Abstract
The steroidal alkaloid tomatidine is an aglycone of α-tomatine, which is abundant in tomato leaves and has several biological activities. Tomatidine has been reported to inhibit the growth of cultured cancer cells in vitro, but its anti-cancer activity in vivo and inhibitory effect against gastric cancer cells remain unknown. We investigated the efficacy of tomatidine using human gastric cancer-derived 85As2 cells and its tumor-bearing mouse model and evaluated the effect of tomatidine-rich tomato leaf extract (TRTLE) obtained from tomato leaves. In the tumor-bearing mouse model, tumor growth was significantly inhibited by feeding a diet containing tomatidine and TRTLE for 3 weeks. Tomatidine and TRTLE also inhibited the proliferation of cultured 85As2 cells. Microarray data of gene expression analysis in mouse tumors revealed that the expression levels of mRNAs belonging to the type I interferon signaling pathway were altered in the mice fed the diet containing tomatidine and TRTLE. Moreover, the knockdown of one of the type I interferon-stimulated genes (ISGs), interferon α-inducible protein 27 (IFI27), inhibited the proliferation of cultured 85As2 cells. This study demonstrates that tomatidine and TRTLE inhibit the tumor growth in vivo and the proliferation of human gastric cancer-derived 85As2 cells in vitro, which could be due to the downregulation of ISG expression.
Keywords: tomatidine, tomato leaves, gastric cancer, IFI27, type I interferon signaling pathway, type I interferon-stimulated genes
1. Introduction
Gastric cancer had the fourth highest incidence rate and the third highest mortality rate among cancers worldwide in 2020 [1]. Gastric cancer risk is closely related to dietary habits, including an increased risk associated with salt intake [2,3] and preventive effects from fruit and vegetable consumption [4,5]. Accumulating evidence has shown that certain nutritional components, such as curcumin and resveratrol, have preventive effects against gastric cancer [6,7].
A steroidal glycoalkaloid α-tomatine is abundant in the flowers, leaves, calyxes, and unripe fruits of tomatoes (1.10, 0.98, 0.80, and 0.47 g/kg of fresh weight, respectively) [8]. Among these, leaves are unused resources that are discarded during tomato harvesting. α-Tomatine has various bioactivities, such as anti-cancer, anti-viral [9], and anti-inflammatory activities [10]. α-Tomatine also reduces the growth of several cultured cancer cell lines, such as LNCaP, VCaP, and PC-3 (human prostate cancer), K562 (human chronic myeloid leukemia), and HL60 (human acute promyelocytic leukemia) cells [11,12]. The anti-cancer activity of α-tomatine has also been shown in vivo, such as in the suppression of tumor formation in tumor-bearing mice using CT-26 (mouse colon cancer) cells via the intraperitoneal administration of α-tomatine (5 mg/kg body weight) [13]. The aglycone of α-tomatine, a steroid alkaloid tomatidine, has been reported to have similar bioactivity [14,15]. For example, incubation with 100 μM tomatidine for 48 h inhibited the growth of HT-29 (colon adenocarcinoma), HeLa (cervical carcinoma), and MCF-7 (breast adenocarcinoma) cells in vitro by 70, 60, and 80%, respectively [16]. Incubation with 30 μM tomatidine for 24 h also inhibited the growth of HBL-100 (breast cancer) cells by approximately 75% [17]. However, whether tomatidine inhibits tumor growth in vivo, similar to α-tomatine, has not yet been reported. Additionally, the effect of tomatidine on gastric cancer cells is unclear, as tomatidine inhibits the growth of the gastric cancer cell line KATO-III [18] but has no effect on another cancer cell line, AGS [19].
Therefore, we investigated whether tomatidine shows anti-cancer activity against human gastric carcinoma-derived 85As2 cells in vitro and its tumor in vivo and whether the same effect can be obtained with the tomatidine-rich tomato leaf extract (TRTLE) prepared from tomato leaves.
2. Materials and Methods
2.1. Preparation of TRTLE
Hot-air-dried tomato leaves (provided by Smart Agriculture Iwata Co., Ltd., Shizuoka, Japan) were added to a 15-fold volume (v/w) of water. After the pH was adjusted to approximately 3.5 with hydrochloric acid (Wako, Osaka, Japan), the sample was heated at 80 °C for 30 min, and diatomaceous earth filtration was performed to obtain the extract. Trisodium citrate dihydrate (Wako) was added to 4% (w/w) concentration of the extract, and the pH was adjusted to 8.5 with sodium hydroxide (Wako). After centrifugation at 4000× g for 10 min at 25 °C, the precipitate was resuspended in water, and the solution was centrifuged again at 4000× g for 10 min at 25 °C. To hydrolyze α-tomatine in the extract, the water-washed precipitate was dissolved in 1.3 mol/L of hydrochloric acid, and the solution was heated at 80 °C for 2 h. After hydrolysis, the pH was adjusted to 8.5, and the precipitate containing hydrolyzed α-tomatine was obtained after centrifugation at 4000× g for 10 min at 25 °C and washing with water as described above. TRTLE was obtained by freeze-drying the precipitate. The tomatidine and α-tomatine contents in TRTLE were determined by high-performance liquid chromatography (HPLC) analysis using SPD-10A (detector) and SCL-10 (pump) (Shimadzu, Kyoto, Japan) as described by Taveira et al. [20]. Samples were dissolved in methanol, diluted with the same solvent, and filtered through a membrane filter (0.45 µm) (Pall corporation, Port Washington, New York, NY, USA). Ten microliters of the sample were injected into the HPLC system, and quantification was carried out using a calibration curve prepared with the tomatidine standard. The HPLC conditions are listed in Table 1. A-Tomatine and tomatidine hydrochloride were purchased from the Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich Japan (Tokyo, Japan), respectively.
Table 1.
High-performance liquid chromatography (HPLC) conditions for tomatidine and α-tomatine concentration measurements.
| HPLC | |
|---|---|
| Column | CAPCELL PAK C18-ACR column 4.6 × 250 mm (OSAKA SODA, Osaka, Japan) |
| Guard column | CAPCELL PAK C18-ACR guard column 4.0 × 10 mm (OSAKA SODA) |
| Wavelength | 205 nm |
| Mobile phase A | 25 mM Triethylammonium phosphate |
| Mobile phase B | Acetonitrile |
| Gradient | B% = 20–45–55–57–20 (0–12, 12–17, 17–20, 20–21, 21 min) |
| Column temperature | 25 °C |
| Flow rate | 0.8 mL/min |
HPLC: High-performance liquid chromatography.
2.2. Cell Culture
Human gastric cancer-derived 85As2 cells were established from the human gastric cancer (MKN-45) cell line as described previously [21] and cultured in Roswell Park Memorial Institute-1640 (Wako) medium with 10% fetal bovine serum (FBS; Sigma-Aldrich) and 1% penicillin-streptomycin solution (stabilized) (Nacalai Tesque, Kyoto, Japan). The cells were cultured in a humidified atmosphere at 5% CO2 and 37 °C, and the medium was replaced every two days. 85As2 cells were seeded at 1.5 × 104 or 3.0 × 103 cells/well in a 24- or 96-well plate (As One, Osaka, Japan), respectively. One day after seeding, the cells were treated with the vehicle, 6.5 μg/mL tomatidine (hydrochloride) (Cayman chemical, Ann Arbor, MI, USA), or 10 μg/mL TRTLE (equivalent to 6.5 μg/mL tomatidine) for 24 h, 48 h, or 72 h. Dimethyl sulfoxide (DMSO; Wako) was used to dissolve the compounds, and the final concentration of DMSO in the cells was 0.1%.
2.3. Cancer Model Induced by Implantation of 85As2 Cells
Eight-week-old male BALB/c-AJcl-nu/nu mice were purchased from Clea Japan, Inc. (Tokyo, Japan) and acclimatized for a week. The mice were provided free access to water and a normal chow diet (MF; Clea, Japan) and maintained under a 12 h light/dark cycle at a constant temperature of 22 °C. To create a syngeneic mouse model, 85As2 cells were harvested from subconfluent cultures and resuspended in phosphate-buffered saline (PBS). Mice anesthetized by inhalation of 1–2.5% isoflurane were subcutaneously inoculated with 1 × 106 cells at each site in the left and right flanks. After cell transplantation, the mice were fed a control diet (Control group), a diet containing 0.05% (w/w) tomatidine (Tomatidine group), or 0.077% (w/w) TRTLE (equivalent to 0.05% (w/w) tomatidine) (TRTLE group) for 3 weeks.
All components of the diet are listed in Table 2. After mixing all the powders together, oil and an appropriate amount of water were added while stirring to solidify the contents. The diets were stored at −30 °C.
Table 2.
Composition of the diet containing tomatidine or tomatidine-rich tomato leaf extract (TRTLE).
| Ingredients | Control | Tomatidine | TRTLE |
|---|---|---|---|
| g/100 g (Except Water) | |||
| Tomatidine (hydrochloride) | - | 0.05 | - |
| Tomatidine-rich tomato leaf extract (TRTLE) | - | - | 0.077 |
| L(-)-Cystine (Wako) | 0.30 | 0.30 | 0.30 |
| AIN93 Vitamin mix (Without choline bitartrate) (Oriental Yeast, Tokyo, Japan) |
1.00 | 1.00 | 1.00 |
| AIN93 Mineral mix (Oriental Yeast) | 3.50 | 3.50 | 3.50 |
| Cellulose (Oriental Yeast) | 5.00 | 5.00 | 5.00 |
| Casein (Oriental Yeast) | 20.00 | 20.00 | 20.00 |
| α-Starch (Oriental Yeast) | 66.20 | 66.20 | 66.20 |
| Safflower oil (Benihana Olein Ichiban Shibori) (Benibana foods, Tokyo, Japan) |
4.00 | 4.00 | 4.00 |
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Shizuoka (protocol codes 195240 and 215299; date of approval: 13 June 2019, and 25 March 2021, respectively).
2.4. Microarray Analysis
RNA samples were extracted from tumors from the 85As2 syngeneic mouse model using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Venlo, The Netherlands). The obtained RNA samples were pooled for each group (Control group, n = 4; Tomatidine group, n = 5; TRTLE group, n = 5) and used for microarray analysis. Microarray analysis was performed by the DNA Chip Research Inc. (Tokyo, Japan) using SurePrint G3 Human GE microarray 8 × 60 K Ver3.0 chips.
From the microarray data, we identified 90 genes in the Tomatidine group and 115 genes in the TRTLE group with an absolute expression level of at least 500 in the Control group and a fold change of at least 1.3 both in the Tomatidine and TRTLE groups as compared to the Control group. To find a common set of differentially expressed gene clusters, these genes were uploaded to the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org/, 28 January 2022), and gene ontology (GO) analysis was performed.
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was extracted from the tumor-derived 85As2 syngeneic mouse model or cultured 85As2 cells after 72 h of incubation using an RNA Extraction kit (Takara Bio Inc., Shiga, Japan), according to the manufacturer’s protocol. The obtained RNA was subjected to gDNA removal and reverse transcription using the PrimerScriptTM RT Reagent Kit (Takara Bio Inc.) to produce cDNA. PCR reactions were performed with TB Green® Premix Ex Taq™ II (TliRNaseH Plus) and Bulk (Takara Bio Inc.) using the Thermal Cycler Dice® Real Time System (Takara Bio Inc.). cDNA (7.5 ng) and 10 nmol gene-specific primers were used for amplification in 12.5 μL qRT-PCR reaction mixture. The qPCR reactions were carried out at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s, and then terminated with a dissociation step for 15 s at 92 °C, 30 s at 60 °C, and 15 s at 95 °C. Target gene expression was calculated using the Ct value (second derivative maximum) methods normalized to β-actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The primer sequences used for qRT-PCR are listed in Table 3.
Table 3.
Sequences of primers used for quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| β-actin | TGGCACCCAGCACAATGA | CTAAGTCATAGTCCGCCTAGAAGCA |
| GAPDH | TGGACCTGACCTGCCGTCTAG | GTGGGTGTCGCTGTTGAAGTC |
| IFI27 | TGCTCTCACCTCATCAGCAGT | CACAACTCCTCCAATCACAACT |
| IFI6 | GATGAGCTGGTCTGCGATCC | TCGAGATACTTGTGGGTGGC |
| IFITM1 | TCGCCTACTCCGTGAAGTCTA | TGTCACAGAGCCGAATACCAG |
| ISG15 | TGTCCCTGAGCAGCTCCATG | TGTCCTGCAGCGCCACACC |
| MX1 | GCCAGGACCAGGTATACAG | GCCTGCGTCAGCCGTGC |
| BST2 | GAGCTTGAGGGAGAGATCACTAC | ATTCTCACGCTTAAGACCTGGTT |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFI27, interferon-induced protein 27; IFI6, interferon-induced protein 6; IFITM1, interferon-induced transmembrane protein 1; ISG15, interferon-stimulated gene 15; MX1, Mx dynamin-like GTPases; BST2, bone marrow stromal cell antigen 2.
2.6. Cell Proliferation and Cytotoxicity Assays
Cell proliferation assays were performed using a cell counting kit-8 (CCK-8) (Dojindo, Tokyo, Japan), and cytotoxicity was assessed using the Cytotoxicity lactate dehydrogenase (LDH) Assay Kit-WST (Dojindo). The 85As2 cells (3 × 103 cells per well) were seeded into a 96-well plate. After incubation for the time shown in the figure, the cell proliferation and cytotoxicity were evaluated according to the manufacturer’s protocol. Cytotoxicity (%) = [absorbance] (sample—negative control)/(positive control—negative control) × 100. The positive control was cells treated with the lysis buffer, and the negative control was untreated cells.
2.7. Small Interfering RNA (siRNA) Transfection
Transfection of siRNA into 85As2 cells was performed according to a standard protocol. The cells were transfected with 10 nM siRNA using Lipofectamine RNAiMAX (Invitrogen, Tokyo, Japan) the day after seeding. The cells were collected after 72 h of incubation and analyzed using qRT-PCR to determine the knockdown efficiency. siRNA oligonucleotides were purchased from Japan Bio Service (JBioS, Saitama, Japan). Two independent siRNAs for each gene were tested, and the oligo sequences of all siRNAs are listed in Table 4. The lowercase “d” at the 3’ terminus indicates an overhang in the DNA sequence.
Table 4.
Sequences of small interfering RNAs (siRNAs).
| siRNA | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| GFP | GCAGCACGACUUCUUCAAGdTdT | CUUGAAGAAGUCGUGCUGCdTdT |
| IFI27 #1 | GUGAAAUAUACCAAAUUCUdTdT | AGAAUUUGGUAUAUUUCACdCdC |
| IFI27 #2 | GAAAUAAAGAUGAAUUGUUdTdT | AACAAUUCAUCUUUAUUUCdTdT |
GFP, green fluorescent protein; IFI27, interferon-induced protein 27.
2.8. Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using JMP software (JMP 5.1.2; SAS, Cary, NC, USA). One-way analysis of variance followed by Dunnett’s test was performed for comparison with the control sample.
3. Results
3.1. HPLC Analysis of TRTLE
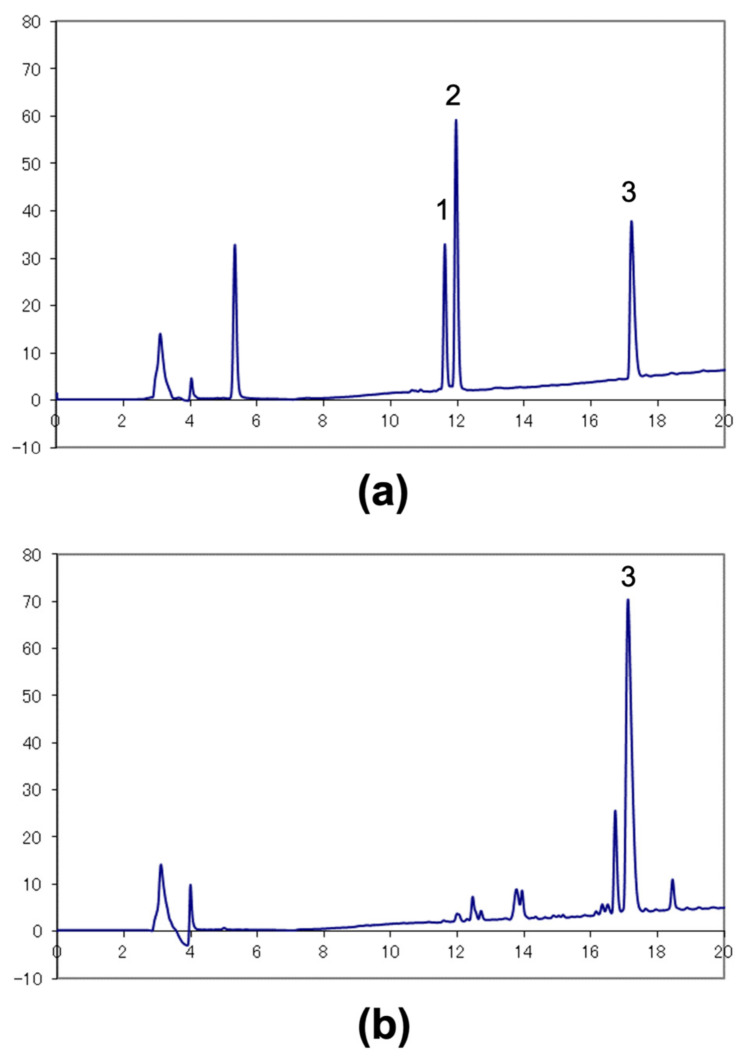
Commercial α-tomatine and tomatidine standards were simultaneously analyzed. The HPLC chromatogram showed peaks (1) and (2), at retention times of 11.6 and 12.0 min, respectively (Figure 1a), similar to that reported by Taveira et al. [20]. They reported that the former peak was dehydrotomatine, and the latter was α-tomatine. Peak (3) at 17.2 min was tomatidine. Peaks corresponding to dehydrotomatine and α-tomatine were not detected in the HPLC chromatogram of TRTLE (Figure 1b), suggesting that these tomatines are almost completely aglyconated by acid hydrolysis. The concentration of tomatidine in TRTLE was 65% (w/w), and α-tomatine was not detected (Figure 1b). Taveira et al. reported that two peaks were detected in the HPLC chromatogram of the tomatidine standard, eluted in the order of: tomatidenol, aglycone of dehydrotomatine, and tomatidine [20]. Although for the HPLC chromatogram of the commercial tomatidine standard used in this study, only one peak (3) was detected (Figure 1a), two peaks were detected in the HPLC chromatogram of the acid-hydrolyzed commercial α-tomatine standard (retention time 16.8 min and 17.2 min, results not shown). These results suggest that the peak detected at a retention time of 16.8 min in TRTLE was most likely to be tomatidenol (Figure 1b).
Figure 1.
High-performance liquid chromatography (HPLC) chromatograms of α-tomatine and tomatidine standards (a) and the tomatidine-rich tomato leaf extract (TRTLE) (b). Peaks from (1) dehydrotomatine, (2) α-tomatine, and (3) tomatidine.
3.2. Tomatidine and TRTLE Inhibit Tumor Growth in a Syngeneic Mouse Model
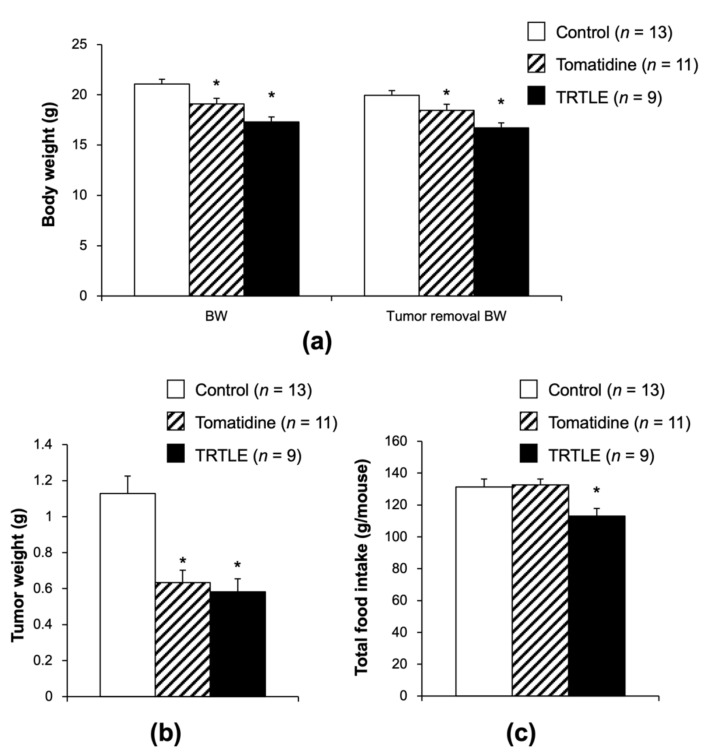
We investigated the effect of tomatidine and TRTLE on tumor formation using a cancer model involving the implantation of 85As2 cells. Body weight and tumor removal body weight were decreased in the Tomatidine and TRTLE groups (Figure 2a). Tumor weight was remarkably reduced to 56.1% and 51.6% in the Tomatidine and TRTLE groups, respectively (Figure 2b). Total food intake for 3 weeks was reduced only in the TRTLE group. These data suggest that the administration of tomatidine and TRTLE inhibited tumor growth in vivo.
Figure 2.
Effect of tomatidine and TRTLE on the tumor growth of an 85As2 syngeneic mouse model. Eight-week-old male BALB/c-AJcl-nu/nu mice were subcutaneously injected with 85As2 cells derived from human gastric cancer tissues. The mice were fed control diets (Control) or diets containing tomatidine (Tomatidine) or TRTLE (TRTLE) for 3 weeks. (a) Body weight (BW), BW after tumor removal (Tumor removal BW), and (b) tumor weight after 3-week experimental period are shown. (c) Total food intake during the 3 weeks of the experimental period is also shown. n = 9–13, means ± standard error (SE). * p < 0.05 vs. Control.
3.3. Tomatidine Suppresses the Expression Levels of Type I Interferon-Stimulated Genes
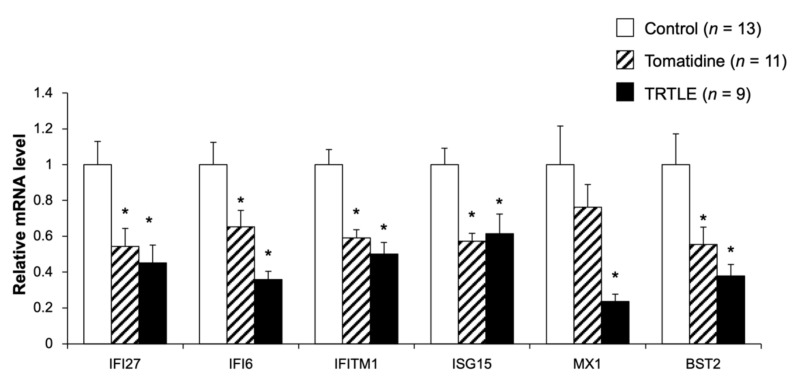
To investigate the mechanism of tomatidine- and TRTLE-induced suppression of tumor growth, microarray analysis was performed on excised tumor tissues, and GO analysis of the obtained data showed that the expression of mRNAs belonging to the type I interferon signaling pathway was altered in the mice fed the diet containing tomatidine or TRTLE (Table 5 and Table 6). The results of Tomatidine versus Control indicated only four descriptions (Table 5). On the other hand, TRTLE vs. Control showed 48 descriptions; the top 10 in the order of decreasing p-value are shown (Table 6). Among these were six genes, namely, interferon-induced protein 27 (IFI27), interferon-induced protein 6 (IFI6), interferon-induced transmembrane protein 1 (IFITM1), interferon-stimulated gene 15 (ISG15), Mx dynamin-like GTPases (MX1), and bone marrow stromal cell antigen 2 (BST2), that were commonly altered in both the Tomatidine and TRTLE groups. When the expression of these genes was measured using qRT-PCR, all genes except MX1 showed significant changes in both groups (Figure 3). These genes are known as type I interferon-stimulated genes (ISGs), and ISGs may be involved in the suppression of tumor growth by tomatidine.
Table 5.
Results of gene ontology (GO) analysis in biological processes (Tomatidine vs. Control).
| Biological Process (Gene Ontology) | |||
|---|---|---|---|
| GO-Term | Description | Count in Network | p-Value |
| GO:0060337 | Type I interferon signaling pathway | 6 of 67 | 0.0062 |
| GO:0045069 | Regulation of viral genome replication | 6 of 99 | 0.0114 |
| GO:1903900 | Regulation of viral life cycle | 7 of 153 | 0.0114 |
| GO:0045071 | Negative regulation of viral genome replication | 5 of 61 | 0.0154 |
Table 6.
Top 10 results of GO analysis in biological processes (TRTLE vs. Control).
| Biological Process (Gene Ontology) | |||
|---|---|---|---|
| GO-Term | Description | Count in Network | p-Value |
| GO:0040029 | Regulation of gene expression, epigenetic | 12 of 202 | 4.63 × 10−6 |
| GO:0060968 | Regulation of gene silencing | 10 of 137 | 1.12 × 10−5 |
| GO:0045814 | Negative regulation of gene expression, epigenetic | 9 of 103 | 1.28 × 10−5 |
| GO:0097549 | Chromatin organization involved in negative regulation of transcription | 9 of 108 | 1.42 × 10−5 |
| GO:0006334 | Nucleosome assembly | 9 of 135 | 5.89 × 10−5 |
| GO:0045653 | Negative regulation of megakaryocyte differentiation | 5 of 18 | 0.00012 |
| GO:0060337 | Type I interferon signaling pathway | 7 of 67 | 0.00012 |
| GO:0006323 | DNA packaging | 10 of 215 | 0.00012 |
| GO:0051253 | Negative regulation of RNA metabolic process | 23 of 1422 | 0.00012 |
| GO:0051172 | Negative regulation of nitrogen compound metabolic process | 31 of 2429 | 0.00012 |
Figure 3.
Expression levels of the type I interferon-stimulated genes in tumors derived from the cancer mouse model induced by the implantation of 85As2 cells. To confirm the effects of tomatidine and TRTLE, gene expression levels in tumors from the cancer mouse model fed control diet (Control) or diets containing tomatidine (Tomatidine) or TRTLE for 3 weeks were measured using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). n = 9–13, means ± SE. * p < 0.05 vs. Control.
3.4. Tomatidine Inhibits the Proliferation of 85As2 Cells without Cytotoxicity
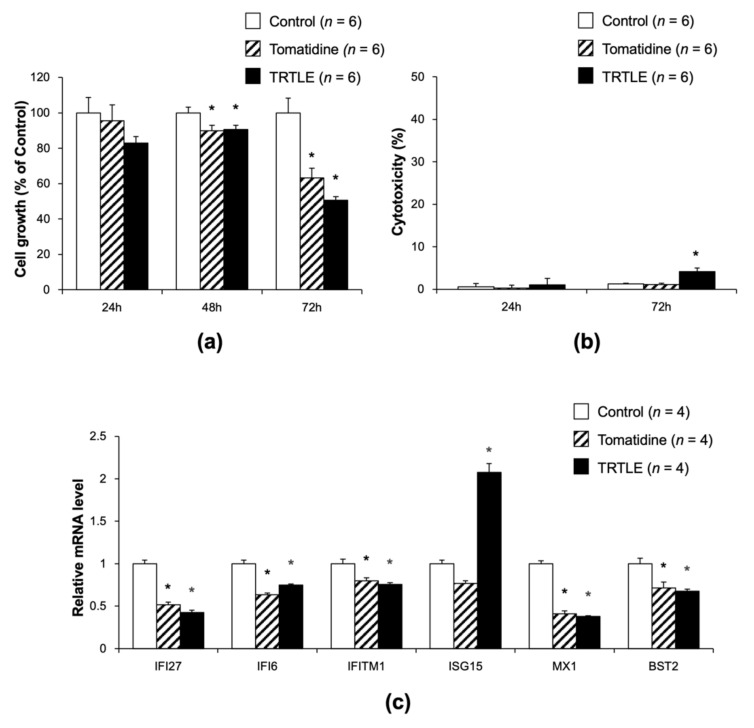
Next, we examined whether tomatidine directly affected the proliferation of 85As2 cells and/or was cytotoxic in these cells. Although 24 h of incubation with tomatidine or TRTLE did not show any significant effect on cell proliferation, tomatidine and TRTLE inhibited cell growth after 48 and 72 h incubation. After 72 h, tomatidine and TRTLE inhibited cell growth to 63.3% and 50.7%, respectively (Figure 4a). Cytotoxicity was not observed after 72 h of incubation with tomatidine (Figure 4b). TRTLE showed cytotoxicity; however, the 50.7% suppression of cell growth after 72 h incubation with TRTLE could not be explained by only a 4.1% cytotoxicity. We also measured the expression levels of ISGs in 85As2 cells cultured in a medium containing tomatidine and TRTLE for 72 h. The expression levels of these genes, except ISG15, were significantly decreased in the cells cultured with tomatidine and TRTLE (Figure 4c). These results indicate that tomatidine and TRTLE inhibit tumor growth in vivo and proliferation of 85As2 gastric cancer cells in vitro. Additionally, the expression levels of ISGs, except ISG15, were also decreased both in vivo and in vitro.
Figure 4.
Effects of tomatidine and TRTLE on the proliferation of 85As2 gastric cancer cells. After incubation of 85As2 cells for 24 h, 48 h or 72 h without (Control) or with tomatidine (Tomatidine) or TRTLE, their (a) cell growth and (b) cytotoxicity were measured. (c) mRNA levels of type I interferon-stimulated genes (ISGs) were measured in the 85As2 cells treated with tomatidine or TRTLE for 72 h. n = 4–6, means ± SE. * p < 0.05 vs. Control.
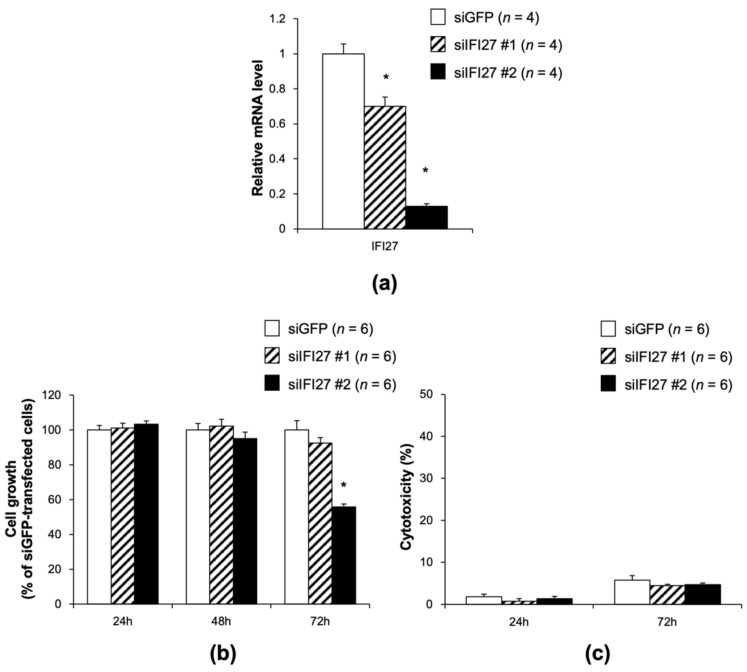
3.5. Knocking Down of IFI27 Inhibits the Proliferation of 85As2 Cells
To investigate whether tomatidine- and TRTLE-induced suppression of ISG expression was required for the inhibition of 85As2 cell proliferation, two types of siIFI27 (#1, #2) were prepared and transfected into 85As2 cultured cells to generate IFI27 knockdown cells. Both siIFI27s (#1, #2) showed a significant decrease in IFI27 gene expression after 72 h of incubation (Figure 5a), with the knockdown efficiency higher in cells treated with siIFI27 #2 than in those treated with #1. Knockdown of IFI27 after 72 h incubation with siIFI27 #2 inhibited cell growth to 55.7% (Figure 5b). Knockdown of IFI27 showed no cytotoxicity in 85As2 cells (Figure 5c), suggesting that cytotoxicity was not involved in the decrease in cell growth by knocking down IFI27. These results were at a similar level to that of the changes caused by tomatidine and TRTLE. Therefore, tomatidine and TRTLE may contribute to the inhibition of cancer cell growth and tumor formation through the downregulation of expression of ISGs, such as IFI27.
Figure 5.
Effect of the knockdown of interferon α-inducible protein 27 (IFI27) on the proliferation of 85As2 gastric cancer cells. (a) Seventy-two hours after transfection with siIFI27, IFI27 mRNA levels in 85As2 cells were measured. After 24 h, 48 h or 72 h of transfection with siIFI27, (b) cell growth and (c) cytotoxicity were measured. siGFP was transfected as a control. n = 4–6, means ± SE. * p < 0.05 vs. Control.
4. Discussion
In this study, we attempted to elucidate the anti-cancer effects of tomatidine and TRTLE and their underlying mechanisms. We have shown that tomatidine and TRTLE have anti-cancer effects on human gastric cancer-derived 85As2 cells in vivo and in vitro, using a syngeneic mouse model and growth assays with cultured cells, respectively. Furthermore, microarray analysis suggested that tomatidine and TRTLE could regulate ISGs.
The results of the present study are the first to demonstrate the anti-cancer activity of tomatidine in vivo (Figure 2b). Similar results were obtained with TRTLE. Previously, it was reported that the administration of tomatidine (subcutaneously, 25 mg/kg/day for 10 days) did not show anti-cancer effects in vivo [22]. In this study, the administration of tomatidine was initiated when a maximum tumor diameter of 5 mm was observed. However, in our present study, approximately 160 mg/kg/day of tomatidine was administered orally for 3 weeks, and administration was started on the day of 85As2 cell transplantation. The difference in results might be attributable to the starting time, dose, method, schedule of administration, or the cell line used.
Administration of tomatidine and TRTLE reduced tumor weight but also resulted in a significant decrease in body weight. Although the decrease in body weight observed in the TRTLE group might be caused by reduced total food intake, a decrease in body weight in the tomatidine group was observed without a reduction in total food intake. This may be related to the effect of tomatidine on lipid metabolism. It has been reported that tomatidine suppresses high-fat diet-induced increases in body weight and fat accumulation in white adipose tissue [23]. Additionally, tomatidine suppresses lipid accumulation in HepG2 hepatocytes [24] and reduces hepatic lipid accumulation in mice fed a high-fat diet, by suppressing the expression of fatty acid synthases and transcription factors involved in lipogenesis [23]. One might hypothesize that tomatidine-induced changes in lipid metabolism may cause a decrease in body weight.
The contribution of cytotoxicity to the effect of tomatidine seemed to be low, compared to that of the anti-cancer effect of α-tomatine. When C6 rat glioma cells were treated for 4 h at 10 and 20 μM of α-tomatine, 40 and 80% cytotoxicity based on LDH activity were observed, respectively [25]. The cytotoxicity of α-tomatine has been suggested to be due to a membrane-disrupting effect, as it can bind to cholesterol [26]. On the other hand, in the present study, an increase in LDH activity was not observed when 85As2 cells were treated with 6.5 μg/mL (14.4 μM) of tomatidine for 72 h (Figure 4b). Therefore, we suggest that tomatidine may exert its anti-cancer effects through a mechanism other than α-tomatine-induced cytotoxicity.
Microarray results showed that tomatidine affected ISGs. Of the six genes identified, IFI27, IFI6, IFITM1, ISG15, and BST2 have been reported to be associated with cancer cell proliferation and tumor growth. Overexpression of IFI27 promotes cancer cell proliferation and tumor growth [27], while its knockdown exhibits the opposite effect [28,29]. IFI6 depletion inhibits esophageal squamous cell carcinoma progression [30]. IFITM1 deficiency suppresses the proliferation, metastasis, and invasion of lung cancer cells [31]. Upregulation of ISG15 expression promotes colon cancer proliferation and metastasis, and when ISG15 is knocked down, there is an opposite effect [32]. Knocking down BST2 suppresses gastric cancer cell proliferation and promotes apoptosis [33]. Therefore, it is suggested that the downregulation of ISG expression contributes to tomatidine- and TRTLE-induced suppression of 85As2 cell proliferation and tumor growth. In the present study, we focused on IFI27. IFI27 is highly expressed in several cancers, such as ovarian cancer [34,35], hepatocellular carcinoma [36], and breast cancer [37]. In fact, IFI27 is a potential prognostic marker and therapeutic target for cancer and tumor growth [38,39,40]. High expression of IFI27 inhibits apoptosis [27,28], and its deficiency promotes apoptosis [39]. IFI27 is also involved in the transition from the S phase to the G2 phase in the cell cycle [29,35] and affects the expression of VEGF-A, which is required for the promotion of angiogenesis [29]. Furthermore, IFI27 was found to be closely associated with the clinical progression of pancreatic adenocarcinoma (PAAD), tumor glycolysis, and M2 macrophage infiltration [40]. Downregulation of IFI27 expression might be partially involved in tomatidine- and TRTLE-induced inhibition of 85As2 cancer cell proliferation and tumor growth. Additionally, the expression of ISGs is upregulated in tumors and are positively correlated with resistance to radiotherapy [41,42]. The immune-checkpoint protein PD-L1 has been reported to protect cancer cells by suppressing DNA damage through the inhibition of the acute response to IFN-1 and the maintenance of ISG expression [43]. Thus, we hypothesize that tomatidine could reduce the resistance of cancer cells to treatment by downregulating ISG expression and may be useful as an adjuvant therapy for radiotherapy. As the relationship between gastric cancer and IFI27 and other ISGs is still unclear, further studies are required.
5. Conclusions
Tomatidine and TRTLE inhibited the tumor formation and growth of cultured 85As2 cells derived from human gastric cancer tissues. This is the first demonstration of the anti-cancer activity of tomatidine in vivo. Furthermore, the results suggest that the anti-cancer activities of tomatidine and TRTLE are mediated by the downregulation of expression of ISGs, such as IFI27.
6. Patents
The preparation method for TRTLE used in this study is the subject of a patent application (JP 2018-184394A) by Tokai Bussan Co., Ltd.
Acknowledgments
We are indebted to the members of the Laboratory of Nutritional Biochemistry (Graduate School of Nutritional and Environmental Sciences, University of Shizuoka) for their technical assistance.
Author Contributions
Conceptualization, J.F., A.M., T.S. (Tomoki Sato) and S.M.; Methodology, J.F., N.S., S.S., T.S. (Takanori Suzuki), M.N., Y.U. and A.M.; Software, Y.K. and M.O.; Validation, J.F., A.M., T.S. (Tomoki Sato) and S.M.; Formal analysis, J.F.; Investigation, J.F.; Resources, S.S., T.S. (Takanori Suzuki), M.N., Y.U., H.N. and K.W.; Data curation, J.F., T.S. (Tomoki Sato) and S.M.; Writing—Original draft preparation, J.F. and S.M.; Writing—Review and editing, K.W., T.S. and S.M.; Visualization, J.F.; Supervision, S.M.; Project administration, T.S. and S.M.; Funding acquisition, T.S. (Tomoki Sato) and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Princess Takamatsu Cancer Research Fund (grant number 19-25134) and the University of Shizuoka Grant for Scientific and Educational Research (grant number is not available).
Institutional Review Board Statement
The study was conducted according to the guidelines of the National Institutes of Health Guide and approved by the Institutional Animal Care and Use Committee of the University of Shizuoka (protocol codes 195240 and 215299; dates of approval: 13 June 2019, and 25 March 2021, respectively).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data obtained in this study will be shared upon reasonable request to the corresponding author.
Conflicts of Interest
Shinji Miura received a research grant from Tokai Bussan Co., Ltd. Shigenobu Shiotani and Takanori Suzuki are inventors of the patent-pending JP 2018-184394A applied by Tokai Bussan Co., Ltd.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Shikata K., Kiyohara Y., Kubo M., Yonemoto K., Ninomiya T., Shirota T., Tanizaki Y., Doi Y., Tanaka K., Oishi Y., et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: The Hisayama study. Int. J. Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 3.Joossens J.V., Hill M.J., Elliott P., Stamler R., Lesaffre E., Dyer A., Nichols R., Kesteloot H. Dietary salt, nitrate, and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int. J. Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 4.Larsson S.C., Bergkvist L., Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: A prospective study. Cancer Epidemiol. Biomark. Prev. 2006;15:1998–2001. doi: 10.1158/1055-9965.EPI-06-0402. [DOI] [PubMed] [Google Scholar]
- 5.Riboli E., Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003;78:559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 6.Bahrami A., Ferns A.G. Effect of curcumin and its derivates on gastric cancer: Molecular mechanisms. Nutr. Cancer. 2021;73:1553–1569. doi: 10.1080/01635581.2020.1808232. [DOI] [PubMed] [Google Scholar]
- 7.Kim S., Kim W., Kim D.H., Jang J.H., Kim S.J., Park S.A., Hahn H., Han B.W., Na H.K., Chun K.S., et al. Resveratrol suppresses gastric cancer cell proliferation and survival through inhibition of PIM-1 kinase activity. Arch. Biochem. Biophys. 2020;689:108413. doi: 10.1016/j.abb.2020.108413. [DOI] [PubMed] [Google Scholar]
- 8.Friedman M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002;50:5751–5780. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- 9.Thorne H.V., Clarke G.F., Skuce R. The inactivation of herpes simplex virus by some Solanaceae glycoalkaloids. Antivir. Res. 1985;5:335–343. doi: 10.1016/0166-3542(85)90003-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B., Zhou B., Bao L., Yang Y., Guo K. Alpha-tomatine exhibits anti-inflammatory activity in lipopolysaccharide-activated macrophages. Inflammation. 2015;38:1769–1776. doi: 10.1007/s10753-015-0154-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang H., Chen X., Li D., He Y., Li Y., Du Z., Zhang K., DiPaola R., Goodin S., Zheng X. Combination of α-tomatine and curcumin inhibits growth and induces apoptosis in human prostate cancer cells. PLoS ONE. 2015;10:e0144293. doi: 10.1371/journal.pone.0144293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao M.W., Chen C.H., Chang Y.L., Teng C.M., Pan S.L. α-Tomatine-mediated anti-cancer activity in vitro and in vivo through cell cycle- and caspase-independent pathways. PLoS ONE. 2012;7:e44093. doi: 10.1371/journal.pone.0044093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.P., Nam S.H., Friedman M. The tomato glycoalkaloid α-tomatine induces caspase-independent cell death in mouse colon cancer CT-26 cells and transplanted tumors in mice. J. Agric. Food Chem. 2015;63:1142–1150. doi: 10.1021/jf5040288. [DOI] [PubMed] [Google Scholar]
- 14.Diosa-Toro M., Troost B., van de Pol D., Heberle A.M., Urcuqui-Inchima S., Thedieck K., Smit J.M. Tomatidine, a novel antiviral compound towards dengue virus. Antivir. Res. 2019;161:90–99. doi: 10.1016/j.antiviral.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Chiu F.L., Lin J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-κB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008;582:2407–2412. doi: 10.1016/j.febslet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 16.Koduru S., Grierson D.S., Van De Venter M., Afolayan A.J. Anticancer activity of steroid alkaloids isolated from Solanum aculeastrum. Pharm. Biol. 2007;45:137. doi: 10.1080/13880200701538690. [DOI] [Google Scholar]
- 17.Chiang C.T., Way T.D., Tsai S.J., Lin J.K. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007;581:5735–5742. doi: 10.1016/j.febslet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Choi S.H., Ahn J.B., Kozukue N., Kim H.J., Nishitani Y., Zhang L., Mizuno M., Levin C.E., Friedman M. Structure–activity relationships of α-, β1-, γ-, and δ-tomatine and tomatidine against human breast (MDA-MB-231), gastric (KATO-III), and prostate (PC3) cancer cells. J. Agric. Food Chem. 2012;60:3891–3899. doi: 10.1021/jf3003027. [DOI] [PubMed] [Google Scholar]
- 19.Friedman M., Levin C.E., Lee S.U., Kim H.J., Lee I.S., Byun J.O., Kozukue N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J. Agric. Food Chem. 2009;57:5727–5733. doi: 10.1021/jf900364j. [DOI] [PubMed] [Google Scholar]
- 20.Taveira M., Ferreres F., Gil-Izquierdo A., Oliveira L., Valentão P., Andrade P.B. Fast determination of bioactive compounds from Lycopersicon esculentum Mill. leaves. Food Chem. 2012;135:748–755. doi: 10.1016/j.foodchem.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara K., Takigahira M., Mihara K., Kubo T., Morimoto C., Morita Y., Terawaki K., Uezono Y., Seyama T. Inhibitory effects of isoflavones on tumor growth and cachexia in newly established cachectic mouse models carrying human stomach cancers. Nutr. Cancer. 2013;65:578–589. doi: 10.1080/01635581.2013.776089. [DOI] [PubMed] [Google Scholar]
- 22.Watkins D.N., Berman D.M., Burkholder S.G., Wang B., Beachy P.A., Baylin S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 23.Wu S.J., Huang W.C., Yu M.C., Chen Y.L., Shen S.C., Yeh K.W., Liou C.J. Tomatidine ameliorates obesity-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2021;91:108602. doi: 10.1016/j.jnutbio.2021.108602. [DOI] [PubMed] [Google Scholar]
- 24.Kusu H., Yoshida H., Kudo M., Okuyama M., Harada N., Tsuji-Naito K., Akagawa M. Tomatidine reduces palmitate-induced lipid accumulation by activating AMPK via vitamin D receptor-mediated signaling in human HepG2 hepatocytes. Mol. Nutr. Food Res. 2019;63:e1801377. doi: 10.1002/mnfr.201801377. [DOI] [PubMed] [Google Scholar]
- 25.Yamashoji S., Onoda E. Detoxification and function of immature tomato. Food Chem. 2016;209:171–176. doi: 10.1016/j.foodchem.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Sucha L., Hroch M., Rezacova M., Rudolf E., Havelek R., Sispera L., Cmielova J., Kohlerova R., Bezrouk A., Tomsik P. The cytotoxic effect of α-tomatine in MCF-7 human adenocarcinoma breast cancer cells depends on its interaction with cholesterol in incubation media and does not involve apoptosis induction. Oncol. Rep. 2013;30:2593–2602. doi: 10.3892/or.2013.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervantes-Badillo M.G., Paredes-Villa A., Gómez-Romero V., Cervantes-Roldán R., Arias-Romero L.E., Villamar-Cruz O., González-Montiel M., Barrios-García T., Cabrera-Quintero A.J., Rodríguez-Gómez G., et al. IFI27/ISG12 downregulates estrogen receptor α transactivation by facilitating its interaction with CRM1/XPO1 in breast cancer cells. Front. Endocrinol. 2020;11:568375. doi: 10.3389/fendo.2020.568375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., Qiu X., Lin S., Chen X., Wang T., Liao T. Knockdown of IFI27 inhibits cell proliferation and invasion in oral squamous cell carcinoma. World J. Surg. Oncol. 2018;16:64. doi: 10.1186/s12957-018-1371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang K.C., Huang S.T., Wu R.C., Huang S.C., Yeh T.S., Chen M.H., Hsu J.T., Chen L.W., Kuo S.F., Chueh H.Y., et al. Interferon α-inducible protein 27 is an oncogene and highly expressed in cholangiocarcinoma patients with poor survival. Cancer Manag. Res. 2019;11:1893–1905. doi: 10.2147/CMAR.S196485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z., Gu S., Lu T., Wu K., Li L., Dong C., Zhou Y. IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress. J. Exp. Clin. Cancer Res. 2020;39:144. doi: 10.1186/s13046-020-01646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J., Jiang Y., Lu J., Wu J., Zhang M. Inhibiting of proliferation, migration, and invasion in lung cancer induced by silencing interferon-induced transmembrane protein 1 (IFITM1) BioMed Res. Int. 2019;2019:9085435. doi: 10.1155/2019/9085435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S., Ren M., Xu H., Xia H., Tang Q., Liu M. Inhibition of ISG15 enhances the anti-cancer effect of trametinib in colon cancer cells. OncoTargets Ther. 2019;12:10239–10250. doi: 10.2147/OTT.S226395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W., Cao Y., Guan Y., Zheng C. BST2 promotes cell proliferation, migration and induces NF-κB activation in gastric cancer. Biotechnol. Lett. 2018;40:1015–1027. doi: 10.1007/s10529-018-2562-z. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y.S., Do Hwan J., Bae S., Bae D.H., Ahn Shick W. Identification of differentially expressed genes using an annealing control primer system in stage III serous ovarian carcinoma. BMC Cancer. 2010;10:576. doi: 10.1186/1471-2407-10-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S., Xie Y., Zhang W., Gao J., Wang M., Zheng G., Yin X., Xia H., Tao X. Interferon alpha-inducible protein 27 promotes epithelial–mesenchymal transition and induces ovarian tumorigenicity and stemness. J. Surg. Res. 2015;193:255–264. doi: 10.1016/j.jss.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 36.Budhu A., Chen Y., Kim J.W., Forgues M., Valerie K., Harris C.C., Wang X.W. Induction of a unique gene expression profile in primary human hepatocytes by hepatitis C virus core, NS3 and NS5A proteins. Carcinogenesis. 2007;28:1552–1560. doi: 10.1093/carcin/bgm075. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen U.B., Wolf C., Mattel M.G., Chenard M.P., Bellocq J.P. Identification of a new interferon-α-inducible gene (p27) on human chromosome 14q32 and its expression in breast carcinoma. Cancer Res. 1993;53:4096–4101. [PubMed] [Google Scholar]
- 38.Lao M., Zhang X., Ma T., Xu J., Yang H., Duan Y., Ying H., Zhang X., Guo C., Qiu J., et al. Regulator of calcineurin 1 gene isoform 4 in pancreatic ductal adenocarcinoma regulates the progression of tumor cells. Oncogene. 2021;40:3136–3151. doi: 10.1038/s41388-021-01763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang S., Zhao J., Song J., Li Y., Zuo R., Sa Y., Ma Z., OuYang H. Interferon alpha-inducible protein 27 (IFI27) is a prognostic marker for pancreatic cancer based on comprehensive bioinformatics analysis. Bioengineered. 2021;12:8515–8528. doi: 10.1080/21655979.2021.1985858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu N., Zuo C., Wang X., Chen T., Yang D., Wang J., Zhu H. miR-942 decreases TRAIL-induced apoptosis through ISG12a downregulation and is regulated by AKT. Oncotarget. 2014;5:4959–4971. doi: 10.18632/oncotarget.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erdal E., Haider S., Rehwinkel J., Harris A.L., McHugh P.J. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31:353–369. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L., Liang H., Fu S., Weichselbaum R.R., Fu Y.X. From DNA damage to nucleic acid sensing: A strategy to enhance radiation therapy. Clin. Cancer Res. 2016;22:20–25. doi: 10.1158/1078-0432.CCR-14-3110. [DOI] [PubMed] [Google Scholar]
- 43.Cheon H.J., Holvey-Bates E.G., McGrail D.J., Stark G.R. PD-L1 sustains chronic, cancer cell-intrinsic responses to type I interferon, enhancing resistance to DNA damage. Proc. Natl. Acad. Sci. USA. 2021;118:e2112258118. doi: 10.1073/pnas.2112258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data obtained in this study will be shared upon reasonable request to the corresponding author.