Abstract
Objectives
The objective of this systematic review was to explore and report the evidence and gaps in the literature for randomized controlled trials (RCTs) studying the effects of motivational interviewing (MI)-based telehealth interventions on outcomes among persons with diabetes (PWD) or prediabetes.
Methods
Following a modified Cochrane approach, we searched Pubmed, CENTRAL, CINAHL, PsycINFO, and Clinicaltrials.gov. Included studies were RCTs published in English before March 25, 2021 evaluating MI-based telehealth on outcomes for adults with diabetes or prediabetes.
Results
A total of 21 retained articles captured results for 6,436 PWD. Among the most commonly investigated outcomes, 60% of articles documented A1C reductions (ranging from <1% to >3%), 56% documented systolic blood pressure reductions, 57% documented diabetes self-efficacy/empowerment improvements, and 40% documented physical activity improvements. Conversely, diastolic blood pressure, lipid panels, body mass index, depressive symptoms, and quality of life were frequently measured outcomes, where MI-based telehealth yielded minor effects (<30% of articles demonstrating improvements).
Conclusions
MI-based telehealth seems most effective for improving A1C, systolic blood pressure, diabetes self-efficacy, and physical activity behaviors. Variability in outcome assessment and intervention heterogeneity were key challenges impeding comparisons across retained articles.
Practice implications
MI-based telehealth interventions demonstrate promising results for improving outcomes in PWD.
Keywords: motivational interviewing, diabetes, telehealth, review, outcomes
1. Introduction
1.1. Background
In the U.S., diabetes is a prevalent chronic condition affecting nearly 34.2 million people [1], and prediabetes affects an expanding population of approximately 88 million adults [1], where an estimated 70% will progress to diabetes [2]. Persons with diabetes (PWD) are at increased risk of cardiovascular mortality and microvascular complications, especially when considering the effects of chronically unmanaged hyperglycemia [3–5]. Along with glycemic stability, blood pressure and lipid goals are also key in reducing the full spectrum of health risks from diabetes [6]. Despite the availability of medications to treat each of these conditions [7], in addition to education and support, only 40–53% of PWD meet the risk-reducing glycemic goal of <7.0% A1C, 37–51% meet the blood pressure goal of <130/80 mmHg, and 46–56% meet the LDL-cholesterol goal of <100 mg/dL [8, 9]. Thus, many PWD not meeting evidence-based therapeutic goals are more prone to developing complications from the disease.
Diabetes requires consistent attention to an array of self-management behaviors; these can be affected by various personal factors including motivators and barriers for using treatment(s) as recommended, attitudes, beliefs, and knowledge level, among others [10]. To improve diabetes self-management, behavioral interventions have demonstrated improvements in diabetes control by targeting eating habits [11], physical activity [11], and medication taking [12], among others. Thus, interventions utilizing behavioral strategies may support and help improve diabetes management among the significant proportion of the U.S. adult population affected by diabetes.
Motivational interviewing (MI) interventions have been explored in various target behaviors, culminating in expansive applications related to disease management behaviors and within healthcare settings [13]. MI is an evidence-based skills set and approach, delivered intentionally by a person trained in its application, to help people uncover their motivations for change and to help facilitate individuals deciding for themselves to make lifestyle and behavioral changes [13]. While MI has shown benefits in improving outcomes for multiple diseases, its potential effectiveness for impacting outcomes in varied populations of adults with type 2 diabetes is particularly noteworthy [14]. However, intensive, evidence-based training is required to achieve a proficiency level in MI skills, and variability among MI study outcomes is often attributed to inadequate amount or length of training and/or non-evidenced methods for MI training [15]. Therefore, MI training and fidelity assessments are important aspects of valid MI intervention and should be considered in studies assessing the impact of an intervention claiming an MI basis.
When delivering behavioral interventions for PWD, telehealth is a mode of delivery that has historically improved access to care [16], and telehealth utilization has rapidly increased due to the COVID-19 pandemic [17, 18] and expansions in reimbursements from the Centers for Medicare and Medicaid Services (CMS) for telehealth-delivered diabetes education [19]. Telehealth has been shown to demonstrate equally efficacious impacts on outcomes compared to face-to-face interventions [20], and promising outcomes have been documented for the effects of telehealth on facilitating and supporting self-management [21]. In addition to one-on-one or group interactions, the American Diabetes Association (ADA) recommends telehealth delivery of lifestyle interventions, such as diabetes self-management education and support (DSMES) [22], and incorporation of person-centered communication, like MI, when addressing diabetes self-management behaviors [23].
MI has been feasibly implemented through various technology-based modes, including computer, video, phone, and animation [24]. Literature synthesis resulting in definitive claims have yet to be established for behavioral interventions like health coaching (i.e., MI) delivered through telehealth [25]. Yet, further systematic examination is warranted to explore and report the effects of telehealth delivery of MI, which we will simply refer to as MI-based telehealth in this report. The operationalization of ‘telehealth’ in this review aligns with the definition provided by the U.S. Department of Health and Human Services, which is “the use of electronic information and telecommunications technologies to support and promote long-distance clinical health care, patient and professional health-related education, public health and health administration” [26].
1.2. Objective
Exploration and synthesis of the literature is needed since MI has shown outcomes-impacting potential, even when this counseling method and approach has been delivered through means other than face-to-face encounters [27]; rigorous evaluation and report of design, intervention methods, and results from randomized controlled trials (RCTs) is warranted [24]. To address this general gap in the literature, this systematic review explores and reports the evidence and gaps in the literature for RCTs studying the effects of MI-based telehealth interventions on diabetes management or prevention across the full spectrum of outcomes for persons with diabetes or prediabetes. This is the first systematic review evaluating this specific realm of research.
2. Methods
This systematic review followed a modified Cochrane approach, as used in previously published systematic reviews [14, 27–29], to explore and report the evidence and gaps in the literature for MI-based telehealth interventions and their impact on diabetes and cardiometabolic outcomes.
2.1. Data sources and searches
To identify studies from peer-reviewed journals, multiple databases were searched, including Pubmed, CENTRAL, CINAHL, and PsycINFO. Next, Clinicaltrials.gov was searched for any unpublished studies relevant to this review topic to address potential for publication bias. Search terms were initially determined through a Pubmed search utilizing MeSH terms, and this search string was altered accordingly to fit other database search engines. Search strings (see Appendix) included variations of the following terms: (“diabetes” OR “prediabetes”) AND “motivational interviewing” AND (“telehealth” OR “telemedicine” OR “telephone” OR “video”). In addition, the reference lists of included articles were hand-searched for relevant studies. Articles reporting relevant protocols were also used to identify subsequent publication of results, and these articles are reported in the number of hand-searched studies. The ‘Preferred Reporting Items for Systematic reviews and Meta-Analyses’ (PRISMA) checklist was used as the guideline for reporting in this systematic review [30].
2.2. Study selection
The study design of focus in the present review was RCTs to ensure that retained study findings would have minimal bias and highest likelihood for causal relationships, especially to provide justification of outcomes impact in complex behavioral intervention studies. Eligibility criteria required inclusion of adults aged 18 years or older with diabetes (type 1 diabetes and type 2 diabetes) or at risk of developing diabetes (prediabetes). Studies including persons with gestational diabetes were excluded from this review. Studies must have been published in English and prior to March 25, 2021, which was the date of article extraction from databases to the Endnote citation manager. However, it was not expected that many studies would be published prior to the 1990’s since this was the time of emerging research for publications reporting use of MI for self-management behaviors in chronic disease contexts.
To achieve the study objective, included studies must have presented results related to diabetes and associated cardiometabolic outcomes, including intermediate, clinical, humanistic/psychosocial, and/or economic [31]. The outcomes were intentionally left broadly defined to identify any evidence and gaps in the literature based on the modified Cochrane approach used. Due to the focus on RCTs, studies were required to be experimental in nature and to apply an intervention focused on MI-based telehealth (i.e., telephone, telecommunication, video, or any other technology-based means). Studies that only included telehealth delivered care with MI-trained interventionists as a supporting component of specific or multiple study interventions or objective(s) were therefore excluded from this review.
2.3. Data collection process
Studies were directly abstracted from the database to the first author’s Endnote library. The initial list of studies was examined for duplicates, which were removed. The first author screened titles and abstracts simultaneously and excluded irrelevant studies. Study inclusion or exclusion was based strictly on the eligibility criteria stated above; any articles that could not be certainly excluded based on the information presented in the abstract were retained for full-text review. To support claims for validity and reliability of findings, full-texts retained for the next review tier were then independently examined by two authors for decision to reject or retain for inclusion in the final synthesis of studies; retain/reject disagreements were discussed to consensus in making the final decision for study retention results.
2.4. Data synthesis and data items
Results for retained studies were qualitatively synthesized into an evidence table. Study details included in the evidence table were first author, year of publication, study design, sample size and characteristics, MI training (if reported), intervention methods and details, targeted outcome(s), and results. To communicate the predominance of positive versus null effects from MI-based telehealth, the percentage of RCT articles demonstrating positive effects was determined for each outcome based on the number of studies with significant effects and the total number of studies evaluating each outcome. Along with these percentages, the effects for each outcome were also summarized across retained studies. These percentages represent the synthesis of findings in this systematic review but do not replace a meta-analysis and should be interpreted with caution.
2.5. Methodological quality assessment
Studies included in the full-text review were also assessed for methodological quality during review using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [32]. This tool evaluates six domains of bias risk, including selection, performance, detection, attrition, selective reporting, and other. Furthermore, the possible bias risk assessment tool ratings range from ‘High Risk of Bias,’ ‘Unclear Risk,’ or ‘Low Risk.’ Assessments were made by the first and second author independently and subsequently discussed until consensus was reached.
3. Results
3.1. Study selection
The PRISMA flow diagram was used to document the number of studies retained at each tier of the review process [30]. Initially, 298 studies were identified in the search. For the full-text review, 37 studies were assessed for eligibility resulting in 21 retained for the final qualitative synthesis of results. Because this review aimed to document the evidence on MI-based telehealth, studies not assessing these specific effects through rigorous RCTs were therefore rejected. The PRISMA flow diagram (Figure 1) depicts specific details for rejection reasons at each review tier. Although various reasons were documented for rejecting studies during full-text reviews, the most common reason was a lack of central study focus on telehealth and/or MI (n=8), especially since many studies included MI as a part of a larger multicomponent program, service, or intervention. Other reasons for rejection during full-text review were inability to extract results specific to eligible study participants (n=3), inappropriate study design (n=3), no results included (n=1), and not published in English (n=1).
Fig. 1.
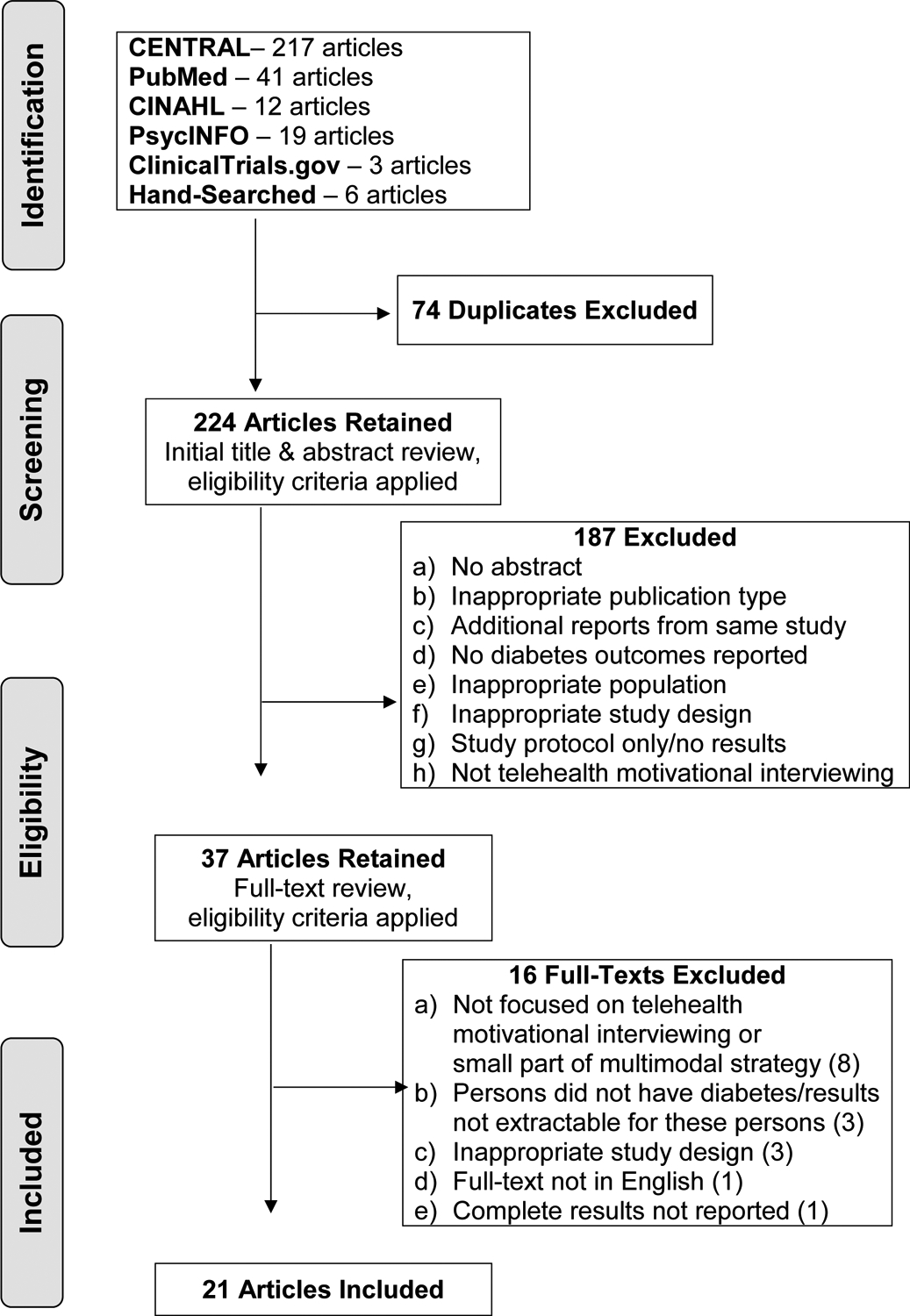
PRISMA Flow Diagram.
3.2. Study characteristics
The 21 retained articles in this review reported results from 19 individual, original research studies. Browning et al. 2016 [33] and Chapman et al. 2018 [34] reported results from one RCT at 12- and 18-month periods, respectively; Swoboda et al. 2016 [35] and 2017 [36] reported results from one RCT for different outcomes. Results from a total of 6,436 individual persons with diabetes, including type 1 and type 2, or prediabetes were captured. All persons were adults (≥18 years old), and five studies focused specifically on older populations (≥50 years old [33, 34, 37], ≥55 years old [38], ≥60 years old [39], and patients with Medicare Advantage [40]). High variability was present in the intervention design, methods, measures, and target outcomes as will be detailed further in sections 3.2.1 and 3.3 below. See Table 1 for the characteristics of studies retained in this review, including study design, setting, sample, MI training, intervention details and interventionist type.
Table 1.
Characteristics of retained randomized controlled trial articles.
| First author, country, year | Design, setting | Sample | MI training | Intervention |
|---|---|---|---|---|
| Abughosh, USA, 2017 [40] | Prospective RCT, telephonic | Medicare Advantage patients with DM and HTN non-adherent (PDC<0.80) to ACEI/ARB I: 250 (A1C=6.59%) C: 500 (A1C=6.47%) |
3 days by MI Trainer; beginning MI proficiency through evaluation scores ranging from 3–4 on 7-point scale | I: 6 MI phone calls made by 4th year pharmacy students monthly over a 6-month period C: No details reported |
| Browning, China, 2014 [38] | Pilot RCT, multi-site | Adults (≥55years old) with T2D I: 50 (A1C=7.16%) C: 50 (A1C=7.00%) |
Intensive 2-day MI workshop + half-day advanced training after 1 month + refreshers | I: Face-to-face (≤18) and telephone (≤19) counseling from health coach (doctor or nurse), decreasing in frequency over 12-month period C: Usual care |
| Browning, China, 2016 [33] | Pragmatic cluster RCT, multi-site | Patients ≥50 years old with T2D I: 385 (A1C=10.60%) C: 345 (A1C=10.29%) |
Intensive 2-day MI workshop + half-day advanced training after 1 month + refreshers | I: Two face-to-face and two telephone sessions of counseling from health coach (doctors, nurses, and psychologists) per month, decreasing in frequency over 12-month period C: Usual care |
| Chapman, China, 2018 [34] | Pragmatic cluster RCT, multi-site | Patients ≥50 years old with T2D I: 385 (A1C=10.60%) C: 345 (A1C=10.29%) |
Intensive 2-day MI workshop + half-day advanced training after 1 month + refreshers | I: Two face-to-face and two telephone sessions of counseling from health coach (doctors, nurses, and psychologists) per month, decreasing in frequency over 18-month period C: Usual care |
| Clark, United Kingdom, 2004 [41] | RCT, single site | Adults (40–70 years old) with T2D and BMI>25 I & C: 100; # NR for I and C (A1C=8.4%) |
No details. | I: Meeting with interventionists (research psychologists) based on MI at baseline, 12, and 24 weeks; follow-up calls at 1, 3, and 7 weeks C: Usual care |
| Collins, USA, 2019 [37] | RCT, single site | Latino adults ≥50 years old with diabetes I: 16 (A1C=NR) C: 10 (A1C=NR) |
2-day MI workshop from MI expert | All received educational printed handout for modifying risk of CVD and pedometer with smartphone app I: Encouraging PA through tailored text messages (5 days/week) and MI phone calls provided by a research assistant (biweekly for 1 month then monthly for 2 months); 3-month period C: Handout only |
| Dale, United Kingdom, 2009 [45] | RCT, multi-site | Patients with T2D (A1C >7.4%) advised to change behavior I1: 90 (A1C=8.4%) I2: 44 (A1C=8.9%) C: 97 (A1C=8.7%) |
2-day program that included MI in addition to various topics | Up to 6 Telecare calls; decreasing in frequency over 150 days; 6-month period I1: Telecare support from peers I2: Telecare support from diabetes specialist nurse C: Single call; usual care |
| Döbler, Germany, 2018 [43] | RCT, single site | Patients 18–70 years old with T2D discharged from diabetes rehabilitation program I: 123 (A1C=7.8%) C: 126 (A1C=7.6%) |
3-day MI workshop | 1-hr face-to-face interview at discharge with individualized behavior planning; monthly telephone calls focused on behavior plans over 1 year, delivered by nonmedical dietitians; assessed intervention fidelity (not MI specific) I: Telephone calls monthly C: Usual care + written information about diet and exercise at 3 and 9 months |
| Fischer, USA, 2012 [46] | RCT, single site | Adult patients with DM in a vulnerable population I: 381 (A1C=8.5%) C: 381 (A1C=8.3%) |
Trained by a CDCES nurse; 10-hr training over 6 weeks | Nurse telephone outreach program over 20 months I: Initial call to adjust medication; 2-week follow-up call to check side effects; 6-week follow-up call to check lipids C: Usual care |
| Hawkins, USA, 2010 [39] | RCT, single site | Rural older adults ≥60 years old with poorly managed DM (A1C>7 mg/dL) I: 40 (A1C=9%) C: 36 (A1C=8.9%) |
MI self-instructional class | Videophone nurse practitioner-delivered MI DSMES over 6 months; assessed intervention fidelity (not MI specific) I: Educational diabetes-related handouts, DSMES curriculum; 15 min weekly calls for 3 months, 15 min monthly calls for 3 months C: Health habits handouts; monthly 5 min calls |
| Hokanson, USA, 2006 [47] | Prospective RCT, single site | Adult (21–80 years old) smokers or recent quitters with T2D I: 57 (A1C=8.9%) C: 57 (A1C=8.3%) |
12-hr training that included MI | Follow-up at 3 and 6 months I: Diabetes education, smoking cessation using MI (1 face-to-face nurse visit followed by 3–6 telephone calls; frequency varied by patient) C: Diabetes education, printed smoking information |
| Huffman, USA, 2021 [42] | Pilot RCT, single site | Patients with T2D and low PA I: 35 (A1C=NR) C: 35 (A1C=NR) |
No details; MI fidelity assessed but NR | All received 2 initial in-person meetings and pedometer; psychologist interventionists I: Positive psychology and MI weekly phone calls to promote PA following treatment manual and assigned activities; 14 sessions over 16-week period C: Time- and attention-matched with weekly phone calls for general diabetes counseling following treatment manual and assigned activities |
| Ingersoll, USA, 2015 [48] | RCT, online | Adults (18–70 years old) with T1D I1: 156 (A1C=NR) I2: 160 (A1C=NR) C: not included in analysis |
4 2-hr sessions and a 2-hr practice session; MI fidelity assessed in 10% with MITI =good/ excellent | I1: Diabetes Driving internet program (DD.com) I2: DD.com + 2 MI telephone calls at baseline and 10 weeks from researchers (undergrad., grad., and post doc. students) C: No content |
| Jansink, Netherlands, 2013 [49] | Cluster RCT, multi-site | Adults (<80 years old) with T2D, A1C>7%, and BMI>25 I: 229 (A1C=7.8%) C: 292 (A1C=7.7%) |
16-hr training that included MI over 6 months | I: MI trained nurses cared for patients and follow-up telephone calls monthly for 6 months C: Nurse-delivered usual care Follow-up at 14 months for all |
| Lauffenburger, USA, 2019 [52] | Pragmatic RCT, telephonic | Horizon BCBS patients (18–64 years old) with A1C ≥8% who filled an oral hypoglycemic agent I: 700 (A1C=9.3%) C: 700 (A1C=9.4%) |
General training program with “script development and role-playing exercises” | I: MI and SDM behaviorally tailored telephone calls from pharmacist (initial consultation call with up to 3 “booster” calls over 12 months) C: No contact |
| Safford, USA, 2015 [50] | Cluster RCT, multi-site with community clusters | Adults with DM I: 198 (A1C=8.0%) C: 226 (A1C=7.9%) |
12-hr training included MI | All received 1-hr group diabetes education, 5-min counseling session, and diabetes report card; 10-month intervention; assessed intervention fidelity (not MI specific) I: Peer coaching (1st in-person or telephone; weekly calls for 2 months; then ≥monthly calls for next 8 months) C: Usual care |
| Swoboda, USA, 2016 [35] | Pragmatic pilot RCT, single site | Adults (40–75 years old) with T2D and risk for CVD I1: 21 (A1C=7.1%) I2: 20 (A1C=6.9%) C: 19 (A1C=7.1%) |
No training details | 4-month intervention; assessed intervention fidelity (not MI specific) I1: Multiple goal/behavior change (face-to-face meeting + 7 biweekly phone calls with dietitian) I2: Single goal/behavior change (same contacts as I1) C: No coaching, resource information provided by nurse (same contacts as I1) |
| Swoboda, USA, 2017 [36] | Pragmatic pilot RCT, single site | Adults (40–75 years old) with T2D and risk for CVD, not loss to follow-up in Swoboda, 2016 [35] I (I1+I2): 37 (A1C=NR) C: 17 (A1C=NR) |
No training details | 4-month intervention I1: Multiple goal/behavior change (face-to-face meeting + 7 biweekly phone calls with dietitian) I2: Single goal/behavior change (same contacts as I1) C: No coaching, resource information (same contacts as I1) |
| Young, USA, 2014 [51] | RCT, multi-site | Adults (>18 years old) with T1D or T2D I: 61 (A1C=NR) C: 60 (A1C=NR) |
6-hr MI training from CDCES + practice | 16-week intervention; assessed intervention fidelity (not MI specific) I: 2-hr face-to-face session with nurse coach about MI; 5 biweekly telephone or videoconference calls with coach (MI goal setting) C: Usual care |
| Young, USA, 2019 [44] | Pilot RCT, telephonic | Adults (18–74 years old) with prediabetes or diabetes who were physically inactive I: 32 (A1C=6.8%) C: 35 (A1C=6.9%) |
MI training via 30-hr web-based program, activities, and 2-day in-person training with MI expert | All received accelerometers via mail I: Encourage PA through 7 MI telephone calls from research associate delivered with decreasing frequency over 24-week intervention and information packet C: PA resource handout |
| Young, USA, 2020 [53] | RCT, multi-site | Adults (≥18 years old) with T2D and A1C≥6.5% I: 158 (A1C=NR) C: 161 (A1C=NR) |
MI-based coaching training; MI fidelity assessed in 5% with MITI but NR | I: MI-based health coaching + mHealth (in-person orientation, 6 biweekly MI phone calls from nurse for 3 months, wearable tracking device with smartphone app for 9 months with data integration into EHR) C: Usual care |
RCT=Randomized controlled trial; DM=Diabetes mellitus; HTN=Hypertension; PDC=Proportion of days covered; ACEI=Angiotensin-converting enzyme inhibitors; ARB=Angiotensin II receptor blockers; I=Intervention group; C=Control group; A1C=Hemoglobin A1C; MI=Motivational interviewing; T2D=Type 2 diabetes; BMI=Body mass index; NR=Not reported; CVD=Cardiovascular disease; PA=Physical activity; CDCES=Certified Diabetes Care and Education Specialist; DSMES=Diabetes self-management education and support; T1D=Type 1 diabetes; MITI=Motivational Interviewing Treatment Integrity; BCBS=Blue Cross Blue Shield; SDM=Shared decision-making; EHR=Electronic health record.
3.2.1. Intervention heterogeneity
The reported interventions were heterogeneous across study characteristics, including type of settings, type of interventionists, PWD sampled, length or duration of intervention periods, frequency of patient encounters, and mode of intervention deliveries. While all 19 studies were RCTs, eight were conducted based from single sites, seven were conducted based from multiple sites, and the remaining four were entirely conducted virtually (online/telephone).
The type of interventionists ranged broadly from nurses (n=8), psychologists (n=3), research personnel (n=3), dietitians (n=2), peers (n=2), pharmacy students (n=1), doctors (n=2), pharmacists (n=1), and nurse practitioners (n=1). The length of interventions was also variable with the shortest intervention lasting 10 weeks and the longest lasting 20 months; most interventions were implemented across a duration of 6–12 months (n=13). The frequency of patient encounters decreased over the intervention period in most studies (n=9). Three studies reported monthly calls, three reported biweekly calls, one reported weekly calls, and the remaining studies (n=3) either used a frequency specific to program completion or based on individual patients. Lastly, the mode of intervention delivery was mostly implemented through a combination of in-person visits and telephone calls (n=10) or only telephone calls (n=5). Additionally, two studies used online website/app supplemented by telephone calls, one solely used videophone calls, and one used text messages and phone calls.
3.2.2. Motivational interviewing training and fidelity assessment
In looking at MI training across all 19 retained studies, three did not describe MI training at all [35, 36, 41, 42], six studies reported details about intensive 2–3 day training programs [33, 34, 37, 38, 40, 43, 44], and the remaining 10 studies gave varying details pertaining to the MI training provided (in person or self-instructed; with or without practice) and lengths of training [39, 45–53]. While three of the 19 studies assessed MI fidelity [42, 48, 53], only one of these reported results for MI fidelity, which was rated as good/excellent using the Motivational Interviewing Treatment Integrity (MITI) assessment tool during the intervention [48]. Although not a strict fidelity assessment, another study evaluated the interventionists’ skills after MI training through practice calls prior to intervention implementation, with scores ranging from 3–4 on a 7-point Likert-type scale [40]. Lastly, some studies reported that MI training was provided by Certified Diabetes Educators [46, 51], now referred to as Certified Diabetes Care and Education Specialists (CDCES).
3.3. Outcomes
Table 2 includes documented findings within each of the 21 retained articles across the full spectrum of target outcomes evaluated, including intermediate behavior change or knowledge gain (n=16), clinical (n=18), humanistic or psychosocial (n=15), and economic (n=2). Table 3 provides a summary of effects for each individual outcome assessed in retained articles, along with the percentage of RCT articles demonstrating positive effects for each outcome. Due to the wide range of evaluated outcomes, the discussion focuses on statistically significant differences to highlight impactful MI-based telehealth interventions.
Table 2.
Targeted outcomes in retained randomized controlled trial articles.
| First author, country, year | Intermediate behavior change outcomes | Clinical outcomes | Humanistic or psychosocial outcomes | Economic or utilization outcomes |
|---|---|---|---|---|
| Abughosh, USA, 2017 [40] |
|
|||
| Browning, China, 2014 [38] |
|
|
|
|
| Browning, China, 2016 [33] |
|
|
|
|
| Chapman, China, 2018 [34] |
|
|
|
|
| Clark, United Kingdom, 2004 [41] |
|
|
||
| Collins, United States, 2019 [37] |
|
|
||
| Dale, United Kingdom, 2009 [45] |
|
|
||
| Döbler, Germany, 2018 [43] |
|
|
|
|
| Fischer, USA, 2012 [46] |
|
|
||
| Hawkins, USA, 2010 [39] |
|
|
|
|
| Hokanson, USA, 2006 [47] |
|
|
|
|
| Huffman, USA, 2021 [42] |
|
|
|
|
| Ingersoll, USA, 2015 [48] |
|
|||
| Jansink, Netherlands, 2013 [49] |
|
|
|
|
| Lauffenburger, USA, 2019 [52] |
|
|
||
| Safford, USA, 2015 [50] | Results from all participants:
|
Results from all participants:
|
||
| Swoboda, USA, 2016 [35] |
|
|
||
| Swoboda, USA, 2017 [36] | NSD; I1+I2=I
|
|
NSD, except for empowerment; I1+I2=I
|
|
| Young, USA, 2014 [51] | At 9 months:
|
At 9 months:
|
||
| Young, USA, 2019 [44] |
|
|
|
|
| Young, USA, 2020 [53] |
|
|
|
ACEI=Angiotensin-converting enzyme inhibitors; ARB=Angiotensin II receptor blockers; PDC=Proportion of days covered; MI=Motivational interviewing; SS=Statistically significant, p<0.05; I=Intervention group; C=Control group; A1C=Hemoglobin A1C; BP=Blood pressure; NSD=No significant differences between groups, p≥0.05; BMI=Body mass index; QoL=Quality of life; FBG=Fasting blood glucose; HDL=High-density lipoproteins; LDL=Low-density lipoproteins; CVD=Cardiovascular disease; NS=Nonsignificant, p≥0.05; MVPA=Moderate to vigorous physical activity.
Table 3.
Summary of outcome effects.
| Outcome | Percentage of RCT articles demonstrating positive effect % (n) n=Total # RCTs assessing each outcome | Summary of outcome effects |
|---|---|---|
| Intermediate behavior change outcomes | ||
| Medication taking | 33% (3) | PDC significantly higher in I than C (0.66 vs. 0.57), respectively [40]; while 2 studies demonstrated no effects [43, 52]. |
| Overall diet | 25% (4) | Diet was significantly improved [33]. Remaining studies found no effect between groups [36, 43, 49], but one of these found SS improvements within I [36]. |
| Fat eating habits | 100% (2) | Fat intake and fat-related eating habits were significantly improved [41], and total, saturated, and monosaturated fat intakes were reduced [35]. |
| Vegetable, fruit, and grain eating habits | 100% (1) | Vegetable and fruit intake was significantly increased, while refined grain intake was significantly reduced [35]. |
| Eating habit goals | 0% (1) | No effect between groups, but SS improvements in confidence with diet goals within I [36]. |
| Physical activity/behaviors | 40% (5) | Two studies found SS improvements in physical activity [41, 43], while the remaining 3 studies found no between group effects [35, 37, 49]. |
| Steps walked | 100% (3) | Steps walked per day improved between groups [42]. Steps walked per week improved among I only [37, 53]. |
| Exercise action planning | 100% (1) | Exercise action planning occurred more in I than C [43]. |
| Exercise coping planning | 0% (1) | No effect [43]. |
| Physical activity goals | 0% (1) | No effect between groups, but SS increased decisional conflict with goals among I [36]. |
| MVPA | 0% (2) | No effect [42, 44]. |
| Diabetes self-care behaviors | 0% (3) | Outside of physical activity and eating habit improvements already captured above, no SS effects on subscales between I and C [33, 34, 41]. SS within group improvements in blood glucose monitoring and foot care were also found [33, 34]. |
| Diabetes knowledge | 100% (1) | Diabetes knowledge improved more in I than C [39]. |
| Smoking cessation behaviors | 50% (2) | SS reductions in daily smoking [47], but no effect on smoking in another study [43]. |
| Efficiency completing tasks | 100% (1) | Program tasks were completed earlier among group receiving MI, but NSD in program adherence or completion [48]. |
| Blood pressure checks | 100% (1) | SS increase in patients having blood pressure checked [49]. |
| Readiness to change | 0% (1) | No effect [49]. |
| Hypoglycemia-related driving mishaps | 0% (1) | No effect [48]. |
| Clinical and anthropometric outcomes | ||
| A1C | 60% (15) | SS reductions in A1C were found in the following 9 studies:
|
| Fasting blood glucose | 0% (2) | No effect between groups, but SS improved within groups [33, 34]. |
| Systolic blood pressure | 56% (9) | SS reductions were found in the following 5 studies:
|
| Diastolic blood pressure | 13% (8) | While majority showed no effect [33, 34, 38, 39, 42, 44, 49], one study found SS reduction within I (−2.50 mmHg) [35]. |
| Blood pressure goals | 0% (1) | No effect on proportion of patients reaching <130/80 mmHg [46]. |
| Total cholesterol | 0% (7) | No effect between groups [33–35, 39, 41, 44, 49], but 2 of these studies found SS improvements within groups [33, 34]. |
| Triglycerides | 0% (6) | No effect between groups [33–35, 39, 41, 47], but 3 of these studies found SS improvements within groups [33, 34, 47]. |
| LDL | 11% (9) | SS improvement in proportion with LDL <100 mg/dL in I (58.5%) than C (46.7%) [46]. Others found no effects between groups [33–35, 39, 41, 47, 49, 50], but 3 of these studies found SS improvements within groups [33, 34, 47]. |
| HDL | 20% (5) | SS improvement of 2 mg/dL in 1 study [35]. Others found no effects between groups [33, 34, 39, 41], but 2 of these studies found SS improvements within groups [33, 34]. |
| CVD risk | 100% (1) | Significantly reduced CVD risk in I compared to C [43]. |
| BMI | 9% (11) | BMI significantly improved over time [50], while other studies found no effect [33–35, 38, 39, 41–44, 49]. |
| Weight | 0% (4) | No effect between groups [33–35, 47], but 2 of these studies found SS improvements within groups [34, 47]. |
| Waist circumference | 25% (4) | SS reductions among males in I (−0.88 cm) compared to C (+0.56 cm) [33]. Others found no effect between groups [34, 38, 41], but 2 of these studies found SS improvements within groups [38, 41]. |
| Hip circumference | 0% (2) | No effect between groups [33, 34]. |
| Physical and mental health | 0% (1) | No effect between groups [51]. |
| Depressive symptoms | 20% (5) | One study found a SS reduction in I compared to C [53]. Others found no effect between groups [36, 42, 43, 47], but one of these studies documented SS improvements within I [36]. |
| Humanistic or psychosocial outcomes | ||
| Quality of life | 29% (7) | SS improvements in I compared to C [38, 50]; others found no between group effect for overall quality of life [33, 34, 37, 44, 49]. |
| General distress | 25% (4) | SS worse in C than I [33]. Three studies found no effect between groups [34, 38, 53], but 1 of these found within group changes [38]. |
| Diabetes distress | 0% (4) | No studies found between group improvements [36, 45, 47, 50], but 2 of these found within group improvements [36, 45]. One study found increased distress in I with SS changes in distress between groups across time [50]. |
| Perceived stress | 100% (1) | SS improvements in I compared to C [53]. |
| Diabetes self-efficacy/empowerment | 57% (7) | SS improvements in I compared to C was found in 4 studies [36, 39, 51, 53], while the remaining found no between group effects, only within group effects [33, 34, 45]. |
| Well-being | 100% (1) | SS improvements in I compared to C [43]. |
| Diabetes-related disability days | 100% (1) | SS less diabetes-related disability days in I compared to C [43]. |
| Self-efficacy for exercise | 33% (3) | SS improvements in I compared to C in 1 study [42], but other 2 studies found no effect [43, 44]. |
| Motivation for physical activity | 100% (1) | SS improvements in I compared to C for enjoyment, competence, appearance, and fitness subscales, but not the social subscale [44]. |
| Physical activity self-regulation | 100% (1) | SS improvements in I compared to C for identified regulation, intrinsic regulation, and relative autonomy index, but not for external or introjected regulation [44]. |
| Physical activity outcome expectations | 100% (1) | SS improvements in I compared to C [44]. |
| Patient activation | 100% (1) | SS changes between groups were found across time [50]. |
| Intervention satisfaction | 100% (1) | Higher satisfaction when delivered by diabetes specialist nurses (94%) compared to peers (77%) [45]. |
| Positive affect | 0% (1) | No effect [42]. |
| Optimism | 0% (1) | No effect [42]. |
| Resilience | 0% (1) | No effect [42]. |
| Illness burden | 0% (1) | No effect [43]. |
| Self-efficacy to quit smoking | 0% (1) | No effect [47]. |
| Self-reported physical function | 0% (2) | No effect [42, 53]. |
| Social support for physical activity | 0% (1) | No effect [44]. |
| Satisfaction with diabetes care | 0% (1) | No effect; only NS improvements in I compared to C [51]. |
| Economic or utilization outcomes | ||
| Cost | 100% (1) | SS lower cost per patient in I ($6600) than C ($9033) [46]. |
| Doctor visits | 0% (1) | No effect; number of doctor visits increased in both groups [38]. |
| Hospital admissions | 0% (1) | No effect [46]. |
RCT=Randomized controlled trial; PDC=Proportion of days covered; I=Intervention group; C=Control group; SS=Statistically significant, p<0.05; MVPA=Moderate to vigorous physical activity; NSD=No significant differences between groups, p≥0.05; A1C=Hemoglobin A1C; LDL=Low-density lipoproteins; HDL=High-density lipoproteins; CVD=Cardiovascular disease; BMI=Body mass index; NS=Nonsignificant, p≥0.05.
Among the four articles reporting more than three statistically significant between-group differences [33, 39, 43, 50], healthcare practitioners (n=3) and peers (n=1) delivered the interventions. In these four, the sample sizes ranged from 76 to 730 PWD, and the PWD were from four different populations: adults ≥50 years old with type 2 diabetes, adults 18–70 years old with type 2 diabetes discharged from diabetes rehabilitation program, rural older adults (≥60 years old) with poorly managed diabetes, and general adult population with diabetes. The MI training reported in the four studies ranged from self-instruction, 12-hour training, and 2 to 3-daylong trainings, none of which conducted MI fidelity assessments. Although not specific to MI, three out of the four studies reported evaluating intervention fidelity. The lengths of intervention periods were 12 months (n=2), 10 months (n=1), and 6 months (n=1). The frequencies of encounters with PWD were either decreasing frequencies of patient contact over the intervention period (n=3) or monthly (n=1). Lastly, there was less variability in the modes of intervention with three interventions utilizing face-to-face and telephone contacts, and the other study used videophones to contact PWD.
3.3.1. Intermediate behavior change or knowledge gain outcomes
See Table 3 for a summary of effects for the intermediate behavior change or knowledge gain outcomes. Medication taking according to prescription improved in 33% of studies assessing this outcome [40, 43, 52]. In some studies, eating habits were significantly affected regarding improved overall diet [33, 36, 43, 49], reduced fat intake and fat-related eating habits [35, 41], and increased vegetable and fruit intake [35], but eating habit goals were not significantly different between groups [36]. Significant improvements were demonstrated for physical activities/behaviors [35, 37, 41, 43, 49], steps walked [37, 42, 53], and exercise action planning [43]; however, exercise coping planning [43], physical activity goals [36], and moderate to vigorous physical activity [42, 44] were not affected.
Diabetes self-care behaviors, such as glucose monitoring and foot care were not significantly different between groups [33, 34, 41]. Diabetes knowledge was significantly improved [39]. One of the two studies assessing smoking cessation behaviors documented significant improvements [43, 47], where the improvement occurred during an MI-based telehealth intervention tailored for smoking cessation. Lastly, intervention specific measures that demonstrated significant improvements included efficiency in completion of program tasks [48] and higher proportion of patients receiving blood pressure checks [49]; however, no statistically significant effects were demonstrated for persons’ readiness to change [49] or hypoglycemia-related driving mishaps [48].
3.3.2. Clinical and anthropometric outcomes
Most studies targeted one or more outcomes related to cardiometabolic conditions; see Table 3 for a summary of these effects. Among studies targeting A1C, 60% demonstrated statistically significant A1C reductions, either reaching A1C goals of <7% (n=1) or attaining reductions of <1.0% (n=5), 1.0–2.9% (n=1), and >3.0% (n=2) in PWD receiving MI-based telehealth. However, fasting blood glucose was not affected [33, 34]. MI-based telehealth significantly improved systolic blood pressure in 56% of studies (improvement range: 1.6–7.0 mmHg) [33–35, 38, 39, 42, 44, 49, 50] but not diastolic blood pressure [33–35, 38, 39, 42, 44, 49] or proportion of patients within blood pressure goal (<130/80 mmHg) [46]. Lipid panel measures of total cholesterol [33–35, 39, 41, 44, 49], triglycerides [33–35, 39, 41, 47] low-density lipoproteins (LDL) [33–35, 39, 41, 46, 47, 49, 50], and high-density lipoproteins (HDL) [33–35, 39, 41] were not largely impacted, but one study reported an overall improvement in CVD risk reduction [43]. Physical and mental health were not significantly different between groups [51]. Lastly, 20% of studies demonstrated significant improvements for depressive symptoms [36, 42, 43, 47, 53], but these improvements were not sustainable [53].
For anthropometrics, only 9% of studies [33–35, 38, 39, 41–44, 49, 50] investigating body mass index (BMI) documented a statistically significant improvement in BMI across time, and weight alone was not significantly changed [33–35, 47]. Waist circumference was significantly improved among males (decreased 0.9 cm) in one study [33, 34, 38, 41], but hip circumference was not significantly changed [33, 34].
3.3.3. Humanistic or psychosocial outcomes
Table 3 summarizes the effects for all humanistic or psychosocial outcomes studied. As a result of MI-based telehealth interventions in retained studies, statistically significant improvements were documented between intervention and control groups for quality of life [33, 34, 37, 38, 44, 49, 50], psychological distress [33, 34, 38, 53], perceived stress [53], diabetes self-efficacy/empowerment [33, 34, 36, 39, 45, 51, 53], well-being [43], diabetes-related disability days [43], self-efficacy for exercise [42–44], motivation for physical activity [44], physical activity self-regulation [44], physical activity outcome expectations [44], and patient activation [50]. Additionally, diabetes self-efficacy was found to be associated with larger reductions in A1C [39]. No statistically significant improvements were found for diabetes distress [36, 45, 47, 50], positive affect [42], optimism [42], resilience [42], illness burden [43], self-efficacy to quit smoking [47], self-reported physical function [42, 53], social support for physical activity [44] or satisfaction with diabetes care [51]. Intervention satisfaction was significantly higher among PWD receiving MI-based telehealth from diabetes specialist nurses compared to peers [45].
3.3.4. Economic or utilization outcomes
Only two studies examined the impact of MI-based telehealth on economic or utilization outcomes (Table 3). While costs were only targeted in one study, a statistically significant economic impact was found for MI-based telehealth with a resultant lower cost per patient receiving the intervention ($6600) versus control ($9033) [46]. However, the number of doctor visits [38] and inpatient hospital admissions [46] were not significantly different between groups.
3.4. Assessment of methodological quality of retained articles
Among the domains in the Cochrane Risk of Bias Tool (Table 4), the majority of retained articles demonstrated low risk of selection bias through sound randomization strategies (n=12), attrition bias (n=12), and selective reporting bias (n=12). Based on the nature of the behavioral interventions studied among MI-trained interventionists, performance bias risk was mostly high or unclear, but three achieved low risk of bias through methods aimed at blinding of participants or personnel. Detection bias risk was also relatively high or unclear among the studies because the majority (n=14) did not address the blinding of allocation from the persons assessing outcomes.
Table 4.
Risk of bias assessment for retained randomized controlled trial articles (Cochrane method).
| Study (first author, year) | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Detection bias | Incomplete outcome data (attrition bias) | Selective reporting bias | Other bias* | |
|---|---|---|---|---|---|---|---|---|
| Participants | Personnel | |||||||
| Abughosh, 2017 [40] | + | ? | ? | − | − | + | + | + |
| Browning, 2014 [38] | ? | ? | ? | − | − | + | + | − |
| Browning, 2016 [33] | + | + | ? | − | − | + | + | − |
| Chapman, 2018 [34] | + | + | ? | − | − | + | + | − |
| Clark, 2004 [41] | + | ? | ? | − | + | ? | − | + |
| Collins, 2019 [37] | + | + | ? | − | + | + | + | − |
| Dale, 2009 [45] | ? | + | − | − | + | + | ? | − |
| Döbler, 2018 [43] | + | + | − | − | ? | ? | + | − |
| Fischer, 2012 [46] | ? | ? | ? | − | − | ? | + | + |
| Hawkins, 2010 [39] | + | + | − | − | ? | + | + | − |
| Hokanson, 2006 [47] | + | ? | ? | − | − | ? | ? | + |
| Huffman, 2021 [42] | ? | ? | ? | − | + | + | + | + |
| Ingersoll, 2015 [48] | ? | ? | ? | − | ? | ? | ? | + |
| Jansink, 2013 [49] | ? | ? | ? | ? | − | ? | ? | − |
| Lauffenburger, 2019 [52] | + | + | − | − | + | + | + | + |
| Safford, 2015 [50] | + | ? | − | − | − | ? | ? | − |
| Swoboda, 2016 [35] | ? | + | + | − | − | + | + | + |
| Swoboda, 2017 [36] | ? | + | + | − | − | ? | + | + |
| Young, 2014 [51] | ? | ? | ? | − | − | ? | ? | − |
| Young, 2019 [44] | + | ? | − | − | + | + | ? | + |
| Young, 2020 [53] | + | ? | + | + | + | + | ? | + |
Abbreviations: + =Low risk of bias, − =High risk of bias, ? = Unclear risk of bias.
Comments (study: comments): (Browning, 2014:Hawthorne effect); (Browning, 2016: Hawthorne effect; contamination due to media coverage of study); (Chapman, 2018: Hawthorne effect); (Collins, 2019: Hawthorne effect); (Dale: additional selection bias imposed by not including patients unlikely to value additional support); (Döbler: social desirability bias based on patients self-report for follow-up outcomes); (Hawkins: sampling bias through convenience sampling); (Jansink: participation bias); (Safford: sampling bias through convenience sampling); (Young, 2014: self-reporting bias).
4. Discussion and conclusion
4.1. Discussion
In synthesizing the body of work represented in the 19 RCTs reported in the 21 retained articles in this systematic review, MI-based telehealth demonstrated mixed, yet promising results for impact on outcomes in PWD. While results are not consistent across all retained studies, MI-based telehealth made a statistically significant impact on various outcomes (Table 3). Each investigation generally documented improved clinical markers for the care of patients with diabetes or prediabetes. Most commonly documented was decline in A1C in majority of studies targeting A1C. Because studies have documented significant reductions in the microvascular event rate (25–35%) for each 1% decline in A1C [3], in the current investigations, this was likely mediated by improvements in eating habits [33, 35], physical activity [35, 43], diabetes knowledge [39], and other intermediate outcomes impacted by MI-based telehealth.
The wide range of intervention and MI training differences across the retained RCTs likely impacted the varied results examined in the synthesis of findings. In other literature, patterns of heterogeneity among MI interventions have been reported [14, 28, 54], and different training settings and methods may impact both the quality and delivery of MI [15, 55, 56]. Therefore, the quality and amount of MI training reported may have impacted the effectiveness of MI-based telehealth interventions, but the overall effect of training in this systematic review cannot be established since many retained studies did not report extensive details about MI training.
Among the selected studies detecting multiple significant effects of MI-based telehealth, characteristics that appeared to foster intervention impact on outcomes may inform current practice. These included implementation by a healthcare practitioner and intervention time periods lasting between 6 to 12 months, with patient encounters occurring at a monthly or decreasing frequency over the intervention period. As was reported in this review, behavioral interventions, such as DSMES and MI-based telehealth interventions, may require direction from trained health professionals for effectiveness, but other persons (peers or care managers) may aid in the program implementation [23]. Further, efficacious modes of intervention delivery among studies detecting multiple significant effects of MI-based telehealth include video calls or a face-to-face visit with telephone call follow-ups.
Essential to improvement of the care and management of PWD is the delivery of DSMES. The ADA, the Association of Diabetes Care & Education Specialists (ADCES), and additional national professional organizations jointly encourage providers to establish access to DSMES for all PWD through innovative modalities [23], especially through the efficiency that technology can provide in bringing these beneficial programs to PWD who have limited access, such as rural populations or during public health emergencies like the COVID-19 pandemic. In addition, the ADA endorses deliberately attending to various areas of psychosocial care [57], and many established measures used in these respective areas were applied in the measurement of outcomes across the reviewed studies, such as the Diabetes Distress Scale (DDS) [36] and the Patient Health Questionnaire (PHQ-9) [43, 53].
The overall results from this review suggest that further study is warranted, using study designs, methods, and measures that are more clearly and standardly articulated in order to more consistently synthesize the impact of MI-based telehealth on outcomes. Future studies should incorporate MI fidelity assessments as a research standard in order to ensure that what is actually delivered in the intervention is MI-consistent; this is important in being able to make claims of validity for MI as the intervention component that affected outcomes. In addition, modes of telehealth delivery warrant a focus on methods emerging in prevalence. Specifically, most studies in this review incorporated telephone calls, but further research should increase examination of MI-based telehealth delivery through videoconferencing on computers, tablets, or smartphones.
Further, minimal research examining the impact of MI-based telehealth on key economic indicators was an identified gap in the literature, and the documented cost savings of about $2400 per patient from MI-based telehealth in one of the retained studies of this review is particularly remarkable. However, these cost savings have low generalizability because this study was conducted in a safety-net health organization caring for mostly indigent and Latino populations [46].
In addition, while this review intended to include studies of persons with prediabetes, only one study was identified that evaluated MI-based telehealth in this population. The very recent emergence of the National Diabetes Prevention Program (DPP), and the Medicare DPP, as well as recent adaptations to an online version of the DPP, suggest that prediabetes will be a research context to watch for in MI-based telehealth for support of risk reducing behaviors in the future.
4.1.1. Limitations
Although this review was able to capture an adequate number of well-conducted studies examining effects of MI-based telehealth, there are some limitations that need to be described. First, search procedures may not have captured all relevant studies ever conducted, although the included databases are directly relevant to the central topic of this review. In addition, publication bias was minimized through including CENTRAL and Clinicaltrials.gov, which capture many registered clinical trials not yet published. Second, the initial title and abstract review was only conducted by the first author; thus, there is a potential of bias in this review tier. However, this bias was minimized in the next tier through inclusion of a second author during the independently conducted full-text review process. In assessing methodological quality, the determination of low, high, or unclear risk of bias may be subjective, but the independent evaluation by two researchers speaks to the confidence in these assessments. Lastly, the qualitative synthesis of findings from this systematic review that are presented in Table 3 should be interpreted with caution. Given the variability in outcomes targeted in retained studies, Table 3 served to summarize the effects for the multitude of outcomes studied. However, the percentages in Table 3 do not replace a meta-analysis. The lack of meta-analysis could be a potential limitation in this systematic review. Based on the broad scope of outcomes captured in retained studies of this systematic review, a meta-analysis was considered to not fully support the aim of our study: to explore and report the evidence for MI-based telehealth. Despite these limitations, this review achieved the objective to explore and report the evidence and gaps in the current science of MI-based telehealth in diabetes care.
4.2. Conclusion
Collectively, this systematic review underscored the overall health gains achieved via MI-based telehealth, which translate into risk reductions for PWD. This review of RCTs found evidence of benefit for MI-based telehealth in a variety of populations with diabetes when implemented for 6–12 months with decreasing frequency of encounters or monthly frequency of encounters over the intervention period. Most demonstrated statistically significant, and clinically meaningful improvements in A1C (reductions of 1–3%) [33–35, 38, 39, 43, 45, 47, 52], which corresponded to diabetes self-efficacy [39]. Other RCTs showed improvement in clinical outcomes of systolic blood pressure (reductions of 1.6–7.0 mmHg) [33, 38, 50] and LDL (reaching goal of <100 mg/dL) [46]. Some investigations demonstrated improvements in dietary behaviors [33, 35, 41] and physical activity [41–43, 53]. Further, evidence also supported improvements across multiple humanistic and psychosocial outcomes, such as quality of life [38, 50] and diabetes self-efficacy [36, 39, 51, 53]. Although less often evaluated, a $2,400 cost savings per PWD receiving MI-based telehealth [46] is a noteworthy outcome worth further investigation. Future research to address gaps in the literature could focus on expanding the populations of investigation to prediabetes, evaluating differences in outcomes between telehealth delivery modalities (telephone versus videoconferencing technology), and outcomes including emergency and urgent care utilization.
4.3. Practice implications
Merging the efficiency of telehealth with the historical effectiveness of appropriately trained and delivered MI emerges as a theme worth integrating into real world practice settings, and sheds light on the impact that healthcare professionals can have on diabetes care through integrating telehealth and MI. Use of telehealth offers PWD the convenience of engagement without the requirement of travel, which may significantly benefit those with limited mobility and/or living in rural locations. Furthermore, multi-point telehealth encounters can accommodate a larger number of guests beyond the healthcare practitioner and PWD alone, such as others essential to the PWD’s care who are not otherwise available to attend in-person appointments.
Telehealth can also offer other unexpected benefits. It allows easy retrieval of important documents, such as home blood glucose logs or medications that people may otherwise have forgotten at home when attending in-person office encounters. Unique to the telehealth setting, videoconferencing connection allows persons to easily show the healthcare practitioner important aspects not typically available during in-person encounters, such as their lunch plate, kitchen or pantry, method of preparing meals, or exercise equipment and use thereof, in addition to their blood glucose monitor or other devices for demonstration of use.
Lastly, MI-based telehealth provides a person-centered approach with nonjudgmental accountability that is better received by individuals compared to traditional healthcare practitioners’ communication styles [58]. When using a videoconferencing connection, it is critical to help persons gain confidence and comfort with use by providing a brief overview at the beginning about viewing and listening options. Other good practices in using videoconferencing include maintaining good eye contact with the camera and not just looking at the individual’s face on the screen, in addition to using the chat feature to clarify terms or medication names. When connecting by telephone alone, a practitioner can maintain person-centered communication by taking time for introductions of all engaged participants and documenting their attendance at the PWD’s request in the medical record. Regardless of using videoconferencing or telephone alone, it is critical for the practitioners to give the PWD their full attention, as some body language or telling nuances in tone can otherwise be overlooked. Lastly, using shorter, more straightforward language can help avoid miscommunication via telehealth. To maintain the person-oriented nature of the encounter, incorporating shared decision-making can empower the PWD to be the ultimate decision-maker for his/her own goal setting.
Supplementary Material
Acknowledgements:
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Prior presentation: Portions of this work were presented as an oral presentation at the annual meeting for the Association of Diabetes Care and Education Specialists (ADCES), August 2020.
We would like to acknowledge our health sciences librarian, Adelia Grabowsky, for aiding with identification of search terms in databases.
Footnotes
- McDaniel: Cassidi C. McDaniel was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR003106. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. McDaniel was also supported by the American Foundation for Pharmaceutical Education (AFPE) under the AFPE Pre-Doctoral Fellowship.
- Kavookjian: Dr. Kavookjian discloses that she is on the Merck Speakers Bureau for non-product medical education for the topics of motivational interviewing, shared decision-making, and health literacy communication; she also served as a consultant for Merck as motivational interviewing content expert in the person-centered communication line of education materials; she also consults for MediMergent, LLC for its US Food and Drug Administration (FDA)- funded project in training pharmacists in motivational interviewing for medication taking in diabetes.
- Whitley: Nothing to disclose.
CRediT Author Statement:
McDaniel: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing - Original Draft, Writing - Review & Editing. Kavookjian: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing - Review & Editing, Supervision. Whitley: Methodology, Investigation, Visualization, Writing - Review & Editing.
Appendix. Supplementary data
Appendix. Search strings.
References
- [1].Centers for Disease Control and Prevention, National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention. (https://www.cdc.gov/diabetes/data/statistics/statistics-report.html) (accessed October 25, 2020). [Google Scholar]
- [2].Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M, Prediabetes: a high-risk state for diabetes development, Lancet (London, England). 379 (2012) 2279–90. DOI: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group, Lancet. 352 (1998) 837–53. [PubMed] [Google Scholar]
- [4].Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes, N Engl J Med. 353 (2005) 2643–53. DOI: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ, Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: Evidence on health outcomes and antidiabetic treatment in United States adults, World J Diabetes. 7 (2016) 449–61. DOI: 10.4239/wjd.v7.i18.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].American Diabetes Association, 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2021, Diabetes Care. 44 (2021) S125–S150. DOI: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- [7].American Diabetes Association, 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021, Diabetes Care. 44 (2021) S111–S124. DOI: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- [8].Whitley HP, Fermo JD, Ragucci K, Chumney EC, Assessment of patient knowledge of diabetic goals, self-reported medication adherence, and goal attainment, Pharm Pract (Granada). 4 (2006) 183. DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC, The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010, Diabetes Care. 36 (2013) 2271–9. DOI: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nam S, Chesla C, Stotts NA, Kroon L, Janson SL, Barriers to diabetes management: patient and provider factors, Diabetes Res Clin Pract. 93 (2011) 1–9. DOI: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [11].Cradock KA, Ólaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KA, Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis, Int J Behav Nutr Phys Act. 14 (2017) 18. DOI: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gonzalez JS, Tanenbaum ML, Commissariat PV, Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice, Am Psychol. 71 (2016) 539–51. DOI: 10.1037/a0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rollnick SR, Miller WR, Butler CC, Motivational interviewing in health care, The Guilford Press, New York, 2008. [Google Scholar]
- [14].Ekong G, Kavookjian J, Motivational interviewing and outcomes in adults with type 2 diabetes: a systematic review, Patient Educ Couns. 99 (2016) 944–52. DOI: 10.1016/j.pec.2015.11.022. [DOI] [PubMed] [Google Scholar]
- [15].Madson MB, Loignon AC, Lane C, Training in motivational interviewing: a systematic review, J Subst Abuse Treat. 36 (2009) 101–9. DOI: 10.1016/j.jsat.2008.05.005. [DOI] [PubMed] [Google Scholar]
- [16].Vadheim LM, Patch K, Brokaw SM, Carpenedo D, Butcher MK, Helgerson SD, Harwell TS, Telehealth delivery of the diabetes prevention program to rural communities, Transl Behav Med. 7 (2017) 286–91. DOI: 10.1007/s13142-017-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caballero AE, Ceriello A, Misra A, Aschner P, McDonnell ME, Hassanein M, Ji L, Mbanya JC, Fonseca VA, COVID-19 in people living with diabetes: An international consensus, J Diabetes Complications. 34 (2020) 107671. DOI: 10.1016/j.jdiacomp.2020.107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sacks LJ, Pham CT, Fleming N, Neoh SL, Ekinci EI, Considerations for people with diabetes during the Coronavirus Disease (COVID-19) pandemic, Diabetes Res Clin Pract. 166 (2020) 108296. DOI: 10.1016/j.diabres.2020.108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Center for Medicare and Medicaid Services, COVID-19 Frequently Asked Questions (FAQs) on Medicare Fee-for-Service (FFS) Billing. Baltimore, MD: Center for Medicare and Medicaid Services. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf (accessed September 2, 2020). [Google Scholar]
- [20].Speyer R, Denman D, Wilkes-Gillan S, Chen YW, Bogaardt H, Kim JH, Heckathorn DE, Cordier R, Effects of telehealth by allied health professionals and nurses in rural and remote areas: a systematic review and meta-analysis, J Rehabil Med. 50 (2018) 225–35. DOI: 10.2340/16501977-2297. [DOI] [PubMed] [Google Scholar]
- [21].So CF, Chung JW, Telehealth for diabetes self-management in primary healthcare: a systematic review and meta-analysis, J Telemed Telecare. 24 (2018) 356–64. DOI: . [DOI] [PubMed] [Google Scholar]
- [22].5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2021, Diabetes Care. 44 (2021) S53–S72. DOI: 10.2337/dc21-S005. [DOI] [PubMed] [Google Scholar]
- [23].Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, Hooks B, Isaacs D, Mandel ED, Maryniuk MD, Norton A, Rinker J, Siminerio LM, Uelmen S, Diabetes Self-management Education and Support in Adults With Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association, Diabetes Care. 43 (2020) 1636–49. DOI: 10.2337/dci20-0023. [DOI] [PubMed] [Google Scholar]
- [24].Shingleton RM, Palfai TP, Technology-delivered adaptations of motivational interviewing for health-related behaviors: a systematic review of the current research, Patient Educ Couns. 99 (2016) 17–35. DOI: 10.1016/j.pec.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dwinger S, Dirmaier J, Herbarth L, Konig HH, Eckardt M, Kriston L, Bermejo I, Harter M, Telephone-based health coaching for chronically ill patients: study protocol for a randomized controlled trial, Trials. 14 (2013) 337. DOI: 10.1186/1745-6215-14-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Telemedicine and telehealth. Washington, DC: The Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth (accessed April 22, 2019). [Google Scholar]
- [27].Teeter BS, Kavookjian J, Telephone-based motivational interviewing for medication adherence: a systematic review, Transl Behav Med. 4 (2014) 372–81. DOI: 10.1007/s13142-014-0270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Poudel N, Kavookjian J, Scalese MJ, Motivational interviewing as a strategy to impact outcomes in heart failure patients: a systematic review, Patient. [Epub ahead of print] (2019). DOI: 10.1007/s40271-019-00387-6. [DOI] [PubMed] [Google Scholar]
- [29].Schaefer MR, Kavookjian J, The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: A systematic review, Patient Educ Couns. 100 (2017) 2190–99. DOI: 10.1016/j.pec.2017.05.037. [DOI] [PubMed] [Google Scholar]
- [30].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration, J Clin Epidemiol. 62 (2009) e1–34. DOI: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [31].Kozma CM, Reeder CE, Schulz RM, Economic, clinical, and humanistic outcomes: a planning model for pharmacoeconomic research, Clin Ther. 15 (1993) 1121–32; discussion 20. [PubMed] [Google Scholar]
- [32].Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials, Brit Med J. 343 (2011) d5928. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Browning C, Chapman A, Yang H, Liu S, Zhang T, Enticott JC, Thomas SA, Management of type 2 diabetes in China: the Happy Life Club, a pragmatic cluster randomised controlled trial using health coaches, Brit Med J Open. 6 (2016) e009319. DOI: 10.1136/bmjopen-2015-009319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chapman A, Browning CJ, Enticott JC, Yang H, Liu S, Zhang T, Thomas SA, Effect of a Health Coach Intervention for the Management of Individuals With Type 2 Diabetes Mellitus in China: A Pragmatic Cluster Randomized Controlled Trial, Frontiers in public health. 6 (2018) 252–52. DOI: 10.3389/fpubh.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Swoboda CM, Miller CK, Wills CE, Setting single or multiple goals for diet and physical activity behaviors improves cardiovascular disease risk factors in adults with type 2 diabetes: a pragmatic pilot randomized trial, Diabetes educator. 42 (2016) 429–43. DOI: 10.1177/0145721716650043. [DOI] [PubMed] [Google Scholar]
- [36].Swoboda CM, Miller CK, Wills CE, Impact of a goal setting and decision support telephone coaching intervention on diet, psychosocial, and decision outcomes among people with type 2 diabetes, Patient education and counseling. 100 (2017) 1367–73. DOI: 10.1016/j.pec.2017.02.007. [DOI] [PubMed] [Google Scholar]
- [37].Collins TC, Lu L, Valverde MG, Silva MX, Parra-Medina D, Efficacy of a multi-component intervention to promote physical activity among Latino adults: a randomized controlled trial, Preventive medicine reports. 16 (2019). DOI: 10.1016/j.pmedr.2019.100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Browning CJ, Yang H, Zhang T, Chapman A, Liu S, Enticott J, Thomas SA, Implementing a chronic disease self-management program into china: the happy life club, Front Public Health. 2 (2014) 181. DOI: 10.3389/fpubh.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hawkins SY, Improving glycemic control in older adults using a videophone motivational diabetes self-management intervention, Research and theory for nursing practice. 24 (2010) 217–32. DOI: 10.1891/1541-6577.24.4.217. [DOI] [PubMed] [Google Scholar]
- [40].Abughosh S, Wang X, Serna O, Esse T, Mann A, Masilamani S, Holstad MM, Essien EJ, Fleming M, A motivational interviewing intervention by pharmacy students to improve medication adherence, Journal of managed care & specialty pharmacy. 23 (2017) 549–60. DOI: 10.18553/jmcp.2017.23.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clark M, Hampson SE, Avery L, Simpson R, Effects of a tailored lifestyle self-management intervention in patients with type 2 diabetes, Br J Health Psychol. 9 (2004) 365–79. DOI: 10.1348/1359107041557066. [DOI] [PubMed] [Google Scholar]
- [42].Huffman JC, Golden J, Massey CN, Feig EH, Chung WJ, Millstein RA, Brown L, Gianangelo T, Healy BC, Wexler DJ, et al. , A positive psychology-motivational interviewing program to promote physical activity in type 2 diabetes: the BEHOLD-16 pilot randomized trial, General hospital psychiatry. 68 (2021) 65–73. DOI: 10.1016/j.genhosppsych.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Döbler A, Herbeck Belnap B, Pollmann H, Farin E, Raspe H, Mittag O, Telephone-delivered lifestyle support with action planning and motivational interviewing techniques to improve rehabilitation outcomes, Rehabilitation psychology. 63 (2018) 170–81. DOI: 10.1037/rep0000224. [DOI] [PubMed] [Google Scholar]
- [44].Young DR, Nguyen MK, Yamamoto A, Pomichowski M, Cornejo M, Paz S, Coleman KJ, Sallis RE, Fortmann SP, Telephone-based motivational interviewing versus usual care in primary care to increase physical activity: a randomized pilot study, Pilot and feasibility studies. 5 (2019). DOI: 10.1186/s40814-019-0390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dale J, Caramlau I, Sturt J, Friede T, Walker R, Telephone peer-delivered intervention for diabetes motivation and support: the telecare exploratory RCT, Patient Educ Couns. 75 (2009) 91–8. DOI: 10.1016/j.pec.2008.09.014. [DOI] [PubMed] [Google Scholar]
- [46].Fischer HH, Eisert SL, Everhart RM, Durfee MJ, Moore SL, Soria S, Stell DI, Rice-Peterson C, MacKenzie TD, Estacio RO, Nurse-run, telephone-based outreach to improve lipids in people with diabetes, American journal of managed care. 18 (2012) 77–84. [PubMed] [Google Scholar]
- [47].Hokanson JM, Anderson RL, Hennrikus DJ, Lando HA, Kendall DM, Integrated tobacco cessation counseling in a diabetes self-management training program: a randomized trial of diabetes and reduction of tobacco, Diabetes educator. 32 (2006) 562–70. DOI: 10.1177/0145721706289914. [DOI] [PubMed] [Google Scholar]
- [48].Ingersoll KS, Banton T, Gorlin E, Vajda K, Singh H, Peterson N, Gonder-Frederick L, Cox DJ, Motivational interviewing support for a behavioral health internet intervention for drivers with type 1 diabetes, Internet interventions. 2 (2015) 103–09. DOI: 10.1016/j.invent.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jansink R, Braspenning J, Keizer E, van der Weijden T, Elwyn G, Grol R, No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial, Scand J Prim Health Care. 31 (2013) 119–27. DOI: 10.3109/02813432.2013.797178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Safford MM, Andreae S, Cherrington AL, Martin MY, Halanych J, Lewis M, Patel A, Johnson E, Clark D, Gamboa C, Richman JS, Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial, Ann Fam Med. 13 Suppl 1 (2015) S18–26. DOI: 10.1370/afm.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Young H, Miyamoto S, Ward D, Dharmar M, Tang-Feldman Y, Berglund L, Sustained effects of a nurse coaching intervention via telehealth to improve health behavior change in diabetes, Telemedicine journal and e-health. 20 (2014) 828–34. DOI: 10.1089/tmj.2013.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lauffenburger JC, Ghazinouri R, Jan S, Makanji S, Ferro CA, Lewey J, Wittbrodt E, Lee J, Haff N, Fontanet CP, Choudhry NK, Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial, PLoS One. 14 (2019) e0214754. DOI: 10.1371/journal.pone.0214754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Young HM, Miyamoto S, Dharmar M, Tang-Feldman Y, Nurse Coaching and Mobile Health Compared With Usual Care to Improve Diabetes Self-Efficacy for Persons With Type 2 Diabetes: Randomized Controlled Trial, JMIR Mhealth Uhealth. 8 (2020) e16665. DOI: 10.2196/16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Resnicow K, Sonneville KR, Naar S, The heterogeneity of MI interventions studies for treatment of obesity, Pediatrics. 142 (2018). DOI: 10.1542/peds.2018-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schwalbe CS, Oh HY, Zweben A, Sustaining motivational interviewing: a meta-analysis of training studies, Addiction. 109 (2014) 1287–94. DOI: 10.1111/add.12558. [DOI] [PubMed] [Google Scholar]
- [56].Söderlund LL, Madson MB, Rubak S, Nilsen P, A systematic review of motivational interviewing training for general health care practitioners, Patient Educ Couns. 84 (2011) 16–26. DOI: 10.1016/j.pec.2010.06.025. [DOI] [PubMed] [Google Scholar]
- [57].Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M, Psychosocial care for people with diabetes: a position statement of the American Diabetes Association, Diabetes Care. 39 (2016) 2126–40. DOI: 10.2337/dc16-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dellasega C, Añel-Tiangco RM, Gabbay RA, How patients with type 2 diabetes mellitus respond to motivational interviewing, Diabetes Res Clin Pract. 95 (2012) 37–41. DOI: 10.1016/j.diabres.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cox DJ, Gonder-Frederick LA, Singh H, Ingersoll KS, Banton T, Grabman JH, Schmidt K, Clarke W, Predicting and reducing driving mishaps among drivers with type 1 diabetes, Diabetes Care. 40 (2017) 742–50. DOI: 10.2337/dc16-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


