Abstract
Due to the coronavirus disease 2019 (COVID-19) pandemic, the development of antiviral drugs has attracted increasing attention. Clinical antiviral drugs show weak solubility, low bioavailability, adverse side effects, or only limited targets. With the advancement of nanotechnology and material science, biosafety nanomaterials have been constructed for drug delivery systems of antiviral disease therapy, such as liposomes, polymers, gold nanoparticles, and graphene. These nanodrug systems can either deliver synthesized antiviral drugs siRNA/miRNA and small molecular compounds, deliver bioactive large molecular drug proteins and mRNA, or show antiviral activity by themselves. Nanodelivery systems could effectively enhance the efficiency of antiviral drugs by increasing drug loading and host cell uptake with a small size and high specific surface area. This review focused on the biosafety nanomaterials used for antiviral therapy and discussed the options for the design of antiviral drugs in the future.
Keywords: Antivirus therapy, Nanodelivery systems, Compound drugs, Biologically active molecule drugs
1. Introduction
Viral infection is a serious threat to global public health. It is estimated that viruses cause approximately two million deaths each year [1]. Human immunodeficiency virus (HIV), hepatitis virus, varicella zoster virus (VZV), human papillomavirus (HPV), influenza A virus (IAV), herpes simplex virus (HSV) and coronaviruses are the main pathogenic viruses associated with human morbidity and mortality. They can spread through the nose, mouth, eyes, skin, etc. Vaccines can protect people from viral infection. However, with the spread of viruses and their accelerated rate of mutation, there are currently no sufficiently effective vaccines to combat all of the various viruses and their variants, as best exemplified by the coronavirus disease 2019 (COVID-19) global pandemic. Therefore, the development of antiviral drugs is an effective way to combat viral infections [1].
Currently, drugs such as enfuvirtide, maraviroc, indinavir, acyclovir, peptides and nucleic acids have been used in the treatment of viral infection in the clinic. Moreover, a number of natural small molecule drugs have been applied in clinical studies, such as curcumin, a natural phenolic compound that has been shown to have antiviral activity [2]. Biologically active antiviral drugs are also being investigated. The antiviral mechanisms of small molecule drugs and biologically active molecules are different. The antiviral mechanism of small molecule drugs is mostly to inhibit virus infection in the body by interfering with virus adsorption and penetration, while biologically active molecules inhibit virus infection by interfering with virus DNA translation and transcription in the body. Although all of these drugs have shown excellent antiviral activity in in vitro studies, there are still many issues that limit their efficacy in the clinic, such as poor solubility, poor stability during storage or application, low bioavailability, the emergence of medication resistance, potential adverse side effects or toxicity [3]. With the development of nanomaterials, these drug delivery systems have shown great potential for antiviral drug encapsulation, stability and activity [4]. Accordingly, the application of nanobiosafety materials is becoming increasingly widespread.
Recently, nanoparticles such as liposomes (LNP) and polymers have attracted attention due to their wide range of functions, low biotoxicity, high biocompatibility and good biodegradability. Due to differences in the composition, structure and functional properties of nanodelivery systems, researchers have designed nanocarrier systems suitable for different antiviral drugs. The small particle size and high specific surface area of antiviral nanodelivery carriers facilitate increased drug loading and entry into host cells, improving the efficacy of antiviral drugs (Table 1 ). Moreover, there are metal-based and carbon-based nanomaterials that have inherent antiviral properties. Some of them have shown antiviral efficiency by stopping viruses from entering host cells for replication or inhibiting virus replication [5]. Therefore, this review summarizes the use of nanomedicines in antiviral therapy using different material-based drug delivery systems developed in recent years (Fig. 1 ) and it discusses the prospects and challenges of antiviral nanostructures in the future.
Table 1.
LNP- and polymer-based nanodelivery systems used for antiviral therapy.
| Drug delivery system | Loading drugs | Mechanism of action | References |
|---|---|---|---|
| LNP* | —— | LNP containing heparan octasaccharide sulfate can binds to pathogens and inhibits herpes simplex virus (HSV) infection. | [9] |
| —— | Cationic LNP containing stearylamine (SA) can inhibit recombinant baculovirus (BV) infection | [11] | |
| Anti-MARV nucleoprotein (NP)–targeting small interfering RNA (siRNA) | Targeting the Marburg virus (MARV) nucleoprotein. | [12] | |
| Lymphocyte function-associated antigen-1 and anti-CCR5 siRNA | Reducing the expression of CCR5, lightening HIV infectivity and reducing viral load in mice. | [13] | |
| Polymers | Chloroquine diphosphate | Achieving an effective therapeutic concentration in the cell to effectively improve the antiviral activity of the drug. | [15] |
| Sofosbuvir (SOF) | Reduced the release rate of SOF to achieve the sensitivity and high entrapment efficiency of drug inside the cells. | [16] | |
| Encapsulated miR323a in the core and favipiravir in the exterior layer | Combining treatment with polymersomes containing PBA functional groups by synergistic impacting against H1N1 virus infection. Combined administration for H1N1 virus by miR323a (interfering with RNA virus polymerase and binding to the PB1 gene) and favipiravir (a pseudopurine nucleotide). |
[17] | |
| α4β7 monoclonal antibodies and tipranavir | Delivering antiretroviral drugs and monoclonal antibodies to HIV host cells to reduce their viral load. | [23] |
*LNP: liposome.
Fig. 1.
Summative scheme of antiviral nanosystems.
2. LNP-based antiviral nanodelivery system
LNP is often widely used as a US FDA-approved drug carrier for gene and drug delivery, nanotechnology, small interfering RNAs (siRNAs), and tumor therapy. LNP has been extremely well researched over the past decades, with 12,627 papers dealing with LNP from 2011 to 2021 based on data from the Science Citation Index extended database and 1,068 papers on LNP as carriers alone, with 31 of these papers related to viruses. The British hematologist Alec D Bangham first discovered LNP in 1961 at the Babraham Institute in Cambridge [6] and first recognized phospholipids as closed bilayers in aqueous systems [7]. The use of LNP was also documented as early as 1973, when the use of LNP encapsulated with ethylenediaminetetraacetic acid and diethylenetriaminepentaacetic acid to alleviate plutonium poisoning increased urinary excretion of plutonium and prolonged survival in mice [8].
2.1. LNP for compounds delivery
Several small molecule compound drugs have been approved for the treatment of human infectious diseases. Iodoside was the first drug approved for the treatment of HSV infection, primarily for human herpetic keratoconjunctivitis, but it cannot be used for systemic antiviral therapy because it cannot distinguish between viral and host cell function. Acyclovir was the first antiviral drug to be successfully administered intravenously. It inhibits DNA viruses such as herpesvirus, VZV and adenovirus but is ineffective against many RNA viruses; currently, it is used to treat herpes keratitis, herpes encephalitis and herpes zoster. It is also toxic and has effects on liver and kidney function and bone marrow. Acyclovir is the drug of choice for the treatment of herpesvirus infections but has the disadvantage of low oral bioavailability and the development of drug resistance with long-term use, so there is an urgent need to develop new antiviral drugs with better effectiveness and less toxicity.
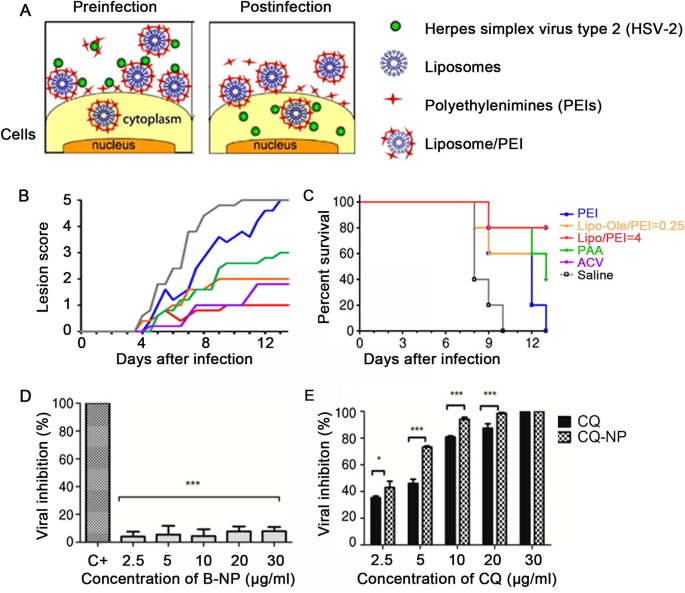
Therefore, researchers have conducted numerous studies to address this feature of LNP. Hendricks et al. [9] generated LNP containing heparan octasaccharide sulfate, which binds to pathogens and inhibits HSV infection. Maitani et al. [10] investigated a novel application by developing LNP without antiviral drugs but containing branched polyethyleneimine (Fig. 2 A-C). When applied topically, these particles inhibited HSV-2 by a unique mechanism. Tahara [11] demonstrated that cationic LNP containing stearylamine can inhibit recombinant baculovirus infection without preloading with an active drug component.
Fig. 2.
Construction of liposomes for antiviral therapy. A) Schematic representation of the antiviral mechanism of liposome/PEI. [10]; B and C) Therapeutic effects of PEI, Lipo–Ole/PEI = 0.25, Lipo/PEI = 4, PAA, ACV and saline on the severity of HSV-2 infection in mouse genital model. [10]; D and E) Antiviral activity of blank nanoparticles (B-NP) (D), chloroquine (CQ), and chloroquine-loaded PLA nanoparticles (CQ-NP) (E) [15]. Copyright 2013 J. Controll Release & 2018 Pharmaceutics.
Most in vivo gene editing studies have relied on adenovirus (AAV) for local delivery of CRISPR components to the retina, skeletal muscle or liver. However, the application of AAV is limited by its low carrying capacity, immune response, hepatotoxicity and lack of cellular targeting. In contrast, LNP have the advantages of low toxicity and low immunogenicity and are suitable for the delivery of small molecule nucleic acids. Thi et al. [12] designed an LNP (NP-718m-LNP) encapsulated with siRNA targeting Ebola virus (MARV), which was shown to inhibit MARV replication in vitro and exhibited broad-spectrum activity against three different MARV strains in an infected guinea pig model. Compared with the control group, viral load and viral transmission were reduced in the organs of the group treated with LNP. The RNAi-based nanoparticle treatment modality was able to downgrade the expression of viral receptors in cells. Lymphocyte function-associated antigen-1 (LFA-1) is a common ligand that can target T cells and macrophages. Based on this background, Kim et al. modified LFA-1 on the surface of LNP and wrapped anti-CCR5 siRNA into the interior of LNP. This design was able to reduce the expression of CCR5, thereby lightening HIV infectivity and reducing viral load in mice [13].
2.2. LNP for biological active molecules delivery
VZV is a neurophilic and lymphophilic alpha herpesvirus that causes varicella and herpes zoster (HZ). The approved live attenuated VZV vaccine is ineffective, whereas the recombinant subunit vaccine provides high protection rates and long protection periods. Wui et al. [14] developed a new adjuvant system, CIA09A, which consists of cationic LNP, Toll-like receptor 4 (TLR4) agonist deacylated low-fat sugar and cholagogue saponin fraction QS-21. The experimental results demonstrated that the CIA09A-adjuvanted IgE vaccine was highly effective in provoking humoral and cellular immune responses against the recombinant IgE protein and the VZV mouse model. In addition, the frequency of IgE-specific activation of the multifunctional CD4+ T cell cytokines interferon (IFN)-c, tumor necrosis factor (TNF)-α and interleukin (IL)-2 was significantly increased after adjuvant vaccine immunization of mice.
3. Polymers-based antiviral nanodelivery system
Among the antiviral drug delivery systems, polymers have been widely used due to their unique structural characteristics (Table 1). They are synthesized by solvent evaporation, spontaneous emulsification and solvent diffusion, and made of biodegradable polymers (e.g., poly(lactide-co-glycolide), PLGA) or natural polymers (e.g., chitosan and alginate). Amphiphilic block copolymers are a major manifestation of polymers as nanodrug delivery systems. Polymer micelles are formed when amphiphilic polymer molecules associate spontaneously to form a core–shell structure in an aqueous medium. The core of the hydrophobic micelles is surrounded by a hydrophilic block shell, such as polyethylene glycol (PEG). The hydrophobic core supports poorly water-soluble drugs. Meanwhile, the hydrophilic shell stabilizes the core, prolongs the blood circulation time, and increases accumulation in tumor tissues. Based on this, researchers have further developed smart polymers (stimulus-responsive polymers) that can change their physical and chemical properties in response to environmental stimuli by changing their conformation. The stimulations include physical (light and heat), chemical (pH) and biological molecules (enzymes). The versatility of polymers and their ease of combination makes it possible to achieve more accurate and programmable drug delivery in specific environments.
3.1. Polymers for compounds delivery
Polymers can protect small molecule compound drugs from degradation and immune escape. Chloroquine diphosphate (CQ) has attracted much attention due to its anti-HSV-1 virus activity, but it is difficult to achieve an effective therapeutic concentration in the cell. CQ-loaded D,L-poly(lactic acid) nanoparticles were prepared by an improved emulsification-solvent evaporation method (Fig. 2D and E), which effectively improved the antiviral activity of the drug [15]. To achieve the sensitivity and high entrapment efficiency of drug inside the cells, Shaymaa Shawky et al. synthesized chitosan nanoparticles followed by polyvinyl alcohol nanoparticles and loaded sofosbuvir (SOF, a hepatitis C virus inhibitor) onto each of the two carriers, which showed that the release rate of SOF could be effectively reduced [16].
IAV is seasonal and highly infectious, and although research and development of related drugs has been ongoing, the rapid mutation of the virus and the development of drug resistance have kept the problem of influenza unresolved. Against this background, Chun et al. [17] proposed amphiphilic polymeric delivery vehicles (PBASomes) modified by conjugation with phenylboronic acid (PBA, which interacts with sialic acid residues on the cell surface through the formation of hydrogen bonds between diol groups [18], [19] and which plays an important role in the attachment of IAV and the binding of PBA [20]) could be used to deliver miR323a (an intracellular drug that interferes with RNA virus polymerase and binds to the PB1 gene of the H1N1 virus [21]) and favipiravir (a pseudopurine nucleotide) into the host cell simultaneously, and through this combined administration thereby improving antiviral efficacy. The polymer was self-assembled from a mixture of a methoxy-poly (ethylene glycol)-block-poly(phenylalanine) (mPEG-b-Phe) amphiphilic copolymer and a PBA conjugated polymer (PBA-PEG-b-pPhe). MiR323a and favipiravir were encapsulated in the core and bilayer of PBASomes, respectively. Their results showed that miR323a and favipiravir significantly enhanced the therapeutic effect of miR323a and favipiravir against H1N1 virus infection. In addition, RME induction and cell structure simulation demonstrated that PBASomes had the advantages of good intracellular uptake, slow release and low cytotoxicity, and could be used as efficient combination drug carriers for IAV treatment.
3.2. Polymers for biologically active molecule delivery
A neutralizing monoclonal antibody can specifically neutralize a virus and prevent it from entering the cell to proliferate. It can be used as short-term prevention among high-risk populations and for the treatment of diseases after viral infections such as HIV. During combined antiretroviral therapy of HIV, it has been found that intestinal-related lymphoid tissues can quickly establish HIV virus reservoirs within HIV-infected patients, which can lead to drug resistance that is hard to treat [22]. Cao designed a core–shell nanosystem comprised of a PLGA core and a phospholipid bilayer shell [23]. For coupling α4β7 monoclonal antibodies (α4β7-LCNPs), researchers coated PEG on the surface of the shell. To prevent infected cells from producing new HIV particles in GALT, protease inhibitors were also encapsulated in LCNPs. Additionally, the nanosystem was loaded with tipranavir (TPV). The results showed that the LCNP system can deliver antiretroviral drugs and monoclonal antibodies to HIV host cells to reduce their viral load.
4. Other nanomaterials for antivirus
In addition to the two main classes of nanocarriers, LNP and polymers, researchers have proposed a number of other structured nanocarriers for the delivery of viral infections. Moreover, some nanomaterials showed antiviral effects themselves (Table 2 ). For instance, silver nanoparticles (AgNPs) are able to bind to viral surface proteins, thus preventing the virus from entering the cell by contacting receptor proteins on the surface of the cell membrane [24]. For the purpose of this review, we classified these carriers into metal nanocarriers, carbon-based nanocarriers, protein carriers and hybrid nanocarriers.
Table 2.
Other nanomaterials used for antiviral therapy.
| Drug delivery system | Loading drugs | Mechanism of action | References |
|---|---|---|---|
| AgNPs | —— | Inhibiting the interaction between viruses and cell membrane receptors | [25] |
| —— | Inactivating viruses by denaturing surface proteins containing cysteine and methionine residues on the viral capsid | [26] | |
| AuNPs | —— | Hindering the membrane fusion of the host cell with the virus | [29], [30], [31] |
| TiO2 | Nucleic acids | Protecting partial modification of oligonucleotides and inter-nucleotide bonds | [32] |
| Carbon dots | —— | Modulating IFN to inhibit viral replication at an early stage of infection | [38] |
| Albumin | Saponins | Reducing plasma histone H4 and NETosis-associated factors and alleviating SARS-CoV-2 ICU patients' PBMCs in SREBP2-mediated systemic inflammation | [40] |
| Exosomes | Interferon-induced transmembrane protein 3 (IFITM3) | Suppressing ZIKV viremia by a 2-log reduction in pregnant mice | [50] |
| HIV-1 promoter | Suppressing continuously epigenetic inheritance of HIV-1 | [48] | |
| Hybrid nanocarriers | Zanamivir | Binding with IAV virus and inhibiting viral infection | [51] |
IFN: Interferon; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; PBMC: Peripheral blood mononuclear cell; ZIKV: Zika virus; HIV: Human immunodeficiency virus; IAV: Influenza A virus.
4.1. Metal nanocarriers
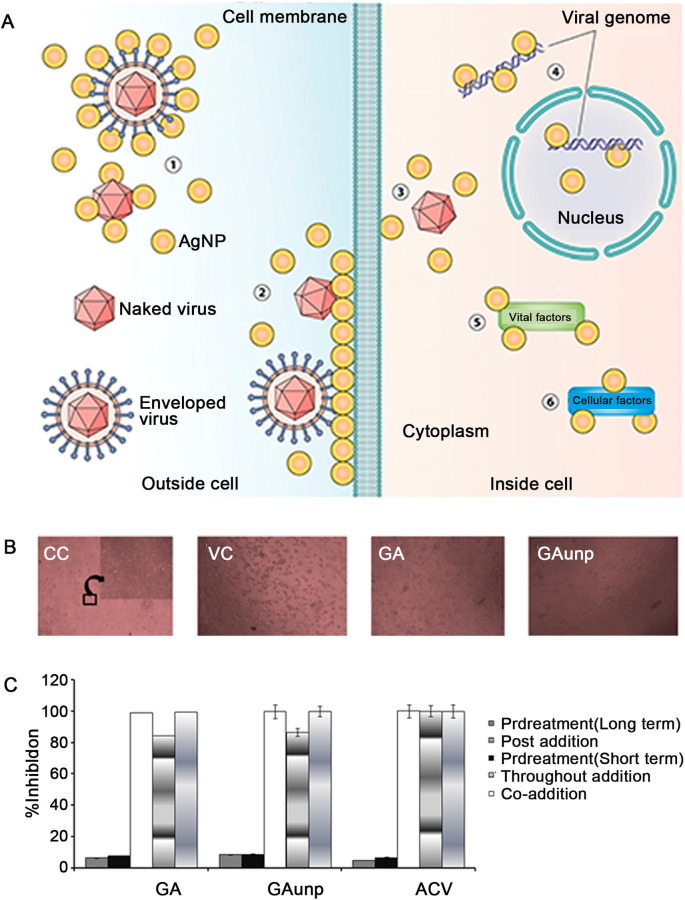
AgNPs have shown promising efficacy in the antibacterial and antiviral fields [25]. AgNPs can bind viral surface proteins and inhibit the interaction between viruses and cell membrane receptors (Fig. 3 A). It has also been reported that AgNPs can inactivate viruses by denaturing surface proteins containing cysteine and methionine residues on the viral capsid. For instance, AgNPs smaller than 10 nm have been shown to interact with the sulfur-containing residues of the gp120 glycoprotein, which is distributed on the lipid membrane of HIV-1 virus and can prevent the virus from binding to the CD4 receptor site on the host cell, thereby suppressing viral infection [26]. However, it is essential to note that the antiviral properties of AgNPs are determined by their specific particle structure in solution and not by the silver ions themselves [27].
Fig. 3.
Antiviral mechanism of AgNPs and antiviral activity of GAunps. A) Potential antiviral mechanism of AgNPs. [26]; B) Optical microscopic images captured from cell control (CC), virus control (VC), infected cells treated with gallic acid (GA) and gold nanoparticles (GAunps) [30]; C) Effect of GAunp treatment over time on HSV-1 [30]. Copyright 2005. J Nanobiotechnology & 2018 Mater Sci. Eng. C Mater Biol. Appl.
Based on this background, researchers have successively applied silver nanoparticles with different structures for antiviral therapy. Lin et al. [28] designed zanamivir-modified AgNPs (Ag@ZNV) for the inhibition of H1N1 virus neuraminidase activity. Moreover, Ag@ZNV inhibited caspase-3-mediated apoptosis through ROS production, confirming that Ag@ZNV could effectively reduce apoptosis due to H1N1 infection.
Gold nanoparticles (AuNPs) are less toxic to healthy cells and more conducive to translation to clinical applications than AgNPs. In fact, researchers have successfully demonstrated that AuNPs could inhibit virus entry into host cells by interacting with hemagglutinin and oxidizing the disulfide bond of this glycoprotein, leading to its inactivation and thereby hindering the membrane fusion of the host cell with the virus. Particularly for pandemic viruses with surface proteins capable of rapid mutation, new treatment strategies for targeted hemagglutinin are more applicable than conventional therapies [29]. Moreover, the antiviral activity of AuNPs correlates with the surface area exposed, with the larger the surface area, the higher the antiviral activity. Thus, the size and modality of these AuNPs play an important role in their antiviral activity.
Chattopadhyay et al. [30] prepared highly monodisperse spherical gold nanoparticles for the inhibition of HSV. The experimental results showed that the monodisperse gold nanoparticles effectively prevented HSV from attaching and entering Vero cells, and their cytotoxicity was lower than that of acyclovir (Fig. 3B and C). Kim et al. [31] found that porous AuNPs were more effective in inhibiting influenza A virus infection than nonporous AuNPs. This was related to the higher surface area of the porous material, with the higher surface area facilitating the interaction of AuNPs with the envelope, thereby increasing their antiviral activity.
Titanium-based nanoparticles have become a popular material for nanodelivery due to their low toxicity, good stability and cell membrane permeability. In recent years, nucleic acid delivery has become a major area of research in antiviral therapy. How to achieve efficient delivery of nucleic acids has become an issue of great interest to researchers in various countries. The ability of titanium dioxide nanoparticles to protect the modified portion of oligonucleotides has attracted attention. Levina et al. [32] developed TiO2PL-ODN, a crystalline form of titanium dioxide nanoparticles containing oligonucleotides. They first synthesized phosphodiester-modified oligonucleotides, which were then combined with titanium dioxide to obtain TiO2PL-ODN nanoparticles. The experimental results showed that the mouse-targeted oligonucleotides in the nanocomposites had the ability to interact with complementary RNAs, which verified that titanium dioxide nanoparticles provide protection against partial modification of oligonucleotides and internucleotide bonds in nanocomposites.
4.2. Carbon-based nanomaterials
The DC-SIGN (dendritic cell (DC)-specific intercellular adhesion molecule-grabbing nonintegrin) receptor is a critically important cohesion receptor through which viruses can efficiently recognize glycoproteins (GPs) containing mannose and fucose in a multivalent manner and adhere to the cell surface to enter the host cell. Thus, blocking this receptor at an early stage of infection to inhibit entry of the pathogen enables the design of new antiviral drugs. To achieve this, researchers have designed various multivalent scaffold materials. Fullerenes are also considered antiviral scaffold materials due to their ability to functionalize multiple molecules on their convex surface. Antonio Muñoz et al. [33] synthesized spherical topological molecules formed from hexadecane adducts of fullerenes by introducing 120 sugar units using efficient CuAAC click chemistry, with the centrally located fullerene molecule covalently linked to 12 surrounding fullerene molecules, each of which was sequentially assigned 10 monosaccharides. Infection analysis showed that these nanospheres effectively inhibited artificial Ebola virus infection of cells and suppressed virus concentrations to half of the subnanomolar range.
In addition to adhesion to host cells through recognition of GPs, viruses can also bind heparan sulfate (HS) on the surface of host cells [34] to achieve attachment of viral particles and entry into susceptible cells (HS is a negatively charged linear polymer consisting of alternating hexoses and glucosamine, which are sulfated at different locations). [35]. Whereas graphene has excellent thermal conductivity, it has been demonstrated that graphene can be used in photothermal therapy and photodynamic therapy for bacterial inactivation [36]. Therefore, the functionalized modification of graphene with HS not only enables the capture of viruses but also their inactivation by photothermal conversion. Archana R. Deokar et al. [37] designed a sulfonated magnetic nanoparticle prepared using functionalized reduced graphene oxide (SMRGO) to achieve the capture and photothermal inactivation of HSV-1 viruses (Fig. 4 A-C).
Fig. 4.
Construction and antiviral activity of carbon-based materials. A) Stepwise preparation of SMRGO [37]; B) Cell cytotoxicity assay; C) Relative percentage of cell infection before and after near-infrared (NIR) irradiated photothermal treatment with Magnetic oxide nanoparticles (MNPs) and SMRGO [37]; D&E) Replication process of porcine reproductive and respiratory syndrome virus (PRRSV) (D and E) in the absence and presence of 0.125 mg/mL CDs. The virus titer of intracellular (D) and the virus titer of supernatant (E) [38]. Copyright 2017 Bioconjug. Chem. & 2016 Carbon.
Surface-functionalized carbon dots, because they can modulate the response of antiviral type I interferon (IFN), can play a role in inhibiting viral replication at an early stage of infection (Fig. 4D and E) [38]. Curcumin (CCM), a polyphenolic compound obtained from turmeric roots, has received wide attention in several fields due to its antioxidant, antiviral, anti-inflammatory and antibacterial functions. Free CCM is insoluble in physiological media, and its bioavailability in vivo is poor, preventing its full therapeutic effect. Therefore, Ting et al. [39] prepared antiviral cationic carbon dots (CCM-CDs) using CCM as a precursor, thereby enhancing the bioavailability of CCM and achieving synergistic antiviral effects. CCM-CDs first altered the protein structure on the surface of porcine epidemic diarrhea virus (PEDV), thereby inhibiting the entry of PEDV; after this, CCM-CDs further suppressed the synthesis of viral negative-strand RNA, viral outgrowth and the accumulation of reactive oxygen species (ROS) in cells infected by virus, thereby inhibiting the progression of the infection process.
4.3. Albumin
Albumin is the most abundant plasma protein and it plays a crucial role in regulating the colloid osmotic pressure of plasma and transporting various lipid-soluble endogenous compounds. Over the past decades, albumin has been widely used as a nanodrug carrier due to its better biocompatibility, low immunogenicity and wide availability. A wide range of drugs can be bound to albumin via covalent interactions, electrostatic interactions or hydrophobic interactions. A prime example is the approval of albumin paclitaxel by the US FDA in 2005 for the treatment of breast cancer. In addition, different sizes of albumin nanoparticles (20–300 nm) can be prepared under mild conditions, thus considerably expanding their application range. For example, Park et al. [40] designed PEGylated nanoparticle albumin-bound (PNAB)-steroidal ginsenoside derivatives to modulate the hyperinflammatory response and sepsis in critically ill patients with COVID-19. Saponins have a steroid-like structure and are able to inhibit TLR-4-mediated systemic inflammatory responses and they possess biological activities, including immunostimulant and antioxidant effects [41]. Experimental data showed that the application of PNAB-steroidal ginsenosides effectively reduced plasma histone H4 and NETosis-associated factors and alleviated PBMCs in ICU patient with COVID-19 in SREBP2-mediated systemic inflammation (Fig. 5 A and B).
Fig. 5.
Construction of hybrid nanostructure PNAB-ginsenosides and Hetero-MNB for antiviral therapy. A) Scheme illustration of PNAB-ginsenosides [40]; B) The time-course survival rate of CLP-operated septic mice models after the treatment of PNAB-Rg6 and PNAB-Rgx365 [40]; C) ①&② Design and synthesis of a heteromultivalent nanobowl (Hetero-MNB) for influenza A virus (IAV) inhibition. ③ Proposed binding patterns between IAV and the Hetero-MNB [51]; D-F) Inhibition of influenza A virus (IAV) infection by the heteromultivalent topography-matching nanostructure [51]. Copyright 2021 Biomaterials & Sci. Adv.
4.4. Exosomes
Exosomes are nanoscale (40–100 nm) vesicles secreted by cells that play an important role in intercellular material transport and signaling [42]. Exosomes are similar in size and function to synthetic nanostructures, but as natural endogenous transport carriers, they have the advantages of low toxicity, nonimmunogenicity and good penetration and therefore may become more viable drug delivery vehicles [43]. In addition, exosomes can be extracted from patient fluids or cells and they have a higher biosafety profile than other nanodelivery vehicles [44]. Additionally, exosomes carrying cell surface molecules have a high adsorption capacity, are able to overcome various biological barriers and are naturally targeted, making exosome delivery systems well suited as delivery vehicles for antiviral therapy [45], [46], [47].
To achieve long-term suppression of HIV-1 virus and to reduce the side effects of treatment, Surya Shrivastava et al. [48] designed an HIV-1 promoter targeting zinc finger protein (ZFP-362), which was integrated into the active region of DNA methyltransferase 3a and could continuously suppress epigenetic inheritance of HIV-1. The modified cells produced exosomes containing RNA encoding this HIV-1 suppressor protein. The results show that this HIV-1 suppressor can effectively inhibit viral gene expression, demonstrating the potential of exosomes as a vehicle for the delivery of specific gene epigenetic regulators. In addition, it has been shown that exosomes can cross the placental barrier and build a communication bridge between mother and fetus [49]. Based on this, Zou et al. [50] designed an exosome (IFITM3-Exos) containing IFITM3 (interferon-inducible transmembrane protein 3) for the treatment of Zika virus (ZIKV virus, which causes neurological complications and fetal defects) infection. The results showed that exosomes delivered IFITM3 protein across the placental barrier, significantly inhibited ZIKV expression and alleviated viremia in major fetal organs.
4.5. Hybrid nanocarriers
Although, in the above discussion of carriers, we have detailed their respective structural characteristics and therapeutic advantages, single structural types of carriers tend to have their own drawbacks. Therefore, researchers have developed the concept of hybrid nanocarriers to maximize the therapeutic effects.
The binding of viral glycoproteins to receptors on the surface of cell membranes is responsible for the infection of organisms with viruses. In the case of IAV, hemagglutinin and neuraminidase (NA) on its surface can bind to sialic acid receptors on the cell membrane for recognition and thus enter the host cell. For this reason, researchers have designed polysialylated structures with synthetic scaffolds for the inhibition of IAV infection. However, different IAV subtypes do not bind to cells in the same way, and it is difficult to achieve broad-spectrum inhibition of IAV infection with a common trimeric structure. In contrast, cell membranes are highly stable self-assembling structures and naturally possess viral binding receptors, which are ideally suitable for the design of inhibitors with multivalent binding capacity. Based on this background, Nie et al. [51] designed and synthesized a heteropolyvalent topological nanostructure based on host cell membranes that acted as a sialic acid donor and loaded it with zanamivir as an NA inhibitor to achieve efficient binding of the nanosystem to the IAV virus and inhibit viral infection (Fig. 5C–F).
5. Conclusion and discussion
Advances in biomaterials promote the development of nanomedicine. In the above discussion, it is easy to see that each type of nanodrug delivery system has its own advantages. LNPs, which are mainly formed from phospholipids, are nontoxic, biocompatible and biodegradable and can also reduce the toxic effects of carrier molecules, which means they are particularly suitable for oral or other routes of drug delivery. Polymers have various compositions, sizes, morphologies and surface properties that can be adjusted by applying different ingredients and manufacturing methods for specific applications. Tailoring polymers can improve organ selectivity by introducing targeting groups. Meanwhile, some inorganic nanoparticles, such as AgNPs and AuNPs, can bind to viral surface receptors and prevent viruses from entering the host cell. However, it is important to note that LNP has been shown to induce immune reactions on several previous occasions, suggesting that potential immune reactions should be considered. In addition, although inorganic nanoparticles or nanodelivery systems have good antiviral activity, they are difficult to degrade in living organisms and tend to accumulate in the body, causing organ toxicity. In contrast, cationic polymers can bind to negatively charged cell membranes to increase permeability and facilitate endocytosis, but they also show cytotoxicity by damaging the cell membranes and reducing the DNA release rate.
Therefore, some factors need to be considered when developing nanodrugs for antiviral therapy. First, the choice of nanodelivery systems depends on the characteristics of the antiviral drug. For example, the activity of the antiviral drug suramin is often limited by its difficulty in penetrating cell membranes and poor cellular internalization, and the use of cationic LNP can overcome this obstacle very well. To inhibit the adhesion of LNP to mucus and make it easier to pass through epithelial cells, LNP can be modified with PEG to improve their molecularly neutral nature. Second, as a drug delivery vehicle, the efficiency of drug release at the targeted site is also a key problem that needs to be considered. Nucleic acid drugs, for example, are of great interest because of their ability to act directly on disease-causing target genes or target mRNAs to treat disease at the genetic level. However, nucleic acid drugs are susceptible to degradation by RNase enzymes in vivo, and it is difficult to achieve lysosomal escape, resulting in a failure to achieve effective drug concentrations at specific target sites. Multitailed ionizable phospholipids can facilitate membrane transformation in acidic endosomal environments and subsequently release nucleic acid drugs from endosomes [52]. Finally, the development of universal vectors for different virus species also needs to be considered. With so many different types of viruses and variants, a single antiviral system is no longer the answer to today's pandemic dilemma, and the development of universal antiviral nanodelivery systems to achieve broad-spectrum antiviral therapy is a major trend for the future.
Acknowledgements
This work was supported by the National Key Research & Development Program of China (No. 2021YFA1201000), the Government Guided Local Science and Technology Development Fund Projects of Hebei Province (No. 216Z2403G), Natural Science Foundation of Hebei Province (No. B2019201449, H2019201466), the Priority Strategy Project of the Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education (ts2020003), and the Hebei Province “Three Three Three Talents Program” (A202003001).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Li Wang: Conceptualization, Data Curation, Writing – Original Draft. Zhaoshuo Wang: Data Curation, Writing – Original Draft. Lingzhi Cao: Writing – Original Draft. Kun Ge: Funding Acquisition, Supervision, Writing – Review & Editing.
References
- 1.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29(3):695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teymouri M., Pirro M., Johnston T.P., Sahebkar A. Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. BioFactors. 2017;43(3):331–346. doi: 10.1002/biof.1344. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 2002;1(1):13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 4.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Vukovic L., Tapparel C., Král P., Krol S., Lembo D., Stellacci F., et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17(2):195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 5.Huang X., Xu W., Li M., Zhang P., Zhang Y.S., Ding J., Chen X. Antiviral biomaterials. Matter. 2021;4(6):1892–1918. doi: 10.1016/j.matt.2021.03.016. [DOI] [Google Scholar]
- 6.Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8(5):660–668. doi: 10.1016/S0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 7.Glauert A.M., Dingle J.T., Lucy J.A. Action of saponin on biological cell membranes. Nature. 1962;196(4858):953–955. doi: 10.1038/196953a0. [DOI] [PubMed] [Google Scholar]
- 8.Rahman Y.E., Rosenthal M.W., Cerny E.A. Intracellular plutonium: removal by liposome-encapsulated chelating agent. Science. 1973;180:300–302. doi: 10.1126/science.180.4083.300. [DOI] [PubMed] [Google Scholar]
- 9.Hendricks G.L., et al. Heparin octasaccharide decoy LNP inhibit replication of multiple cross mark viruses. Antiviral Res. 2015;116:34–44. doi: 10.1016/j.antiviral.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maitani Y., et al. Polyethylenimine combined with LNP and with decreased numbers of primary amine residues strongly enhanced therapeutic antiviral efficiency against herpes simplex virus type 2 in a mouse model. J. Control. Release. 2013;166(2):139–146. doi: 10.1016/j.jconrel.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Tahara K., et al. Effects of cationic LNP with stearylamine against virus infection. Int. J. Pharm. 2018;543(1–2):311–317. doi: 10.1016/j.ijpharm.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Thi E.P., Mire C.E., Ursic-Bedoya R., Geisbert J.B., H. Lee A.C., Agans K.N., Robbins M., Deer D.J., Fenton K.A., MacLachlan I., Geisbert T.W. Marburg virus infection in nonhuman primates: Therapeutic treatment by lipid-encapsulated siRNA. Sci. Transl. Med. 2014;6(250):250ra116. doi: 10.1126/scitranslmed.3009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.-S., Peer D., Kumar P., Subramanya S., Wu H., Asthana D., Habiro K., Yang Y.-G., Manjunath N., Shimaoka M., Shankar P. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 2010;18(2):370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wui S.R., Kim K.S., Ryu J.I., Ko A., Do H.T.T., Lee Y.J., Kim H.J., Lim S.J., Park S.A., Cho Y.J., Kim C.-G., Lee N.G. Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice. Vaccine. 2019;37(15):2131–2141. doi: 10.1016/j.vaccine.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Lima T., Feitosa R., dos Santos-Silva E., dos Santos-Silva A., Siqueira E., Machado P., Cornélio A., do Egito E., Fernandes-Pedrosa M., Farias K., da Silva-Júnior A. Improving encapsulation of hydrophilic chloroquine diphosphate into biodegradable nanoparticles: A promising approach against Herpes virus simplex-1 infection. Pharmaceutics. 2018;10(4):255. doi: 10.3390/pharmaceutics10040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shawky S., El-Shafai N.M., El-Mehasseb I.M., Shoueir K.R., El-Kemary M.A., et al. Spectroscopic study of self-assembly of anti-hepatitis C virus sofosbuvir drug with bio-polymeric nanoparticles for improving the drug release effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;261 doi: 10.1016/j.saa.2021.120008. [DOI] [PubMed] [Google Scholar]
- 17.Chun H., Yeom M., Kim H.O., Lim J.W., Na W., Park G., Park C., Kang A., Yun D., Kim J., Song D., Haam S. Efficient antiviral co-delivery using polymersomes by controlling the surface density of cell-targeting groups for influenza A virus treatment. Polym. Chem. 2018;9(16):2116–2123. doi: 10.1039/C8PY00116B. [DOI] [Google Scholar]
- 18.Springsteen G., Wang B. A detailed examination of boronic acid–diol complexation. Tetrahedron. 2002;58(26):5291–5300. [Google Scholar]
- 19.Lacina K., Skladal P., James T.D. Boronic acids for sensing and other applications - a mini-review of papers published in 2013. Chem. Cent. J. 2014;8(1):60. doi: 10.1186/s13065-014-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumlin U., Olofsson S., Dimock K., Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir. Viruses. 2008;2(5):147–154. doi: 10.1111/j.1750-2659.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L., Liu H., Gao S., Jiang W., Huang W. Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J. Virol. 2010;84(17):8849–8860. doi: 10.1128/JVI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun T.-W., Nickle D., Justement J., Meyers J., Roby G., Hallahan C., Kottilil S., Moir S., Mican JoAnn M., Mullins J., Ward D., Kovacs J., Mannon P., Fauci A. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 2008;197(5):714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 23.Cao S., Jiang Y., Zhang H., Kondza N., Woodrow K.A. Core-shell nanoparticles for targeted and combination antiretroviral activity in gut-homing T cells. Nanomedicine. 2018;14(7):2143–2153. doi: 10.1016/j.nano.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai M., Deshmukh S.D., Ingle A.P., Gupta I.R., Galdiero M., Galdiero S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016;42(1):46–56. doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- 25.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3(1):6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khandelwal N., Kaur G., Chaubey K.K., Singh P., Sharma S., Tiwari A., Singh S.V., Kumar N. Silver nanoparticles impair Peste des petits ruminants virus replication. Virus Res. 2014;190:1–7. doi: 10.1016/j.virusres.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Lin Z., Li Y., Guo M., Xu T., Wang C., Zhao M., Wang H., Chen T., Zhu B. The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 2017;7(2):742–750. doi: 10.1039/C6RA25010F. [DOI] [Google Scholar]
- 29.Thompson C.I., Barclay W.S., Zambon M.C. Changes in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host. J. Antimicrob. Chemother. 2004;53(5):759–765. doi: 10.1093/jac/dkh155. [DOI] [PubMed] [Google Scholar]
- 30.Halder A., Das S., Ojha D., Chattopadhyay D., Mukherjee A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;89:413–421. doi: 10.1016/j.msec.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Kim J., Yeom M., Lee T., Kim H.O., Na W., Kang A., Lim J.W., Park G., Park C., Song D., Haam S. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020;18(1):54. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levina A., Repkova M., Shikina N., Ismagilov Z., Kupryushkin M., Pavlova A., Mazurkova N., Pyshnyi D., Zarytova V. Pronounced therapeutic potential of oligonucleotides fixed on inorganic nanoparticles against highly pathogenic H5N1 influenza A virus in vivo. Eur. J. Pharm. Biopharm. 2021;162:92–98. doi: 10.1016/j.ejpb.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz A., Sigwalt D., Illescas B.M., Luczkowiak J., Rodríguez-Pérez L., Nierengarten I., Holler M., Remy J.S., Buffet K., Vincent S.P., Rojo J., Delgado R., Nierengarten J.F., Martín N. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016;8(1):50–57. doi: 10.1038/nchem.2387. [DOI] [PubMed] [Google Scholar]
- 34.Mårdberg K., Trybala E., Glorioso J.C., Bergström T. Mutational analysis of the major heparan sulfate-binding domain of herpes simplex virus type 1 glycoprotein C. J. Gen. Virol. 2001;82(8):1941–1950. doi: 10.1099/0022-1317-82-8-1941. [DOI] [PubMed] [Google Scholar]
- 35.Szunerits S., Barras A., Khanal M., Pagneux Q., Boukherroub R. Nanostructures for the inhibition of viral infections. Molecules. 2015;20(8):14051–14081. doi: 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M.C., Deokar A.R., Liao J.H., Shih P.Y., Ling Y.C. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano. 2013;7(2):1281–1290. doi: 10.1021/nn304782d. [DOI] [PubMed] [Google Scholar]
- 37.Deokar A.R., Nagvenkar A.P., Kalt I., Shani L., Yeshurun Y., Gedanken A., Sarid R. Graphene-based “Hot Plate” for the capture and destruction of the herpes simplex virus type 1. Bioconjug. Chem. 2017;28(4):1115–1122. doi: 10.1021/acs.bioconjchem.7b00030. [DOI] [PubMed] [Google Scholar]
- 38.Du T., Liang J., Dong N., Liu L., Fang L., Xiao S., Han H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. doi: 10.1016/j.carbon.2016.09.032. [DOI] [Google Scholar]
- 39.Ting D.u., Dong N., Fang L., Lu J., Bi J., Xiao S., Han H. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1(10):5451–5459. doi: 10.1021/acsanm.8b00779. [DOI] [PubMed] [Google Scholar]
- 40.Park, H.H., et al., PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses. Biomaterials 273 (2021), 120827. 10.1016/j.biomaterials.2021.120827. [DOI] [PMC free article] [PubMed]
- 41.Kingstad-Bakke B., Toy R., Lee W., Pradhan P., Vogel G., Marinaik C.B., Larsen A., Gates D., Luu T., Pandey B., Kawaoka Y., Roy K., Suresh M. Polymeric pathogen-like particles-based combination adjuvants elicit potent mucosal T cell immunity to influenza A virus. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.559382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trams E.G., et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta, Biomembr. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 43.Ogorevc E., Kralj-Iglic V., Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol. Oncol. 2013;47(3):197–205. doi: 10.2478/raon-2013-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu W., Rana S., Zoeller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou X., Yuan M., Zhang T., Wei H., Xu S., Jiang N.a., Zheng N., Wu Z. Extracellular vesicles expressing a single-chain variable fragment of an HIV-1 specific antibody selectively target Env (+) tissues. Theranostics. 2019;9(19):5657–5671. doi: 10.7150/thno.33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M., Zang X., Wang M., Li Z., Qiao M., Hu H., Chen D. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J. Mater. Chem. B. 2019;7(15):2421–2433. doi: 10.1039/C9TB00170K. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21(10):585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrivastava S., Ray R.M., Holguin L., Echavarria L., Grepo N., Scott T.A., Burnett J., Morris K.V. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun. 2021;12(1):5541. doi: 10.1038/s41467-021-25839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheller-Miller S., et al. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci. Rep. 2019;9(1):608. doi: 10.1016/j.ymthe.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou X., Yuan M., Zhang T., Zheng N., Wu Z. EVs containing host restriction factor IFITM3 inhibited ZIKV infection of fetuses in pregnant mice through trans-placenta delivery. Mol. Ther. 2021;29(1):176–190. doi: 10.1016/j.ymthe.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nie C., Stadtmüller M., Parshad B., Wallert M., Ahmadi V., Kerkhoff Y., Bhatia S., Block S., Cheng C., Wolff T., Haag R. Heteromultivalent topology-matched nanostructures as potent and broad-spectrum influenza A virus inhibitors. Sci. Adv. 2021;7(1) doi: 10.1126/sciadv.abd3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S., Cheng Q., Wei T., Yu X., Johnson L.T., Farbiak L., Siegwart D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021;20(5):701–710. doi: 10.1038/s41563-020-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]