Abstract
There has been much effort to provide eco-friendly and biodegradable materials for the next generation of composite products owing to global environmental concerns and increased awareness of renewable green resources. This review article uniquely highlights the use of green composites from natural fiber, particularly with regard to the development and characterization of chitosan, natural-fiber-reinforced chitosan biopolymer, chitosan blends, and chitosan nanocomposites. Natural fiber composites have a number of advantages such as durability, low cost, low weight, high specific strength, non-abrasiveness, equitably good mechanical properties, environmental friendliness, and biodegradability. Findings revealed that chitosan is a natural fiber that falls to the animal fiber category. As it has a biomaterial form, chitosan can be presented as hydrogels, sponges, film, and porous membrane. There are different processing methods in the preparation of chitosan composites such as solution and solvent casting, dipping and spray coating, freeze casting and drying, layer-by-layer preparation, and extrusion. It was also reported that the developed chitosan-based composites possess high thermal stability, as well as good chemical and physical properties. In these regards, chitosan-based “green” composites have wide applicability and potential in the industry of biomedicine, cosmetology, papermaking, wastewater treatment, agriculture, and pharmaceuticals.
Keywords: natural fiber, chitosan, chitosan blends, chitosan nanocomposites, cellulose, nanocellulose
1. Introduction
Nowadays, ecological concerns have resulted in renewed interest in natural materials. Recyclability and environmental safety are becoming increasingly important in the consideration of a better future in sustainability [1,2,3,4]. Hence, the need for more versatile polymer-based materials has led to increasing interest in polymer composites filled with natural, organic fillers, for example, fillers that are biodegradable and come from renewable sources [5,6,7,8]. Day by day, biomaterials are crucial in the development of a sustainable environment. Even though biomaterials are newly in development for the delivery of drugs, tissue engineering, and medical diagnostics, but there has been good improvement for both physical and chemical methods that can manage biological responses [9].
The most suitable definition for a biomaterial is any substance that is not a drug or synthetic substance that can be used any time to partially or totally replace any tissue or other parts of body, which can improve the quality of life for an individual [10]. There are four different types of biomaterials that are commonly known in industries, which are polymers, metals, ceramics, and composites.
In general, chitosan is a sugar that is contained in the hard outer skeleton of shellfish such as crab, lobster, and shrimp, which is used in medication [11,12]. In fact, chitosan is a derivative from chitin, and it is one of the most bountiful natural biopolymers as opposed to cellulose [10]. The composition of chitin in seashell waste consists of proteins (30–40%) calcium carbonate and calcium phosphate (30–50%), and chitin (20–30%) [10,13]. This review paper discusses green composites of natural fibers in detail and also discusses the development and characterization of chitosan, natural-fiber-reinforced chitosan biopolymers, chitosan blends, as well as chitosan nanocomposites.
2. Natural Fiber
Natural fiber is a lignocellulosic material which is mainly composed of cellulose, hemicelluloses, lignin, pectin, wax, ash, and moisture [14,15,16]. It is important to understand the composition because the mechanical properties of natural fibers are dependent on it, as stated by Farok et al. [17]. From previous study, Sinha et al. [18] stated that natural fibers are hair-like or thread-like naturally existing substances with a high aspect ratio, and the application of these fibers is in high demand due to their advantages such as low cost, low weight, and biodegradability. However, natural fibers also come with drawbacks, which includes their hydrophilic nature [19,20,21,22]. The hydroxyl group will absorb the moisture and prevent from damage and degradation [23,24,25,26,27,28].
According to Sinha et al. [18], natural fiber is classified into three categories, which are animal, vegetable, and mineral fibers. Natural fibers such as abaca, cotton, jute, flax, hemp, and coir are deployed for different industrial applications. Recently, there has been in rapid growth in research and innovation in natural fiber composites in multidisciplinary areas [29,30,31,32,33]. The characteristics of natural fiber composites are durability, low cost, low weight, high specific strength, non-abrasiveness, equitably good mechanical properties, eco-friendliness, and biodegradability [6,29,34,35]. These materials possess promising potential for a wide range of industries, including medical, structural and construction, packaging, military, aerospace, and automobile industries [36,37,38,39,40,41,42,43,44,45,46,47].
2.1. Types of Green Composites and Chemical Composition of Natural Fibers
Green composites are a type of biocomposites in which natural fibers are used to strengthen a bio-based polymer matrix [48]. Animal fiber is extracted from the fur of animals, mineral fiber is a naturally occurring fiber or modified fiber produced from minerals, while the main content of plant fiber is cellulose [49,50]. Examples of these classifications are shown in Table 1.
Table 1.
Examples of natural fibers [49].
| Natural Fibers | Example |
|---|---|
| Mineral | Asbestos |
| Fibrous brucite | |
| Wollastonite | |
| Plant | Bast
|
Leaf
| |
Fruit
| |
Grass
| |
Straw
| |
| Wood pulp | |
| Animal | Silk |
| Wool | |
| Feathers |
Natural fiber manufacturing is expanding worldwide as the product base expands. Table 2 lists the major manufacturers as well as the yearly outputs of these fibers across the globe [51]. Chemical composition refers to the arrangement of particles and the types and ratios of atoms in chemical substances where the composition is different when there is an addition or subtraction of chemicals, and when the ratios changes. Table 3 shows the chemical composition of natural fibers. The common chemical constituents in green or natural fibers are cellulose, hemicelluloses, lignin, pectin, and wax. All natural fibers have similar components but different compositions, which make them behave differently [52,53,54,55].
Table 2.
Production amount of natural fiber produced [56].
| Fiber | Producer | Production Amount (×103 ton) |
|---|---|---|
| Abaca | Philippines (85%), Ecuador | 70 |
| Alpaca | Peru, Bolivia, Chile | 7 |
| Angora wool | China, Argentina, Chile, Czech Republic, Hungary, France | 3 |
| Bagasse | Brazil, China, India, Thailand, Australia, USA | 75,000 |
| Bamboo | China, Japan, India, Chile, Ecuador, Indonesia, Myanmar, Nigeria, Sri Lanka, Philippines, Pakistan | 30,000 |
| Camel hair | China, Mongolia, Afghanistan, Iran | 2 |
| Cashmere wool | China, Mongolia, Australia, India, Iran, Pakistan, New Zealand, Turkey, USA | 20 |
| Coir | India, Sri Lanka, Thailand, Vietnam, Philippines, Indonesia, Brazil | 1200 |
| Cotton | China, Brazil, India, Pakistan, USA, Uzbekistan, Turkey | 25,000 |
| Flax | France, Belgium, Netherland, Poland, Russian Federation, China | 830 |
| Hemp | China (80%), Chile, France, Germany, UK | 214 |
| Jute | India (60%), Bangladesh, Myanmar, Nepal | 3450 |
| Kapok | Philippine, Malaysia, China, South America, Indonesia, Thailand | 101 |
| Kenaf | India (45%), China, Malaysia, USA, Mexico, Thailand, Vietnam | 970 |
| Mohair wool | South Africa, USA | 5 |
| Ramie | China, Brazil, Lao PDR, Philippines, India | 280 |
| Silk | China (70%), Brazil, Bulgaria, Egypt, Madagascar, India, Thailand, Vietnam, Uzbekistan, Turkmenistan | 150 |
| Sisal | Brazil (40%), Kenya, Tanzania, China, Cuba, Haiti, Madagascar, Mexico, Sri Lanka, India | 378 |
| Wool | Australia, Argentina, China, Iran, New Zealand, Russia, UK, Uruguay | 2100 |
Table 3.
Chemical composition of lignocellulosic fiber [18].
| Type of Fiber | Cellulose | Hemi Cellulose | Lignin | Pectin | Wax | Ash | Moisture | Others |
|---|---|---|---|---|---|---|---|---|
| Abaca | 56–64 | 25–29 | 11–14 | - | - | - | - | - |
| Jute | 64.4 | 12 | 0.2 | 11.8 | 0.5 | 0.5–2.1 | 10 | - |
| Sisal | 65.8 | 12 | 0.8 | 9.9 | 1.2 | 0.3 | 10 | - |
| Kenaf | 44.4 | - | 20.1 | - | - | 4.6 | - | - |
| Coconut | 37–43 | 24–28 | 26–28 | - | - | - | - | 7 |
| Bamboo | 78.83 | - | 10.15 | - | - | - | - | - |
According to Table 3, different types of fiber consist of different compositions of cellulose, hemicellulose, lignin, pectin, wax, ash, moisture, and other contents. For cellulose, bamboo [57] contains the highest composition, which is 78.83%, followed by sisal, which is 65.8%, and the lowest one is coconut, which only contains 37–43% cellulose. For hemicellulose, coconut and abaca are the highest, the percentages of which are around 24% to 29%. Coconut also has the highest content of lignin, up until 28%, and is followed by kenaf, which is 20.1%, and the lowest is jute, which is only 0,2%. Meanwhile for pectin, wax, ash, moisture, and others, there are only a few fibers that contain these compositions, such as jute, sisal, kenaf, and coconut [58,59].
2.2. Mechanical Properties of Green Fibers
Generally, mechanical properties give meaning to the physical properties which the materials show as forces are applied; examples of these properties are elasticity, strength of tensile, elongation, hardness, and fatigue limit. Nowadays, natural or green fibers are widely used for production in a few types of applications, including automotive, aircraft, construction, and building [29,60,61,62,63]. Thus, mechanical properties of natural fibers are crucial so that benefits can be utilized as much as possible. As well as advantages, natural fibers also have major drawbacks such as their hydrophilic nature, which means they have a high degree of moisture absorption and poor dimensional stability. Few studies have comprehensively discussed the limitations of natural fiber composites such as compatibility with polymers, low thermal properties, as well as irregular properties [64,65,66]. Table 4 shows the mechanical and physical properties of natural fibers.
Table 4.
Physical and mechanical properties of lignocellulosic fibers [18].
| Type of Fiber | Diameter |
Density (g/cm3) |
Tensile Strength (MPa) |
Young’s Modulus (GPa) |
|---|---|---|---|---|
| Abaca | 250–300 | 1.5 | 717 | 18.6 |
| Jute | 250–2500 | 1.3–1.49 | 393–800 | 13–26.5 |
| Sisal | 205–230 | 1.41 | 350–370 | 12.8 |
| Kenaf | 83.5 | 1.2 | 282.60 | 7.13 |
| Coconut | 396.98 | 1.2 | 140–225 | 3–5 |
| Bamboo | - | 1.2–1.5 | 500–575 | 27–40 |
The physical and mechanical properties of natural fibers consist of the diameter of fiber, density of the fibers, tensile strength, and the Young’s modulus value of the fiber. For diameter [67], jute fiber has the greatest diameter, which is around 250 to 2500 . This is followed by coconut. which has a value of 396.98, and kenaf has the lowest value, which is 83.5 . Abaca, bamboo and jute fibers have the highest density, which is around 1.5 g/cm3. For the tensile strength which represents the resistance of fibers, jute’s tensile strength can reach 800 MPa. This is followed by abaca and bamboo, with values of 717 MPa and 500–575 MPa, respectively [68]. The Young’s Modulus represents the elasticity of fiber, and bamboo has the highest value of Young’s Modulus, with the value of 27–40 GPa [69]. Since the tensile strength is higher, it used as reinforcement in order to improve or upgrade the mechanical properties of a composite such as cement mortar and polymer-reinforced composites [70,71,72]. Abaca fiber also has higher tensile strength, which is about 717 MPa, and its strength is good for any natural fibers. Thus, it is usually used in the production of the exterior of passenger vehicles, where it can resist stone strikes [73,74] However, abaca fibers have not been fully explored to their fullest potential as a composite [18].
3. Chitosan
3.1. Advantages and Disadvantages of Chitosan
As a biomaterial, chitosan provides many advantages and disadvantages. As a biomaterial form, chitosan can be made into a few forms, such as hydrogels, sponges, films which appear in 3D forms, and also porous membrane which appears in 2D form, and each of them have specific applications in industries [75,76]. The advantages and disadvantages for each of the type are shown in Table 5.
Table 5.
Advantages and disadvantages of biomaterials for chitosan [75].
| Type | Descriptions | Advantages | Disadvantages |
|---|---|---|---|
| Hydrogels (3D) |
|
|
|
| Sponges (3D) |
|
|
|
| Films (2D) |
|
|
|
| Porous Membrane (2D) |
|
|
|
3.2. Chemical and Physical properties of Chitosan
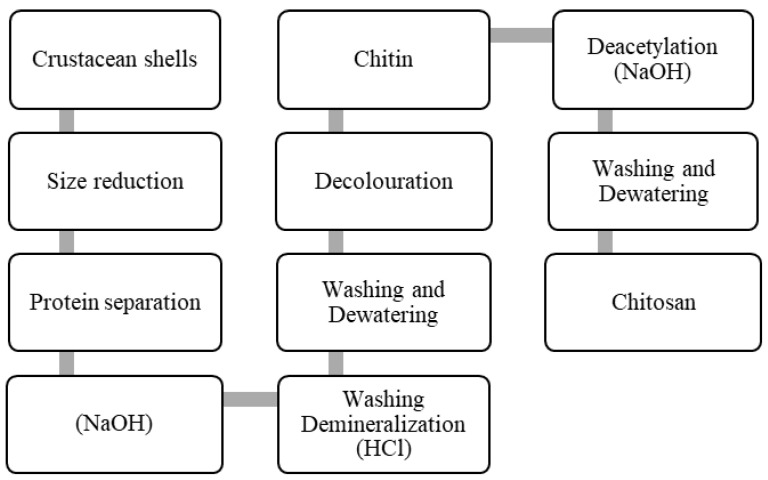
After cellulose, chitin is the second most ubiquitous natural polysaccharide on Earth and is composed of β(1→4)-linked 2-acetamido-2-deoxy-β-D-glucose1 (N-acetylglucosamine) (Figure 1). It is often considered as a cellulose derivative, even though it does not occur in organisms producing cellulose. It is structurally identical to cellulose, but it has acetamide groups (−NHCOCH3) at the C-2 positions. Similarly, the principle derivative of chitin, chitosan, is a linear polymer of α (1→4)-linked 2-amino-2-deoxy-β-D-glucopyranose and is easily derived by N-deacetylation, to a varying extent that is characterized by the degree of deacetylation, and is consequently a copolymer of N-acetylglucosamine and glucosamine (Figure 2). Chitin is estimated to be produced annually almost as much as cellulose [77]. It has become of great interest not only as an under-utilized resource but also as a new functional biomaterial of high potential in various fields, and the recent progress in chitin chemistry is quite significant. The production of chitosan from crustacean shells obtained as a food industry waste is economically feasible, especially if it includes the recovery of carotenoids. Figure 3 displays the process of making chitosan from crustacean shells.
Figure 1.
Structure of chitin.
Figure 2.
Partially deacetylated chitin.
Figure 3.
Making of chitosan.
Generally, chitosan is a derivative from chitin, where a certain group of polymers deacetylated from chitin. Hence, chitin and chitosan are different based on the degree of deacetylation [78,79]. In addition, the production of chitosan is mainly based on a further reaction of chitin. Besides, the properties of every chitosan produced is different due to different raw materials [80,81]. However, the qualities can be measured using same properties such as viscosity, deacetylation, molecular weight, and polymorphous structure [82]. The physical properties of chitosan-based polymer are shown in Table 6.
Table 6.
Physical properties of chitosan-based polymer.
| Type of Chitosan-Based | Physical properties | Explanation | References |
|---|---|---|---|
| Chitosan—tapioca starch edible film |
|
|
[83] |
| Chitosan film—natural antioxidants |
|
|
[84] |
| Chitosan—green tea extract |
|
|
[85] |
3.3. Mechanical Properties of Chitosan
To comprehend the mechanical behavior of chitosan-based films, the mechanical properties of chitosan films must be investigated. The efficiency and integrity of the films are determined by their tensile strength and percentage elongation at break during preparation, use, and handling. Since chitin and chitosan have poor mechanical strength, it is necessary to change some of their properties so that they can be used as bioadhesives, nanocomposites, and waste materials that pollute the atmosphere [86]. Based on Table 7, the highest value of tensile strength is for chitosan—spirulina extract (21.24 MPa–29.65MPa) with percentage range between 2.5% and 50.0%. However, the higher elongation is for chitosan—graphene oxide, with 57.34% to 72.70% and less than 2%.
Table 7.
Mechanical properties of chitosan.
| Type | Percentage (%) | Elongation (%) | Tensile Strength (MPa) |
Young’s Modulus (GPa) |
References |
|---|---|---|---|---|---|
| Chitosan (CS) | 2.0–10.0 (CS) | - | 9.0–16.0 | 250–380 | [87] |
| Chitosan—antimicrobial | 2.0–10.0 (CS) | - | 14.0–18.0 | 150–440 | [87] |
| Chitosan—Spirulina Extract (SE) | 2.5–50.0 (SE) | 26.13–39.53 | 21.24–29.65 | - | [88] |
| Chitosan—graphene oxide (GO) | 0.0–2.0 (GO) | 57.34–72.70 | 6.99–15.32 | - | [89] |
| Chitosan—glycerol | 1.0–3.0 (CS) | 9.50–67.93 | 0.281–12.147 | - | [90] |
3.4. Thermal Properties of Chitosan
Chitosan exhibits a high sensitivity to numerous types of degradation, including thermodegradation. Thermal study revealed that this biopolymer cannot resist temperatures beyond 200–220 °C [91,92]. Figure 4 depicts the thermogravimetry (TG) curve of chitosan. In a chitosan polymer, there are two levels of degradation. Weight reduction in the first stage begins at 220 °C and progresses to 320 °C, with a 50 percent weight loss. The overall weight loss rate is measured by derivative equipment. At 295 °C, there is a reaction connected with the TG apparatus. The second stage achieves a mean temperature of 470 °C, with a weight loss of 40%. The activation energy of the degradation of chitosan was found to be 52.2 kJ/mol [93,94].
Figure 4.
Thermogravimetric curves for chitosan [95]. (Mostafa Amin, 2012).
4. Processing of Chitosan Green Composites
As chitosan is created from the derivation of chitin, the usual industrial process that are applied for the extraction of chitin involve three main steps; the deproteinization of raw material with the addition of alkaline solution, followed by demineralization through a treatment using acidic solution, and lastly the discoloration of product obtained through the treatment using alkaline solution [96]. There are different ways of converting chitin to chitosan, such as through an enzymatic method or a process of chemical conversion, but between these two methods, the chemical process is more preferable since the cost is a lot cheaper compared to the other method and also when considering the production capacity of chitosan [96,97].
According to Muxika et al. [96], chitosan is a substance or copolymer that comes from deacetylation using alkaline from chitin, where it formed by D-glucosamine and N-acetyl-D-glucosamine units, which are also linked by ß-1, 4 glycosidic linkages, and the solubility of chitosan allows it to be produced in various forms such as films, nanofibers, hydrogels, or pastes. In the biomaterial industry, chitosan production provides many applications based on the structure and forms [98,99]. There are various types of chitosan processing methods, such as solution casting, dipping and spray coating, compression molding, freeze casting and drying, blending, layer-by-layer processing, and also, rapid prototyping. A few of the techniques or processes are further discussed.
4.1. Solution and Solvent Casting
The solution casting method is the most commonly known method for chitosan processing. This process involves an acidic solution in which the chitosan powder is dissolved and poured into a Petri dish (Figure 5). This process needs to be carried out in dry conditions at room temperature or in an oven at a certain temperature until the film is completely dry and peels off the mold by itself [96]. Through the application of multisolution coatings on glass substrate, this technique is not only appropriate for the production of single-layered film or membrane, but also for the fabrication of multilayered, dense films. The addition of highly volatile solvents to the casting solution followed by an evaporation stage before phase inversion in a non-solvent immersion may aid in the development of a top dense layer. However, even though this method is commonly used as a method or technique to prepare chitosan films at a small scale, further research must be done to analyze the possibility for the manufacturing scale production of chitosan [100]. For the production of different kinds of nanocomposite membranes, the solution casting approach has been widely researched [101].
Figure 5.
General steps in the (a) solution casting and (b) solvent casting method for composite fabrication [102,103].
4.2. Dipping and Spray Coating
In industry, coating is usually applied in edible active packaging systems, where the system must preserve the quality of products and extend the lifetime of products [104,105]. According to Muxika et al. [96], chitosan edible coating is used and tested on vegetables and fruits, and there are two ways to apply the coating: dipping and spraying. Dipping is introduced in the food product through acidic solution forming and spraying, introducing the method of pulverizing the film-forming solution [106] (Figure 6). There is difference between them, as they are affected by coating properties or thickness. The formation of polymeric coatings via spraying systems is influenced by factors such as drying time, temperature, and technique, due to the protonation of chitosan amine groups in acidic environments, which gives chitosan caustic properties that eventually decreases certain sensory qualities [107]. However, even dipping and spraying have their own advantages in controlling the quality of food and bacteria growth, but there are still drawbacks present, and these must be considered before implementing dipping and spray coating [96].
Figure 6.
(i) Dipping and (ii) spray coating method [16].
4.3. Freeze Casting and Drying
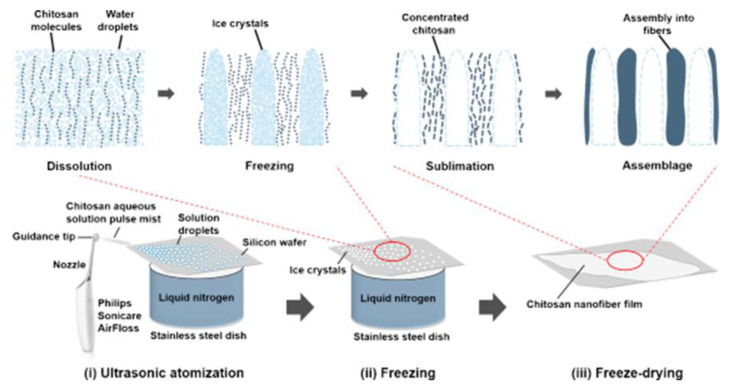
Freeze casting, also known as ice templating, is one of the most common methods that is used in tissue engineering applications in order to obtain chitosan scaffolds. The structure obtained from this technique gives a proper environment for the attachment of cells, growth, and the final form of new production tissue [108]. In addition, this method was created to manipulate the degree of porosity, pore size, pore shape, and pore orientation to modify the pore structure of porous materials [109]. According to Muxika et al. [96], the freeze casting process involves dissolution in a small quantity of acidic aqueous solution followed by freezing the solution in a copper or stainless-steel mold which cools down the solution in a very short time and results in the formation of two distinct phases: the frozen solvent and polymer phase. Wang and Wakisaka [110] used this method in combination with ultrasonic atomization in the production of uniformly oriented chitosan nanofibers (Figure 7). The good mixing of chitosan powder, formic acid, acetic acid, and/or l-lactic acid in distilled water followed by ultrasonic atomization–freeze casting and drying resulted in excellent fiber formability, as well as the minimization of volatile organic solvent use, which made the obtained chitosan nanofibers safe, environmentally friendly and compatible. Because it is a flexible process for manufacturing porous materials, that has attracted a lot of interest in recent years [111,112,113].
Figure 7.
The processes involved in the ultrasonic atomization–freeze casting of chitosan nanofibers [110].
4.4. Layer-by-Layer
The layer-by-layer (LbL) deposition technique, which involves building up successive layers of oppositely charged species, has been extensively utilized for producing multilayer, thin films. The electrostatic force of attraction, hydrogen bonding, and affinity between synthetic polymers, proteins, polysaccharides, and other molecules are used in the layer-by-layer technique. According to Costa and Mano [114], because of its cationic character, chitosan has been utilized to create LbL-based films and coatings. Figure 8 shows general processes involved in the LbL method. In this method, substrate is immersed in the chitosan solution, resulting in the formation of a very thin layer on the surface. Numerous properties of the multifunctional chitosan-based films produced can be controlled, namely the thickness difference, permeability to gases and glucose, film strength, as well as film flammability. The characteristics of these films are determined by a few factors such as pH, type of chemical, and ionic cross-linking during deposition, which may influence mechanical film performance [115,116].
Figure 8.
Layer-by-layer technique used in the production of edible coatings based on chitosan, pullulan, linseed, nopal cactus, and aloe mucilage [117].
4.5. Extrusion
Biodegradable, chitosan-based packaging has also been extensively produced via extrusion due to it having a higher productivity and requiring less space. One of the most common extrusion methods is melt extrusion, which produces a film with excellent mechanical characteristics and thermal stability (Figure 9). Basically, this method can produce a final product according to a specific formulation and composition based on requirements. The process involved in this method is simple and involves a continuous flow, where the materials blend in the mixer, followed by an extruder to produce a pellet. The pellets are then used in the production of a film through the twin-screw extruder, usually for medical [118,119], 3D-printing [120], and packaging applications [121,122].
Figure 9.
Extrusion method of chitosan film production [123].
5. Mechanical Properties of Chitosan-Based Green Composites
Over the past decade, significant efforts have been made in the development of green composites by utilizing chitosan. This advancement opened the path for future natural-fiber composites (NFCs) with improved mechanical characteristics to be developed by engineers and researchers. Natural fibers such as plant fibers offer a number of benefits, including low weight, cheap cost, and biodegradability. The mechanical characteristics of chitosan-based green composites are important to allow them to be used to their full potential in specific applications. Table 8 lists the mechanical properties of chitosan-based green composites from varying source of fibers.
Table 8.
Mechanical properties of chitosan-based green composites.
| Polymers | Fibers | Processing Technique | Mechanical Properties | References | |
|---|---|---|---|---|---|
| Tensile Strength | Tensile Modulus | ||||
| Chitosan | Cellulose-modified | Ionic liquid treatment | 22–80 MPa | 236–3316 MPa | [91] |
| Chitosan | Bamboo charcoal | Blending | 25–75 MPa | 4600–5400 MPa | [124] |
| Chitosan | Modified bamboo charcoal | Blending | 75–110 MPa | 5400–7000 MPa | [124] |
| Chitosan | Thyme | Dissolution | 5.59–12.2 MPa | - | [125] |
| Chitosan | Clove | Dissolution | 6.54–12.2 MPa | - | [125] |
| Chitosan | Cinnamon | Dissolution | 12.2–21.35 MPa | - | [125] |
| Chitosan | PLA/CS | Solution casting | 30.95 MPa | 4.10 MPa | [126] |
| Chitosan | PLA/CS/ENR | Solution casting | 10.0 MPa | 4.70 MPa | [126] |
6. Thermal Properties of Chitosan-Based Green Composites
The effect of filler form on composite thermal stability was investigated using a thermogravimetric analyzer (Jupiter STA 449F3, Netzsch). With an initial sample weight of approximately 5 mg, measurements were taken in a nitrogen atmosphere (flow rate 20 cm3 min−1) at a heating rate of 10 K min−1 over a temperature range of 30–1100 °C. Previous research by Grząbka-Zasadzińska et al. [91] used the solvent casting technique to make chitosan/nanocrystalline cellulose composites. To begin, chitosan was dissolved in CH3COOH at a concentration of 2% (v/v). Next, various amounts of nanocrystalline celluloses were applied to chitosan to produce mixtures of CNC mass/mass ratios of 1, 3, and 5% (in comparison to the dry mass of chitosan). Composites of 5% cellulose I and cellulose II were also made as a reference. All of the mixtures were ultrasonically homogenized for 20 min, then added to Petri dishes and dried for 12 h at 35 °C.
The samples were named using the following convention: CHT stands for chitosan, the number represents the percentage of filler added, and C I, C II, CNC I, or CNC II represent the filler form. CHT/5 CNC II, for example, denotes chitosan containing 5% nanocrystalline cellulose II. Thermogravimetric (TG) curves of chitosan and its composites with micro- and nanometric celluloses are given in Figure 10.
Figure 10.
Thermogravimetric curves for chitosan and its composites [91].
The TG findings indicate that the sample containing CNC II has greater thermal stability than the film containing CNC I. However, CNC II composites proved to be more thermally stable than C II composites. A similar trend was observed in composites dependent on (nano)cellulose I, but only when high mass loss was taken into account. In terms of thermal stability, it appears that not only polymorphic variation but also filler size is essential.
7. Chitosan-Blend Composites
Despite several benefits of chitosan such as biodegradability, lack of toxicity, and abundance in nature, chitosan-based materials have poor water barrier capabilities owing to their hydrophilic nature, which mainly impacts their mechanical, gas permeability, and thermal properties. Thus, one method for reducing the hydrophilic nature of chitosan is to blend biopolymers to make composites. Blending chitosan with other polysaccharides was found to generate improved barrier, mechanical characteristics, and aesthetic composite properties [127]. This section provides an overview of the research on chitosan-blend composites divided by property characterization, namely mechanical and thermal properties. Table 9 summarizes some of the mechanical properties of chitosan-blend composites for many applications, including packaging and medical applications.
Table 9.
Mechanical properties of chitosan-blend composites.
| Polymers | Polymers Blend | Processing Technique | Mechanical Properties | References | |
|---|---|---|---|---|---|
| Tensile Strength | Tensile Modulus | ||||
| Chitosan | Polyhydroxybutyrate | Melting | 7.5–11 MPa | 1044–2499 MPa | [128] |
| Chitosan | Deacetylated chitosan | Gel spinning | 59.8–117.1 MPa | 2.1–4.1 GPa | [128] |
| Chitosan | CMC-CH-OL | Magnetically stirring | 7.0 ± 0.8 MPa | - | [129] |
| Chitosan | CMC-CH-OL-CEO | Magnetically Stirring | 4.8 ± 0.9 MPa | - | [129] |
| Chitosan | Carbon nanotubes | Magnetically Stirring | - | - | [130] |
| Chitosan | Cellulose nano whiskers | Solution casting | 21.6–31.25 MPa | 399.5–535.76 MPa | [131] |
| Chitosan | Cellulose nano whiskers | Solution casting | 21.6–38.25 MPa | 399.5–644 MPa | [131] |
| Chitosan | Glycerol-free | Solution casting | 28–44.5 MPa | 1.05–1.15 GPa | [132] |
| Chitosan | Glycerol-plasticized | Solution casting | 22.5–33 MPa | 0.6–1.0 GPa | [132] |
| Chitosan | Nano diamond (4.5–1%) | Solution casting | 100 ± 2.5 MPa | 3314 ± 416 MPa | [133] |
| Chitosan | Biogenic silver nanoparticles | Ultra sonication | 65.04 ± 1.46 MPa | - | [134] |
| Chitosan | Poly vinyl alcohol (PVA) | Film-forming dispersions and casting | 24–43 MPa | - | [135] |
| Low and high molecular weight (LMw/HMw) chitosan | Glycerol | Solution casting | LMw CS: 31.89–61.82 MPa HMw CS: 23.87–55.83 MPa |
- | [106] |
7.1. Chitosan-Blend Composites
As reported by Rajan et al. [126], the addition of a high amount of chitosan lowers the crystallinity of Poly(hydroxybutyrate) (PHB) composites, thus decreasing its thermal stability. Similarly, the tensile and impact strengths of composites decrease with the addition of chitosan. On the other hand, some essential oils (such as cinnamon and ginger) can enhance the characteristics of bio-based films of chitosan–carboxymethyl cellulose [128]. These green materials could be used to improve food safety and quality by preserving it.
Thou et al. [129] also pointed out that the mechanical and surface properties of chitosan can be improved by blending it with some carbonaceous materials such as cellulose and multiwall carbon nanotubes [130]. The study also showed that the composites experienced a significant improvement in thermal stability by delaying the degradation time. Cobos et al. [131] synthesized chitosan/graphene (CS/GS) nanocomposites and evaluated their thermal and mechanical properties. CS/GS nanocomposites showed an enhancement in mechanical and thermal properties when compared with native chitosan. This might be due to strong interaction between both polymers through covalent/non-covalent functionalization [136,137,138]. Furthermore, a high weight percent of chitosan matrix dissolved in acetic acid, on the other hand, was able to contribute to the integration of a large quantity of nanofiller reinforcement [139]. Delavar and Shojaei [132] reported that nanodiamond/chitosan composites (ND/CS) possessed good mechanical properties (20% better than unmodified materials) through the dispersion of nanodiamond in chitosan matrix and strong intermolecular interactions between them. Therefore, chitosan-blend composites could be an option to be used industrial applications.
7.2. Thermal Properties of Chitosan-Blend Composite
The effect of cinnamon and ginger essential oils (EO) on thermal properties of chitosan–carboxymethyl cellulose films emulsified with oleic acid was studied by Noshirvani et al. [129]. Various cinnamon and ginger EO levels were used in the production of biobased films. From the thermogravimetric results, it was found that the combination of oleic acid and EO causes a reduction in thermal stability; the thermal stability decreased as the volume of EO increased, as shown in Figure 11. This is due to the fact that the polymer network’s composition changes, resulting in the formation of a discontinuous structure. This leads to a reduction in the density of the framework and an expansion of free volume locations. Given the presence of EOs, this result may be ascribed to a potential link between cinnamaldehyde and chitosan, which was destroyed as a consequence of the second event.
Figure 11.
(a) TGA for chitosan–essential oil for the first event and (b) TGA for chitosan–essential oil and cinnamon essential oil for the second event [129].
The incorporation of chitosan into PVA films seems to be a viable approach for obtaining antibacterial and biodegradable food packaging [140]. Bonilla et al. [135] found that the thermal stability was increased in the chitosan blend films. From the TGA analysis, the addition of chitosan increased the Tmax of the blends, indicating that the PVA films were more thermally stable as a consequence of the chitosan addition. This is due to the fact that the thermal behavior of chitosan, and particularly its glass transition temperature (Tg), is higher. Furthermore, polymer interactions result in an increase in effective mean molecular weight, and therefore, Tg values as a consequence of hydrogen bond formation. The increase in Tg values in the blends also indicates that the two macromolecules are miscible. The excellent miscibility of the polymers has an impact on the PVA crystallization process. This decrease in crystallization or melting temperature is indicated the compatibility of the chitosan blend.
8. Chitosan Hybrid Composite
This section reviews and discusses the chitosan hybrid composite. The sources of hybrid materials could be synthetic fiber, natural fiber, clay, mineral, polymer, and nanomaterials.
8.1. Mechanical Properties of Chitosan Hybrid Composites
Table 10 shows the mechanical properties of chitosan hybrid composites. Arumugam et al. [141] investigated the hybridization of a glass fiber (GF)/sisal fiber (SF)/chitosan (CTS) hybrid composite for orthopedic bone fracture plate applications in future. The composites possessed high mechanical properties due to a unique sandwich structure. It exhibited the bending strength of 343 MPa, ultimate tensile strength of 146 MPa, and compressive strength of 380 MPa with a higher Young’s modulus in the bending tests (21.56 GPa) compared to the tensile (6646 MPa) and compressive modulus (2046 MPa). On the contrary, green composites of chitosan and calcium phosphate were hybridized and resulted in the significant reduction in strength and modulus [141]. The optimal composition (in terms of initial strength and degradation behavior) weight to volume ratio of chitosan/calcium phosphate was 10 wt/v%. In addition, a hybrid composite of chitosan and clay was successfully synthesized via electrostatic interaction between positively charged chitosan and negatively charged clay [142]. The hybridization of both materials improved the mechanical strength and anti-fatigue properties of the composites. Guo et al. [143] incorporated nanostructured hydroxyapatite with chitosan (HA–CS) and investigated their mechanical properties. They pointed out that the hybrid composites have great potential for bone tissue engineering due to excellent biocompatibility and mechanical properties.
Table 10.
Mechanical properties of chitosan hybrid composites.
| Polymers | Fiber | Processing Technique | Mechanical Properties | References | |
|---|---|---|---|---|---|
| Tensile Strength | Tensile Modulus | ||||
| Chitosan | Sisal fiber reinforced with hybrid polymer sandwich composite | Layer-by-layer | 110–146 MPa | 5800–6646 MPa | [141] |
| Chitosan | Calcium phosphate-flexible chitosan | Mixing and heating | 45.7 MPa | - | [142] |
| Chitosan | Clay–chitosan hybrid | Electro-stimulus-responsive | 2.25–2.70 MPa | 0.2–1.5 MPa | [143] |
| Chitosan | Bioactive calcium phosphate-flexible chitosan | Mixing and heating | 1.6–45.7 MPa | 10.2–77.3 MPa | [142] |
| Chitosan | Hydroxyapatite | Dip-coating and bio inspired mineralization | 3.12 MPa | 73.67 MPa | [144] |
| Chitosan | CS fiber porous scaffold | Dip-coating and bio inspired mineralization | 0.68 MPa | 3.40 MPa | [144] |
| Chitosan | Trabecular bone | Dip-coating and bio inspired mineralization | - | - | [144] |
| Chitosan | Sodium montmorillonite and zinc oxide nanoparicles | Polymer intercalation | 22.34 MPa ± 1.75 | 1.750 MPa ± 0.06 | [145] |
| Chitosan | Nano-ZnO nanocomposite | Polymer intercalation | 30.49 MPa ± 1.17 | 2.190 MPa ± 0.02 | [145] |
| Chitosan | Nano-ZnO and organoclay nanocomposite-C4 | Polymer intercalation | 38.86 MPa ± 1.49 | 2.410 MPa ± 0.01 | [145] |
| Chitosan | Grape pomace extract | Solvent casting | 9.89–13.58 MPa | 0.13–0.20 MPa | [146] |
| Chitosan | Potato starch | Solution blending/casting | 9.27–12.5 MPa | - | [147] |
| Chitosan | Cellulose nanocrystal (CNC) | Solution casting | 79.3–104.7 MPa | 1607–2068 MPa | [148] |
| Chitosan | Galangal rhizome extract | Chitosan film forming solution | 46.1–67.5 MPa | - | [149] |
| Chitosan as a coating material | Soy protein isolated and human hair fibers | Hot pressed and compression molding | 11.67–24.54 MPa | - | [150] |
| Chitosan | Viscose rayon filaments | Film molding | 105–151 MPa | 1.94–2.43 GPa | [151] |
| Chitosan | Corn starch and flax fabric | Compression molding | 17.64–24.03 MPa | 0.63–0.66 GPa | [152] |
| Chitosan as a coating material | Soy protein and sisal fiber |
Hand lay-up and solution casting method | 11.67–23.70 MPa | - | [153] |
8.2. Thermal Properties of Chitosan Hybrid Composites
An experiment that was performed by Yeh et al. [154] showed the thermal properties of chitosan hybrid materials with different weights of tetraethoxysilane/vinyltriethoxysilane (VTES/ TEOS). Table 11 indicates that the hybrid materials all possessed better thermostability and thermal decomposition. They improved with an increase in the amount of VTES and TEOS as reticular inorganic SiO2 was formed. The hybrid material that was made of TEOS was higher in thermostability than that of VTES because it is difficult for SiO2 to take shape in poorly soluble VTES.
Table 11.
Thermal properties of chitosan and hybrid materials [154].
| Weight of VTES/TEOS (g) | Thermal Properties | ||
|---|---|---|---|
| Td (°C) | Tm (°C) | Char Yield (%) | |
| 0/0 | 245 | 303 | 34.1 |
| 0/0.8 | 249 | 306 | 37.2 |
| 0.8/0.8 | 253 | 308 | 40.4 |
| 0.8/1.6 | 257 | 310 | 43.8 |
| 0.8/2.4 | 260 | 313 | 45.6 |
| 0.8/3.2 | 263 | 315 | 47.1 |
| 1.2/0 | 247 | 304 | 36.3 |
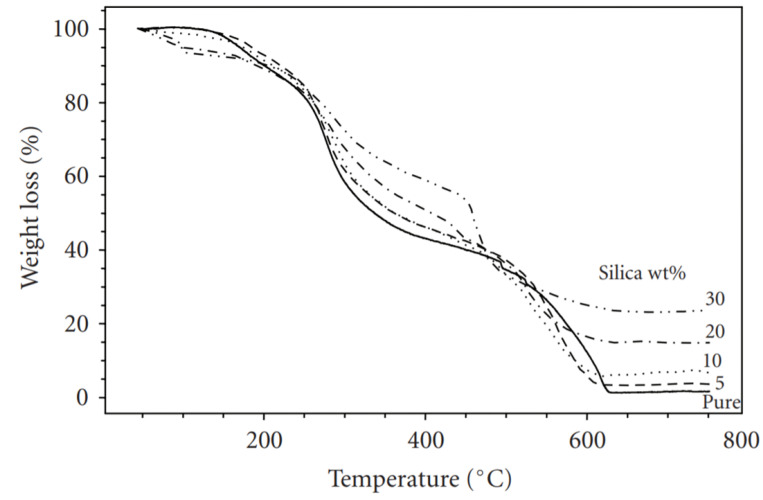
Because of the decomposition of low-molecular-weight species, chitosan loses weight more slowly between 160 °C and 270 °C. Thermal decomposition is more pronounced between 170 °C and 450 °C, owing to the complex dehydration of the saccharide rings, depolymerization, and decomposition of the polymer’s acetylated and deacetylated units [155]. Yeh, Chen, and Huang [154] studied the effect of silica with chitosan and analyzed the thermogravimetric of nanocomposites, and it was under synthetic air in the temperature range of 50–750 °C. Figure 12 shows the result of TGA.
Figure 12.
Thermogravimetric curves for chitosan-Si nanocomposites [156].
The initial weight loss observed between 100 and 160 °C tends to be caused by the loss of absorbed water on the surface of chitosan as well as a byproduct of subsequent condensation of the Si–OH groups. Because of the decomposition of low-molecular-weight species, chitosan loses weight more slowly between 160 °C and 270 °C. Thermal decomposition is more pronounced between 170 °C and 450 °C, owing to the complex dehydration of saccharide rings, depolymerization, and the decomposition of the polymer’s acetylated and deacetylated units.
The incorporation of a silica network and its contact with the polymer increases the hybrids’ thermal tolerance and, as a result, the thermal decomposition temperature. The amount of silica material incorporated in the hybrids correlated to the amount of residue retained at 750 °C, suggesting that the sol–gel reaction was active [157].
Thermal properties of potato starch mixed with chitosan films were found to be higher with the addition of citric acid (CA) [147]. The existence of a crosslinking effect with CA addition added to the thermal stability of this film, which may further improve the interactions between molecules. When CA was included in the films, the maximum temperature corresponding to each step in the TGA analysis appeared to emerge at higher temperatures, which may indicate greater intermolecular interactions among the components. On the other hand, Yadav, Behera, Chang, and Chiu [148] studied the thermal properties of cellulose nanocrystal/chitosan (CNC/CS) composite films by varying the CS loading of 2, 4, 6, and 8 wt.%. The TGA results showed that the incorporation of CS at different loadings showed almost the same thermal stability. Due to interactions between the CNC and the CS matrix, the thermal stability of CNC-reinforced CS composite films improved marginally after CNC insertion.
Thakhiew, Devahastin, and Soponronnarit [149] developed a blended film of chitosan and galangal rhizome extract at different drying methods, i.e., hot-air drying (HAD) and low-pressure, superheated steam drying (LPSSD), and different loadings of galangal rhizome extract, i.e., 0%, 0.6%, 0.9%, 1.2%, and 1.5%. They found that the usage of a higher temperature in the LPSSD may have resulted in more widespread thermal cross-linkage compared to the HAD method. In the case of the incorporation of galangal extract, a higher number of chemical cross-linkage interactions between the galangal extract and chitosan was observed. These phenomena were due to the electrostatic interactions and hydrogen bonding that may have caused conformational changes when the galangal extract was integrated into the chitosan matrix. From the DMA analysis, the storage modulus, loss modulus, and tan δ of the chitosan films across a temperature range of −120 to 230 °C were obtained, which show evidence of cross-linkage interactions.
Deepmala, Jain, Singh, and Chauhan [150] manufactured chitosan-coated, human-hair-reinforced, phytagel-modified, soy-protein-based composite for packaging and coating applications. Various wt.% of human hair were used with coated and non-coated chitosan. The experimental data prove that the tensile stress was enhanced to 24.54 MPa with the application of chitosan coating, due to the fact that the maximum surface cracks and voids were filled with the chitosan solution and the stress concentration was decreased. In the view of thermal properties, chitosan coating was found to improve the storage modulus and the glass transition temperature; 2 wt.% human hair had the highest value of storage modulus but the lowest value of tan δ. The stiffness of the manufactured samples was enhanced as a result of the chitosan coating application on the final composites, as the non-coated surface contained many cracks, while chitosan coating covered all gaps and surface cracks of the composite’s surface. Because the energy dissipation process was ineffective in this case, composites started to behave like brittle materials, thus increasing stiffness and storage modulus.
Gorade, Chaudhary, Parmaj, and Kale [151] prepared a composite with viscose rayon filaments and reinforcement with chitosan to produce a chitosan–viscose rayon biocomposite. Various weights of viscose rayon filament (15, 20 and 25 wt.%) were examined in the view of microstructure and thermal properties. As the weights of viscose rayon filament increased, some voids were observed due to the fact that the chitosan solution did not penetrate completely during biocomposite production, as shown in Figure 13. However, in terms of thermal stability, the result of including viscose rayon filament into the chitosan polymeric matrix increased the biocomposite’s thermal stability owing to viscose rayon filament’s greater thermal stability, as shown in Figure 14. The sample decomposition generally involved breaking down of glycosidic units into smaller pieces, followed by the production of volatile gases. In addition, the glycosyl unit was completely decomposed and depolymerized, resulting in the production of char [158].
Figure 13.
The micrograph images of (a) chitosan film, chitosan–viscose rayon biocomposite with different loadings; (b) 12 wt.%, (c) 20 wt.%, and (d) 25 wt.%.
Figure 14.
The TG curves of (a) chitosan film, (b) viscose rayon filament, and (c) chitosan–viscose rayon biocomposite.
Prabhakar and Song [152] examined the composite prepared by hybridizing corn starch, chitosan, and flax fabric to produce effective flame-retardant, eco-biodegradable composites for industrial applications. They concluded that the decomposition at higher temperatures from the TGA analysis shows that corn starch has a beneficial effect on the composites’ thermal stability due to the multihydroxyl groups in corn starch, which may create additional molecular hydrogen bonds. In terms of flammability, the flame retardancy improved substantially as the amount of corn starch increased. In addition, the thick char produced by the carbonaceous ingredient corn starch was thought to be responsible for the composites’ flame-retardant characteristics.
Sabzevari et al. [159] found that the graphene oxide (GO) chitosan composite has greater thermal stability over GO, as shown by its stability upon heating to the upper-temperature limit of 500 °C (Figure 15). In this study, low-molecular-weight chitosan was cross-linked with GO to produced GO–chitosan composite. The higher thermal stability of the GO–chitosan composite was due to higher crystallinity observed from the X-ray diffraction (XRD) and scanning electron microscope (SEM) analyses. The chitosan chains were well introduced and firmly bound to the oxygen functional groups of GO, while preserving the stacked structure of GO sheets in the material, which indicated excellent cross-linking occurred.
Figure 15.
The TGA curves of graphene oxide (GO), chitosan (LCTS), and GO-LCTS composite [159].
Verma, Singh, Singh, and Jain [153] conducted an experiment on hybrid composites reinforced with soy protein and sisal fiber by varying sisal fiber weight percentages of 0, 3, 4, 5, 6, 7, and 10. In this study, chitosan was used as a coating material, where the composites were coated with chitosan by immersing the samples in chitosan solution. They reported that the inclusion of sisal fiber at a higher weight percentage and chitosan coating to the thermal tests resulted in an improvement in thermal stability. The DMA analysis also demonstrated that the storage modulus and glass transition temperature for different compositions were greater for chitosan-coated specimens than for non-coated specimens, with the maximum values observed at 5 wt. percent sisal fiber composites in both instances.
9. Application of Chitosan-Based Green Composites
Chitosan has many usages or applications in industries, for example, biomedicine, cosmetology, papermaking, wastewater treatment, agriculture, or pharmaceutical applications, and others. The applications are further explained in the next section, which consists of an explanation for biomedical applications and the specific usage of chitosan in the industry. Chitosan has many benefits towards biomedical applications such as biocompatibility and control biodegradability, which lead to the degradation of products. In addition, it is not harmful and does not produce any dangerous reactions [96]. Besides that, chitosan can be modified by blending it with other polymeric materials such as cellulose because it has modifiable functional groups; thus, the stability of blends enhances. Some of the potential applications of chitosan–cellulose blends are tabulated in Table 12. It can be summarized that the stability of chitosan-based materials can be improved by blending them with other compatible biopolymers that can be commercially utilized.
Table 12.
Some of the potential applications of chitosan–cellulose/nanocellulose composites.
| Potential Applications | References |
|---|---|
| Adsorbent for the removal of heavy metal ions | [160,161,162] |
| Adsorbent for the removal of acidic reagents, metals, amino acids, proteins, and other compounds | [163] |
| Biocomposite films | [164,165] |
| Biomedical applications | [166] |
| Coronary artery bypass graft | [167] |
| Drug delivery | [168] |
| Electronic | [169] |
| Food packaging | [170] |
| Medical material | [171] |
| Odor treatment | [172] |
| Self-healing | [173] |
| Textiles | [174] |
| Wound dressing | [175,176] |
| Wound healing (good antibacterial effect) | [177] |
9.1. Drug Delivery
Nowadays, controlled drug delivery provides many advantages to humans, e.g., it can enhance efficacy and reduce or eliminate unwanted side effects and also the level of drugs. Chitosan has some special properties that make it ideal to be used for drug delivery functions. Furthermore, a chitosan nanoparticle system that is conjugated by anti-bradykinin B2 can enhance the inhibition of HIV replication. Chitosan used in the drug industry is solely used to reduce side effects from cancer treatment such as cardiotoxicity. The drug is confined with chitosan nanoparticles. These nanoparticles of chitosan can enhance the absorption of a chemical known as doxorubicin in the small intestine. The type of nanoparticles of chitosan that is commonly used in this specific industry is chitosan tripolyphosphate (TPP) nanoparticles [178]. Chitosan nanoparticles also can improve the tea polyphenols stabilities and prevent their reactions of oxidation or degradation in the gastrointestinal tract. Hence, chitosan that synthesized from polyethylene glycol is probably suitable for use as a drug controlled release carrier [96]. According to Bernkop-Schnürch and Dünnhaupt [179] and Ahsan et al. [180], the chemical stability, particle sizes, toxicity, release kinetic profiles, and type of delivery system are important elements that must be considered when it comes to processing chitosan for this purpose. Chitosan can be used in various forms depending on the function and applications of the carrier. Figure 16 shows that chitosan can be employed in a variety of ways, depending on the carrier’s function and uses.
Figure 16.
Drug delivery systems based on chitosan and various techniques for their manufacture [181].
9.2. Wound Dressing
Chitosan is a natural antibacterial polymer with features that make it excellent for wound dressing, such as being cheap to make, stable for long-term use, biodegradable, non-toxic, and having a biocidal impact on a wide range of pathogens. Besides, chitosan is a suitable material or substance for wound dressing, mainly for the prevention of wound infection. It is suitable or compatible due to inherent antibacterial activity and other benefits such as analgesic effects and hemostatic activity. Plus, chitosan can function as a mechanical barrier on blood that causes immediate clotting. Chitosan efficacy was tested and carried out in vitro toxicity evaluation on 3T3 cells, and the result showed that it is an agent suitable for the treatment of internal and external bleeding. Additionally, chitosan-based products vary from typical dressings such as gauze or cotton wool in that chitosan actively participates in wound healing processes [182]. Chitosan-based wound dressing materials have been formed into a number of shapes, including films, sponges, hydrogels, particles, and fibers, due to its ease of processing, as shown in Table 13. Matica et al. [183] comprehensively discussed chitosan as a wound dressing in medical sectors.
Table 13.
The benefits and drawbacks of major wound dressing types [183].
| Type | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Sponges |
|
|
[183,184,185,186,187,188] |
| Films |
|
|
[189,190,191,192] |
| Fibers |
|
|
[75,193,194,195,196,197,198] |
| Membranes |
|
|
[199,200,201] |
| Hydrogels |
|
|
[202,203,204,205,206] |
| Hydrocolloids |
|
|
[183] |
9.3. Food Packaging
In the food industry, chitosan is considered as a bioactive polymer that can be used for food packaging manufacture based on its antioxidant, antimicrobial, mechanical, and barrier properties. The use of chitosan as a material for packaging can reduce the risk on human health and also environmental problems. In fact, the mechanical and barrier properties of pure chitosan films are suitable for food and active packaging. Chitosan has also been made a reference polymer in order to manufacture active packaging with the improvement or capability to prevent the growth of microorganisms and upgrade the safety of food [178]. It is feasible to create food packaging items that are safe against a broad range of modifying and pathogenic microbes by mixing chitosan with other natural antimicrobial agents [178,207,208].
Chitosan also can be used as a thin edible film/coating with food several methods such as by dipping, spraying, and pulverizing the film forming solution with an aerosol spray coating [214,215,216], as shown in Figure 17. An edible coating is a thin layer generated as a coating on a food product that is applied in liquid form, while chitosan film is a prefabricated thin coating that may be applied on or between food components once produced. Because chitosan regulates gas exchange, inhibits the respiration rate, is fungistatic, is capable of eliciting host defense responses, and lowers the rate of ethylene generation compared to the control fruit throughout the storage period, it has the potential to extend the shelf life of perishable fruits and vegetables as a post-harvest treatment [106,217].
Figure 17.
(A) Development of chitosan/gelatin-based polymeric films with inclusion of citrus essential oils [209]; (B) preservation mechanism of chitosan-based coating to maintain quality of vegetables and fruits [210]; (C) multifunctional coating composed of Eryngium campestre essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry [211]; (D) edible film’s antimicrobial activity against E. coli O157:H7 on cherry tomatoes [212]. Reproduced from Zhang et al. [213].
Researchers found that chitosan coating significantly preserves the qualitative qualities of sliced mango fruit and extends its shelf life by reducing water loss and sensory degradation, boosting soluble solid content, titratable acidity, vitamin C, and preventing microbe development [218,219,220,221]. Table 14 shows studies made with chitosan films and chitosan coatings in food products.
Table 14.
Applications of chitosan-based films and coatings in different food products.
| Chitosan Based | Combination | Food | References |
|---|---|---|---|
| Film | Gelatin/grape seed extract/Ziziphora clinopodioides essential oil | Minced trout fillets | [222] |
| Chitosan powder/glycerol/NaOH solution | Chilled meat | [115] | |
| Cassava starch/glycerol/polyethylene glycol | Meat slices | [223] | |
| Zataria multiflora essential oil/Cinnamomum zeylanicum | Green chili | [224] | |
| Chitosan powder/glycerol | Chilled meat | [225] | |
| Chitosan/ Basil Essential Oil | Cooked ham | [226] | |
| Apricot (Prunus armeniaca) kernel essential oil/glycerol | Spiced beef | [227] | |
| Coatings | Agar/Artemisia annua oil | Cherry tomato | [212] |
| Apple peel polyphenols (APP)/glycerol | Strawberry | [228] | |
| Essential oils (EO) of Elettaria Cardamomum/glycerol | Chicken drumsticks | [229] |
9.4. Dermatology and Skin Care
In cosmetic applications, chitosan is a natural cationic polymer which turns viscous when being neutralized with acid and works as a cationic humectant in cosmetics and topical formulations. It is used in the manufacture of creams, lotions, and other cosmetic preparations. Additionally, chitosan is known for its application as a film-forming and hydrating agent. Besides, chitosan also has benefits as sun protection, as the emulsions of sun protection that were mixed with chitosan have good effects on water resistance, which improve the safety of skin [230]. By changing the keratin structure, chitosan is extensively employed as a skin permeability enhancer in drug delivery systems, and it is absorbed to the negative charges of the skin surface [231]. Chitosan also improves the water content of the stratum corneum and increases the fluidity of the cell membrane, aided by its hydrophilic hydroxyl groups, which enable chitosan to interact with water molecules [232]. Chitosan adheres to the skin due to its positive charges and relatively high molecular weight, allowing it to be used as a percutaneous drug delivery vehicle. In recent years, there has been increased interest in using chitosan in the creation of nanoparticles as a carrier for active ingredients in cosmetics and medicine delivery to the skin. Chitosan-based nanoparticles have been used to treat local problems including skin malignant melanoma and infection [233].
9.5. Cosmetics for Oral Care Products
As oral care products affect human health, the cosmetics industry tends to use and focus on natural compounds such as chitin, chitosan, and their derivatives. Since chitosan has a lower molecular weight, it shows inhibition on the oral adsorption of streptococci and is proposed as a potential anticavity agent. Chitosan also able to interfere with microorganisms’ adherence and other factors [230]. Achmad et al. [234] evaluated the efficacy of chitosan against dental plaque development in actual formulations. In the study, chitosan was incorporated into toothpastes, rinses, and other vehicles. Chitosan, which is used in toothpaste and mouthwashes to prevent biofilm development in the mouth owing to the presence of S. mutans, has been shown to reduce S. mutans colonies. Chitosan has a broad antibacterial spectrum; thus, its efficacy against various bacterial strains associated with dental caries has been studied by a number of researchers [235,236,237].
10. Challenges and Opportunities
Chitosan captures a special position as a natural source in the composite industry due to its appealing characteristics such as antibacterial and film-forming properties. Currently, many products and applications are utilizing chitosan, primarily in the food and medical lines. Extensive research and manufacturing efforts have supported the development of chitosan-based products, which has been aided by rising customer demand for natural and safer additives with useful qualities, as well as rising environmental concerns [238,239]. However, there are few challenges that need to be focused on to widen the application of chitosan-based composites. The methods or techniques involved in chitosan-based products are mostly for small-scale production. To move closer to industrial production, these current technologies may be improved or integrated with other beneficial technology in order to produce mass numbers of chitosan-based products with the necessary characteristics for various applications. Due to some chitosan limitation properties such as low thermal stability to fulfil specific needs, more chitosan derivatives must be researched, and the degradation and environmental impact of chitosan-based products must be studied. Furthermore, more research is needed, especially in terms of toxicity and antibacterial properties, to improve chitosan-based products for food packaging before they may be used commercially. Improvements in thermal and strength characteristics, as well as the ability to bind contaminants, are also required.
11. Conclusions
Nature provides a variety of biomaterials that can be obtained easily from animals and plants. Chitosan is one natural fiber with promising characteristics as a composite material. As a biomaterial form, chitosan can be made into a few forms such as 3D (hydrogels and sponges) and 2D (films and porous membranes); each of them have their own set of industrial applications. On the other hand, chitosan has relatively poor mechanical, thermal, and barrier properties. For example, chitosan experienced weight reduction (50%) at 220–320 °C and a further 40% weight loss at the temperature of 470 °C. With the combination of two or more polymers, biomaterials can significantly increase the properties of composites. Therefore, chitosan-based composites are extensively investigated and explored. There are various methods available in the literature according to applications for the development of chitosan-based composites, including solution and solvent casting, dipping and spray coating, freeze casting and drying, layer-by-layer processes, and extrusion. The mechanical properties and thermal properties of different types of composites have been discussed from different resources. It was reported that the developed chitosan-based green composites, chitosan-blend composites, and chitosan hybrid composites showed thermal stability improvement due to the highly crystalline structure of the composites observed by XRD and SEM analysis. Not only that, but their mechanical properties also enhanced with the increase in tensile strength. As chitosan green composites offer many advantages, they have a wide range of applications and potential in the biomedicine, cosmetology, papermaking, wastewater treatment, agriculture, and pharmaceutical industries. In future, the use of chitosan-based composites in industrial applications may be able to completely replace synthetic fibers, thus reducing environmental pollution. In-depth research is still needed in terms of investigating the environmentally friendly chemical treatment used, as well as the toxicity and antibacterial properties of materials, so that composite materials reinforced with natural fibers can perform better in the future.
Author Contributions
Conceptualization, R.A.I., H.A.A.; validation, R.A.I., H.A.A.; investigation, R.A.I.; writing—original draft preparation, R.A.I., H.A.A., A.H.N., N.N., M.Y.M.Z., M.R.M.A., S.M.S., E.S.Z., S.S., H.A., M.A., E.S., N.H.S., M.R., S.Z.S.Z., M.R.R., N.A.M., Z.R., A.A., S.P.B., R.I.; writing—review and editing, R.A.I., H.A.A., A.H.N., N.N., M.Y.M.Z., M.R.M.A., S.M.S., E.S.Z., S.S., H.A., M.A., E.S., N.H.S., M.R., S.Z.S.Z., M.R.R., N.A.M., Z.R., A.A., S.P.B., R.I.; funding acquisition, S.Z.S.Z., M.R.R., N.A.M., Z.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to express their gratitude for the financial support received from the Universiti Teknologi Malaysia, project CRG 30.3, “Retardant coating using graphene/bamboo aerogel mixtures on SAR robotics system, grant number PY/2020/03495—R.J130000.7351.4B534”. The research has been carried out under the program Research Excellence Consortium (JPT (BPKI) 1000/016/018/25 (57)) provided by the Ministry of Higher Education Malaysia (MOHE). This work was funded by Universiti Kebangsaan Malaysia (UKM) for the financial support through research grants, Dana Pecutan Penerbitan—LESTARI UKM: PP/LESTARI/2022, XX-2020-010 and XX-2021-002. In addition, this work was also funded by Universiti Putra Malaysia, and Fundamental Research Grant Scheme FRGS/1/2021/TK0/UPM/02/21 provided by Ministry of Higher Education Malaysia (MOHE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roslan Z., Ramli Z., Razman M., Asyraf M., Ishak M., Ilyas R., Nurazzi N. Reflections on Local Community Identity by Evaluating Heritage Sustainability Protection in Jugra, Selangor, Malaysia. Sustainability. 2021;13:8705. doi: 10.3390/su13168705. [DOI] [Google Scholar]
- 2.Ali S., Razman M., Awang A., Asyraf M., Ishak M., Ilyas R., Lawrence R. Critical Determinants of Household Electricity Consumption in a Rapidly Growing City. Sustainability. 2021;13:4441. doi: 10.3390/su13084441. [DOI] [Google Scholar]
- 3.Rozilah A., Jaafar C.N.A., Sapuan S.M., Zainol I., Ilyas R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers. 2020;12:2605. doi: 10.3390/polym12112605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapuan S.M., Aulia H.S., Ilyas R.A., Atiqah A., Dele-Afolabi T.T., Nurazzi M.N., Supian A.B.M., Atikah M.S.N. Mechanical Properties of Longitudinal Basalt/Woven-Glass-Fiber-reinforced Unsaturated Polyester-Resin Hybrid Composites. Polymers. 2020;12:2211. doi: 10.3390/polym12102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Mantia F.P., Morreale M. Green composites: A brief review. Compos. Part A Appl. Sci. Manuf. 2011;42:579–588. doi: 10.1016/j.compositesa.2011.01.017. [DOI] [Google Scholar]
- 6.Ilyas R., Sapuan S., Harussani M., Hakimi M., Haziq M., Atikah M., Asyraf M., Ishak M., Razman M., Nurazzi N., et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers. 2021;13:1326. doi: 10.3390/polym13081326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramamoorthy S.K., Skrifvars M., Persson A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015;55:107–162. doi: 10.1080/15583724.2014.971124. [DOI] [Google Scholar]
- 8.Tarique J., Sapuan S., Khalina A., Sherwani S., Yusuf J., Ilyas R. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021;13:1191–1219. doi: 10.1016/j.jmrt.2021.05.047. [DOI] [Google Scholar]
- 9.Mitragotri S., Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakshi P.S., Selvakumar D., Kadirvelu K., Kumar N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020;150:1072–1083. doi: 10.1016/j.ijbiomac.2019.10.113. [DOI] [PubMed] [Google Scholar]
- 11.Hu X., Ricci S., Naranjo S., Hill Z., Gawason P. Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications. Molecules. 2021;26:4499. doi: 10.3390/molecules26154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail M.I., Roslan A., Saari N.S., Hashim K.H., Kalamullah M.R. AIP Conference Proceedings, Proceedings of the 3rd Electronic and Green Materials International Conference 2017, Krabi, Thailand, 29–30 April 2017. Volume 1885. AIP Publishing; Melville, NY, USA: 2017. Ethanolic extract of propolis for biodegradable films packaging enhanced with chitosan; p. 020231. [Google Scholar]
- 13.Alabaraoye E., Achilonu M., Hester R. Biopolymer (Chitin) from Various Marine Seashell Wastes: Isolation and Characterization. J. Polym. Environ. 2018;26:2207–2218. doi: 10.1007/s10924-017-1118-y. [DOI] [Google Scholar]
- 14.Komuraiah A., Kumar N.S., Prasad B.D. Chemical Composition of Natural Fibers and Its Influence on Their Mechanical Properties. Mech. Compos. Mater. 2014;50:359–376. doi: 10.1007/s11029-014-9422-2. [DOI] [Google Scholar]
- 15.Paridah M.T., Basher A.B., SaifulAzry S., Ahmed Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources. 2011;6:5260–5281. [Google Scholar]
- 16.Aisyah H.A., Paridah M.T., Sapuan S.M., Khalina A., Berkalp O.B., Lee S.H., Lee C.H., Nurazzi N.M., Ramli N., Wahab M.S., et al. Thermal Properties of Woven Kenaf/Carbon Fibre-Reinforced Epoxy Hybrid Composite Panels. Int. J. Polym. Sci. 2019;2019:5258621. doi: 10.1155/2019/5258621. [DOI] [Google Scholar]
- 17.Faruk O., Bledzki A.K., Fink H.-P., Sain M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012;37:1552–1596. doi: 10.1016/j.progpolymsci.2012.04.003. [DOI] [Google Scholar]
- 18.Sinha A.K., Narang H.K., Bhattacharya S. Mechanical properties of natural fibre polymer composites. J. Polym. Eng. 2017;37:879–895. doi: 10.1515/polyeng-2016-0362. [DOI] [Google Scholar]
- 19.Lau K.-T., Hung P.-Y., Zhu M.-H., Hui D. Properties of natural fibre composites for structural engineering applications. Compos. Part B Eng. 2018;136:222–233. doi: 10.1016/j.compositesb.2017.10.038. [DOI] [Google Scholar]
- 20.Asyraf M.R.M., Rafidah M., Azrina A., Razman M.R. Dynamic mechanical behaviour of kenaf cellulosic fibre biocomposites: A comprehensive review on chemical treatments. Cellulose. 2021;28:2675–2695. doi: 10.1007/s10570-021-03710-3. [DOI] [Google Scholar]
- 21.Asyraf M., Ishak M., Norrrahim M., Nurazzi N., Shazleen S., Ilyas R., Rafidah M., Razman M. Recent advances of thermal properties of sugar palm lignocellulosic fibre reinforced polymer composites. Int. J. Biol. Macromol. 2021;193:1587–1599. doi: 10.1016/j.ijbiomac.2021.10.221. [DOI] [PubMed] [Google Scholar]
- 22.Halimatul M., Sapuan S., Jawaid M. Water absorption and water solubility properties of sago starch biopolymer composite films filled with sugar palm particles. Polimery. 2019;64:596–603. doi: 10.14314/polimery.2019.9.4. [DOI] [Google Scholar]
- 23.Nurazzi N.M., Asyraf M.R.M., Athiyah S.F., Shazleen S.S., Rafiqah S.A., Harussani M.M., Kamarudin S.H., Razman M.R., Rahmah M., Zainudin E.S., et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers. 2021;13:2170. doi: 10.3390/polym13132170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aisyah H.A., Paridah M.T., Sapuan S.M., Ilyas R.A., Khalina A., Nurazzi N.M., Lee S.H., Lee C.H. A Comprehensive Review on Advanced Sustainable Woven Natural Fibre Polymer Composites. Polymers. 2021;13:471. doi: 10.3390/polym13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C., Khalina A., Lee S. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers. 2021;13:438. doi: 10.3390/polym13030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N. Comparison of Static and Long-term Creep Behaviors between Balau Wood and Glass Fiber Reinforced Polymer Composite for Cross-arm Application. Fibers Polym. 2021;22:793–803. doi: 10.1007/s12221-021-0512-1. [DOI] [Google Scholar]
- 27.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N. Influence of Additional Bracing Arms as Reinforcement Members in Wooden Timber Cross-Arms on Their Long-Term Creep Responses and Properties. Appl. Sci. 2021;11:2061. doi: 10.3390/app11052061. [DOI] [Google Scholar]
- 28.Asyraf M., Ishak M., Sapuan S., Yidris N., Ilyas R. Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies. J. Mater. Res. Technol. 2020;9:6759–6776. doi: 10.1016/j.jmrt.2020.01.013. [DOI] [Google Scholar]
- 29.Ilyas R.A., Zuhri M.Y.M., Norrrahim M.N.F., Misenan M.S.M., Jenol M.A., Samsudin S.A., Nurazzi N.M., Asyraf M.R.M., Supian A.B.M., Bangar S.P., et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers. 2022;14:182. doi: 10.3390/polym14010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asyraf M., Ishak M., Syamsir A., Nurazzi N., Sabaruddin F., Shazleen S., Norrrahim M., Rafidah M., Ilyas R., Rashid M.Z.A., et al. Mechanical properties of oil palm fibre-reinforced polymer composites: A review. J. Mater. Res. Technol. 2022;17:33–65. doi: 10.1016/j.jmrt.2021.12.122. [DOI] [Google Scholar]
- 31.Nurazzi N.M., Asyraf M.R.M., Rayung M., Norrrahim M.N.F., Shazleen S.S., Rani M.S.A., Shafi A.R., Aisyah H.A., Radzi M.H.M., Sabaruddin F.A., et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers. 2021;13:2710. doi: 10.3390/polym13162710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurazzi N.M., Asyraf M.R.M., Khalina A., Abdullah N., Aisyah H.A., Rafiqah S.A., Sabaruddin F.A., Kamarudin S.H., Norrrahim M.N.F., Ilyas R.A., et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers. 2021;13:646. doi: 10.3390/polym13040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurazzi N., Khalina K., Sapuan S. Mechanical properties of sugar palm yarn/woven glass fiber reinforced unsaturated polyester composites: Effect of fiber loadings and alkaline treatment. Polimery. 2019;64:665–675. doi: 10.14314/polimery.2019.10.3. [DOI] [Google Scholar]
- 34.Alsubari S., Zuhri M.Y.M., Sapuan S.M., Ishak M.R., Ilyas R.A., Asyraf M.R.M. Potential of Natural Fiber Reinforced Polymer Composites in Sandwich Structures: A Review on Its Mechanical Properties. Polymers. 2021;13:423. doi: 10.3390/polym13030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahaya R., Sapuan S., Jawaid M., Leman Z., Zainudin E. Mechanical performance of woven kenaf-Kevlar hybrid composites. J. Reinf. Plast. Compos. 2014;33:2242–2254. doi: 10.1177/0731684414559864. [DOI] [Google Scholar]
- 36.Asyraf M.R.M., Rafidah M., Ishak M.R., Sapuan S.M., Yidris N., Ilyas R.A., Razman M.R. Integration of TRIZ, morphological chart and ANP method for development of FRP composite portable fire extinguisher. Polym. Compos. 2020;41:2917–2932. doi: 10.1002/pc.25587. [DOI] [Google Scholar]
- 37.Sharma S., Sudhakara P., Singh J., Ilyas R.A., Asyraf M.R.M., Razman M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers. 2021;13:2623. doi: 10.3390/polym13162623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amir A., Ishak M., Yidris N., Zuhri M., Asyraf M. Potential of Honeycomb-Filled Composite Structure in Composite Cross-Arm Component: A Review on Recent Progress and Its Mechanical Properties. Polymers. 2021;13:1341. doi: 10.3390/polym13081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amir A., Ishak M., Yidris N., Zuhri M., Asyraf M. Advances of composite cross arms with incorporation of material core structures: Manufacturability, recent progress and views. J. Mater. Res. Technol. 2021;13:1115–1131. doi: 10.1016/j.jmrt.2021.05.040. [DOI] [Google Scholar]
- 40.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N., Ilyas R.A., Rafidah M., Razman M.R. Potential Application of Green Composites for Cross Arm Component in Transmission Tower: A Brief Review. Int. J. Polym. Sci. 2020;2020:8878300. doi: 10.1155/2020/8878300. [DOI] [Google Scholar]
- 41.Azman M.A., Asyraf M.R.M., Khalina A., Petrů M., Ruzaidi C.M., Sapuan S.M., Nik W.B.W., Ishak M.R., Ilyas R.A., Suriani M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers. 2021;13:1917. doi: 10.3390/polym13121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naveen J., Jawaid M., Zainudin E.S., Sultan M.T.H., Yahaya R. Evaluation of ballistic performance of hybrid Kevlar®/Cocos nucifera sheath reinforced epoxy composites. J. Text. Inst. 2019;110:1179–1189. doi: 10.1080/00405000.2018.1548801. [DOI] [Google Scholar]
- 43.Supian A., Jawaid M., Rashid B., Fouad H., Saba N., Dhakal H.N., Khiari R. Mechanical and physical performance of date palm/bamboo fibre reinforced epoxy hybrid composites. J. Mater. Res. Technol. 2021;15:1330–1341. doi: 10.1016/j.jmrt.2021.08.115. [DOI] [Google Scholar]
- 44.Asyraf M., Ishak M., Sapuan S., Yidris N. Conceptual design of multi-operation outdoor flexural creep test rig using hybrid concurrent engineering approach. J. Mater. Res. Technol. 2020;9:2357–2368. doi: 10.1016/j.jmrt.2019.12.067. [DOI] [Google Scholar]
- 45.Asyraf M., Ishak M., Sapuan S., Yidris N. Conceptual design of creep testing rig for full-scale cross arm using TRIZ-Morphological chart-analytic network process technique. J. Mater. Res. Technol. 2019;8:5647–5658. doi: 10.1016/j.jmrt.2019.09.033. [DOI] [Google Scholar]
- 46.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N., Ilyas R.A., Rafidah M., Razman M.R. Evaluation of Design and Simulation of Creep Test Rig for Full-Scale Crossarm Structure. Adv. Civ. Eng. 2020;2020:6980918. doi: 10.1155/2020/6980918. [DOI] [Google Scholar]
- 47.Asyraf M.R.M., Ishak M.R., Sapuan S.M., Yidris N. Utilization of Bracing Arms as Additional Reinforcement in Pultruded Glass Fiber-Reinforced Polymer Composite Cross-Arms: Creep Experimental and Numerical Analyses. Polymers. 2021;13:620. doi: 10.3390/polym13040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asrofi M., Sapuan S.M., Ilyas R.A., Ramesh M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type) Mater. Today Proc. 2020;46:1626–1630. doi: 10.1016/j.matpr.2020.07.254. [DOI] [Google Scholar]
- 49.Alias A.H., Norizan M.N., Sabaruddin F.A., Asyraf M.R.M., Norrrahim M.N.F., Ilyas A.R., Kuzmin A.M., Rayung M., Shazleen S.S., Nazrin A., et al. Hybridization of MMT/Lignocellulosic Fiber Reinforced Polymer Nanocomposites for Structural Applications: A Review. Coatings. 2021;11:1355. doi: 10.3390/coatings11111355. [DOI] [Google Scholar]
- 50.Hazrol M.D., Sapuan S.M., Ilyas R.A., Othman M.L., Sherwani S.F.K. Electrical properties of sugar palm nanocrystalline cellulose, reinforced sugar palm starch nanocomposites. Polimery. 2020;65:363–372. doi: 10.14314/polimery.2020.5.4. [DOI] [Google Scholar]
- 51.Madyaratri E.W., Ridho M.R., Aristri M.A., Lubis M.A.R., Iswanto A.H., Nawawi D.S., Antov P., Kristak L., Majlingová A., Fatriasari W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers. 2022;14:362. doi: 10.3390/polym14030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poletto M., Ornaghi J.H.L., Zattera A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials. 2014;7:6105–6119. doi: 10.3390/ma7096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopattananon N., Panawarangkul K., Sahakaro K., Ellis B. Performance of pineapple leaf fiber–natural rubber composites: The effect of fiber surface treatments. J. Appl. Polym. Sci. 2006;102:1974–1984. doi: 10.1002/app.24584. [DOI] [Google Scholar]
- 54.Baley C., Gomina M., Breard J., Bourmaud A., Davies P. Variability of mechanical properties of flax fibres for composite reinforcement. A review. Ind. Crop. Prod. 2020;145:111984. doi: 10.1016/j.indcrop.2019.111984. [DOI] [Google Scholar]
- 55.Karimah A., Ridho M.R., Munawar S.S., Adi D.S., Ismadi, Damayanti R., Subiyanto B., Fatriasari W., Fudholi A. A review on natural fibers for development of eco-friendly bio-composite: Characteristics, and utilizations. J. Mater. Res. Technol. 2021;13:2442–2458. doi: 10.1016/j.jmrt.2021.06.014. [DOI] [Google Scholar]
- 56.Gholampour A., Ozbakkaloglu T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020;55:829–892. doi: 10.1007/s10853-019-03990-y. [DOI] [Google Scholar]
- 57.Wahab R., Mustafa M.T., Salam M.A., Sudin M., Samsi H.W., Rasat M.S.M. Chemical Composition of Four Cultivated Tropical Bamboo in Genus Gigantochloa. J. Agric. Sci. 2013;5:66. doi: 10.5539/jas.v5n8p66. [DOI] [Google Scholar]
- 58.Omran A.A.B., Mohammed A.A.B.A., Sapuan S.M., Ilyas R.A., Asyraf M.R.M., Koloor S.S.R., Petrů M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers. 2021;13:231. doi: 10.3390/polym13020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurazzi N.M., Shazleen S., Aisyah H.A., Asyraf M., Sabaruddin F., Mohidem N., Norrrahim M.N.F., Kamarudin S., Ilyas R.A., Ishak M., et al. Effect of silane treatments on mechanical performance of kenaf fibre reinforced polymer composites: A review. Funct. Compos. Struct. 2021;3:045003. doi: 10.1088/2631-6331/ac351b. [DOI] [Google Scholar]
- 60.Ilyas R.A., Zuhri M.Y.M., Aisyah H.A., Asyraf M.R.M., Hassan S.A., Zainudin E.S., Sapuan S.M., Sharma S., Bangar S.P., Jumaidin R., et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers. 2022;14:202. doi: 10.3390/polym14010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girijappa Y.G.T., Rangappa S.M., Parameswaranpillai J., Siengchin S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019;6:226. doi: 10.3389/fmats.2019.00226. [DOI] [Google Scholar]
- 62.Sari N.H., Pruncu C.I., Sapuan S.M., Ilyas R.A., Catur A.D., Suteja S., Sutaryono Y.A., Pullen G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020;91:106751. doi: 10.1016/j.polymertesting.2020.106751. [DOI] [Google Scholar]
- 63.Ilyas R. Biopolymers and Biocomposites: Chemistry and Technology. Curr. Anal. Chem. 2020;16:500–503. doi: 10.2174/157341101605200603095311. [DOI] [Google Scholar]
- 64.Mahmud S., Hasan K.M.F., Jahid A., Mohiuddin K., Zhang R., Zhu J. Comprehensive review on plant fiber-reinforced polymeric biocomposites. J. Mater. Sci. 2021;56:7231–7264. doi: 10.1007/s10853-021-05774-9. [DOI] [Google Scholar]
- 65.Vinod A., Sanjay M., Suchart S., Jyotishkumar P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020;258:120978. doi: 10.1016/j.jclepro.2020.120978. [DOI] [Google Scholar]
- 66.Ilyas R. The Preparation Methods and Processing of Natural Fibre Bio-polymer Composites. Curr. Org. Synth. 2019;16:1068–1070. doi: 10.2174/157017941608200120105616. [DOI] [PubMed] [Google Scholar]
- 67.Das S., Singha A.K., Chaudhuri A., Ganguly P.K. Lengthwise jute fibre properties variation and its effect on jute–polyester composite. J. Text. Inst. 2019;110:1695–1702. doi: 10.1080/00405000.2019.1613735. [DOI] [Google Scholar]
- 68.Defoirdt N., Biswas S., de Vriese L., Tran L.Q.N., van Acker J., Ahsan Q., Gorbatikh L., van Vuure A., Verpoest I. Assessment of the tensile properties of coir, bamboo and jute fibre. Compos. Part A Appl. Sci. Manuf. 2010;41:588–595. doi: 10.1016/j.compositesa.2010.01.005. [DOI] [Google Scholar]
- 69.Osorio L., Trujillo E., van Vuure A.W., Verpoest I. Morphological aspects and mechanical properties of single bamboo fibers and flexural characterization of bamboo/epoxy composites. J. Reinf. Plast. Compos. 2011;30:396–408. doi: 10.1177/0731684410397683. [DOI] [Google Scholar]
- 70.Shahinur S., Hasan M., Ahsan Q., Saha D.K., Islam S. Characterization on the Properties of Jute Fiber at Different Portions. Int. J. Polym. Sci. 2015;2015:262348. doi: 10.1155/2015/262348. [DOI] [Google Scholar]
- 71.Chakraborty S., Kundu S.P., Roy A., Basak R.K., Adhikari B., Majumder S. Improvement of the mechanical properties of jute fibre reinforced cement mortar: A statistical approach. Constr. Build. Mater. 2013;38:776–784. doi: 10.1016/j.conbuildmat.2012.09.067. [DOI] [Google Scholar]
- 72.Jariwala H., Jain P. A review on mechanical behavior of natural fiber reinforced polymer composites and its applications. J. Reinf. Plast. Compos. 2019;38:441–453. doi: 10.1177/0731684419828524. [DOI] [Google Scholar]
- 73.Sabri S., Fuadi Z., Kurniawan R., Rizal S., Homma H., Kosukegawa H., Takagi T., Miki H. Tensile Strength and Fracture Behavior of Single Abaca Fiber. J. Nat. Fibers. 2021:1–15. doi: 10.1080/15440478.2021.1967832. [DOI] [Google Scholar]
- 74.Cai M., Takagi H., Nakagaito A.N., Li Y., Waterhouse G.I. Effect of alkali treatment on interfacial bonding in abaca fiber-reinforced composites. Compos. Part A Appl. Sci. Manuf. 2016;90:589–597. doi: 10.1016/j.compositesa.2016.08.025. [DOI] [Google Scholar]
- 75.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- 76.Sharma S., Batra S. Materials for Biomedical Engineering. Elsevier; Amsterdam, The Netherlands: 2019. Recent advances of chitosan composites in artificial skin: The next era for potential biomedical application; pp. 97–119. [Google Scholar]
- 77.Kumar M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. doi: 10.1016/S1381-5148(00)00038-9. [DOI] [Google Scholar]
- 78.Yeul V.S., Rayalu S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013;21:606–614. doi: 10.1007/s10924-012-0458-x. [DOI] [Google Scholar]
- 79.Potivas T., Laokuldilok T. Deacetylation of Chitin and the Properties of Chitosan Films with Various Deacetylation Degrees. Food Appl. Biosci. 2014;13:559–568. doi: 10.12982/CMUJNS.2014.0058. [DOI] [Google Scholar]
- 80.Avelelas F., Horta A., Pinto L.F., Marques S.C., Nunes P.M., Pedrosa R., Leandro S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs. 2019;17:239. doi: 10.3390/md17040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo Q., Wang Y., Han Q., Ji L., Zhang H., Fei Z., Wang Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019;209:266–275. doi: 10.1016/j.carbpol.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 82.Li Q., Dunn E.T., Grandmaison E.W., Goosen M.F.A. Applications of Chitin and Chitosan. CRC Press; Boca Raton, FL, USA: 2020. Applications and Properties of Chitosan; pp. 3–29. [DOI] [Google Scholar]
- 83.Vásconez M.B., Flores S., Campos C.A., Alvarado J., Gerschenson L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009;42:762–769. doi: 10.1016/j.foodres.2009.02.026. [DOI] [Google Scholar]
- 84.Souza V.G.L., Fernando A.L., Pires J.R.A., Rodrigues P.F., Lopes A.A.S., Fernandes F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017;107:565–572. doi: 10.1016/j.indcrop.2017.04.056. [DOI] [Google Scholar]
- 85.Siripatrawan U., Harte B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010;24:770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- 86.Peter S., Lyczko N., Gopakumar D., Maria H.J., Nzihou A., Thomas S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass Valorization. 2021;12:4777–4804. doi: 10.1007/s12649-020-01244-6. [DOI] [Google Scholar]
- 87.Isa S.A.B.M., Mohamed R. Physical and Mechanical Properties of Chitosan and Polyethylene Blend for Food Packaging Film. Int. J. Mech. Prod. Eng. 2015;3:51–55. [Google Scholar]
- 88.Balti R., Mansour M.B., Sayari N., Yacoubi L., Rabaoui L., Brodu N., Massé A. Development and characterization of bioactive edible films from spider crab (Maja crispata) chitosan incorporated with Spirulina extract. Int. J. Biol. Macromol. 2017;105:1464–1472. doi: 10.1016/j.ijbiomac.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed J., Mulla M., Arfat Y.A., Lidia A.T.T. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017;71:141–148. doi: 10.1016/j.foodhyd.2017.05.013. [DOI] [Google Scholar]
- 90.Fundo J.F., Quintas M.A.C., Silva C.L.M. Influence of film forming solutions on properties of chitosan/glycerol films; Proceedings of the 11th International Congress Engineering and Food (ICEF 11); Athens, Greece. 22–26 May 2011; pp. 963–964. [Google Scholar]
- 91.Grząbka-Zasadzińska A., Amietszajew T., Borysiak S. Thermal and mechanical properties of chitosan nanocomposites with cellulose modified in ionic liquids. J. Therm. Anal. Calorim. 2017;130:143–154. doi: 10.1007/s10973-017-6295-3. [DOI] [Google Scholar]
- 92.Diab M., El-Sonbati A., Bader D. Thermal stability and degradation of chitosan modified by benzophenone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011;79:1057–1062. doi: 10.1016/j.saa.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 93.Diab M.A., El-Sonbati A.Z., Al-Halawany M.M., Bader D.M.D. Thermal stability and degradation of chitosan modified by cinnamic acid. Open J. Polym. Chem. 2012;2:17555. doi: 10.4236/ojpchem.2012.21003. [DOI] [Google Scholar]
- 94.Diab M.A., El-Sonbati A.Z., Bader D.M.D., Zoromba M.S. Thermal Stability and Degradation of Chitosan Modified by Acetophenone. J. Polym. Environ. 2012;20:29–36. doi: 10.1007/s10924-011-0330-4. [DOI] [Google Scholar]
- 95.Harish Prashanth K.V., Tharanathan R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007;18:117–131. doi: 10.1016/j.tifs.2006.10.022. [DOI] [Google Scholar]
- 96.Muxika A., Etxabide A., Uranga J., Guerrero P., de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017;105:1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 97.Schmitz C., Auza L.G., Koberidze D., Rasche S., Fischer R., Bortesi L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs. 2019;17:452. doi: 10.3390/md17080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venkatesan J., Kim S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs. 2010;8:2252–2266. doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shoueir K.R., El-Desouky N., Rashad M.M., Ahmed M., Janowska I., El-Kemary M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021;167:1176–1197. doi: 10.1016/j.ijbiomac.2020.11.072. [DOI] [PubMed] [Google Scholar]
- 100.Matet M., Heuzey M.-C., Pollet E., Ajji A., Avérous L. Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydr. Polym. 2013;95:241–251. doi: 10.1016/j.carbpol.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 101.Adoor S.G., Prathab B., Manjeshwar L.S., Aminabhavi T.M. Mixed matrix membranes of sodium alginate and poly(vinyl alcohol) for pervaporation dehydration of isopropanol at different temperatures. Polymer. 2007;48:5417–5430. doi: 10.1016/j.polymer.2007.06.064. [DOI] [Google Scholar]
- 102.Roy S., Singha N.R. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes. 2017;7:53. doi: 10.3390/membranes7030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moura D., Mano J.F., Paiva M.C., Alves N.M. Chitosan nanocomposites based on distinct inorganic fillers for biomedical applications. Sci. Technol. Adv. Mater. 2016;17:626–643. doi: 10.1080/14686996.2016.1229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Langroodi A.M., Tajik H., Mehdizadeh T., Moradi M., Kia E.M., Mahmoudian A. Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT. 2018;98:372–380. doi: 10.1016/j.lwt.2018.08.063. [DOI] [Google Scholar]
- 105.Assanti E., Karabagias V.K., Karabagias I.K., Badeka A., Kontominas M.G. Shelf life evaluation of fresh chicken burgers based on the combination of chitosan dip and vacuum packaging under refrigerated storage. J. Food Sci. Technol. 2021;58:870–883. doi: 10.1007/s13197-020-04601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leceta I., Molinaro S., Guerrero P., Kerry J., de la Caba K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015;100:142–150. doi: 10.1016/j.postharvbio.2014.09.022. [DOI] [Google Scholar]
- 107.Nambiar R.B., Sellamuthu P.S., Perumal A.B., Sadiku E.R., Adeyeye O.A. Green Biopolymers and Their Nanocomposites. Springer; Berlin/Heidelberg, Germany: 2019. The Use of Chitosan in Food Packaging Applications; pp. 125–136. [Google Scholar]
- 108.Pourhaghgouy M., Zamanian A., Shahrezaee M., Masouleh M.P. Physicochemical properties and bioactivity of freeze-cast chitosan nanocomposite scaffolds reinforced with bioactive glass. Mater. Sci. Eng. C. 2016;58:180–186. doi: 10.1016/j.msec.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 109.Farhangdoust S., Zamanian A., Yasaei M., Khorami M. The effect of processing parameters and solid concentration on the mechanical and microstructural properties of freeze-casted macroporous hydroxyapatite scaffolds. Mater. Sci. Eng. C. 2013;33:453–460. doi: 10.1016/j.msec.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y., Wakisaka M. Chitosan nanofibers fabricated by combined ultrasonic atomization and freeze casting. Carbohydr. Polym. 2015;122:18–25. doi: 10.1016/j.carbpol.2014.12.080. [DOI] [PubMed] [Google Scholar]
- 111.Liaw B.-S., Chang T.-T., Chang H.-K., Liu W.-K., Chen P.-Y. Fish scale-extracted hydroxyapatite/chitosan composite scaffolds fabricated by freeze casting—An innovative strategy for water treatment. J. Hazard. Mater. 2020;382:121082. doi: 10.1016/j.jhazmat.2019.121082. [DOI] [PubMed] [Google Scholar]
- 112.Wang C., Chen X., Wang B., Huang M., Wang B., Jiang Y., Ruoff R.S. Freeze-Casting Produces a Graphene Oxide Aerogel with a Radial and Centrosymmetric Structure. ACS Nano. 2018;12:5816–5825. doi: 10.1021/acsnano.8b01747. [DOI] [PubMed] [Google Scholar]
- 113.Yin K., Divakar P., Wegst U.G. Freeze-casting porous chitosan ureteral stents for improved drainage. Acta Biomater. 2019;84:231–241. doi: 10.1016/j.actbio.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Costa R.R., Mano J.F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014;43:3453–3479. doi: 10.1039/c3cs60393h. [DOI] [PubMed] [Google Scholar]
- 115.Chang W., Liu F., Sharif H.R., Huang Z., Goff H., Zhong F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019;90:50–61. doi: 10.1016/j.foodhyd.2018.09.026. [DOI] [Google Scholar]
- 116.Panda P.K., Yang J.-M., Chang Y.-H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (γ-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021;171:457–464. doi: 10.1016/j.ijbiomac.2020.12.226. [DOI] [PubMed] [Google Scholar]
- 117.Treviño-Garza M.Z., Garcia S., Heredia N., Alanís-Guzmán M.G., Arévalo-Niño K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus) Postharvest Biol. Technol. 2017;128:63–75. doi: 10.1016/j.postharvbio.2017.01.007. [DOI] [Google Scholar]
- 118.Sadeghianmaryan A., Naghieh S., Sardroud H.A., Yazdanpanah Z., Soltani Y.A., Sernaglia J., Chen X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020;164:3179–3192. doi: 10.1016/j.ijbiomac.2020.08.180. [DOI] [PubMed] [Google Scholar]
- 119.Deng X., Gould M., Ali M.A. Fabrication and characterisation of melt-extruded chitosan/keratin/PCL/PEG drug-eluting sutures designed for wound healing. Mater. Sci. Eng. C. 2021;120:111696. doi: 10.1016/j.msec.2020.111696. [DOI] [PubMed] [Google Scholar]
- 120.Cleymand F., Poerio A., Mamanov A., Elkhoury K., Ikhelf L., Jehl J., Kahn C., Ponçot M., Arab-Tehrany E., Mano J.F. Development of novel chitosan/guar gum inks for extrusion-based 3D bioprinting: Process, printability and properties. Bioprinting. 2021;21:e00122. doi: 10.1016/j.bprint.2020.e00122. [DOI] [Google Scholar]
- 121.Monika, Pal A.K., Bhasney S.M., Bhagabati P., Katiyar V. Effect of dicumyl peroxide on a poly(lactic acid)(PLA)/poly (butylene succinate)(PBS)/functionalized chitosan-based nanobiocomposite for packaging: A reactive extrusion study. ACS Omega. 2018;3:13298–13312. doi: 10.1021/acsomega.8b00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang H., Qian J., Ding F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018;66:395–413. doi: 10.1021/acs.jafc.7b04528. [DOI] [PubMed] [Google Scholar]
- 123.Suhag R., Kumar N., Petkoska A.T., Upadhyay A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020;136:109582. doi: 10.1016/j.foodres.2020.109582. [DOI] [PubMed] [Google Scholar]
- 124.Nitayaphat W., Jiratumnukul N., Charuchinda S., Kittinaovarat S. Mechanical properties of chitosan/bamboo charcoal composite films made with normal and surface oxidized charcoal. Carbohydr. Polym. 2009;78:444–448. doi: 10.1016/j.carbpol.2009.04.027. [DOI] [Google Scholar]
- 125.Hosseini S.M.H., Razavi S.H., Mousavi M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009;33:727–743. doi: 10.1111/j.1745-4549.2008.00307.x. [DOI] [Google Scholar]
- 126.Zakaria Z., Islam S., Hassan A., Haafiz M.K.M., Arjmandi R., Inuwa I.M., Hasan M. Mechanical Properties and Morphological Characterization of PLA/Chitosan/Epoxidized Natural Rubber Composites. Adv. Mater. Sci. Eng. 2013;2013:629092. doi: 10.1155/2013/629092. [DOI] [Google Scholar]
- 127.Vargas M., Albors A., Chiralt A., González-Martínez C. Water interactions and microstructure of chitosan-methylcellulose composite films as affected by ionic concentration. LWT Food Sci. Technol. 2011;44:2290–2295. doi: 10.1016/j.lwt.2011.02.018. [DOI] [Google Scholar]
- 128.Rajan R., Sreekumar P.A., Joseph K., Skrifvars M. Thermal and mechanical properties of chitosan reinforced polyhydroxybutyrate composites. J. Appl. Polym. Sci. 2012;124:3357–3362. doi: 10.1002/app.35341. [DOI] [Google Scholar]
- 129.Noshirvani N., Ghanbarzadeh B., Gardrat C., Rezaei M.R., Hashemi M., Le Coz C., Coma V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017;70:36–45. doi: 10.1016/j.foodhyd.2017.03.015. [DOI] [Google Scholar]
- 130.Zarate-Triviño D., Prokhorov E., Luna-Bárcenas G., Mendez-Nonell J., González-Campos J., Elizalde-Peña E., Mota-Morales J.D., Santiago-Jacinto P., Terrones M., Gómez-Salazar S., et al. The effect of CNT functionalization on electrical and relaxation phenomena in MWCNT/chitosan composites. Mater. Chem. Phys. 2015;155:252–261. doi: 10.1016/j.matchemphys.2015.02.041. [DOI] [Google Scholar]
- 131.Thou C.Z., Khan F.S.A., Mubarak N., Ahmad A., Khalid M., Jagadish P., Walvekar R., Abdullah E., Khan S., Khan M., et al. Surface charge on chitosan/cellulose nanowhiskers composite via functionalized and untreated carbon nanotube. Arab. J. Chem. 2021;14:103022. doi: 10.1016/j.arabjc.2021.103022. [DOI] [Google Scholar]
- 132.Cobos M., González B., Fernández M.J., Fernández M.D. Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int. J. Biol. Macromol. 2018;114:599–613. doi: 10.1016/j.ijbiomac.2018.03.129. [DOI] [PubMed] [Google Scholar]
- 133.Delavar Z., Shojaei A. Enhanced mechanical properties of chitosan/nanodiamond composites by improving interphase using thermal oxidation of nanodiamond. Carbohydr. Polym. 2017;167:219–228. doi: 10.1016/j.carbpol.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 134.Kadam A., Momin B., Palamthodi S., Lele S. Physicochemical and functional properties of chitosan-based nano-composite films incorporated with biogenic silver nanoparticles. Carbohydr. Polym. 2019;211:124–132. doi: 10.1016/j.carbpol.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 135.Bonilla J., Fortunati E., Atarés L., Chiralt A., Kenny J. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014;35:463–470. doi: 10.1016/j.foodhyd.2013.07.002. [DOI] [Google Scholar]
- 136.Shang S., Gan L., Yuen M.C.-W. Improvement of carbon nanotubes dispersion by chitosan salt and its application in silicone rubber. Compos. Sci. Technol. 2013;86:129–134. doi: 10.1016/j.compscitech.2013.07.010. [DOI] [Google Scholar]
- 137.Nurazzi N.M., Sabaruddin F.A., Harussani M.M., Kamarudin S.H., Rayung M., Asyraf M.R.M., Aisyah H.A., Norrrahim M.N.F., Ilyas R.A., Abdullah N., et al. Mechanical Performance and Applications of CNTs Reinforced Polymer Composites—A Review. Nanomaterials. 2021;11:2186. doi: 10.3390/nano11092186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kadier A., Ilyas R.A., Huzaifah M.R.M., Harihastuti N., Sapuan S.M., Harussani M.M., Azlin M.N.M., Yuliasni R., Ibrahim R., Atikah M.S.N., et al. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers. 2021;13:3365. doi: 10.3390/polym13193365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lewandowska K. Characterization of chitosan composites with synthetic polymers and inorganic additives. Int. J. Biol. Macromol. 2015;81:159–164. doi: 10.1016/j.ijbiomac.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Abral H., Atmajaya A., Mahardika M., Hafizulhaq F., Kadriadi, Handayani D., Sapuan S., Ilyas R. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020;9:2477–2486. doi: 10.1016/j.jmrt.2019.12.078. [DOI] [Google Scholar]
- 141.Arumugam S., Kandasamy J., Shah A.U.M., Sultan M.T.H., Safri S.N.A., Majid M.S.A., Basri A.A., Mustapha F. Investigations on the Mechanical Properties of Glass Fiber/Sisal Fiber/Chitosan Reinforced Hybrid Polymer Sandwich Composite Scaffolds for Bone Fracture Fixation Applications. Polymers. 2020;12:1501. doi: 10.3390/polym12071501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ding S.-J. Biodegradation behavior of chitosan/calcium phosphate composites. J. Non-Cryst. Solids. 2007;353:2367–2373. doi: 10.1016/j.jnoncrysol.2007.04.020. [DOI] [Google Scholar]
- 143.Liu K.-H., Liu T.-Y., Chen S.-Y., Liu D.-M. Effect of clay content on electrostimulus deformation and volume recovery behavior of a clay–chitosan hybrid composite. Acta Biomater. 2007;3:919–926. doi: 10.1016/j.actbio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 144.Guo Y.-P., Guan J.-J., Yang J., Wang Y., Zhang C.-Q., Ke Q.-F. Hybrid nanostructured hydroxyapatite–chitosan composite scaffold: Bioinspired fabrication, mechanical properties and biological properties. J. Mater. Chem. B. 2015;3:4679–4689. doi: 10.1039/C5TB00175G. [DOI] [PubMed] [Google Scholar]
- 145.Rodrigues C., de Mello J.M.M., Dalcanton F., Macuvele D.L.P., Padoin N., Fiori M.A., Soares C., Riella H.G. Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J. Polym. Environ. 2020;28:1216–1236. doi: 10.1007/s10924-020-01678-y. [DOI] [Google Scholar]
- 146.Ferreira A., Nunes C., Castro A., Ferreira P., Coimbra M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohydr. Polym. 2014;113:490–499. doi: 10.1016/j.carbpol.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 147.Wu H., Lei Y., Lu J., Zhu R., Xiao D., Jiao C., Xia R., Zhang Z., Shen G., Liu Y., et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019;97:105208. doi: 10.1016/j.foodhyd.2019.105208. [DOI] [Google Scholar]
- 148.Yadav M., Behera K., Chang Y.-H., Chiu F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers. 2020;12:202. doi: 10.3390/polym12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thakhiew W., Devahastin S., Soponronnarit S. Physical and mechanical properties of chitosan films as affected by drying methods and addition of antimicrobial agent. J. Food Eng. 2013;119:140–149. doi: 10.1016/j.jfoodeng.2013.05.020. [DOI] [Google Scholar]
- 150.Deepmala K., Jain N., Singh V.K., Chauhan S. Fabrication and characterization of chitosan coated human hair reinforced phytagel modified soy protein-based green composite. J. Mech. Behav. Mater. 2018;27:1–8. doi: 10.1515/jmbm-2018-0007. [DOI] [Google Scholar]
- 151.Gorade V., Chaudhary B., Parmaj O., Kale R. Preparation and Characterization of Chitosan/viscose Rayon Filament Biocomposite. J. Nat. Fibers. 2020:1–12. doi: 10.1080/15440478.2020.1764442. [DOI] [Google Scholar]
- 152.Prabhakar M.N., Song J.-I. Fabrication and characterisation of starch/chitosan/flax fabric green flame-retardant composites. Int. J. Biol. Macromol. 2018;119:1335–1343. doi: 10.1016/j.ijbiomac.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Verma A., Singh C., Singh V., Jain N. Fabrication and characterization of chitosan-coated sisal fiber–Phytagel modified soy protein-based green composite. J. Compos. Mater. 2019;53:2481–2504. doi: 10.1177/0021998319831748. [DOI] [Google Scholar]
- 154.Yeh J.-T., Chen C.-L., Huang K.-S. Synthesis and properties of chitosan/SiO2 hybrid materials. Mater. Lett. 2007;61:1292–1295. doi: 10.1016/j.matlet.2006.07.016. [DOI] [Google Scholar]
- 155.Covas C.A.P., Argüelles-Monal W., Román J.S. A kinetic study of the thermal degradation of chitosan and a mercaptan derivative of chitosan. Polym. Degrad. Stab. 1993;39:21–28. doi: 10.1016/0141-3910(93)90120-8. [DOI] [Google Scholar]
- 156.Al-Sagheer F., Muslim S. Thermal and Mechanical Properties of Chitosan/SiO2 Hybrid Composites. J. Nanomater. 2010;2010:490679. doi: 10.1155/2010/490679. [DOI] [Google Scholar]
- 157.Kumar A.A.J., Srinivasan V. Thermal characteristics of chitosan dispersed poly lactic acid/basalt hybrid composites. Mater. Express. 2016;6:337–343. doi: 10.1166/mex.2016.1310. [DOI] [Google Scholar]
- 158.Celebi H., Kurt A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015;133:284–293. doi: 10.1016/j.carbpol.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 159.Sabzevari M., Cree D.E., Wilson L.D. Graphene Oxide–Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega. 2018;3:13045–13054. doi: 10.1021/acsomega.8b01871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li N., Bai R. Copper adsorption on chitosan–cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005;42:237–247. doi: 10.1016/j.seppur.2004.08.002. [DOI] [Google Scholar]
- 161.Liu Z., Wang H., Liu C., Jiang Y., Yu G., Mu X., Wang X. Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem. Commun. 2012;48:7350–7352. doi: 10.1039/c2cc17795a. [DOI] [PubMed] [Google Scholar]
- 162.Zhou Y., Fu S., Zhang L., Zhan H., Levit M.V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II) Carbohydr. Polym. 2014;101:75–82. doi: 10.1016/j.carbpol.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 163.Rogovina S., Akopova T., Vikhoreva G., Gorbacheva I. Solid state production of cellulose–chitosan blends and their modification with the diglycidyl ether of oligo(ethylene oxide) Polym. Degrad. Stab. 2001;73:557–560. doi: 10.1016/S0141-3910(01)00141-0. [DOI] [Google Scholar]
- 164.Stefanescu C., Daly W.H., Negulescu I.I. Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose. Carbohydr. Polym. 2012;87:435–443. doi: 10.1016/j.carbpol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 165.Azizi S., Ahmad M.B., Hussein M.Z., Ibrahim N.A., Namvar F. Preparation and properties of poly(vinyl alcohol)/chitosan blend bionanocomposites reinforced with cellulose nanocrystals/ZnO-Ag multifunctional nanosized filler. Int. J. Nanomed. 2014;9:1909–1917. doi: 10.2147/IJN.S60274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu Y.-B., Yu S.-H., Mi F.-L., Wu C.-W., Shyu S.-S., Peng C.-K., Chao A.-C. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohydr. Polym. 2004;57:435–440. doi: 10.1016/j.carbpol.2004.05.013. [DOI] [Google Scholar]
- 167.Azevedo E.P., Retarekar R., Raghavan M.L., Kumar V. Mechanical properties of cellulose: Chitosan blends for potential use as a coronary artery bypass graft. J. Biomater. Sci. Polym. Ed. 2013;24:239–252. doi: 10.1080/09205063.2012.690273. [DOI] [PubMed] [Google Scholar]
- 168.Feng H., Zhang L., Zhu C. Genipin crosslinked ethyl cellulose–chitosan complex microspheres for anti-tuberculosis delivery. Colloids Surf. B Biointerfaces. 2013;103:530–537. doi: 10.1016/j.colsurfb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 169.Trigueiro J.P.C., Silva G.G., Pereira F.V., Lavall R.L. Layer-by-layer assembled films of multi-walled carbon nanotubes with chitosan and cellulose nanocrystals. J. Colloid Interface Sci. 2014;432:214–220. doi: 10.1016/j.jcis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 170.Bao W., Xu C., Song F., Wang X., Wang Y. Preparation and properties of cellulose/chitosan transparent films. Acta Polym. Sin. 2015;1:49–56. doi: 10.11777/j.issn1000-3304.2015.14170. [DOI] [Google Scholar]
- 171.Yu S.-H., Hsieh H.-Y., Pang J.-C., Tang D.-W., Shih C.-M., Tsai M.-L., Tsai Y.-C., Mi F.-L. Active films from water-soluble chitosan/cellulose composites incorporating releasable caffeic acid for inhibition of lipid oxidation in fish oil emulsions. Food Hydrocoll. 2013;32:9–19. doi: 10.1016/j.foodhyd.2012.11.036. [DOI] [Google Scholar]
- 172.Twu Y.-K., Huang H.-I., Chang S.-Y., Wang S.-L. Preparation and sorption activity of chitosan/cellulose blend beads. Carbohydr. Polym. 2003;54:425–430. doi: 10.1016/j.carbpol.2003.03.001. [DOI] [Google Scholar]
- 173.Duan J., Han C., Liu L., Jiang J., Li J., Li Y., Guan C. Binding Cellulose and Chitosan via Intermolecular Inclusion Interaction: Synthesis and Characterisation of Gel. J. Spectrosc. 2015;2015:179258. doi: 10.1155/2015/179258. [DOI] [Google Scholar]
- 174.Xu X., Zhuang X., Cheng B., Xu J., Long G., Zhang H. Manufacture and properties of cellulose/O-hydroxyethyl chitosan blend fibers. Carbohydr. Polym. 2010;81:541–544. doi: 10.1016/j.carbpol.2010.03.011. [DOI] [Google Scholar]
- 175.Tran C.D., Duri S., Harkins A.L. Recyclable synthesis, characterization, and antimicrobial activity of chitosan-based polysaccharide composite materials. J. Biomed. Mater. Res. Part A. 2013;101A:2248–2257. doi: 10.1002/jbm.a.34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harkins A.L., Duri S., Kloth L.C., Tran C.D. Chitosan-cellulose composite for wound dressing material. Part Antimicrobial activity, blood absorption ability, and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;102:1199–1206. doi: 10.1002/jbm.b.33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abou-Zeid N., Waly A., Kandile N., Rushdy A., El-Sheikh M., Ibrahim H. Preparation, characterization and antibacterial properties of cyanoethylchitosan/cellulose acetate polymer blended films. Carbohydr. Polym. 2011;84:223–230. doi: 10.1016/j.carbpol.2010.11.026. [DOI] [Google Scholar]
- 178.Aider M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010;43:837–842. doi: 10.1016/j.lwt.2010.01.021. [DOI] [Google Scholar]
- 179.Bernkop-Schnürch A., Dünnhaupt S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 180.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 181.Ali A., Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018;109:273–286. doi: 10.1016/j.ijbiomac.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 182.Dhivya S., Padma V.V., Santhini E. Wound dressings—A review. BioMedicine. 2015;5:22. doi: 10.7603/s40681-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matica M.A., Aachmann F.L., Tøndervik A., Sletta H., Ostafe V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019;20:5889. doi: 10.3390/ijms20235889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramos-e-Silva M., de Castro M.C.R. New dressings, including tissue-engineered living skin. Clin. Dermatol. 2002;20:715–723. doi: 10.1016/S0738-081X(02)00298-5. [DOI] [PubMed] [Google Scholar]
- 185.Jayakumar R., Prabaharan M., Kumar P.T.S., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 186.Xue Y., Mou Z., Xiao H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale. 2017;9:14758–14781. doi: 10.1039/C7NR04994C. [DOI] [PubMed] [Google Scholar]
- 187.Pourshahrestani S., Zeimaran E., Kadri N.A., Gargiulo N., Jindal H.M., Naveen S.V., Sekaran S.D., Kamarul T., Towler M.R. Potency and Cytotoxicity of a Novel Gallium-Containing Mesoporous Bioactive Glass/Chitosan Composite Scaffold as Hemostatic Agents. ACS Appl. Mater. Interfaces. 2017;9:31381–31392. doi: 10.1021/acsami.7b07769. [DOI] [PubMed] [Google Scholar]
- 188.Huang N., Lin J., Li S., Deng Y., Kong S., Hong P., Yang P., Liao M., Hu Z. Preparation and evaluation of squid ink polysaccharide-chitosan as a wound-healing sponge. Mater. Sci. Eng. C. 2018;82:354–362. doi: 10.1016/j.msec.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 189.Zahedi P., Rezaeian I., Ranaei-Siadat S.-O., Jafari S.H., Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2009;21:77–95. doi: 10.1002/pat.1625. [DOI] [Google Scholar]
- 190.Brás T., Rosa D., Gonçalves A.C., Gomes A.C., Alves V.D., Crespo J.G., Duarte M.F., Neves L.A. Development of bioactive films based on chitosan and Cynara cardunculus leaves extracts for wound dressings. Int. J. Biol. Macromol. 2020;163:1707–1718. doi: 10.1016/j.ijbiomac.2020.09.109. [DOI] [PubMed] [Google Scholar]
- 191.Moeini A., Pedram P., Makvandi P., Malinconico M., d’Ayala G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020;233:115839. doi: 10.1016/j.carbpol.2020.115839. [DOI] [PubMed] [Google Scholar]
- 192.Kaczmarek B., Nadolna K., Owczarek A., Michalska-Sionkowska M., Sionkowska A. The characterization of thin films based on chitosan and tannic acid mixture for potential applications as wound dressings. Polym. Test. 2019;78:106007. doi: 10.1016/j.polymertesting.2019.106007. [DOI] [Google Scholar]
- 193.Cheng F., He J., Yan T., Liu C., Wei X., Li J., Huang Y. Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose. RSC Adv. 2016;6:94429–94436. doi: 10.1039/C6RA15983D. [DOI] [Google Scholar]
- 194.Dumont M., Villet R., Guirand M., Montembault A., Delair T., Lack S., Barikosky M., Crepet A., Alcouffe P., Laurent F., et al. Processing and antibacterial properties of chitosan-coated alginate fibers. Carbohydr. Polym. 2018;190:31–42. doi: 10.1016/j.carbpol.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 195.Gu B.K., Park S.J., Kim M.S., Lee Y.J., Kim J.-I., Kim C.-H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int. J. Biol. Macromol. 2016;82:89–96. doi: 10.1016/j.ijbiomac.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 196.Pilehvar-Soltanahmadi Y., Dadashpour M., Mohajeri A., Fattahi A., Sheervalilou R., Zarghami N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2018;18:414–427. doi: 10.2174/1389557517666170308112147. [DOI] [PubMed] [Google Scholar]
- 197.Seon G.M., Lee M.H., Kwon B.-J., Kim M.S., Koo M.-A., Seomun Y., Kim J.-T., Kim T.H., Park J.-C. Recombinant batroxobin-coated nonwoven chitosan as hemostatic dressing for initial hemorrhage control. Int. J. Biol. Macromol. 2018;113:757–763. doi: 10.1016/j.ijbiomac.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 198.He J.M., Wu Y.D., Wang F.W., Cheng W.L., Huang Y.D., Fu B. Hemostatic, antibacterial and degradable performance of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Fibers Polym. 2014;15:504–509. doi: 10.1007/s12221-014-0504-5. [DOI] [Google Scholar]
- 199.Bhattarai S.R., Bhattarai N., Yi H.K., Hwang P.H., Cha D.I., Kim H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials. 2004;25:2595–2602. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 200.Moghadas B., Solouk A., Sadeghi D. Development of chitosan membrane using non-toxic crosslinkers for potential wound dressing applications. Polym. Bull. 2021;78:4919–4929. doi: 10.1007/s00289-020-03352-8. [DOI] [Google Scholar]
- 201.Yang C., Dan N., You W., Huang Y., Chen Y., Yu G., Dan W., Wen H. Modification of collagen-chitosan membrane by oxidation sodium alginate and in vivo/in vitro evaluation for wound dressing application. Int. J. Polym. Anal. Charact. 2019;24:619–629. doi: 10.1080/1023666X.2019.1648637. [DOI] [Google Scholar]
- 202.Fan L., Yang H., Yang J., Peng M., Hu J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016;146:427–434. doi: 10.1016/j.carbpol.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 203.Song H.-F., Chen A.-Z., Wang S.-B., Kang Y.-Q., Ye S.-F., Liu Y.-G., Wu W.-G. Preparation of Chitosan-Based Hemostatic Sponges by Supercritical Fluid Technology. Materials. 2014;7:2459–2473. doi: 10.3390/ma7042459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Patrulea V., Applegate L.A., Ostafe V., Jordan O., Borchard G. Optimized synthesis of O-carboxymethyl-N,N,N-trimethyl chitosan. Carbohydr. Polym. 2015;122:46–52. doi: 10.1016/j.carbpol.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 205.Ahmed E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ryu J.H., Lee Y., Kong W.H., Kim T.G., Park T.G., Lee H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules. 2011;12:2653–2659. doi: 10.1021/bm200464x. [DOI] [PubMed] [Google Scholar]
- 207.Falguera V., Quintero J.P., Jiménez A., Muñoz J.A., Ibarz A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- 208.Kaur P., Sandhu K., Bangar S., Purewal S., Kaur M., Ilyas R., Asyraf M., Razman M. Unraveling the Bioactive Profile, Antioxidant and DNA Damage Protection Potential of Rye (Secale cereale) Flour. Antioxidants. 2021;10:1214. doi: 10.3390/antiox10081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mahato N., Sharma K., KoteswaraRao R., Sinha M., Baral E.R., Cho M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019;59:611–625. doi: 10.1080/10408398.2017.1384716. [DOI] [PubMed] [Google Scholar]
- 210.Xing Y., Xu Q., Li X., Chen C., Ma L., Li S., Che Z., Lin H. Chitosan-Based Coating with Antimicrobial Agents: Preparation, Property, Mechanism, and Application Effectiveness on Fruits and Vegetables. Int. J. Polym. Sci. 2016;2016:4851730. doi: 10.1155/2016/4851730. [DOI] [Google Scholar]
- 211.Arabpoor B., Yousefi S., Weisany W., Ghasemlou M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocoll. 2021;111:106394. doi: 10.1016/j.foodhyd.2020.106394. [DOI] [Google Scholar]
- 212.Cui H., Yuan L., Li W., Lin L. Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157: H7 on cherry tomato. Int. J. Food Sci. Technol. 2017;52:687–698. doi: 10.1111/ijfs.13322. [DOI] [Google Scholar]
- 213.Zhang X., Ismail B.B., Cheng H., Jin T.Z., Qian M., Arabi S.A., Liu D., Guo M. Emerging chitosan-essential oil films and coatings for food preservation-A review of advances and applications. Carbohydr. Polym. 2021;273:118616. doi: 10.1016/j.carbpol.2021.118616. [DOI] [PubMed] [Google Scholar]
- 214.Shariatinia Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019;263:131–194. doi: 10.1016/j.cis.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 215.Singla A.K., Chawla M. Chitosan: Some pharmaceutical and biological aspects-An update. J. Pharm. Pharmacol. 2010;53:1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 216.Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 217.Liu X.-Y., Chen C., Xu H.-H., Zhang Y.-S., Zhong L., Hu N., Jia X.-L., Wang Y.-W., Zhong K.-H., Liu C., et al. Integrated printed BDNF/collagen/chitosan scaffolds with low temperature extrusion 3D printer accelerated neural regeneration after spinal cord injury. Regen. Biomater. 2021;8:rbab047. doi: 10.1093/rb/rbab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cazón P., Vázquez M. Sustainable Agriculture Reviews 36. Springer; Berlin/Heidelberg, Germany: 2019. Applications of Chitosan as Food Packaging Materials; pp. 81–123. [DOI] [Google Scholar]
- 219.Chien P.-J., Sheu F., Yang F.-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007;78:225–229. doi: 10.1016/j.jfoodeng.2005.09.022. [DOI] [Google Scholar]
- 220.Moreira M.D.R., Roura S.I., Ponce A. Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT Food Sci. Technol. 2011;44:2335–2341. doi: 10.1016/j.lwt.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 221.Taheri A., Behnamian M., Dezhsetan S., Karimirad R. Shelf life extension of bell pepper by application of chitosan nanoparticles containing Heracleum persicum fruit essential oil. Postharvest Biol. Technol. 2020;170:111313. doi: 10.1016/j.postharvbio.2020.111313. [DOI] [Google Scholar]
- 222.Kakaei S., Shahbazi Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016;72:432–438. doi: 10.1016/j.lwt.2016.05.021. [DOI] [Google Scholar]
- 223.Valencia-Sullca C.E., Atarés L., Vargas M., Chiralt A. Physical and Antimicrobial Properties of Compression-Molded Cassava Starch-Chitosan Films for Meat Preservation. Food Bioprocess Technol. 2018;11:1339–1349. doi: 10.1007/s11947-018-2094-5. [DOI] [Google Scholar]
- 224.Mohammadi A., Hashemi M., Hosseini S.M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 2016;33:580–588. doi: 10.1016/j.ifset.2015.10.015. [DOI] [Google Scholar]
- 225.Liu F., Chang W., Chen M., Xu F., Ma J., Zhong F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019;212:150–159. doi: 10.1016/j.carbpol.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 226.Amor G., Sabbah M., Caputo L., Idbella M., de Feo V., Porta R., Fechtali T., Mauriello G. Basil Essential Oil: Composition, Antimicrobial Properties, and Microencapsulation to Produce Active Chitosan Films for Food Packaging. Foods. 2021;10:121. doi: 10.3390/foods10010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang D., Dong Y., Chen X., Liu Y., Wang J., Wang X., Wang C., Song H. Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 2020;162:838–844. doi: 10.1016/j.ijbiomac.2020.06.220. [DOI] [PubMed] [Google Scholar]
- 228.Riaz A., Aadil R.M., Amoussa A.M.O., Bashari M., Abid M., Hashim M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021;45:e15018. doi: 10.1111/jfpp.15018. [DOI] [Google Scholar]
- 229.Khorshidi S., Mehdizadeh T., Ghorbani M. The effect of chitosan coatings enriched with the extracts and essential oils of Elettaria Cardamomum on the shelf-life of chicken drumsticks vacuum-packaged at 4 °C. J. Food Sci. Technol. 2021;58:2924–2935. doi: 10.1007/s13197-020-04794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jimtaisong A., Saewan N. Use of Chitosan and its Derivatives in Cosmetics. Househ. Pers. Care Today. 2015;9:20–24. [Google Scholar]
- 231.Qin C., Du Y., Xiao L., Liu Y., Yu H. Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J. Appl. Polym. Sci. 2002;86:1724–1730. doi: 10.1002/app.11080. [DOI] [Google Scholar]
- 232.Sakulwech S., Lourith N., Ruktanonchai U., Kanlayavattanakul M. Preparation and characterization of nanoparticles from quaternized cyclodextrin-grafted chitosan associated with hyaluronic acid for cosmetics. Asian J. Pharm. Sci. 2018;13:498–504. doi: 10.1016/j.ajps.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Misak H., Zacharias N., Song Z., Hwang S., Man K.-P., Asmatulu R., Yang S.-Y. Skin cancer treatment by albumin/5-Fu loaded magnetic nanocomposite spheres in a mouse model. J. Biotechnol. 2013;164:130–136. doi: 10.1016/j.jbiotec.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 234.Achmad H., Ramadhany Y.F. Effectiveness of chitosan tooth paste from white shrimp (Litopenaeusvannamei) to reduce number of Streptococcus mutans in the case of early childhood caries. J. Int. Dent. Med. Res. 2017;10:358–363. [Google Scholar]
- 235.İkinci G., Şenel S., Akıncıbay H., Kaş S., Erciş S., Wilson C.G., Hıncal A.A. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 2002;235:121–127. doi: 10.1016/S0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- 236.Ji Q.X., Zhong D.Y., Lü R., Zhang W.Q., Deng J., Chen X.G. In vitro evaluation of the biomedical properties of chitosan and quaternized chitosan for dental applications. Carbohydr. Res. 2009;344:1297–1302. doi: 10.1016/j.carres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 237.Costa E.M., Silva S., Veiga M., Tavaria F.K., Pintado M.M. A review of chitosan’s effect on oral biofilms: Perspectives from the tube to the mouth. J. Oral Biosci. 2017;59:205–210. doi: 10.1016/j.job.2017.07.001. [DOI] [Google Scholar]
- 238.Johari A.N., Ishak M.R., Leman Z., Yusoff M.Z.M., Asyraf M.R.M. Influence of CaCO3 in pultruded glass fiber/unsaturated polyester resin composite on flexural creep behavior using conventional and time-temperature superposition principle methods. Polimery. 2020;65:792–800. doi: 10.14314/polimery.2020.11.6. [DOI] [Google Scholar]
- 239.Ilyas R., Sapuan S., Asyraf M., Dayana D., Amelia J., Rani M., Norrrahim M., Nurazzi N., Aisyah H., Sharma S., et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers. 2021;13:1701. doi: 10.3390/polym13111701. [DOI] [PMC free article] [PubMed] [Google Scholar]