helpful in allowing us to predict that a combination of the small and large microsphere formulations would effectively control an M. tuberculosis infection. That prediction proved to be correct. In addition, the bioassay was able to demonstrate that bioactive rifampin was still being released in infected animals at the end of the 26-day treatment period. In fact, the drug levels in mice receiving small- and large-microsphere formulations (approximately 3.5- to 14-fold greater than the MICs) were high enough to suggest that reduction of levels of viable mycobacteria would have continued if the experiment had been prolonged.
These results reveal that by using microsphere technology, it is possible to effectively treat an M. tuberculosis infection in a mouse model. It was demonstrated that a single injection of a large-microsphere formulation can significantly reduce CFUs in the lungs of infected mice up to 26 days postinfection. By using a combination of small and large formulations, it is possible to achieve significant reduction in CFUs in the lungs of infected mice at 26 days postinfection, with 40% of those mice showing no CFUs. This was accomplished by using only one or two treatments during the 1st week of infection. The large microspheres were injected once, and the small were injected only twice. In order to accomplish similar significant reductions in CFUs with an oral regimen, it was necessary to administer 10 mg/kg daily for 25 days, an amount that has been equated to the clinically tolerated dosage for rifamycin derivatives in humans (20). Thus, the microsphere formulations, when given only twice (i.e., at 0 and 7 days), were able to achieve results similar to those achieved with the drug given every day for 25 days. These developments are quite encouraging and indicate that microsphere technology can be used to deliver sufficient levels of rifampin in the blood to treat mycobacterial infections.
To put these results into perspective with regard to their potential usefulness in human therapy, it is important to evaluate the quantities used in the mouse model. If one assumes that mice weigh approximately 0.03 kg, then the mice that were injected with both small and large microspheres would have received an equivalent dose of about 947 mg/kg (28.4 mg of total rifampin given in the 1.4% [wt/wt] small plus 27% [wt/wt] large microsphere treatment). We know from other studies, as well as those presented here (Fig. 4), that the small microspheres tend to release their drug content almost completely by 30 days. However, we know from other studies that the large microspheres will continue to release rifampin for at least 50 days (data not shown). Although it is not possible to calculate the total amount of rifampin that had been released from the large microspheres at the end of 26 days, we can make a reasonable prediction from other work that about half of the total amount of drug was still encapsulated. This would mean that a dose equivalent to about 474 mg/kg had been released during the 26-day experiment. That amount would be between the amounts given to the groups that received 10 and 20 mg/kg/day (i.e., 10 to 20 mg/kg/day × 26 days = 260 to 540 mg/kg). This is not an unreasonable amount, especially considering that dosing was limited to two injection points (i.e., days 0 and 7) instead of the daily regimen necessary for oral administration.
Studies are currently being conducted in order to increase core loadings, improve release kinetics, and optimize delivery of small- and large-microsphere formulations. These refinements should result in microsphere formulations that can be used for improved therapy of tuberculosis by allowing for targeting and sustained release of antimycobacterial drugs, with minimal dosing. In order to identify an optimal microsphere formulation, any number of variables can be manipulated, including the type and molecular weight of polymer, the quantity of drug encapsulated, and the size and porosity of the microspheres. By varying these parameters, a formulation can be designed so that the desired performance criteria, such as the delivery of a predetermined amount of drug at an acceptable rate, over a predetermined period of time, are met. Obviously, the physical and chemical properties of the drug to be delivered will have a substantial effect on how the formulation parameters are manipulated in order to achieve the desired performance criteria. We have demonstrated in a wide range of controlled-release products that once an optimal formulation for a particular product has been established, the formulation can be easily reproduced. We have even demonstrated for a number of formulations that they can be reproducibly scaled up. These factors are important in considering the use of microsphere technology in humans.
ACKNOWLEDGMENTS
This work was supported by the NIH, NIAID grant RO-1 AI38185, to Southern Research Institute.
The technical assistance for the animal model work by Anne Brazier, Reginald Harris, Beth Taylor, Gloria Triggs, and Frank Vance is greatly appreciated. We also thank Tom Tice for helpful suggestions and Lloyd Carlson for the scanning electron micrographs of microsphere preparations.
REFERENCES
- 1.Barrow E L W, Winchester G A, Staas J K, Quenelle D C, Barrow W W. Use of microsphere technology for sustained and targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob Agents Chemother. 1998;42:2682–2689. doi: 10.1128/aac.42.10.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E M, Wu M, Young L S. Intracellular killing of Mycobacterium avium complex by rifapentine and liposome-encapsulated amikacin. J Infect Dis. 1987;156:510–513. doi: 10.1093/infdis/156.3.510. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Nosocomial transmission of multidrug-resistant tuberculosis to health-care workers and HIV-infected patients in an urban hospital. Florida Morbid Mortal Weekly Rep. 1990;39:718–722. [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons. Florida and New York Morbid Mortal Weekly Rep. 1991;40:649–652. [PubMed] [Google Scholar]
- 5.Chadwick M, Nicholson G, Gaya H. Brief report: combination chemotherapy with ciprofloxacin for infection with Mycobacterium tuberculosis in mouse models. Am J Med. 1989;87:35S–36S. doi: 10.1016/0002-9343(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 6.Collins F M. Mycobacterial pathogenesis: a historical perspective. Front Biosci. 1998;3:123–132. doi: 10.2741/a285. [DOI] [PubMed] [Google Scholar]
- 7.Cowsar D R, Tice T R, Gilley R M, English J P. Poly(lactide-co-glycolide) microcapsules for controlled release of steroids. Methods Enzymol. 1985;112:101–116. doi: 10.1016/s0076-6879(85)12010-0. [DOI] [PubMed] [Google Scholar]
- 8.Cynamon M H, Swenson C E, Palmer G S, Ginsberg R S. Liposome-encapsulated-amikacin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1989;33:1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deol P, Khuller G K, Joshi K. Therapeutic efficacies of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrob Agents Chemother. 1997;41:1211–1214. doi: 10.1128/aac.41.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duzgunes N, Perumal V K, Kesavalu L, Goldstein J A, Debs R J, Gangadharam P R J. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988;32:1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dooley S W, Castro K G, Jarvis W R, Holmberg S D. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers S, Bucke W, Leitzke S, Fortmann L, Smith D, Hansch H, Hahn H, Bancroff G, Muller R. Liposomal amikacin for treatment of M. avium infections in clinically relevant experimental settings. Zentbl Bakteriol. 1996;284:218–231. doi: 10.1016/s0934-8840(96)80097-1. [DOI] [PubMed] [Google Scholar]
- 13.Eldridge J H, Hammond C J, Meulbroek J A, Staas J K, Gilley R M, Tice T R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11:205–214. [Google Scholar]
- 14.Eldridge J H, Staas J K, Meulbroek J A, Tice T R, Gilley R M. Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangadharam P R, Ashtekar D R, Flasher D L, Duzgunes N. Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically stabilized liposomes. Antimicrob Agents Chemother. 1995;39:725–730. doi: 10.1128/AAC.39.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilding D K, Reed A M. Biodegradable polymers for use in surgery: polyglycolic/poly(lactic acid) homo- and copolymers. Polymer. 1979;20:1459–1464. [Google Scholar]
- 17.Goble M. Drug resistance. In: Friedman L N, editor. Tuberculosis. Current concepts and treatment. Boca Raton, Fla: CRC Press; 1994. pp. 259–284. [Google Scholar]
- 18.Hora M S, Rana R K, Nunberg J H, Tice T R, Gilley R M, Hudson M E. Release of human serum albumin from poly(lactide-co-glycolide) microspheres. Pharm Res. 1990;7:1190–1194. doi: 10.1023/a:1015948829632. [DOI] [PubMed] [Google Scholar]
- 19.Jacob E, Setterstrom J A, Bach D E, Heath J R, McNiesh L M, Cierny I G. Evaluation of biodegradable ampicillin anhydrate microcapsules for local treatment of experimental staphylococcal osteomyelitis. Clin Orthop Relat Res. 1991;267:237–244. [PubMed] [Google Scholar]
- 20.Ji B, Truffot-Pernot C, Lacroix C, Raviglione M C, O’Brien R J, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 21.Khor M, Lowrie D B, Coates A R M, Mitchison D A. Recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin in experimental murine tuberculosis. Br J Exp Pathol. 1986;67:587–596. [PMC free article] [PubMed] [Google Scholar]
- 22.Kradolfer F, Schnell R. Incidence of resistant pulmonary tuberculosis in relation to initial bacterial load. Chemotherapy. 1970;15:242–249. doi: 10.1159/000220688. [DOI] [PubMed] [Google Scholar]
- 23.Kradolfer F, Schnell R. The combination of rifampicin and other antituberculous agents in chronic murine tuberculosis. Chemotherapy. 1971;16:173–182. doi: 10.1159/000220725. [DOI] [PubMed] [Google Scholar]
- 24.Leitzke S, Bucke W, Borner K, Muller R, Hahn H, Ehlers S. Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrob Agents Chemother. 1998;42:459–461. doi: 10.1128/aac.42.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nightingale S D, Saletan S L, Swenson C E, Lawrence A J, Watson D A, Pilkiewicz F G, Silverman E G, Cal S X. Liposome-encapsulated gentamicin treatment of Mycobacterium avium-Mycobacterium intracellulare complex bacteremia in AIDS patients. Antimicrob Agents Chemother. 1993;37:1869–1872. doi: 10.1128/aac.37.9.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orozco L C, Quintana F O, Beltrán R M, deMoreno I, Wasserman M, Rodriguez G. The use of rifampicin and isoniazid entrapped in liposomes for the treatment of murine tuberculosis. Tubercle. 1986;67:91–97. doi: 10.1016/0041-3879(86)90002-4. [DOI] [PubMed] [Google Scholar]
- 27.Petersen E A, Grayson J B, Hersh E M, Dorr R T, Chiang S M, Oka M, Proffitt R T. Liposomal amikacin: improved treatment of Mycobacterium avium complex infection in the beige mouse model. J Antimicrob Chemother. 1996;38:819–828. doi: 10.1093/jac/38.5.819. [DOI] [PubMed] [Google Scholar]
- 28.Redding T W, Schally A V, Tice T R, Meyers W E. Long-acting delivery systems for peptides: inhibition of rat prostate tumors by controlled release of [d-Trp6]luteinizing hormone-releasing hormone from injectable microcapsules. Proc Natl Acad Sci USA. 1984;81:5845–5848. doi: 10.1073/pnas.81.18.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tice T R, Cowsar D R. Biodegradable controlled-release parenteral systems. J Pharm Technol. 1984;8:26–35. [Google Scholar]
- 30.Tice T R, Rowe C E, Gilley R M, Setterstrom J A, Mirth D D. Development of microencapsulated antibiotics for topical administration. Lincolnshire, Ill: Controlled Release Society, Inc.; 1986. pp. 169–170. [Google Scholar]
- 31.Toosi Z, Ellner J J. Host response to Mycobacterium tuberculosis. Front Biosci. 1998;3:133–140. doi: 10.2741/a286. [DOI] [PubMed] [Google Scholar]
- 32.United States Pharmacopeial Convention, Inc. U.S. Pharmacopeia, XXII ed. Rockville, Md: United States Pharmacopeial Convention, Inc.; 1990. p. 1226. [Google Scholar]
- 33.Visscher G E, Robison R L, Argentieri G I. Tissue response to biodegradable injectable microcapsules. J Biomater Applications. 1987;2:118–131. doi: 10.1177/088532828700200103. [DOI] [PubMed] [Google Scholar]
- 34.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to 50:50 poly(dl-lactide-co-glycolide) microcapsules. J Biomed Mater Res. 1985;19:349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 35.Visscher G E, Robison R L, Maulding H V, Fong J W, Pearson J E, Argentieri G I. Biodegradation of and tissue reaction to microcapsules. J Biomed Mater Res. 1986;20:667–676. doi: 10.1002/jbm.820200510. [DOI] [PubMed] [Google Scholar]
- 36.Vladimirsky M A, Ladigina G A. Antibacterial activity of liposome-entrapped streptomycin in mice infected with Mycobacterium tuberculosis. Biomedicine. 1982;36:375–377. [PubMed] [Google Scholar]
- 37.Wright E L, Quenelle D C, Suling W J, Barrow W W. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimycobacterial drug studies. Antimicrob Agents Chemother. 1996;40:2206–2208. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]