Abstract
An important mechanism of bacterial resistance to β-lactam antibiotics is inactivation by β-lactam-hydrolyzing enzymes (β-lactamases). The evolution of the extended-spectrum β-lactamases (ESBLs) is associated with extensive use of β-lactam antibiotics, particularly cephalosporins, and is a serious threat to therapeutic efficacy. ESBLs and broad-spectrum β-lactamases (BDSBLs) are plasmid-mediated class A enzymes produced by gram-negative pathogens, principally Escherichia coli and Klebsiella pneumoniae. MK-0826 was highly potent against all ESBL- and BDSBL-producing K. pneumoniae and E. coli clinical isolates tested (MIC range, 0.008 to 0.12 μg/ml). In E. coli, this activity was associated with high-affinity binding to penicillin-binding proteins 2 and 3. When the inoculum level was increased 10-fold, increasing the amount of β-lactamase present, the MK-0826 MIC range increased to 0.008 to 1 μg/ml. By comparison, similar observations were made with meropenem while imipenem MICs were usually less affected. Not surprisingly, MIC increases with noncarbapenem β-lactams were generally substantially greater, resulting in resistance in many cases. E. coli strains that produce chromosomal (Bush group 1) β-lactamase served as controls. All three carbapenems were subject to an inoculum effect with the majority of the BDSBL- and ESBL-producers but not the Bush group 1 strains, implying some effect of the plasmid-borne enzymes on potency. Importantly, MK-0826 MICs remained at or below 1 μg/ml under all test conditions.
An important mechanism of bacterial resistance to β-lactam antibiotics is inactivation by both existing and evolving β-lactamases. The extended-spectrum β-lactamases (ESBLs) (functional group 2be [3]) and broad-spectrum β-lactamases (BDSBLs) (functional group 2b [3]) are plasmid-mediated class A enzymes produced by gram-negative pathogens, principally Escherichia coli and Klebsiella pneumoniae, which occur primarily as the causative agents of nosocomial infections contracted in intensive care, burn, oncology, and neonatal units. MK-0826 (Fig. 1) (4, 6–8, 10–13, 17, 18, 22, 23) is a novel 1-β-methyl carbapenem with clear pharmacokinetic advantages over currently available carbapenems (6–8, 13, 22, 23) and with excellent antibacterial activity against multiple-drug-resistant isolates of E. coli and Klebsiella spp. (10–12) (but not Pseudomonas spp. [17]). Its activity against BDSBL- and ESBL-producing clinical isolates of E. coli and K. pneumoniae exceeds that of extended-spectrum cephalosporins. In addition, MK-0826 generally exhibits lower MICs than does imipenem against these isolates regardless of the presence of the enzymes, presumably due to differences in penicillin-binding protein (PBP)-binding affinity. As with other β-lactam antibiotics, the target of activity of MK-0826 is interference in bacterial cell wall synthesis by binding to specific PBPs, leading to growth inhibition and, with few exceptions, cell lysis. In E. coli, the high-molecular-weight PBPs 1a/1b, 2, and 3 are enzymes essential for cell wall biosynthesis and thus are lethal targets of β-lactams (19, 21, 24). The interaction of MK-0826 with E. coli PBPs was investigated and associated with its substantial potency.
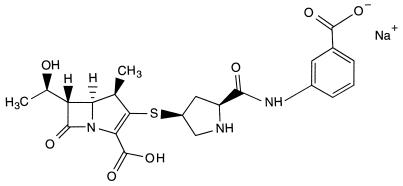
FIG. 1.
Chemical structure of MK-0826: (4R,5S,6S,8R,2′S,4′S)-3-[[2-[[(3-car-boxyphenyl)amino]carbonyl]pyrrolidin-4-yl]thio]-4-methyl-6-(1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-en-2-carboxylic acid monosodium salt.
E. coli clinical isolates that produce either SHV-1, TEM-10, TEM-1, TEM-7, or TEM-12 β-lactamase and K. pneumoniae clinical isolates that produce TEM-10 and SHV-1, TEM-5, TEM-10, or an uncharacterized ESBL were evaluated for an inoculum effect. Inoculum effects are known to be widespread among β-lactam antibiotics when activity is directed against bacteria that produce β-lactamase. Wild-type E. coli cells normally produce little or no β-lactamase. Data for MICs at two inoculum levels for the strains that produce the plasmid-encoded enzymes were compared to those obtained for wild-type E. coli cells that have no evident plasmids but produce a basal level of chromosomal β-lactamase.
MATERIALS AND METHODS
Antibiotics.
Stock solutions of carbapenem antibiotics MK-0826 (L-749,345), imipenem (Merck Research Laboratories [MRL]), and meropenem (ICI Pharmaceuticals/Stuart Pharmaceuticals) were prepared in 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, pH 7.0, and quantitated by a differential spectrophotometric assay in which hydroxylamine-extinguishable absorbance of the intact carbapenem nucleus was monitored. To determine absorbance spectra, a carbapenem sample (ca. 0.2 to 0.5 mg) was weighed by using a microbalance accurate to 0.001 mg. The sample was dissolved in 10.0 ml of 100 mM MOPS buffer, pH 7.0, and the native UV spectrum was determined at room temperature. The carbapenem solution was hydrolyzed (extinguished) to completion (reaction time may be >30 min, particularly for 1-β-methyl carbapenems) with 20 mM NH2OH · HCl, and the spectrum of the extinguished β-lactam was determined. The spectrum for the extinguished β-lactam was subtracted from the native spectrum, and the resulting wavelength (λmax ext) with the greatest change in absorbance (change in optical density [Δ OD]) and the molar extinction coefficient (ɛext) for each carbapenem were used for further quantitation of carbapenem concentrations. For example, immediately prior to use, a carbapenem solution was diluted in 100 mM MOPS buffer, pH 7.0, and the absorbance at the previously determined λmax ext was measured at room temperature for native and fully extinguished (accomplished by addition of 10 to 20 mM NH2OH · HCl) carbapenem. The Δ OD at the λmax ext, the molecular weight, and the ɛext of the compound were used to determine the antibiotic concentration. For maximum stability, antibiotic concentration was kept at ≤2 mg/ml and solutions were stored at 0°C or frozen and stored at −80°C.
Noncarbapenem antibiotics were prepared on a weight per volume basis in water or appropriate buffer, taking active component analyses into account. Ceftriaxone, ceftazidime, cefotaxime, and penicillin G were obtained from Sigma Chemical Company, cefepime and aztreonam were obtained from Bristol-Myers Squibb, and amoxicillin and clavulanic acid were obtained from Smith-Kline Beecham Pharmaceuticals.
Antibiotic stock solutions were diluted to the desired concentration in water or buffer as appropriate and sterilized by filtration using Sterile Acrodisc 0.45-μm-pore-size syringe filters (filter no. 4184; Gelman Sciences). [3H]benzylpenicillin N-ethylpiperidinium salt (∼30 Ci/mmol) was prepared at MRL.
Organisms and conditions of culture.
The strains included in these studies are listed in Table 1. Trypticase soy broth (TSB) (BBL, Becton Dickinson Microbiology Systems, or Difco Laboratories) was inoculated from cultures grown on brain heart infusion (BHI) agar (Difco Laboratories) slants maintained at 4°C and grown 18 h at 35°C with shaking at 220 rpm. Cultures diluted in physiological saline were used for determination of broth microdilution MICs. The numbers of CFUs per milliliter in all overnight cultures were obtained on BHI agar plates incubated 18 to 24 h at 35°C.
TABLE 1.
Bacterial strains
| Species and strain | β-lactamase(s) produceda | Description | Sourcec or reference |
|---|---|---|---|
| K. pneumoniae | |||
| CL 5188 | TEM-10, SHV-1 | Clinical isolate | J. P. Quinn |
| CL 5189 | TEM-10, SHV-1 | Clinical isolate | J. P. Quinn |
| CL 5128 | TEM-5 | Clinical isolate | G. Jacoby |
| CL 5196 | TEM-10 | Clinical isolate | J. P. Quinn |
| CL 5203 | Unchar. ESBLb | Clinical isolate | C. Urban |
| CL 5212 | Unchar. ESBLb | Clinical isolate | C. Urban |
| E. coli | |||
| CL 5121 | SHV-1 | Clinical isolate | G. Jacoby |
| CL 5190 | TEM-10 | Clinical isolate | J. P. Quinn |
| CL 5115 | TEM-1 | Clinical isolate | G. Jacoby |
| CL 5120 | TEM-7 | Clinical isolate | G. Jacoby |
| CL 5197 | TEM-12 | Clinical isolate | J. P. Quinn |
| MB5503 | Bush group 1 | Strain LS619 (envA+) | 28 |
| DH5α | Bush group 1 | F− φ80 ΔlacZ ΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 λ− thi-1 gyrA96 relA1 | BRL |
| LS641 | Bush group 1 | Resistant to fusidic acid | K. Young and L. L. Silver, MRL |
| MB4903 | Bush group 1 | Strain LS584 | 28 |
| KN126 (MB4303) | Bush group 1 | 14 |
No additional β-lactamases are known to be produced.
Enzymes have not been characterized; however, since these strains and those included in studies by Urban et al. (26) have a common origin, it is inferred that they carry the TEM-26 enzyme (26a). Unchar., uncharacterized.
J. P. Quinn is at Columbus Hospital, Chicago, Ill., G. Jacoby is at Massachusetts General Hospital, Boston, Mass., and C. Urban is at New York Hospital Medical Center, New York, N.Y.
MIC determinations.
MICs were determined by broth microdilution according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines (15), with cell concentrations as indicated. For these experiments, Mueller-Hinton broth (MHB) was obtained from BBL. The MIC was defined as the lowest concentration of antibiotic inhibiting visible growth after incubation for 20 h at 35°C (24 h at 37°C for strain KN126).
β-Lactamase characterization.
BDSBLs and ESBLs had been characterized by each investigator prior to strain deposit in the Merck Clinical Culture Collection.
Confirmation of β-lactamase production.
BBL Cefinase nitrocefin discs for the detection of β-lactamases were cut into quarters. One piece was placed in a growth control well of the 20-h microdilution assay plate for each strain and observed for a color change, thereby indicating the presence or absence of the enzyme. Wells that contained sub-MICs of antibiotics were tested to detect enzyme induction if growth controls yielded poor or no color change.
Preparation of bacterial membranes.
KN126 cells were grown at 37°C in 15 liters of Antibiotic Medium 3 (Difco) to a final absorbance at 600 nm of approximately 2. Cells were chilled to 10°C, concentrated by filtration, collected by centrifugation at 10,000 × g for 10 min, and resuspended in 220 ml of 20 mM sodium phosphate buffer, pH 7.0. Cells were disrupted by two cycles of sonication and cooling on ice; unbroken cells and debris were removed by centrifugation at 8,000 × g for 10 min. Membranes, collected by centrifugation at 40,000 × g for 60 min, were washed and resuspended in 20 mM sodium phosphate buffer, pH 7.0. Protein concentration was determined by the Bio-Rad microassay method, and membranes were stored at −80°C.
Competitive binding assays.
Binding affinity for the E. coli PBPs was determined in a competition assay with [3H]benzylpenicillin by using a modification of the procedure described by Spratt (20). Membrane proteins (total protein, 225 μg) from strain KN126 were incubated in 100-μl reaction mixtures with various concentrations of unlabelled test compound or buffer control for 10 min at 30°C. Subsequently, [3H]benzylpenicillin (25 μg/ml) (specific activity, ∼30 Ci/mmol) was added and incubation was continued for an additional 10 min. Reactions were terminated and bacterial inner membranes were solubilized in one step by the addition of excess unlabelled penicillin (4 mg/ml) and 1.3% Sarkosyl (Sigma Chemical Company). Insoluble outer membranes were pelleted by centrifugation at 40,000 × g. Supernatant fluid was combined with an equal volume of sample loading buffer and heated at 100°C for 4 min.
Reagents for electrophoresis were purchased from Bio-Rad Laboratories. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at a constant current of 18 mA through a 10% acrylamide–0.13% bisacrylamide gel (16 cm by 16 cm by 0.75 mm) prepared in 0.375 M Tris-hydrochloride buffer, pH 8.8. Gels were stained with 0.1% (wt/vol) Coomassie blue in 50% (vol/vol) methanol–10% (vol/vol) acetic acid and destained in methanol-acetic acid-H2O (5:10:85). Following incorporation of En3Hance (New England Nuclear), gels were dried and exposed to Kodak XAR-5 film, and radiolabelled benzylpenicillin-protein complexes were quantitated by scanning densitometry. Binding affinity was determined graphically and expressed as the concentration of test compound that reduced the binding of [3H]benzylpenicillin to 50% of that for a drug-free control (IC50).
RESULTS
Confirmation of β-lactamase production.
All clinical isolates received as β-lactamase-positive when grown in unmedicated medium were strongly and rapidly positive for the production of β-lactamase at both inoculum levels tested in the microdilution assay. The presence of plasmids was confirmed by agarose gel electrophoresis (data not shown). The Bush group 1 strains MB4903, MB5503, LS641, and DH5α were weakly positive only after several hours’ reaction time; a positive response for β-lactamase production with cultures of all four strains was stronger and more rapid in the presence than in the absence of all β-lactam antibiotics tested except aztreonam.
Binding to E. coli PBPs.
MK-0826 showed high-affinity binding to the essential PBPs of E. coli KN126 (Table 2). Its binding to PBP 2 (IC50, 0.01 μg/ml) was identical to that for imipenem and 30- to 40-fold superior to those for cefepime and ceftriaxone, respectively. The MK-0826 IC50 for PBP 3 (0.04 μg/ml) was similar to that of cefepime and ceftriaxone (0.03 μg/ml in both cases).
TABLE 2.
Binding of MK-0826 and other agents to PBPs of E. coli KN126
| PBP | IC50 (μg/ml) of:
|
|||
|---|---|---|---|---|
| MK-0826a | Imipenemb | Ceftriaxonec | Cefepimed | |
| 1a | 0.1 | 0.02 | 0.03 | 3.8 |
| 1b | 0.2 | 0.3 | 0.4 | 1.9 |
| 2 | 0.01 | 0.01 | 0.4 | 0.3 |
| 3 | 0.04 | 2.9 | 0.03 | 0.03 |
| 4 | 0.1 | 0.2 | 40 | 73 |
| 5 | 0.1 | 0.1 | >75 | >75 |
| 6 | 5.3 | 0.2 | 23 | 74 |
At the doses of 7.5 × 104 and 7.5 × 105 CFU/ml, the MICs were both 0.016 μg/ml.
At the doses of 7.5 × 104 and 7.5 × 105 CFU/ml, the MICs were both 0.5 μg/ml.
At the doses of 7.5 × 104 and 7.5 × 105 CFU/ml, the MICs were 0.03 and 0.06 μg/ml, respectively.
At the doses of 7.5 × 104 and 7.5 × 105 CFU/ml, the MICs were 0.03 and 0.06 μg/ml, respectively.
In vitro activity against K. pneumoniae and E. coli clinical isolates and against Bush group 1 E. coli laboratory strains.
Determination of susceptibility to control antibiotics included in this study was based on NCCLS criteria (15) with parameters for imipenem applied to meropenem. Determination of susceptibility to MK-0826 was based on provisional/tentative breakpoints recently approved by the NCCLS; correlation with clinical outcome data is necessary prior to finalization of the interpretive criteria (1). The MK-0826 MICs used for determination of susceptible, intermediate, and resistant strains or isolates were ≤4.0, 8.0, and ≥16.0 μg/ml, respectively.
The carbapenem antibiotics MK-0826, meropenem, and imipenem were highly active against all K. pneumoniae and E. coli clinical isolates (MIC ranges, 0.008 to 0.12, 0.016 to 0.06, and 0.06 to 0.5 μg/ml, respectively) in a broth microdilution assay employing approximately the inoculum level (3 × 105 to 7 × 105 CFU/ml) recommended by the NCCLS (Tables 3 and 4). This recommended inoculum level (hereinafter referred to as 1×, or standard, inoculum) is designated as the lower of the two levels tested, although a cell concentration of slightly greater than 7 × 105 CFU/ml was actually present in some cases.
TABLE 3.
Antibiotic susceptibility profiles of K. pneumoniae isolates at two cell densities
| Strain (enzyme[s]) | Cell density (CFU/ml) | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbapenems
|
Cephalosporins
|
Monobactam: ATM | Penicillin with or without oxapenam
|
|||||||||
| MK-0826 | MERc | IPM | CTRX | CAZ | CTX | CPM | AM | AM/CA | CA | |||
| CL 5188 (TEM-10, SHV-1) | 1.5 × 106 | 0.12 | 0.06 | 0.25 | 32 | >128 | 16 | 16 | >128 | >128 | 16/8 | 32 |
| 1.5 × 107 | 1 [8] | 1 [16] | 1 [4] | >128 [>4] | >128 | >128 [>8] | >128 [>8] | >128 | >128 | 64/32 [4] | >256 [>8] | |
| CL 5189 (TEM-10, SHV-1) | 1.8 × 106 | ≤0.03 | ≤0.03 | 0.06 | 4 | >128 | 1 | 2 | 64 | >128 | 8/4 | 16 |
| 1.8 × 107 | 0.25 [≥8] | 0.25 [≥8] | 0.25 [4] | 128 [32] | >128 | 32 [32] | 128 [64] | >128 [>2] | >128 | 16/8 [2] | 32 [2] | |
| CL 5128 (TEM-5) | 1.1 × 106 | 0.12 | ≤0.03 | 0.06 | 8 | 64 | 4 | 2 | 16 | >128 | 32/16 | 16 |
| 1.1 × 107 | 1 [8] | 0.12 [≥4] | 0.5 [8] | 64 [8] | 64 [1] | >128 [>32] | 128 [64] | >128 [>8] | >128 | 32/16 [1] | 4 [0.25] | |
| CL 5196 (TEM-10) | 1.2 × 106 | 0.06 | ≤0.03 | 0.5 | 2 | >128 | 1 | 4 | 128 | >128 | 8/4 | 32 |
| 1.2 × 107 | 0.5 [8] | 1 [≥32] | 1 [2] | >128 [>64] | >128 | 128 [128] | 128 [32] | >128 | >128 | 16/8 [2] | >256 [>8] | |
| CL 5203 (unchar. ESBLb) | 9.9 × 105 | ≤0.03 | ≤0.03 | 0.25 | 0.5 | 64 | 0.5 | 0.5 | 8 | >128 | 8/4 | 64 |
| 9.9 × 106 | 0.5 [≥16] | 1 [≥32] | 0.5 [2] | 32 [64] | >128 [>2] | 0.5 [1] | 64 [128] | >128 [>16] | >128 | 16/8 [2] | >256 [>4] | |
| CL 5212 (unchar. ESBLb) | 9.6 × 105 | 0.12 | 0.06 | 0.5 | >128 | >128 | >128 | >128 | >128 | >128 | 16/8 | >256 |
| 9.6 × 106 | 0.5 [4] | 0.5 [8] | 0.5 [1] | >128 | >128 | >128 | >128 | >128 | >128 | 32/16 [2] | >256 | |
Fold MIC increases at 10× inoculum are shown in brackets. Fold MIC changes were calculated based on serial twofold antibiotic dilutions prior to rounding off of MIC values.
ESBL presumed to be TEM-26 (see Table 1, footnote b). unchar., uncharacterized.
Antibiotic abbreviations: MER, meropenem; IPM, imipenem; CTRX, ceftriaxone; CAZ, ceftazidime; CTX, cefotaxime; CPM, cefepime; ATM, aztreonam; AM, amoxicillin; AM/CA, amoxicillin-clavulanic acid, 2:1; CA, clavulanic acid.
TABLE 4.
Antibiotic susceptibility profiles of E. coli isolates at two cell densities
| Strain (enzyme) | Cell density (CFU/ml) | MIC (μg/ml)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbapenems
|
Cephalosporins
|
Monobactam: ATM | Penicillin with or without oxapenam
|
|||||||||
| MK-0826 | MERb | IPM | CTRX | CAZ | CTX | CPM | AM | AM/CA | CA | |||
| CL 5121 (SHV-1) | 9.6 × 105 | 0.016 | 0.03 | 0.12 | 0.06 | 0.25 | 0.06 | 0.06 | 0.06 | >512 | 16/8 | 32 |
| 9.6 × 106 | 0.25 [16] | 0.5 [16] | 0.5 [4] | 2 [32] | 8 [32] | 4 [64] | 4 [64] | >128 [>2,048] | >512 | 16/8 [1] | 32 [1] | |
| CL 5190 (TEM-10) | 1.4 × 106 | 0.06 | 0.016 | 0.06 | 4 | >128 | 1 | 4 | 64 | >512 | 8/4 | 16 |
| 1.4 × 107 | 0.06 [1] | 0.25 [16] | 0.12 [2] | >128 [>32] | >128 | 32 [32] | 128 [32] | >128 [>2] | >512 | 8/4 [1] | 64 [4] | |
| CL 5115 (TEM-1) | 1.0 × 106 | 0.008 | 0.03 | 0.25 | 0.03 | 0.25 | 0.06 | 0.03 | 0.06 | >512 | 16/8 | 32 |
| 1.0 × 107 | 0.008 [1] | 0.03 [1] | 0.25 [1] | 0.25 [8] | 0.5 [2] | 0.12 [2] | 0.12 [4] | 0.12 [2] | >512 | 16/8 [1] | 32 [1] | |
| CL 5120 (TEM-7) | 6.0 × 105 | 0.016 | 0.03 | 0.12 | 0.5 | 32 | 0.25 | 2 | 1 | >512 | 16/8 | 32 |
| 6.0 × 106 | 0.03 [2] | 0.12 [4] | 0.25 [2] | 16 [32] | 128 [4] | 4 [16] | 128 [64] | 32 [32] | >512 | 16/8 [1] | 32 [1] | |
| CL 5197 (TEM-12) | 1.3 × 106 | 0.12 | 0.03 | 0.12 | 1 | 64 | 0.5 | 4 | 2 | >512 | 16/8 | 16 |
| 1.3 × 107 | 0.12 [1] | 0.12 [4] | 0.25 [2] | 64 [64] | >128 [>2] | 8 [16] | >128 [>32] | >128 [>64] | >512 | 16/8 [1] | 64 [4] | |
| MB5503 (Bush group 1 β-lactamase) | 6.9 × 105 | 0.008 | 0.016 | 0.12 | 0.06 | 0.12 | 0.03 | 0.016 | 0.03 | 2 | 2/1 | 32 |
| 6.9 × 106 | 0.008 [1] | 0.03 [2] | 0.12 [1] | 0.06 [1] | 0.25 [2] | 0.06 [2] | 0.03 [2] | 0.06 [2] | 4 [2] | 4/2 [2] | 32 [1] | |
| DH5α (Bush group 1 β-lactamase) | 1.1 × 105 | 0.004 | 0.03 | 0.12 | ≤0.06 | 0.12 | ≤0.06 | ≤0.06 | ≤0.06 | 4 | 2/1 | 32 |
| 1.1 × 106 | 0.008 [2] | 0.06 [2] | 0.25 [2] | ≤0.06 | 0.25 [2] | ≤0.06 | ≤0.06 | 0.12 [≥2] | 4 [1] | 4/2 [2] | 32 [1] | |
| LS641 (Bush group 1 β-lactamase) | 8.2 × 105 | 0.008 | 0.03 | 0.25 | ≤0.06 | 0.25 | ≤0.06 | ≤0.06 | 0.12 | 8 | 4/2 | 32 |
| 8.2 × 106 | 0.12 [16] | 0.12 [4] | 0.5 [2] | 1 [≥16] | 1 [4] | 0.5 [≥8] | 0.5 [≥8] | 4 [32] | 8 [1] | 8/4 [2] | 64 [2] | |
| MB4903 (Bush group 1 β-lactamase) | 6.6 × 105 | 0.008 | 0.03 | 0.25 | ≤0.06 | 0.25 | 0.12 | ≤0.06 | 0.12 | 4 | 4/2 | 32 |
| 6.6 × 106 | 0.008 [1] | 0.03 [1] | 0.25 [1] | 0.25 [≥4] | 2 [8] | 0.25 [2] | 0.25 [≥4] | 2 [16] | 8 [2] | 8/4 [2] | 32 [1] | |
Fold MIC increases at 10× inoculum are shown in brackets. Fold MIC changes were calculated based on serial twofold antibiotic dilutions prior to rounding off of MIC values.
Antibiotic abbreviations are the same as for Table 3.
At 1× inoculum, the majority of clinical isolates were susceptible to ceftriaxone, cefotaxime, and cefepime. All six K. pneumoniae isolates and three of five E. coli isolates were resistant to ceftazidime. Approximately half of the clinical isolates were resistant to aztreonam. As expected, in the absence of a β-lactamase inhibitor, all clinical isolates were resistant to amoxicillin. All but one of the isolates were susceptible or intermediate to amoxicillin plus clavulanic acid. The MIC of clavulanic acid, which is a potent inhibitor of β-lactamase but itself has low intrinsic antimicrobial activity, ranged from 16 to >256 μg/ml (Tables 3 and 4). The Bush group 1 strains were susceptible to all agents tested except clavulanic acid (Table 4).
Many of the BDSBL- and ESBL-producing strains became highly resistant (MICs, ≥128 μg/ml) to a number of the noncarbapenem β-lactam antibiotics when the inoculum level was increased by 10-fold (10× inoculum) (increasing the amount of β-lactamase present). MK-0826 and meropenem MICs against the BDSBL- and ESBL-producing strains at 10× inoculum were increased to ≥16-fold and ≥32-fold, respectively, but not to resistant levels (highest MK-0826 and meropenem MICs observed, 1 μg/ml). (In some strains the fold increase could not be established due to undetermined endpoints at 1× inoculum.) On the whole, imipenem MICs were affected to a lesser extent; MIC increases of one- to eightfold in the presence of 10× inoculum (maximum MIC observed, 1 μg/ml) were observed. However, imipenem MICs were higher than those of either MK-0826 or meropenem at the lower inoculum level (Tables 3 and 4).
By comparison, carbapenem MICs against the majority of E. coli strains that produced a low, basal level of chromosomal β-lactamase increased only one- to twofold at the higher inoculum level (Table 4). MICs of each antibiotic against MB5503 and DH5α and MICs of the carbapenems as well as amoxicillin and clavulanic acid alone and in combination against MB4903 were similar at the two inoculum levels (occasional twofold differences were noted). However, against MB4903, MICs of the four cephalosporins tested were higher by at least 2- to 8-fold while the MIC of the monobactam aztreonam was higher by 16-fold at the higher inoculum level. Among these strains MIC increases at 10× standard inoculum were generally greatest for strain LS641 with all antibiotics except imipenem, amoxicillin, clavulanic acid, and amoxicillin plus clavulanic acid.
DISCUSSION
BDSBLs and ESBLs are produced chiefly by enterobacterial pathogens such as E. coli and K. pneumoniae and have broad substrate profiles. MK-0826 was highly active against all ESBL- and BDSBL-producing E. coli and K. pneumoniae clinical isolates tested (MIC range, 0.008 to 0.12 μg/ml). The potency of MK-0826 compared favorably to those of meropenem (MIC range, 0.016 to 0.06 μg/ml) and imipenem (MIC range, 0.06 to 0.5 μg/ml). Its activity was superior to those of ceftriaxone, ceftazidime, cefotaxime, cefepime, and aztreonam as well as amoxicillin alone or in combination with clavulanic acid. The potency of MK-0826 against E. coli was associated with excellent binding to essential PBPs, the lethal target of β-lactam antibiotics. Using strain KN126, the binding of MK-0826 to PBP 2 was found to be identical to that for imipenem and 30- to 40-fold superior to those for cefepime and ceftriaxone. The MK-0826 IC50 for PBP 3 was similar to those of cefepime and ceftriaxone and superior to that of imipenem. Thus, although MK-0826 and imipenem demonstrated identical and high-affinity binding to E. coli PBP 2, the affinity of PBP 3 for MK-0826 approached that for PBP 2. Yang et al. (27) showed similar and high-affinity binding of both imipenem and meropenem to E. coli PBP 2 and superior binding by meropenem to PBP 3 compared to that by imipenem. Therefore, although MK-0826, meropenem, and imipenem were potent against all E. coli strains tested, the somewhat superior intrinsic potency of MK-0826 and meropenem may be related to their improved binding to PBP 3 relative to that of imipenem.
Not surprisingly, the extended-spectrum cephalosporins and aztreonam appear to be highly susceptible to plasmid-mediated enzymes since notable increases in MIC were observed in a number of strains when the inoculum level was increased 10-fold. Among strains not resistant to these agents at the standard inoculum, MICs generally increased to resistant levels, often with MICs of ≥128 μg/ml at higher inoculum. The carbapenems MK-0826, meropenem, and imipenem were found in this study to be subject to a relatively small inoculum effect in the presence of BDSBLs and ESBLs, with imipenem manifesting the least effect. There did, however, appear to be some level of activity of the BDSBL and ESBL enzymes against the carbapenems that was evident in the presence of increased amounts of enzyme. When the inoculum levels of five E. coli and six K. pneumoniae strains were increased 10-fold, increasing the total amounts of β-lactamase present, the MK-0826 MICs increased, but to no greater than 1 μg/ml. Similar observations were made with meropenem, while imipenem MICs were less affected. The generally smaller fold increases at 10× inoculum of imipenem MIC indicate that imipenem may be less susceptible to these enzymes. Thus, MK-0826 and meropenem appear to be better substrates than imipenem, but this has not been directly tested. Alternatively, differences in MIC increases may be related to rate of cell entry. Permeability alterations in gram-negative bacteria are expected to play a role in resistance to β-lactamase-resistant as well as β-lactamase-susceptible β-lactam antibiotics. Since even a minor degree of hydrolysis may be effective at reducing the amount of antibiotic that reaches its target due to a decreased amount of antibiotic entering the periplasm (16), the observed carbapenem MIC differences at different inoculum sizes may be a result of differences in rate of cell entry and may be related to molecular size, conformation, and/or charge of the antibiotics. Even though MICs at the standard inoculum of imipenem were the highest among those of the three carbapenems tested, the fold MIC increases of this small molecule at higher inoculum were less than those of the larger molecules meropenem and MK-0826. In no case was the MK-0826, imipenem, or meropenem MIC raised to a resistant level.
Results obtained in this study with aztreonam, cefotaxime, and ceftazidime and the E. coli isolate that produces the TEM-1 β-lactamase were consistent with those obtained by Sykes et al. (25) using a Pseudomonas aeruginosa strain that produces TEM-1. Our observation of moderate to large inoculum effects with the E. coli strain that produces the SHV-1 β-lactamase was inconsistent with their observation of a minimal to absent inoculum effect with a P. aeruginosa strain that produces SHV-1. Our results were unexpected since Sykes et al. showed the efficiency of hydrolysis by both TEM-1 and SHV-1 to be low for aztreonam, cefotaxime, and ceftazidime. It should be noted, however, that our higher inoculum density was 10-fold their highest level tested, and the differences observed may be due to bacterial filamentation resulting from inhibition of PBP 3 because, in addition to drug destruction by β-lactamases, filamentous transformation with continued growth has been suggested to help explain the mechanism of inoculum effect, particularly for antibiotics (notably, aztreonam) with marked inoculum effects yet high stability to β-lactamases (9). Eng et al. (5), testing clinical isolates of Enterobacteriaceae, including E. coli and K. pneumoniae, observed large inoculum effects with aztreonam, ceftazidime, and cefoperazone, moderate effects with cefotaxime and ceftriaxone, and a minimal to absent inoculum effect with imipenem or cefoxitin. The authors argued that (chromosomal) β-lactamase could not account for the observed inoculum effects due to the β-lactamase stability of the antibiotics studied (no plasmid-mediated β-lactamases were reported for these strains). Instead, these effects appeared to be a manifestation of increased OD secondary to the development of filamentous bacterial forms with an increase in bacterial mass during exposure to antibiotics which are not rapidly bactericidal. As stated above, in the present study MK-0826 showed binding to PBP 3 similar to those of the two cephalosporins tested (ceftriaxone and cefepime), but like imipenem, it also exhibited high-affinity binding to PBP 2, which may help to account for its inoculum effects being lower than those of the cephalosporins and aztreonam independently of the effects of β-lactamase.
It has been suggested that all gram-negative bacteria contain a species-specific chromosomal β-lactamase and that these enzymes preferentially hydrolyze cephalosporins (2). In order to compare any effect of plasmid-mediated enzymes to that of chromosomal β-lactamase, the Bush group 1 β-lactamase-producing strains MB4903, MB5503, LS641, and DH5α were similarly tested for an inoculum effect. These strains had been found to produce some basal level of chromosomal β-lactamase and an enhanced but unmeasured amount of β-lactamase in the presence of all β-lactam antibiotics (except aztreonam) tested, implying a capacity for enhanced expression (induction by β-lactams) of the chromosomal β-lactamase genes in these strains. Carbapenem MICs against three wild-type E. coli strains with no evident plasmids increased no more than 2-fold at the higher inoculum level; against a fourth strain (LS641), meropenem MICs increased 4-fold and MK-0826 MICs increased 16-fold. The generally small MIC increases observed for the other β-lactams tested did not result in NCCLS-defined resistance in these strains. Against MB4903, MICs of the four cephalosporins tested were higher by 2- to at least 8-fold at 10× inoculum; against LS641, they were higher by 4- to ≥16-fold. Cephalosporin MICs appeared to be increased to a lesser degree against MB5503 and DH5α, but an MIC endpoint for DH5α was obtained only with ceftazidime, which exhibited a twofold increase in MIC at the higher inoculum level. The inoculum effects observed with these strains may be due to PBP binding patterns and filamentous transformation. While the clinical relevance of inoculum effects due to filamentous transformation is unclear, these effects should nevertheless be considered as potentially important to clinical therapeutic decisions (9).
Thus, it is concluded that MK-0826, like the other carbapenems tested, may be inactivated to a small extent by BDSBLs and ESBLs present in clinical isolates of both E. coli and K. pneumoniae when increasing amounts of enzyme are present; however, under these conditions resistance to MK-0826 (based on provisional NCCLS criteria) was not observed, even though MICs increased.
The results confirmed that the antibacterial activity (particularly at high inoculum density) of MK-0826, a new long-acting injectable carbapenem antibiotic, exceeds those of the extended-spectrum cephalosporins, including cefepime and ceftriaxone, a long-acting cephalosporin with reported stability to some bacterial β-lactamases. Therefore, clinical failure of MK-0826 due to the presence of BDSBLs and ESBLs in strains of both K. pneumoniae and E. coli would not be expected, as it might be with cephalosporins. Moreover, the activity of MK-0826 compared favorably with those of the carbapenems imipenem and meropenem and is expected to offer pharmacokinetic advantages.
ACKNOWLEDGMENTS
We gratefully acknowledge our associates at MRL for their essential help in this work: we thank Daniel Shungu for the time and effort spent in gathering clinical isolates and information, Patricia Scott, Barbara Pelak, and Lynn Gerckens for cataloging and providing working sources of the clinical isolates, Avery Rosegay for synthesizing the [3H]benzylpenicillin used in PBP affinity studies, and Charles Hirsch and Ronald Ratcliffe for their technical expertise.
REFERENCES
- 1.Barry A L, Fuchs P C, Brown S D. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. In vitro activity of the carbapenem, MK-0826 (L-749,345), and provisional criteria for disk tests, abstr. D-38; p. 138. [Google Scholar]
- 2.Bush K. Characterization of β-lactamases. Antimicrob Agents Chemother. 1989;33:259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorso K, Kohler J, Silver L L, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Bactericidal effect of L-749,345 on Staphylococcus aureus and Serratia marcescens in the presence and absence of human serum, abstr. F-123; p. 121. [Google Scholar]
- 5.Eng R H K, Cherubin C, Smith S M, Buccini F. Inoculum effect of β-lactam antibiotics on Enterobacteriaceae. Antimicrob Agents Chemother. 1985;28:601–606. doi: 10.1128/aac.28.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerckens L S, Pelak B A, Thompson R, Rosen H, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Pharmacokinetic evaluation of L-749,345 (ZD-4433), a long-acting parenteral carbapenem, in rodents, abstr. F127; p. 122. [Google Scholar]
- 7.Gill C J, Jackson J J, Gerckens L S, Pelak B A, Thompson R K, Sundelof J G, Kropp H, Rosen H. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749,345) Antimicrob Agents Chemother. 1998;42:1996–2001. doi: 10.1128/aac.42.8.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill C J, Jackson J J, Sundelof J G, Rosen H, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vivo activity of a novel long-acting carbapenem antibiotic, L-749,345, in mouse models of localized and systemic bacterial infections, abstr. F125; p. 121. [Google Scholar]
- 9.Goldstein E J C, Citron D M, Cherubin C E. Comparison of the inoculum effects of members of the family Enterobacteriaceae on cefoxitin and other cephalosporins, β-lactamase inhibitor combinations, and the penicillin-derived components of these combinations. Antimicrob Agents Chemother. 1991;35:560–566. doi: 10.1128/aac.35.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby G, Han P, Tran J. Comparative in vitro activities of carbapenem L-749,345 and other antimicrobials against multiresistant gram-negative clinical pathogens. Antimicrob Agents Chemother. 1997;41:1830–1831. doi: 10.1128/aac.41.8.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler J, Dorso K, Pelak B A, Gerckens L S, Hammond G G, Silver L L, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of the potent, broad-spectrum carbapenem L-749,345 against BDSβL- and ESβL-producing Escherichia coli and Klebsiella pneumoniae clinical isolates, abstr. F122; p. 121. [Google Scholar]
- 12.Kohler J, Dorso K, Young K, Hirsch C, Silver L L, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Evaluation of resistance selection potential of the new carbapenem L-749,345 in β-lactamase-producing Escherichia coli clinical isolates, abstr. F124; p. 121. [Google Scholar]
- 13.Majumdar A, Birk K, Blum R A, Cairns A M, Conroy J, Mendel C M, Muson D, Rogers J D. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Pharmacokinetics of L-749,345, a carbapenem antibiotic, in healthy male and female volunteers, abstr. F130; p. 122. [Google Scholar]
- 14.Nagata T, Horiuchi T. Isolation and characterization of a temperature-sensitive amber suppressor mutant of Escherichia coli K12. Mol Gen Genet. 1973;123:77–88. doi: 10.1007/BF00282991. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. p. 13–17. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 16.Nikaido H, Normark S. Sensitivity of Escherichia coli to various β-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic β-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987;1:29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 17.Pelak B A, Gerckens L S, Scott P M, Gill C, Pacholok C, Lynch L, Dorso K, Kohler J, Shungu D, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Antibacterial profile of L-749,345 (ZD-4433), a new potent 1-β-methyl carbapenem, abstr. F119; p. 120. [Google Scholar]
- 18.Scott P M, Gill C J, Shungu D L, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Comparative in vitro activity of L-749,345 against respiratory tract bacterial pathogens, abstr. F121; p. 121. [Google Scholar]
- 19.Spratt B G. Distinct penicillin-binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spratt B G. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem. 1977;72:341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- 21.Spratt B G, Cromie K D. Penicillin-binding proteins of Gram-negative bacteria. Rev Infect Dis. 1988;10:699–711. doi: 10.1093/clinids/10.4.699. [DOI] [PubMed] [Google Scholar]
- 22.Sundelof J G, Gill C J, Thompson R, Rosen H, Kropp H. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Disposition of an extended half-life carbapenem in rhesus monkeys, chimpanzees and humans, abstr. F128; p. 122. [Google Scholar]
- 23.Sundelof J G, Hajdu R, Gill C J, Thompson R, Rosen H, Kropp H. Pharmacokinetics of L-749,345, a long-acting carbapenem antibiotic, in primates. Antimicrob Agents Chemother. 1997;41:1743–1748. doi: 10.1128/aac.41.8.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Nishimura Y, Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci USA. 1978;75:664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykes R B, Bommer D P, Bush K, Georgopapadakou N H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982;21:85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban C, Meyer K S, Mariano N, Rahal J J, Flamm R, Rasmussen B A, Bush K. Identification of TEM-26 β-lactamase responsible for a major outbreak of ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 1994;38:392–395. doi: 10.1128/aac.38.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Urban, C. Personal communication.
- 27.Yang Y, Bhachech N, Bush K. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by β-lactamases. J Antimicrob Chemother. 1995;35:75–84. doi: 10.1093/jac/35.1.75. [DOI] [PubMed] [Google Scholar]
- 28.Young K, Silver L L. Leakage of periplasmic enzymes from envA1 strains of Escherichia coli. J Bacteriol. 1991;173:3609–3614. doi: 10.1128/jb.173.12.3609-3614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]