Abstract
This randomized, open-label, crossover study was conducted to investigate whether the coadministration of zafirlukast would affect the pharmacokinetics of azithromycin, clarithromycin, or 14-hydroxyclarithromycin (14-OHC). Twelve healthy subjects (six males and six females) received single 500-mg doses of azithromycin and clarithromycin with and without zafirlukast given to a steady-state concentration. Blood was collected prior to all macrolide doses and for 3 and 10 days after each clarithromycin and azithromycin dose, respectively. Serum was assayed for azithromycin, clarithromycin, and 14-OHC concentrations by validated high-performance liquid chromatography assay systems. Data analyses were done by noncompartmental and nonparametric methods. Analysis of the patients indicated that the addition of steady-state concentrations of zafirlukast did not significantly alter the pharmacokinetic parameters of or overall exposure (based on the area under the concentration-time curve) to azithromycin, clarithromycin, and 14-OHC. While zafirlukast is a known inhibitor of CYP3A4, it does not appear to exert a clinically or statistically significant pharmacokinetic effect on azithromycin, clarithromycin, or 14-OHC.
Azithromycin and clarithromycin are macrolide antibiotics used extensively for the treatment of outpatient bacterial infections (1, 6). Although the pharmacokinetics and activity of azithromycin do not appear to be affected by alterations in the CYP systems, the activity of clarithromycin against Haemophilus influenzae, Helicobacter pylori, and other organisms is enhanced by the formation of an active metabolite, 14-hydroxyclarithromycin (14-OHC) (1, 2). The metabolism of clarithromycin to this active metabolite occurs via the CYP3A4 enzyme system. Past studies with clarithromycin suggest that not only can clarithromycin have an inhibitory effect on the CYP3A4 metabolism of other drugs, but its own metabolism by this pathway can be both induced (e.g., rifabutin) and inhibited (e.g., delavirdine) (2, 8, 11). Zafirlukast, a leukotriene receptor antagonist recently approved for the treatment of mild to moderate asthma, is a known inhibitor of CYP3A4/2C9; however, its effect on the pharmacokinetics of azithromycin and clarithromycin have not been studied (5, 12). Asthmatics have a high incidence of bacterial infections and thus the potential for the concurrent use of zafirlukast with either of these commonly used macrolides is high (10). This randomized, open-label, crossover study was conducted to investigate whether steady-state concentrations of zafirlukast would affect the pharmacokinetics of either azithromycin, clarithromycin, or 14-OHC.
MATERIALS AND METHODS
Twelve nonsmoking, healthy volunteers (six males and six females), aged 39 ± 5 years (mean ± standard deviation), with a mean weight of 68 ± 12 kg, were recruited, and they gave written informed consent for this study, which was approved by the Institutional Review Board of Bassett Healthcare, Cooperstown, N.Y. Based on an α probability level of 0.10 and a standard deviation of 9 mg/liter (for area under the concentration-time curve [AUC] of clarithromycin) (3), the use of the 12 subjects resulted in a power of 0.70 to identify an estimated clinically significant change of 25%. Each subject underwent a complete history and physical examination and had laboratory evaluations to determine liver and kidney functions. None had a known allergy to the study medications or related compounds or a serious allergy to any medication. No exposure to drugs, including alcohol and caffeine (with the exception of occasional acetaminophen use), was permitted during the study arms. Women were required to undergo a pregnancy test prior to entering and before each arm of the study and to use a barrier method of birth control during the entire length of, and for 3 months past the conclusion of, the study.
The volunteers, with at least a 1-week or 4-week washout period between the clarithromycin and azithromycin arms, randomly received the following four treatments in a crossover manner: (i) a single oral dose of 500 mg of azithromycin, (ii) 20 mg of zafirlukast (Accolate; Zeneca Pharmaceuticals) (lot no. FAA021; expiration date, June 98) twice a day for 12 days with a single oral dose of 500 mg of azithromycin given 2 h after the fifth dose, (iii) a single oral dose of 500 mg of clarithromycin, and (iv) 20 mg of zafirlukast twice a day for 4 days with a single oral dose of 500 mg of clarithromycin given 2 h after the fifth dose. Volunteers were required to take each dose of zafirlukast 1 h before or 2 h after food ingestion and were required to fast for 12 h prior to and 4 h after the administration of macrolide doses. The investigators observed the administration of the macrolides and the morning doses of zafirlukast. Volunteers were also required to return the unit dose packaging of the prior evening’s zafirlukast dose and to record the time the drug was taken. On the study mornings, an indwelling catheter was inserted into a forearm vein and was kept open with dilute solutions of heparin and normal saline. Blood samples were drawn just before (baseline) each macrolide dose and aggressively for 3 and 10 days (during which time zafirlukast administration was continued during the test arms) after each clarithromycin and azithromycin dose, respectively. Serum was separated by centrifugation, and all samples were subsequently frozen at −80°C until analysis. All macrolide doses were administered with 240 ml of tap water in each study period, and volunteers were questioned concerning adverse effects at the time of each blood collection.
Samples, packaged frozen in dry ice, were shipped to the Infectious Diseases Pharmacokinetics Laboratory at the National Jewish Medical and Research Center in Denver, Colo. All serum specimens were assayed for azithromycin, clarithromycin, and 14-OHC by using validated high-performance liquid chromatography (HPLC) assay procedures. All HPLC was performed with the following equipment: a Waters (Milford, Mass.) model 510 pump and model 680 gradient controller and solvent select valve, a Spectra Physics (San Jose, Calif.) model 8875 fixed-volume autosampler, and an ESA (Bedford, Mass.) Coulochem II electrochemical detector, a Macintosh 7100 computer (Apple Computers, Inc., Cupertino, Calif.), and the Rainin (Woburn, Mass.) Dynamax HPLC data management system. The standard curves for serum azithromycin ranged from 0.05 to 5.00 mg/liter. The best fits of the standard curves were achieved with a weight of 1/Y2. The coefficients of determination (R2) for the standard curves all exceeded 0.99. The median recovery of azithromycin from serum was 85.8% (range, 70.7 to 93.9%). Testing for azithromycin within-day precision produced a median coefficient of variation (CV) of 1.9% (low, 0% at 0.25 mg/liter; high, 3.9% at 1.00 mg/liter). Testing for azithromycin overall-assay precision produced a median CV of 5.2% (low, 3.1% at 5.00 mg/liter; high, 9.5% at 0.05 mg/liter). Validation quality control sample CV values varied from 5.1% (0.21 mg/liter) to 11.4% (1.50 mg/liter).
The standard curves for serum clarithromycin ranged from 0.20 to 10.00 mg/liter. The best fits of the standard curves were achieved with a weight of 1/Y2. The R2 values for the standard curves all exceeded 0.99. The median recovery of clarithromycin from serum was 102.6% (range, 94.8 to 104.5%). Testing for clarithromycin within-day precision produced a median CV of 2.8% (low, 0.9% at 10.00 mg/liter; high, 10.3% at 0.05 mg/liter). Testing for clarithromycin overall-assay precision produced a median CV of 3.7% (low, 2.2% at 10.00 mg/liter; high, 6.5% at 0.20 mg/liter). Validation quality control sample CV varied from 6.8% (7.5 mg/liter) to 13.3% (1.60 mg/liter). Similar results were seen with 14-OHC.
The pharmacokinetics of azithromycin, clarithromycin, and 14-OHC were assessed by standard noncompartmental methods by using the TopFit version 2.0 software packet (4, 9). The maximum concentration in serum (Cmax) and the time to reach the maximum concentration in serum (Tmax) were read by the program directly from the serum concentration-time data for each volunteer. The area under the serum concentration-time curves from 0 h to the final time point (AUCT) or infinity (AUC0–∞) were calculated for azithromycin and for clarithromycin and 14-OHC, respectively, by using the linear trapezoidal rule.
Data sets were assessed for distribution normality by the Kolmogorov-Smirnov test for normality with the SigmaStat version 2.03 software package (SPSS, Inc.). Since a portion of the data sets failed normality analyses, the Wilcoxon signed-rank test was utilized to assess for statistically significant differences between control and test phases with the SYSTAT version 7.0 software package (SPSS, Inc.). P values of less than 0.05 were considered significant.
RESULTS
Twelve volunteers completed both arms of this trial; however, the clarithromycin data of one volunteer were not utilized due to a technical error during a study arm. Most adverse events were rated as mild to moderate by the study investigators and were gastrointestinal in nature, which was attributed by the study volunteers to the lack of food and caffeine, although the macrolide doses could not be ruled out as potential causes. A minority of volunteers also experienced headaches, which were thought to be secondary to caffeine withdrawal, and chest tightness or lightheadedness, which were thought to be secondary to zafirlukast dosing.
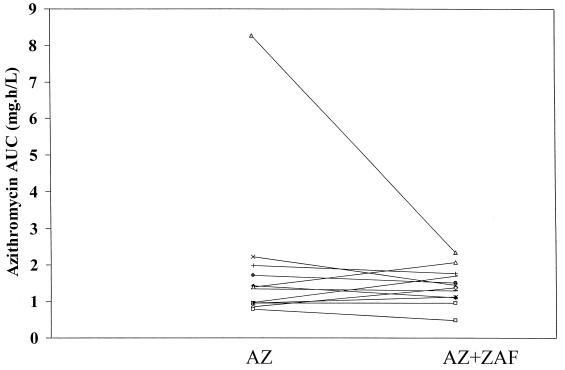
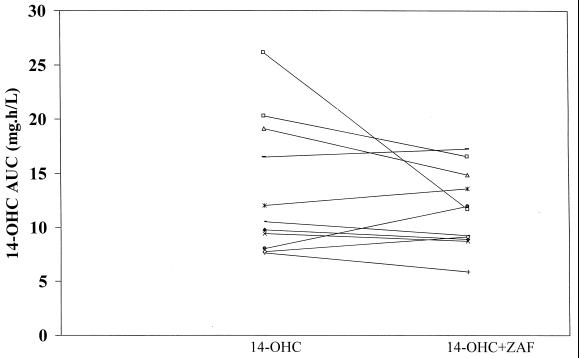
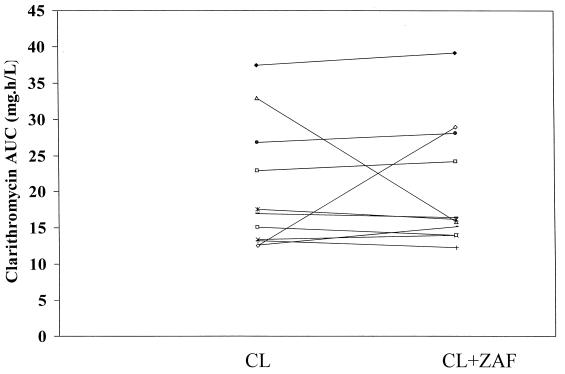
A summary of the pharmacokinetic parameters for azithromycin, clarithromycin, and 14-OHC is presented in Table 1. Analysis of the data indicated that the addition of steady-state concentrations of zafirlukast did not significantly alter the pharmacokinetic parameters of either azithromycin or clarithromycin and its metabolite. Additionally, exposure to zafirlukast did not significantly change the AUC of the macrolides, although the AUCs of azithromycin and 14-OHC were slightly less when these macrolides were given with zafirlukast (see individual data in Fig. 1 to 3).
TABLE 1.
Pharmacokinetic results of the macrolides alone and with zafirlukasta
| Treatment phase | Cmax (mg/liter) | Tmax (mg/liter) | AUCb (mg · h/liter) |
|---|---|---|---|
| Azithromycin | |||
| Alone | 0.33 ± 0.14 | 3.12 ± 1.33 | 1.91 ± 2.05 |
| With zafirlukast | 0.38 ± 0.15c | 2.68 ± 1.35d | 1.43 ± 0.51d |
| Clarithromycin | |||
| Alone | 2.47 ± 0.75 | 1.56 ± 0.46 | 20.15 ± 8.74 |
| With zafirlukast | 2.35 ± 0.47e | 1.52 ± 0.53f | 20.41 ± 8.53d |
| 14-OHC | |||
| Alone | 1.12 ± 0.41 | 2.01 ± 1.40 | 13.37 ± 6.22 |
| With zafirlukast | 1.10 ± 0.37g | 1.49 ± 0.85h | 11.62 ± 3.63c |
Values are means ± standard deviations.
AUC denotes AUCT data for azithromycin and AUC0–∞ data for clarithromycin 14 and OHC.
Not significantly different from preceding value at P = 0.33.
Not significantly different from preceding value at P = 0.48.
Not significantly different from preceding value at P = 0.72.
Not significantly different from preceding value at P = 0.88.
Not significantly different from preceding value at P = 0.59.
Not significantly different from preceding value at P = 0.28.
FIG. 1.
Individual azithromycin AUCT values during both the control (azithromycin [AZ]) and test (AZ plus zafirlukast [ZAF]) phases.
FIG. 3.
Individual 14-OHC AUC∞ values during both the control (14-OHC) and test (14-OHC plus zafirlukast [ZAF]) phases.
DISCUSSION
The minor change in the AUCs of azithromycin and 14-OHC most likely represent normal fluctuations in macrolide pharmacokinetics or mild interactions whose significance the study was not designed to appropriately identify. Based on the literature, the lack of interaction of azithromycin with CYP systems suggests that the change in exposure is attributable to intersubject variations and intrasubject fluctuations (2). For 14-OHC, clarithromycin’s metabolite, one would have to wonder whether the lack of interaction is due to either of these previously mentioned reasons. The relatively minor change in AUCs could easily be explained by inherent metabolism fluctuations. However, the literature supports not only that clarithromycin is a strong inhibitor of CYP enzyme systems but that its own metabolism is also susceptible to inhibition. One study of six human immunodeficiency virus-positive patients who were coadministered 500 mg of clarithromycin twice daily and 300 mg of delavirdine three times a day noted that the concurrent use of these agents not only increased the AUC of delavirdine by 44% but also increased the AUC of clarithromycin by 100%, thereby resulting in a 75% decrease in the AUC of 14-OHC (8). This has been noted with protease inhibitors as well, but the mechanism of the interaction is not as clear. Despite zafirlukast being a known inhibitor of CYP3A4, albeit a weak one, it did not have either a statistically or a clinically significant effect on clarithromycin or its metabolite. Arguments that the zafirlukast was not administered long enough, prior to macrolide administration, to fully inhibit CYP enzymes may be valid but is doubtful. Zafirlukast was administered to steady-state concentrations in this study, and there are indications that zafirlukast begins CYP inhibition immediately upon the start of dosing. One report describes an elderly patient that had been stabilized on warfarin for several months. Shortly after the start of zafirlukast (20 mg twice daily), the patient’s prothrombin time was noted to have more than doubled and her international normalized ratio had more than quadrupled. These changes in clotting indices normalized once the zafirlukast was discontinued (7). The reverse of this interaction, clarithromycin or azithromycin with zafirlukast, still needs to be investigated as there have been questions concerning erythromycin administration potentially resulting in decreased mean concentrations of zafirlukast in plasma (12).
FIG. 2.
Individual clarithromycin AUC∞ values during both the control (clarithromycin [CL]) and test (CL plus zafirlukast [ZAF]) phases.
ACKNOWLEDGMENTS
We thank Ruth Blackman, Laura Cabelus, and Anne Menhinick for valuable nursing support and Jennifer Chen, Alison Killen, and Roberta Steere for technical assistance.
REFERENCES
- 1.Amsden G W. Erythromycin, clarithromycin, and azithromycin: are the differences real? Clin Ther. 1996;18:56–72. doi: 10.1016/s0149-2918(96)80179-2. [DOI] [PubMed] [Google Scholar]
- 2.Amsden G W. Macrolides versus azalides: a drug interaction update. Ann Pharmacother. 1995;29:906–917. doi: 10.1177/106002809502900913. [DOI] [PubMed] [Google Scholar]
- 3.Cheng K L, Nafziger A N, Peloquin C A, Amsden G W. Effect of grapefruit juice on clarithromycin pharmacokinetics. Antimicrob Agents Chemother. 1998;42:927–929. doi: 10.1128/aac.42.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibaldi M, Perrier D. Pharmacokinetics. New York, N.Y: Marcel Dekker, Inc.; 1975. [Google Scholar]
- 5.Kelloway J S. Zafirlukast: the first leukotriene-receptor antagonist approved for the treatment of asthma. Ann Pharmacother. 1997;31:1012–1021. doi: 10.1177/106002809703100912. [DOI] [PubMed] [Google Scholar]
- 6.Langtry H D, Brogden R N. Clarithromycin. A review of its efficacy in the treatment of respiratory tract infections in immunocompetent patients. Drugs. 1997;53:973–1004. doi: 10.2165/00003495-199753060-00006. [DOI] [PubMed] [Google Scholar]
- 7.Morkunas A, Graeme K. Zafirlukast-warfarin drug interaction with gastrointestinal bleeding. J Toxicol Clin Toxicol. 1997;35:501. . (Abstract.) [Google Scholar]
- 8.Pharmacia-Upjohn. Product information, Rescriptor. Bridgewater, N.J: Pharmacia-Upjohn; 1997. [Google Scholar]
- 9.Tanswell P, Koup J. TopFit: a PC based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993;31:514–520. [PubMed] [Google Scholar]
- 10.Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112:591–596. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace R J, Jr, Brown B A, Griffith D E, Girard W, Tanaka K. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis. 1995;171:747–750. doi: 10.1093/infdis/171.3.747. [DOI] [PubMed] [Google Scholar]
- 12.Zeneca Pharmaceuticals. Prod information, Accolate. Wilmington, Del: Zeneca Pharmaceuticals; 1998. [Google Scholar]