Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multiple organs. Infectious agents have been implicated in the pathogenesis of SLE. The emergent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces a pro-inflammatory cytokine storm and has been linked to autoimmune phenomena, which can lead to the onset of autoimmune diseases. We report the case of a 70-year-old patient who developed a toxic epidermal necrolysis (TEN)-like subacute cutaneous lupus (SCL) as a severe presentation of SLE, 1 month after SARS-CoV-2 infection. After excluding other causes of SLE, treatment was initiated with a successful outcome.
Keywords: SARS-CoV-2, COVID-19, Systemic lupus erythematous, Subacute cutaneous lupus, Toxic epidermal necrolysis
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that affects multiple organs, resulting in a remarkable diversity of clinical symptoms, including cutaneous, musculoskeletal, hematological, renal and neurological manifestations. It mainly affects young women, with a female/male ratio of 9:1. The natural history of SLE ranges from an insidious and progressive disease, with periodic exacerbations and remissions, to an acute and rapidly fatal condition. The leading causes of death include renal disease, infection and severe disease flares [1]. The etiology of SLE remains unknown, although both exogenous and endogenous factors have been implicated, with infectious agents playing an important role. Multiple viruses have been associated with the onset of SLE, such as Epstein-Barr virus, cytomegalovirus and human immunodeficiency virus type 1, probably due to immunologic features that favor a self-reactive response [2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently emerged agent that causes coronavirus disease 2019 (COVID-19). This acute viral infection, with clinical manifestations ranging from a flu-like syndrome to life-threatening pneumoniae, induces an increase in pro-inflammatory serum cytokines and chemokines, and appears to produce a more severe clinical course in patients with underlying medical conditions. Although there is a possible link between COVID-19 pathophysiology and new presentations or flares of autoimmune disorders, the role of SARS-CoV-2 is still not clear. To date, there have been several reports about SLE patients who have been infected with SARS-CoV-2, but the data regarding the onset of clinical manifestations of SLE in the context of COVID-19 are sparse [3]. We report the case of a patient who developed SLE following SARS-CoV-2 infection.
Case Report
Investigations
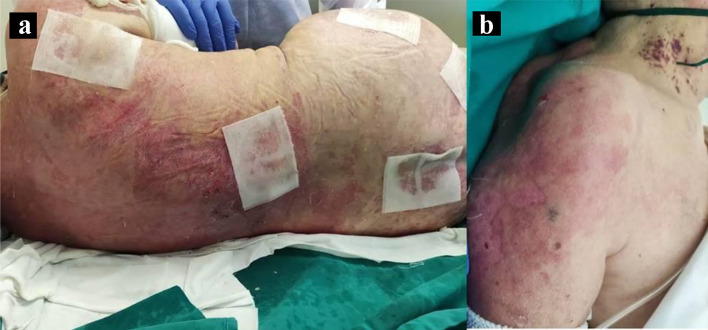
A 70-year-old female patient developed photo-distributed erythematous and purpuric macules on the upper trunk and shoulders that gradually progressed to superficial flaccid bullae extending to non-photoexposed areas, culminating into large sheets of detached epidermis, over a period of 4 weeks (Fig. 1). The Nikolsky sign was positive and the skin lesions extended to 70% of her body surface. She was also found to have malar erythema and inflammatory small joints polyarthralgias on both hands. As relevant medical history, the patient had high blood pressure, managed with telmisartan 40 mg daily for the previous 5 years. The diagnosis of a lower respiratory SARS-CoV-2 infection was performed 29 days before the onset of these symptoms, leading to her hospitalization for 15 days due to hypoxemic respiratory failure; she required supplemental oxygen and the administration of dexamethasone 6 mg/day, ipratropium bromide 40 µg q6h, enoxaparin 40 mg/day and paracetamol 1 g prn. At discharge, she was reported to be fully recovered from COVID-19.
Figure 1.
Toxic epidermal necrolysis (TEN)-like lesions in subacute cutaneous lupus (SCL). Large sheets of detached epidermis along the patient’s dorsum (a) and left shoulder (b).
Diagnosis
Considering the severe cutaneous manifestations, Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was the first diagnostic hypothesis to be considered. However, drug causality seemed improbable and the ALDEN score for each drug previously prescribed revealed an unlikely result (Table 1) [4]. Regarding the referred polyarthralgias and the malar erythema, SLE was also taken into account. The initial laboratory tests showed a mild normocytic anemia, no eosinophilia and elevated inflammatory parameters. A thorough immune and autoimmune study was performed, demonstrating high antinuclear antibody titre and positive anti-SSA and SSB autoantibodies. Blood cultures and serological tests for multiple infectious agents were negative (Table 2). A skin biopsy of the upper left thigh showed peripheral scattered apoptotic keratinocytes, vacuolar degeneration of the basal layer and a mild mononuclear infiltrate in the superficial dermis (Fig. 2). Direct immunofluorescence (DIF) showed granular IgM and C3 deposits along the dermoepidermal junction, suggesting lupus. Considering the subacute presentation, the autoimmune results and the histological findings, a diagnosis of TEN-like subacute cutaneous lupus (SCL) as a severe presentation of SLE was assumed.
Table 1. Calculation of ALDEN Score [4] for Each Drug Recently or Chronically Administrated.
| Drug | ALDEN score |
||||||
|---|---|---|---|---|---|---|---|
| Causal link | Total ALDEN score | Type of drug | Dechallenge | Prechallenge/rechallenge | Drug present in the body on index day | Delay from initial drug component intake to onset of reaction | |
| Paracetamol | Very unlikely | -2 | Associated: 2 Drug with definite but lower risk |
Negative: -2 Drug continued without harm |
Negative: -2 Exposure without reaction |
Excluded: -3 Drug stopped |
Suggestive: +3 From 5 to 28 days |
| Sodium enoxaparin | Very unlikely | -5 | Not suspected: -1 No evidence of association |
Negative: -2 Drug continued without harm |
Negative: -2 Exposure without reaction |
Excluded: -3 Drug stopped |
Suggestive: +3 From 5 to 28 days |
| Ipratropium bromide | Very unlikely | -3 | Not suspected: -1 No evidence of association |
Neutral: 0 Drug stopped or unknown |
Negative: -2 Exposure without reaction |
Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Dexamethasone | Very unlikely | -1 | Not suspected: -1 No evidence of association |
Neutral: 0 Drug stopped or unknown |
Not done/unknown: 0 No known previous exposure |
Excluded: -3 Drug stopped | Suggestive: +3 From 5 to 28 days |
| Telmisartan | Very unlikely | -3 | Unknown: 0 | Neutral: 0 Drug stopped (or unknown) |
Negative: -2 Exposure without reaction |
Definite: 0 Drug continued |
Unlikely: -1 > 56 days |
Table 2. Full Blood Workup Alterations.
| Result (normal range) | |
|---|---|
| Blood test | |
| Leucocytes | 14.3 × 109/L (3.6 - 10.5 × 109/L) |
| Neutrophils | 9.3 × 109/L (1.5 - 7.7 × 109/L) |
| Hemoglobin | 9.9 g/dL (12 - 16 g/dL) |
| Mean corpuscular volume | 84 fL (80 - 100 fL) |
| Erythrocyte sedimentation rate | 51 mm/h (1 - 20 mm/h) |
| C-reactive protein | 20.1 mg/dL (0.5 - 1 mg/dL) |
| Procalcitonin | 0.67 ng/mL (0 - 0.5 ng/mL) |
| Lactate dehydrogenase | 585 U/L (< 247 U/L) |
| Ferritin | 2,790 ng/mL (30 - 300 ng/mL) |
| Albumin | 1.6 g/dL (3.5 - 5.2 g/dL) |
| Immune and autoimmune tests | |
| Complement component 3 | 0.66 g/L (0.83 - 1.93 g/L) |
| Complement component 4 | 0.1 (0.15 - 0.57) |
| Antinuclear antibodies | Positive (1:1,280, nuclear homogeneous pattern) |
| Extractable nuclear antigens antibodies | SSA60 (Ro60)/SSB (La) positive |
| Urine analysis | |
| 24 h proteinuria | 642 mg/24 h (50 - 80 mg/24 h) |
| Blood serologies | |
| Cytomegalovirus | Negative (IgM and IgG) |
| Herpes simplex virus 1 and 2 | Negative (IgM and IgG) |
| Epstein-Barr virus | Negative (IgM, IgG and EBNA) |
| Hepatitis B virus | Negative (HBsAg, HBsAb and HBcAb) |
| Hepatitis C virus | Negative (IgM and IgG) |
| Hepatitis A virus | Negative (IgM and IgG) |
| Human immunodeficiency virus | Negative (Ag/Ab) |
| Treponema pallidum | Negative (IgM and IgG) |
| Rickettsia conorii | Negative (IgM and IgG) |
| Coxiella burnetii | Negative (IgM and IgG) |
IgG: immunoglobulin G; IgM: immunoglobulin M; EBNA: Epstein-Barr nuclear antigen; HBsAg: hepatitis B surface antigen; HBsAb: hepatitis B surface antibody; HBcAb: hepatitis B core antibody; Ag: antigen; Ab: antibody.
Figure 2.
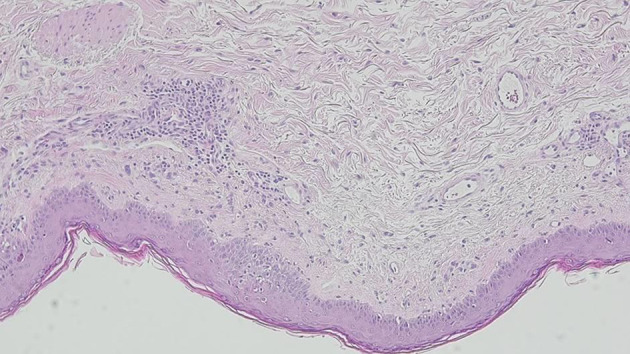
Skin biopsy showing vacuolar degeneration of the basal layer with scattered apoptotic keratinocytes and a dermal mononuclear cell infiltrate.
Treatment
The patient was admitted to the Burn Intensive Care Unit for continuous surveillance and balneotherapy for 10 days. She was later transferred to the Internal Medicine Department and started on hydroxychloroquine 400 mg/day and prednisolone 1 mg/kg/day.
Follow-up and outcomes
After 34 days of hospitalization and considering the marked improvement of the skin lesions, the patient was discharged. Due to the advanced age, she underwent a chest-abdomen-pelvis computed tomography scan, positron emission tomography and endoscopic study that excluded malignancy as the possible trigger of SLE. After ruling out other possible causes, a possible trigger for the development of SLE was the SARS-CoV-2 infection. After 1 year of follow-up, the patient is asymptomatic under a maintenance dose of prednisolone (5 mg/day) and hydroxychloroquine (400 mg/day), and the antinuclear antibodies titre decreased to a value of 1:640.
Discussion
We report the case of a patient with SLE, presenting as the severe form of TEN-like SCL. One month after SARS-CoV-2 infection, a 70-year-old female patient developed photoexposed macules that progressed to flaccid bullae and generalized epidermis detachment. Regarding the skin lesions, SJS/TEN was the first diagnostic hypothesis to be equated, but the application of the ALDEN score revealed improbable drug causality. A thorough autoimmune investigation suggested SLE, later confirmed by the histological findings of a skin biopsy.
TEN-like lesions may occur in the context of acute or SCL, leading to diagnostic challenges, particularly in patients with no previously known SLE. Several factors may help distinguish between classic SJS/TEN and TEN-like SLE. The absence of a relevant drug exposure and the initial involvement of photo-exposed areas favor TEN-like SLE. The presence of an interface dermatitis on the skin biopsy and the identification of IgM and C3 deposits at the basal layer suggest SLE [5]. Additionally, the progression of the skin lesions in a period of a few weeks instead of hours/days, as well as the identification of SSA and SSB autoantibodies, was concordant with the SCL presentation, and not acute cutaneous lupus [6]. The SARS-CoV-2 infection was a possible trigger for SLE due to the temporal relationship between COVID-19 and the onset of the skin lesions. No other infectious agents were identified, malignancy was excluded and drug-induced SLE seemed unlikely. Of note, DIF is helpful in achieving a likely diagnosis, especially when the histopathology results are not clarifying. DIF positivity may be found in various disorders, but besides lupus erythematous, IgM and C3 deposits are only observed in immune complex vasculitis, lichen planus and pemphigus erythematosus [7]. These conditions are not associated with the clinical and autoimmune findings described in this case.
Coronaviruses have previously been linked to autoimmune conditions and, in the case of SARS-CoV-2, to immune thrombocytopenia [8]. The possible mechanisms of autoimmunity in the context of a SARS-CoV-2 infection include molecular mimicry (cross reacting epitope between the virus and the host), bystander killing (virus-specific CD8+ T cells migrating to the target tissues and exerting cytotoxicity), epitope spreading, viral persistence (polyclonal activation due to the constant presence of viral antigens driving immune-mediated injury) and formation of neutrophil extracellular traps [9]. Other theories have been proposed: 1) The COVID-19-related lymphopenia may lead to the failure in maintaining peripheral tolerance, resulting in an activation of effector T cells with autoimmune potential; 2) Both COVID-19 and SLE patients present with changes in the microbiome, which may weaken the immune system, leading to increased disease severity and autoimmune manifestations [10].
Several cases of SLE flares following COVID-19 have been described. Only two reports depicted SLE induction in the context of SARS-CoV-2 infection, with the limitation that on both, despite the COVID-19 diagnosis preceding the SLE symptoms, the most frequent infectious agents and other causes associated with SLE were not excluded [11, 12]. Although single-patient reports have limitations, the authors hope to offer insight into the possibility of COVID-19-related changes to the immune system leading to autoimmune diseases, such as SLE.
Learning points
SLE is a systemic autoimmune condition that can be triggered by multiple infectious agents. SARS-CoV-2 has been linked to autoimmune phenomena, constituting a possible trigger for autoimmune disorders. SLE disease flare-ups after SARS-CoV-2 infection may have a severe and life-threatening course.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Patient’s informed consent for publication of this report was obtained, including any accompanying photographs.
Author Contributions
Ana Luisa Nunes, Leonor Simoes, Carolina Figueiredo and Ruben Carvalho contributed to the writing of this manuscript; all the authors were involved in the patient’s treatment and in the review of this report.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illescas-Montes R, Corona-Castro CC, Melguizo-Rodriguez L, Ruiz C, Costela-Ruiz VJ. Infectious processes and systemic lupus erythematosus. Immunology. 2019;158(3):153–160. doi: 10.1111/imm.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawalha AH, Manzi S. Coronavirus Disease-2019: Implication for the care and management of patients with systemic lupus erythematosus. Eur J Rheumatol. 2020;7(Suppl 2):S117–S120. doi: 10.5152/eurjrheum.2020.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, Haustein UF. et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 5.Shahidi-Dadras M, Araghi F, Ahmadzadeh A, Rakhshan A, Tabary M, Dadkhahfar S. TEN/SJS-like lupus erythematosus presentation complicated by COVID-19. Dermatol Ther. 2021;34(1):e14612. doi: 10.1111/dth.14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peric J, Lekic B, Bosic M, Skiljevic D. Toxic epidermal necrolysis-like subacute cutaneous lupus erythematosus: a case report. Serbian Journal of Dermatology and Venereology. 2019;11(4):129–132. doi: 10.2478/sjdv-2019-0018. [DOI] [Google Scholar]
- 7.Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6(3):172–180. doi: 10.4103/2229-5178.156386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavan S, Gonakoti S, Asemota IR, Mba B. A case of systemic lupus erythematosus flare triggered by severe coronavirus disease 2019. J Clin Rheumatol. 2020;26(6):234–235. doi: 10.1097/RHU.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracia-Ramos AE, Saavedra-Salinas MA. Can the SARS-CoV-2 infection trigger systemic lupus erythematosus? A case-based review. Rheumatol Int. 2021;41(4):799–809. doi: 10.1007/s00296-021-04794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz-Agranov N, Zandman-Goddard G. Autoimmunity and COVID-19 - The microbiotal connection. Autoimmun Rev. 2021;20(8):102865. doi: 10.1016/j.autrev.2021.102865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonometti R, Sacchi MC, Stobbione P, Lauritano EC, Tamiazzo S, Marchegiani A, Novara E. et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci. 2020;24(18):9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 12.Zamani B, Moeini Taba SM, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. 2021;15(1):29. doi: 10.1186/s13256-020-02582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.