Abstract
The clinical implications of hypertension in addition to a high prevalence of both uncontrolled blood pressure and medication nonadherence promote interest in developing device-based approaches to hypertension treatment. The expansion of device-based therapies and ongoing clinical trials underscores the need for consistency in trial design, conduct, and definitions of clinical study elements to permit trial comparability and data poolability. Standardizing methods of blood pressure assessment, effectiveness measures beyond blood pressure alone, and safety outcomes are paramount. The Hypertension Academic Research Consortium (HARC) document represents an integration of evolving evidence and consensus opinion among leading experts in cardiovascular medicine and hypertension research with regulatory perspectives on clinical trial design and methodology. The HARC document integrates the collective information among device-based therapies for hypertension to better address existing challenges and identify unmet needs for technologies proposed to treat the world’s leading cause of death and disability. Consistent with the Academic Research Consortium charter, this document proposes pragmatic consensus clinical design principles and outcomes definitions for studies aimed at evaluating device-based hypertension therapies.
Keywords: clinical trials, hypertension, outcomes, renal denervation
Despite its recognition as the leading cause of death and disability worldwide, the awareness, treatment, and control of blood pressure (BP) have plateaued if not modestly declined.1,2 In the United States alone, for example, more than half of individuals with hypertension—representing >29 million people—are estimated to have BP exceeding professional society and guideline-recommended treatment goals.3,4 As the most commonly diagnosed condition and largest contributor to mortality in industrialized countries, hypertension is present in >1 in 3 individuals, and the risk of cardiovascular mortality doubles for every 20 mm Hg and 10 mm Hg increase in the systolic BP (SBP) and diastolic BP (DBP), respectively.5 With population growth and aging, the global prevalence of hypertension and associated adverse outcomes are expected to continue to escalate.6
Irrespective of baseline hypertension severity, even moderate reductions in BP translate into clinically meaningful reductions in cardiac, renal, and cerebral vascular-related adverse events.7,8 Recent large randomized clinical trials have also demonstrated the clinical benefits of more intensive BP reduction,9,10 leading to revision of guideline-directed treatment goals to further lower SBP and DBP standards.11,12 Countering evidence-based efforts to improve BP with a broad spectrum of pharmaceutical therapies are routine challenges in clinical practice that include patient intolerance of medication-related adverse effects, general nonadherence with prescribed therapy, and physician inertia for treatment of an illness that remains largely silent until onset of clinically irreversible conditions.
Device-Based Therapies for Hypertension
Over the past decade, the potential utility of device-based hypertension therapies has evolved from enthusiasm to skepticism and then renewed promise. After several observational studies and an unblinded randomized trial13 suggesting clinically significant reductions in BP among patients with severe treatment-resistant hypertension,14–16 renal denervation (RDN) was positioned as an important new treatment strategy with global public health effect, and early enthusiasm for clinical adoption outpaced the available data. After the lack of demonstrable effectiveness compared with a sham procedure and antihypertensive therapy in the SYMPLICITY HTN-3 trial (Renal Denervation in Patients With Uncontrolled Hypertension),17 however, the prospect of RDN’s role in clinical practice diminished and appeared to validate earlier criticisms of trial conduct and, more broadly, the prospects for device-based hypertension therapies.
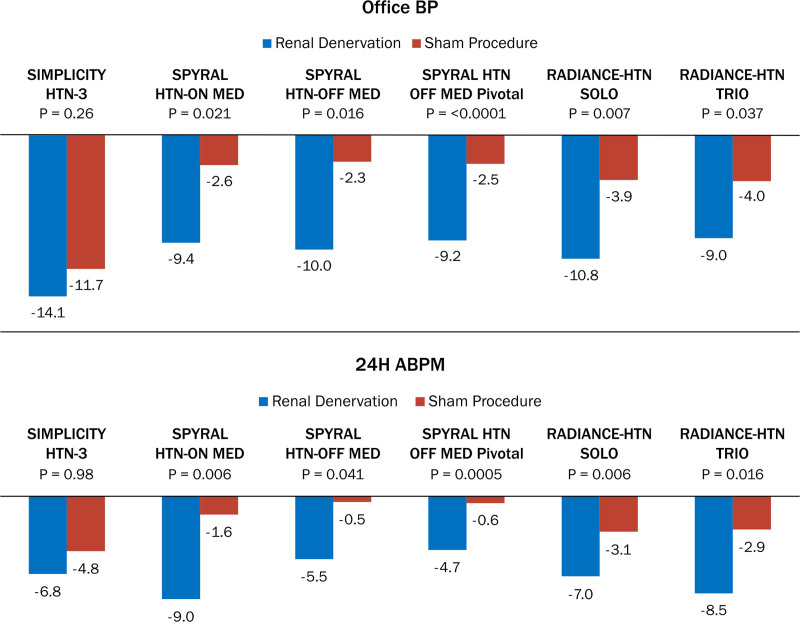
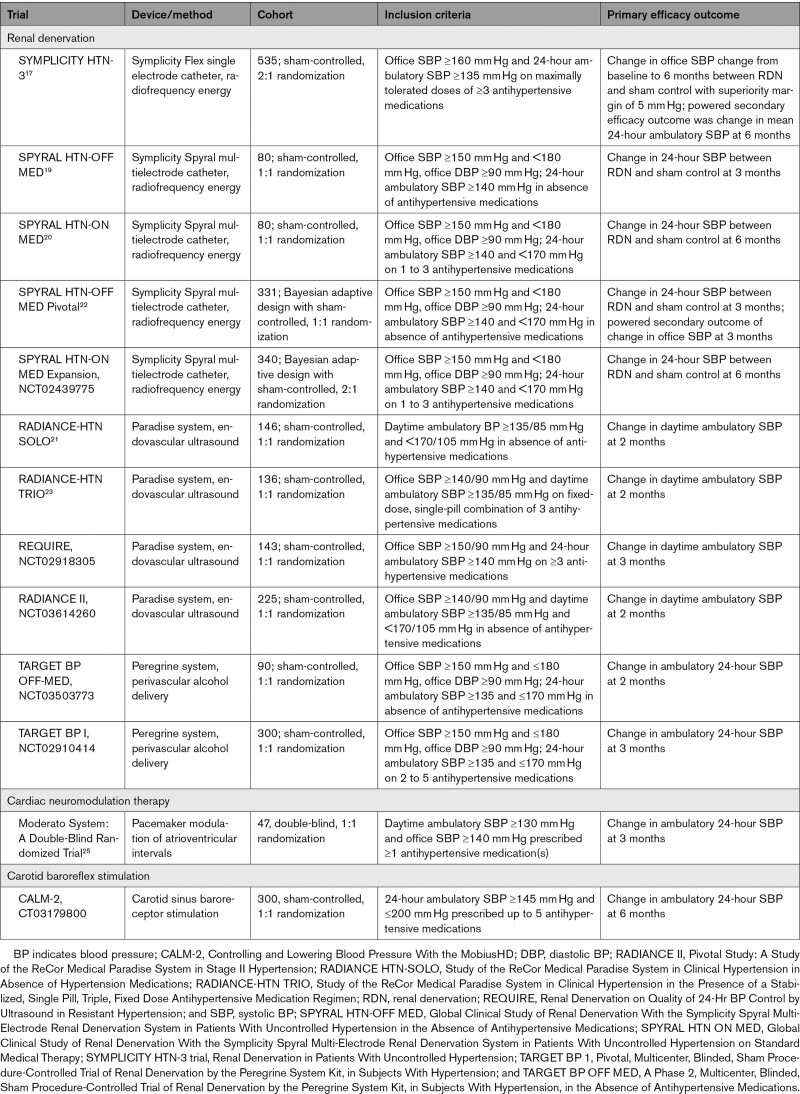
Advances in trial design and conduct to account for confounding variables of procedural technique, medication variability, and selection of both patients and outcomes have substantially informed subsequent studies.18 Additional exploratory preclinical and clinical studies continued, and signals of effectiveness with RDN persisted, motivating further investigations. To date, results from 5 sham-controlled, randomized RDN trials have demonstrated significant BP reductions in patients on or off concomitant antihypertensive therapies (Figure 1),19–23 supporting a biological proof of principle for this novel treatment. In addition to RDN, device-based therapies for which the effectiveness and safety are being evaluated in randomized controlled trials include those targeting carotid sinus baroreceptors, and pacemaker modulation of atrioventricular intervals.19–21,24,25 Table 1 summarizes completed and ongoing clinical trials of these device-based therapies. Given this background, experience with device-based therapies for hypertension warrants a careful approach to clinical evidence generation with a thoughtful consideration of patient population, trial methods, and procedural safety and effectiveness outcomes.
Figure 1.
Change in systolic blood pressure (mm Hg) after renal denervation in 6 prospective, randomized, sham-controlled trials.
ABPM indicates ambulatory blood pressure measurement; BP, blood pressure; RADIANCE HTN SOLO, Study of the ReCor Medical Paradise System in Clinical Hypertension in Absence of Hypertension Medications; RADIANCE-HTN TRIO, Study of the ReCor Medical Paradise System in Clinical Hypertension in the Presence of a Stabilized, Single Pill, Triple, Fixed Dose Antihypertensive Medication Regimen; SYMPLICITY HTN-3 trial, Renal Denervation in Patients With Uncontrolled Hypertension; SPYRAL HTN-OFF MED, Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications; SPYRAL HTN-OFF PIVOTAL, Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications Pivotal; and SPYRAL HTN ON MED, Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy. Adapted from references 15, 17–21.
Table 1.
Contemporary Randomized Trials of Device Therapies for Hypertension
Hypertension Academic Research Consortium
Rationale and Methods
Consistent, pragmatic definitions that support key clinical trial processes such as independent adjudication of study outcomes and safety oversight provide informative benefit/risk evidence and can promote efficient innovation of safe and effective therapies. The Hypertension Academic Research Consortium (HARC) was initiated to create consensus among experts involved in developing device-based therapies for hypertension following the process defined in the ARC charter.26 In February 2020, HARC participants met in in Silver Spring, MD. The meeting was organized by Cardialysis (Rotterdam, The Netherlands) and was attended by experts from Europe, the United States, Canada, and Australia, as well as representatives from the US Food and Drug Administration, a European Notified Body (DEKRA Certification BV, Arnhem, The Netherlands), and observers from the cardiovascular device industry (participant listing in the Supplemental Material). After the meeting, focused writing groups were charged to develop individual sections on outcomes definitions and trial design principles presented in this document.
Clinical Trial Design Consideratons
Trial Design, Conduct, and Presence or Absence of Medications
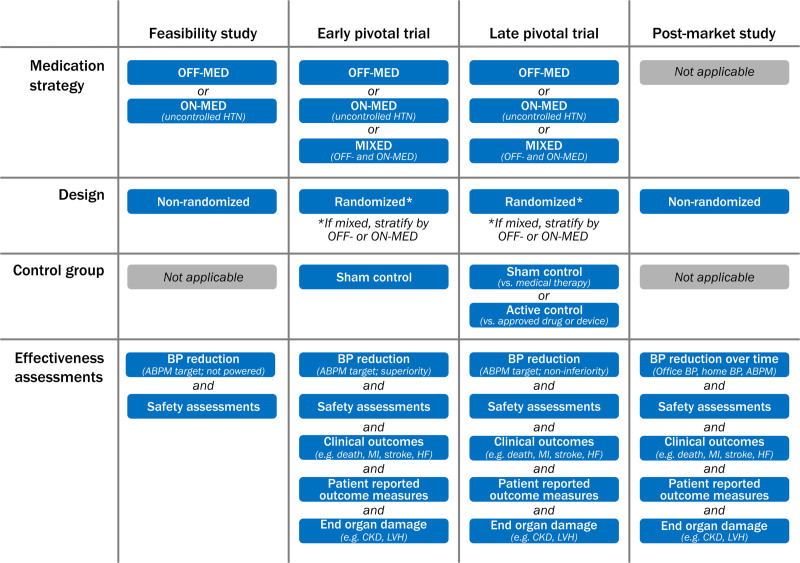
The design for clinical trials of device-based therapies for hypertension treatment will vary depending on the regulatory phase of strategy. Figure 2 outlines many of the specifics recommended by HARC for the various stages of hypertension device therapy clinical studies.
Figure 2.
Clinical investigation recommendations for novel devices for hypertension management.
ABPM indicates ambulatory blood pressure measurement; BP, blood pressure; CKD, chronic kidney disease; HF, heart failure; HTN, hypertension; LVH, left ventricular hypertrophy; and MI, myocardial infarction.
Initial studies to test proof of concept and feasibility are typically single-arm or open-label randomized trials.14,24,27 Notable features of feasibility studies include a target population similar to that planned for pivotal studies and an adequate number of participants to demonstrate some evidence of effectiveness and assessment for major safety concerns, although statistical significance is not required. During feasibility and pilot studies, modifications of the technology or the procedure may be tested with finalization of the device design and procedural protocol before a pivotal trial.
HARC recommends a staged off medications (OFF-MED) then on medications (ON-MED) progression of randomized trials. After a first stage of addressing safety and biological plausibility (OFF-MED), recruitment of an ON-MED population of patients with uncontrolled hypertension is logical to document the magnitude of the antihypertensive effect of the procedure and its durability in the presence of medications. In practice, recruitment of patients with uncontrolled hypertension prescribed a single agent of the same class would be impractical, and thus most ON-MED studies allow patients on 1 to 3 (or more) antihypertensive medications. These designs are prone to large between-patient variability in BP response after the procedure. An alternative, used in the RADIANCE HTN TRIO study (Study of the ReCor Medical Paradise System in Clinical Hypertension in the Presence of a Stabilized, Single Pill, Triple, Fixed Dose Antihypertensive Medication Regimen),23 is to switch at enrollment all eligible patients with apparent resistant hypertension prescribed multiple medications to a triple combination single pill to be taken for the remainder of the study. This strategy promotes medication adherence and a more consistently treated cohort of patients with resistant hypertension confirmed by ambulatory BP (ABP).
Early phase studies may be followed by randomized, blinded, sham-controlled trials to compare effectiveness and to assess device safety (OFF-MED and ON-MED). Although a 1:1 allocation of experimental treatment versus control is the most efficient design, a 2:1 (study device versus control) randomization ratio may be considered to enlarge the size of the study cohort (achieving learning curves faster and affording more patients exposed to the device-based therapy to identify adverse safety outcomes), although this will necessarily increase sample size to achieve statistical power to demonstrate effectiveness in BP lowering. Additional safety data can be obtained by “crossing over” control group patients after measurement of the primary effectiveness outcome to receive the device-based therapy or allowing for access to the device-based therapy in either the premarket or the postmarket interval.
The US Food and Drug Administration requires sham-controlled trials whenever feasible and ethical, and pivotal clinical trials for at least the first generation of device-based therapies must therefore include a sham procedure group as the control.28 Results from a recent meta-analysis suggested that in most interventional trials, sham procedures are safe, and the use of the sham procedure when applied in a symmetrical way without cointervention after randomization produces a robust estimate of the effect, albeit smaller than in nonsham trials and more likely closer to the true effect.29 The most appropriate placebo control for device-based therapies is an invasive sham procedure, mimicking the active treatment closely. For RDN trials, typically a sham involves a renal angiogram but no denervation device advanced into the artery. The use of invasive sham procedures is generally associated with a higher degree of complexity of trial execution, introducing ethical concerns of performing a procedure conferring an immediate risk of adverse events and potential harm without potential benefit to the patient. However, by blinding the patient and all postprocedure assessors, sham-controlled trials reduce confounders and bias, allowing the true treatment effects of any interventional therapy to be discerned. Although nearly all recent hypertension trials of device-based therapies have been sham-controlled,19–23 it is important to recognize this 1 design attribute does not always exclude other flaws in trial design or performance.
Once safety and effectiveness in both on and off medication blinded, pivotal trials has been proven, subsequent randomized trials may be open-label with a conventionally treated control group13 or include a control group with strict protocol-specified medical therapy.30 For trials involving medical therapy, adherence testing using urinary or plasma metabolite assays may be useful to interpretation of results. Among blinded studies, effectiveness of maintaining the blind must be demonstrated through patient questionnaires administered immediately after the procedure and at the time of the primary outcome assessment. For open-label studies, a Prospective Randomized Open-label Blinded End Point (PROBE) design should be used with assessment of BP response by an observer-independent BP measurement such as ABP monitoring (ABPM) and by evaluators blinded to the randomization.30
Once RDN technologies are approved and are available in clinical practice, sham or medical therapy controls may no longer be required or feasible. Instead, a shift from device versus medical therapy to investigational device versus approved device controls is anticipated. Once this occurs, HARC advises that it is reasonable to propose an active device control using a noninferiority design. In RDN device versus device trials, however, treatment assignment should remain blinded to the patients, research staff, and subsequent caregivers, if possible. Such noninferiority designs must be rigorous, requiring evidence of effectiveness of the active comparator on the primary outcome of interest with strict noninferiority margins as well as adequate clinical outcome and safety data.
Single-arm studies are generally not considered as proof of safety or effectiveness for a device-based therapy, particularly in the early phases of developing breakthrough technologies such as current RDN methods. More frequently, single-arm studies are performed as postapproval device-based studies to provide broader insight into the effectiveness of a therapy in a less selected patient population and in scenarios common to clinical practice. Well-designed registries can recruit larger volumes of patients than randomized trials, identifying signals of rare but serious adverse events that might provide guidance, especially in the postmarketing phase. They may also allow subgroup hypotheses to be generated for effectiveness.
Medication Burden and Adherence
The BP response to device-based therapies cannot be reliably quantified without accounting for antihypertensive treatments prescribed in terms of the number of different classes of antihypertensive medications as well as doses, and patient adherence to the prescribed medications in both blinded and unblinded periods of a trial. Accounting for medication changes is particularly challenging after unblinding, because differential behavior of health care providers or patients when knowing treatment allocation may lead to a potential bias, thus obscuring the intervention’s effect per se, especially in the long term.
Medication burden is usually evaluated using simplified dichotomous measures (either on- or off-treatment), or a simple measure of the number of antihypertensive medications (disregarding the dose and the class), not accounting for the dose-dependency of the BP-lowering effect of the medications. Besides these simple measures, medication burden can also be evaluated using the sum of defined daily dose of each individual antihypertensive medication and by using the antihypertensive load index,31 which is the sum of the ratio of the current daily dosage divided by the maximum recommended daily dosage for each medication. The maximum daily dosage of each agent as indicated for hypertension is obtained from pharmaceutical databases.31,32 Although alternative models may be developed, medication indices should account for both number and dose of prescribed medications.
Nonadherence to medications is recognized as a major factor in reducing BP treatment effectiveness and contributes to perceived “treatment resistance” in patients with hypertension. Indeed, nonadherence is highly prevalent (in up to 50% of patients), especially in patients diagnosed with severe hypertension.33 In individuals with perceived treatment-resistant hypertension, 50% of treatment resistance may be explained by nonadherence (“pseudoresistance”).34 In addition to adverse clinical events, there are numerous adverse consequences of suboptimal adherence to antihypertensive medications including a confounding effect on BP outcomes in clinical trials of device-based therapy for hypertension. Moreover, the time course of poor adherence can be variable, with fluctuating adherence over time, further confounding measurements of effectiveness.20
Reasons for poor adherence to treatment are multifactorial. Treatment-, patient-, provider- and health care system–related factors affect medication nonadherence in patients with hypertension, but predicting nonadherence remains challenging. Several indirect and direct methods have been developed to assess treatment adherence (Table S1). Assessing medication adherence helps guide medical treatment, avoid unnecessary and potentially harmful treatment intensification, decrease the number of medical visits at specialized clinics, and allow implementation of strategies to improve medication adherence. Medication monitoring and informing patients about testing have been shown to improve BP control, and adherence to antihypertensive medications is associated with lower cardiovascular risk.35 Inclusion of adherence assessment in clinical trials as described in this section is important for interpreting study outcomes, at least in the early development phase of device-based therapies, to inform the treatment effect.
Medication Reinstatement Algorithms
Except for patient-related safety requiring deviation from study protocol, baseline medications should remain without change (or absent in OFF-MED trials) for both treatment and control groups until timing of the primary BP outcome measure. There are 2 main reasons for starting, resuming, or enhancing medical therapy after the study’s primary outcome is reached: (1) an ethical need to control BP in the trial’s study participants, and (2) to compare medication requirements between the device-treated and the control groups to learn whether the procedure affects the intensity of medical treatment required to achieve the BP target.
A typical medication algorithm, already used in device-based hypertension trials for patients initially OFF-MED,19,21,22 is described in Table S2. The selection of medication classes, and their order of use in this stepwise titration, conforms with widely used current international hypertension guidelines,11,12 with further recommendations provided in the Table S2 footnote how to individualize therapy in patients with difficult-to-control hypertension. One shortcoming in trials completed to date19,30 is that using the algorithm has been left to the discretion of the clinical investigators and not mandatory. It is recommended that future trials include rigorous protocols for the intensification of medical therapy by the clinical staff or treating physicians, as tolerated, to achieve target BP, while verifying adherence to medications by patients.
BP Outcomes for Device-Based Therapies for Hypertension Trials
The following section addresses the specific advantages and disadvantages of each method to measure and report BP and potential complementary use of multiple BP assessment strategies.
Office BP
When properly performed, office BP (OBP) measurement is inexpensive and accurate, and reflects what has been used in landmark clinical trials and routine clinical practice for decades. In early RDN trials, however, OBP results as a primary outcome often demonstrated higher variability than in traditional medical therapy studies, leading to uncertainty about consistency in measurement and method, susceptibility to regression to the mean, and inability to confirm treatment effect. This variability may have been amplified by inconsistent medication regimens and adherence.
The importance of using proper technique cannot be overstated and has been described elsewhere.36 Strict adherence to measurement protocols makes choice of “attended” or “unattended” (ie, with or without a health care provider present) readings less important, but the same approach should be consistently applied throughout a clinical trial. Averaging data from >1 visit increases precision and reduces sample size but may also increase trial complexity.37 Effects associated with unblinded assessment and white-coat effect should be minimized.
In randomized trials, the mean difference in BP from baseline to specific posttreatment time points (eg, 3 or 6 months) compared between treatment and control groups is a measure of effectiveness. SBP reduction is generally the key clinical target, but DBP is of particular interest in trials that enroll younger patients or use elevated DBP as an entry criterion.
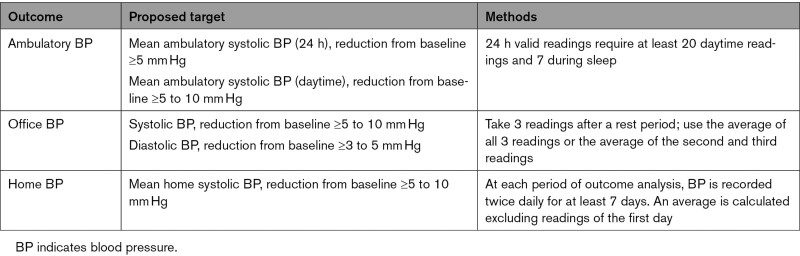
Data from both observational and randomized studies, including meta-analyses of medical therapy trials, demonstrate that decrements in office SBP of 5 and 10 mm Hg are associated with ≈10% and ≈20% reductions in cardiovascular disease events, respectively, and independent of comorbidities such as cardiovascular disease.7 As such, a mean difference in OBP of at least 5 to 10 (preferably closer to 10) mm Hg for SBP compared with baseline, or 3 to 5 mm Hg for DBP, may be considered a clinically meaningful reduction. In addition, BP response rates (ie, responder analysis) and time within target range can provide a complementary effectiveness assessment.
Ambulatory BP
ABPM yields more precise and reproducible BP profiles than conventional OBP assessments.38 ABPM is less susceptible to bias and placebo effect and eliminates the white-coat effect.39 It estimates short-term BP variability and provides better prognostic value for end-organ damage and cardiovascular events versus OBP.40 In addition, the relationship between cardiovascular events and mortality is stronger for ABPM than OBP, in particular when including nocturnal BP.40–42
For these reasons, ABPM has been adopted as the preferred BP outcome in major randomized RDN trials. ABPM has proven to be particularly well suited because the BP-lowering effects of RDN have been apparent throughout a 24-hour period.43 Although elevated SBP and DBP are associated with increased cardiovascular risk, the association with SBP is more consistent and persists after adjustment for DBP. Therefore, SBP has been preferred in hypertension clinical trials as the primary outcome.12 In RDN trials, both ambulatory daytime and 24-hour SBP assessments have been used.
A reduction in mean 24-hour ambulatory SBP of ≥5 mm Hg relative to baseline can be considered a clinically meaningful response to RDN,28,44,45 recognizing that a 10-mm Hg reduction in office SBP is associated with a significant reduction in the risk of major cardiovascular events and mortality across varying baseline BPs.7 The procedures to measure ABPM have been previously described.36 To address issues of adherence and missing data, 24-hour ABPM has been considered valid with a minimum of 20 daytime readings and 7 during sleep.46,47 Independent core laboratories are desirable to provide feedback on data quality, maintain uniform process across sites, conserve confidentiality, and reduce potential bias.
Home BP
Home BP measurement has emerged as an important complement to OBP and ABPM in both clinical practice and research. Self-measured home BP avoids the white-coat response, allows for multiple measurements over time, identifies patients with masked hypertension, and has been shown to correlate better with target organ damage and cardiovascular events than OBP.36 However, home BP measurement is highly operator-dependent and is reliant on equipment accuracy; home BP also misses nighttime BP, orthostatic hypotension, and short-term BP variability. Awareness of home BP results may also bias patient behaviors and medication adherence. On the basis of these limitations, home BP measurement can be a secondary outcome for device-based therapy trials, but assessment issues limit its use as a primary outcome.
Optimal home BP measurement methods have been previously detailed.11,12,36,48 At each outcome assessment time, it is important to record BP twice daily (morning and evening) for at least 7 consecutive days with the readings from the first day discarded in clinical trials,46 and at least 70% of expected readings at each time period should be available (ie, up to 30% missing data are not ideal but currently acceptable). Electronic transfer of readings to an independent core laboratory is encouraged and may enable long-term effectiveness follow-up without requiring participants to attend a study visit.49
Evidence from the relatively few observational studies relating cardiovascular risk with home BP measurement suggest that each 10-mm Hg increase in SBP is associated with an ≈15% to 20% increase in adverse events.50 Given their similar profiles on determination of cardiovascular risk,51 similar thresholds for clinically significant response may be considered for daytime ABP and home BP in device-based therapy trials. Table S3 describes the advantages and disadvantages ABPM and home BP measurements.
Consensus Summary: Selection of BP Measurement Modalities as Outcomes
Clinically relevant BP reduction considerations proposed by consensus are represented in Table 2. All BP assessments—ambulatory, office, and home—may be considered for trials evaluating device-based hypertension treatment because each modality offers different but complementary information on the BP response (Table S4). As a practical matter, however, all randomized trials of hypertension therapies may use 2 or more of these measures, typically with ABPM specified as the primary effectiveness outcome measure.
Table 2.
Blood Pressure Outcome Considerations in Device-Based Therapies for Hypertension Trials
Additional Outcomes Relevant to Device-Based Therapies for Hypertension Trials
Clinical Outcomes
HARC recommends to ascertain and classify death,52,53 neurological events,54 myocardial infarction,55 bleeding,56 access and vascular complications,56 acute kidney injury,56 and heart failure,47 following standardized definitions (Tables S5–S8).52,53,57 Although complications occur infrequently with current technologies, procedure-specific criteria are proposed on the basis of existing ARC standards and available in the Supplemental Material. Independent adjudication of clinical events (both safety and effectiveness) is a quality marker in clinical trials, enhances the validity of study results, and is often requested by regulatory authorities.
Target-Organ Damage
Regression of hypertension-mediated organ damage is associated with improved cardiovascular outcomes.58 Several prospective observational studies have suggested that RDN is associated with regression of echocardiographic or cardiac magnetic resonance imaging–based left ventricular mass index,59,60 improvement in left ventricular function,59–61 decrease in left atrial volume index,61 and augmentation index or pulse wave velocity,59 irrespective of BP reduction.62 These studies, however, are limited because of sample size and lack of independent, blinded core laboratory assessment. Available data, limited by sample size and short-term follow-up, suggest that RDN is safe and effective in patients with chronic kidney disease, although it remains to be established whether RDN leads to improvement in albuminuria or kidney function, or at least attenuates the decline in glomerular filtration rate.63
BP Targets and Variability
Clinical guidelines for hypertension treatment share an OBP target of <130/80 mm Hg (when tolerated and on the basis of risk profiles), with an OBP of <140/90 mm Hg for those >65 years of age per the European guidelines.11,12,47 The proportion of patients achieving these targets is an alternative to targeting a specified absolute BP reduction from baseline,11,44 considering that relative percentage reduction from baseline after device-based therapies is influenced by initial BP levels and might be less applicable in milder forms of hypertension.19
Long-term visit-to-visit variability in BP offers prognostic information in patients with hypertension and can be used as a secondary efficacy outcome. High BP variability has been associated with increased cardiovascular risk,64 and device-based therapies have been shown to reduce such variability after correction of baseline BP values, thus offering potential as an effectiveness outcome (Table S9).65 A more consistent reduction in BP would expectedly mitigate BP variability and improve time in target range that has been associated with a lower risk of adverse events.66
Heart Rate
Aside from reductions in both sympathetic activity and BP after RDN, a decline in resting heart rate has also been shown in several studies.67,68 In the SPYRAL HTN-OFF MED pilot study (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications), for example, resting heart rate was reduced after RDN both in the office and during daytime and nighttime ABPM.68 From 2 reports, an elevated resting heart rate (above the median 73.5 beats per minute) has also been suggested to predict BP reduction after RDN.68,69 Other reports, however, have not demonstrated this association. At present, heart rate reduction after device-based therapy should be considered an exploratory outcome.
Predictors of Treatment Response
The inability to accurately identify individual therapy responders undermines efforts to identify broadly applicable predictors of response and inform patient selection. To date, post hoc “responder” analyses on the basis of single-point paired comparisons may not appropriately characterize a treatment response given (1) day-to-day BP variability, (2) the lack of consensus about follow-up time point or meaningful reduction in BP, (3) dependency of the change in BP from the pretreatment BP, (4) the suggestion that even lesser declines in BP may not imply absence of a treatment effect, and (5) susceptibility of treatment bias.70 Unlike the population mean effect that statistically compensates for BP variability, an individual patient can only be compared against a random time point that could be higher or lower than the patient’s long-term mean BP. In the SPYRAL HTN-OFF and ON MED (Global Clinical Study of Renal Denervation With the Symplicity Spyral Multi-Electrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy) trials19,20 and the RADIANCE HTN-SOLO21 (Study of the ReCor Medical Paradise System in Clinical Hypertension in Absence of Hypertension Medications) trials, for example, approximately half of patients in the sham group at outcome follow-up experienced a BP increase, whereas half experienced a decrease, and few patients had no change. Although often impractical, averaging multiple BP measurements at baseline and follow-up might be required to control for BP variability and for regression to the mean, and specifically identify individual treatment responders.
A broad list of potential predictors of response has been proposed to date but is limited with little if any external validation. No individual predictor, except baseline BP, has been consistently identified across multiple trials. In the SPYRAL HTN-OFF MED Pilot and Pivotal trials, ambulatory heart rate above the median (>73.5 bpm) was predictive of reduction in average daytime SBP, daytime DBP, and office SBP.69 Recently, both plasma renin activity and aldosterone levels were significantly reduced after RDN compared with sham control; higher baseline plasma renin activity was associated with a significantly greater reduction in both office and 24-hour SBP.71
Last, because no reliable procedural measure exists to confirm effective nerve ablation, the extent to which incomplete denervation contributes to clinical nonresponsiveness is uncertain. HARC endorses incorporation of exploratory substudies as part of larger trials confirming safety and effectiveness.
Patient-Related Outcomes and Preference
Patient perspectives and preferences are important in assessing the benefits and risks of antihypertensive therapy. In general, patient-reported outcomes, quality of life, and patient preferences have until recently rarely been the main objective of clinical trials in device-based medicine. Patient-reported outcomes instruments rely on patients’ response to questionnaires (eg, quality-of-life questionnaires), and on measurements of patients’ well-being (eg, cognitive function) or outcomes self-assessed through medical diaries/ trackers. To date, the European Quality of Life 5-Dimension 3 Level (EQ-5D-3 L) questionnaires and the short form health survey (SF36) are considered to be reliable and validated measures of quality of life and have been used in hypertension research.72 In a recent survey, patient and physician attitudes toward pharmaceutical therapies and RDN for hypertension may be differ relative to severity of hypertension and medication burden.73 Specifically, preference for device-based therapy among patients may be highest among those not taking medications and may be independent of BP severity.
HARC encourages incorporating such assessments in device-based hypertension trials and developing health status models specific to this area of study. For patient preference, Discrete Choice Experiments may be useful to quantify patients’ preferences for attributes of interventional and pharmaceutical treatments for hypertension that include both effectiveness and risks of treatment-related adverse events.74 For self-reported outcomes, the first step is the selection of an appropriate patient-reported outcome measure (PROM), presenting the patient’s perspective about how an interventional procedure for hypertension will affect their well-being. The selection of a PROM is based on its appropriateness, acceptability to patients, interpretability, validity, and ability to detect changes over time.
For future trials, HARC recommends the following: (1) the validation of PROMs in the field of hypertension that account for the heterogeneity of patients’ perception according to culture or health care systems, (2) systematic inclusion of PROMs in prospective trials, and (3) inclusion of patient’s perspective and preferences at an early stage of clinical trial design.
Timing of Assessments Related to Late-Term Safety and Effectiveness
Timing of Outcome Selection
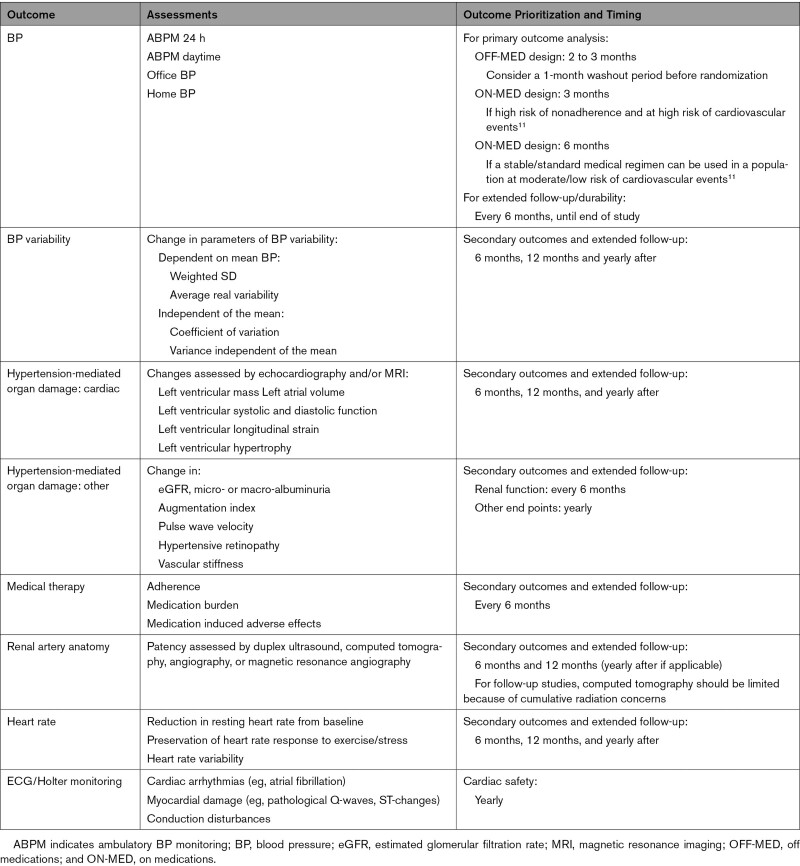
Four key factors may be considered when determining the optimal timing for outcome assessment in device-based hypertension trials: (1) what the study population is (eg, on versus off medications, mild versus severe hypertension); (2) what the primary assessment is (BP versus measures of end-organ damage); (3) when the treatment effect is expected; and (4) what the likelihood is of confounders interfering with the treatment effect. Timing of outcome recommendations is provided in Table 3.
Table 3.
Timing for Analysis of Primary and Secondary Outcomes in Hypertension Trials With Device-Based Therapies
Available evidence supporting the use of different technologies to inhibit the sympathetic nervous system reveals significant reductions in both OBP and ABPM within the first 3 months of treatment. Of note, the risk for confounding by medication adherence issues rapidly increases within the first 6 months, supporting a rationale for primary outcomes sooner rather than later within a 6-month window. In patients who are enrolled in device-based therapy trials and prescribed antihypertensive medications using stable and standardized concomitant medication regimens, selection of BP outcomes between 2 and 6 months after intervention is reasonable. For proof-of-concept studies in medication-naive patients (including participants in whom antihypertensive medications are discontinued before enrollment, for example, after a prespecified washout period), 2 to 3 months for the timing of outcome assessment may be acceptable. A 4- to 6-week washout period was shown to be safe in relatively low-risk patients with hypertension enrolled in 3 sham-controlled randomized trials,21,22 consistent with safety of antihypertensive medication withdrawal reported in a meta-analysis of 66 pharmaceutical trials.75
For trial outcomes other than BP, later time intervals may be reasonable considering the type of event assessed; for instance, atrial fibrillation burden could be assessed (ie, Holter) at 3 months, whereas assessment of left ventricular remodeling may require 6 to 12 months.60 Extension of the follow-up beyond 12 months is likely to be necessary for clinical outcomes (eg, death, stroke, myocardial infarction) in adequately powered studies.7
Late-Term BP Outcomes and Durability of Treatment Effect
The demonstration of a sustained reduction in BP that can be solely attributed to an interventional procedure can be challenging because of medication changes, comorbidities, and patient behavior (eg, weight loss, exercise, diet, adherence).76 Although BP assessment after a medication washout period at late-term follow-up (eg, 1 year) has been proposed to assess durability in randomized trials, comparisons can nevertheless be confounded by factors unrelated to medical therapy.
Aside from measurement of BP, quantification of antihypertensive medication use at baseline and during longitudinal follow-up is important to patients, providers, and the health care ecosystem. Against the background of sustained reductions in BP, the number of medications and doses are expected to remain stable, if not decline. The number of prescribed medications is 1 important factor that limits adherence. If the procedure is successful, decreased medication burden may lead to fewer side effects and improve adherence to the remaining medications. Just as with BP, however, medication burden might be confounded by successful healthy lifestyle changes such as weight loss, exercise, and introduction of low-fat and low-salt diets supporting withdrawal of medications. However, as recurrence of hypertension is commonplace, serial assessment during long-term follow-up for the need for medication reinstatement is an important consideration. For assessment of durability, measurement of BP at least 12 months after intervention and compared with baseline (and a control group, if possible) can provide an assurance of a durable treatment effect. Alternatively, time in a targeted BP range during serial follow-up may provide some insight into durability and has been predictive of cardiovascular risk.66
Late-Term Safety Outcomes
Kidney function is a critical safety issue associated with both hypertension and RDN. After RDN, measurements of kidney function (ie, estimated glomerular filtration rate) are advocated at 6-month intervals for up to 3 years. A secondary consideration is the measurement of the urine albumin/creatinine ratio, because increases in micro- or macro-albuminuria are associated with an adverse kidney and cardiovascular prognosis.77
A more challenging issue is the assessment of renal artery anatomy after an endovascular procedure because of variability in noninvasive assessment procedures and the need to differentiate morphological changes from the natural history of atherosclerotic disease progression. Currently, duplex ultrasound, computed tomographic angiography, and magnetic resonance angiography have been applied with reasonable success in the existing device-based hypertension trials.19–23 With current RDN therapies, development of device-related proximal renal artery stenosis has been rarely observed.20–22,78 Among such cases reported in the context of both clinical trials and routine practice, the majority occur within the first year after treatment.78 Therefore, assessment of renal vasculature at 6 or preferably 12 months is recommended. On the imaging modality (eg, ultrasound, computed tomographic angiography, magnetic resonance angiography, catheter-based angiography), it is recommended to use the imaging procedure that is most feasible and is associated with high levels of study interpretation expertise within the particular health system. Escape of BP control or an unexplained decrease in the estimated glomerular filtration rate can be a useful clinical indicator of renal artery damage or dysfunction, prompting further study. Duplex ultrasound will detect only hemodynamically significant lesions and is not optimal for assessment of distal branch lesions. Pending the establishment of long-term safety associated with RDN, patient follow-up for at least several years is reasonable.
Clinical Trial Target Populations
Severity of Hypertension
Incorporating the methodological lessons learned from SYMPLICITY HTN-3,17 contemporary randomized RDN trials have enrolled patients with moderate but uncontrolled hypertension (eg, average OBP ~155/100 mm Hg) in the presence and absence of medical therapy.19–23 In contrast, the SYMPLICITY HTN-3 trial enrolled patients with severe, treatment-resistant hypertension (average OBP ~180/98 mm Hg despite >5 prescribed antihypertensive medications).17 On the basis of more contemporary trials, RDN may have utility as an adjunct to tailored medical therapy to improve BP control and reduce pill burden.44 Among contemporary randomized trials, treatment with RDN has been associated with at least similar reduction in office SBP to what has been achieved with a single antihypertensive medication.8 However, whereas SYMPLICITY HTN-3 was performed in truly treatment-resistant severe hypertension, several subsequent studies were performed either OFF-MED or in more moderate hypertension ON-MED scenarios of uncontrolled hypertension. Thus, robust evidence for the utility of RDN in severe resistant hypertension is lacking and needs to be explored in further randomized controlled trials considering the associated poor medication adherence. For instances of severe hypertension, RDN may achieve BP control with existing medications or potentially reduce the medication burden, but in most cases will be complementary rather than exclusionary to medications for BP control. Alternatively, preference for nonpill or reduced lifetime medication burden and associated side effects are characteristics of RDN candidates in the earlier stages of the hypertensive disease.44 In these settings, RDN could become a secondary alternative to starting with medications in patients with mild hypertension in the short term, but medication(s) may also be needed to control BP during long-term follow-up.
Isolated Systolic Hypertension
Isolated systolic hypertension (ISH) has been associated with a reduced response to RDN,79 which, if confirmed in future trials, may be related to an important irreversible vascular component of hypertension in these patients. On the basis of this earlier observation, patients with ISH were excluded from recent trials. However, real-world registry data80 as well as a subanalysis of the RADIOSOUND trial (A Randomized Comparison of Ultrasound Based Versus Radiofrequency Based Catheter Ablation Techniques in Patients With Therapy Resistant Arterial Hypertension With Large Renal Arteries)81 demonstrated comparable effectiveness of RDN in both ISH and combined hypertension in patients <75 years old. Also, recent insights into the BP-lowering mechanism of RDN propose reduced vascular stiffness82 as well as normalization of high stroke volume83 as additional contributors to improved BP control after RDN. These modes of action could equally apply in patients with ISH. The presumption of a reduced BP-lowering effect of RDN in patients with ISH may have been overestimated and might also not hold true for newer denervation technologies and techniques.
White-Coat Hypertension and Other Hyperadrenergic Hypertensive Conditions
Primary hypertension is characterized by an increase in sympathetic activity combined with other neuroendocrine, kidney, vascular, central, genetic, and environmental mechanisms. However, in specific hypertensive conditions, sympathetic activation appears to be more pronounced and represents a hallmark of the high BP state. This is the case for white-coat hypertension, in which increased OBP with normal 24-hour ABP or home BP has been associated with a marked adrenergic activity as assessed by microneurographic recording of sympathetic nerve traffic.84,85 The influence of RDN on these various BP phenotypes is not well characterized and represents an opportunity for dedicated study.
Hypertension-Mediated Organ Damage and Patients at High Cardiovascular Risk
Data from observational studies suggest that RDN may be associated with favorable changes in diastolic function indices and left ventricular mass.60,61 Kidney function is neutrally or beneficially influenced by RDN, whereas for arterial structure and function, the data are scarce.86 Given the close link of left ventricular mass changes with improved outcomes in hypertension,11 patients with left ventricular hypertrophy and unfavorable remodeling could be a target RDN population. High cardiovascular risk settings such as coronary artery disease, diabetes, and patients with chronic kidney disease with uncontrolled hypertension11 would be expected to have the greatest benefit from BP control by RDN.44 In addition, hypertension with other comorbidities such as heart failure and arrhythmia burden (ie, atrial fibrillation) are particularly relevant populations for future studies.44,80 Because difficult-to-control hypertension is related to cerebrovascular events (of high concern for patients) and BP control is linked to better outcomes,7 improvement of cognitive decline and stroke risk could be core outcomes for inclusion in RDN trials.
Indications Beyond Hypertension
Because RDN interrupts both renal efferent and afferent nerve activity, effects on reducing the sympathetic signature within the body may have pluripotent effects beyond BP reduction (Figure S1). Although hypertension has remained the focus of major clinical trials for RDN, several observational and small randomized studies have explored its potential in disease states other than hypertension, including arrhythmias,67,87 glucose metabolism,88 obstructive sleep apnea,89 chronic kidney disease,90 and heart failure.91 Although such studies remain preliminary, HARC advocates similar trial design, conduct, and attention to safety as with hypertension trials, although primary outcomes may differ.
Statistical Considerations
Randomized controlled trials of device-based therapies for hypertension have consistently used change in BP measurement (generally 24-hour or daytime systolic ABP change from baseline to 2–6 months) as the primary outcome. ANCOVA adjusted on baseline BP is preferable to a simple paired t test to test the primary hypothesis (as reported in the next generation trials),92 and standard statistics are used to report binary variables, multilevel categorical variables, and time-to-event clinical outcomes. Four considerations are essential during the design phase. First, given the general use of a sham control in current trials, the assumed difference in treatment outcomes and the associated sample size considerations must account for an expected reduction in the sham group. Second, the intention-to-treat population should be considered as the primary analysis cohort; however, there are legitimate reasons to analyze a per-protocol population in addition (eg, excluding untreated patients, those with major protocol violations, those missing ABPM, or those fulfilling escape criteria requiring change in medications), and the as-treated cohort. Third, given uncertainty about the effects of novel device-based therapies for hypertension, Bayesian adaptive designs have been proposed as a more efficient method that avoids unnecessary enrollment of patients.22 This approach allows for prespecified interim analyses with predetermined stopping rules for effectiveness or futility.22 However, it increases statistical complexity and reduces sample size, but a larger sample might be important for the detection of infrequent safety outcomes. Fourth, in an effort to incorporate clinical outcomes in the primary outcome of device-based therapies for hypertension trials, generalized pairwise comparison methods can be used to hierarchically assess components of a composite. Offering advantages beyond traditional time-to-event analyses, generalized pairwise comparison methods, which include the Finkelstein-Schoenfeld and win-ratio methods, offer the advantages of prioritizing most severe outcomes, incorporating recurrent events, and combining time-to-event with quantitative outcomes.93 In addition, such methods allow the combination of BP outcomes with patient-centered outcomes, such as medication burden.94
Conclusions
The advancement of device-based approaches to hypertension has been motivated by
clinical implications associated with a persistently high prevalence of both uncontrolled BP and medication nonadherence. The expansion of device technologies and emerging clinical trials imparts the need for consistency in trial design, conduct, and definitions of clinical study elements to allow for trial comparability and data poolability. The HARC program represented an integration of evolving evidence and consensus opinion among leading experts in cardiovascular medicine and hypertension research with regulatory perspective on clinical trial design and methodology. From this program, clinical design principles and outcomes definitions for studies aimed at evaluating device-based hypertension therapies were established, and standardization of BP assessment, effectiveness measures beyond BP, and safety outcomes have been proposed.
Article Information
HARC Steering Committee: David E. Kandzari, MD; Felix Mahfoud, MD; Michael A. Weber, MD; Konstantinos Tsioufis, MD; Donald Cutlip, MD; and Ernest Spitzer, MD.
Acknowledgments
The authors are grateful to Hans Jonker at Cardialysis for preparation of the figures.
Sources of Funding
HARC was funded through grants from Medtronic, ReCor Medical, Boston Scientific, BackBeat Medical, Ablative Solutions, Vascular Dynamics, and Metavention.
Disclosures
Dr Kandzari reports institutional research/grant support from Ablative Solutions and Medtronic; and personal consulting honoraria from Medtronic. Dr Mahfoud receives research support from the Deutsche Gesellschaft für Kardiologie and Deutsche Forschungsgemeinschaft and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical. Dr Weber reports consulting honoraria from Urovant, Johnson & Johnson, Ablative Solutions, Regeneron, Medtronic, Omron, and ReCor Medical. Dr Townsend receives consultant honoraria from Medtronic and Regeneron and royalties from UpToDate and is a data and safety monitoring member at AXIO. Dr Parati declares speaking honoraria from Omron Healthcare, Servier, and Novartis. Dr Fisher receives consultant and research funding from Recor Medical and consulting honoraria from Medtronic and Aktiia. Dr Lobo receives consulting honoraria from Medtronic, ReCor Medical, Ablative Solutions, CVRx, Rox Medical, and Vascular Dynamics and reports educational grants from Medtronic and ReCor Medical. Dr Bloch reports consulting honoraria from Recor and Medtronic. Dr Böhm reports research support from the Deutsche Forschungsgemeinschaft and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Daiichii-Sankyo, Medtronic, Novartis, ReCor, Servier, and Vifor. Dr Azizi receives research grants from the French Ministry of Health, Quantum Genomics, and the European Horizon 2020 program; grant support and nonfinancial support from ReCor Medical and Idorsia; and personal fees from CVRx, AstraZeneca, Alnylam Pharmaceutical, and Poxel Pharma. Dr Schlaich receives research supported from the NHMRC Research Fellowship and has received consulting fees or travel and research support from Medtronic, Abbott, Otsuka, Novartis, Servier, Pfizer, and Boehringer Ingelheim. Dr Kirtane reports institutional funding to Columbia University or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, CSI, Siemens, Philips, ReCor Medical, Neurotronic, and Biotronik. In addition to research grants, institutional funding includes fees paid to Columbia University or Cardiovascular Research Foundation for consulting or speaking engagements in which Dr Kirtane controlled the content. Dr Kirtane also reports consulting for IMDS; and travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr Daemen receives institutional grant/research support from AstraZeneca, Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic, Pie Medical, and ReCor Medical. Dr Pathak declares consulting honoraria from Medtronic, ReCor Medical, Ablative Solutions, and CVRx and educational and research grants from Medtronic, ReCor Medical, and Ablative Solutions. Dr Ukena reports consulting and lecture honoraria from Bayer, Boehringer Ingelheim, Medtronic, and Pfizer. Dr Lurz declares institutional grants from ReCor Medical. Dr Grassi declares lecture honoraria from Medtronic and Merck. Dr Finn receives research grants from ReCor Medical and Medtronic through CVPath, and honoraria from Medtronic. Dr Morice declares being the chief executive officer and shareholder of CERC. Dr Jüni serves as an unpaid steering committee member of trials funded by Abbott Vascular, AstraZeneca, Biotronik, Biosensors, St Jude Medical, Terumo, and The Medicines Company; receives institutional research grants from Appili Therapeutics, AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and The Medicines Company; and receives institutional honoraria for participation in advisory boards or consulting from Amgen, Ava, and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer. Dr Stone has received speaker or other honoraria from Terumo, Cook, and Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Reva, MAIA Pharmaceuticals, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, and Gore; and has equity/options from Ancora, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and the MedFocus family of funds. The other authors report no conflicts.
Supplemental Material
HARC Participants
Tables S1–S9
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ABP
- ambulatory blood pressure
- ABPM
- ambulatory blood pressure monitoring
- BP
- blood pressure
- DBP
- diastolic blood pressure
- HARC
- Hypertension Academic Research Consortium
- ISH
- isolated systolic hypertension
- OBP
- office blood pressure
- PROM
- patient-reported outcome measure
- RDN
- renal denervation
- SBP
- systolic blood pressure
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.057687.
For Sources of Funding and Disclosures, see page 860.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Felix Mahfoud, Email: felix.mahfoud@uks.eu.
Michael A. Weber, Email: michaelwebermd@cs.com.
Raymond Townsend, Email: townsend@upenn.edu.
Gianfranco Parati, Email: gianfranco.parati@unimib.it.
Naomi D.L. Fisher, Email: nfisher@partners.org.
Melvin D. Lobo, Email: m.d.lobo@qmul.ac.uk.
Michael Bloch, Email: Michael.Boehm@uks.eu.
Michael Böhm, Email: Michael.Boehm@uks.eu.
Andrew S.P. Sharp, Email: drandrewsharp@gmail.com.
Roland E. Schmieder, Email: roland.schmieder@uk-erlangen.de.
Michel Azizi, Email: michel.azizi@aphp.fr.
Markus P. Schlaich, Email: markus.schlaich@uwa.edu.au.
Vasilios Papademetriou, Email: vasilios.papademetriou@va.gov.
Ajay J. Kirtane, Email: ak189@cumc.columbia.edu.
Joost Daemen, Email: j.daemen@erasmusmc.nl.
Atul Pathak, Email: atul.pathak@chpg.mc.
Christian Ukena, Email: Christian.Ukena@uks.eu.
Philipp Lurz, Email: Philipp.Lurz@medizin.uni-leipzig.de.
Guido Grassi, Email: guido.grassi@unimib.it.
Martin Myers, Email: martin.myers@sunnybrook.ca.
Aloke V. Finn, Email: afinn@cvpath.org.
Marie-Claude Morice, Email: mc.morice@icps.com.fr.
Roxana Mehran, Email: roxana.mehran@mountsinai.org.
Peter Jüni, Email: peter.juni@utoronto.ca.
Gregg W. Stone, Email: gstone@crf.org.
Mitchell W. Krucoff, Email: mitchell.krucoff@dm.duke.edu.
Paul K. Whelton, Email: pkwhelton@gmail.com.
Konstantinos Tsioufis, Email: ktsioufis@gmail.com.
Donald E. Cutlip, Email: dcutlip@bidmc.harvard.edu.
Ernest Spitzer, Email: ESpitzer@cardialysis.nl.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005; 365:217–223. doi: 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014; 130:1692–1699. doi: 10.1161/CIRCULATIONAHA.114.010676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018; 137:109–118. doi: 10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020; 324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012; 125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. ; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020; 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016; 387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 8.Blood Pressure Lowering Treatment Trialists’ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021; 397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015; 373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, Yang J, Jiang Y, Xu X, Wang TD, et al. ; STEP Study Group. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021; 385:1268–1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018; 138:e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 13.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9 [DOI] [PubMed] [Google Scholar]
- 14.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009; 373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3 [DOI] [PubMed] [Google Scholar]
- 15.Sievert H, Schofer J, Ormiston J, Hoppe UC, Meredith IT, Walters DL, Azizi M, Diaz-Cartelle J, Cohen-Mazor M. Renal denervation with a percutaneous bipolar radiofrequency balloon catheter in patients with resistant hypertension: 6-month results from the REDUCE-HTN clinical study. EuroIntervention. 2015; 10:1213–1220. doi: 10.4244/EIJY14M12_01 [DOI] [PubMed] [Google Scholar]
- 16.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013; 34:2132–2140. doi: 10.1093/eurheartj/eht197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, et al. ; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014; 370:1393–1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 18.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015; 36:219–227. doi: 10.1093/eurheartj/ehu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, et al. ; SPYRAL HTN-OFF MED T. rial I. nvestigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017; 390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 20.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, et al. ; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018; 391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 21.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, et al. ; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018; 391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 22.Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020; 395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 23.Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, Rump LC, Persu A, Basile J, Bloch MJ, et al. ; RADIANCE-HTN investigators. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021; 397:2476–2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 24.Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, Moelker A, Kroon A, Reuter H, Ansel G, et al. ; CALM-FIM_EUR investigators. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet. 2017; 390:2655–2661. doi: 10.1016/S0140-6736(17)32337-1 [DOI] [PubMed] [Google Scholar]
- 25.Kalarus Z, Merkely B, Neužil P, Grabowski M, Mitkowski P, Marinskis G, Erglis A, Kaźmierczak J, Sturmberger T, Sokal A, et al. Pacemaker-based cardiac neuromodulation therapy in patients with hypertension: a pilot study. J Am Heart Assoc. 2021; 10:e020492. doi: 10.1161/JAHA.120.020492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krucoff MW, Mehran R, van Es GA, Boam AB, Cutlip DE. The academic research consortium governance charter. JACC Cardiovasc Interv. 2011; 4:595–596. doi: 10.1016/j.jcin.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol. 2010; 56:1254–1258. doi: 10.1016/j.jacc.2010.03.089 [DOI] [PubMed] [Google Scholar]
- 28.FDA Executive Summary. Circulatory System Devices Panel Meeting. December 5, 2018 General Issues Panel Clinical Evaluation of Anti-Hypertensive Devices. Accessed December 1, 2021. https://www.fda.gov/media/123043/download
- 29.Lauder L, da Costa BR, Ewen S, Scholz SS, Wijns W, Lüscher TF, Serruys PW, Edelman ER, Capodanno D, Böhm M, et al. Randomized trials of invasive cardiovascular interventions that include a placebo control: a systematic review and meta-analysis. Eur Heart J. 2020; 41:2556–2569. doi: 10.1093/eurheartj/ehaa495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, et al. ; Renal Denervation for Hypertension (DENERHTN) I. nvestigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015; 385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 31.Wan SH, Hart M, Hajjar I. A novel measurement index for antihypertensive medication burden and its use. Hypertension. 2009; 54:e135–e136. doi: 10.1161/HYPERTENSIONAHA.109.140681 [DOI] [PubMed] [Google Scholar]
- 32.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, Sharp ASP, Bloch MJ, Basile J, Wang Y, et al. Six-month results of treatment-blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO Trial. Circulation. 2019; 139:2542–2553. doi: 10.1161/CIRCULATIONAHA.119.040451 [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013; 34:2940–2948. doi: 10.1093/eurheartj/eht295 [DOI] [PubMed] [Google Scholar]
- 34.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014; 35:3267–3276. doi: 10.1093/eurheartj/ehu364 [DOI] [PubMed] [Google Scholar]
- 35.Gupta P, Patel P, Štrauch B, Lai FY, Akbarov A, Gulsin GS, Beech A, Marešová V, Topham PS, Stanley A, et al. Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension. 2017; 70:1042–1048. doi: 10.1161/HYPERTENSIONAHA.117.09631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019; 73:e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giorgini P, Weder AB, Jackson EA, Brook RD. A review of blood pressure measurement protocols among hypertension trials: implications for “evidence-based” clinical practice. J Am Soc Hypertens. 2014; 8:670–676. doi: 10.1016/j.jash.2014.07.024 [DOI] [PubMed] [Google Scholar]
- 38.Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circ Res. 2015; 116:1034–1045. doi: 10.1161/CIRCRESAHA.116.303755 [DOI] [PubMed] [Google Scholar]
- 39.Patel HC, Hayward C, Ozdemir BA, Rosen SD, Krum H, Lyon AR, Francis DP, di Mario C. Magnitude of blood pressure reduction in the placebo arms of modern hypertension trials: implications for trials of renal denervation. Hypertension. 2015; 65:401–406. doi: 10.1161/HYPERTENSIONAHA.114.04640 [DOI] [PubMed] [Google Scholar]
- 40.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999; 282:539–546. doi: 10.1001/jama.282.6.539 [DOI] [PubMed] [Google Scholar]
- 41.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005; 46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a [DOI] [PubMed] [Google Scholar]
- 42.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005; 111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B [DOI] [PubMed] [Google Scholar]
- 43.Kario K, Weber MA, Mahfoud F, Kandzari DE, Schmieder RE, Kirtane AJ, Bohm M, Hettrick DA, Townsend RR, Tsioufis KP. Changes in 24-hour patterns of blood pressure in hypertension following renal denervation therapy. Hypertension. doi: 10.1161/HYPERTENSIONAHA.119.13081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahfoud F, Azizi M, Ewen S, Pathak A, Ukena C, Blankestijn PJ, Böhm M, Burnier M, Chatellier G, Durand Zaleski I, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020; 41:1588–1599. doi: 10.1093/eurheartj/ehaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandzari DE, Townsend RR, Bakris G, Basile J, Bloch MJ, Cohen DL, East C, Ferdinand KC, Fisher N, Kirtane A, et al. Renal denervation in hypertension patients: proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv. 2021; 98:416–426. doi: 10.1002/ccd.29884 [DOI] [PubMed] [Google Scholar]
- 46.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, et al. ; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014; 32:1359–1366. doi: 10.1097/HJH.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 47.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020; 75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 48.Stergiou GS, Palatini P, Parati G, O’Brien E, Januszewicz A, Lurbe E, Persu A, Mancia G, Kreutz R; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021; 39:1293–1302. doi: 10.1097/HJH.0000000000002843 [DOI] [PubMed] [Google Scholar]
- 49.Bobrie G, Weber JL, Postel-Vinay N, Menard J, Plouin PF. Teletransmission of home blood pressure monitoring: making it easier. J Hypertens. 2008; 26:1046–1047. discussion 1048. doi: 10.1097/HJH.0b013e3282f76792 [DOI] [PubMed] [Google Scholar]
- 50.Sheikh S, Sinha AD, Agarwal R. Home blood pressure monitoring: how good a predictor of long-term risk? Curr Hypertens Rep. 2011; 13:192–199. doi: 10.1007/s11906-011-0193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016; 10:224–234.e17. doi: 10.1016/j.jash.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, et al. ; Academic Research Consortium. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Eur Heart J. 2018; 39:2192–2207. doi: 10.1093/eurheartj/ehy223 [DOI] [PubMed] [Google Scholar]
- 53.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI). 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018; 137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 54.Lansky AJ, Messé SR, Brickman AM, Dwyer M, van der Worp HB, Lazar RM, Pietras CG, Abrams KJ, McFadden E, Petersen NH, et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: an Academic Research Consortium Initiative. J Am Coll Cardiol. 2017; 69:679–691. doi: 10.1016/j.jacc.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 55.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on Behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018; 138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 56.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012; 33:2403–2418. doi: 10.1093/eurheartj/ehs255 [DOI] [PubMed] [Google Scholar]
- 57.Spitzer E, McFadden E, Vranckx P, Garcia-Garcia HM, Seltzer JH, Held C, de Vries T, Menon V, Brown KJ, Soliman OII, et al. Critical appraisal of contemporary clinical endpoint definitions in coronary intervention trials: a guidance document. JACC Cardiovasc Interv. 2019; 12:805–819. doi: 10.1016/j.jcin.2018.12.031 [DOI] [PubMed] [Google Scholar]
- 58.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlöf B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004; 292:2350–2356. doi: 10.1001/jama.292.19.2350 [DOI] [PubMed] [Google Scholar]
- 59.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012; 59:901–909. doi: 10.1016/j.jacc.2011.11.034 [DOI] [PubMed] [Google Scholar]
- 60.Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014; 35:2224–31b. doi: 10.1093/eurheartj/ehu093 [DOI] [PubMed] [Google Scholar]
- 61.Tsioufis C, Papademetriou V, Dimitriadis K, Tsiachris D, Thomopoulos C, Kasiakogias A, Kordalis A, Kefala A, Koutra E, Lau EO, et al. Effects of multielectrode renal denervation on cardiac and neurohumoral adaptations in resistant hypertension with cardiac hypertrophy: an EnligHTN I substudy. J Hypertens. 2015; 33:346–353. doi: 10.1097/HJH.0000000000000408 [DOI] [PubMed] [Google Scholar]
- 62.Kordalis A, Tsiachris D, Pietri P, Tsioufis C, Stefanadis C. Regression of organ damage following renal denervation in resistant hypertension: a meta-analysis. J Hypertens. 2018; 36:1614–1621. doi: 10.1097/HJH.0000000000001798 [DOI] [PubMed] [Google Scholar]
- 63.Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, Veelken R, Ukena C, Uder M, Böhm M, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015; 33:1261–1266. doi: 10.1097/HJH.0000000000000556 [DOI] [PubMed] [Google Scholar]
- 64.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013; 10:143–155. doi: 10.1038/nrcardio.2013.1 [DOI] [PubMed] [Google Scholar]
- 65.Persu A, Gordin D, Jacobs L, Thijs L, Bots ML, Spiering W, Miroslawska A, Spaak J, Rosa J, de Jong MR, et al. ; European Network COordinating research on Renal Denervation (ENCOReD). Blood pressure response to renal denervation is correlated with baseline blood pressure variability: a patient-level meta-analysis. J Hypertens. 2018; 36:221–229. doi: 10.1097/HJH.0000000000001582 [DOI] [PubMed] [Google Scholar]
- 66.Fatani N, Dixon DL, Van Tassell BW, Fanikos J, Buckley LF. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol. 2021; 77:1290–1299. doi: 10.1016/j.jacc.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B, Laufs U, Neuberger HR, Böhm M. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol. 2013; 167:2846–2851. doi: 10.1016/j.ijcard.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 68.Böhm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, Weber MA, Hoshide S, Patel M, Tyson CC, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019; 40:743–751. doi: 10.1093/eurheartj/ehy871 [DOI] [PubMed] [Google Scholar]
- 69.Böhm M, Tsioufis K, Kandzari DE, Kario K, Weber MA, Schmieder RE, Townsend RR, Kulenthiran S, Ukena C, Pocock S, et al. Effect of heart rate on the outcome of renal denervation in patients with uncontrolled hypertension. J Am Coll Cardiol. 2021; 78:1028–1038. doi: 10.1016/j.jacc.2021.06.044 [DOI] [PubMed] [Google Scholar]
- 70.Kandzari DE, Mahfoud F, Bhatt DL, Böhm M, Weber MA, Townsend RR, Hettrick DA, Schmieder RE, Tsioufis K, Kario K. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020; 76:1410–1417. doi: 10.1161/HYPERTENSIONAHA.120.15745 [DOI] [PubMed] [Google Scholar]
- 71.Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, Pocock S, David S, Patel K, Rao A, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021; 77:2909–2919. doi: 10.1016/j.jacc.2021.04.044 [DOI] [PubMed] [Google Scholar]
- 72.Kindermann I, Wedegärtner SM, Mahfoud F, Weil J, Brilakis N, Ukena J, Ewen S, Linz D, Fahy M, Mancia G, et al. ; Global SYMPLICITY Registry Investigators. Improvement in health-related quality of life after renal sympathetic denervation in real-world hypertensive patients: 12-month outcomes in the Global SYMPLICITY Registry. J Clin Hypertens (Greenwich). 2017; 19:833–839. doi: 10.1111/jch.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmieder RE, Kandzari DE, Wang TD, Lee YH, Lazarus G, Pathak A. Differences in patient and physician perspectives on pharmaceutical therapy and renal denervation for the management of hypertension. J Hypertens. 2021; 39:162–168. doi: 10.1097/HJH.0000000000002592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, Bresnahan BW, Kanninen B, Bridges JF. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013; 16:3–13. doi: 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- 75.van der Wardt V, Harrison JK, Welsh T, Conroy S, Gladman J. Withdrawal of antihypertensive medication: a systematic review. J Hypertens. 2017; 35:1742–1749. doi: 10.1097/HJH.0000000000001405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu J, Liu Y, Zhang L, Zhou L, Li D, Quan H, Zhu L, Hu F, Li X, Meng S, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020; 9:e016804. doi: 10.1161/JAHA.120.016804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harrison TG, Tam-Tham H, Hemmelgarn BR, Elliott M, James MT, Ronksley PE, Jun M. Change in proteinuria or albuminuria as a surrogate for cardiovascular and other major clinical outcomes: a systematic review and meta-analysis. Can J Cardiol. 2019; 35:77–91. doi: 10.1016/j.cjca.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 78.Townsend RR, Walton A, Hettrick DA, Hickey GL, Weil J, Sharp ASP, Blankestijn PJ, Böhm M, Mancia G. Review and meta-analysis of renal artery damage following percutaneous renal denervation with radiofrequency renal artery ablation. EuroIntervention. 2020; 16:89–96. doi: 10.4244/EIJ-D-19-00902 [DOI] [PubMed] [Google Scholar]
- 79.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, et al. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2017; 38:93–100. doi: 10.1093/eurheartj/ehw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahfoud F, Mancia G, Schmieder R, Narkiewicz K, Ruilope L, Schlaich M, Whitbourn R, Zirlik A, Zeller T, Stawowy P, et al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol. 2020; 75:2879–2888. doi: 10.1016/j.jacc.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 81.Fengler K, Rommel KP, Lapusca R, Blazek S, Besler C, Hartung P, von Roeder M, Kresoja KP, Desch S, Thiele H, et al. Renal denervation in isolated systolic hypertension using different catheter techniques and technologies. Hypertension. 2019; 74:341–348. doi: 10.1161/HYPERTENSIONAHA.119.13019 [DOI] [PubMed] [Google Scholar]
- 82.Ott C, Franzen KF, Graf T, Weil J, Schmieder RE, Reppel M, Mortensen K. Renal denervation improves 24-hour central and peripheral blood pressures, arterial stiffness, and peripheral resistance. J Clin Hypertens (Greenwich). 2018; 20:366–372. doi: 10.1111/jch.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lurz P, Kresoja KP, Rommel KP, von Roeder M, Besler C, Lücke C, Gutberlet M, Schmieder RE, Mahfoud F, Thiele H, et al. Changes in stroke volume after renal denervation: insight from cardiac magnetic resonance imaging. Hypertension. 2020; 75:707–713. doi: 10.1161/HYPERTENSIONAHA.119.14310 [DOI] [PubMed] [Google Scholar]
- 84.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol. 2002; 40:126–132. doi: 10.1016/s0735-1097(02)01931-9 [DOI] [PubMed] [Google Scholar]
- 85.Grassi G, Seravalle G, Trevano FQ, Dell’oro R, Bolla G, Cuspidi C, Arenare F, Mancia G. Neurogenic abnormalities in masked hypertension. Hypertension. 2007; 50:537–542. doi: 10.1161/HYPERTENSIONAHA.107.092528 [DOI] [PubMed] [Google Scholar]
- 86.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012; 60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870 [DOI] [PubMed] [Google Scholar]
- 87.Ukena C, Becker N, Pavlicek V, Millenaar D, Ewen S, Linz D, Steinberg JS, Böhm M, Mahfoud F. Catheter-based renal denervation as adjunct to pulmonary vein isolation for treatment of atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2020; 38:783–790. doi: 10.1097/HJH.0000000000002335 [DOI] [PubMed] [Google Scholar]
- 88.Verloop WL, Spiering W, Vink EE, Beeftink MM, Blankestijn PJ, Doevendans PA, Voskuil M. Denervation of the renal arteries in metabolic syndrome: the DREAMS-study. Hypertension. 2015; 65:751–757. doi: 10.1161/HYPERTENSIONAHA.114.04798 [DOI] [PubMed] [Google Scholar]
- 89.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995; 96:1897–1904. doi: 10.1172/JCI118235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, Böhm M, Lambert EA, Krum H, Sobotka PA, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013; 168:2214–2220. doi: 10.1016/j.ijcard.2013.01.218 [DOI] [PubMed] [Google Scholar]
- 91.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013; 162:189–192. doi: 10.1016/j.ijcard.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 92.Pocock SJ, Bakris G, Bhatt DL, Brar S, Fahy M, Gersh BJ. Regression to the mean in SYMPLICITY HTN-3: implications for design and reporting of future trials. J Am Coll Cardiol. 2016; 68:2016–2025. doi: 10.1016/j.jacc.2016.07.775 [DOI] [PubMed] [Google Scholar]
- 93.Verbeeck J, Spitzer E, de Vries T, van Es GA, Anderson WN, Van Mieghem NM, Leon MB, Molenberghs G, Tijssen J. Generalized pairwise comparison methods to analyze (non)prioritized composite endpoints. Stat Med. 2019; 38:5641–5656. doi: 10.1002/sim.8388 [DOI] [PubMed] [Google Scholar]
- 94.Kandzari DE, Hickey GL, Pocock SJ, Weber MA, Böhm M, Cohen SA, Fahy M, Lamberti G, Mahfoud F. Prioritised endpoints for device-based hypertension trials: the win ratio methodology. EuroIntervention. 2021; 16:e1496–e1502. doi: 10.4244/EIJ-D-20-01090 [DOI] [PMC free article] [PubMed] [Google Scholar]