Abstract
Salicylic acid (SA) has been shown to ameliorate drought stress. However, physiological and biochemical mechanisms involved in drought stress tolerance induced by SA in plants have not been well understood. Thus, this study aimed to study the role of SA application on enzymatic and non-enzymatic antioxidants, photosynthetic performance, and plant growth in A. chilensis plants subjected to moderate drought stress. One-year-old A. chilensis plants were subjected to 100% and 60% of field capacity. When plants reached moderate drought stress (average of stem water potential of −1.0 MPa, considered as moderate drought stress), a single SA application was performed on plants. Then, physiological and biochemical features were determined at different times during 14 days. Our study showed that SA application increased 13.5% plant growth and recovered 41.9% AN and 40.7% gs in drought-stressed plants on day 3 compared to drought-stressed plants without SA application. Interestingly, SOD and APX activities were increased 85% and 60%, respectively, in drought-stressed SA-treated plants on day 3. Likewise, SA improved 30% total phenolic content and 60% antioxidant capacity in drought-stressed A. chilensis plants. Our study provides insight into the SA mechanism to tolerate moderate drought stress in A. chilensis plants.
Keywords: superoxide dismutase activity, ascorbate peroxidase activity, CO2 assimilation, total phenolics, plant growth
1. Introduction
Drought has become one of the most important environmental stress factors for plants, reducing yields by more than 50% [1,2,3,4]. This stress negatively affects carbon accumulation, tissue expansion, and plant development [5,6,7]. Stomatal closure is among the first physiological processes affected by drought stress, decreasing photosynthesis and plant growth [8,9,10,11]. Although it has been reported that photosynthesis is mainly inhibited by stomatal closure, it might be also affected by metabolic impairment. Thus, studies have reported that drought stress decreases ribulose-1,5-bifosfate carboxylase/oxygenase (Rubisco) activity, Calvin and Benson cycle enzymes inactivation, low adenosine triphosphate (ATP), and damage to photosystem II [8,12,13,14,15]. However, the mechanisms by which metabolic impairment causes photosynthesis inhibition under drought stress have not been fully elucidated [16,17]. Plants subjected to drought stress increase reactive oxygen species (ROS) production in different cellular organelles such as chloroplasts, mitochondria, and peroxisomes, generating oxidative stress, damaging DNA, proteins, and lipids [18,19,20]. Thus, González-Villagra et al. [21] showed that oxidative stress increased about 50% after 20 days of drought stress compared to well-watered Aristotelia chilensis plants under greenhouse conditions.
On the other hand, plants have developed morphological, physiological, and molecular mechanisms to tolerate drought stress. Stomatal closure is among the most important physiological mechanisms to tolerate drought stress by preventing water loss by transpiration [22]. Other important physiological mechanisms to tolerate drought stress are the biosynthesis of enzymatic and non-enzymatic antioxidants [23,24,25,26]. The primary defense line of antioxidant enzymes is superoxide dismutase (SOD) and ascorbate peroxidase (APX), which scavenge ROS such as superoxide anions (O2.) and hydrogen peroxide (H2O2), forming water and oxygen [27,28,29]. Among non-enzymatic antioxidants, phenolic compounds are highly reactive as hydrogen or electron donors and have the ability to chelate transition metal ions, inhibit lipoxygenase activity, and scavenge ROS, which is considered as an adaptive response to drought stress [30,31,32,33].
Among plant hormones, salicylic acid (SA) is involved in plant defense against microbial pathogens, activating the systemic acquired resistance (SAR) [34,35]. Under non-stressed conditions, SA plays an important role in nutrient uptake, cell elongation, photosynthetic pigment levels, photosynthetic activity, and source-to-sink regulation, regulating plant growth and development [36,37,38,39]. Currently, SA has been suggested as an important molecule involved in plant tolerance to drought stress [38,40]. For example, Chen et al. [41] showed that SA application increased CO2 assimilation and plant growth in drought-stressed Zoysia japonica plants. Currently, Zafar et al. [42] reported that leaf gas exchange, SOD and APX activities, and dry weight accumulation were improved by SA application in Conocarpus erectus and Populus deltoides plants subjected to drought stress. Therefore, SA is a critical plant hormone that regulates the activation of biotic and abiotic stress defense. However, the physiological and biochemical mechanisms involved in drought stress tolerance induced by SA in plants have not been well understood [42,43].
Aristotelia chilensis (Mol.), also known as “maqui”, is an important endemic berry that grows in Southern Chile [26,44,45,46,47,48]. Currently, several studies related to crop management and morphological and physiological characterization have been performed in this species considering their antioxidant properties [21,47,48,49,50,51].
Therefore, we hypothesized that SA application triggers enzymatic and non-enzymatic antioxidant mechanisms, which ameliorate oxidative stress, improving photosynthesis performance and plant growth of A. chilensis plants subjected to moderate drought stress. Thus, the aim of this study was to study the role of SA application on enzymatic and non-enzymatic antioxidants, photosynthetic performance, and plant growth in A. chilensis plants subjected to moderate drought stress. Our results provide new knowledge into the role of SA on the antioxidant defense system in A. chilensis to tolerate moderate drought stress.
2. Results
2.1. Relative Growth Rate and Plant Water Status in A. chilensis
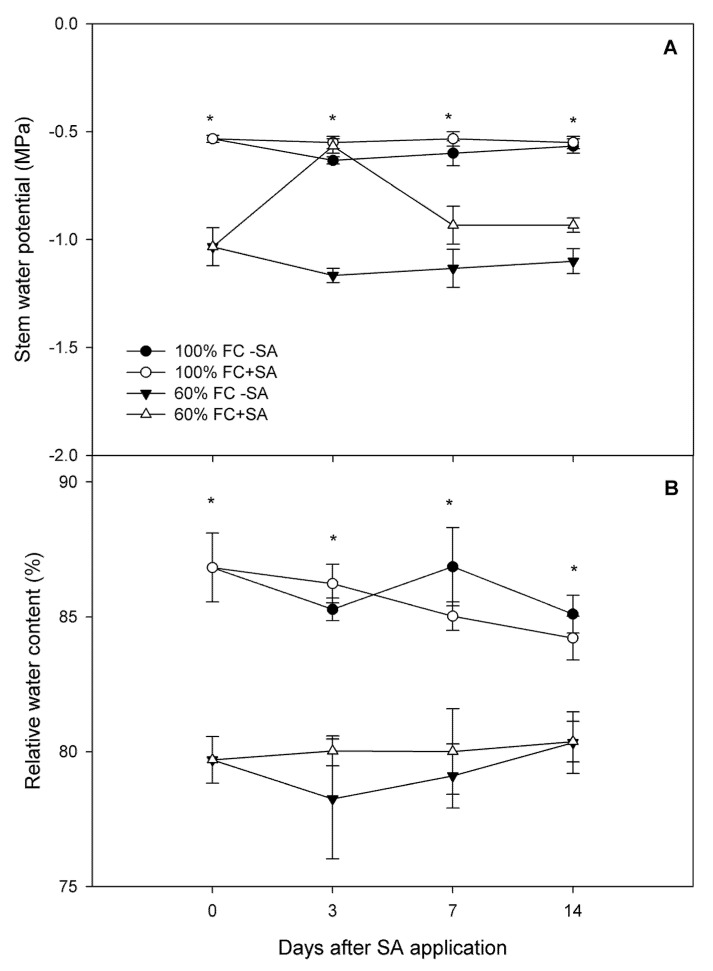
Our results revealed that moderate drought stress negatively affected plant growth in A. chilensis plants, reducing about 27% relative growth rate (RGR) compared to well-irrigated plants (control, 100% FC) at days 3 and 7 of the experiment (Table 1). However, SA application stimulated plant growth in moderate drought-stressed A. chilensis plants, increasing 13.5%, 7.8%, and 5.4% RGR with respect to moderate drought-stressed plants without SA-treated plants at days 3, 7, and 14, respectively. A similar tendency was observed in well-irrigated plants, where RGR was increased 10.6% and 15% at days 3 and 7 of the experiment in plants with SA application, showing no differences at day 14 of the experiment with respect to plants without SA application. In our study, plants subjected to moderate drought stress showed significantly lower stem water potential (Ψw) (around −1.3 MPa) compared to well-irrigated plants at day 3 of the experiment (−0.5 MPa) (Figure 1A). Interestingly, when SA application was performed, Ψw increased in moderate drought-stressed plants, reaching well-irrigated plant levels at day 7. However, on days 7 and 14 of the experiment, Ψw in moderate drought-stressed plants with SA application were reduced, reaching similar values as drought-stressed plants without SA application. In contrast, no changes were observed in well-irrigated plants throughout the experiment. Concerning relative water content (RWC), moderate drought-stressed plants showed slightly lower levels (6%) than well-irrigated plants (Figure 1B). Meanwhile, moderate drought-stressed plants with SA application showed similar RWC values compared to plants subjected to drought stress without SA application. With respect to control plants (100% FC) with and without exogenous SA, no variations were shown in RWC during the experiment (Figure 1B).
Table 1.
Relative growth rates of A. chilensis plants grown under 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times post SA application.
| Relative Growth Rate (mg Dry Weight Day−1) | |||
|---|---|---|---|
| Treatment | Day 3 | Day 7 | Day 14 |
| 100% FC-SA | 47.14 ± 1.14 Ab * | 45.96 ± 0.39 Ab * | 45.71 ± 2.34 Aa * |
| 100% FC + SA | 52.08 ± 1.29 Aa * | 52.90 ± 1.69 Aa * | 44.70 ± 2.85 Ba * |
| 60% FC-SA | 37.87 ± 2.18 Ab | 38.54 ± 1.07 Ab | 37.65 ± 0.77 Ab |
| 60% FC + SA | 42.70 ± 1.43 Aa | 41.21 ± 1.09 Aa | 39.63 ± 0.65 Aa |
The bars are means ± SE (n = 3). Different uppercase letters indicate significant differences among post SA application time for the same SA and irrigation treatment according to Tukey’s test (p ≤ 0.05). Different lowercase letters indicate significant differences between SA treatment for the same irrigation treatment and day according to Tukey’s test (p ≤ 0.05). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
Figure 1.
(A) Stem water potential and (B) relative water content in A. chilensis plants grown under 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times post SA application. The bars are means ± SE (n = 3). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
2.2. Photosynthetic Performance in A. chilensis Subjected to Moderate Drought Stress
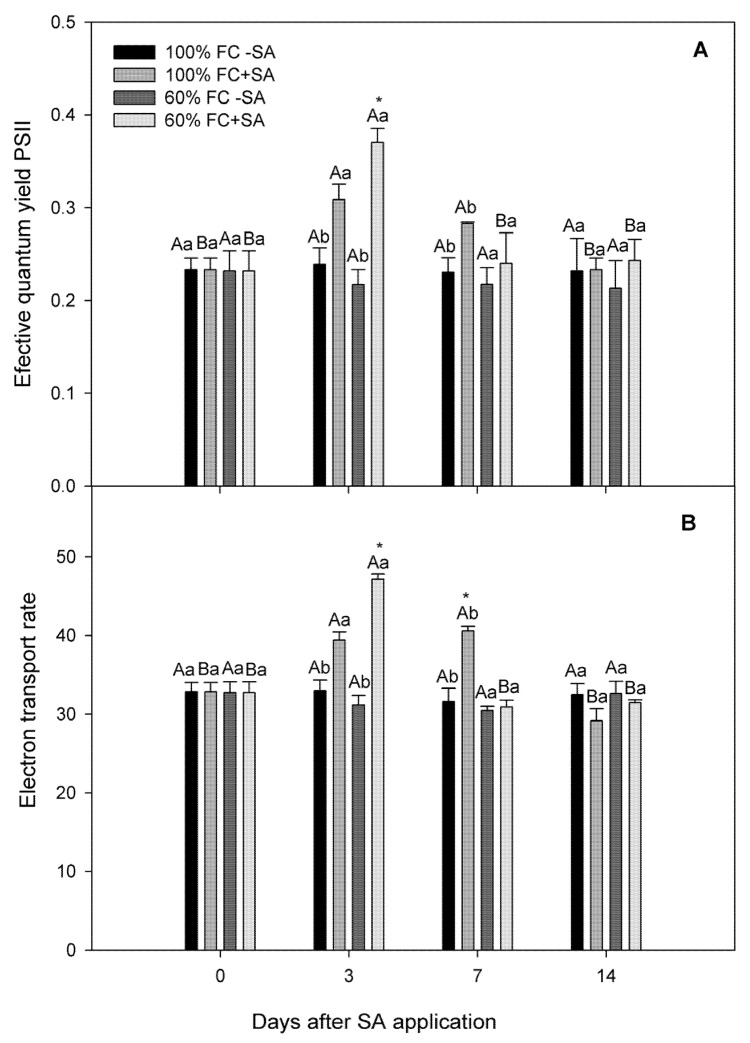
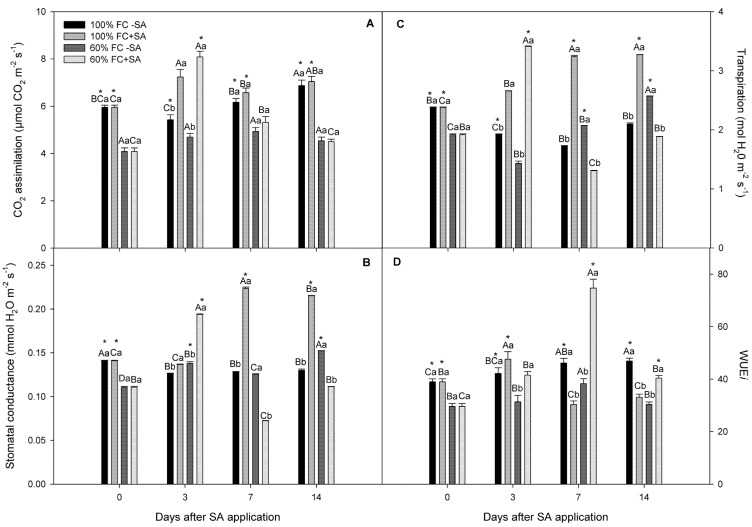
We observed a significant interaction among time, irrigation treatments, and SA levels for electron transport rate (ETR), effective quantum yield (ΦPSII), CO2 assimilation, stomatal conductance, and transpiration (p ≤ 0.05), except WUEi. In our study, moderate drought-stressed plants had similar levels as well-irrigated plants in terms of ΦPSII and ETR, remaining unchanged during the experiment (Figure 2). Surprisingly, exogenous SA application increased 41% ΦPSII and 33% ETR in plants subjected to moderate drought stress at day 3, recovering values at days 7 and 14 of the experiment compared to drought-stressed plants without SA application, showing a transitory improvement of this parameter. A similar tendency showed SA-treated well-irrigated plants, where ΦPSII and ETR were increased at day 3 and 7, declining at day 14. On the other hand, moderate drought stress negatively affected net CO2 assimilation (AN) in A. chilensis plants, showing 30.8% lower AN at the beginning of the experiment compared with plants subjected to 100% FC (Figure 3A). SA application recovered and increased AN in moderate drought-stressed plants at day 3, being 41.9% higher than moderate drought-stressed plants without SA application. However, AN decreased in moderate drought-stressed plants treated with SA at days 7 and 14, reaching levels of plants without SA. A similar tendency was observed in 100% FC plants where exogenous SA increased 33.8% AN compared with plants without SA at day 3; however, AN reached control values from day 7 of the experiment, reaching 100% FC without SA. In our study, lower stomatal conductance (gs) levels were observed in moderate drought-stressed plants than well-irrigated ones (Figure 3B). Exogenous SA increased 41% gs in moderately stressed plants compared to those without SA at day 3, decreasing at days 7 and 14. Well-irrigated SA-treated plants progressively increased gs from day 7 and 14 compared to plants without SA treatment. Concerning transpiration (E), this parameter was significantly reduced in plants (60% FC) throughout the experiment compared to well-irrigated plants (Figure 3C). A similar tendency was observed when SA was applied to plants, where moderate drought-stressed plants increased E levels compared to non-SA-treated plants at day 3 of the experiment, while well-irrigated SA-treated plants showed an increase in E levels at days 7 and 14 of the experiment. Furthermore, we observed that SA application improved WUEi in moderate drought-stressed plants at day 3, reaching the highest level at day 7 of the experiment, while well-irrigated plants had a slight increase in WUEi only at day 3 (Figure 3D).
Figure 2.
Photochemical parameters in A. chilensis plants grown under 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times. (A) effective quantum yield (ΦPSII) and (B) electron transport rate (ETR). The bars are means ± SE (n = 3). Different uppercase letters indicate significant differences among days for the same SA and irrigation treatment according to Tukey’s test (p ≤ 0.05). Different lowercase letters indicate significant differences between SA treatment for the same irrigation treatment and time post SA application according to Tukey’s test (p ≤ 0.05). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
Figure 3.
Gas-exchange measurements in A. chilensis plants grown under 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times. (A) CO2 assimilation, (B) stomatal conductance, (C) transpiration, and (D) intrinsic water-use efficiency (WUEi). The bars are means ± SE (n = 3). Different uppercase letters indicate significant differences among days for the same SA and irrigation treatment according to Tukey’s test (p ≤ 0.05). Different lowercase letters indicate significant differences between SA treatment for the same irrigation treatment and time post SA application according to Tukey’s test (p ≤ 0.05). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
2.3. Antioxidant Capacity and Total Phenolic Content Determination
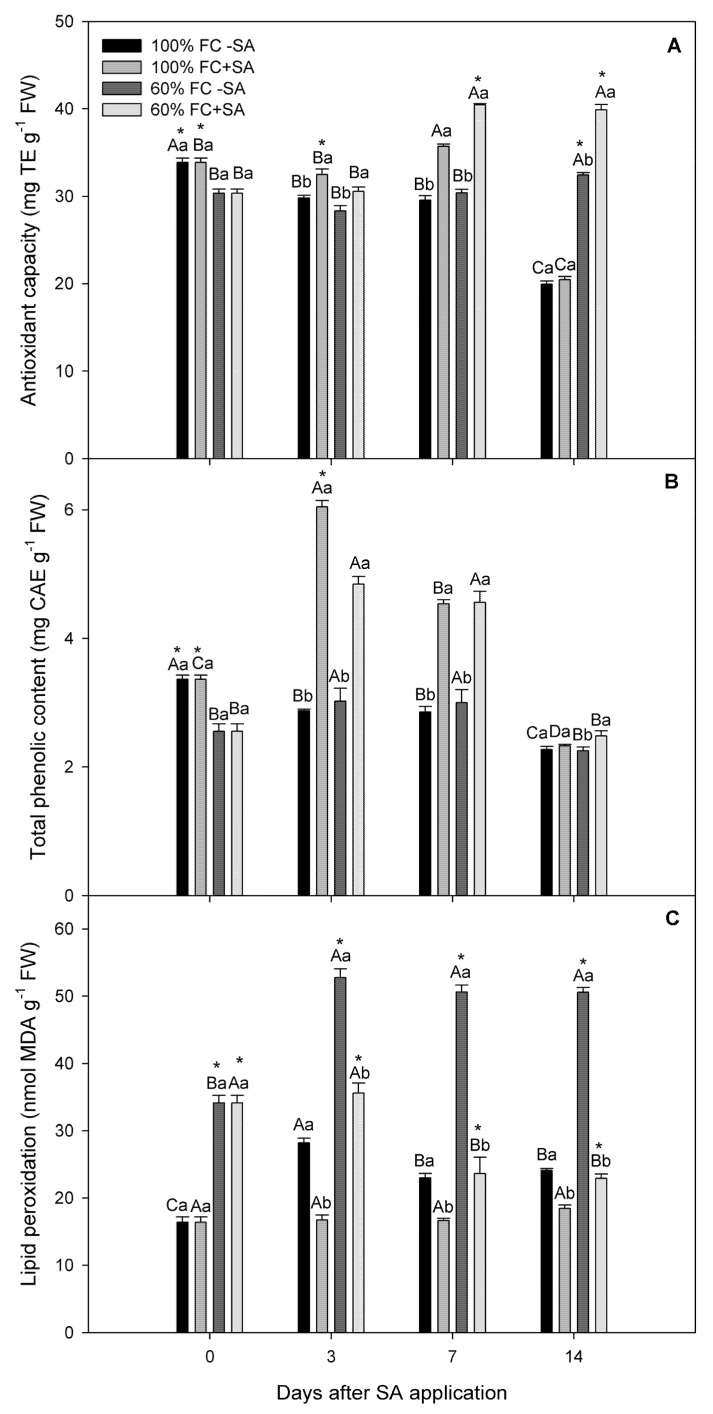
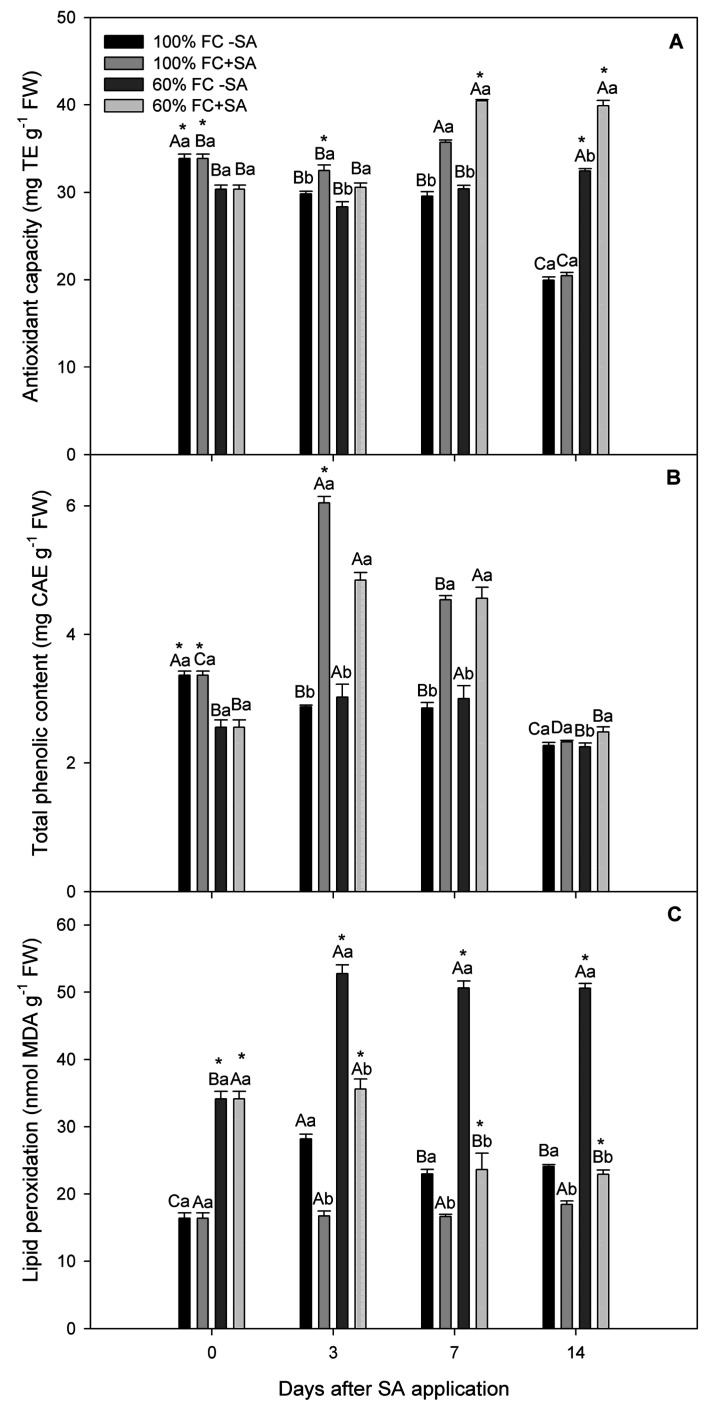
In our study, antioxidant capacity (AC) and total phenolic content (TPC) showed statistically significant interaction among day, irrigation treatment, and SA treatment (p ≤ 0.05). Our results showed that moderate drought-stressed plants had lower antioxidant capacity (AC) compared to well-irrigated plants at day 3, unchanging until day 14 of the experiment, where a slight increase (about 12%) was observed (Figure 4A). However, SA application significantly increased (around 30%) AC in moderate drought-stressed plants on days 7 and 14 of the study. Likewise, well-irrigated plants showed a slightly higher AC level at day 7 when exogenous SA was applied; however, their AC levels decreased at day 14 of the experiment. In TPC, plants subjected to moderate drought stress had similar levels as well-irrigated plants during the experiment, except day 0 (Figure 4B). Interestingly, exogenous SA application significantly improved TPC levels (about 60%) at days 3 and 7 in plants subjected to drought stress, while well-irrigated plants showed the highest TPC levels at day 3, which was 2-fold greater than non-SA-treated plants (Figure 4B).
Figure 4.
Non-enzymatic antioxidant system and oxidative stress in A. chilensis plants grown 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times. (A) Antioxidant capacity, (B) total phenolic content, and (C) lipid peroxidation. The bars are means ± SE (n = 3). Different uppercase letters indicate significant differences among times post SA application for the same SA and irrigation treatment according to Tukey’s test (p ≤ 0.05). Different lowercase letters indicate significant differences between SA treatment for the same irrigation treatment and time post SA application according to Tukey’s test (p ≤ 0.05). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
2.4. Lipid Peroxidation
As an indicator of oxidative stress, lipid peroxidation was determined in A. chilensis plants. Our results showed that lipid peroxidation was higher in moderate drought-stressed A. chilensis plants, where a significant increase (around 2.5-fold) compared to well-irrigated plants was observed throughout the experiment (Figure 4C). Meanwhile, drought-stressed plants subjected to SA application significantly decreased their MDA content from day 7, reaching the lowest levels at day 14. On the other hand, well-irrigated SA-treated plants remained unchanged in their MDA content during the experiment, showing a mean value of 17 nmol g−1 fresh weight.
2.5. Superoxide Dismutase and Ascorbate Peroxidase Activity in A. chilensis
Superoxide dismutase (SOD) and ascorbate peroxidase (APX) activities were determined as an enzymatic antioxidant defense mechanism in A. chilensis. We observed a significant interaction among time, irrigation treatments, and SA levels for SOD and APX activities (p ≤ 0.05). Plants subjected to drought stress showed an evident increment in SOD activity compared to well-irrigated A. chilensis plants throughout the experiment (Figure 5A). Meanwhile, well-irrigated plants maintained relatively constant SOD activity in our study. Exogenous SA application significantly increased SOD activity in moderate drought-stressed and well-irrigated plants. The highest SOD activity was observed in drought-stressed SA-treated plants at day 3, where it increased about 85% with respect to drought-stressed plants without SA application (Figure 5A). However, SOD activity decayed in SA-treated plants subjected to drought, reaching the same levels as non-SA-treated plants at day 14 of the experiment. Concerning APX, we observed that drought-stressed plants had significantly higher levels than well-irrigated plants, exhibiting around 2-fold greater activity throughout the experiment (Figure 5B). Interestingly, exogenous SA application increased (around 60%) APX activity in moderate drought-stressed A. chilensis plants at day 3. Then, APX activity decreased to reach similar levels as non-SA-treated plants at day 14 of the experiment. Similar behavior was observed in well-irrigated plants, where APX activity slightly increased from day 3, reaching the highest level at day 7, around 30% greater than well-irrigated plants without SA application (Figure 5B).
Figure 5.
Enzymatic antioxidant system in A. chilensis plants grown under 100% and 60% FC and two SA doses (0 and 0.5 mM) at different times after SA application. (A) Superoxide dismutase (SOD) and (B) ascorbate peroxidase (APX) activity. The bars are means ± SE (n = 3). Different uppercase letters indicate significant differences among times post SA application for the same SA and irrigation treatment according to Tukey’s test (p ≤ 0.05). Different lowercase letters indicate significant differences between SA treatment for the same irrigation treatment and time post SA application according to Tukey’s test (p ≤ 0.05). According to Tukey’s test, asterisks indicate significant differences between irrigation treatments of the experiment for the same SA treatment and time post SA application (p ≤ 0.05).
3. Discussion
Drought stress is the most severe manifestation of climate change, causing a severe threat to food security [52,53]. The results from our study revealed that the relative growth rate was decreased by 27% in A. chilensis plants subjected to moderate drought stress compared to well-irrigated plants at days 3 and 7 of the experiment (Table 1). Likewise, plant water status, including stem water potential (Ψw) and relative water content (RWC), was reduced concomitantly with plant growth in moderate drought-stressed A. chilensis plants (Figure 1). In fact, Ψw reached a stable lowest value of −1.3 MPa in drought-stressed plants (irrigation at 60% FC), while well-irrigated plants maintained their Ψw around −0.5 MPa during the experiment. Similarly, moderate drought-stressed A. chilensis plants had a slightly lower (6%) RWC level compared to well-irrigated plants. The negative effects of moderate drought stress on plant growth and plant water status have been reported for several species such as Phaseolus vulgaris [54], Vaccinium corymbosum [55], Malus domestica [56], and Punica granatum [57]. We previously reported that severe drought stress reduced 5-fold Ψw and 71% plant growth in A. chilensis plants after 20 days of water restriction [21]. In this context, it is well-known that plants induce stomatal closure, which is among the earliest response to drought stress, as a mechanism for preventing water loss, decreasing CO2 assimilation (AN), and inhibiting plant growth [58,59,60]. Here, we observed that AN and gs were significantly decreased by 30.8% and 21.4%, respectively, in plants subjected to drought stress contrasted with well-irrigated plants, which exhibited the highest and steady AN and gs values throughout the experiment (Figure 3). Thus, plant growth inhibition could be explained due to lower gs and AN levels in drought-stressed A. chilensis plants in our experiment. Nonetheless, photosynthesis might be also affected by metabolic impairment. Thus, it has been reported that reactive oxygen species (ROS) are produced in different cellular compartments such as chloroplasts, mitochondria, and peroxisomes [19,61]. ROS production in chloroplasts is mediated by photoreduction of O2 and the ground-state oxygen to singlet state in the reaction centers of Photosystem I (PSI) and Photosystem II (PSII), and then generating different types of ROS such as 1O2, O2.-, H2O2, and OH., decreasing photosynthetic performance [62]. Under drought stress, ROS production induces damage to DNA, proteins, carbohydrates, and lipids, triggering oxidative stress [18]. Thus, lipid peroxidation was measured as an indicator of oxidative stress in our study (Figure 4C). Our results showed a significant increase (around 2.5-fold) in lipid peroxidation in moderate drought-stressed A. chilensis plants, which was similar to our previous study in the same species [21]. Therefore, A. chilensis plants were biochemically and physiologically affected by drought stress, increasing lipid peroxidation and decreasing gs, AN, and plant growth.
Salicylic acid (SA) is a plant hormone regulating plant growth and development under non-stressed conditions [37,38,39]. Currently, SA has been suggested as an important molecule involved in ameliorating plant drought stress [38,39,40,63]. However, the physiological and biochemical mechanisms involved on drought stress tolerance induced by SA in plants have not been well understood [42,43]. Some reports have shown that SA improves SOD and APX activities and leaf gas exchange in Conocarpus erectus, Populus deltoids, and Olea europaea plants subjected to drought stress [38,42]. Therefore, we hypothesized that SA application triggers enzymatic and non-enzymatic antioxidant mechanisms, ameliorating oxidative stress and improving photosynthesis performance and plant growth in A. chilensis plants subjected to drought stress. We observed that three days post SA application, AN recovered in drought-stressed plants compared to drought-stressed plants without SA application (Figure 3A). A similar tendency was observed in well-watered plants where exogenous SA increased AN three days post application compared with plants without SA. Concomitantly, exogenous SA increased gs in stressed plants compared to those without SA (Figure 3B). Interestingly, we observed that SA application improved WUEi in drought-stressed plants at day 3, reaching the highest level at day 7 of the experiment (Figure 3D). In addition, we observed that higher CO2 assimilation in SA-treated plants was concomitant with greater plant growth and better plant water status in A. chilensis plants subjected to drought stress in our experiment. Thus, SA application recovered plant growth in drought-stressed A. chilensis plants, with respect to drought-stressed plants without SA treatment (Table 1). Our results agree with Chen et al. [39], where they showed that SA application increased CO2 assimilation and plant growth in drought-stressed Zoysia japonica plants. Recently, Shemi et al. [64] showed that SA treatment increased about 25% plant growth and crop yield in Zea mays plants subjected to drought stress due to higher CO2 assimilation and stomatal conductance. Interestingly, the authors also showed that the intercellular CO2 concentration remained unchanged between SA-treated and non-SA-treated plants, which could indicate a better metabolic efficiency triggered by SA. We observed that exogenous SA application increased electron transport rate (ETR) and effective quantum yield (ΦPSII) in plants subjected to moderate drought stress at day 3 of the experiment (Figure 3). In fact, Khalvandi et al. [65] suggested that SA may also regulate some physiological responses related to carbon uptake and/or fixation in the chloroplasts, such as high ribulose-1,5-bifosfate carboxylase/oxygenase (Rubisco) concentration and activity, increasing CO2 assimilation and plant growth. Likewise, Shao et al. [66] showed that Rubisco and Rubisco activase enzyme activities increased due to SA application in Zea mays plants subjected to drought stress. The authors also reported that SA induced high transcript levels of Rubisco large subunit (Rbc L), α-form Ribulose-1,5-bisphosphate carboxylase/oxygenase (ZmRCAα), and β-form Ribulose-1,5-bisphosphate carboxylase/oxygenase (ZmRCAβ) mRNA, which may confirm the positive effects of SA on CO2 assimilation in our study. It has been reported that SA induces stomatal closure under biotic stress, preventing pathogen invasion into plants [35]. However, we observed a stomatal aperture in SA-treated A. chilensis plants, where gs increased 41% in moderate drought-stressed plants compared to moderate drought-stressed plants without SA at day 3 (Figure 3B). Our results were similar to those reported by Shemi et al. [64] and Habibi et al. [67], where they showed that SA induced stomatal aperture in Z. mays and Hordeum vulgare plants subjected to drought stress. Recently, Zamora et al. [68] showed that Arabidopsis and Solanum lycopersicum plants did not reduce stomatal aperture after SA spraying. The authors also suggested that SA-induced stomatal closure apparently requires high concentrations or prolonged SA treatments. Therefore, we can suggest that SA-induced stomatal closure seems dependent of the species, SA concentration, and/or biotic or abiotic stress specific. On the other hand, Nazar et al. [37] also suggested that SA helps to maintain the integrity of light-harvesting apparatus by enzymatic and non-enzymatic antioxidants, being an important mechanism for increasing photosynthesis under drought stress. In our study, we observed that SA application had a transitory stimulus of photosynthetic performance including ETR, ΦPSII, gs, AN, and plant growth at day 3 post SA application. It has been reported that SA may undergo different chemical modifications such as glycosylation, methylation, and amino acid conjugation, metabolizing into inactive or storage forms to modulate its activity and fine-tune its levels [69,70]. Thus, the transitory improvement of photosynthetic performance and plant growth in A. chilensis plants could be explained by SA metabolism, which modulate its activity and levels in plants.
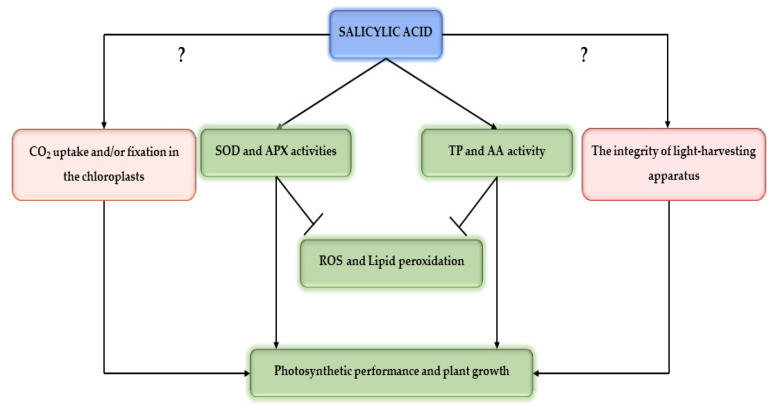
As we mentioned before, plants have developed complex mechanisms to scavenge ROS and avoid oxidative stress under drought stress, such as enzymatic and non-enzymatic antioxidants [24,25,26]. Thus, SOD and APX activities were determined as an enzymatic antioxidant defense mechanism in A. chilensis. In our study, exogenous SA application significantly increased (85%) SOD activity in drought-stressed plants at day 3 with respect to drought-stressed plants without SA application (Figure 5A). However, SOD activity in SA-treated plants reached levels of control (non-SA-treated plants) on day 14 of the experiment. The same tendency showed APX activity, where SA application increased (around 60%) its levels in drought-stressed A. chilensis plants at day 3, decreasing to reach similar levels as non-SA-treated plants at day 14 of the experiment (Figure 5B). Similar results were reported by Mohi-Ud-Din et al. [63] and Sharma et al. [71], where SA application increased SOD and APX activities in Glycine max and Phaseolus vulgaris plants subjected to drought stress. Wang et al. [72] showed that Hordeum vulgare plants overexpressing the isochorismate synthase gene, which is a key gene for SA biosynthesis and which significantly increased SOD and APX activities, decreasing ROS and lipid peroxidation induced by drought stress, could partly explain our results. In addition, as a non-enzymatic antioxidant, exogenous SA application significantly increased TPC levels (60%) at days 3 and 7 in plants subjected to drought stress, while the same treatment increased 30% AC in drought-stressed plants at days 7 and 14 of the study (Figure 4). Phenolic compounds are important molecules to counteract oxidative stress, which are highly reactive as hydrogen or electron donors and have the ability to chelate transition metal ions, scavenging ROS, are being considered as an adaptive response to drought stress [30,31,32,33]. Interestingly, we observed that drought-stressed A. chilensis plants subjected to SA application significantly decreased their lipid peroxidation, measured as MDA content. This result suggests that both enzymatic and non-enzymatic antioxidant mechanisms could contribute to coping with ROS, decreasing oxidative stress, and improving photosynthetic performance and plant growth in A. chilensis plants subjected to drought stress. Thus, a schematic representation is shown in Figure 6, where we suggested that SA induces enzymatic and non-enzymatic antioxidant mechanisms to tolerate drought stress, decrease oxidative stress, and improve photosynthesis performance and plant growth in A. chilensis plants. However, SA molecular regulation mechanisms to induce enzymatic and non-enzymatic antioxidant mechanisms in A. chilensis plants under drought stress is still unknown.
Figure 6.
Schematic representation of SA-induced mechanism to tolerate drought stress in A. chilensis plants. We suggested that SA induces enzymatic and non-enzymatic antioxidant mechanisms to tolerate drought stress, decrease oxidative stress, and improve photosynthesis performance and plant growth in A. chilensis plants. Abbreviations: superoxide dismutase (SOD), ascorbate peroxidase (APX), total phenolic content (TPC), antioxidant capacity (AC), reactive oxygen species (ROS).
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
One-year-old A. chilensis plants obtained in vitro conditions were used in this study, which Plangen Co., Máfil, Chile donated. Plants of uniform size were conditioned in plastic pots containing 1.5 L of Andisol soil under greenhouse conditions for 2 weeks according to González-Villagra et al. [21,26]. Greenhouse conditions were a 16/8 h light/dark photoperiod, 23 ± 2 °C temperature, 70% relative humidity, and a mean 300 µmol photons m−2 s−1. Plants were acclimated for two weeks. Then, plants were divided into two groups; plants daily irrigated (DI), maintained at 100% of field capacity (100% FC) and plants non-irrigated (NI), maintained at 60% of FC reaching an average stem water potential of −1.0 MPa after 10 days of treatment (considered as moderate drought stress for this species, according to our previous study; González-Villagra et al. [21,26]). When NI plants reached moderate drought stress, a single application of 0.5 mM salicylic acid (SA) (Sigma, St. Louis, MO, USA) was performed, spraying homogeneously for both irrigation treatments (+SA) [73,74]. The SA was dissolved in ultrapure water containing 0.05% (v/v) of Tween 20 used as the surfactant wetting agent. Control solution only contained ultrapure water with Tween 20 (-SA). The experiment was carried out for 14 days. At different time points post SA application (0, 3, 7, and 14 days), in vivo gas-exchange determination was performed, and leaf samples were collected in the middle of the light period, frozen in liquid nitrogen, and stored at −80 °C until biochemical analyses.
4.2. Plant Growth and Water Status Measurements in A. chilensis
4.2.1. Relative Growth Rate (RGR)
In order to measure growth rate during the experiment, the Hoffmann and Poorter [75] protocol was used. It was calculated by relative growth rate (RGR) from the mean natural logarithm-transformed dry weight (DW). The t1 was time 0 and t2 were the times 3, 7, and 14 days. RGR was calculated by Formula (1).
| RGR = [(lnDW2) − (lnDW1)/(t2 − t1)] | (1) |
4.2.2. Plant Water Status
Stem water potential (Ψw) was measured between 08:00 and 10:00 on the leaf petiole using a Scholander chamber Model 1000 (PMS, Instruments Co., Corvallis, OR, USA) based on the Begg and Turner [76] protocol. For this, 90 min before measurement, leaves were covered with aluminum foil in a plastic bag. In addition, relative water content (RWC) was determined following the Rahimi et al. [77] method. Two leaves were removed, weighed, and immersed into double distilled water for the next 24 h at 4 °C in dark conditions. Then, leaves were oven-dried to a constant weight at 60 °C. Formula (2) was used to determine RWC:
| RWC = [(fresh weight − dry weight)/(turgid weight − dry weight)] × 100 | (2) |
4.3. Photosynthetic Performance
Electron transport rate (ETR), effective quantum yield (ΦPSII), CO2 assimilation (AN), stomatal conductance (gs), and transpiration (E) were measured in order to determine the photosynthetic performance of A. chilensis plants. Thus, in vivo measurements were performed using a portable infrared CO2 analyzer equipped with a Li-Cor LR6400 cuvette measurement with its light source (LI-COR Inc., Lincoln, NE, USA), during the light period (08:00 to 10:00 h), as described by Reyes-Díaz et al. [78]. The portable photosynthesis system controlled the light source (400 µmol photons m−2 s−1), temperature (25 °C), humidity, and CO2 concentration. External air with CO2 was used to obtain a concentration reference of 400 µmol mol−1, with a flow rate of 300 mL min−1 and 80% external relative humidity. Four measurements per plant were performed.
4.4. Lipid Peroxidation
Leaf samples were macerated with a mixture of trichloroacetic acid (TCA) and thiobarbituric acid (TBA). Then, the homogenate was centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatant was collected and used for lipid peroxidation (LP) determination. The LP was measured based on the formation of thiobarbituric acid-reactive substances (TBARS) according to Du and Bramalage [79]. In order to correct the interference generated by TBARS-sugars complexes, absorbance was measured at 440, 532, and 600 nm (UV/VIS Unico SpectroQuest 2800). The TBARS content was expressed as nmol of malondialdehyde (MDA) per gram of fresh weight (nmol MDA g−1 FW). Formula (3) was used to determine the content of TBARS in terms of MDA equivalent:
| MDA equivalent (nmol/mL) = [(A532 − A600)/157,000] × 106 | (3) |
4.5. Antioxidant Capacity and Total Phenolic Content Determination
Leaf samples were ground in liquid nitrogen and macerated with ethanol (80% v/v). The homogenate was centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatant was collected and used for antioxidant capacity and total phenolic content determinations. Antioxidant capacity was determined by the DPPH (2.2-diphenyl-1-picryl-hydrazyl) assay [80], measuring the absorbance at 515 nm (UV/VIS Unico SpectroQuest 2800). Antioxidant capacity was expressed as mg of Trolox equivalents per gram of fresh weight (mg TE g−1 FW). Total phenolic content was determined using the Folin–Ciocalteu method (Singleton and Rossi [81]), measuring the absorbance at 765 nm (UV/VIS Unico SpectroQuest 2800) using caffeic acid as standard. Total phenolic content was expressed as mg of caffeic acid equivalents per gram of fresh weight (mg CAE g−1 FW).
4.6. Superoxide Dismutase (SOD) and Ascorbate Peroxidase (APX) Activities in A. chilensis
Leaf samples were ground in liquid nitrogen and macerated with 50 mM potassium phosphate buffer (K2HPO4–KH2PO4, 50 mM, pH 7.0). Then, the homogenate was centrifuged at 11,000 g for 15 min at 4 °C, and the supernatant (crude extract) was used for SOD and APX determinations. Superoxide dismutase (SOD) (EC. 1.15.1.1) activity was determined by measuring the photochemical inhibition of nitroblue tetrazolium (NBT) as reported by Giannopolitis et al. [82]. Briefly, crude extract (20 µL) was added to a reaction mixture containing potassium phosphate buffer, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 13 mM methionine, and 322 µM NBT. Riboflavin was added to start the reaction. Reaction mixtures were illuminated for 15 min, and the absorbance was measured at 560 nm. One SOD unit was defined as the amount of enzyme corresponding to 50% inhibition of the NBT reduction (Donahue et al. [83]). The enzyme activity was standardized for the protein content. Protein in the crude enzyme extract was determined by the Bradford method (Bradford [84]).
Ascorbate peroxidase (APX) (EC. 1.11.1.11) activity was determined by Nakano and Asada [85]. For this, the crude extract (40 µL) was diluted in a reaction mixture containing 1 mL of extraction buffer, 5 µL of H2O2 (30% v/v), and 40 µL of 10 mM ascorbic acid. Enzyme activity was calculated using a molar extinction coefficient of 2.8 mM cm−1.
4.7. Experimental Design and Statistical Analyses
A completely randomized design was used with three replicates for each treatment and time. Data were tested for homogeneity and normality of variances using the Levene and Kolmogorov–Smirnov tests, respectively. Then, data were analyzed using three-way ANOVA, where factors were drought treatments, SA application, and time post SA application. Tukey’s multiple comparison test p ≤ 0.05 was used. Sigma Stat v.2.0 (SPSS, Chicago, IL, USA) was used to perform the statistical analysis.
5. Conclusions
Our study demonstrated that moderate drought stress negatively affects the physiological and biochemical features of A. chilensis plants. Our study provides insight into the SA mechanism to tolerate drought stress in A. chilensis plants. Our results showed that SA application increases SOD and APX activities, total phenolic content, and antioxidant capacity concomitant with decreased oxidative stress, and improved photosynthesis performance and plant growth in A. chilensis plants subjected to moderate drought stress. However, further molecular studies are needed to understand the SA mechanism to improve moderate drought stress tolerance in A. chilensis.
Acknowledgments
We would like to thank Plangen Co., Máfil, Chile for providing the maqui plants.
Author Contributions
J.G.-V. and L.A.B. designed and coordinated the experiment. R.T.-N. carried out photosynthetic performance analyses. J.G.-V. performed plant water status, plant growth and enzymatic antioxidant analyses. M.M.R.-D. carried out non-enzymatic antioxidant analyses. J.G.-V. performed statistical analyses and formulated the manuscript. J.G.-V., M.M.R.-D., C.I.-B., R.T.-N., A.L.E. and L.A.B. revised and corrected it. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo (ANID, ex CONICYT) for the FONDECYT POSTDOCTORAL N° 3200594 project, internal project from UC Temuco (2019EM-JG-06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the results section.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyer J. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 2.Pessarakli M. Plant and Crop Stress. 3rd ed. CRC Press; Boca Raton, FL, USA: 2010. [Google Scholar]
- 3.IPCC . In: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Shukla P.R., Skea J., Buendia E.C., Masson-Delmotte V., Pörtner H.-O., Roberts D.C., Zhai P., Slade R., Connors S., van Diemen R., et al., editors. IPCC; Geneva, Switzerland: 2019. in press. [Google Scholar]
- 4.Molnár I., Cozma L., Dénes T.É., Vass I., Vass I.Z., Rakosy-Tican E. Drought and saline stress tolerance induced in somatic hybrids of Solanum chacoense and potato cultivars by using mismatch repair deficiency. Agriculture. 2021;11:696. doi: 10.3390/agriculture11080696. [DOI] [Google Scholar]
- 5.Tadeo F., Gómez-Cadenas A. Fisiología de las plantas y el estrés. In: Azcón-Bieto J., Talón M., editors. Fundamentos de Fisiología Vegetal. 2nd ed. McGraw Hill Interamericana; Mexico City, Mexico: 2008. [Google Scholar]
- 6.Tardieu F., Granier C., Muller B. Water deficit and growth. Co-ordinating processes without an orchestrator. Curr. Opin. Plant Biol. 2011;14:283–289. doi: 10.1016/j.pbi.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Wen L., Shi Y., Su D., Lu W., Cheng Y., Li Z. Stress-responsive tomato gene SlGRAS4 function in drought stress and abscisic acid signaling. Plant Sci. 2021;304:110804. doi: 10.1016/j.plantsci.2020.110804. [DOI] [PubMed] [Google Scholar]
- 8.Flexas J., Bota B., Loreto F., Cornic G., Sharkey T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- 9.Flexas J., Ribas-Carbó M., Bota J., Galmés J., Henkle M., Martínez-Cañellas S., Medrano H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006;172:73–82. doi: 10.1111/j.1469-8137.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 10.Galmés J., Ribas-Carbó M., Medrano H., Flexas J. Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J. Exp. Bot. 2011;62:653–665. doi: 10.1093/jxb/erq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piveta L.B., Roma-Burgos N., Noldin J.A., Viana V.E., Oliveira C.D., Lamego F.P., Avila L.A.D. Molecular and physiological responses of rice and weedy rice to heat and drought stress. Agriculture. 2021;11:9. doi: 10.3390/agriculture11010009. [DOI] [Google Scholar]
- 12.Flexas J., Bota J., Galmés J., Medrano H., Ribas-Carbó M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006;127:343–352. doi: 10.1111/j.1399-3054.2006.00621.x. [DOI] [Google Scholar]
- 13.Li P., Li Y.J., Zhang F.J., Zhang G.Z., Jiang X.Y., Yu H.M., Hou B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017;89:85–103. doi: 10.1111/tpj.13324. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-San Millán A., Aranjuelo I., Douthe C., Nadal M., Ancín M., Larraya L., Farran I., Flexas J., Veramendi J. Physiological performance of transplastomic tobacco plants overexpressing aquaporin AQP1 in chloroplast membranes. J. Exp. Bot. 2018;69:3661–3673. doi: 10.1093/jxb/ery148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He W., Yan K., Zhang Y., Bian L., Mei H., Han G. Contrasting photosynthesis, photoinhibition and oxidative damage in honeysuckle (Lonicera japonica Thunb.) under iso-osmotic salt and drought stresses. Environ. Exp. Bot. 2021;182:104313. doi: 10.1016/j.envexpbot.2020.104313. [DOI] [Google Scholar]
- 16.Hasanuzzaman M., Nahar K., Rahman A., Al-Mahmud J., Alharby H., Fujita M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018;13:203–212. doi: 10.1080/17429145.2018.1458913. [DOI] [Google Scholar]
- 17.Zhang S., Xu X., Sun Y., Zhang J., Li C. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018;17:336–347. doi: 10.1016/S2095-3119(17)61758-1. [DOI] [Google Scholar]
- 18.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 19.You J., Chan Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denaxa N.K., Damvakaris T., Roussos P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020;259:108812. doi: 10.1016/j.scienta.2019.108812. [DOI] [Google Scholar]
- 21.González-Villagra J., Rodrigues-Salvador A., Nunes-Nesi A., Cohen J.D., Reyes-Díaz M.M. Age-related mechanism and its relationship with secondary metabolism and abscisic acid in Aristotelia chilensis plants subjected to drought stress. Plant Physiol. Biochem. 2018;124:136–145. doi: 10.1016/j.plaphy.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., Zhang L., Dong F., Gao J., Galbraith D.W., Song C.P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida R., Hobo T., Ichimura K., Mizoguchi T., Takahashi F., Aronso J., Ecker J.R., Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- 24.Singh D., Laxmi A. Transcriptional regulation of drought responses: A tortuous network of transcriptional factors. Front. Plant Sci. 2015;6:895. doi: 10.3389/fpls.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guajardo E., Correa J.A., Contreras-Porcia L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta. 2016;243:767–781. doi: 10.1007/s00425-015-2438-6. [DOI] [PubMed] [Google Scholar]
- 26.González-Villagra J., Cohen J.D., Reyes-Díaz M.M. Abscisic acid is involved in phenolic compounds biosynthesis, mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol. Plant. 2019;165:855–866. doi: 10.1111/ppl.12789. [DOI] [PubMed] [Google Scholar]
- 27.Mittler R., Vanderauwera S., Golley M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Silva E.N., Silveira J., Aragao R.M., Vieira C.F., Carvalho F. Photosynthesis impairment and oxidative stress in Jatropha curcas exposed to drought are partially dependent on decreased catalase activity. Acta Physiol. Plant. 2019;41:4. doi: 10.1007/s11738-018-2794-5. [DOI] [Google Scholar]
- 29.Saed-Moucheshi A., Sohrabi F., Fasihfar E., Baniasadi F., Riasat M., Mozafari A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021;21:148. doi: 10.1186/s12870-021-02919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice-Evans C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 31.Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010;30:161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- 32.Nakabayashi R., Yonekura-Sakakibara K., Urano K., Suzuki M., Yamada Y., Nishizawa T., Matsuda F., Kojima M., Sakakibara H., Shinozaki K., et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014;77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi F., Kuromori T., Urano K., Yamaguchi-Shinozaki K., Shinozaki K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020;11:556972. doi: 10.3389/fpls.2020.556972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durrant W.E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 35.Zeier J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 2021;62:102050. doi: 10.1016/j.pbi.2021.102050. [DOI] [PubMed] [Google Scholar]
- 36.Khodary S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004;6:5–8. [Google Scholar]
- 37.Nazar R., Umar S., Khan N.A., Sareer O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015;98:84–94. doi: 10.1016/j.sajb.2015.02.005. [DOI] [Google Scholar]
- 38.Brito C., Dinis L.T., Meijón M., Ferreira H., Pinto G., Moutinho-Pereira J., Correia C. Salicylic acid modulates olive tree physiological and growth responses to drought and re-watering events in a dose dependent manner. J. Plant Physiol. 2018;230:21–32. doi: 10.1016/j.jplph.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Khan F.S., Gan Z.M., Li E.Q., Ren M.K., Hu C.G., Zhang J.Z. Transcriptomic and physiological analysis reveals interplay between salicylic acid and drought stress in citrus tree floral initiation. Planta. 2022;255:24. doi: 10.1007/s00425-021-03801-2. [DOI] [PubMed] [Google Scholar]
- 40.Khan M.I., Fatma M., Per T.S., Anjum N.A., Khan N. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant. Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z.L., Li X.M., Zhang L.H. Effect of salicylic acid pretreatment on drought stress response of zoysiagrass (Zoysia japonica). Russ. J. Plant Physiol. 2014;61:619–625. [Google Scholar]
- 42.Zafar Z., Rasheed F., Atif R.M., Javed M.A., Maqsood M., Gailing O. Foliar application of salicylic acid improves water stress tolerance in Conocarpus erectus L. and Populus deltoides L. saplings: Evidence from morphological, physiological, and biochemical changes. Plants. 2021;10:1242. doi: 10.3390/plants10061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saheri F., Barzin G., Pishkar L., Akbar-Boojar M.M., Babaeekhou L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia. 2020;75:2189–2200. doi: 10.2478/s11756-020-00571-2. [DOI] [Google Scholar]
- 44.Hoffman A. Flora Silvestre de Chile, Zona Araucana. 5th ed. Fundación Claudio Gay; Santiago, Chile: 2005. [Google Scholar]
- 45.Fredes C., Montenegro G., Zoffoli J., Robert P. Polyphenol content and antioxidant activity of maqui (Aristotelia chilensis Molina Stuntz) during fruit development and maturation in central Chile. Chil. J. Agric. Res. 2012;72:582–589. doi: 10.4067/S0718-58392012000400019. [DOI] [Google Scholar]
- 46.Céspedes C., El-Hafidi M., Pavon N., Alarcon J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008;107:820–829. doi: 10.1016/j.foodchem.2007.08.092. [DOI] [Google Scholar]
- 47.Fredes C., Yousef G., Robert P., Grace M., Lila M.A., Gómez M., Gebauer M., Montenegro G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014;94:2639–2648. doi: 10.1002/jsfa.6602. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez L., Trostchansky A., Wood I., Mastrogiovanni M., Vogel H., González B., Maróstica M., Fuentes E., Palomo I. Antiplatelet activity and chemical analysis of leaf and fruit extracts from Aristotelia chilensis. PLoS ONE. 2021;16:e0250852. doi: 10.1371/journal.pone.0250852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel H., Peñailillo P., Doll U., Contreras G., Catenacci G., González B. Maqui (Aristotelia chilensis): Morpho-phenological characterization to design high-yielding cultivation techniques. J. Appl. Res. Med. Arom. Plants. 2014;1:123–133. doi: 10.1016/j.jarmap.2014.09.001. [DOI] [Google Scholar]
- 50.Bastías A., Correa F., Rojas P., Almada R., Muñoz C., Sagredo B. Identification and characterization of microsattelite Loci in maqui (Aristotelia chilensis [Molina] Stuntz) using Next-Generation Sequencing (NGS) PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0159825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuentealba-Sandoval V., Fisher S., Pinto A.A., Bastías R., Peña-Rojas K. Maqui (Aristotelia chilensis (Mol.) Stuntz), towards sustainable canopy management: A review. Ind. Crops Prod. 2021;170:113735. doi: 10.1016/j.indcrop.2021.113735. [DOI] [Google Scholar]
- 52.Gupta A., Rico-Medina A., Caño-Delgado A.I. The physiology of plant responses to drought. Science. 2020;17:266–269. doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- 53.Seleiman M.F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., Dindaroglu T., Abdul-Wajid H.H., Battaglia M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10:259. doi: 10.3390/plants10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyashita K., Tanakamaru S., Maitani T., Kimura K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005;53:205–214. doi: 10.1016/j.envexpbot.2004.03.015. [DOI] [Google Scholar]
- 55.Lobos T.E., Retamales J.B., Ortega-Farías S., Hanson E., López-Olivari R., Mora M.L. Regulated deficit irrigation effects on physiological parameters, yield, fruit quality and antioxidants of Vaccinium corymbosum plants cv. Brigitta. Irrig. Sci. 2018;36:49–60. doi: 10.1007/s00271-017-0564-6. [DOI] [Google Scholar]
- 56.Bhusal N., Han S.G., Yoon T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.) Sci. Hortic. 2019;246:535–543. doi: 10.1016/j.scienta.2018.11.021. [DOI] [Google Scholar]
- 57.Zahedi S.M., Hosseini M., Meybodi N., Abadía J., Germ M., Gholami R., Abdelrahman M. Evaluation of drought tolerance in three commercial pomegranate cultivars using photosynthetic pigments, yield parameters and biochemical traits as biomarkers. Agric. Water Manag. 2022;261:107357. doi: 10.1016/j.agwat.2021.107357. [DOI] [Google Scholar]
- 58.Flexas J., Medrano H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitation revisited. Ann. Bot. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galmés J., Medrano H., Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- 60.Woolfenden H.C., Baillie A.L., Gray J.E., Hobbs J.K., Morris R.J., Fleming A.J. Models and mechanisms of stomatal mechanics. Trends Plant Sci. 2018;23:822–832. doi: 10.1016/j.tplants.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Cruz de Carvalho M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohi-Ud-Din M., Talukder D., Rohman M., Ahmed J.U., Jagadish S.V.K., Islam T., Hasanuzzaman M. Exogenous Application of methyl jasmonate and salicylic acid mitigates drought-induced oxidative damages in french bean (Phaseolus vulgaris L.) Plants. 2021;10:2066. doi: 10.3390/plants10102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shemi R., Wang R., Gheith E.S.M.S., Hussain H.A., Hussain S., Irfan M., Cholidah L., Zhang K., Zhang S., Wang L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021;11:3195. doi: 10.1038/s41598-021-82264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khalvandi M., Siosemardeh A., Rooji E., Keramati S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon. 2021;7:e05908. doi: 10.1016/j.heliyon.2021.e05908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao R.X., Xin L.F., Guo J.M., Zheng H.F., Mao J., Han X.P., Jia L., Jia S.J., Du C.G., Song R., et al. Salicylic acid-induced photosynthetic adaptability of Zea mays L. to polyethylene glycol-simulated water deficit is associated with nitric oxide signaling. Photosynthetica. 2018;56:1370–1377. doi: 10.1007/s11099-018-0850-4. [DOI] [Google Scholar]
- 67.Habibi G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012;56:57–63. [Google Scholar]
- 68.Zamora O., Schulze S., Azoulay-Shemer T., Parik H., Unt J., Brosche M., Schroeder J., Yarmolinski D., Kollist H. Jasmonic acid and salicylic acid play minor roles in stomatal regulation by CO2, abscisic acid, darkness, vapor pressure deficit and ozone. Plant J. 2021;108:134–150. doi: 10.1111/tpj.15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dempsey D.A., Vlot A.C., Wildermuth M.C., Klessig D.F. Salicylic Acid biosynthesis and metabolism. Arab. Book. 2011;9:e0156. doi: 10.1199/tab.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefevere H., Bauters L., Gheysen G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020;11:338. doi: 10.3389/fpls.2020.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma M., Gupta S., Majumder B., Maurya V., Deeba F., Alam A., Pandey V. Proteomics unravel the regulating role of salicylic acid in soybean under yield limiting drought stress. Plant Physiol. Biochem. 2018;130:529–541. doi: 10.1016/j.plaphy.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Wang W., Zhang G., Yang S., Zhang J., Deng Y., Qi J., Wu J., Fu D., Wang W., Hao Q. Overexpression of isochorismate synthase enhances drought tolerance in barley. J. Plant Physiol. 2021;260:153404. doi: 10.1016/j.jplph.2021.153404. [DOI] [PubMed] [Google Scholar]
- 73.Giménez M.J., Serrano M., Valverde J.M., Martínez-Romero D., Castillo S., Valero D., Guillén F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017;97:1220–1228. doi: 10.1002/jsfa.7853. [DOI] [PubMed] [Google Scholar]
- 74.Iqbal N., Fatma M., Gautam H., Sehar Z., Rasheed F., Khan M., Sofo A., Khan N.A. Salicylic acid increases photosynthesis of drought grown mustard plants effectively with sufficient-N via regulation of ethylene, abscisic acid, and nitrogen-use efficiency. J. Plant Growth Regul. 2022 doi: 10.1007/s00344-021-10565-2. [DOI] [Google Scholar]
- 75.Hoffmann W.A., Poorter H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002;90:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Begg J.E., Turner N.C. Water potential gradients in field tobacco. Plant Physiol. 1970;46:343–346. doi: 10.1104/pp.46.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahimi A., Husseini S.M., Pooryoosef M., Fateh I. Variation of stem water potential, relative water content and SPAD under gradual drought stress and recovery in two medicinal species of Plantago ovate and P. psyllium. Plant Ecophysiol. 2010;2:53–60. [Google Scholar]
- 78.Reyes-Díaz M., Meriño-Gergichevich C., Alarcón E., Alberdi M., Horst W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.) J. Soil Sci. Plant Nutr. 2011;11:59–78. doi: 10.4067/S0718-95162011000400005. [DOI] [Google Scholar]
- 79.Du Z., Bramalage W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992;40:1566–1570. doi: 10.1021/jf00021a018. [DOI] [Google Scholar]
- 80.Chinnici F., Bendini A., Gaiani A., Riponi C. Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004;52:4684–4689. doi: 10.1021/jf049770a. [DOI] [PubMed] [Google Scholar]
- 81.Singleton V., Rossi J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 82.Giannopolitis C., Ries S. Superoxide dismutases: Purification and quantitative relationship with-soluble protein in seedlings. Plant Physiol. 1977;59:315–318. doi: 10.1104/pp.59.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donahue J.L., Okpodu C.M., Cramer C.L., Grabau E.A., Alscher R.G. Responses of antioxidants to paraquat in pea leaves (Relationships to Resistance) Plant Physiol. 1997;113:249–257. doi: 10.1104/pp.113.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 85.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the results section.