This study assessed long-term safety and effectiveness of spinal cord stimulation using a passive recharge burst stimulation design for chronic intractable pain in the trunk and/or limbs. Significant improvements in pain, physical, mental, and emotional functioning observed after 6 months of treatment were maintained at 24 months.
Keywords: catastrophizing, chronic pain, depression, device recharging, long-term outcomes, neuromodulation, pain medication, passive recharge burst, quality of life, spinal cord stimulation
Study Design.
Prospective, international, multicenter, single-arm, post-market study.
Objective.
The aim of this study was to assess long-term safety and effectiveness of spinal cord stimulation using a passive recharge burst stimulation design for chronic intractable pain in the trunk and/or limbs. Herein we present 24-month outcomes from the TRIUMPH study (NCT03082261).
Summary of Background Data.
Passive recharge burst spinal cord stimulation (B-SCS) uniquely mimics neuronal burst firing patterns in the nervous system and has been shown to modulate the affective and attentional components of pain processing.
Methods.
After a successful trial period, subjects received a permanent SCS implant and returned for follow-up at 6, 12, 18, and 24 months.
Results.
Significant improvements in physical, mental, and emotional functioning observed after 6 months of treatment were maintained at 2 years. Pain catastrophizing scale (PCS) scores dropped below the population norm. Health-related quality of life on EQ-5D improved across all domains and the mean index score was within one standard deviation of norm. Pain reduction (on NRS) was statistically significant (P < 0.001) at all timepoints. Patient reported pain relief, a stated percentage of improvement in pain, was consistent at all timepoints at 60%. Patients reported significant improvements across all measures including activity levels and impact of pain on daily life. At 24 months, 84% of subjects were satisfied and 90% would recommend the procedure. Subjects decreased their chronic pain medication intake for all categories; 38% reduced psychotropic and muscle relaxants, 46% reduced analgesic, anti-convulsant and NSAIDs, and 48% reduced opioid medication. Adverse events occurred at low rates without unanticipated events.
Conclusion.
Early positive results with B-SCS were maintained long term. Evidence across multiple assessment tools show that B-SCS can alleviate pain intensity, psychological distress, and improve physical function and health-related quality of life.
Level of Evidence: 3
Chronic pain of moderate-to-severe intensity affects as many as 20% of the population.1 Of these, pain is disabling for approximately 12%,2 limiting them from work productivity or participating in life roles.3,4 Chronic pain is often emotionally distressing; resulting in a strong co-occurrence of mood issues such as depression and anxiety.2,5 Health-related quality of life ratings among chronic pain patients have been reported as 28% lower than that of the general population.6
Spinal cord stimulation (SCS) is a valuable pain management option for patients with intractable neuropathic pain of the trunk and/or limbs.7,8 A commonly-used metric for assessing therapeutic response is to calculate the percentage of pre-implant baseline pain that is relieved with treatment, using a visual analog scale (VAS) or numeric rating scale (NRS). Pain ratings captured in tightly controlled studies may be inconsistent with those captured in studies with real-world observational designs (e.g.,9–11). The International Association for the Study of Pain released a revised definition of pain in 2020 saying, in part, that, “Pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors…. Although pain usually serves an adaptive role, it may have adverse effects on function and social and psychological well-being.”12 Because the pain experience is multi-factorial, so should be its assessment. The IMMPACT group recommends the incorporation of multiple nonpain measures such as physical and emotional functioning in pain studies,13 and this approach is endorsed by the International Neuromodulation Society.14
Passive recharge burst (BurstDR; Abbott, Plano, TX) SCS has emerged in the past decade as an important innovation.15,16 This stimulation design is characterized by a five-pulse train with internal frequency of 500Hz delivered at 40Hz, with a 1 ms pulse width, utilizing a passive recharge pattern. Several randomized controlled trials have demonstrated superiority of this waveform to conventional tonic SCS.16–20 The mechanisms of action of passive recharge burst (hereafter referred to as burst) are different than that of tonic, as demonstrated in animal models.21–24 Burst firing patterns occur naturally in the medial thalamic and intra-laminar nuclei and this complex is believed to potentiate anterior cingulate cortex (ACC) neuronal activity.25 Brain imaging studies in humans have confirmed that, unlike tonic stimulation, burst stimulation modulates the medial pain pathway in the brain that projects to the ACC and anterior insula. These regions are believed to process the emotional and affective aspects of pain. Burst stimulation may therefore exert a greater effect by not only modulating the lateral and the descending pain-inhibitory pathways, similar to tonic SCS, but also the medial aspect of the spinothalamic tract.26–29
Focusing on pain intensity for the assessment and care of chronic pain patients results in incomplete goal setting, incorrect patient selection for treatment, and limits our understanding of therapy response and effectiveness.30 A systematic literature review of burst SCS clinical outcomes identified that a wide range of nonpain measures have been employed in the assessment of burst SCS outcomes, and together, strongly point to holistic improvement.31 One year results from the prospective TRIUMPH study corroborated these findings, in particular pointing to improvements in mental health and reductions in the use of opioid medications.32 Herein we report 2-year outcomes from the TRIUMPH study.
MATERIALS AND METHODS
TRIUMPH (clinicaltrials.gov registration NCT03082261) was a prospective, post-market, single-arm study that enrolled 269 participants across 22 sites in the United States, Canada, and Europe. The purpose of TRIUMPH was to assess long-term safety and effectiveness of SCS for chronic pain in the trunk and/or limbs using a burst enabled spinal cord stimulation (B-SCS) system. The study began enrolling in 2016 and the final follow up was completed in August 2020. The study was conducted in compliance with principles of Good Clinical Practice, the Declaration of Helsinki, and present regional local laws and regulations. Study oversight was provided by local institutional review boards or ethics committees, and subjects gave their written informed consent before any study activities.
Centers were instructed to approach all eligible patients and ask for their interest in participating in the study. Patients (≥18 years of age) with chronic, intractable pain of the trunk, and/or lower limbs, recommended by a physician for SCS therapy, were recruited for this study. Patients had to pass a psychological screening according to the standard of care of individual sites. Eligible patients had a baseline score on the Numeric Rating Scale (NRS) ≥6 over the past 24 hours for average pain specific to the area(s) of chronic pain being treated with SCS. Patients with an existing SCS system, who previously failed SCS, were planning to have a different neurostimulation system or drug pump implanted, or had a primary diagnosis of peripheral vascular disease, angina pectoris, or chronic migraine were excluded from the study.
Design and Intervention
Subject demographics and medical histories were captured at baseline. Subjects completed questionnaires regarding pain intensity (NRS), patient-reported pain relief (PRPR; a stated percentage of improvement in pain), quality of life (EQ-5D),33 mood and affect (Pain Catastrophizing Scale [PCS],34 State-Trait Anxiety Inventory [STAI],35 Patient Health Questionnaire-9 [PHQ-9, a depression scale],36 Tampa Scale for Kinesiophobia [TSK, a fear avoidance scale]37), sleep (Medical Outcome Study [MOS] Sleep Scale),38 and physical function (Patient-Reported Outcome Measure Information System [PROMIS] Physical Function Scale)39 at baseline and at follow-up intervals (6, 12, 18, and 24 months post-permanent implant).
Before receiving a permanent SCS implant, participants underwent a trial evaluation period. Following a successful trial evaluation (defined as ≥50% patient reported pain relief and a willingness to have a permanent implant), a permanent SCS system was implanted. The implant was activated and programmed with both tonic and burst settings. Tonic stimulation works by emitting electric pulses delivered at a consistent frequency, pulse width, and amplitude. This waveform is dependent on paresthesia to cover painful areas and can therefore not be used at subperception levels. Burst is a waveform that delivers groups of pulses at a high frequency and at amplitudes much lower than tonic stimulation. During the interburst interval passive repolarization occurs before the next burst. This stimulation design does not use paresthesia to mask the pain sensation. Subjects were instructed in the use of their patient programmer and determined the schedule and intensity of their treatment, using tonic, burst, or a combination of waveforms. Participants with an unsuccessful trial were withdrawn from the study.
Activity level and usage of pain-related medication (including analgesics, non-steroidal anti-inflammatory drugs [NSAIDs], opioids, anticonvulsants, muscle relaxants, and psychotropic medication) were assessed at baseline and follow-up. In addition, subjects were asked about their satisfaction with treatment, overall change in their health state (Patient Global Impression of Change [PGIC]), and waveform preference at follow up. Device-related information such as programming parameters, battery consumption, and recharging activities were also collected at follow-up. Adverse events were captured throughout the study.
Data Analysis
Statistical analysis was completed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Data are presented as means ± standard deviation (SD), and proportions. Two-tailed paired t test was used for continuous variables. All statistical analyses were performed with significance accepted at P < 0.05. No adjustment for multiplicity has been made. Imputation methods were used as appropriate to account for missing data. The datasets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
RESULTS
This report includes data from 128 subjects (Figure 1). These subjects underwent a 3- to 30-day trial period utilizing burst stimulation and an external trial stimulator, were implanted with a permanent implantable pulse generator (IPG), used burst stimulation at least sometimes, and completed the 24-month assessments. Three subjects in this cohort had a tonic salvage trial after the initial burst trial failed. Twelve patients who were trialed with an all-in-one procedure (or “on-the-table” trial) and one patient who only used tonic stimulation throughout the follow-up period were excluded from analysis.
Figure 1.
Subject flow chart.
Average age was 58.4 ± 13.2 years and 65.6% were female. The average (SD) duration of chronic pain was 9.8 ± 8.1 years. Pain started after an accident (motor vehicle or other) in 35.1% of subjects, due to a medical condition in 29.7%, and following surgery in 14.1%. Radiculopathy and persistent spinal pain syndrome (formerly failed back surgery syndrome), diagnosed separately or combined with another chronic pain condition, were the most frequent pain diagnoses in the study. Only 12 subjects (9%) had a diagnosis that did not include one of these two indications; they were diagnosed with complex regional pain syndrome or intervertebral disc disorder. Most subjects categorized as “Other" had nonsurgical back pain (e.g., spinal stenosis, spondylosis) (Table 1).
TABLE 1.
Demographics and Baseline Characteristics
| All Enrolled Subjects (N = 128) | |
| Sex | |
| Male | 34.4% (44/128) |
| Female | 65.6% (84/128) |
| Age, y | |
| Mean ± SD | 58.4 ± 13.2 |
| (Min, max) | (18.0, 86.0) |
| Height, cm | |
| Mean ± SD | 168.9 ± 9.5 |
| Weight, kg | |
| Mean ± SD | 90.7 ± 20.0 |
| Duration of experiencing chronic pain, y | |
| Mean ± SD | 9.8 ± 8.1 |
| How did pain start | |
| Motor vehicle accident | 7.8% (10/128) |
| Other accident | 27.3% (35/128) |
| Surgery | 14.1% (18/128) |
| Medical condition | 29.7% (38/128) |
| Other | 21.1% (27/128) |
| Occupational status: subjects could select more than one category | |
| Working full-time | 21.9% (28/128) |
| Working part-time | 3.1% (4/128) |
| Home maker | 4.7% (6/128) |
| Volunteer | 1.6% (2/128) |
| Retired | 39.1% (50/128) |
| Disabled | 25.8% (33/128) |
| Other | 7.0% (9/128) |
| Pain diagnosis: A subject might have up to two pain diagnoses | |
| Causalgia | 0.8% (1/128) |
| Complex regional pain syndrome | 2.3% (3/128) |
| Intervertebral disc disorder with/without radiculopathy | 7.8% (10/128) |
| Lumbosacral plexus disorders | 0.8% (1/128) |
| Persistent spinal pain syndrome | 50.0% (64/128) |
| Radiculopathy | 59.4% (76/128) |
| Other | 5.5% (7/128) |
Percutaneous Octrode leads were implanted in 64.4% of subjects, and a paddle lead was used in 36.6%. A nonrechargeable IPG was implanted in 82.8% of subjects. At 6 months, in participants with a rechargeable IPG, 82.6% (19/23) charged their devices on a weekly basis or less frequently and this increased to nearly all subjects (21/22) at 24 months. Greater than 21% (5/23) recharged once every 3 weeks, or less often at 6 months and this proportion remained stable until 24 months.
At each study visit, ≥86% of participants reported using burst stimulation. Of these, ≥70% used it intermittently with a duty cycle of 30 seconds on and 90 seconds off. The majority of subjects preferred burst stimulation at all follow-up timepoints, ranging from 90.2% of subjects at 6 months to 85.0% at 24 months. About half of subjects at each timepoint indicated that burst was the only waveform they used to control their pain.
Pain reduction (on NRS) was statistically significant (P < 0.001) at all timepoints. PRPR was 60.3% (± 27.6) at 6 months, 63.1% (± 25.1) at 12 months, 61.7% (± 26.2) at 18 months, and 60% (± 24.1) at 24 months (Figure 2).
Figure 2.
Longitudinal mean (standard error) for pain intensity (NRS) and patient reported pain relief (PRPR) presented as box-and-whisker plots. Pain ratings on NRS and PRPR improved with treatment and were robustly maintained through 24 months.
At baseline, 78.1% of subjects rated that pain had a major impact in their life. At 24 months, this proportion decreased to 27.3%. With regards to the subject's activity level, the percentage reporting being moderately or very active increased from 32% at baseline to 53.1% at 24 months. The proportion of subjects who reported that they were “moderately better," “better," or “a great deal better" on the PGIC was 77.3% at 24 months. Fewer than 5% of subjects did not report a change in their condition with treatment. Figure 3 presents the proportions of these three analyses at all timepoints. Subjects decreased their chronic pain medication intake for all categories with 38% reducing psychotropic medication and muscle relaxants, 46% reducing analgesic, anti-convulsant and NSAIDs, and 48% reducing opioid medications at 24 months (Table 2).
Figure 3.
Impact of pain on subject's life and physical activity level improved during the study and results were maintained through 24 months. Similarly, global impression of change was sustained until end of the study.
TABLE 2.
Proportion of Subjects Who Decreased Chronic Pain-related Medication Intake
| 6 Mo (N = 123) | 12 Mo (N = 119) | 18 Mo (N = 122) | 24 Mo (N = 128) | |
| Decrease in medication intake of | ||||
| Analgesic | 38.2% (29/76) | 41.7% (30/72) | 46.7% (35/75) | 46.2% (36/78) |
| Anti-convulsant | 30.9% (17/55) | 37.7% (20/53) | 42.6% (23/54) | 46.4% (26/56) |
| Muscle relaxant | 17.4% (4/23) | 21.7% (5/23) | 40.9% (9/22) | 37.5% (9/24) |
| Nonsteroidal anti-inflammatory drug | 36.0% (9/25) | 40.0% (10/25) | 46.2% (12/26) | 46.2% (12/26) |
| Opioid medication | 44.4% (44/99) | 45.2% (42/93) | 51.1% (48/94) | 48.0% (47/98) |
| Psychotropic medication | 26.1% (6/23) | 22.7% (5/22) | 50.0% (12/24) | 37.5% (9/24) |
There were statistically significant (P < 0.001) improvements across all evaluated psychosocial domains at all follow-up timepoints. Most notably, catastrophizing measured on PCS decreased to normal population levels and were sustained at all timepoints.34 At 24 months, 79% (34/43) were no longer clinically catastrophizing and 61% (38/62) were no longer clinically depressed.36,40 The improvements observed at 6 and 12 months were maintained through 24 months (Figure 4). Similarly, health-related quality of life as per the EQ-5D improved across all domains. At 24 months, the EQ-5D index score was within one SD of the population norm (Figure 5) and 64% (82/128) met the clinically important change.41
Figure 4.
Longitudinal mean (standard error) for catastrophizing (PCS), depression (PHQ-9), fear avoidance (TSK), anxiety (STAI), sleep (MOS Sleep Scale), and physical function (PROMIS-8; presented as T-scores). All psychosocial measure improvements were maintained through 24 months.
Figure 5.
Longitudinal mean (standard error) for EQ-5D index scores. The proportion of participants with no problems (dark green) improved over time in all EQ-5D domains.
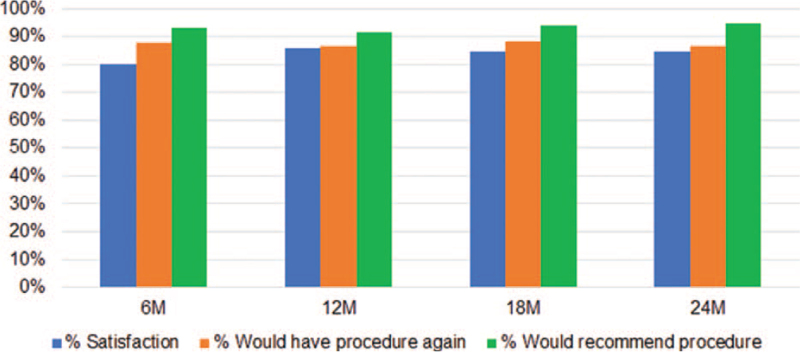
Subject satisfaction was high with 84.4% rating themselves as ‘satisfied’ or ‘very satisfied’ with treatment at 24 months. At all timepoints, >85% of subjects expressed that they would be willing to do the procedure again for the same results, and >90% would recommend the treatment to a family member with the same condition (Figure 6).
Figure 6.
Satisfaction ratings with treatment were maintained at high levels (>80% for all measures) at all time points throughout the study.
No unanticipated adverse events have been reported. Among the 269 enrolled subjects, there were a total of 53 events in 41 subjects (15.2%). Twenty-one events were considered serious and were reported in 18 subjects (6.7%). The most common serious event was infection (n = 5; 1.9%). There were also 32 nonserious events in 27 subjects. Most common nonserious events were changes in stimulation or reduced pain relief due to lead failure (n = 4) or lead migration (n = 4). No event occurred at a rate higher than 3%. There were no neurological injuries.
DISCUSSION
We report here that significant improvements in physical, mental, and emotional functioning can be sustained through 24 months of treatment with B-SCS. In particular, pain-related catastrophizing and depression improved with treatment. Given that psychological distress portends poor treatment outcomes,42,43 the capacity to shift pain-related beliefs and behaviors toward more positive outlooks suggests that burst SCS, through its unique mechanism of action, is a therapy suitable to manage patients thought of as difficult to treat and to salvage nonresponders.
This study enrolled patients who were broadly representative of the SCS population.44 This report follows those who had standard multi-day trials and who used burst at least some of the time after their permanent implant, thus giving insight into usage patterns in the real world. In addition to pain descriptors, a range of non-pain measures were collected, in alignment with best-practice recommendations.13,14
Subjects used and preferred burst over tonic stimulation. When using burst stimulation, patients most often employed a cycling pattern (commonly 30 seconds on and 90 seconds off), whereas tonic stimulation was more often used continuously. Good clinical outcomes have been reported to be maintained while cycling burst stimulation, a setting that substantially reduces the overall electrical dosage and battery consumption.45,46 Similar results have been achieved with lower-energy on:off cycling patterns such as 30:180 or 30:360 seconds.45–47
A minority of subjects in this study (<20%) used rechargeable devices based on physician choice. Data on subjects’ patterns of recharging their IPG batteries indicated that recharging fit within their lives, either as part of a normal routine or upon the device becoming depleted. Recharging was usually accomplished weekly within a few hours and with a minimum of complications. This is considerably less than the daily recharge burden that has been reported for devices using high-energy stimulation designs.10
Pain intensity (on NRS) was statistically significant reduced at all timepoints. The PRPR was 60% and this showed maintenance of the 12-month cohort's PRPR of 59%.32 A recent single-center retrospective review of 174 SCS patients indicated that PRPRs are strongly correlated with calculated percentage pain reductions, and that the former are consistently higher.48 Similar trends have been observed across a variety of pain populations.49–51 Thus, stated vs. calculated pain reduction percentages may not be equivalent; stated percentages, as a momentary measure in the context of current background pain, may be the more personally salient descriptor. In addition, >50% of the subjects in this report who initially said that pain had a major impact in their lives improved by at least one category, whereas nearly half of opioid users maintained their dosage reduction through 24 months. Taken together, pain has a significantly smaller footprint in patients’ lives with B-SCS.
Many SCS studies have reported on reductions in opioid usage given the crisis of misuse, addiction, and overdose in the US and other countries.32,52,53 However, chronic pain is usually addressed with multiple therapies including so-called “rational” polypharmacy, indicating medications across different classes. Starting at 6 months and continuing to 24 months, many patients saw a reduction in antidepressants, anticonvulsants, NSAIDs, and other analgesics. This is a substantial finding in many ways, including financial and cognitive side-effect improvements in these subjects.
In addition to successful reduction in pain intensity, all psychometric data including anxiety, depression, kinesiophobia, catastrophizing, sleep, and physical function showed statistically significant improvements. The most impactful effect of the therapy was observed on catastrophizing and depression. Interestingly, a recent multivariate regression analysis found that, after controlling for other variables, these two psychological factors were found to be significantly associated with chronic, severe low back pain and disability. This highlights their importance in persistent pain when negative beliefs about pain may become solidified, in contrast to kinesiophobia or anxiety which may be more relevant during the earlier stages leading up to chronic pain.54 Additionally, quality of life in chronic patients is strongly associated with catastrophizing and depression, more than with pain intensity.55,56 We observed improvements across all EQ-5D domains. Subjects reported that they were significantly improved as compared to before the implant, and rates of satisfaction with therapy were high. The magnitude and nature of these outcomes were consistent with previous reports20,32 and were maintained through 24 months.
Limitations and caveats of this study have been previously discussed.32 Briefly, this study was not designed to compare tonic and burst groups and, due to its real-world nature, programming and stimulation configurations were according to subject and physician preference. Twelve subjects who underwent an on-the-table trial (4% of enrollments) were excluded from current analysis. As burst is a subperception modality, paresthesia mapping was performed with tonic stimulation in these patients. The FDA recently issued a letter emphasizing the importance of a trial stimulation period before implant.57 Medical device reports of SCS devices for pain show that failure to achieve or maintain adequate pain control is the most common reported problem, occurring in 28% of cases. Moreover, most failed trials occur in spite of sufficient paresthesia over the painful area with trial stimulation.58
CONCLUSION
This study shows that subjects treated with B-SCS show no decrease in therapeutic effect over time; they improve baseline to 6 months and consecutively remain at a similar level in terms of pain, quality of life, and psychological profile up to 2 years after permanent implant. At the end of the study, subjects were highly satisfied with the therapy across the multiple symptom domains of chronic pain, feeling better, more active, while taking less pain and adjuvant medication. This provides converging evidence across multiple assessment tools that B-SCS can address the intensity of pain while also improving biopsychosocial issues.
Key Points
The objective of this study was to assess long-term safety and effectiveness of spinal cord stimulation using a passive recharge burst stimulation design for chronic intractable pain in the trunk and/or limbs.
Significant improvements in pain, physical, mental, and emotional functioning observed at 6 months of treatment were maintained at 2 years after implant.
Participants reported a significant decrease in the impact of pain on life and in the amount of medication consumed across all drug classes.
Burst spinal cord stimulation treatment had high patient satisfaction: more than 85% of subjects expressed that they would be willing to do the procedure again and >90% would recommend the treatment.
The magnitude and nature of these outcomes were consistent with previous reports and maintained through 24 months.
Acknowledgments
The authors thank all of the TRIUMPH study investigators, research coordinators, and study participants. Third-party writing assistance was provided by Allison Foster, PhD, of Foster Medical Communications. The authors also acknowledge Curtis Klager, biostatistician, for conducting the statistical analyses and checking the statistical language in the manuscript.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
Abbott funds were received in support of this work.
Relevant financial activities outside the submitted work: board membership, consultancy, stocks, patents, employment.
References
- 1.Bouhassira D, Lantéri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008; 136:380–387. [DOI] [PubMed] [Google Scholar]
- 2.Raftery MN, Sarma K, Murphy AW, et al. Chronic pain in the Republic of Ireland—community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain 2011; 152:1096–1103. [DOI] [PubMed] [Google Scholar]
- 3.Latham J, Davis BD. The socioeconomic impact of chronic pain. Disabil Rehabil 1994; 16:39–44. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CJ. The cost and burden of chronic pain. Rev Pain 2009; 3:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med 2006; 68:262–268. [DOI] [PubMed] [Google Scholar]
- 6.Poder TG, Wang L, Carrier N. EQ-5D-5L and SF-6Dv2 utility scores in people living with chronic low back pain: a survey from Quebec. BMJ Open 2020; 10:e035722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Overview | Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin | Guidance | NICE. Available at https://www.nice.org.uk/guidance/ta159. Accessed May 5, 2021. [Google Scholar]
- 8.Prager J. Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation 2010; 13:68–69. [DOI] [PubMed] [Google Scholar]
- 9.Kapural L, Yu C, Doust MW, et al. Novel 10-khz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology 2015; 123:851–860. [DOI] [PubMed] [Google Scholar]
- 10.Stauss T, Majdoub FE, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol 2019; 6:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayed D, Salmon J, Khan TW, et al. Retrospective analysis of real-world outcomes of 10 kHz SCS in patients with upper limb and neck pain. J Pain Res 2020; 13:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. IASP Announces Revised Definition of Pain - IASP. Available at https://www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=10475. Accessed May 20, 2021. [Google Scholar]
- 13.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003; 106:337–345. [DOI] [PubMed] [Google Scholar]
- 14.Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: IMMPACT/ION/INS recommendations. Pain 2021; 162:1935–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ridder D, Vanneste S, Plazier M, et al. Burst spinal cord stimulation. Neurosurgery 2010; 66:986–990. [DOI] [PubMed] [Google Scholar]
- 16.De Ridder D, Plazier M, Kamerling N, et al. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013; 80:642–649. [DOI] [PubMed] [Google Scholar]
- 17.Kriek N, Groeneweg JG, Stronks DL, et al. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain 2017; 21:507–519. [DOI] [PubMed] [Google Scholar]
- 18.Schu S, Slotty PJ, Bara G, et al. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014; 17:443–450. [DOI] [PubMed] [Google Scholar]
- 19.Tjepkema-Cloostermans MC, de Vos CC, Wolters R, et al. Effect of burst stimulation evaluated in patients familiar with spinal cord stimulation. Neuromodulation 2016; 19:492–497. [DOI] [PubMed] [Google Scholar]
- 20.Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018; 21:56–66. [DOI] [PubMed] [Google Scholar]
- 21.Tang R, Martinez M, Goodman-Keiser M, et al. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation 2014; 17:143–151. [DOI] [PubMed] [Google Scholar]
- 22.Crosby ND, Goodman Keiser MD, Smith JR, et al. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation 2015; 18:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Gong W-Y, Johanek LM, Sluka KA. A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physical activity in an animal model of neuropathic pain. Anesth Analg 2016; 122:1178–1185. [DOI] [PubMed] [Google Scholar]
- 24.Kent AR, Weisshaar CL, Venkatesan L, et al. Burst & high-frequency spinal cord stimulation differentially effect spinal neuronal activity after radiculopathy. Ann Biomed Eng 2020; 48:112–120. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarthy K, Fishman M, Zuidema X, et al. Mechanism of action in burst spinal cord stimulation: review and recent advances. Pain Med 2019; 20:S13–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation 2016; 19:47–59. [DOI] [PubMed] [Google Scholar]
- 27.De Ridder D, Vanneste S. Does tonic spinal cord stimulation really influence the medial pain system? Neuromodulation 2016; 19:227–228. [DOI] [PubMed] [Google Scholar]
- 28.Yearwood T, De Ridder D, Yoo HB, et al. Comparison of neural activity in chronic pain patients during tonic and burst spinal cord stimulation using fluorodeoxyglucose positron emission tomography. Neuromodulation 2020; 23:63. [DOI] [PubMed] [Google Scholar]
- 29.Bocci T, De Carolis G, Paroli M, et al. Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: novel insights into spinal and brain mechanisms of action. Neuromodulation 2018; 21:480–488. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain 2016; 157:65–69. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarthy K, Malayil R, Kirketeig T, et al. Burst spinal cord stimulation: a systematic review and pooled analysis of real-world evidence and outcomes data. Pain Med 2019; 20:S47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falowski SM, Moore GA, Cornidez EG, et al. Improved psychosocial and functional outcomes and reduced opioid usage following burst spinal cord stimulation. Neuromodulation 2021; 24:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990; 16:199–208. [DOI] [PubMed] [Google Scholar]
- 34.Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 2000; 23:351–365. [DOI] [PubMed] [Google Scholar]
- 35.Metzger RL. A reliability and validity study of the State-Trait Anxiety Inventory. J Clin Psychol 1976; 32:276–278. [Google Scholar]
- 36.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French DJ, France CR, Vigneau F, et al. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa scale for kinesiophobia (TSK). Pain 2007; 127:42–51. [DOI] [PubMed] [Google Scholar]
- 38.Allen RP, Kosinski M, Hill-Zabala CE, et al. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep Med 2009; 10:531–539. [DOI] [PubMed] [Google Scholar]
- 39.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010; 63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. PCSManual_English.pdf. Available at http://sullivan-painresearch.mcgill.ca/pdf/pcs/PCSManual_English.pdf. Accessed May 20, 2021. [Google Scholar]
- 41.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005; 14:1523–1532. [DOI] [PubMed] [Google Scholar]
- 42.Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry 2008; 53:224–234. [DOI] [PubMed] [Google Scholar]
- 43.Truchon M, Côté D. Predictive validity of the Chronic Pain Coping Inventory in subacute low back pain. Pain 2005; 116:205–212. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RS, Desai MJ, Rigoard P, et al. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract 2014; 14:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vesper J, Slotty P, Schu S, et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation 2019; 22:190–193. [DOI] [PubMed] [Google Scholar]
- 46.Deer TR, Patterson DG, Baksh J, et al. Novel intermittent dosing burst paradigm in spinal cord stimulation. Neuromodulation 2020; 24:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leong SL, De Ridder D, Deer T, et al. Potential therapeutic effect of low amplitude burst spinal cord stimulation on pain. Neuromodulation 2021; 24:574–580. [DOI] [PubMed] [Google Scholar]
- 48.Hagedorn JM, Deer TR, Canzanello NC, et al. Differences in calculated percentage improvement versus patient-reported percentage improvement in pain scores: a review of spinal cord stimulation trials. Reg Anesth Pain Med 2021; 46:293–297. [DOI] [PubMed] [Google Scholar]
- 49.Cushman D, McCormick Z, Casey E, et al. Discrepancies in describing pain: is there agreement between numeric rating scale scores and pain reduction percentage reported by patients with musculoskeletal pain after corticosteroid injection? Pain Med 2015; 16:870–876. [DOI] [PubMed] [Google Scholar]
- 50.Pratici E, Nebout S, Merbai N, et al. An observational study of agreement between percentage pain reduction calculated from visual analog or numerical rating scales versus that reported by parturients during labor epidural analgesia. Int J Obstet Anesth 2017; 30:39–43. [DOI] [PubMed] [Google Scholar]
- 51.Cepeda MS, Africano JM, Polo R, et al. Agreement between percentage pain reductions calculated from numeric rating scores of pain intensity and those reported by patients with acute or cancer pain. Pain 2003; 106:439–442. [DOI] [PubMed] [Google Scholar]
- 52.North B, Sharan A. Does SCS help reduce opioid usage? Pain Med 2021; 22:772–773. [DOI] [PubMed] [Google Scholar]
- 53.Al-Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: Post hoc analysis of data from two prospective studies. Sci Rep 2019; 9:11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranger TA, Cicuttini FM, Jensen TS, et al. Catastrophization, fear of movement, anxiety, and depression are associated with persistent, severe low back pain and disability. Spine J 2020; 20:857–865. [DOI] [PubMed] [Google Scholar]
- 55.Lame IE, Peters ML, Vlaeyen JW, et al. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. Eur J Pain 2005; 9:15–24. [DOI] [PubMed] [Google Scholar]
- 56.Offenbaecher M, Kohls N, Ewert T, et al. Pain is not the major determinant of quality of life in fibromyalgia: results from a retrospective “real world” data analysis of fibromyalgia patients. Rheumatol Int 2021; 41:1995–2006. [DOI] [PubMed] [Google Scholar]
- 57. U.S. Food and Drug Administration. Available at https://www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers. Accessed May 20, 2021. [Google Scholar]
- 58.Jang H, Kim M, Chang C, et al. Analysis of failed spinal cord stimulation trials in the treatment of intractable chronic pain. J Korean Neurosurg Soc 2008; 43:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]