Abstract
In December 2019, coronavirus disease 2019 (COVID-19), a severe respiratory illness caused by the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. The greatest impact that COVID-19 had was on intensive care units (ICUs), given that approximately 20% of hospitalized cases developed acute respiratory failure (ARF) requiring ICU admission. Based on the assumption that COVID-19 represented a viral pneumonia and no anti-coronaviral therapy existed, nearly all national and international health care societies recommended “supportive care only” avoiding other therapies outside of randomized controlled trials, with a specific prohibition against the use of corticosteroids in treatment. However, early studies of COVID-19-associated ARF reported inexplicably high mortality rates, with frequent prolonged durations of mechanical ventilation (MV), even from centers expert in such supportive care strategies. These reports led the authors to form a clinical expert panel called the Front-Line COVID-19 Critical Care Alliance (www.flccc.net). The panel collaboratively reviewed the emerging clinical, radiographic, and pathological reports of COVID-19 while initiating multiple discussions among a wide clinical network of front-line clinical ICU experts from initial outbreak areas in China, Italy, and New York. Based on the shared early impressions of “what was working and what wasn’t working”, the increasing medical journal publications and the rapidly accumulating personal clinical experiences with COVID-19 patients, a treatment protocol was created for the hospitalized patients based on the core therapies of methylprednisolone, ascorbic acid, thiamine, heparin and non-antiviral co-interventions (MATH+). This manuscript reviews the scientific and clinical rationale behind MATH+ based on published in-vitro, pre-clinical, and clinical data in support of each medicine, with a special emphasis of studies supporting their use in the treatment of patients with viral syndromes and COVID-19 specifically.
Keywords: MATH plus, Methylprednisolone, Ascorbic acid, Thiamine, Heparin
Introduction
In December 2019, coronavirus disease 2019 (COVID-19), an illness characterized by pneumonia associated with the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. By March 11, 2020, the World Health Organization (WHO) had characterized the novel coronavirus outbreak as a pandemic, with confirmed cases in 213 countries. The greatest impact this malady had was on intensive care units (ICUs), given approximately 20% of hospitalized cases developed acute respiratory failure (ARF) requiring ICU admission [1, 2].
Since COVID-19 was initially defined as a primary viral syndrome and no validated anti-coronavirus therapy existed, nearly all national and international health care societies advocated a primary focus on supportive care with avoidance of other therapies outside of randomized controlled trials (RCTs), and with specific recommendations to avoid the use of corticosteroids [3-5].
The pervasive belief amongst world health care societies that corticosteroids were harmful in COVID-19 respiratory illness was surprising for several reasons. First, as will be detailed in this manuscript, contrary to the WHO and CDC’s interpretation of prior pandemic data, a review of the same data by a group including one of the authors (GUM) was both published and publicized by the Society for Critical Care Medicine in early April 2020 which concluded that the largest and most well-controlled studies from the severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and H1N1 pandemics found that the mortality of patients with moderate to severe illness was significantly reduced when treated with corticosteroids [6]. Second, reports from the “front-line” clinicians in Italy and New York reported on rapidly observable, positive impacts when corticosteroids were used in treatment. Further, an expert panel of US radiologists had published an tragically little-noticed review of the early computed tomography (CT) scans from Wuhan, China in March of 2020, where they concluded that the “most common pattern of lung injury in COVID-19 is of an organizing pneumonia” (OP), a condition accurately identifiable by CT scan and whose first-line therapy is corticosteroids. The presence of OP likely explains both the seemingly baffling clinical presentation of early COVID-19 respiratory disease as well as the efficacy of corticosteroids as evidenced in a recent review by one of the authors (PK) [7, 8].
However, in that period prior to the now-widespread use of corticosteroids, multiple early studies of COVID-19-associated ARF reported inexplicably high mortality rates, with frequent prolonged durations of mechanical ventilation (MV), even from centers expert in such supportive care strategies [9]. These reports led many physicians, including the authors of this manuscript, to question the widely recommended supportive care-only approach, and to review the evidence behind therapies that could counteract the well-recognized syndrome of severe hypoxemia, hyper-inflammation, and hypercoagulability, with the rationale that interventions targeted at these pathophysiologies could decrease dependence on mechanical ventilators and mortality in COVID-19 patients, and thus, have an immediate significant global impact on this public health emergency [9, 10].
As a group of clinical researchers in critical care with over a 100-year collective front-line, bedside ICU experience in the treatment of severe infections and acute respiratory distress syndrome (ARDS), the authors formed a clinical expert panel which we called the Front-Line COVID-19 Critical Care Alliance (www.flccc.net). The panel collaboratively reviewed the emerging clinical, radiographic, and pathological reports of COVID-19 while initiating multiple discussions among a wide clinical network of front-line clinical ICU experts from initial outbreak areas in China, Italy, and New York. Based on the shared early impressions of “what was working and what wasn’t working”, the increasing medical journal publications and the rapidly accumulating personal clinical experiences with COVID-19 patients, a treatment protocol was created for hospitalized patients, adapted from a protocol created by one of the authors (PEM) at their home institution. The protocol consisted of the four “core” therapies of methylprednisolone, ascorbic acid (AA), thiamine, heparin, and a number of co-interventions and thus was called “MATH+” (Table 1). The core medicines were all highly familiar, low-cost, FDA-approved medications with known therapeutic mechanisms, well-established safety profiles and multiple clinical trials showing benefit in similar disease models such as ARDS. The additional co-interventions were also supported by either promising early clinical data, strong scientific rationale, and/or a pre-existing clinical evidence base for similar critical care conditions as those in COVID-19. Since the development of MATH+ early in the pandemic, the treatment efficacy of the majority of the protocol components (corticosteroids, AA, heparin, statins, vitamin D, and melatonin) has now been either validated in subsequent RCTs or more strongly supported with large observational data sets [11-16].
Table 1. MATH+ Hospital Treatment Protocol for COVID-19 (www.flccc.net).
| Medication | Indication/initiation | Recommended dosing | Titration/duration |
|---|---|---|---|
| Methylprednisolone | A. Mild hypoxemia: requires O2 via NC to maintain saturation > 92%. | 40 mg IV bolus, then 20 mg IV twice daily | A1. Once off O2, then taper with 20 mg daily for 3 days then 10 mg daily for 3 days, monitor CRP response. A2. If FiO2, or CRP increase move to B. |
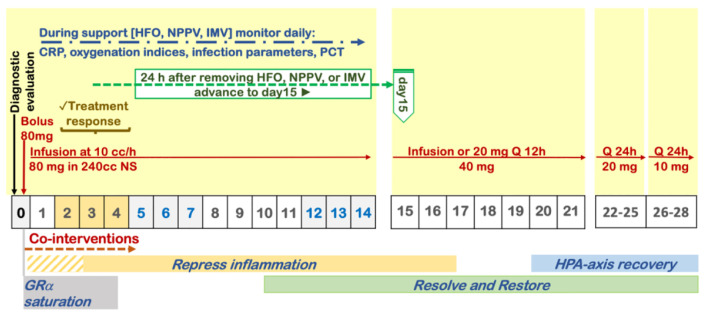
| B. Moderate-severe hypoxemia (high-flow O2, NIPPV, IMV). | COVID-19 respiratory failure protocol (Fig. 2). Preferred: 80 mg IV bolus, followed by 80 mg/240 mL normal saline IV infusion at 10 mL/h. Alternate: 40 mg IV twice daily | B1. Once off IMV, NPPV, or high-flow O2, decrease to 20 mg twice daily. Once off O2, then taper with 20 mg/day for 3 days then 10 mg/day for 3 days. B2. If no improvement in oxygenation in 2 - 4 days, double dose to 160 mg daily. B3. If no improvement and increase in CRP/ferritin, move to “pulse dose” below. | |
| C. Refractory illness/cytokine storm | “Pulse” dose with 125 mg IV every 6 - 8 h | Continue for 3 days then decrease to 80 mg IV daily dose above (B). If still no response or CRP/ferritin high/rising, consider “Salvage therapy” below | |
| Ascorbic acid | O2 < 4 L on hospital ward | 500 - 1,000 mg oral every 6 h | Until discharge |
| O2 > 4 L or in ICU | 1.5 - 3 g intravenously every 6 h | Sooner of 7 days or discharge from ICU, then switch to oral dose above | |
| Thiamine | ICU patients | 200 mg IV twice daily | Sooner of 7 days or discharge from ICU |
| Heparin (low molecular weight) | Hospital ward patients with moderate severity not requiring high-flow oxygen | 1 mg/kg twice daily. Monitor anti-Xa, target 0.2 - 0.5 IU/mL. Adjust dose and monitor for eGFR | Until discharge then start DOAC at half dose for 4 weeksb |
| ICU patientsa | 0.5 mg/kg twice daily. Monitor anti-Xa levels, target 0.6 - 1.1 IU/mL. Adjust dose and monitor for eGFR | Later of: discharge from ICU or off oxygen, then decrease to hospital ward dosing above | |
| Vitamin D | Hospital ward patients on ≤ 4 L O2 | Calcifediol preferred: 0.532 mg PO day 1, then 0.266 mg PO day 3 and 7 and weekly thereafter. Cholecalciferol: 10,000 IU/day PO or 60,000 IU day 1, 30,000 IU days 3 and 7 and then weekly | Until discharge from ICU |
| ICU patients or on > 4 L O2 | Cholecalciferol 480,000 IU (30 mL) PO on admission, then check vitamin D level on day 5, if < 20 ng/mL, 90,000 PO IU/day for 5 days | Until discharge from ICU | |
| Spironolactone | All patients | 100 mg twice daily | 10 days |
| Dutasteride | All patients (except pregnant patients) | 2 mg on day 1, then 1 mg daily (finasteride 10 mg daily | 10 days |
| Fluvoxamine | All patients | 50 - 100 mg twice daily | |
| Atorvastatin | ICU patients | 80 mg PO daily | Until discharge |
| Melatonin | Hospitalized patients | 6 - 12 mg PO at night | Until discharge |
| Zinc | Hospitalized patients | 75 - 100 mg PO daily | Until discharge |
| Famotidine | Hospitalized patients | 40 - 80 PO mg twice daily | Until discharge15 days |
| Therapeutic plasma exchange | Patients refractory to pulse dose steroids | Five sessions, every other day | Completion of five exchanges |
aContinue low molecular weight heparin 1 mg/kg twice daily in ward patients transferred to intensive care units unless contraindicated. For patients with end-stage renal disease, use unfractionated heparin. bConsider extended thromboprophylaxis. CRP: C-reactive protein; DOAC: direct oral anti-coagulant; ICU: intensive care unit; IMV: invasive mechanical ventilation; IU: International Units; IV: intravenous; NIPPV: non-invasive positive pressure ventilation; O2: oxygen; PO (per os): oral administration; eGFR: estimated glomerular filtration rate.
Many centers similarly attempted to develop “treatment guidelines” for COVID-19, and although they primarily emphasized supportive respiratory care techniques, many also included approaches either quickly retracted as obviously harmful, such as “early intubation” or therapeutic agents and interventions whose mechanisms of action held only theoretical anti-SARS-CoV-2 activity [17-21].
The authors were troubled by editorials published in major peer-reviewed medical journals which argued that all treatments used in a “novel” disease were “experimental” and thus use should be restricted to only within RCTs [22]. “Experimental” therapies, best defined as those with either no clinical evidence to support or near nil clinical familiarity with use in similar disease states, were indeed adopted and widely used, particularly in the early weeks of the pandemic when drugs such as hydroxychloroquine, remdesivir, lopinavir/ritonavir and tocilizumab were employed. However, these agents stand in marked contrast to the core MATH+ therapies of which there was extensive clinical experience and expertise amongst the authors along with published clinical evidence showing positive outcomes when used in the treatment of patients with similar diseases and conditions. In some instances, several were already incorporated into standard ICU treatment protocols for conditions such as severe pneumonia, ARDS, and sepsis in their institutions. Each element of MATH+ has been extensively studied in critical illness, almost all sufficiently so that meta-analyses have been published on their use and indications, thus none could be viewed as an “experimental therapy”, given they are considered more in line with “standard” or “supportive care” for many critical illness states.
Although the authors place immense value and importance on the need for well-conducted prospective observational and/or RCTs, in such a novel disease syndrome, it must be recognized that not all institutions possess the necessary experience, resources, or infrastructure to design and conduct such trials, especially during a pandemic. Further, the group decided against a randomized, placebo-controlled trial design given that such trials require investigators to possess “clinical equipoise”, which is the belief by the investigator that neither intervention in the control or experimental group is “better”. With respect to each of the individual “core” therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit such that use was favored, with these judgments based on not only the rapidly accumulated evidence and insight into COVID-19 but also from our collective knowledge, research, and experience with each of the component medications in critical illness and other severe infections.
Conversely, the authors believe it is within the immense power and resources of large research institutions to conduct such trials where clinical equipoise exists. A powerful example of such an accomplishment is the RECOVERY trial conducted by researchers at Oxford University [11]. Specifically, the design and execution of the RECOVERY trial depended on investigators with clinical equipoise around the use of corticosteroids in the treatment of a severe coronavirus syndrome. The MATH+ authors did not possess such equipoise, as we held a collective belief as to the critical importance of corticosteroid therapy in COVID-19, as evidenced above [6, 8, 23].
Thus, it came as no surprise to the authors that the RECOVERY trial was stopped early due to excess deaths in a control group consisting of over 4,000 patients treated with placebo. A conservative estimate of avoidable death in the placebo group if they had instead received corticosteroids is that over 200 lives would have been saved, 109 in patients requiring oxygen and 84 in those on MV [11].
The scientific and clinical rationale supporting the MATH+ treatment protocol will be reviewed in the following sections through a review of the published in-vitro, pre-clinical, and clinical data in support of each medicine, with a special emphasis on studies involving the treatment of viral syndromes and COVID-19 specifically.
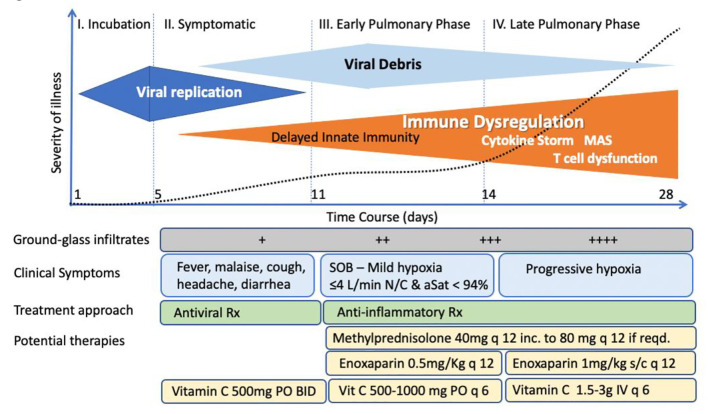
COVID-19 has several phases, a viremic asymptomatic and symptomatic phase of the disease which roughly occurs during the first 8 days of infection followed by pulmonary phases of the disease which in a small percentage of patient may lead to severe respiratory failure [24]. The major manifestations of the pulmonary phases of disease are not a result of direct viral effects, rather, it is hyper-inflammatory innate immune host response mainly driven by activated macrophages associated with thrombophilia and hypercytokinemia [24]. Thus, by the time that patients present with severe disease requiring hospitalization they generally are past the viremic phase and anti-viral therapy will most likely be ineffective. Therefore, the MATH plus protocol focuses on the management of the hyperinflammatory and coagulopathic manifestations of the disease.
Methylprednisolone and COVID-19
Methylprednisolone was chosen based on the following criteria: 1) evidence of corticosteroid responsive disease; 2) results of relevant clinical studies, many from prior viral pandemics including more than 10,000 patients; and 3) pharmacological characteristics.
Similar to ARDS, patients with severe COVID-19 have a significant reduction in glucocorticoid receptor expression in bronchoalveolar lavage fluid myeloid cells that negatively related to lung neutrophilic inflammation, NETosis, and disease severity [25, 26]. The dysregulated inflammation and coagulation observed in COVID-19 (see pathophysiology) is also similar to that of multifactorial ARDS where ample evidence has demonstrated the ability of prolonged corticosteroid treatment (CST) to downregulate systemic and pulmonary inflammation-coagulation-fibroproliferation and accelerate disease resolution [25, 27]. Additionally, the CT findings of ground-glass opacities and the histological findings of organizing pneumonia, hyaline membranes, inflammatory exudates, and acute fibrinous and organizing pneumonia are all compatible with CST-responsive interstitial inflammatory lung disease [8, 28, 29].
Relevant clinical studies at the time of the creation of MATH+ included RCTs in adult patients with non-viral ARDS, large-scale observational studies in patients with SARS-CoV (n = 7,008), H1N1 (n = 2,141), influenza, and early results from multiple COVID-19 observational studies [30-36]. In non-viral ARDS, aggregate data from 10 RCTs (n = 1,093) showed that CST was associated with a sizable increase by day 28 in MV-free days (weighted mean difference (WMD): 6.18 days, 95% confidence interval (CI): 3.45 - 8.90 days), ICU-free days (WMD: 8.12 days, 95% CI: 3.87 - 12.37 days) and a reduction in hospital mortality (risk ratio (RR): 0.67, 95% CI: 0.52 - 0.870) with the greatest impact observed with methylprednisolone treatment [6, 33, 37]. Importantly, the survival benefit observed during hospitalization persisted after hospital discharge with follow-up observations extending up to 1 year [6]. Except for transient hyperglycemia (mostly within the 36 h following an initial bolus), CST was not associated with increased risk for neuromuscular weakness, gastrointestinal bleeding, or nosocomial infections (RR: 0.83 (95% CI: 0.67 - 1.02)).
The evidence of benefit in viral pneumonia (SARS and H1N1) relies on large-scale studies (n = 9,149) which included adjustment for confounders and analysis of CST variables (type, timing, dose, and duration) on the outcome [32, 33]. These studies reported a significant reduction in mortality with dosage and duration of CST similar to the one recommended by the Corticosteroid Guideline Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) (Fig. 1) [6, 38]. In the largest SARS-CoV study, after adjustment for possible confounders, methylprednisolone 80 mg/day was safe and decreased the risk for death by 63% (hazard ratio (HR): 0.37, 95% CI: 0.24 - 0.56) [32]. In the H1N1 study, subgroup analysis among patients with PaO2/FiO2 < 300 mm Hg (535 vs. 462), low-to-moderate-dose CST (methylprednisolone 25 - 150 mg/day) significantly reduced both 30-day mortality (adjusted hazard ratio (aHR): 0.49 (95% CI: 0.32 - 0.77)) and 60-day mortality (aHR: 0.51 (95% CI: 0.33 - 0.78)) despite having a higher rate of nosocomial infections [33].
Figure 1.
Protocol for prolonged corticosteroid treatment recommended by the Corticosteroid Guideline Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM).
Methylprednisolone, for its greater penetration in lung tissue, longer residence time, and greater inhibitory activity of transcription factor nuclear factor-κB (NF-κB) (driver of lung inflammation) is the most frequently used intravenous corticosteroid for the treatment of severe acute inflammatory lung diseases [39-41]. The initial daily dose of 1 mg/kg of ideal body weight (approximately 80 mg) was the one shown to be associated with the highest mortality reduction in RCTs of non-viral ARDS and large observational studies in SARS-CoV and H1N1 pneumonia [6, 32, 33]. A recent study that matched the expression changes induced by SARS-CoV-2 in human lung tissue tissues and A549 lung cell line against the expression changes triggered by 5,694 FDA-approved drugs, found methylprednisolone to be the drug with the greatest potential to revert the changes induced by COVID-19, while other closely related corticosteroids, such as dexamethasone or prednisone, were not [42].
The risk for decreased viral clearance with CST is overstated and the most frequently quoted article by Arabi et al reported that in patients that received greater than 7 days CST, there was a strong trend toward lower 90-day mortality (aOR: 0.51, 95% CI: 0.26 - 1.00; P = 0.05) and no impact on viral clearance (aOR: 0.94, 95% CI: 0.36 - 2.47; P = 0.90) [43]. Contrary to the widespread, unfounded fears of delayed viral clearance which unfortunately influenced the multiple national and international society recommendations against use of CST in COVID-19, the reality is that there is no evidence linking delayed viral clearance to worsened outcomes in critically ill COVID-19 patients, and further, it is unlikely that it would have a greater negative impact than the hosts own “cytokine storm” [27].
Subsequent to the introduction of the MATH+ protocol, even more definitive support for CST was provided by a large randomized trial along with prospective observational studies. The RECOVERY trial investigated dexamethasone (6 mg once daily for up to 10 days) in a randomized, controlled, open-label, adaptive, platform trial with a primary outcome of 28-day mortality [11]. The RCT studied 2,104 patients randomly allocated to receive dexamethasone compared to 4,321 patients concurrently allocated to usual care. CST was associated with a significant reduction in mortality (21.6% vs. 24.6%) with an age-adjusted rate ratio): 0.83; 95% CI: 0.74 - 0.92; P < 0.001). Dexamethasone reduced deaths by one-third in the subgroup of patients receiving invasive MV (29.0% vs. 40.7%, rate ratio: 0.65 (95% CI: 0.51 - 0.82); P < 0.001), by one-fifth in patients receiving oxygen without invasive MV (21.5% vs. 25.0%, rate ratio: 0.80 (95% CI: 0.70 - 0.92); P = 0.002), but did not reduce mortality in patients not receiving respiratory support at randomization (17.0% vs. 13.2%, rate ratio: 1.22 (95% CI: 0.93 - 1.61); P = 0.14). However, it should be noted that dexamethasone is the corticosteroid associated with greater suppression of the adrenal gland. Notably, the RECOVERY RCT utilized a small dose of dexamethasone and did not incorporate tapering to prevent rebound inflammation.
An Italian multicenter, prospective observational study explored the association between exposure to prolonged CST (a pre-designed protocol: methylprednisolone 80 mg for 9 days followed by tapering based on improvement in predefined laboratory parameters) and the need for ICU referral, intubation or death within 28 days (composite primary endpoint) in patients (83 on CST vs. 90 matched control) with severe COVID-19 pneumonia admitted to Italian respiratory high-dependency units [44]. The composite primary endpoint was met by 19 vs. 40 (aHR: 0.41; 95% CI: 0.24 - 0.72). Transfer to ICU and need for invasive MV was necessary in 15 vs. 27 (P = 0.07) and 14 vs. 26 (P = 0.10), respectively. By day 28, the methylprednisolone group had fewer deaths (6 vs. 21, aHR: 0.29; 95% CI: 0.12 - 0.73) and more days off invasive MV (24.0 ± 9.0 vs. 17.5 ± 12.8; P = 0.001). Study treatment was associated with rapid improvement in PaO2/FiO2 and C-reactive protein (CRP) levels without affecting lymphocyte count. The complication rate was similar for the two groups (P = 0.84). No difference was observed in viral shedding, determined as the number of days between hospital referral and the first negative nasopharyngeal swab.
A Spanish semi-randomized study investigated methylprednisolone (3 days each, 80 mg and 40 mg, respectively) in 85 COVID-19 (56 CST, 29 control) hypoxemic patients; the primary composite outcome was similar to the Italian study [45]. CST was associated with reduced risk of the composite endpoint in the intention-to-treat, age-stratified analysis (combined RR: 0.55 (95% CI: 0.33 - 0.91); P = 0.024).
The Henry Ford COVID-19 Management Task Force conducted a single pre-test, single post-test quasi-experiment in a multicenter health system in Michigan [35]. They investigated 213 patients with confirmed moderate to severe COVID admitted over a 2-week period; the first week 81 patients received standard of care (SOC), and the second week 132 patients also received SOC and early initiation of CST (methylprednisolone 0.5 - 1 mg/kg/day for 3 days, and longer duration if they required MV). In the first week, half of the patients in the SOC group received CST but with a later initiation. The primary composite outcome was similar to the Italian study, and was reached by fewer patients in the CST group (34.9% vs. 54.3%, P = 0.005) [46]. This treatment effect was observed within each individual component of the composite endpoint. Significant reduction in median hospital length of stay was also observed in the early corticosteroid group (8 vs. 5 days, P < 0.001). Hospital length of stay was decreased by 3 days (P < 0.001) [35].
It is noteworthy that the initial MATH plus protocol emphasizing the use of methylprednisolone in patients with severe COVID-19 predated the publication of the RECOVERY groups findings.
In the aftermath of the RECOVERY trial, a total of six additional RCTs investigating CST in patients with severe COVID-19 were published. An updated meta-analysis requested by the WHO included patients randomized to receive systemic dexamethasone, hydrocortisone, or methylprednisolone (678 patients) or to receive usual care or placebo (1,025 patients) [47]. Data on mortality found little inconsistency between the trial results (I2 = 15.6%) and the summary OR was 0.70 (95% CI: 0.48 - 1.01; P = 0.053) based on the random-effects meta-analysis. They reported 222 deaths among patients randomized to corticosteroids (32.7%) and 425 deaths (42.5%) among patients randomized to usual care or placebo (summary OR: 0.66 (95% CI: 0.53 - 0.82); P < 0.001). As a result of these findings, the WHO updated their “Corticosteroids for COVID-19: Living Guidance” document recommending “systemic corticosteroids rather than no corticosteroids for the treatment of patients with severe and critical COVID-19 (strong recommendation, based on moderate certainty evidence)” [48]. Additional rationale in support of CST was recently reviewed and presented in Table 2 [35, 36, 43, 44, 49-54].
Table 2. Review of Corticosteroid Therapy in Patients With COVID-19.
| Published RCTs/cohort studies of corticosteroid therapy in COVID-19 | Absolute difference in mortality rate (Rx group vs. control group) | Estimated number needed to treat to save one life |
|---|---|---|
| Methylprednisolone - hospital patients [54] | 5.9% vs. 42.9% | 2.7 |
| Methylprednisolone - ICU patients [44] | 7.2% vs. 23.3% | 6.2 |
| Methylprednisolone - hospital patients [35] | 13.6% vs. 26.3% | 7.8 |
| Methylprednisolone - ARDS patients [53] | 46.0% vs. 61.8% | 6.3 |
| Methylprednisolone - percent on oxygen [36] | 13.9% vs. 23.9% | 10.0 |
| CoDEX - dexamethasone - mechanical ventilation [52] | 56.3% vs. 61.5% | 19.2 |
| RECOVERY trial (dexamethasone) [51] | ||
| Percent on oxygen | 23.3% vs. 26.2% | 28.6 |
| Percent on mechanical ventilation | 29.3% vs. 41.4% | 8.4 |
| Hydrocortisone - CAPE COVID - ICU patients [60] | 14.7% vs. 27.4% | 7.9 |
| Hydrocortisone - REMAP-CAP - ICU patients [49] | 28% vs. 33% | 20.0 |
Table is provided by Pierre Kory Flccc.org. COVID-19: coronavirus disease 2019.
AA and COVID-19
Approximately 15% of patients with COVID-19 infection progress to a respiratory illness, which in its early phase is consistent with OP, and if either not treated or insufficiently treated with corticosteroids progresses to a more severe pneumonitis, with about 5-10% requiring MV which then further injures the lung and causes ARDS often coincident with a cytokine storm characterized by vasoplegia, hypercoagulability and multiorgan failure [10, 25, 27]. AA is the most potent and important anti-oxidant in mammals with pleiotropic modes of action targeting multiple molecules and biological pathways involved in inflammatory states such as sepsis, ARDS, trauma, and burns [55-57].
A significant body of preclinical and clinical evidence in septic shock and other types of stress responses demonstrate that intravenous AA (IVAA) can attenuate many of the life-threatening complications of a dysregulated immune system during COVID-19 infection [27, 57, 58]. In contrast to influenza and other respiratory viruses, there is a blunted antiviral response with low interferon (IFN) production and increase in pro-inflammatory cytokines. In a minority of patients, cytokine storm ensues with overwhelming production of pro-inflammatory cytokines and reactive oxygen species (ROS) leading to progressive organ failure [25, 27, 59-61].
The innate immune and adaptive response provides an essential role in the antiviral response and is mediated by the release of type I IFN-α/β by macrophages, lymphocytes and infected immune cells [59, 62]. Several experiments employing H1N1 infected knockout mice unable to synthesize AA found that administration of AA increases IFN production, restores expression of genes necessary for production of IFNs and decreases proinflammatory gene expression with a subsequent decrease in the release of proinflammatory cytokines [62, 63]. AA is thus an essential factor in the anti-viral immune response during the early phase of virus infection through the production of type I IFNs [62].
AA is also a co-factor for the production of endogenous catecholamines and corticosteroid synthesis [57-70]. Given that humans, due to an evolutionary mutation, are almost unique among all mammals in their inability to synthesize AA, in states of stress plasma AA levels are rapidly and markedly decreased as opposed to other mammals such as goats that immediately begin to produce many grams of AA in stressed or infected states [57, 71, 72]. AA reverses ROS-induced oxidant stress impairment of glucocorticoid receptor function [73, 74]. Thus, AA is synergistic with endogenous and exogenous corticosteroids in reversal of shock [57, 74]. In clinical studies AA given with or without steroids results in decreases in vasopressor requirement and reversal of shock [57, 70, 72, 74]. AA antioxidative and ROS scavenging properties may counteract cytokine, chemokine and inflammatory cell-mediated excessive production of ROS which are known to cause decreased vascular tone and endothelial injury [72, 74].
In animal models, IVAA was shown to improve arteriolar responsiveness to vasoconstrictors and decrease microvascular permeability [72, 75]. The hemodynamic effects of AA have been demonstrated in septic shock, trauma, and burns where administration of AA reduced vasopressor and volume resuscitation requirement [55, 57, 76, 77].
Marik et al, in a propensity adjusted study of patients with sepsis, administered IVAA, hydrocortisone, and thiamine in patients with severe sepsis and found a significant survival benefit [55]. CITRIS-ALI, the largest double-blinded placebo-controlled trial of high-dose AA in ARDS patients found that both mortality and decreased ICU length of stay were significantly reduced in the treatment arm [78]. The reasons for the lack of immediate adoption of this therapy in ARDS can only be explained by the fact that the original primary outcome analysis failed to account for all the early excess deaths in the control group, where no severity of illness (SOFA) score was assigned to the patients who died. A subsequent letter to the editor by a group of prominent scientists demanded an analysis accounting for these early deaths. The study authors complied and found that the primary outcome of SOFA score was statistically significantly decreased at 96 h along with the mortality in the treated group [79]. Thus, CITRIS-ALI, although inexplicably portrayed as a negative trial, was instead profoundly positive in terms of both its primary outcome and important secondary outcomes.
Two large meta-analyses involving critically ill patients demonstrated intravenous vitamin C administration showed no adverse reactions, reduced the need for fluids and vasopressor support and reduced the length of time on mechanical ventilators [58, 80].
Most importantly, a prospective, randomized, double-blinded, placebo-controlled trial of high-dose IVAA in COVID-19 respiratory failure was conducted at three hospitals in Hubei, China where the intervention group was treated with 12 g of IV AA every 12 h for 7 days [81]. The trial was stopped early due to control of the epidemic, thus only 56 patients were included. Although the primary endpoint of invasive MV-free days was not significant (26.0 vs. 22.0, P = 0.57), significant improvements in oxygenation and reductions in IL-6 were found in the intervention group over the 7 days and a reduction in 28-day mortality was observed, although the difference was not statistically significant (22.2% vs. 37.9%, P = 0.31). In the sub-group of patients with SOFA scores ≥ 3, the differences in ICU and hospital mortality were statistically significant while the 28-day mortality approached, but did not reach statistical significance (21.7% vs. 52.4%, P = 0.06) [81]. To date, there is only one small RCT involving IVAA demonstrating improved mortality in those patients with an SOFA score ≥ 3, and there has been accumulating evidence that IVAA improves inflammatory markers, coagulopathic parameters, ameliorates cardiac injury, decreases incidence of systemic inflammatory response syndrome (SIRS) and shortens the duration of SIRS (Table 3) [81-89].
Table 3. Clinical Studies Involving IVAA in COVID-19.
| Study type | Intervention | Outcome IVAA vs. control | Comments/level of evidence |
|---|---|---|---|
| RCT of 56 COVID-19 pneumonia and multiple organ injury ICU patients. Age: 67 ± 13 years. Severe COVID-19 PaO2/FIO2 < 300 [81] | IVAA: 24 g/day (n = 27) or placebo (n = 29) for 7 days (*corticosteroid allowed) (time to treatment 11 - 25 days to onset of symptoms) | IVAA: lower ICU and hospital mortality in patients with SOFA scores ≥ 3 (3 - 10 days, P = 0.03). No difference in ventilation-free days (26.5 vs. 10.5 days, P = 0.56) | IVAA group had higher PaO2/FIO2 and lower IL-6 levels on admission. Level of evidence 2 |
| Open label RCT of 150 patients with severe COVID-19. Age: 52 - 53 years [82] | IVAA: 50 mg/kg/day + standard therapy or standard therapy (75 per group) | IVAA: sooner to symptom-free status (7.1 ± 1.8 vs. 9.6 ± 2.1) (P < 0.0001). No difference in mortality or need for mechanical ventilation | Corticosteroids allowed. Level of evidence 2 |
| Open label RCT of 60 patients with severe COVID-19. Age: 57 - 61 years [83] | IVC 6 g/day + standard therapy or standard therapy (n = 30 per group) for 5 days | Lower body temperature on third day of hospitalization (P = 0.001) Improvement in oxygen saturation on third day of hospitalization (P = 0.014). No difference in mortality | Both groups received dexamethasone. Level of evidence 2 |
| Retrospective cohort study of 76 patients with moderate to severe COVID-19. Age: 52 - 71 years [84] | IVAA: 6 g/12 h on first day, 6 g/day for following 4 days + standard therapy (n = 30) or standard therapy (n = 46) for 5 days | Improved oxygenation in treatment group. Decrease in pro-inflammatory biomarkers. Reduced risk of 28-day mortality (HR: 0.14, 95% CI: 0.03 - 0.72, P = 0.037) | Level of evidence 3 |
| Propensity matched before and after retrospective analysis of 110 patients with moderate COVID-19 pneumonia. Age: 31 - 47 years [85] | IVAA: 100 mg/kg/day + standard therapy or standard therapy (55 per group) for 7 days | Fewer patients progressing to severe type of COVID-19 (P = 0.03). Reduction in incidence and progression of SIRS (P = 0.008) | Level of evidence 3 |
| Retrospective analysis of 232 patients with COVID-19 pneumonia. Age: 46 - 74 years [86] | IVAA: 2 g/day + standard therapy (n = 153) or standard therapy (n = 170) | Shorter length of hospital stay (7 vs. 8 days, P = 0.05). No difference in re-admission rate (P = 0.94), admission to ICU, need for advanced oxygen support (P = 0.49), and mortality (P = 0.52). Need for advanced medical treatment (P < 0.001) | Level of evidence 3 |
| Retrospective study of 34 critically ill patients with COVID-19. Age: 53 - 77 years [87] | IVAA: 1.5 g/6 h + standard therapy (n = 8) or standard therapy (n = 24) for 4 days | Higher rate of hospital mortality in treatment group (19 (79%) vs. 7 (88%), P = 0.049) and SOFA score. No difference in daily vasopressor requirement or ICU length of stay | Higher baseline SOFA score in IVAA group. Level of evidence 3 |
| Retrospective study of 236 patients with severe COVID-19. Age: 57 - 73 years [88] | IVAA: 100 mg/kg/6 h on day 1 then 100 mg/kg/12 h for the next 5 days + standard therapy (n = 85) or standard therapy (n = 151 | Reduction in inflammatory markers (CRP, P = 0.032; IL-6, P = 0.005; TNF-α, P = 0.015) | Level of evidence 3 |
| Retrospective study of 113 patients with severe COVID-19 and cardiac injury. Age: 59 - 77 years [89] | IVAA: 100 mg/kg/6 h on day 1 then 100 mg/kg/12 h for the next 5 days + standard therapy (n = 51) or standard therapy (n = 62) | IVAA was associated with ameliorated cardiac injury (P = 0.04). Reduced levels of inflammatory markers (CRP, IL-6, IL-8, and TNF-α) at 21 days of hospitalization | Level of evidence 3 |
Based on level 5 evidence (cited in text) and current studies, we recommend the use of IVAA as adjunctive therapy in patients with COVID-19. IVAA: intravenous ascorbic acid; SOFA: sequential organ failure assessment; RCT: randomized controlled trial; HR: hazard ratio; CI: confidence interval; TNF-α: tumor necrosis factor alpha; IL-6: interleukin-6; IL-8: interleukin-8; CRP: C-reactive protein; SIRS: systemic inflammatory response syndrome; IVC: intravenous vitamin C.
In summary, IVAA was included based on the pleiotropic effects on important physiologic functions, its properties as powerful antioxidant/ROS scavenger, and reversal of ROS-induced oxidant stress impairment of glucocorticoid receptor function, its impact on outcomes in the treatment of both COVID respiratory failure and non-COVID ARDS as well as other hyperinflammatory conditions along with an impeccable safety profile and low cost. Based on the current preclinical and clinical evidence based on Oxford evidence medicine levels of evidence (Oxford Center for Evidence-Based Medicine Levels of Evidence (http://www.cebm.net/?o=1 116), we recommend the use of IVAA as an adjunctive therapeutic in the management of COVID-19 [90].
Thiamine and COVID-19
Thiamine is a water-soluble vitamin passively absorbed in the small intestine. After ingestion, free thiamine is converted to the active form thiamine pyrophosphate (TPP), commonly known as vitamin B1, by thiamine pyrophosphokinase. The majority of TPP in the body is found in erythrocytes and accounts for approximately 80% of the body’s total storage [91]. TPP is a key co-factor for pyruvate dehydrogenase, the gatekeeper for entry into the Krebs Cycle, without which pyruvate would be converted to lactate as opposed to acetyl-coenzyme A [91].
Multiple other non-cofactor roles of thiamine exist within the immune system, gene regulation, oxidative stress response, cholinergic activity, chloride channel function, and neurotransmission [91]. In experimental rheumatoid arthritis, thiamine increased the ability of corticosteroids to suppress production of tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) [92].
The human adult can store around 30 mg of thiamine in muscle tissue, liver and kidneys; however, these stores can become depleted in as little as 18 days after the cessation of thiamine intake [91]. A thiamine deficiency syndrome, beriberi, bears a number of similarities to sepsis, including peripheral vasodilation, cardiac dysfunction, and elevated lactate levels [57]. In critical illness, the prevalence of thiamine deficiency is in 10-20% upon admission and can increase up to 71% during ICU stay, suggesting rapid depletion of this vitamin [93, 94]. Based on limited data, no association was detected between thiamine levels, markers of oxidative stress and mortality [94, 95].
In one study, a significant negative correlation was reported between thiamine and lactic acid levels in patients with sepsis without liver dysfunction [93]. In a pilot RCT of patients with septic shock (n = 88), the administration of thiamine (200 mg twice a day for 7 days) reduced lactate levels and improved mortality over time in a pre-defined subgroup of patients with thiamine deficiency (35% of cohort) [96]. In a retrospective, single-center, matched cohort study, administration of thiamine within 24 h of septic shock (n = 123) was associated with improved likelihood of lactate clearance and a reduction in 28-day mortality [97]. In a randomized study of patients undergoing gastrointestinal surgery, thiamine administration (200 mg daily for 3 days) was associated with significant reduction in post-operative delirium [98].
It should be noted that the increased secretion of IL-17 by TH17 cells contributes to the proinflammatory cytokine storm characteristic of COVID-19 [99]. In an ex-vivo study, Vatsalya et al demonstrated that 200 mg thiamine/day decreased TH17 cell activation [100].
Sulaiman et al evaluated the use of thiamine as an adjunct to therapy in a propensity matched study involving 738 critically ill patients. Propensity matching included 166 patients of whom 83 received thiamine. There was a statistically significant decrease in mortality and incidence of thrombosis in patients receiving thiamine [101].
Given these promising results and favorable safety profile, the MATH+ protocol included thiamine supplementation as part of the combination therapy in critically ill COVID-19 patients.
Anticoagulation (AC) and COVID-19
From the earliest clinical experiences caring for COVID-19 patients, physician reports of excess clotting emerged from China and Italy [102-104]. Infections are recognized activators of inflammatory and coagulation responses as part of the host defense, and in COVID-19, although patients present with prominent elevation of D-dimer and fibrin/fibrinogen degradation products as is typically seen in traditional disseminated intravascular coagulation (DIC), either little or no abnormalities in prothrombin time (PT), partial thromboplastin time (PTT), and platelet counts are seen initially [102]. The term COVID-19-associated coagulopathy (CAC) was created to describe these abnormalities in tests, although typical impaired clotting that results in increased bleeding is not observed [102]. Conversely, nearly all published clinical reports describe CAC as a “hypercoagulable” condition.
Thromboelastography (TEG) has best elucidated the hypercoagulable nature of CAC given its ability to assess both the pro-thrombotic and hypocoagulable dynamics of whole blood as it forms clot under conditions of low shear stress. A group including one of the authors (PK) recently published a case series of TEG studies from the first wave of COVID-19 patients encountered which consistently revealed hypercoagulability with rapid and large amplitudes of clot formation with little to no fibrinolytic activity present [105, 106]. These early insights, along with the large amount of subsequent investigations reviewed below, served as an initial basis for the more aggressive anti-coagulation regimen incorporated within MATH+.
Given that investigations into CAC found severe hypercoagulability, it is unsurprising that the majority of published data report a higher than previously reported frequency of clotting in critically ill COVID-19 patients despite receiving thromboprophylaxis. Helms et al from France reported an incidence of 16.7% of venous thromboembolism (VTE) (mainly pulmonary embolism (PE)) in their COVID-19 respiratory failure patients, an incidence six-fold higher than a matched population of non-COVID ARDS patients treated a year prior. Equally alarming, 96.6% of patients on continuous renal replacement therapy developed circuit clotting. In two studies from Holland, the incidence of VTE in ICU patients was up to one-third by day 7 and greater than 50% after day 14 [72, 79].
In a lower extremity ultrasound screening study of an ICU population with two-thirds on systemic AC and one-third on thromboprophylaxis, VTE was found in 69% of the patients, with a 100% incidence in those on prophylaxis and 56% in patients on AC [107]. The VTE rates reported in the above ICU populations of COVID-19 patients are magnitudes higher than the approximate 8% rate of VTE reported in previous studies of non-COVID-19 ICU patients receiving thromboprophylaxis [108].
In contrast to COVID-19 ICU patients, the rates of VTE in COVID-19 hospitalized ward patients have been lower. Middeldorp reported a cumulative 9.2% incidence of VTE, similar to pre-COVID-19 incidences in non-ICU patients; however, another study found a cumulative incidence of 27% with 4% arterial thrombosis resulting in a composite incidence of 29% [109, 110]. However, not all studies of hospital ward patients found such high incidences, for instance Lodigiani et al reported a 6.6% incidence in this population while Cattaneo et al found that in a population of 388 COVID-19 patients, 64 underwent screening leg ultrasound, and no patient developed VTE [111].
In regards to PE incidences alone, a recent systematic review of PE prevalence in COVID-19 analyzed 52 studies which included 20,523 patients and reported a markedly increased pooled prevalence of 9% in non-ICU patients and 19% among ICU patients [112].
In addition to the markedly elevated incidence of “macrovascular” thrombosis, autopsies have also revealed extensive microvascular thromboses, with one report finding severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes and widespread thrombosis with microangiopathy [113]. Another found that alveolar capillary microthrombi were nine times higher prevalent in COVID-19 patients than patients with influenza (P < 0.001) [114]. Microvascular thrombosis is also a prominent feature in multiple organs, in some cases despite full anticoagulation and regardless of timing of the disease course, suggesting that it plays an early role in causing illness [115]. A recent autopsy series found that in 17 of 25 examined lungs, intravascular fibrin thrombi were found within medium sized arteries or arterioles while in 23 of the 25, platelet aggregates and/or thrombi were found in medium sized arteries, arterioles and capillaries [116]. Even more worrisome were the brain findings where a widespread presence of microthrombi and acute infarction was observed in six of 20 cases. In two of the cases with clinical infarction, there was global anoxic brain injury. Further, in a recent systematic review examining the incidence of stroke in COVID-19, the proportion of COVID-19 patients with stroke (1.8%, 95% CI: 0.9-3.7%) was eight times higher than that reported among hospitalized patients with influenza (0.2%) [117]. More concerning was the suggestion that these estimates were almost certainly a gross underestimate due to: 1) missed stroke diagnoses in those not extubated and who died; 2) the restrictions on and therefore lack of autopsies; and 3) the well-recognized drop in the number of patients with acute cerebrovascular symptoms seeking medical attention in the COVID-19 era.
Given such high and devastating incidences of macro- and micro-vascular thrombosis in multiple organs among COVID-19 patients, a major clinical question is whether anti-coagulant therapy can improve the outcomes of COVID-19 patients. Tang first reported on 449 patients with “severe” COVID-19 and found that low-molecular weight heparin (LMWH), the majority of the time in prophylactic doses, was associated with a large mortality benefit in the sub-group of patients meeting sepsis-induced coagulopathy score ≥ 4 (40.0% vs. 64.2%, P = 0.029), or D-dimer more than six-fold of upper limit of normal (32.8% vs. 52.4%, P = 0.017) [118]. A large study from Mt. Sinai in New York City on 2,777 patients reported a mortality of 29.1% in those treated with therapeutic AC compared to 62.7% who did not receive treatment dose [119]. Another study found that among 49 mechanically ventilated patients, 33% were diagnosed with PE and that the use of high-intensity thromboprophylaxis was associated with a lower occurrence of PE (2/18; 11%) than a standard regimen (11/22; 50%; OR 0.13 (0.02 - 0.69); P = 0.02) [120].
A retrospective study of 468 hospitalized patients also found that the initial use of high-intensity thromboprophylaxis was associated with improved 30-day mortality (adjusted RR 0.26; 95% CI: 0.07 - 0.97; P = 0.04) without a significant increase of bleeding [121]. The now largest cohort study of 4,389 patients found that both prophylactic and therapeutic ACs were associated with an absolute decrease of in-hospital mortality and intubation by almost 50% and 30%, respectively [122]. Among the sub-group of patients (n = 1,860) initiated on AC within 48 h of admission, therapeutic AC was associated with lower in-hospital mortality than prophylactic AC, although the difference was not statistically significant (aHR 0.86; 95% CI: 0.73 - 1.02; P = 0.08). Interestingly, rates of major bleeding were similar on therapeutic AC (27/900, 3.0%) as compared to patients on prophylactic AC (33/1,959, 1.7%) and no AC (29/1,530, 1.9%). Jonmarker et al compared the outcomes of COVID-19 ICU patients treated with standard, intermediate, and full-dose AC [123]. They found that mortality was lower in high dose (13.5%) vs. medium dose (25.0%) and low dose thromboprophylaxis (38.8%) groups (P = 0.02).
The first RCT comparing therapeutic AC to standard thromboprophylaxis in COVID-19 ICU patients on MV (HESACOVID) was recently published, and although small, reported statistically significant improvements in oxygenation, liberation from MV (HR: 4.0 (95% CI: 1.035 - 15.053), P = 0.031), and ventilator-free days (15 days (IQR 6 - 16) versus 0 days (IQR 0 - 11), P = 0.028) in patients treated with therapeutic doses of AC [124].
In contrast the REMAP-CAP, ACTIV-4a and the ATTACC, the largest prospective international platform studies employing Bayesian analytics demonstrated that in critically ill patients defined as ICU level of respiratory-cardiac support either in an ICU or non-ICU floor, the administration of systemic AC with therapeutic dose heparin compared to heparin thromboprophylaxis resulted in no improvement in clinical outcomes [125]. Conversely, in non-critically ill COVID-19 patients, systemic AC with therapeutic dose heparin compared to thromboprophylaxis resulted in improved survival, and decreased need for cardiorespiratory support [126]. Based on these findings, the initial MATH+ has been modified to recommend unless otherwise indicated that systemic AC with therapeutic dose heparin be reserved for non-critically ill COVID-19 patients and thromboprophylaxis be instituted in critically ill. Systemic heparinization should be continued if the non-critically progress to requiring ICU level of care.
Although it is encouraging that the initial MATH+ protocol-recommended treatment dose AC for COVID-19 ICU patients has now been strongly associated with improved survival, what is worrisome are the multiple reports of “coagulation failure” in which severe thrombotic complications occurred in COVID-19 patients despite therapeutic AC [107, 109, 127]. A possible explanation for this phenomenon was provided by Maier et al, where they used capillary viscometry in 15 severely ill COVID-19 ICU patients, almost all in ARDS, and found that all patients had a blood viscosity exceeding 95% of normal, a condition they termed “COVID-19 associated hyperviscosity” [128]. The four patients with the highest viscosity all suffered thrombotic complications despite the majority of patients having been on either systemic AC or intermediate dose prophylaxis. Given that hyperviscosity is thought due to increased plasma proteins such as fibrinogen or immunoglobulin which then damage endothelium, this suggests that therapeutic plasma exchange (TPE) may play a role [129]. The growing body of evidence strongly supporting the role of TPE in COVID-19 is reviewed below in the section “Salvage therapy”.
To the best of our knowledge, no major national or international medical society to date has recommended therapeutic AC be administered as standard practice in any sub-group of COVID-19 patients. Many have instead recommended standard thromboprophylaxis for all hospitalized patients with COVID-19 while also avoiding a recommendation for even high-intensity thromboprophylaxis. This therapeutic conservatism is puzzling, given that, based on the best available evidence to date, the incidence and risks of the now well-described severe hypercoagulability appear to far outweigh the risks of even a slightly more aggressive AC regimen, based on the large magnitude of survival associated with therapy and the paucity of reports of significantly increased bleeding complications [118, 119]. Thus we believe that, in hospitalized patients, an aggressive thromboprophylaxis in critically ill patients and therapeutic dose AC be administered in non-critically ill patients unless specifically contraindicated.
The “intermediate” dose thromboprophylaxis we recommend in ICU patients is based on pharmacokinetic and anti-Xa level monitoring studies and suggests use of weight-based prophylaxis with 0.5 mg/kg twice daily of LMWH [130]. In regards to patients with impaired renal function particularly in those with creatinine clearances of less than 30 mL/min, there is currently conflicting literature on dosing as in some preparations, there is risk of accumulation [131]. We therefore recommend monitoring anti-Xa levels in this subset of patients and the use of unfractionated heparin in patients on dialysis or those with creatinine clearances of less than 10 mL/min.
In non-ICU patients, we recommend treatment dose AC be provided using 1 mg/kg LMWH twice daily. Further, we recommend monitoring of anti-Xa levels aiming for an anti-Xa activity of 0.6 - 1.1 IU/mL due to reports that heparin resistance appears to be common in COVID-19 [132]. In addition, due to augmented renal clearance, COVID-19 patients may have reduced anti-Xa activity despite standard dosages of LMWH [133].
Melatonin and COVID-19
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized from tryptophan in the pineal gland and in the mitochondria of almost all cells in the body [134]. Melatonin is released from the pineal gland into the systemic circulation, achieving plasma concentration between 80 and 120 pg/mL at night and 10 - 20 pg/mL during the day. Melatonin binds to two receptor subtypes: MT1 and MT2 [135]. The melatonin receptors are G-protein coupled receptors (GPCRs) which both activate and inhibit a constellation of intracellular signaling pathways.
In addition to its role in regulating the circadian rhythm, melatonin is a potent anti-oxidant and immune regulator that controls both the innate and adaptive immune response [134, 136]. The anti-oxidative effect of melatonin cooperates with its anti-inflammatory actions by up-regulating anti-oxidative enzymes (e.g. superoxide dismutase), down-regulating pro-oxidative enzymes (e.g. nitric oxide synthase), and by interacting directly with free radicals, functioning as free radical scavenger [134, 137]. Melatonin plays an important role in protecting the mitochondria from oxidative injury, thereby playing a critical role in maintaining energy production [134]. Melatonin has significant anti-inflammatory, anti-apoptotic properties, and anti-NF-κB activation and has been demonstrated to reduce pro-inflammatory cytokines levels [138-141].
Melatonin levels fall off dramatically after age 40; these are also the patients at highest risk of developing COVID-19 and from dying from the disease [142, 143].
SARS-CoV-2-induced endothelial dysfunction is initiated by increases in the phosphorylation levels of JAK2 and STAT3, producing increased amounts of ROS [144]. These changes can be reversed by administration of melatonin by abating the production of superoxide anion, hydrogen peroxide and peroxynitrite [138]. The clinical utility of melatonin in COVID-19 was first demonstrated in a large prospective registry created to identify risk factors for the development of a positive SARS-CoV-2 test [16]. Researchers found that the most potentially impactful intervention to lower risk of testing positive was if patients were taking melatonin, paroxetine, or carvedilol, all medications that had been previously identified in drug-repurposing studies to have specific activity and potential benefit against SARS-CoV-2 [16, 141].
Oral melatonin use by humans is exceedingly safe, with only minor side effects such as headache and drowsiness. The lethal dose 50 (LD 50) of melatonin is reported to be infinity, i.e., it is impossible to administer a large enough dose of melatonin to kill an animal. It should be noted that there is marked variability in first-pass hepatic metabolism, resulting in marked unpredictability in serum levels [144]. Furthermore, the optimal dose of melatonin in “healthy individuals” and those with inflammatory disorders is unknown. For patients with COVID-19 we suggest a dose of 6 - 12 mg, taken at night [138]. However, a dose of up to 400 mg has been suggested [140]. To date, there have been three randomized trials demonstrating that melatonin use leads to quicker time to recovery, decrease in CRP levels and improved oxygenation level (Table 4) [145-147]. In addition, a retrospective study of 791 patients revealed that melatonin exposure post intubation was associated with improved survival (Table 4) [148].
Table 4. Clinical Studies Involving Melatonin in the Treatment of COVID-19.
| Trial details | Dose | Outcomes | Comments/level of evidence |
|---|---|---|---|
| Randomized single blind trial. Treatment: n = 14. Control: n = 17. Age: 25 - 65 years. Hospitalized mild moderate COVID-19 [145] | 6 mg melatonin at bedtime for 14 days | Improved declined CRP in treatment group. Percentage of recovery (based on symptoms) in patients who took melatonin was higher than that of patients in the control group (85.7% vs. 47.1%) | Corticosteroids given. Level of evidence 2 |
| Double blind randomized controlled trial. Treatment: n = 24. Control: n = 20. Age: 36 - 64 years. Mild to moderate COVID-19 [146] | 3 mg melatonin three times a day for 14 days | Faster time to symptom resolution, faster time to discharge. Improvement in CRP | Unclear if standard of care included corticosteroids. Level of evidence 2 |
| Randomized open label study. Treatment: n = 48. Control: n = 48. Age: 36 - 69 years. Mild to moderate COVID-19 [147] | 3 mg at bedtime for 7 days | Improvement in oxygenation | Higher usage of methylprednisolone in control group. Level of evidence 2 |
| Retrospective study. Patients: n = 791. Mean age: 56 years. Patients received melatonin: n = 112 [148] | 112 patients received melatonin | Improved survival in those patients who received melatonin | Study adjusted for comorbidities and therapy including corticosteroids. Level of evidence 3 |
Based on level 5 evidence (cited in text) and current studies, we recommend the use of melatonin as adjunctive therapy in patients with COVID-19. CRP: C-reactive protein.
Based on the level of evidence (Oxford Center for Evidence-Based Medicine Levels of Evidence (http://www.cebm.net/?o=1116), we recommend the use of melatonin as adjunct to therapy.
Zinc and COVID-19
Zinc likely plays an important role in the prophylaxis of COVID-19, in the treatment of the early symptomatic phase, and in limiting the immune dysregulation and associated cytokine storm in the pulmonary phase [149]. Zinc is a nutritionally fundamental trace element and is the second most abundant trace metal in the human body after iron. Since zinc does not have a major storage depot in the body, zinc deficiency is easily and rapidly produced. It should be recognized that the same dietary factors leading to deficiency of zinc frequently result in the deficiency of other micronutrients. Zinc plays an important role in the host’s anti-viral (and antibacterial) immune response. In addition, zinc is directly viricidal. Zinc is a component of over 1,000 transcription factors, including DNA binding proteins and is required in over 300 metalloenzymes. Zinc plays a central role in cellular differentiation and proliferation, and its deficiency causes impaired immune response, increased susceptibility to infections and impaired wound healing [150, 151]. Zinc is necessary for optimal functioning of both innate and adaptive immunity. Zinc status strongly affects T- and B-lymphocyte function and antibody formation [150]. Impaired immune function due to inadequate zinc status may be the most common cause of secondary immunodeficiency in humans. Zinc deficiency is an important public health problem affecting 2 billion people worldwide, including a considerable proportion of the Western population [150, 152-154]. Zinc levels are reported to be very low in critically ill patients, particularly those with sepsis and acute respiratory failure [151, 155, 156]. Low zinc levels have been reported to be associated with recurrent infections and increased hospital mortality [157]. In addition, zinc deficiency has been demonstrated to potentiate ventilator-induced lung injury [158].
Previous studies have demonstrated the benefit of zinc supplementation in viral infections, most notably upper respiratory tract infections. Meta-analyses of RCTs have demonstrated that zinc lozenges at a dose of ≥ 75 mg/day (elemental zinc) administered within 24 h of onset of symptoms and taken for at least 5 days significantly reduced the duration of common cold symptoms, school absence and the use of antibiotic [159, 160]. Trials of low dose zinc lozenges (< 75 mg/day zinc) found no effect on the duration of colds. However, when combined with vitamin C, low-dose zinc was reported to reduce the duration of symptoms of the common cold [154]. When used prophylactically for at least 5 months, zinc lozenges at a dose ≥ 75 mg/day reduced the risk of developing a common cold. Zinc supplementation of nursing home elderly patients was reported to reduce the incidence of pneumonia [161]. Adverse events of zinc lozenges include a bad taste and increased incidence of nausea.
Te Velthuis and colleagues demonstrated that zinc together with the zinc ionophore pyrithione inhibited the activity of the SARS-CoV RNA-dependent RNA polymerase blocking viral replication in a cell culture [162]. It should be noted that both hydroxychloroquine and the plant phytochemical quercetin are zinc ionophores [163, 164]. However, the role of zinc with or without the addition of zinc ionophores in the treatment of COVID-19 remains speculative [165].
Selective Serotonin Reuptake Inhibitors (SSRIs) and COVID-19
SSRIs, particularly fluvoxamine and fluoxetine have demonstrated promise as repurposed agents in the management of COVID-19. The administration of these agents is associated with a decrease in the release of pro-inflammatory cytokines IL-6, IL-10, TNF-α and CCL2 [166]. SSRIs particularly fluvoxamine and fluoxetine possess pleotropic effects such as inhibiting the SR1 potentially attenuating the cytokine storm. Some SSRIs inhibit acid sphingomyelinase activity which may prevent viral entry into epithelial cells [166-168]. In addition, SSRIs inhibit platelet activation thus potentially preventing endothelial injury and microthrombosis [166]. A placebo control double-blinded randomized double-blinded study demonstrated that the administration of fluvoxamine at a dose of 300 mg per day decreased hospitalization rates [169].
The large randomized placebo-controlled platform TOGETHER trial revealed that 100 mg of fluvoxamine two times a day for 10 days was effective in reducing the need for hospitalization in high risk outpatients with COVID-19 [170]. Recently, a large retrospective study conducted by Hoertel et al revealed that use of antidepressants particularly SSRIs was significantly associated with a reduction in mortality and need for MV [167].
Anti-Androgen Therapy and COVID-19
Male sex, particularly those with androgenetic alopecia (AGA) [171-175], users of anabolic-androgenic steroids (AAS) [176], hypersensitivity of the androgen receptor (AR) [177, 178], and women with hyperandrogenic states are known independent risk factors for COVID-19 [179, 180]. In contrast, users of anti-androgen agents, in particular those under androgen deprivation therapy (ADT) for castration-resistant prostate cancer, and males with prostate cancer have lower risk compared to age-adjusted males without prostate cancer [181-185]. These observations are supported by the molecular mechanisms of SARS-CoV-2 cell entry is highly dependent on androgen activity [186, 187]. In addition, there is a strong correlation between SARS-CoV-2 concentration and damage, and androgen activity [188, 189]. These findings support the hypothesis that anti-androgens could be effective against COVID-19 with observational and RCTs in early COVID-19 have demonstrated that dutasteride, spironolactone and proxalutamide could protect against COVID-19 disease progression [190-195]. These benefits have also been observed in hospitalized patients through RCTs for finasteride and proxalutamide [191, 193, 194, 196, 197]. Thus, the strong biological plausibility of androgen-dependent mechanisms of SARS-CoV-2 cell invasion, the multiple corresponding epidemiological observations in both excess and blockage of androgen activity, observational, and several RCTs demonstrating the efficacy of a variety of anti-androgens in preventing COVID-19 disease progression support the recommendation for their use during hospitalization.
Anti-androgen treatment during hospitalization must be done for at least 12 - 14 days, since earlier interruptions can lead to severe relapse, rapid disease progression and high mortality rate [198].
Vitamin D and COVID-19
Vitamin D is obtained via the diet or produced in the skin by UVB light. Aside from its known role in calcium metabolism and bone health, it also has important roles in the immune system including support of endothelial barriers, and innate and adaptive immunity [199]. The innate immune system in COVID-19 produces both pro-inflammatory and anti-inflammatory cytokines while vitamin D reduces the production of pro-inflammatory Th1 cytokines such as TNF-α and IFN-γ and increases the expression of anti-inflammatory cytokines by macrophages [200-202].
Given its important roles in immune function, many have hypothesized that vitamin D deficiency increases susceptibility to infections and that supplementation may improve outcomes, particularly in COVID-19 [203, 204]. Data supportive of the theory that deficiency leads to infections largely rest on the fact that seasonal influenza infections generally peak in conjunction with times of the year when 25(OH)D concentrations are lowest [205]. Further, the onset of the epidemic and higher case load in countries during the winter season also raises the possible association with low vitamin D status [206]. Rhodes et al first identified this link by comparing the mortality of COVID-19 in relation to country latitude and found that, even after adjusting for age, there was a 4.4% increase in mortality for each degree latitude north of 28°. Further, ethnic minorities in both the United States of America and the United Kingdom have high rates of vitamin D deficiency, potentially explaining why the mortality rates in these populations are much higher. Recently, strong evidence supporting a prophylactic role of vitamin D supplementation in COVID-19 comes from a large observational analysis of de-identified tests from a national laboratory which included over 190,000 patients from all 50 states. They analyzed SARS-CoV-2 test results among patients with a vitamin D level drawn at some point in the previous 12 months. The SARS-CoV-2 positive test rates among three vitamin D range levels were as follows: 12.5% if “deficient” (< 20 ng/mL), 8.1% if “adequate” (30 - 34 ng/mL), and 5.9% if the level was above 55 ng/mL [207].
Given the strong associations of vitamin D deficiency with higher rates of viral infections, multiple studies have tested whether vitamin D supplementation can reduce this risk. Although studies have conflicted in their findings, a recent meta-analysis from 2018 found that regular supplementation with vitamin D decreased the risk of acute respiratory tract infections, with the most profound effects in patients with severe vitamin D deficiency [208].
The risk of vitamin D insufficiency and the benefits of pre-illness supplementation were most recently highlighted in an Iranian study of 235 patients with vitamin D levels measured on admission [209]. They found that of the patients with severe COVID-19, 67.2% had vitamin D insufficiency. Further, the mortality rates of patients over 40 with and without sufficient vitamin D were 9.7% vs. 20%, suggesting that vitamin D serves an important role in modulating the immune response.
In the ICU, vitamin D deficiency is common and levels decrease rapidly after admission [210, 211]. Further, deficiency has strong negative correlations with outcomes, namely higher mortality [212, 213]. Multiple, initial studies of vitamin D supplementation in critically ill populations were conducted and included in a 2017 meta-analysis that found a statistically significant effect in reducing mortality [214, 215]. However, more recently, the results of a large, prospective, multi-national, double-blinded, placebo-controlled trial (VIOLET) on the effects of cholecalciferol supplementation in vitamin D deficient critically ill patients were published [216]. The study results, surprisingly, were profoundly negative in that no benefits were found of giving a large single dose supplement given around the time of admission into the ICU. Further, no benefits were found in any individual sub-group, even among those with more severe illness or with more severe deficiency.
Although the findings of the VIOLET trial strongly suggest that vitamin D supplementation alone has no benefit as an intervention in the critically ill, our inclusion of vitamin D in COVID-19 treatment, aside from the evidence suggesting the possibility of a more potent therapeutic role in both viral syndromes and COVID-19 (likely few patients with viral syndromes were included in the VIOLET study), is largely based on the therapeutic enhancement of corticosteroid effect when co-administered with vitamin D, similar to the synergistic effects of corticosteroids with vitamin C [217]. Investigators have demonstrated that vitamin D up-regulates glucocorticoid receptors which leads to increased T-cell apoptosis while it can also enhance the corticosteroid effect on and suppression of cytokine production in peripheral blood cell monocytes [218-220].
Recently, in a pilot RCT of vitamin D therapy in hospitalized COVID-19 patients using calcifediol, the direct precursor to the active form of vitamin D in the serum, patients were treated on the day of admission with an oral dose of 0.532 mg (roughly equivalent in potency to a dose of 68,000 IU of vitamin D3), then they gave half the dose on day 3, day 7, and weekly thereafter. They found that of the 50 patients treated with calcifediol, one required admission to the ICU (2%) while of 26 untreated patients, 13 required admission to the ICU (50%) (P < 0.001, OR 0.02 (0.002 - 0.17)) [14]. None of the treated patients died while two control group patients died. The authors concluded that calcifediol seems to reduce the severity of the disease, but larger trials will be required to provide a more definitive answer.
Thus, available data suggest that high-dose vitamin D supplementation is beneficial not only in the prevention of viral infections but also in COVID-19 and in improving the effects of corticosteroid therapy.
Although the impact of supplementation varies by deficiency status as well as severity of illness, vitamin D supplementation is safe; one meta-analysis of healthy patients found no adverse events, while in the critically ill, mild hypercalcemia was the most common adverse effect [208, 221].
Serum levels greater than 50 nmol/L (20 ng/mL) are thought sufficient for protection against acute respiratory tract infections [208]. It should be noted that the predominant form of supplementation in North America is vitamin D2 (ergocalciferol) and in Europe it is vitamin D3 (cholecalciferol), although the dosing is the same. One report found that “doses up to 10,000 IU/day is safe, although well above what is needed” and that “only 1,000 - 2,000 IU may be needed to obtain optimal effects on bone and immunity” [222]. Thus to reduce the risk of infection, one expert recommended that people at risk of COVID-19 consider taking 10,000 IU/day of vitamin D3 for a few weeks to rapidly raise 25(OH)D concentrations, followed by 5,000 IU/day. The goal should be to raise 25(OH)D concentrations above 40 - 60 ng/mL (100 - 150 nmol/L) [222].
In the critically ill, the doses used from published RCTs ranged from 200,000 to 600,000 IU of vitamin D3, generally in a single enteral dose [214, 223, 224]. Based on the Castillo et al’s trial of calcifediol in COVID-19, in hospitalized patients, we recommend either the same doses of calcifediol be used or the equivalent doses with cholecalciferol. In the ICU, we favor a single large dose of 480,000 IU (30 mL) similar to the prior ICU trials above (Table 1). The vitamin D level should then be re-checked on day 5, if < 20 ng/mL, a supplemental dose of 96,000 IU/day for 5 days should be given. In summary, clinical data have demonstrated that in some studies vitamin D administration during infection may reduce inflammatory markers, more rapid viral clearance, and decrease admission to the ICU (Table 5) [14, 225-227].
Table 5. Clinical Studies Involving Vitamin D Therapy in COVID-19.
| Trial details | Dose | Outcomes | Comments |
|---|---|---|---|
| Randomized single blind trial. Treatment: n = 14 Control: n = 17. Age: 25 - 65 years. Hospitalized mild COVID-19. Mean age 51 ± 15 years, healthy cohorts no comorbidities. Asymptomatic to mild COVID-19 [225] |
Oral cholecalciferol 60,000 IU daily for 7 days (if target 25(OH)D concentration > 50 ng/mL not achieved on day 7; same dose continued, if target achieved weekly 60,000 IU) | Significant rapid disappearance of viral mRNA in treatment group at 21 days (62%vs. 20%) significant decrease in fibrinogen. No difference in procalcitonin, CRP, ferritin or D-dimer | Level of evidence 2. Placebos were not identical |
| Double masked randomized open label trial. Treatment: n = 50. Control: n = 26. Age: 43 - 63 years. Moderately severe COVID-19 [14] | Oral calcifediol 0.532 mg on day of admission and 0.266 on day 3, 7 and weekly until discharge | Need for ICU admission was lower in the group receiving intervention (2% vs. 50%, P < 0.001). Two patients in control group died, none in the intervention group died | Level of evidence 2 |
| Randomized open label study. Treatment: n = 44. Control: n = 43. Age: 20 - 83 years. Vitamin level less than 30. Mild to moderate COVID-19 [226] | Cholecalciferol 60,000 IU daily for 8 days in participants with BMI 18 - 25 kg/m2 and 10 days for participants with BMI > 25 kg/m2 | Significant reduction of inflammatory markers (CRP, LDH, ferritin, IL-6, N/L ratio) in intervention group compared to control group. No difference in hospital stay or mortality | Level of evidence 2. Adjustment made for comorbidities |
| Double blind randomized trial. Treatment: n = 119. Control: n = 118. Mean age: 56 ± 15 years. Moderate to severe COVID-19 [227] | Single dose of oral cholecalciferol 200,000 IU | No significant difference between groups in median length of hospital stay (7 vs. 7, P = 0.94), mortality (7.6% vs. 5.1%, P = 0.43). No significant difference in need for ventilation or length of ventilation. No significant difference in post-hoc analysis on patients with vitamin D deficiency. | Level of evidence 2. Patient could enroll for up to 10 days from development of symptoms; 60% of patients in both groups received corticosteroids |
Based on level 5 evidence (cited in text) and current studies we recommend use of vitamin D as adjunctive therapy. BMI: body mass index; CRP: C-reactive protein; LDH: lactate dehydrogenase; ICU: intensive care unit; IL-6: interleukin-6.
Based on the level of evidence (Oxford Center for Evidence-Based Medicine Levels of Evidence (http://www.cebm.net/?o=1116), we recommend the use of vitamin D as adjunct to therapy.
Statin Therapy and COVID-19
Statins are medicines that lower lipid levels but also have multiple anti-inflammatory actions. Over a decade of observational studies, both matched and non-matched, showed largely consistent benefits in patients with sepsis and/or ARDS [228]. Multiple RCTs were then conducted using various statins and doses; however, in a well-conducted meta-analysis of RCTs in sepsis involving 2,628 patients, no difference in mortality between groups was found [229]. Similarly, in ARDS trials, a meta-analysis from 2016 found no difference in important outcomes [230]. However, in an editorial that reviewed the outcomes from the STATInS and HARP-2 trials, they found that an alteration of just three events would have yielded statistically significant results in favor of statin use based on mortality outcomes [231-233]. This low “fragility index” suggests that benefits in subgroups exist but are then “lost” in the heterogenous populations that are often included in RCTs of critical illness syndromes such as ARDS and sepsis. This hypothesis was seemingly validated by a secondary analysis of the HARP-2 trial in which the authors split patients into two phenotypes of ARDS, a “hyperinflammatory” and “hypoinflammatory” type [234]. The hyperinflammatory group had higher values of sTNFr-1 and IL-6, lower platelet counts, more vasopressor use, fewer ventilator free days and much higher 28-day mortality. When the hyperinflammatory phenotype received simvastatin 80 mg, a large and statistically significant reduction in mortality was found. Further, in COVID-19, two retrospective studies have demonstrated a strong association of statin use with survival. In a large study of 13,981 patients in China, among which 1,219 received statins, the all-cause mortality was almost halved in the statin-treated patients (HR: 0.58 (95% CI: 0.43 - 0.80, P = 0.001) [13]. In a smaller study in the USA, one group found that among a group of 88 patients, 55% of whom died, atorvastatin use was associated with a 73% lower risk of progression to death (aHR: 0.38 (95% CI: 0.18 - 0.77, P = 0.008) [235]. Thus, given the frequent hyperinflammation and elevated levels of IL-6 in COVID-19 respiratory failure, it appears reasonable to employ statin therapy. Atorvastatin is favored due to its more favorable drug-interaction profile and a higher dose of 80 mg should be used, similar to the HARP-2 trial.
Famotidine and COVID-19
Famotidine, a histamine-2 receptor antagonist (H2RA), although commonly used to suppress acid production in the stomach, is also known to have in-vitro properties which not only inhibit viral replication such as in HIV but also exert stimulatory effects on almost all immune cells of the innate and adaptive immune system [236]. It can also prevent H2R cytokine inhibition and prevent inhibition by histamine on Th-1 cytokine release [237, 238].
H2RAs have proven effective in the past against other viruses. Cimetidine, and less so famotidine exhibited reduced viral infection with HIV in vitro, increased the clearance of warts caused by human papilloma virus, and appeared effective in improving the symptoms associated with chronic Epstein-Barr virus infection [239-241]. In fact, ranitidine bismuth citrate effectively inhibited the nucleoside triphosphate hydrolase and DNA unwinding activities of the SARS coronavirus helicase and dramatically reduced its replication levels in infected cells [242].
Given prior evidence of anti-viral, and in particular anti-SARS-CoV and immune system effects, Freedberg et al performed a retrospective cohort study using propensity score matching in COVID-19 patients at a single medical center. The treatment group all received famotidine within 24 h of admission. A total of 1,620 patients were included with 81 having received famotidine. They found that the use of famotidine was associated with a large reduced risk for death or intubation (aHR: 0.42, 95% CI: 0.21 - 0.85) and also with reduced risk for death alone (aHR: 0.30, 95% CI: 0.11 - 0.80) [243]. An interesting associated finding was that in patients on proton pump inhibitors, no reduced risk for any patient outcomes was observed. Although an observational study, propensity score matching was performed between groups, and a large difference in intubation and death was observed. Although such a study should be strictly considered as hypothesis generating only with the need for an RCT to optimally validate, in the interim, given the biologic plausibility, prior efficacy against other viruses along with a well-known safety profile, low cost, high availability and potentially large associated reduction in mortality, use of famotidine in the treatment of COVID-19 appears reasonable. Doses used in the Freedberg study were 10 mg in 17%, 20 mg in 47%, and 40 mg in 35% with a median of 5.8 days of use [243].
Management of Respiratory Failure
Although a comprehensive review of the optimal support of oxygenation and ventilation in COVID-19 respiratory failure is beyond the scope of this manuscript, several key physiologic insights should be recognized.
Early publications quickly highlighted the puzzling discordance between the degree of hypoxemia and modest work of breathing observed in COVID-19 patients, describing it as “silent hypoxemia” and such patients as “happy hypoxemics” [244, 245]. Similarly, soon after MV was instituted, unexpectedly high degrees of lung compliance in conjunction with severe hypoxemia was deemed a new “L” phenotype. Although reasons for lack of dyspnea are multiple, the largest contributors are: 1) early COVID-19 is an “organizing pneumonia” representing a cellular infiltration into the alveoli and ducts rather than alveolar fluid accumulation/edema as in classic ARDS making the lung “dry and light” versus “heavy and fluid-filled” and thus leads to less energy work to inflate and counter-act de-recruitment; 2) the as yet un-explained, paradoxical hyperperfusion of the foci of organizing pneumonia suggesting a failure of typical hypoxic pulmonary vasoconstriction and causing disproportionate hypoxemia (Fig. 2); and 3) the likely early and extensive micro- and/or macrovascular clotting not detected on routine imaging studies [8, 246, 247].
Figure 2.
Spectral computed tomography (CT) image with contrast in a coronavirus disease 2019 (COVID-19) patient. Markedly increased iodine uptake is seen (color scale on right of image), indicating increased perfusion to the ground glass opacities.
These differences from “traditional ARDS” were unfortunately both widely minimized and overlooked as evidenced by frequent recommendations for “early intubation” in what was an unfounded fear of the mechanically well-tolerated hypoxemia. Such approaches likely contributed to not only the unacceptably high mortality first reported but also the widespread shortages of ventilators, ICU beds, ventilators, nurses and medications in some of the earliest hard-hit areas. Such approaches curiously departed from the long held therapeutic principle of instituting MV, “neither too early, nor too late”, with decisions to intubate resting upon an assessment of the patients’ work of breathing (WOB) and their ability to sustain that work rather than solely on a presumed necessary level of oxygen saturation. When WOB is felt excessive or unsustainable despite non-invasive modes, then and only then should initiation of invasive mechanical support be pursued. Our recommended strategy for COVID-19 respiratory failure is illustrated in Figure 3. With similar approaches, many centers quickly learned that adopting such a primary focus on the support of oxygenation using non-invasive means and methods (self-proning) led to less need for ventilators and ICU beds with improved outcomes.
Figure 3.
Therapeutic approach to hypoxemia and respiratory failure in coronavirus disease 2019 (COVID-19).
Salvage Therapy
It has become increasingly recognized that the pathophysiologic mechanisms leading to hospitalization in COVID-19 occur in phases (Fig. 4) largely driven by the systemic host response phase rather than the cytopathic viral replicative phase [248]. Since the host response is now understood as a complex interaction of inflammation, endotheliopathy, cytokine storm, and hypercoagulability, some have argued that TPE could offer unique benefits by removing cytokines, stabilizing endothelial membranes, and reversing the hypercoagulable state [249].
Figure 4.
Phases of coronavirus disease 2019 (COVID-19).
In several of the authors’ clinical experiences, they have encountered a subset of patients who have failed to respond physiologically to the combined therapies that make up the MATH+ protocol, largely thought secondary to advanced disease at the time of presentation or extensive co-morbidity. In the first such cases, TPE was trialed with temporally associated physiologic improvements observed which then led to both extubation and discharge. In two of the authors’ experiences (PEM, PK), at the time of this writing, they encountered a total of 16 patients that demonstrated little physiologic improvement despite being treated with high-dose MATH+ protocol who were then empirically treated with TPE. Thirteen of the 16 were extubated and discharged while three failed to respond and later died. Increasing publications of case series and case reports from centers across the world have now described the efficacy of TPE in over 60 COVID-19 patients that did not respond to initial therapies, with the majority having been treated with corticosteroids [250-261]. Nearly all describe similar positive physiologic and clinical responses temporally associated with initiation or completion of TPE. Further, three retrospective, observational cohort studies including a total of 74 patients treated with plasmapheresis have reported dramatic differences in both extubation and survival [262-264]. The largest, a study from Pakistan of 45 COVID-19 patients treated with plasmapheresis compared to 45 propensity-matched controls, reported that the mortality in the plasmapheresis treated group was 8.9% vs. 38.5% in controls (HR: 0.21, 95% CI: 0.09 - 0.53, log rank 0.002) 263]. Khamis et al in Oman published on 31 COVID-19 patients in moderate to severe respiratory failure where 11 of the more severely ill patients received TPE with a slightly higher proportion of the TPE group also receiving tocilizumab compared to controls [263]. They reported both large improvements in extubation rates (73% vs. 20%, P = 0.018) and mortality (0% vs. 35%, P = 0.03).
Although these studies are strongly suggestive of a role for TPE in the management of COVID-19 patients unresponsive to now standard therapies such as corticosteroids, both prospective and/or randomized studies should be done to better establish the indications, duration, and efficacy of TPE.
Conclusion
In conclusion, the varied pathophysiologic mechanisms identified in COVID-19 likely require multiple therapeutic agents working in concert to counteract the diverse, deleterious consequences of this aberrant immune response. It is exceedingly unlikely that a “magic bullet” will be found, or even a medicine which would be effective at multiple stages of the disease. The MATH+ treatment protocol instead offers an inexpensive combination of medicines with a well-known safety profile based on strong physiologic rationale and an increasing clinical evidence base which potentially offers a life-saving approach to the management of COVID-19 patients.
Acknowledgments
Thanks for Frank Benno Junghans for the creation and design of Tables 1 and 2 and Dr. Gopal Punjabi for providing the image in Figure 2.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author Contributions
Dr. Meduri’s contribution is the result of work supported with the resources and use of facilities at the Memphis VA Medical Center. The contents of this commentary do not represent the views of the US Department of Veterans Affairs or the United States Government.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Author Note
A version of this manuscript was published by The Journal of Intensive Care Medicine and was retracted by the editors after being published and highly cited. The article has been changed, the contested findings have been removed, and we have added the addition of antiandrogen therapy. It is noteworthy that this manuscript emphasizing the benefit of corticosteroid anticoagulant and antioxidant therapy in patients with COVID-19 was conceived of before the results of the recovery trial were known.
References
- 1.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I. et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson KC, Chotirmall SH, Bai C, Rello J. COVID-19: interim guidance on management pending empirical evidence. American Thoracic Society-led International Task Force. 2020. Available from: https://www.lifebridgehealth.org/Uploads/Public/Documents/research/2020%20COVID%20Articles/COVID%20Guidelines/covid-19-guidance.pdf.
- 4. CDC Information for Clinicians on Investigational Therapeutics for Patients with COVID-19. Public Health Media Library. 2020.
- 5. WHO clinical management of severe acute respiratory infection when novel coronavirus (2019-NCoV) infection is suspected. Interim Guidance. WHO. 2020.
- 6.Villar J, Confalonieri M, Pastores SM, Meduri GU. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2(4):e0111. doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kory P, Kanne JP. SARS-CoV-2 organising pneumonia: 'Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?'. BMJ Open Respir Res. 2020;7(1):e000724. doi: 10.1136/bmjresp-2020-000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L. et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, Arvind V. et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, Lei F. et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32(2):176–187.e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, Quesada Gomez JM. "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richmond L, Schneider RP, Steffel J, Sandor PS, Tarnutzer AA. Case report: new-onset retinal migraine after transseptal catheterization. Headache. 2020;60(2):463–468. doi: 10.1111/head.13732. [DOI] [PubMed] [Google Scholar]
- 16.Jehi L, Ji X, Milinovich A, Erzurum S, Rubin BP, Gordon S, Young JB. et al. Individualizing risk prediction for positive coronavirus disease 2019 testing: results from 11,672 patients. Chest. 2020;158(4):1364–1375. doi: 10.1016/j.chest.2020.05.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicastri E, Petrosillo N, Ascoli Bartoli T, Lepore L, Mondi A, Palmieri F, D'Offizi G. et al. National Institute for the Infectious Diseases "L. Spallanzani", IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12(1):8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Z, Rochwerg B, Wang Y, Adhikari NK, Murthy S, Lamontagne F, Fowler RA. et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ. 2020;192(20):E536–E545. doi: 10.1503/cmaj.200648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massachusetts General Hospital. Background: hydroxychloroquine and COVID-19. Mass General Hospital. Published April 25, 2020. Accessed April 26, 2020. https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/background-on-hydroxychloroquine-and-covid-19.PDF.
- 20. Yale New-Haven Hospital System. Initial treatment algorithm for hospitalized adults with severe COVID-19 respiratory failure, including mechanical ventilation and ECMO PLUS confirmed POSITIVE SARS-CoV-2 by PCR. Yale New-Haven Hospital System. Published April 1, 2020. Accessed April 10, 2020. https://medicine.yale.edu/news-article/23611/
- 21. China National Health Commission. Chinese Clinical Guidance for Covid-19 Pneumonia (7th Edition) 2020. China National Health Commission. Published March 16, 2020. Accessed November 11, 2020. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html.
- 22.Waterer GW, Rello J, Wunderink RG. COVID-19: first do no harm. Am J Respir Crit Care Med. 2020;201(11):1324–1325. doi: 10.1164/rccm.202004-1153ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar J, Confalonieri M, Pastores SM, Meduri GU. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2:e0111. doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marik PE, Iglesias J, Varon J, Kory P. A scoping review of the pathophysiology of COVID-19. Int J Immunopathol Pharmacol. 2021;35:20587384211048026. doi: 10.1177/20587384211048026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Lee HK. Re-analysis of single cell transcriptome reveals that the NR3C1-CXCL8-Neutrophil axis determines the severity of COVID-19. Front Immunol. 2020;11:2145. doi: 10.3389/fimmu.2020.02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L, Zhang X, Wang Y, Zeng X. Severe COVID-19 pneumonia: assessing inflammation burden with volume-rendered chest CT. Radiol Cardiothorac Imaging. 2020;2(2):e200044. doi: 10.1148/ryct.2020200044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V. Hong Kong Hospital Authority SARS Collaborative Group (HASCOG) Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54(1):28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long Y, Xu Y, Wang B, Zhang L, Jia D, Xue F, Duan G. et al. Clinical Recommendations from an observational study on MERS: glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med. 2016;9(5):8865–8873. [Google Scholar]
- 33.Li H, Yang SG, Gu L, Zhang Y, Yan XX, Liang ZA, Zhang W. et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses. 2017;11(4):345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Miller J. et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71(16):2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Cruz A, Ruiz-Antoran B, Munoz-Gomez A, Sancho-Lopez A, Mills-Sanchez P, Centeno-Soto GA, Blanco-Alonso S. et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. 2020;64(9):e01168-20. doi: 10.1128/AAC.01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 38.Pastores SM, Annane D, Rochwerg B. The Corticosteroid Guideline Task Force of SCCM and ESICM. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2018;44(4):474–477. doi: 10.1007/s00134-017-4951-5. [DOI] [PubMed] [Google Scholar]
- 39.Greos LS, Vichyanond P, Bloedow DC, Irvin CG, Larsen GL, Szefler SJ, Hill MR. Methylprednisolone achieves greater concentrations in the lung than prednisolone. A pharmacokinetic analysis. Am Rev Respir Dis. 1991;144(3 Pt 1):586–592. doi: 10.1164/ajrccm/144.3_Pt_1.586. [DOI] [PubMed] [Google Scholar]
- 40.Li SMG, Miller DD, Yates CR. Evaluation of AP-1 and NF-KB inhibitory potency for oral glucocorticoids. In proceedings of the American review of respiratory disease. PharmSci. 2003;5(S1):R6173. [Google Scholar]
- 41.Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165(7):983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 42.Draghici S, Nguyen TM, Sonna LA, Ziraldo C, Vanciu RL, Fadel R, Morrison A. et al. COVID-19: disease pathways and gene expression changes predict methylprednisolone can improve outcome in severe cases. Bioinformatics. 2021;37(17):2691–2698. doi: 10.1101/2020.05.06.20076687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J. et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 44.Salton F, Confalonieri P, Meduri GU, Santus P, Harari S, Scala R, Lanini S. et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020;7(10):ofaa421. doi: 10.1093/ofid/ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, Mora V. et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia. Wien Klin Wochenschr. 2021;133(7):303–311. doi: 10.1007/s00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salton F, Confalonieri P, Santus P, Harari S, Scala R, Lanini S, Vertui V. et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.06.17.20134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC. et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murthy SC, Siddalingappa K, Suresh T. Nicolau's syndrome following diclofenac administration: A report of two cases. Indian J Dermatol Venereol Leprol. 2007;73(6):429–431. doi: 10.4103/0378-6323.37070. [DOI] [PubMed] [Google Scholar]
- 49.Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, van Bentum-Puijk W. et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dequin PF, Heming N, Meziani F, Plantefeve G, Voiriot G, Badie J, Francois B. et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recovery Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L. et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A. et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C, Hou D, Du C, Cai Y, Zheng J, Xu J, Chen X. et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit Care. 2020;24(1):643. doi: 10.1186/s13054-020-03340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, Najafizadeh SR. et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135(3):326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 57.Moskowitz A, Andersen LW, Huang DT, Berg KM, Grossestreuer AV, Marik PE, Sherwin RL. et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care. 2018;22(1):283. doi: 10.1186/s13054-018-2217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang M, Jativa DF. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018;6:2050312118807615. doi: 10.1177/2050312118807615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C. et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM. et al. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-alpha/beta at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai Y, Li YF, Tang LP, Tsoi B, Chen M, Chen H, Chen XM. et al. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed Res Int. 2015;2015:675149. doi: 10.1155/2015/675149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front Immunol. 2020;11:1021. doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sang X, Wang H, Chen Y, Guo Q, Lu A, Zhu X, Meng G. Vitamin C inhibits the activation of the NLRP3 inflammasome by scavenging mitochondrial ROS. Inflammasome. 2016;2:13–19. doi: 10.1515/infl-2016-0001. [DOI] [Google Scholar]
- 66.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C. et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lv D, Xu Y, Cheng H, Ke Y, Zhang X, Ying K. A novel cell-based assay for dynamically detecting neutrophil extracellular traps-induced lung epithelial injuries. Exp Cell Res. 2020;394(2):112101. doi: 10.1016/j.yexcr.2020.112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB. et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohammed BM, Fisher BJ, Kraskauskas D, Farkas D, Brophy DF, Fowler AA 3rd, Natarajan R. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5(8):3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418. doi: 10.1186/s13054-015-1131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatterjee IB, Majumder AK, Nandi BK, Subramanian N. Synthesis and some major functions of vitamin C in animals. Ann N Y Acad Sci. 1975;258:24–47. doi: 10.1111/j.1749-6632.1975.tb29266.x. [DOI] [PubMed] [Google Scholar]
- 72.Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35(1):5–13. doi: 10.1002/biof.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okamoto K, Tanaka H, Makino Y, Makino I. Restoration of the glucocorticoid receptor function by the phosphodiester compound of vitamins C and E, EPC-K1 (L-ascorbic acid 2-[3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6 -yl hydrogen phosphate] potassium salt), via a redox-dependent mechanism. Biochem Pharmacol. 1998;56(1):79–86. doi: 10.1016/S0006-2952(98)00121-X. [DOI] [PubMed] [Google Scholar]
- 74.Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther. 2018;189:63–70. doi: 10.1016/j.pharmthera.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Tyml K. Vitamin C and microvascular dysfunction in systemic inflammation. Antioxidants (Basel) 2017;6(3):49. doi: 10.3390/antiox6030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA. et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial. Chest. 2020;158(1):164–173. doi: 10.1016/j.chest.2020.02.049. [DOI] [PubMed] [Google Scholar]
- 78.Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B. et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Grooth HJ, Elbers PWG, Vincent JL. Vitamin C for sepsis and acute respiratory failure. JAMA. 2020;323(8):792. doi: 10.1001/jama.2019.21981. [DOI] [PubMed] [Google Scholar]
- 80.Hemila H, Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care. 2020;8:15. doi: 10.1186/s40560-020-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G. et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, Sohail H. et al. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020;12(11):e11779. doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.JamaliMoghadamSiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani M, Sekhavati Moghadam E. et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26(1):20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao D, Xu M, Wang G, Lv J, Ma X, Guo Y, Zhang D. et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging (Albany NY) 2021;13(5):7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao B, Liu M, Liu P, Peng Y, Huang J, Li M, Wang Y. et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol. 2021;12:638556. doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suna K, Melahat US, Murat Y, Figen OE, Ayperi O. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med Clin (Barc) 2021 doi: 10.1016/j.medcli.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li M, Ching TH, Hipple C, Lopez R, Sahibzada A, Rahman H. Use of intravenous vitamin C in critically ill patients with COVID-19 infection. J Pharm Pract. 2021 doi: 10.1177/08971900211015052. [DOI] [PubMed] [Google Scholar]
- 88.Xia G, Fan D, He Y, Zhu Y, Zheng Q. High-dose intravenous vitamin C attenuates hyperinflammation in severe coronavirus disease 2019. Nutrition. 2021;91-92:111405. doi: 10.1016/j.nut.2021.111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia G, Qin B, Ma C, Zhu Y, Zheng Q. High-dose vitamin C ameliorates cardiac injury in COVID-19 pandemic: a retrospective cohort study. Aging (Albany NY) 2021;13(17):20906–20914. doi: 10.18632/aging.203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glickman LB, Geigle PR, Paleg GS. A systematic review of supported standing programs. J Pediatr Rehabil Med. 2010;3(3):197–213. doi: 10.3233/PRM-2010-0129. [DOI] [PubMed] [Google Scholar]
- 91.Collie JTB, Greaves RF, Jones OAH, Lam Q, Eastwood GM, Bellomo R. Vitamin B1 in critically ill patients: needs and challenges. Clin Chem Lab Med. 2017;55(11):1652–1668. doi: 10.1515/cclm-2017-0054. [DOI] [PubMed] [Google Scholar]
- 92.Menezes RR, Godin AM, Rodrigues FF, Coura GME, Melo ISF, Brito AMS, Bertollo CM. et al. Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund's adjuvant. Pharmacol Rep. 2017;69(5):1036–1043. doi: 10.1016/j.pharep.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, Chou PP. et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25(4):576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Costa NA, Gut AL, de Souza Dorna M, Pimentel JA, Cozzolino SM, Azevedo PS, Fernandes AA. et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care. 2014;29(2):249–252. doi: 10.1016/j.jcrc.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Corcoran TB, O'Neill MA, Webb SA, Ho KM. Prevalence of vitamin deficiencies on admission: relationship to hospital mortality in critically ill patients. Anaesth Intensive Care. 2009;37(2):254–260. doi: 10.1177/0310057X0903700215. [DOI] [PubMed] [Google Scholar]
- 96.Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, Wolfe R. et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. 2016;44(2):360–367. doi: 10.1097/CCM.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woolum JA, Abner EL, Kelly A, Thompson Bastin ML, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46(11):1747–1752. doi: 10.1097/CCM.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 98.Moslemi R, Khalili H, Mohammadi M, Mehrabi Z, Mohebbi N. Thiamine for prevention of postoperative delirium in patients undergoing gastrointestinal surgery: a randomized clinical trial. J Res Pharm Pract. 2020;9(1):30–35. doi: 10.4103/jrpp.JRPP_19_124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L. et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun. 2020;11(1):3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vatsalya V, Li F, Frimodig JC, Gala KS, Srivastava S, Kong M, Ramchandani VA. et al. Therapeutic prospects for Th-17 cell immune storm syndrome and neurological symptoms in COVID-19: thiamine efficacy and safety, in-vitro evidence and pharmacokinetic profile. medRxiv. 2020 doi: 10.1101/2020.08.23.20177501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al Sulaiman K, Aljuhani O, Al Dossari M, Alshahrani A, Alharbi A, Algarni R, Al Jeraisy M. et al. Evaluation of thiamine as adjunctive therapy in COVID-19 critically ill patients: a two-center propensity score matched study. Crit Care. 2021;25(1):223. doi: 10.1186/s13054-021-03648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A. et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, Rosengart TK. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3(6):e2011192. doi: 10.1001/jamanetworkopen.2020.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, Dodek P. et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med. 2015;43(2):401–410. doi: 10.1097/CCM.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 109.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS. et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas W, Varley J, Johnston A, Symington E, Robinson M, Sheares K, Lavinio A. et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G. et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi L, Xu J, Duan G, Yang H, Wang Y. The pooled prevalence of pulmonary embolism in patients with COVID-19. Intensive Care Med. 2020;46(11):2089–2091. doi: 10.1007/s00134-020-06235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F. et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S. et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T. et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34(8):1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fridman S, Bres Bullrich M, Jimenez-Ruiz A, Costantini P, Shah P, Just C, Vela-Duarte D. et al. Stroke risk, phenotypes, and death in COVID-19: Systematic review and newly reported cases. Neurology. 2020;95(24):e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- 118.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW. et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taccone FS, Gevenois PA, Peluso L, Pletchette Z, Lheureux O, Brasseur A, Garufi A. et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48(11):e1087–e1090. doi: 10.1097/CCM.0000000000004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clagett GP. Thrombosis research. Journal of Vascular Surgery. 1989;9:371–373. doi: 10.1016/0741-5214(89)90060-8. [DOI] [Google Scholar]
- 122.Mandal AK, Dam P, Franco OL, Sellami H, Mandal S, Sezgin GC, Biswas K. et al. Response to “MacIntyre et al., 2020: A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients”. Int J Nurs Stud. 2020;109:103714. doi: 10.1016/j.ijnurstu.2020.103714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jonmarker S, Hollenberg J, Dahlberg M, Stackelberg O, Litorell J, Everhov AH, Jarnbert-Pettersson H. et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care. 2020;24(1):653. doi: 10.1186/s13054-020-03375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lemos ACB, do Espirito Santo DA, Salvetti MC, Gilio RN, Agra LB, Pazin-Filho A, Miranda CH. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Investigators R-C, Investigators AC-a, Investigators A, Goligher EC, Bradbury CA, McVerry BJ, Lawler PR. et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Investigators A, Investigators AC-a, Investigators R-C, Lawler PR, Goligher EC, Berger JS, Neal MD. et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ. et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khamis F, Al-Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, Pandak N, Al Khalili H. et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim SY, Jeon K, Kim HJ, Kim SM, Song J, Ha JM, Um SW. et al. Antifactor Xa levels in critically ill Korean patients receiving enoxaparin for thromboprophylaxis: a prospective observational study. J Korean Med Sci. 2013;28(3):466–471. doi: 10.3346/jkms.2013.28.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagge J, Crowther M, Hirsh J. Is impaired renal function a contraindication to the use of low-molecular-weight heparin? Arch Intern Med. 2002;162(22):2605–2609. doi: 10.1001/archinte.162.22.2605. [DOI] [PubMed] [Google Scholar]
- 132.White D, MacDonald S, Bull T, Hayman M, de Monteverde-Robb R, Sapsford D, Lavinio A. et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomasa-Irriguible TM, Martinez-Vega S, Mor-Marco E, Herraiz-Ruiz A, Raguer-Pardo L, Cubells-Larrosa C. Low molecular weight heparins in COVID-19 patients: beware of augmented renal clearance! Crit Care. 2020;24(1):325. doi: 10.1186/s13054-020-03058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Colunga Biancatelli RML, Berrill M, Mohammed YH, Marik PE. Melatonin for the treatment of sepsis: the scientific rationale. J Thorac Dis. 2020;12(Suppl 1):S54–S65. doi: 10.21037/jtd.2019.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernandez-Montesinos R, Guerrero JM, Pozo D. The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs. 2006;7(5):423–431. [PubMed] [Google Scholar]
- 137.Espino J, Pariente JA, Rodriguez AB. Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev. 2012;2012:670294. doi: 10.1155/2012/670294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dominguez-Rodriguez A, Abreu-Gonzalez P. et al. Melatonin, cardiovascular disease and COVID-19: a potential therapeutic strategy? Melatonin Res. 2020;3:318–321. doi: 10.32794/mr11250065. [DOI] [Google Scholar]
- 139.Reiter RJ, Sharma R, Ma Q, Dominquez-Rodriguez A, Marik PE, Abreu-Gonzalez P. Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med Drug Discov. 2020;6:100044. doi: 10.1016/j.medidd.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reiter RJ, Abreu-Gonzalez P, Marik PE, Dominguez-Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front Med (Lausanne) 2020;7:226. doi: 10.3389/fmed.2020.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wurtman RJ. Age-related decreases in melatonin secretion—clinical consequences. J Clin Endocrinol Metab. 2000;85(6):2135–2136. doi: 10.1210/jc.85.6.2135. [DOI] [PubMed] [Google Scholar]
- 143.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C. et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aldhous M, Franey C, Wright J, Arendt J. Plasma concentrations of melatonin in man following oral absorption of different preparations. Br J Clin Pharmacol. 1985;19(4):517–521. doi: 10.1111/j.1365-2125.1985.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alizadeh Z, Keyhanian N, Ghaderkhani S, Dashti-Khavidaki S, Shokouhi Shoormasti R, Pourpak Z. A pilot study on controlling coronavirus disease 2019 (COVID-19) inflammation using melatonin supplement. Iran J Allergy Asthma Immunol. 2021;20(4):494–499. doi: 10.18502/ijaai.v20i4.6959. [DOI] [PubMed] [Google Scholar]
- 146.Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, Rezaie E. et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, double-blind clinical trial. Arch Med Res. 2022;53(1):79–85. doi: 10.1016/j.arcmed.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mousavi SA, Heydari K, Mehravaran H, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Shamshirian A. Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial. J Med Virol. 2022;94(1):263–271. doi: 10.1002/jmv.27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.10.15.20213546. [DOI] [Google Scholar]
- 149.Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA. et al. Zinc and respiratory tract infections: Perspectives for COVID19 (Review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gammoh NZ, Rink L. Zinc in Infection and Inflammation. Nutrients. 2017;9(6):624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heyland DK, Jones N, Cvijanovich NZ, Wong H. Zinc supplementation in critically ill patients: a key pharmaconutrient? JPEN J Parenter Enteral Nutr. 2008;32(5):509–519. doi: 10.1177/0148607108322402. [DOI] [PubMed] [Google Scholar]
- 152.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA. et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 154.Maggini S, Wenzlaff S, Hornig D. Essential role of vitamin C and zinc in child immunity and health. J Int Med Res. 2010;38(2):386–414. doi: 10.1177/147323001003800203. [DOI] [PubMed] [Google Scholar]
- 155.Linko R, Karlsson S, Pettila V, Varpula T, Okkonen M, Lund V, Ala-Kokko T. et al. Serum zinc in critically ill adult patients with acute respiratory failure. Acta Anaesthesiol Scand. 2011;55(5):615–621. doi: 10.1111/j.1399-6576.2011.02425.x. [DOI] [PubMed] [Google Scholar]
- 156.Mertens K, Lowes DA, Webster NR, Talib J, Hall L, Davies MJ, Beattie JH. et al. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br J Anaesth. 2015;114(6):990–999. doi: 10.1093/bja/aev073. [DOI] [PubMed] [Google Scholar]
- 157.Hoeger J, Simon TP, Beeker T, Marx G, Haase H, Schuerholz T. Persistent low serum zinc is associated with recurrent sepsis in critically ill patients - A pilot study. PLoS One. 2017;12(5):e0176069. doi: 10.1371/journal.pone.0176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boudreault F, Pinilla-Vera M, Englert JA, Kho AT, Isabelle C, Arciniegas AJ, Barragan-Bradford D. et al. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight. 2017;2(11):e86507. doi: 10.1172/jci.insight.86507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev. 2013;6:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 160.Hemila H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8(5):2054270417694291. doi: 10.1177/2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meydani SN, Barnett JB, Dallal GE, Fine BC, Jacques PF, Leka LS, Hamer DH. Serum zinc and pneumonia in nursing home elderly. Am J Clin Nutr. 2007;86(4):1167–1173. doi: 10.1093/ajcn/86.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10):e109180. doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, Ortiz M, O'Sullivan CK, Fernandez-Larrea JB. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1-6 cells to a liposome model. J Agric Food Chem. 2014;62(32):8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- 165.Shittu MO, Afolami OI. Improving the efficacy of Chloroquine and Hydroxychloroquine against SARS-CoV-2 may require Zinc additives - A better synergy for future COVID-19 clinical trials. Infez Med. 2020;28(2):192–197. [PubMed] [Google Scholar]
- 166.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front Pharmacol. 2021;12:652688. doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hoertel N, Sanchez-Rico M, Vernet R, Beeker N, Jannot AS, Neuraz A, Salamanca E. et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26(9):5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 168.Carpinteiro A, Edwards MJ, Hoffmann M, Kochs G, Gripp B, Weigang S, Adams C. et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep Med. 2020;1(8):100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, Miller JP. et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, Thabane L, Milagres AC, Ferreira TS, Dos Santos CVQ. et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee J, Yousaf A, Fang W, Kolodney MS. Male balding is a major risk factor for severe COVID-19. J Am Acad Dermatol. 2020;83(5):e353–e354. doi: 10.1016/j.jaad.2020.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wambier CG, Vano-Galvan S, McCoy J, Gomez-Zubiaur A, Herrera S, Hermosa-Gelbard A, Moreno-Arrones OM. et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The "Gabrin sign". J Am Acad Dermatol. 2020;83(2):680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Salazar Arenas MA, Munoz Del Carpio-Toia A, Aybar Galdos J, Rodriguez-Morales AJ. Alopecia and severity of COVID-19: a cross-sectional study in Peru. Infez Med. 2021;29(1):37–45. [PubMed] [Google Scholar]
- 175.Wambier CG, McCoy J, Goren A. Male balding as a major risk factor for severe COVID-19: A possible role for targeting androgens and transmembrane protease serine 2 to protect vulnerable individuals. J Am Acad Dermatol. 2020;83(6):e401–e402. doi: 10.1016/j.jaad.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cadegiani F, Lin EM, Goren A, Wambier CG. Potential risk for developing severe COVID-19 disease among anabolic steroid users. BMJ Case Rep. 2021;14(2):e241572. doi: 10.1136/bcr-2021-241572. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177.Velavan TP, Pallerla SR, Ruter J, Augustin Y, Kremsner PG, Krishna S, Meyer CG. Host genetic factors determining COVID-19 susceptibility and severity. EBioMedicine. 2021;72:103629. doi: 10.1016/j.ebiom.2021.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McCoy J, Wambier CG, Herrera S, Vano-Galvan S, Gioia F, Comeche B, Ron R. et al. Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients. J Eur Acad Dermatol Venereol. 2021;35(1):e15–e17. doi: 10.1111/jdv.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Subramanian A, Anand A, Adderley NJ, Okoth K, Toulis KA, Gokhale K, Sainsbury C. et al. Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study. Eur J Endocrinol. 2021;184(5):637–645. doi: 10.1530/EJE-20-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cadegiani FA, Lim RK, Goren A, McCoy J, Situm M, Kovacevic M, Vano Galvan S. et al. Clinical symptoms of hyperandrogenic women diagnosed with COVID-19. J Eur Acad Dermatol Venereol. 2021;35(2):e101–e104. doi: 10.1111/jdv.17004. [DOI] [PubMed] [Google Scholar]
- 181.Patel VG, Zhong X, Liaw B, Tremblay D, Tsao CK, Galsky MD, Oh WK. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31(10):1419–1420. doi: 10.1016/j.annonc.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM. et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhowmick NA, Oft J, Dorff T, Pal S, Agarwal N, Figlin RA, Posadas EM. et al. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer. 2020;27(9):R281–R292. doi: 10.1530/ERC-20-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee KM, Heberer K, Gao A, Becker DJ, Loeb S, Makarov DV, Gulanski B. et al. A Population-Level Analysis of the Protective Effects of Androgen Deprivation Therapy against COVID-19 Disease Incidence and Severity. medRxiv. 2021 doi: 10.1101/2021.05.10.21255146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Experton B, Tetteh HA, Lurie N, Walker P, Elena A, Hein CS, Schwendiman B. et al. A predictive model for severe COVID-19 in the medicare population: a tool for prioritizing primary and booster COVID-19 vaccination. Biology (Basel) 2021;10(11):1185. doi: 10.3390/biology10111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Afar DE, Vivanco I, Hubert RS, Kuo J, Chen E, Saffran DC, Raitano AB. et al. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001;61(4):1686–1692. [PubMed] [Google Scholar]
- 188.Wang XM, Mannan R, Xiao L. Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series. Commun Med. 2021;1:24. doi: 10.1038/s43856-021-00025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kumar N, Zuo Y, Yalavarthi S, Hunker KL, Knight JS, Kanthi Y, Obi AT. et al. SARS-CoV-2 spike protein S1-mediated endothelial injury and pro-inflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism. Viruses. 2021;13(11):2209. doi: 10.3390/v13112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cadegiani FA, Zimerman RA, Fonseca DN, Correia MN, Muller MP, Bet DL, Slaviero MR. et al. Final results of a randomized, placebo-controlled, two-arm, parallel clinical trial of proxalutamide for hospitalized COVID-19 patients: a multiregional, joint analysis of the Proxa-rescue AndroCoV trial. Cureus. 2021;13(12):e20691. doi: 10.7759/cureus.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cadegiani FA, Fonseca DN, McCoy J, Zimerman RA, Mirza FN, Correia MN, Barros RN. et al. Efficacy of proxalutamide in hospitalized COVID-19 patients: a randomized, double-blind, placebo-controlled, parallel-design clinical trial. medRxiv. 2021 [Google Scholar]
- 192.Cadegiani FA, Goren A, Wambier CG, Zimerman RA. Proxalutamide improves inflammatory, immunologic, and thrombogenic markers in mild-to-moderate COVID-19 males and females: an exploratory analysis of a randomized, double-blinded, placebo-controlled trial early antiandrogen therapy (EAT) with proxalutamide (The EAT-Proxa Biochemical AndroCoV-Trial) medRxiv. 2021 [Google Scholar]
- 193.Cadegiani FA, McCoy J, Gustavo Wambier C, Goren A. Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial - Biochemical) Cureus. 2021;13(2):e13047. doi: 10.7759/cureus.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cadegiani FA, Zimerman RA, Fonseca DN, Correia MC, McCoy J, Wambier CG, Goren A. Proxalutamide (GT0918) reduces the rate of hospitalization in mild-to-moderate COVID-19 female patients: a randomized double-blinded placebo-controlled two-arm parallel trial. medRxiv. 2021 doi: 10.1101/2021.07.06.21260086. [DOI] [Google Scholar]
- 195.McCoy J, Goren A, Cadegiani FA, Vano-Galvan S, Kovacevic M, Situm M, Shapiro J. et al. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front Med (Lausanne) 2021;8:668698. doi: 10.3389/fmed.2021.668698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 196.Zarehoseinzade E, Allami A, Ahmadi M, Bijani B, Mohammadi N. Finasteride in hospitalized adult males with COVID-19: A risk factor for severity of the disease or an adjunct treatment: A randomized controlled clinical trial. Med J Islam Repub Iran. 2021;35:30. doi: 10.47176/mjiri.35.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cadegiani FA, Zimerman RA, A G. Proxalutamide treatment for hospitalized COVID-19 patients in southern brazil: the south arm of a randomized, double-blind, placebo-controlled, parallel clinical trial - the South Proxa-Rescue AndroCoV Trial. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 198.Zimerman RA, Fonseca DN, Correia MN, Barros RN, Onety DC, Israel KCP, Almeida BG, Proxalutamide reduction of mortality rate in hospitalized COVID-19 patients depends on treatment duration - an exploratory analysis of the Proxa-Rescue AndroCoV Trial. medRxiv. 2021. [DOI]
- 199.Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, Faliva MA. et al. Self-care for common colds: the pivotal role of vitamin D, Vitamin C, Zinc, and echinacea in three main immune interactive clusters (Physical Barriers, Innate and Adaptive Immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med. 2018;2018:5813095. doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS. 2019;127(10):681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- 202.Rossi GA, Fanous H, Colin AA. Viral strategies predisposing to respiratory bacterial superinfections. Pediatr Pulmonol. 2020;55(4):1061–1073. doi: 10.1002/ppul.24699. [DOI] [PubMed] [Google Scholar]
- 203.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Quraishi SA, Bittner EA, Blum L, Hutter MM, Camargo CA Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014;149(2):112–118. doi: 10.1001/jamasurg.2013.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF. et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Youssef DA, Ranasinghe T, Grant WB, Peiris AN. Vitamin D's potential to reduce the risk of hospital-acquired infections. Dermatoendocrinol. 2012;4(2):167–175. doi: 10.4161/derm.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. ONS Coronavirus (COVID-19) related deaths by Ethnic Group, England and Wales - Office for National Statistics. 2020;1-10.
- 208.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G. et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, Hadadi A. et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15(9):e0239799. doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Venkatesh B, Nair P. Hypovitaminosis D and morbidity in critical illness: is there proof beyond reasonable doubt? Critical Care. 2014;18:7–9. doi: 10.1186/cc13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360(18):1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 212.Zhang YP, Wan YD, Sun TW, Kan QC, Wang LX. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Crit Care. 2014;18(6):684. doi: 10.1186/s13054-014-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Amrein K, Zajic P, Schnedl C, Waltensdorfer A, Fruhwald S, Holl A, Purkart T. et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;18(2):R47. doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, Landoni G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. 2017;38:109–114. doi: 10.1016/j.jcrc.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 215.Amrein K, Martucci G, McNally JD. When not to use meta-analysis: Analysing the meta-analyses on vitamin D in critical care. Clin Nutr. 2017;36(6):1729–1730. doi: 10.1016/j.clnu.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 216.National Heart Lung Blood Institute Petal Clinical Trials Network. Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM. et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N Engl J Med. 2019;381(26):2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954–962. doi: 10.1016/j.chest.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Journal S, Kuhbandner K, Hammer A, Haase S, Terbrack E, Schippers A, Wagner N. et al. Oral Presentations. 2018;24(S2):24. [Google Scholar]
- 219.Zhang Y, Leung DYM, Goleva E. Vitamin D enhances glucocorticoid action in human monocytes: involvement of granulocyte-macrophage colony-stimulating factor and mediator complex subunit 14. J Biol Chem. 2013;288(20):14544–14553. doi: 10.1074/jbc.M112.427054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Y, Leung DY, Goleva E. Anti-inflammatory and corticosteroid-enhancing actions of vitamin D in monocytes of patients with steroid-resistant and those with steroid-sensitive asthma. J Allergy Clin Immunol. 2014;133(6):1744–1752.1741. doi: 10.1016/j.jaci.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Christopher KB. Vitamin D supplementation in the ICU patient. Curr Opin Clin Nutr Metab Care. 2015;18(2):187–192. doi: 10.1097/MCO.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 222.Bergman P. The link between vitamin D and COVID-19: distinguishing facts from fiction. J Intern Med. 2021;289(1):131–133. doi: 10.1111/joim.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Han JE, Jones JL, Tangpricha V, Brown MA, Brown LAS, Hao L, Hebbar G. et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Amrein K, Schnedl C, Berghold A, Pieber TR, Dobnig H. Correction of vitamin D deficiency in critically ill patients - VITdAL@ICU study protocol of a double-blind, placebo-controlled randomized clinical trial. BMC Endocr Disord. 2012;12:27. doi: 10.1186/1472-6823-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD. et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2022;98(1156):87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 226.Lakkireddy M, Gadiga SG, Malathi RD, Karra ML, Raju I. Ragini. Chinapaka S. et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021;11(1):10641. doi: 10.1038/s41598-021-90189-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, Silva CBR. et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wan YD, Sun TW, Kan QC, Guan FX, Zhang SG. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care. 2014;18(2):R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pertzov B, Eliakim-Raz N, Atamna H, Trestioreanu AZ, Yahav D, Leibovici L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults - A systematic review and meta-analysis. Clin Microbiol Infect. 2019;25(3):280–289. doi: 10.1016/j.cmi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 230.Gao XQ, Li YF, Jiang ZL. Impact of statins on ALI/ARDS: A meta-analysis. Pulm Pharmacol Ther. 2016;39:85–91. doi: 10.1016/j.pupt.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 231.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B. et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187(7):743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 232.Kruger PS, Terblanche M. Statins in patients with sepsis and ARDS: is it over? No. Intensive Care Med. 2017;43(5):675–676. doi: 10.1007/s00134-016-4564-4. [DOI] [PubMed] [Google Scholar]
- 233.McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, Johnston P. et al. Simvastatin in the acute respiratory distress syndrome. New England Journal of Medicine. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 234.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C. et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24(1):429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jafarzadeh A, Nemati M, Khorramdelazad H, Hassan ZM. Immunomodulatory properties of cimetidine: Its therapeutic potentials for treatment of immune-related diseases. Int Immunopharmacol. 2019;70:156–166. doi: 10.1016/j.intimp.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 237.Smolinska S, Groeger D, Perez NR, Schiavi E, Ferstl R, Frei R, Konieczna P. et al. Histamine receptor 2 is required to suppress innate immune responses to bacterial ligands in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(7):1575–1586. doi: 10.1097/MIB.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 238.Morichika T, Takahashi HK, Iwagaki H, Yoshino T, Tamura R, Yokoyama M, Mori S. et al. Histamine inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in an intercellular adhesion molecule-1- and B7.1-dependent manner. J Pharmacol Exp Ther. 2003;304(2):624–633. doi: 10.1124/jpet.102.042515. [DOI] [PubMed] [Google Scholar]
- 239.Bourinbaiar AS, Fruhstorfer EC. The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: identification of a new class of antiviral agents. Life Sci. 1996;59(23):PL365–PL370. doi: 10.1016/S0024-3205(96)00553-X. [DOI] [PubMed] [Google Scholar]
- 240.Goldstein JA. Cimetidine, ranitidine, and Epstein-Barr virus infection. Ann Intern Med. 1986;105(1):139. doi: 10.7326/0003-4819-105-1-139_2. [DOI] [PubMed] [Google Scholar]
- 241.Gooptu C, Higgins CR, James MP. Treatment of viral warts with cimetidine: an open-label study. Clin Exp Dermatol. 2000;25(3):183–185. doi: 10.1046/j.1365-2230.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 242.Yang N, Tanner JA, Zheng BJ, Watt RM, He ML, Lu LY, Jiang JQ. et al. Bismuth complexes inhibit the SARS coronavirus. Angew Chem Int Ed Engl. 2007;46(34):6464–6468. doi: 10.1002/anie.200701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA, Sobieszczyk ME. et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159(3):1129–1131.e3. doi: 10.1101/2020.05.01.20086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am J Respir Crit Care Med. 2020;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Couzin-Frankel J. The Mystery of the Pandemic’s “Happy Hypoxia”. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 246.Cobes N, Guernou M, Lussato D, Queneau M, Songy B, Bonardel G, Grellier JF. Ventilation/perfusion SPECT/CT findings in different lung lesions associated with COVID-19: a case series. Eur J Nucl Med Mol Imaging. 2020;47(10):2453–2460. doi: 10.1007/s00259-020-04920-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, Ledot S. et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marik PE, Varon J, Kory P. Treatment of COVID-19 is critically phase specific. Crit Care Shock. 2020;23(5):10–12. [Google Scholar]
- 249.Keith P, Day M, Perkins L, Moyer L, Hewitt K, Wells A. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care. 2020;24(1):128. doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fernandez J, Gratacos-Gines J, Olivas P, Costa M, Nieto S, Mateo D, Sanchez MB. et al. Plasma exchange: an effective rescue therapy in critically ill patients with coronavirus disease 2019 infection. Crit Care Med. 2020;48(12):e1350–e1355. doi: 10.1097/CCM.0000000000004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Adeli SH, Asghari A, Tabarraii R, Shajari R, Afshari S, Kalhor N, Vafaeimanesh J. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol Arch Intern Med. 2020;130(5):455–458. doi: 10.20452/pamw.15340. [DOI] [PubMed] [Google Scholar]
- 252.Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, Maclaren G. et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Altmayer V, Saheb S, Rohaut B, Marois C, Cao A, Gallo A, Le Guennec L. et al. Therapeutic plasma exchange in a critically ill Covid-19 patient. J Clin Apher. 2021;36(1):179–182. doi: 10.1002/jca.21830. [DOI] [PubMed] [Google Scholar]
- 254.Akkoyunlu Y, Cetin G, Bolukcu S, Okay G, Ogun H, Durdu B, Okyaltirik F. et al. The successful management of an elderly Covid-19 infected patient by plasmapheresis. Transfus Apher Sci. 2020;59(6):102924. doi: 10.1016/j.transci.2020.102924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Faqihi F, Alharthy A, Alodat M, Kutsogiannis DJ, Brindley PG, Karakitsos D. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: A pilot study. J Crit Care. 2020;60:328–333. doi: 10.1016/j.jcrc.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tian H, Sui Y, Tian S, Zou X, Xu Z, He H, Wu T. Case report: clinical treatment of the first critical patient with coronavirus disease (COVID-19) in Liaocheng, Shandong Province. Front Med (Lausanne) 2020;7:249. doi: 10.3389/fmed.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morath C, Weigand MA, Zeier M, Speer C, Tiwari-Heckler S, Merle U. Plasma exchange in critically ill COVID-19 patients. Crit Care. 2020;24(1):481. doi: 10.1186/s13054-020-03171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang L, Zhai H, Ma S, Chen J, Gao Y. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br J Haematol. 2020;190(4):e181–e183. doi: 10.1111/bjh.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shi H, Zhou C, He P, Huang S, Duan Y, Wang X, Lin K. et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int J Antimicrob Agents. 2020;56(2):105974. doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.DePace NL, Soloway S, Roshal D, Soloway AM, Colombo J. Unexpected SARS-CoV-2 cardiorespiratory arrest in a myopathy patient undergoing immunosuppressive treatment: A case report. Medicine (Baltimore) 2020;99(30):e21377. doi: 10.1097/MD.0000000000021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Keith P, Day M, Choe C, Perkins L, Moyer L, Hays E, French M. et al. The successful use of therapeutic plasma exchange for severe COVID-19 acute respiratory distress syndrome with multiple organ failure. SAGE Open Med Case Rep. 2020;8:2050313X20933473. doi: 10.1177/2050313X20933473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gucyetmez B, Atalan HK, Sertdemir I, Cakir U, Telci L. COVID-19 Study Group. Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: a retrospective study. Crit Care. 2020;24(1):492. doi: 10.1186/s13054-020-03215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ren JL, Zhang AH, Wang XJ. Corrigendum to "Traditional Chinese medicine for COVID-19 treatment" [Pharmacol. Res. 155 (2020) 104743] Pharmacol Res. 2020;155:104768. doi: 10.1016/j.phrs.2020.104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.