Abstract
The COVID-19 pandemic has resulted in the rapid development of a range of vaccines against SARS-CoV-2. Vaccine-induced immune thrombocytopenia and thrombosis (VITT) is a rare but life-threatening complication of primarily adenoviral-based vaccines associated with the presence of antibodies to a PF4/polyanion neoepitope and measured by using enzyme-linked immunosorbent assays. Presented are serial anti–PF4/polyanion antibody, platelet, and D-dimer measurements in a large cohort of patients and their relation to relapse. Overall, 51% of patients using the Stago assay had persistently positive anti–PF4/polyanion levels 100 days' postdiagnosis, whereas 94% of patients monitored by using the Immucor assay remain positive. The median duration of positivity of the PF4 assay is 87 days, with 72% of patients remaining positive after a median follow-up of 105 days. The use of plasma exchange seemed to reduce anti–PF4/polyanion levels and increase platelet counts in the acute setting more rapidly than other therapies. The rate of relapse in this study was 12.6%, with all relapsed cases exhibiting persistently positive PF4 antibodies and falling platelet counts. Only one patient had extension of their thrombosis. Overall, despite the persistence of PF4 antibodies in 72% of patients, the rate of relapse was low and did not seem to result in recrudescence of the aggressive clinical picture seen at index presentation. Monitoring of these patients in the UK cohort is ongoing and will aid in definition of the natural history of this novel condition.
Introduction
The COVID-19 pandemic began in Wuhan province in China in December 2019 and has led to 432 505 813 infections and 5 950 376 deaths worldwide as of 25 February 2022.1 The rapid spread of this novel infection across the globe led to widespread institution of sweeping nonpharmacologic interventions such as mask mandates, travel restrictions, and closures of schools and businesses as well as the development of several novel vaccinations against COVID-19 that were developed with unprecedented speed.2, 3, 4 The United Kingdom was the first country to roll out the adenoviral vector–based ChAdOx1 nCoV-19 vaccine developed by AstraZeneca in January 2021. As of 16 February 2022, a total of 24.9 million people have received their first dose and 24.2 million people have received their second dose of the AstraZeneca vaccine in the United Kingdom.5
In March 2021, reports emerged simultaneously from Norway, Germany, and the United Kingdom regarding thrombosis at unusual sites combined with thrombocytopenia in patients who had received the ChAdOx1 nCoV-19 vaccine. Subsequently, a novel condition known as vaccine-induced immune thrombocytopenia and thrombosis (VITT) was described.6, 7, 8 VITT has also been described after administration of the adenoviral-based vaccine Ad26.COV2.S developed by Johnson & Johnson/Janssen.9 This led to several countries introducing restrictions upon who could receive adenoviral-based vaccines. Many of these restrictions were based on age, as the risk has been shown to be higher in people in younger age groups. In the United Kingdom, for example, a decision was made on 7 April 2021, to offer ChAdOx1 nCoV-19 only to those aged >30 years; subsequently, this recommendation was increased on 7 May 2021, to only those aged >40 years.10
A number of large cohort studies have been published describing the initial presentations of patients with confirmed VITT as well as those with the broader condition of cerebral sinus venous thrombosis after administration of the COVID-19 vaccine.11, 12 However, to date, there are only limited descriptions of the natural history of the PF4/polyanion antibodies in VITT.13
Methods
Data were collected on cases that occurred in the United Kingdom between January and August 2021. Data were collected from a combination of sources. These sources included daily meetings of the UK Expert Haematology Panel (EHP), which was established as soon as this condition became apparent and helped define and direct the national response; this was via anonymized electronic forms coordinated by Public Health England and direct correspondence with local hematologists and laboratories reporting the anti–PF4/polyanion antibody results. All cases were reported to the Medicines and Healthcare products Regulatory Agency.
In accordance with the Declaration of Helsinki, informed consent for data collection was obtained from patients included in this study. This consent was usually obtained retrospectively due to the acute nature of the presentation. Ethics board approval for the collection and storage of the data was obtained via a substantial amendment to a preexisting nationwide patient data registry.
The focus of the current article was how the PF4 antibody levels changed over time in patients with VITT and whether this change had any effect on patients' rate of relapse. Given the importance, as per the case definition, of D-dimer and platelet levels, we also compared these levels over time with those of the PF4 antibody optical density (OD) values and the impact of therapy on these antibody levels. In an effort to provide as representative a sample of the UK cohort as possible, permissive inclusion criteria were used. Cases were included in the longitudinal analysis if they had three or more PF4 enzyme-linked immunosorbent assay (ELISA) readings during the course of the study.
Case definition
This data set is an extension of that previously reported in the UK cohort of patients in which the case definition was previously described.11 Relapses were defined as recurrent thrombocytopenia following normalization of platelets that required re-treatment, or extension of existing thrombus, or thrombosis in a new site.
Laboratory tests
The PF4 ELISAs were performed in specialist hemostasis laboratories in the United Kingdom during the study period. The centralization of processing and reporting of PF4 ELISA results was due to many laboratories in the country having converted their heparin-induced thrombocytopenia (HIT) assays to chemiluminescent immunoassays in recent years, which are not sensitive for VITT cases. The 2 assays used by specialist laboratories were the Lifecodes PF4 assay (Immucor) and the Asserachrom HPIA assay (Diagnostica Stago Inc. [hereafter referred to as Stago]). The cutoff for positivity for the Immucor assay was an OD reading of 0.4; for the Stago assay, it was 0.238. Standard blood counts and clotting assays, including Clauss fibrinogen and D-dimer, were performed in the hospital where the patient was being managed. The units for D-dimer were micrograms per liter or nanograms per milliliter fibrinogen-equivalent units (FEU) with a cutoff for positivity of 500 FEU. Due to the retrospective nature of the data and the new presentation of this syndrome, the interval between testing, including PF4 ELISAs, was at the discretion of the treating physician.
Statistical tests
GraphPad Prism (GraphPad Software) was used to illustrate the graphs in this paper. Linear regression modeling was used to compare the change in laboratory values over time, and Wilcoxon matched-pairs signed-rank tests were used to compare mean PF4/polyanion values at different time points, with a P value <.05 taken as the criterion of statistical significance (GraphPad version 9 for Windows). Variables have been described as absolute values, percentages, medians, interquartile ranges, and ranges where specified and as appropriate.
Results
Baseline characteristics and treatment received
A total of 148 patients were included in this analysis; 73 (49%) were female. The median age was 49 years (range, 20-77 years). The median platelet count at presentation was 49 × 109/L (range, 9-311 × 109/L), and the median D-dimer level was 19 795 µg/L (FEU) (range, 700-119 000 µg/L [FEU]). In patients whose platelet levels were normal at admission, all became thrombocytopenic during their admission. D-dimer assays from some laboratories only detected levels up to a maximum value of 5000 or 20 000 µg/L (FEU); if this was the case, those values were taken to be the value quoted although it was understood that this approach may lead to an underestimate in a minority of cases. A total of 143 (96.6%) patients had thrombosis that was confirmed on imaging. Similar to the larger UK cohort from which these data originate, the mortality rate in this group of patients was 17.5%, in which the therapeutic pathway has been described (supplemental Figure 1, available on the Blood Web site).11 Further information on the location of thromboses and the treatments received by patients in this analysis are included in Table 1 . The median presenting anti–PF4/polyanion OD readings for patients presenting with cerebral venous sinus thrombosis or splanchnic vein thromboses were higher than for those patients with deep vein thrombosis or pulmonary embolism alone: 1.29 vs 1.09 and 2.7 vs 2.35 for the Stago and Immucor assays, respectively.
Table 1.
Sites of thrombosis and major hemorrhage and treatment received by patients
| Variable | No. (%) |
|---|---|
| Sites of thrombosis | |
| Cerebral venous sinus thrombosis | 67 (45) |
| Intracerebral/subarachnoid hemorrhage | 35 (23) |
| Arterial (eg, limb ischemia and stroke) | 32 (21) |
| Lower extremity deep venous thrombosis | 17 (11) |
| Pulmonary embolus | 40 (27) |
| Splanchnic vein thrombosis (eg, mesenteric, portal, and hepatic) | 23 (16) |
| Treatment received | |
| Intravenous immunoglobulin | 108 (73) |
| Systemic corticosteroids (oral prednisolone/ dexamethasone or intravenous methylprednisolone) | 85 (57) |
| Nonheparin anticoagulation (eg, fondaparinux, argatroban, and direct-acting oral anticoagulants) | 106 (72) |
| Low-molecular-weight heparin | 24 (16) |
| Unfractionated heparin | 8 (5) |
| Surgical/interventional radiology intervention | 19 (13) |
| Platelet transfusion | 16 (11) |
| PEX | 18 (12) |
Patients may have had thrombus in multiple sites as well as receiving multiple treatment modalities.
Due to the rapid evolution of knowledge around VITT and its management after its discovery, the treatment algorithm for the patients in this study changed throughout the observation period. Treatment was guided by a daily national multidisciplinary team meeting chaired by the EHP, which also produced interim consensus guidelines for the management of VITT that were distributed via the British Society of Haematology Web site. Plasma exchange (PEX) was reserved for patients who were deemed as having the most severe disease according to clinical parameters such as widespread thrombosis and adverse laboratory markers such as severe thrombocytopenia.11 PEX was continued until platelet count was normal. A suggested follow-up protocol for patients at discharge is included in supplemental Figure 2.
Serial anti–PF4/polyanion readings
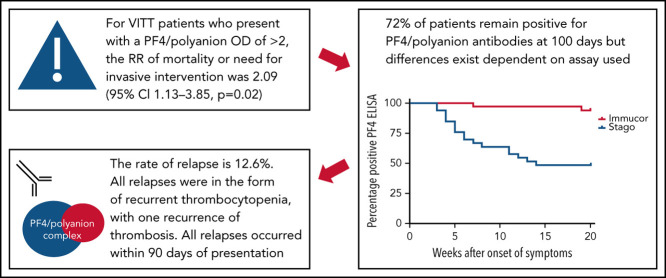
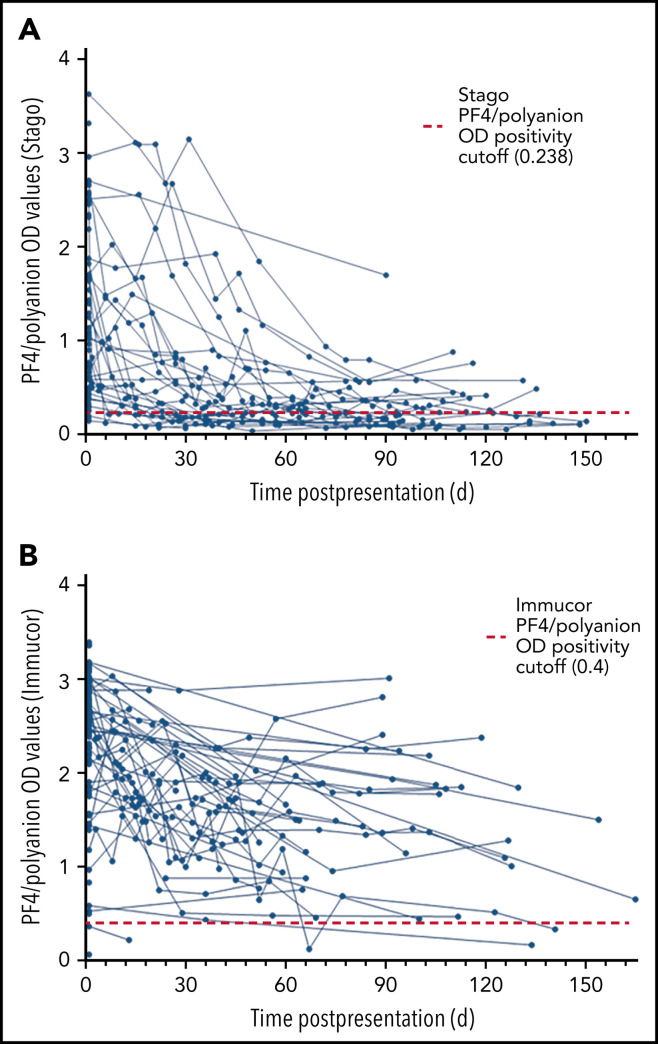
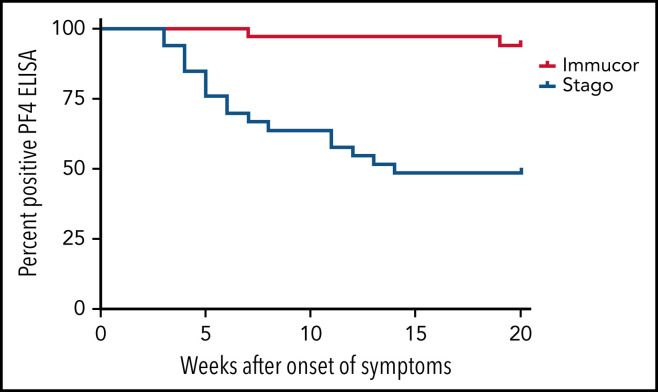
Owing to the different cutoff values for positivity with each assay, the serial anti–PF4/polyanion readings for samples processed via both the Stago and Immucor assays are shown separately in Figure 1 . The x-axis in each panel shows time from presentation, with day 1 being considered the day that the diagnosis of VITT was confirmed via the PF4 ELISA. Due to differences in monitoring between centers as well as early mortality in some patients, serial anti–PF4/polyanion levels (multiple readings beyond 30 days) were available for 70 patients. For this subgroup of patients, the median duration of positive anti–PF4/polyanion readings was 87 days, and 72% of these remained positive after a median follow-up of 105 days. As discussed in the following paragraphs, however, there are differences based on the assay used. This discrepancy is expressed more clearly in Figure 2 .
Figure 1.
Scatter plots of PF4/polyanion OD values of all patients with confirmed or probable VITT over time. (A) All patients monitored using the Stago assay (n = 61 patients). (B) All patients monitored using the Immucor assay (n = 87 patients).
Figure 2.
Kaplan-Meier curve displaying the percentage of patients testing positive on different PF4 assays over time. The positivity cutoff for the Stago and Immucor assays comprised ODs of 0.238 and 0.4, respectively, and an event was recorded as soon as the patient had a negative ELISA. n = 33, Stago assay; n = 34, Immucor assay.
Sixty-one patients were originally confirmed as having VITT via the Stago assay. Serial data from the Stago assay were available for 33 patients. Of these, 17 (52%) have normalized their PF4/polyanion antibody levels by day 100, with almost all patients showing a clear downward trend (Figure 1A). The median duration of persistently positive anti-PF4/polyanion OD values in those patients who remained positive was 139 days (range, 34-157 days).
Eighty-seven patients were confirmed as having VITT according to the Immucor assay. Serial data were available for 37 of these patients; however, as can be seen in Figure 1B, only 2 of these patients have normalized their anti-PF4/polyanion antibody values within 100 days, and indeed 86% (32 of 37) of this group remain strongly positive, with OD values in excess of 1 at 100 days. The median duration of persistently positive anti–PF4/polyanion OD values in those patients who remained positive was 89 days (range, 34-165 days).
Functional testing in the form of heparin-induced platelet activation assays was conducted in 67 of the patient samples. Thirty (45%) of these were considered positive, with platelet activation present at low concentrations of heparin that was abrogated by the addition of heparin in excess. This testing was conducted before the description of novel PF4-induced platelet activation (PIPA) and PF4-induced flow cytometry–based platelet activation assays.14
Serial anti–PF4/polyanion antibody values related to platelets and D-dimers
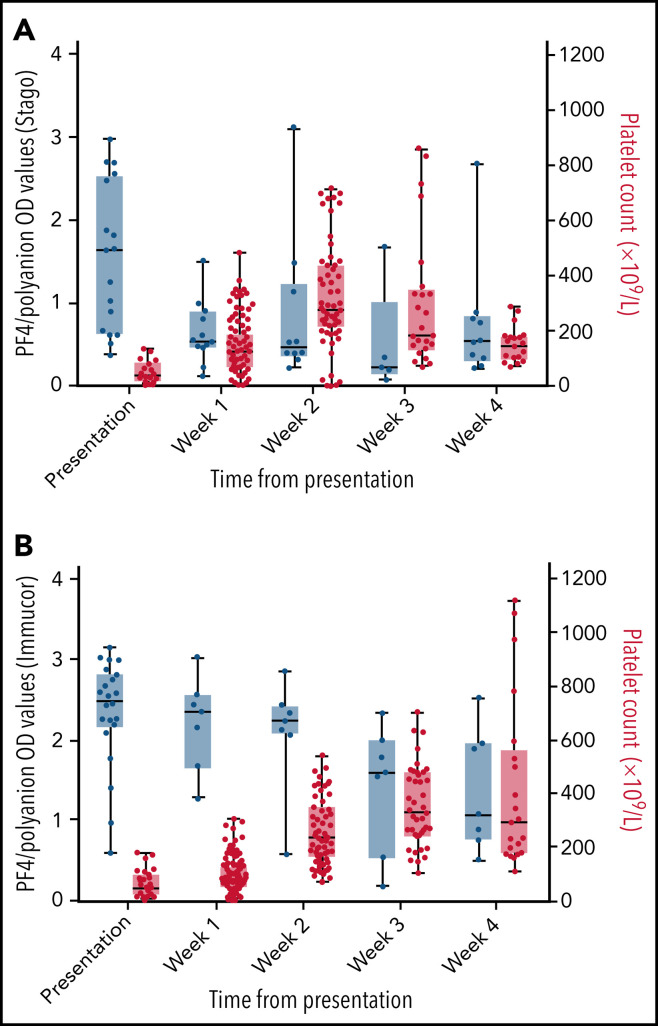
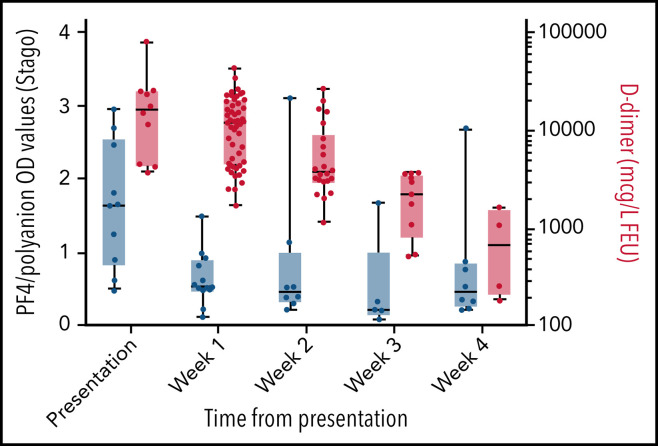
There was a clear increase in platelet counts over the first 3 weeks after presentation (Figure 3 ), with many patients increasing their levels to above the upper limit of normal by the second and third weeks. The increase in platelets occurred at the same time as a decrease in anti–PF4/polyanion OD values from presentation. A decrease in D-dimer values occurred that mirrors the decrease seen in the anti–PF4/polyanion values over time (Figure 4 ). The D-dimer values in this subgroup went from a median value of 16 480 µg/L (range, 4160-80 000 µg/L) at presentation to all values being <2000 µg/L (the cutoff for probable VITT) by week 4 postpresentation.
Figure 3.
Box and whisker plots of PF4/polyanion OD values and platelet counts over time. PF4/polyanion values were measured using the Stago (A) and Immucor (B) assays. The positivity cutoff for the Stago and Immucor assays comprised ODs of 0.238 and 0.4, respectively, with a normal platelet count of 150 to 400 × 109/L. Whiskers extend between the maximum and minimum values, and the middle line represents the median value with all data points displayed as dots. This graph includes only patients for whom serial PF4/polyanion and platelet counts were available, with some patients having multiple measurements in a given week (n = 18 and 22 patients for each graph, respectively).
Figure 4.
Box and whisker plot of PF4/polyanion OD values as measured by using the Stago assay and D-dimer levels. The positivity cutoff for the Stago assay was an OD of 0.238, and D-dimer normal values were 0 to 500 µg/L (FEU). D-dimer values were plotted on a logarithmic scale. Whiskers extend between the maximum and minimum values with all data points displayed as dots and the middle line representing the median value. This graph includes only patients for whom serial PF4/polyanion and D-dimer measurements were available, with some patients having multiple measurements in a given week (n = 10 patients).
Serial anti–PF4/polyanion values and platelets after PEX
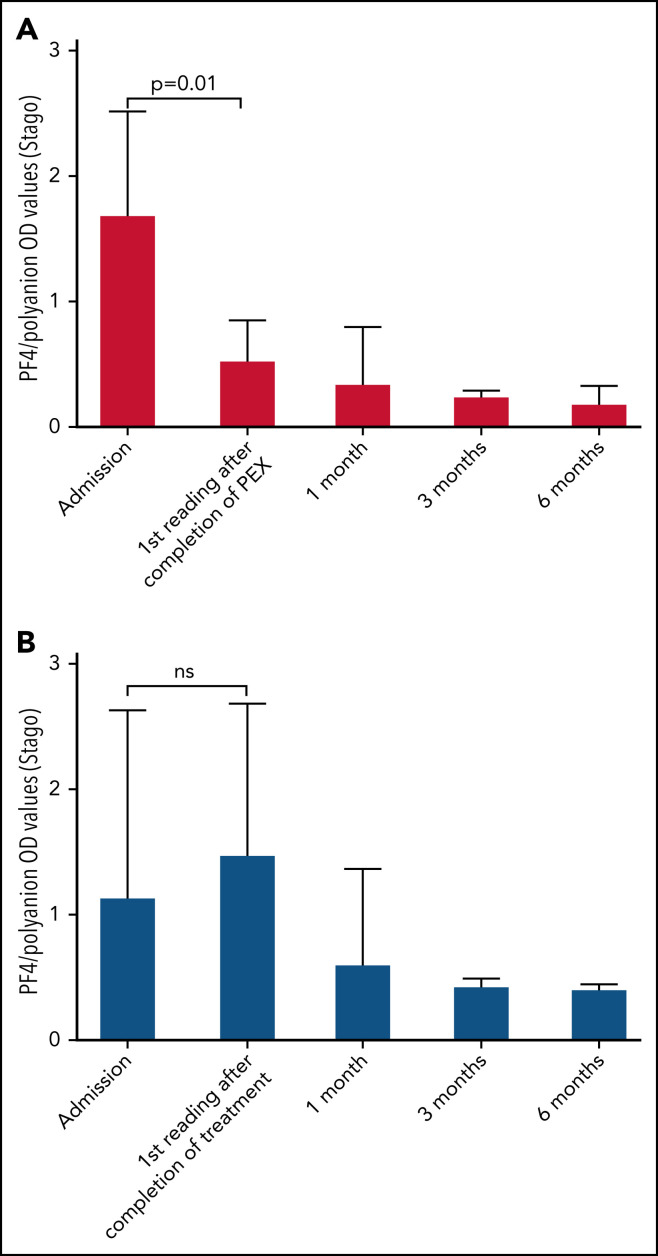
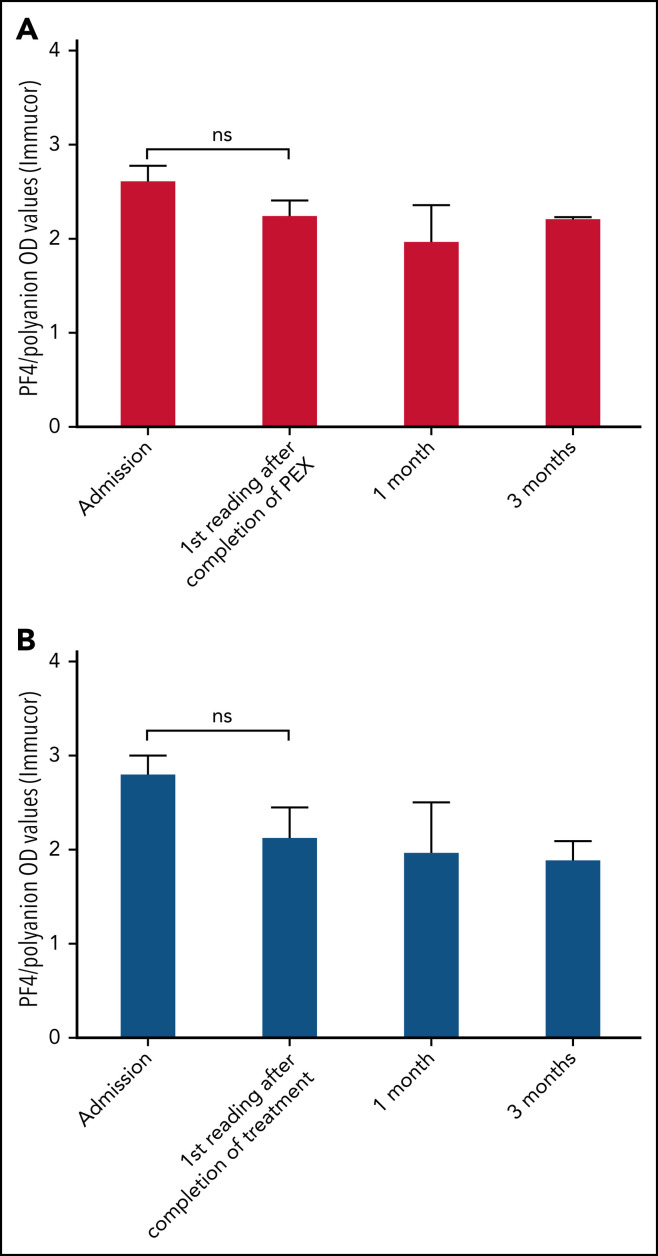
In the group of 9 patients who received PEX and who were monitored by using the Stago assay, patients received a median of 4 PEX treatments (single-volume exchanges; range, 2-11 treatments). Unfortunately, these data were not available for all patients monitored by Immucor who received PEX. Figure 5A shows the early reduction in PF4/polyanion antibodies brought about by PEX as measured by the Stago assay compared with age- and sex-matched control patients who did not receive PEX, as shown in Figure 5B. This rapid reduction in PF4/polyanion antibody levels was not seen in those patients who were monitored by using the Immucor assay (Figure 6 ), although clinical improvements and laboratory improvements were noted in almost all patients who received PEX. Similar to data in Figure 1, the low PF4/polyanion antibody levels seen in patients monitored via the Stago assay persisted over time, whereas once again the antibodies detected by the Immucor assay appeared more persistent.
Figure 5.
Bar charts of patients' PF4/polyanion OD values over time as measured using the Stago assay and based on whether they have received PEX. Median PF4/polyanion OD values for patients who received PEX over time (n = 9) (A) compared with age- and sex-matched control patients who did not receive PEX (n = 10) (B). The tails represent interquartile ranges. The positivity cutoff for the Stago assay was an OD of 0.238. Wilcoxon matched-pairs signed-rank tests were used to determine statistically significant differences between mean PF4/polyanion OD values at different time points, with P < .05 considered to be significant. ns, not significant.
Figure 6.
Bar charts of patients' PF4/polyanion OD values over time as measured by the Immucor assay and based on whether they have received PEX. Median PF4/polyanion OD values for patients who received PEX over time (n = 9) (A) compared with age- and sex-matched control patients who did not receive PEX (n = 11) (B). The tails represent interquartile ranges. The positivity cutoff for the Immucor assay was an OD of 0.4. Wilcoxon matched-pairs signed-rank tests were used to determine statistically significant differences between mean PF4/polyanion OD values at different time points, with P < .05 considered to be significant. ns, not significant.
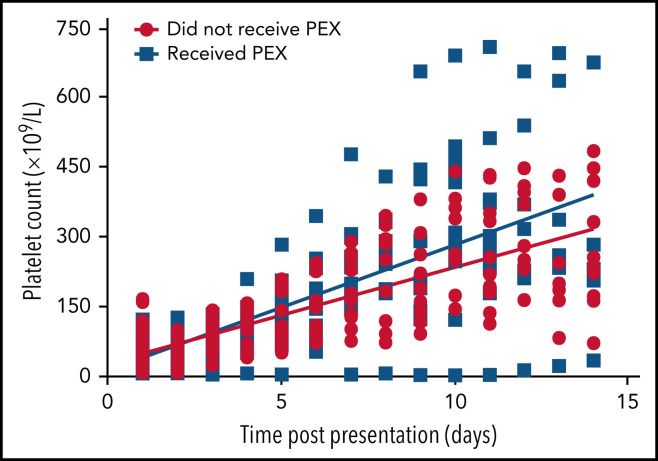
Similarly, there was a statistically significant difference in platelet counts between those who received PEX and those who did not in the first 14 days' postpresentation (P = .0002) (Figure 7 ). One patient whose thrombocytopenia was extremely resistant to PEX was considered an outlier and not included in this analysis; however, this did not change the statistical significance of the finding.
Figure 7.
Scatter plot of patients' platelet counts based on whether they received PEX. Normal platelet values were 150 to 400 × 109/L. Linear regression modeling used to plot lines of best fit and compare rates of change with P < .05 considered significant.
As noted earlier, PEX was introduced as a treatment modality during the UK wave of VITT cases for the most severe forms. Requirement for PEX, therefore, along with mortality and need for neurosurgical or interventional radiology interventions can be seen as a surrogate marker for disease severity. Taking these 3 outcomes as a composite, the relative risk of this outcome for those patients who had a presenting PF4/polyanion OD value >2 on the Stago assay was 2.09 (95% confidence interval, 1.13-3.85; P = .01). The relative risk for the same outcome using a PF4/polyanion OD value >2 on the Immucor assay was 2.47 (95% confidence interval, 0.83-7.37; P = .1). This finding is suggestive, at least, that a higher presenting PF4/polyanion OD value is associated with a worse outcome.
Relapse
Longer term clinical outcome data were gathered on 79 patients, 16 of whom had died. Of the remaining 63 patients, there were 10 episodes of relapse, although 3 of these were in the same patient, yielding a relapse rate of 12.6%. All but one of these relapses presented as a drop in platelet count rather than extension of thrombosis or recurrence of symptoms. The one exception was a patient who re-presented with recurrence of their thrombocytopenia and extension of their pulmonary embolism 1 week after being discharged home with a normal platelet count. One patient also had persistent thrombocytopenia and increased D-dimer levels, with breakthrough thrombosis requiring change of anticoagulation. This latter case, however, was all part of the patient's initial admission and thus was not classified as a relapse. Indeed, this was very early postdiscovery of VITT when treatment pathways were still being developed.
All patients who relapsed had persistently elevated anti–PF4/polyanion antibody levels when the relapse occurred. Representative graphs of trends in anti–PF4/polyanion antibody levels, platelet counts, and D-dimer levels are provided for 1 patient who was monitored via the Stago assay and 1 patient monitored via the Immucor assay (supplemental Figures 3 and 4, respectively). The median anti–PF4/polyanion OD value at the time of relapse was 1.9 (range, 0.69-2.79; positivity cutoff, 0.4) for those patients who were monitored by using the Immucor assay and 0.77 (range, 0.56-3.14; positivity cutoff, 0.238) on the Stago assay. The median time from initial presentation to relapse for these cases was 28 days (range, 15-90 days). All relapses received further intravenous immunoglobulin. In the case of the patient with multiply relapsing disease, the patient also received rituximab and mycophenolate, and the patient with recurrent thrombosis did not respond to an initial trial of rituximab and so proceeded to PEX.
Discussion
We sought to describe the ongoing changes in key laboratory parameters, in particular PF4/polyanion antibody levels, in patients with VITT after ChAdOx1 nCoV-19 vaccination as well as how these laboratory parameters may relate to relapse. Furthermore, the aim was to determine the length of time that PF4/polyanion antibodies could be detected and if therapy for VITT had any impact on this time frame.
The serial anti–PF4/polyanion readings on the Stago assay seemed to normalize more quickly than those analyzed on the Immucor assay, with 86% of patients in the Immucor cohort remaining strongly positive at the time of this analysis with an OD value >1. This outcome may partially be explained by differences in patient cohorts; for example, more patients received PEX in those who were monitored with the Stago assay compared with the Immucor assay. Recent descriptions of longitudinal assays from the German cohort would indicate a divergence between their novel PIPA assays and anti-PF4 ELISAs over time.15 The Stago assay used in the reference laboratory for this study is a polyspecific assay compared with the immunoglobulin G (IgG)–specific Immucor assay used by the other reference laboratory, and differences between these assays, which were developed for HIT, in detecting VITT cases have been well described.16 The IgG–specific assay may be more specific for pathogenesis in HIT, but it is possible that the antibodies detected by the Stago assay in VITT are more closely aligned with the functional platelet activation assay described by the German group.17 It is entirely possible that the patients who tested negative on the heparin induced platelet aggregation (HIPA) assay may, in fact, be positive when a more appropriate VITT-specific assay is used. It would be instructive to run the PIPA assays on future cases as functional testing with the traditional HIPA assays was less informative.
In the largest cohort of patients with HIT followed up longitudinally, PF4 antibodies tended to persist for a median time of between 50 and 85 days,18 and thus it would seem that the PF4 antibodies found in VITT persist for longer given that the median in this cohort is currently 87 days, and follow-up is ongoing and therefore this number is likely to increase. This persistence of antibodies is more akin to the natural history of spontaneous HIT-like syndromes.19, 20 The mechanism by which they cause thrombocytopenia and thrombosis seems to ameliorate over time, which is also a feature of spontaneous HIT-like syndromes.21 It should also be noted that, similar to traditional HIT, the presence of PF4/polyanion antibodies both post–COVID-19 vaccination and in the wider population has recently been described, and not all of these patients subsequently develop overt VITT.22, 23 Despite the persistent positivity on the anti-PF4 ELISA for the patients with VITT, there was early and sustained normalization of platelet and D-dimer levels (as noted later in this section), except in patients who relapsed.
The dichotomy in testing methods described here means it is reasonable to consider the advantages and disadvantages of different anti-PF4 ELISAs when interpreting results in the context of VITT. It should be noted that, from the offset, obviously neither assay was developed for the diagnosis of VITT; however, the authors feel from their experience that either the Immucor or the Stago assay may be used to diagnose VITT in the acute setting. It would also be reasonable to consider Immucor testing over Stago testing in the context of a suspected missed diagnosis as it is possible the result would still be positive months after the acute episode, although a negative result in this context would be less informative. Given the closer correlation with novel functional assays and clinical outcomes to date, the Stago assay seems preferable for monitoring given the lack of widespread availability of the PIPA assay.
There was a clear rise in platelet count with therapy, corresponding to a reduction in PF4 antibody levels, regardless of whether measured by using the Stago or the Immucor assay. In particular, 92.5% of patients had normalized platelet levels by the third week postpresentation. In addition, another key marker of disease activity, D-dimer, which was extremely elevated in all patients on presentation, decreased in conjunction with the anti–PF4/polyanion antibody levels over the first 3 weeks after presentation and treatment, and was <2000 µg/L (the diagnostic cutoff for probable VITT) in all patients by week 4. These data are encouraging; that is, despite the overwhelming prothrombotic response that we have seen in patients with this condition, it can be reversed with appropriate therapy such as nonheparin anticoagulation, intravenous immunoglobulin, steroids, and, in more severe cases, PEX.
PEX was considered as a treatment option for the most severely affected patients, including in the index presentation in this cohort. After identification of the role of PF4/polyanion antibodies in disease pathogenesis along with the observation that patients with extremely low platelet counts (<30 × 109/L) and cerebral venous sinus thrombosis had such a high mortality (>70%),11 it was increasingly recognized that these patients needed to be treated in an aggressive fashion. This lent weight to the use of PEX in severely affected cases given its ability to rapidly reduce the noxious antibodies in a wide range of other autoimmune conditions.
PEX seemed to promptly reduce the level of PF4/polyanion antibodies when measured by using the Stago assay (Figure 5), although, as noted in the broader cohort, the antibodies measured by using the Immucor assay seemed more persistent (Figure 6). This scenario was despite a clinical improvement in most patients who received PEX and improved clinical outcomes as noted previously. Similarly, platelet levels seemed to increase more rapidly than in those who did not receive PEX (Figure 7), at least in the important initial 2 weeks after presentation. However, inferences about therapeutic efficacy were not subject to a clinical trial, and potential adverse effects of PEX24 require monitoring in a center that has experience managing these patients.
The rate of relapse seen in this study was fortunately low, and many of the episodes classified as a relapse re-presented, in fact, with a drop in platelets that was subsequently re-treated due to a lack of knowledge about the natural history of the condition regarding further thrombotic episodes. As noted in the Results, there was extension of thrombus in one case. No clear-cut relationship seems to exist between change in antibody levels and reduction in platelets. There was a slight uptrend in antibody levels, as noted in supplemental Figure 2A, before the drop in platelets; however, as shown in supplemental Figure 2B, platelet counts varied widely despite a fairly static level of anti–PF4/polyanion antibodies, as was characteristic of patients monitored via the Immucor assay. It should be noted, however, that the absolute number of relapses remains small, and it is therefore difficult to draw firm conclusions. Although not described during the duration of this study, late relapses could still conceivably occur in the manner of HIT upon re-exposure to an appropriate provoking antigen, and thus ongoing surveillance is necessary.
Of the many important questions that remain in VITT, two are immediately relevant to the patients' clinical outcomes: that of further COVID-19 vaccinations, given the ongoing pandemic, and duration of anticoagulation. Patients in our cohort25 and the German cohort15, 17 have received a second dose of a COVID-19 vaccine (nonadenoviral-based vaccines) to date. It will be instructive to observe the response of these patients over time regarding platelets, anti–PF4/polyanion levels, and any further episodes of thrombosis; however, the evidence to date would suggest a low rate of recurrence. All the patients who received further vaccines in the UK cohort, thus far, did so while still receiving anticoagulation and have not been associated with recurrence of thrombocytopenia or thrombosis.
Of the 63 patients in this study who are still alive and for whom we have up-to-date clinical outcomes, 7 have stopped anticoagulation as they had a lower level of thrombus burden or more conventional thrombosis and had normalized their PF4/polyanion antibodies. Many of the patients, however, have multiple sites of thrombosis, thrombosis at unusual sites, or persistently positive PF4 antibodies, and the current consensus plan would be to continue anticoagulation for a minimum of 1 year in these patients. This study raises the possibility that a different approach to stopping anticoagulation may be necessary for those patients being monitored with the Immucor assay. Given their persistence, rather than just waiting for their PF4/polyanion antibodies to evanesce, these patients may require a more proactive approach. The low rate of relapse despite antibody persistence suggests a lower level of pathogenicity over time and bodes well for anticoagulation cessation in this cohort.
Conflict-of-interest disclosure: W.L. declares speaker honoraria from Boehringer Ingelheim, Bayer, Bristol Myers Squibb, LEO Pharma, and Pfizer; and has been on advisory boards for Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Daiichi Sankyo, and Pfizer. W.T. has been on advisory boards for Daiichi Sankyo, Sanofi, and Ablynx; received speakers fees from Takeda, Bayer, Alexion, Pfizer, Bayer, Portola, Sobi, and Novo Nordisk; and received support to attend educational events from Novo Nordisk and Sobi. S.P. has received honoraria for educational lectures, chairing and participation in advisory boards, or sponsorship for running courses from SOBI, Amgen, Alexion, Sanofi, CSL Behring, Novartis, Pharmacosmos, and Vifor Pharma, outside the submitted work. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank all the hematologists and allied health care professionals who contributed to the data collection from sources nationwide, who helped them attain a greater understanding of this novel and challenging condition.
Authorship
Contribution: B.C. and M.S. originally conceived the study; B.C., W.L., S.B., W.T., A.K., C.D., J.H., M.M., E.B.-S., S.A., and P.C. contributed clinical and follow-up data of patients at their respective sites; D.S. and A.C. provided laboratory data from the 2 major reference laboratories used to process the anti-PF4 ELISAs; S.P., W.L., M.M., B.J.H., and M.S. were part of the UK EHP, which directed the national response to VITT and assisted with oversight of the data collection; B.C. wrote the original manuscript; M.S. provided initial feedback; and all authors contributed to and viewed the manuscript before final submission.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
REFERENCES
- 1.COVID Live–Coronavirus Statistics–Worldometer https://www.worldometers.info/coronavirus/
- 2.Baden LR, El Sahly HM, Essink B, et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus vaccine–weekly summary of Yellow Card reporting. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- 6.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Office of the Commissioner Joint CDC and FDA Statement on Johnson & Johnson COVID-19 vaccine. https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html Published 13 April 2021.
- 10.MHRA response to JCVI advice on COVID-19 vaccine AstraZeneca for people aged under 40. https://www.gov.uk/government/news/mhra-response-to-jcvi-advice-on-covid-19-vaccine-astrazeneca-for-people-aged-under-40 Published 7 May 2021.
- 11.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet Lond Engl. 2021;398(10306):1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler J, Jilma P, Samadi N, et al. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thromb Res. 2021;207:126–130. doi: 10.1016/j.thromres.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Handtke S, Wolff M, Zaninetti C, et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137(26):3656–3659. doi: 10.1182/blood.2021012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schönborn L, Thiele T, Kaderali L, Greinacher A. Decline in pathogenic antibodies over time in VITT. N Engl J Med. 2021;385(19):1815–1816. doi: 10.1056/NEJMc2112760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost. 2021;19(8):2007–2013. doi: 10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schönborn L, Thiele T, Kaderali L, et al. Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient. Blood. 2022;139(12):1903–1907. doi: 10.1182/blood.2021014214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 19.Warkentin TE, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40–51. doi: 10.1016/j.thromres.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Roberge G, Scarvelis D. Long-term anti-PF4 persistence in autoimmune heparin-induced thrombocytopenia: a glimpse into the natural history of vaccine-induced immune thrombotic thrombocytopenia. Thromb Update. 2021;5:100067. [Google Scholar]
- 21.Poudel DR, Ghimire S, Dhital R, Forman DA, Warkentin TE. Spontaneous HIT syndrome post-knee replacement surgery with delayed recovery of thrombocytopenia: a case report and literature review. Platelets. 2017;28(6):614–620. doi: 10.1080/09537104.2017.1366973. [DOI] [PubMed] [Google Scholar]
- 22.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138(4):299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uaprasert N, Watanaboonyongcharoen P, Vichitratchaneekorn R, et al. Prevalence of thrombocytopenia, anti-platelet factor 4 antibodies and D-dimer elevation in Thai people after ChAdOx1 nCoV-19 vaccination. Res Pract Thromb Haemost. 2021;5(6):e12580. doi: 10.1002/rth2.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J Clin Apher. 2007;22(5):270–276. doi: 10.1002/jca.20143. [DOI] [PubMed] [Google Scholar]
- 25.Lacy J, Pavord S, Brown KE. VITT and second doses of Covid-19 vaccine. N Engl J Med. 2022;386(1):95. doi: 10.1056/NEJMc2118507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.