Abstract
Aim:
Estimate the impact of dynamic motor control on treatment outcomes in children with cerebral palsy.
Method:
Multiple regression on a retrospective cohort of 473 ambulatory children with cerebral palsy who underwent conservative treatment, single-level orthopaedic surgery, single-event multi-level orthopaedic surgery, or selective dorsal rhizotomy. Outcomes included gait pattern, gait speed, energy cost of walking, and Pediatric Outcomes Data Collection Instrument. Explanatory variables considered were pre-treatment levels of each outcome, treatment group, prior treatment, age, and dynamic motor control computed from surface electromyography using synergy analysis. Effect sizes were estimated from the adjusted response.
Results:
Pre-treatment levels had effect sizes 2-13 times larger than the next largest variable. Individuals with milder pre-treatment involvement had smaller gains or actual declines. Dynamic motor control was significant in all domains except energy cost. The effect size of dynamic motor control was second only to pre-treatment level, and was substantially larger than the effect size of treatment group for outcomes where both were significant (gait pattern 2:1, gait speed 4:1). The effect of dynamic motor control was independent of treatment group.
Interpretation:
Dynamic motor control is an important factor in treatment outcomes. Better dynamic motor control is associated with better outcomes, regardless of treatment.
Among children with cerebral palsy (CP), gait outcomes following treatment are highly variable. For example, in a survey of 35 articles describing outcomes after gastrocnemius lengthening Shore found up to 43% of patients had recurrent equinus and up to 36% ended up with a calcaneous gait. Other studies have shown similarly high and disconcerting rates of poor outcomes after highly-invasive surgical procedures including psoas lengthening 1 hamstrings lengthening 2, femoral derotation osteotomy 3; 4, rectus femoris transfer 5, and single-event multi-level orthopaedic surgery for crouch gait 6. Unsatisfactory outcomes are not restricted to orthopaedic surgery. Low rates of positive outcomes have also been reported after Botulinum Toxin Type A injections 7, strength training 8, and orthosis use 9. While some patients benefit from all of these treatments, many do not.
There is a clear need to identify patient-specific preoperative factors that guide treatment decisions and predict outcomes. Unfortunately, reported treatment indications remain frustratingly vague. Many outcomes studies do not test proposed indications, but rather simply survey the performance of the status quo. There has been recent progress in identifying optimal treatment indications from clinical and biomechanical variables using techniques, such as machine learning, biomechanical assessments, and computer simulations. These efforts have successfully identified risk factors in psoas lengthening 1 hamstrings lengthening 2, orthosis prescription 10, stiff-knee gait 5 and crouch gait 6.
Diminishing Returns
A common thread from prior studies is that individuals with greater pre-treatment deformity typically have greater improvements after treatment. A larger Gait Profile Score, indicating greater gait deviation from unimpaired individuals, is associated with larger improvements after orthopaedic surgery, even after accounting for possible regression to the mean effects 11. Dreher showed that patients with greater excessive anteversion were more likely to have a good outcome following femoral derotation osteotomies 12; 3. Hicks identified the amount of pre-operative knee flexion as a predictor of post-operative knee flexion among patients walking in crouch 6. Our own team has shown that the amount of dynamic hip flexion deformity is a factor in predicting good outcome from psoas lengthening 1. Consistently, these prior studies based on kinematics and musculoskeletal factors have indicated that individuals with smaller deviations are more likely to have worse outcomes after treatment, having reached a point of diminishing returns.
Role of Motor Control
CP is caused by an injury to the brain, and poor motor control is a hallmark of CP. However, prior analyses aimed at optimizing treatment decisions have largely focused on biomechanics and gait patterns. In the clinic, physicians and therapists have routinely suggested that motor control is important for predicting outcomes, but quantifying motor control is challenging. There are a variety of motor control assessments that are a part of most clinical evaluations 13. These clinical assessments focus on individual joints, and subjectively evaluate items such as involuntary movement, speed, and force generation. Joint-level motor control assessments are generally graded on a limited ordinal scale such as ‘normal’, ‘impaired’, and ‘unable’, and aggregated to form an overall metric. Among children with CP, these measures have been shown to influence function 14, developmental outcomes 15, and treatment outcomes in the upper extremity 16. The link with outcomes in the lower extremities for CP is not clearly established, though a single case report does exist 17.
Clinical motor control assessments, while valuable, involve only limited, non-functional movements. Further, these clinical measures are subjective, relying on the impression of the assessor. Given the presumed and demonstrated importance of motor control, and the known shortcomings of existing measures, there is a compelling need for an objective measure of motor control that applies directly to walking.
Synergy Analysis and Walking Dynamic Motor Control
During gait, electromyography (EMG) provides the best tool for examining neuromuscular control. However, EMG is notoriously difficult to interpret. Raw EMG data only gives insight into activity of individual muscles, and does not provide a comprehensive view of an individual’s control strategy. Because of the complexity of the musculoskeletal system, the cause-effect relationship between EMG signals and pathological movements is unclear 18; 19. Quantifying neurologic impairment and understanding its contribution to movement is critical to evaluating and treating pathological movement.
Muscle synergies provide a framework for understanding and quantifying motor control. Synergies are defined as “coordinated patterns of muscle activity that flexibly combine to produce functional motor behaviors” 20 and are thought to reflect a simplified control strategy compared to modulating each muscle individually. In unimpaired individuals, a small set of synergies describes most muscle activity during walking 21. Individuals who have had a neurologic injury, such as stroke or CP, often exhibit even fewer synergies in their movement patterns, and altered synergies are theorized to contribute to impaired movement 22. Currently, the clinical utility of synergy analysis for diagnosis and treatment planning remains unclear.
We previously demonstrated that synergies are altered in CP, and developed a measure to quantify complexity of neuromuscular control 23. The measure, called the Walking Dynamic Motor Control index (Walk-DMC), has a strong association with clinically assessed selective motor control. In the present study, we test the hypothesis that Walk-DMC is associated with treatment outcomes in CP. Specifically, we evaluate if Walk-DMC has a significant effect on changes in gait pattern, gait speed, energy cost of walking, and function after orthopaedic surgery or selective dorsal rhizotomy.
Methods
We performed a retrospective analysis of individuals seen at our center from 1994 – 2014, with a diagnosis of CP, GMFCS levels I, II, and III, age < 19 years, who had an initial gait analysis that included surface EMG, and a follow-up gait analysis between 9 and 24 months after a variety of treatments.
Synergy Analysis
To evaluate the impact of altered neuromuscular control on outcomes we computed Walk-DMC from surface EMG data for each participant. The Walk-DMC metric reflects the complexity of the EMG signal; particularly how much independent activation is exhibited by muscles during gait. Surface EMG was collected from four muscles on each leg – the rectus femoris, medial hamstrings, medial gastrocnemius, and anterior tibialis. Nonnegative matrix factorization was used to derive the synergy structure24. Walk-DMC was computed by determining the total variance in EMG accounted for by one synergy, then transforming one minus this variance to a z-score with respect to typically-developing children:
where (1-VAF1) is the variance not accounted for by one synergy in a given individual, and (1-VAF1)TD_AVE and (1-VAF1)TD_SD are the average and standard deviations of the unaccounted variance in 84 typically-developing children. This scales Walk-DMC so that 100 is the typically-developing average and 10 points is one standard deviation. A larger Walk-DMC reflects more complex muscle activation during walking; similar to typically-developing peers. Each individual’s average Walk-DMC was computed from all available trials (3 – 6 per gait analysis session). The entirety of each walking trial, consisting of a concatenation of four or more strides, was used 24.
Treatments
Treatment history was extracted from the participants’ medical records. Treatments were categorized into four levels:
CONS: conservative treatment such as routine physical therapy, excluding botulinum toxin injection
ORTHO-1: single-level orthopaedic surgery
SEMLS: single-event multi-level surgery, consisting of two or more orthopaedic procedures on a limb, and
SDR: selective dorsal rhizotomy.
Outcome Measures
Gait quality was measured by the Gait Deviation Index 25 and dimensionless walking speed. The GDI gives a measure of the overall difference between the kinematics of an individual and the average kinematics of typically developing children. The GDI and Walk-DMC are scaled similarly, so that a GDI of 100 ±10 is equal to the average ± one standard deviation of the kinematic deviations for typically-developing children. Dimensionless walking speed provides a measure of speed accounting for differences in stature to allow for longitudinal comparisons 26.
Energy cost of walking was evaluated using net nondimensional oxygen cost during a 6-minute self-selected speed walking trial 27. Volume flow and oxygen concentration of inspired and expired gases were measured with a breath-by-breath oximeter. Net oxygen cost was calculated as the difference between cost at rest and during steady-state walking, and rendered dimensionless to reduce the effects of age and stature. The net nondimensional oxygen cost was then divided by the nondimensional oxygen cost of speed-matched control data, giving energy cost of walking as a multiple of speed-matched control.
Function was measured using the Pediatric Outcomes Data Collection Instrument, Sports & Physical Fitness scale (PODCI-SPF). This subscale was chosen for relevance to treatments aimed at improving movement and lack of a ceiling effect 28.
Statistical Analysis
A stepwise multiple linear regression model was computed to explain the changes in each outcome measure from pre-treatment to post-treatment gait analysis (Matlab, MathWorks, Natick USA). Starting with a constant model, terms were added (p < .05) and removed (p > .10), including linear and interaction terms. Factors considered were the pre-treatment level of each outcome variable (e.g. Pre-GDI for explaining Post-GDI), Walk-DMC, treatment group (CONS, ORTHO-1, SEMLS, SDR), age, and prior surgery (yes/no). A robust fitting algorithm using a bisquare weighting function was used to reduce the potentially biasing effect of outliers. To assess the influence of individual regressors on the four outcome measures, effect sizes were estimated from the adjusted response, in which an individual regressor is allowed to vary throughout its range while averaging the effects of the other regressors 29.
RESULTS
The query returned 473 individuals who were well matched across treatment groups for age, sex, GMFCS level, prior treatment, and follow-up time (Table I).
Table I.
Participant Demographics
| Treatment | Number | Age [yr+mo] |
Sex F∣M |
GMFCS I | GMFCS II | GMFCS III | Prior | FU time [yr+mo] |
|---|---|---|---|---|---|---|---|---|
| CONS | 76 | 6+8 (2+7) | 35∣41 | 29% | 37% | 34% | 64% | 1+7 (0+7) |
| ORTHO-1 | 39 | 6+11 (3+4) | 16∣ 23 | 41% | 38% | 21% | 87% | 1+6 (0+4) |
| SEMLS | 176 | 10+0 (3+5) | 77∣99 | 14% | 48% | 38% | 71% | 1+5 (0+5) |
| SDR | 182 | 5+7 (2+0) | 82∣100 | 19% | 42% | 39% | 57% | 1+6 (0+4) |
| Overall | 473 | 7+8 (3+4) | 210∣263 | 21% | 43% | 36% | 66% | 1+6 (0+5) |
Gait Deviation Index
Within treatment groups, the pre and post-treatment GDI were 71→74 (CONS), 71→76 (ORTHO-1), 67→76 (SEMLS), and 70→75 (SDR). The minimum clinically important difference (MCID) for GDI has been estimated to be 5 points 30. Within treatment groups, improvements beyond the MCID were seen in 36% CONS, 44% ORTHO-1, 66% SEMLS, and 57% SDR, while worsening beyond the MCID was seen in 14% CONS, 18% ORTHO-1, 6% SEMLS, and 13% SDR.
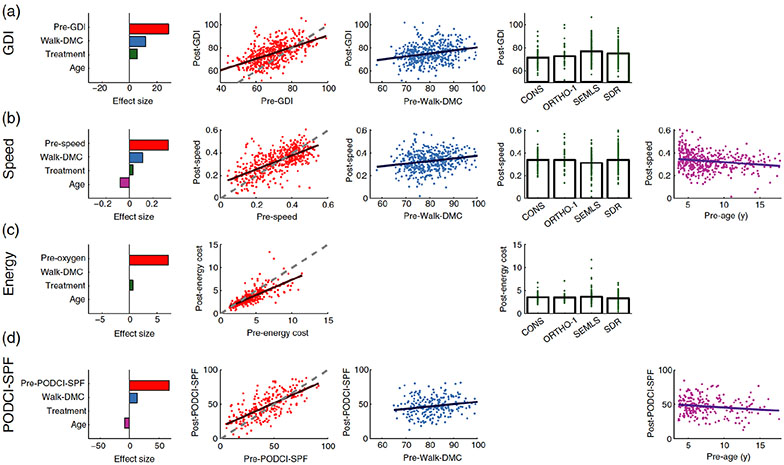
Pre-treatment GDI, walk-DMC, and treatment group were all significant in the post-treatment GDI model Table II). The treatment group main effect indicated a difference in Post-GDI based on treatment compared to the conservatively treated group (CONS). There was also a significant interaction between Pre-GDI and treatment group, indicating that the slopes of Post-GDI versus Pre-GDI lines differed based on treatment. The effect sizes (standard deviations) of pre-GDI, Walk-DMC, and treatment group were 28.5(4.9), 11.7 (4.7), and 5.6 (2.1) respectively (Figure 1A).
Table II.
Regression Results
| Term | Estimate | GDI (r2 =.42) SE |
P | Estimate | Speed (r2=.53) SE |
P | Estimate | Energy (r2=.72) SE |
P | Estimate | PODCI (r2=.54) SE |
P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | −0.7 | 6.3 | 0.91 | −0.010 | 0.042 | 0.81 | 0.72 | 0.14 | <.01 | −5.5 | 9.8 | 0.58 |
| Pre | 0.7 | 0.1 | <.01 | 0.616 | 0.035 | <.01 | 0.67 | 0.02 | <.01 | 0.7 | 0.1 | <.01 |
| DMC | 0.3 | 0.1 | <.01 | 0.002 | 0.001 | <.01 | 0.3 | 0.1 | <.05 | |||
| Age | −0.004 | 0.001 | <.01 | −0.6 | 0.3 | <.05 | ||||||
| Ortho-1 | 9.6 | 10.9 | 0.38 | −0.001 | 0.016 | 1.0 | −0.08 | 0.19 | 0.68 | |||
| SEMLS | 21.6 | 7.0 | <.01 | −0.027 | 0.012 | <.05 | 0.10 | 0.13 | 0.43 | |||
| SDR | 28.2 | 7.1 | <.01 | 0.001 | 0.011 | 0.9 | −0.22 | 0.13 | 0.09 | |||
| GDI x Ortho-1 | −0.1 | 0.2 | 0.42 | |||||||||
| GDI x SEMLS | −0.2 | 0.1 | <.05 | |||||||||
| GDI x SDR | −0.4 | 0.1 | <.01 |
Figure 1.
For each outcome measure (row) the effect sizes and adjusted responses are shown for statistically significant predictors (columns). Plots are only shown for variables that had a significant relationship in the regression model (see Table II). All measures are dimensionless, except for age (years). Energy cost is expressed as a multiple of energy cost for a speed-matched control (×SMC). The dashed line in the post- vs. pre-treatment plots represents no change. Post-treatment levels above this line are improvement, and below the line are worsening, except for energy cost where below the line represents improvement. For each outcome domain, pre-treatment levels closer to those of typically-developing individuals lead to smaller improvements (or worsening) after treatment. We have described this response as “diminishing returns”. In contrast, higher Walk-DMC values, reflecting control more like that of typically developing individuals, lead to larger postoperative improvements, or “increasing returns”. The pre-treatment level of the outcome variable had the largest effect size across all outcomes. Walk-DMC had the next largest effect size – larger than treatment choice or age - except in the case of energy cost, where Walk-DMC was not a significant predictor. It is also noteworthy that the effect size of treatment choice is consistently small, and in the case of the PODCI-SPF, not statistically significant.
The slope of the Pre-GDI term was less than one (slope = 0.7), meaning that after controlling for Walk-DMC and treatment group, individuals with higher Pre-GDI tended to improve less than individuals with lower Pre-GDI. This response can be described as “diminishing returns”; meaning better pre-treatment gait led to smaller improvements, or even worsening, after treatment. In contrast, better dynamic motor control led to larger improvements in gait following treatment (“increasing returns”). It is further noted that the effect of Walk-DMC was independent of treatment group (no interaction term between Walk-DMC and treatment group).
Walking Speed
Within treatment groups, the pre- and post-treatment dimensionless speed changes were 0.33→0.36 (CONS), 0.34→0.36 (ORTHO-1), 0.31→0.29 (SEMLS), and 0.32→0.35 (SDR). We chose a speed change of ±10% of the pre-treatment value to represent a meaningful difference 31. Within treatment groups, improvements were seen in 38% CONS, 44% ORTHO-1, 26% SEMLS, 47% SDR, while worsening was seen in 24% CONS, 28% ORTHO-1, 42% SEMLS, 27% SDR.
Pre-treatment speed, Walk-DMC, age, and treatment group were all significantly associated with post-treatment speed (Table II). The effect sizes (standard deviations) of pre-speed, Walk-DMC, treatment, and age were 0.29(0.04), 0.11 (0.05), 0.03 (0.03), and −0.08 (0.04) respectively (Figure 1B).
The coefficient of the pre-treatment speed term was less than one (0.62), meaning that after controlling for Walk-DMC, treatment, and age, individuals with higher pre-treatment speed tended to improve less than individuals with lower pre-treatment speed (“diminishing returns”). The coefficient of Walk-DMC was positive, implying that better dynamic motor control was associated with better speed outcomes (“increasing returns”). The coefficients of the ORTHO-1 and SEMLS treatment groups were less than zero, indicating that these treatments resulted in worse speed outcomes than conservative treatment (CONS). The negative coefficient of age implied that older individuals improved less than younger individuals.
Energy Cost of Walking
Within treatment groups, the pre- and post-treatment energy cost were 4.2→3.6 (CONS), 3.2→2.8 (ORTHO-1), 3.9→3.7 (SEMLS), and 4.6→3.6 (SDR) times speed matched control (noting that higher energy is a worse outcome). We chose a change of ±10% of the pre-treatment energy cost to represent a meaningful difference in energy cost of walking 31. Within treatment groups, improvements were seen in 37% CONS, 28% ORTHO-1, 34% SEMLS, 53% SDR, while worsening was seen in 9% CONS, 13% ORTHO-1, 16% SEMLS, 4% SDR. It should be noted that despite normalization, energy cost decreases with age. This has an important impact on the results since, on average, 40% of the observed change would be expected due to ageing from 7.7 to 9.2 years 27.
Pre-treatment energy cost and treatment group were significantly associated with post-treatment energy cost (Table II). Walk-DMC was not a significantly associated with post-treatment energy cost. The effect sizes (standard deviations) of pre-energy cost and treatment were 7.1(0.62) and 0.6 (0.26) respectively (Figure 1C).
The coefficient of the pre-treatment energy cost term was less than one (0.67), meaning that after controlling for treatment, individuals with lower pre-treatment energy cost tend to improve less than individuals with higher pre-treatment energy cost (“diminishing returns”). The ORTHO-1 and SDR treatment groups improved more than the conservative treatment, while SEMLS improved less.
PODCI-SPF
Within treatment groups, the pre- and post-treatment PODCI-SPF were 48→51 (CONS), 44→52 (ORTHO-1), 43→44 (SEMLS), and 42→48 (SDR). We chose a change of 6.8 points to represent a meaningful difference in PODCI-SPF score 28. Within treatment groups, improvements were seen in 33% CONS, 42% ORTHO-1, 39% SEMLS, 46% SDR, while worsening was seen in 28% CONS, 10% ORTHO-1, 34% SEMLS, 13% SDR.
Initial PODCI, walk-DMC, and age were all significantly associated with PODCI-SPF at the post-treatment gait analysis (Table II). The effect sizes (standard deviations) of pre-PODCI-SPF, Walk-DMC, and age were 67.7 (17.6), 12.7 (9.0), and −8.8 (7.5) respectively (Figure 1D).
The coefficient of the Pre-PODCI-SPF term was less than one (0.7), meaning that after controlling for Walk-DMC and age, individuals with higher pre-treatment PODCI-SPF (higher functioning) tend to improve less than individuals with lower pre-treatment PODCI-SPF (“diminishing returns”). The coefficient of Walk-DMC was positive (0.3), indicating better dynamic motor control is associated with bigger improvements in PODCI-SPF following treatment (“increasing returns”). The coefficient of age was negative, indicating that older individuals improved less than younger individuals.
Cross-Validation
A 10-fold cross validation was performed in order to estimate the robustness of the four models32 (Table III). The cross-validation procedure consisted of performing replications of regression procedure, while omitting a randomly selected 10% of the samples in each replicate, then testing the resulting models on the omitted observations. The cross-validated errors were all similar to the original errors (<3% higher).
Table III.
Cross-Validation Results
| Outcome | Root Mean Square Error | |
|---|---|---|
| Original | Cross- Validation |
|
| GDI | 7.8 | 7.7 |
| Speed | 0.08 | 0.08 |
| Energy | 1.1 | 0.8 |
| PODCI | 13.1 | 13.5 |
Discussion
Dynamic motor control is an important factor in explaining treatment outcomes. Walk-DMC was a significantly associated with outcomes for gait (GDI), speed, and function (PODCI-SPF). In each of these three domains, the effect size of Walk-DMC was second only to the effect size of the pre-treatment outcome variable value, and notably, was consistently larger than the effect size of treatment group. Walk-DMC was not associated with post-treatment energy cost.
In every instance where Walk-DMC was significant, a higher Walk-DMC (better motor control) was associated with better outcomes. In contrast, better pre-treatment levels of each outcome variable were associated with worse outcomes of that variable (“diminishing returns”). The results of this study concur with the clinical perception that better motor control is associated with better outcomes. In this sense, Walk-DMC does not add fundamentally new information. What Walk-DMC does provide is a method to objectively and quantitatively examine this clinical impression. Walk-DMC can be easily computed from EMG data, and does not require full gait analysis; only EMG data during walking. These advantages make the Walk-DMC index simple and clinically feasible, in addition to being strongly associated with outcomes. It should be noted, however, that Walk-DMC does not provide specific treatment guidance, and thus full gait analysis is still needed for optimal treatment planning.
As an example of the Walk-DMC effect, consider two patients undergoing SEMLS. Assume the patients are at the group mean for pre-treatment GDI, speed, age, and PODCI-SPF (69, 0.32, 7.7, and 43 respectively). If patient 1 is in the 10th percentile of Walk-DMC while patient 2 is in the 90th percentile, then we would expect the following post-treatment differences: GDI - 75 vs. 80, Speed - 0.29 vs. 0.34, and PODCI-SPF - 44 vs. 51. Note that the differences in GDI and PODCI-SPF outcomes between the two patients exceed the MCIDs, and the speed difference exceeds 10%.
This study is limited by its retrospective nature. We analyzed a convenience sample of individuals, and hence sample bias is always a concern. Referrals for post-operative gait analysis are routine at our center. However, we do not see every patient back within two years of treatment. This may be particularly important for our conservative treatment group, which may be biased towards individuals who are seeking care due to emerging gait problems, pain, or loss of function. We are also limited by the variables we have chosen to test. The choice of the pre-treatment outcome variables and Walk-DMC were mandated by our hypothesis. The other variables considered were motivated by experience with clinical practice, and by previous analyses 11.
The results of this study clearly show that motor control, as measured by Walk-DMC, is an important factor in determining outcomes of children with CP over a wide range of common treatments. Individuals with good motor control tended to have good outcomes in gait, speed, and function, while those with poor motor control fared worse in each of these domains, regardless of which treatment was chosen. This raises the question of what treatments are best for individuals with poor motor control. We can think of treatment such as orthopaedic surgery as the removal of biomechanical constraints. For example, muscle lengthening may increase a joint’s range-of-motion. However, reduced motor control may limit the number of movements an individual can execute, restricting the patient’s ability to exploit their new, less constrained biomechanical system.
This study has shown that motor control is associated with treatment outcomes across a broad spectrum of outcome measures. However, this study does not allow us to confirm or refute any specific hypothesis regarding the mechanisms responsible for the observed effect. It is clear that treatments with the ability to improve motor control would be valuable for improving long-term outcomes. Examination of these long-term outcomes, and the discovery or development of motor-control enhancing treatments is a compelling need for future work.
What this paper adds.
Better dynamic motor control (Walk-DMC) is associated with better treatment outcomes.
Dynamic motor control impacts treatment outcomes for gait pattern, gait speed, and sports and physical function.
Milder pre-treatment status is associated with smaller post-treatment gains in gait pattern, gait speed, energy cost of walking, and sports and physical function. For very mild patients, decline in function is commonly observed.
References
- 1.Schwartz MH, Rozumalski A, Truong W, Novacheck TF. (2013) Predicting the outcome of intramuscular psoas lengthening in children with cerebral palsy using preoperative gait data and the random forest algorithm. Gait & Posture 37: 473–9. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Dias LS, Delp SL. (2006) Do the hamstrings operate at increased muscle-tendon lengths and velocities after surgical lengthening? Journal of Biomechanics 39: 1498–506. [DOI] [PubMed] [Google Scholar]
- 3.Dreher T, Wolf SI, Heitzmann D, Swartman B, Schuster W, Gantz S, Hagmann S, Doderlein L, Braatz F. (2012) Long-term outcome of femoral derotation osteotomy in children with spastic diplegia. Gait & Posture 36: 467–70. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MH, Rozumalski A, Novacheck TF. (2013) Femoral derotational osteotomy: Surgical indications and outcomes in children with cerebral palsy. Gait Posture. [DOI] [PubMed] [Google Scholar]
- 5.Reinbolt JA, Fox MD, Schwartz MH, Delp SL. (2009) Predicting outcomes of rectus femoris transfer surgery. Gait Posture 30: 100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicks JL, Delp SL, Schwartz MH. (2011) Can biomechanical variables predict improvement in crouch gait? Gait Posture 34: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ubhi T, Bhakta BB, Ives HL, Allgar V, Roussounis SH. (2000) Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Archives of Disease in Childhood 83: 481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damiano DL, Arnold AS, Steele KM, Delp SL. (2010) Can Strength Training Predictably Improve Gait Kinematics? A Pilot Study on the Effects of Hip and Knee Extensor Strengthening on Lower-Extremity Alignment in Cerebral Palsy. Physical Therapy 90: 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ries AJ, Novacheck TF, Schwartz MH. (2015) The Efficacy of Ankle-Foot Orthoses on Improving the Gait of Children With Diplegic Cerebral Palsy: A Multiple Outcome Analysis. Pm r 7: 922–9. [DOI] [PubMed] [Google Scholar]
- 10.Ries AJ, Novacheck TF, Schwartz MH. (2014) A data driven model for optimal orthosis selection in children with cerebral palsy. Gait & Posture 40: 539–44. [DOI] [PubMed] [Google Scholar]
- 11.Rutz E, Donath S, Tirosh O, Graham HK, Baker R. (2013) Explaining the variability improvements in gait quality as a result of single event multi-level surgery in cerebral palsy. Gait Posture 38: 455–60. [DOI] [PubMed] [Google Scholar]
- 12.Dreher T, Wolf S, Braatz F, Patikas D, Doderlein L. (2007) Internal rotation gait in spastic diplegia - Critical considerations for the femoral derotation osteotomy. Gait & Posture 26: 25–31. [DOI] [PubMed] [Google Scholar]
- 13.Fowler EG, Staudt LA, Greenberg MB, Oppenheim WL. (2009) Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Developmental Medicine and Child Neurology 51: 607–14. [DOI] [PubMed] [Google Scholar]
- 14.Ostensjo S, Carlberg EB, Vollestad NK. (2004) Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Developmental Medicine and Child Neurology 46: 580–9. [DOI] [PubMed] [Google Scholar]
- 15.Chen CM, Hsu HC, Chen CL, Chung CY, Chen KH, Liaw MY. (2013) Predictors for changes in various developmental outcomes of children with cerebral palsy-A longitudinal study. Research in Developmental Disabilities 34: 3867–74. [DOI] [PubMed] [Google Scholar]
- 16.Van Heest AE, House JH, Cariello C. (1999) Upper extremity surgical treatment of cerebral palsy. Journal of Hand Surgery-American Volume 24A: 323–30. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg EJ, Fowler EG, Oppenheim WL. (2012) Case Reports: The Influence of Selective Voluntary Motor Control on Gait After Hamstring Lengthening Surgery. Clinical Orthopaedics and Related Research 470: 1320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinbolt JA, Fox MD, Arnold AS, Ounpuu S, Delp SL. (2008) Importance of preswing rectus femoris activity in stiff-knee gait. Journal of Biomechanics 41: 2362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele KM, Seth A, Hicks JL, Schwartz MS, Delp SL. (2010) Muscle contributions to support and progression during single-limb stance in crouch gait. Journal of Biomechanics 43: 2099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, Kesar TM. (2015) Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron 86: 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. (2006) Motor patterns in human walking and running. Journal of Neurophysiology 95: 3426–37. [DOI] [PubMed] [Google Scholar]
- 22.Cheung VCK, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E. (2012) Muscle synergy patterns as physiological markers of motor cortical damage. Proceedings of the National Academy of Sciences of the United States of America 109: 14652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steele KM, Rozumalski A, Schwartz MH. (2015) Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev Med Child Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira AS, Gizzi L, Farina D, Kersting UG. (2014) Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Frontiers in Human Neuroscience 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz MH, Rozumalski A. (2008) The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture 28: 351–7. [DOI] [PubMed] [Google Scholar]
- 26.Hof AL. (1996) Scaling gait data to body size. 4: 222–3. [Google Scholar]
- 27.Schwartz MH, Koop SE, Bourke JL, Baker R. (2006) A nondimensional normalization scheme for oxygen utilization data. Gait Posture 24: 14–22. [DOI] [PubMed] [Google Scholar]
- 28.Oeffinger DL, Rogers SP, Bagley A, Gorton G, Tylkowski CM. (2009) Clinical Applications of Outcome Tools in Ambulatory Children with Cerebral Palsy. Physical Medicine and Rehabilitation Clinics of North America 20: 549–+. [DOI] [PubMed] [Google Scholar]
- 29.DuMouchel W (1988) Graphical representations of main effects and interaction effects in a polynomial regression on several predictors. In: Wegman EJ, Gantz DT, Miller JJ editors. Computing Science and Statistics Fairfax, Virginia: American Statistical Association. p 127–32. [Google Scholar]
- 30.Baker R, McGinley JL, Schwartz M, Thomason P, Rodda J, Graham HK. (2012) The minimal clinically important difference for the Gait Profile Score. Gait & Posture 35: 612–5. [DOI] [PubMed] [Google Scholar]
- 31.Oeffinger D, Shriners Hospital for Children (SHC) L, KY, Bagley A, Sacramento C, Rogers S, Lexington K, Gorton G, Springfield M, Kryscio R, University of Kentucky L, KY, Abel M, University of Virginia C, VA, Damiano D, Washington University SL, MO, Barnes D, Houston T, Tylkowski C, Lexington K, USA. (2015) Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Developmental Medicine & Child Neurology 50: 918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauerbrei W, University of Freiburg G. (1999) The Use of Resampling Methods to Simplify Regression Models in Medical Statistics. Journal of the Royal Statistical Society: Series C (Applied Statistics) 48: 313–29. [Google Scholar]