Abstract
Objective:
The relationship between cancers and obstructive sleep apnea (OSA) has been discussed for decades. However, the previous meta-analysis led to opposite conclusions. To further investigate this controversial issue, we performed this systematic review and meta-analysis update.
Methods:
PubMed, Embase, and the Cochrane Library were systematically searched and studies on “cancer and OSA” were all included. Two reviewers independently searched articles, extracted data, and assessed the quality of included studies. Moreover, the overall incidence of cancer and OSA in corresponding populations was calculated.
Results:
Of the 1434 titles identified, 22 articles involving more than 32.1 million patients were included in this meta-analysis. An overall incidence of OSA positive individuals in cancer was 46 (95%CI, 27–67)%, and the prevalence of cancers in OSA patients reached 1.53 (95%CI, 1.01–2.31) times higher than non-OSA individuals.
Conclusion:
This meta-analysis indicated that there was a high prevalence of OSA in cancer patients, and individuals with OSA were more likely to develop tumors, and the incidence was related to the severity of OSA.
Keywords: cancer, meta-analysis, obstructive sleep apnea, tumor
1. Introduction
Obstructive sleep apnea (OSA) is a medical disorder characterized by recurrent upper airway collapse caused by the partial or complete collapse of upper airway and cessation of airflow during sleep.[1] It is widespread in the general population. The overall prevalence of symptomatic OSA was 20% to 30% in men, and 10% to 15% in women, and the rates were still increasing.[2] OSA is currently recognized as a systemic disease, and is often associated with multiple comorbidities, including hypertension, myocardial infarction, stroke, mellitus diabetes, pulmonary hypertension, and depression.
Up to now, the pathophysiological mechanism of OSA is considered as intermittent hypoxia caused by apnea,[3] and this intermittent hypoxia may be related to the occurrence of tumors in OSA patients. Some scholars argued that intermittent hypoxia was similar to tissue ischemia-reperfusion.[4–6] Through repeated hypoxia and reoxygenation processes, a large number of transcriptional mediators of the hypoxic and inflammatory responses are generated, which increase oxidative stress, inflammation, and DNA damage, then, followed by tumorigenesis.[5]
Previous meta-analyses also discussed the relationship between cancers and OSA, however, it reached highly conflicting outcomes.[7,8] Recently epidemiological investigations have found that OSA is closely related to the occurrence and development of tumors.[9–11] With the release of some new data, we conducted this meta-analysis to assess the incidence of cancers and OSA in their respective populations, thus, re-evaluate the associating between cancers and OSA.
2. Method
2.1. Literature-search strategy
This literature search performed on March 20, 2020 without any restrictions in region, publication type, journal or language. The databases of PubMed (Medline), Embase (Excerpta Medica Database), Web of Science (Science Citation Index and Social Sciences Citation Index), and the Cochrane Library were thoroughly searched with the following strategy: (“cancer” or “tumor” or “carcinoma” or “neoplasm”) and (“OSA” or “OSAH” or “sleep apnea” or “obstructive sleep apnea” or “obstructive sleep apnea-hypopnea syndrome”).
The inclusion criteria were the following:
-
1.
articles were published as original research,
-
2.
written only in English,
-
3.
tumor located in pharynx, larynx, oral cancer, etc, which may contribute to OSA anatomically.
The exclusion criteria were the following:
-
1.
obstructive sleep apnea diagnosed after surgeon,
-
2.
review article, case report, letter, editorial, commentary, and conference abstract,
-
3.
study with unavailable data,
-
4.
data from duplicate articles in previous studies.
2.2. Data extraction and quality assessment
Two reviewers (YC and PN) independently inspected all candidate articles independently. The following information was extracted from the included studies: author, year of publication, study country, region, sample size, study design, age, gender, BMI, apnea hypopnea index (AHI), diagnostic methods of OSA, follow-up period, and tumor location.
The quality of the included studies was evaluated according to the Newcastle-Ottawa Scale (NOS), and scores ≥6 were defined as high-quality. Two authors independently performed the analyses, and consensuses were reached on all decisions. Discrepancies were resolved by discussion with a senior author (QL).
2.3. Statistical analysis and data synthesis
All data were entered into STATA statistical software 13.0 (Stata Corp LP, College Station, TX) for meta-analysis. Data were presented as mean ± SD to evaluate the relationships between cancer and OSA. The heterogeneity of the studies was measured using the I2 statistic, with the level of significance set at P < .05.
If I2 value > 50%, we considered that substantial heterogeneity among studies and the random-effects model was used to calculate the pooled RR and 95%CI; otherwise, the fixed-effects model was applied. Likewise, subgroup analysis was conducted to compare observed effects between OSA and non-OSA, with P < .05 denoting statistical significance. Publication bias was assessed by Begg test and Egger test.
The conduct of this meta-analysis was consistent with the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. Regarding the quality of studies, studies scored ≥6 stars in NOS which suggested high quality.
2.4. Ethics and dissemination
This study will not need to provide ethical approval, because this study based on the published data. We are expected to publish this study on peer-reviewed journals.
3. Results
3.1. Study characteristics
The characteristics of the included studies are shown in Table 1. There were 22 clinical studies that included more than 32.1 million individuals and met the criteria for meta-analysis.[9–29] The literature selection procedure is presented in Figure S1, Supplemental Digital Content. The included articles were published between 2011 and 2020, among which, 15 articles based on cancers in OSA patients (3 articles covered cancers in terms of OSA severity), and the rest 7 articles reported OSA in cancer patients. Regarding the quality of studies, all studies reached high-quality levels.
Table 1.
Characteristics of the included studies.
| Author | Year | Country | Development | Population | Study design | Age | Gender | BMI | AHI | Diagnostic methods of OSA | Follow-up | Cancer | NOS |
| Gao | 2020 | China | Developing | 2400 | Mendelian randomization study | – | All women | OSA: case 23.97 ± 3.21, control 23.88 ± 3.17; breast cancer: case 23.96 ± 3.20, control 23.90 ± 3.24 | – | PSG | – | Breast cancer | 7 |
| Chen | 2019 | China | Developing | 20,900 | Cohort study | OSA: 45.97 yr, control: 45.64 yrs | OSA: men 2970, women 1210, control: men 11,880, women 4840 | – | – | ICD-9 | 14 yr | Colorectal cancer | 7 |
| Choi | 2019 | Korea | Developed | 274,201 | Retrospective cohort study | OSA: 48.7 ± 14.2; control: 48.7 ± 14.2 | All women | – | – | ICD-10 | 3.7 ± 2.3 yr | Breast cancer | 6 |
| Jara | 2019 | United States | Developed | 1,377,285 | Retrospective matched cohort | 55.2 ± 13.0 | Men 1,294,648, women 82,637 | – | – | ICD-9 | 8.1 ± 4.3 yr | Cancers | 7 |
| Liu | 2019 | China | Developing | 45 | Case-control study | – | – | – | – | PSG | 1 yr | Lung cancer | 6 |
| Polonis | 2019 | United States | Developed | 161 | Cohort study | Non-OSA: 35.6 ± 11.0, OSA: 47.3 ± 11.9 | Non-OSA: men 59, women 29; OSA: men 64, women 9 | Non-OSA: 26.9 ± 5.1; OSA: 33.0 ± | Non-OSA: 1.0 ± 1.3; OSA: 33.9 ± 27.3 | PSG | 12.7 yr | Cancers | 6 |
| Sillah | 2019 | United States | Developed | 1446 | Case-cohort study | Case group: 57.2 ± 10.8; subcohort: 50.4 ± 12.9 | Case group: men 173, women 131; subcohort: men 665, women 497 | – | Case group: 47.0 ± 31.1; subcohort: 41.3 ± 34.7 | PSG | 1851 (IQR: 1002–2835) d | Cancers | 8 |
| Brenner | 2018 | Israel | Developed | 5243 | Retrospective cohort study | 51 ± 13.1 | Men 3905, women 1338 | 29.7 (26.4–33.9) | 18 (10–34) | PSG | 5.9 yr | Cancers | 7 |
| Cabezas | 2018 | Spain | Developed | 60 | Cross-sectional study | 67.8 ± 11 | Men 35, women 25 | – | 15.2 (6.9–29.4) | PSG | 12 mo | Lung cancer | 6 |
| Campos-Rodriguez | 2018 | United States | Developed | 83 | Cross-sectional study | 48.8 ± 8.8 | All women | 27.4 ± 5.4 | 5.1 (2–9.4) | PSG | – | Breast cancer | 6 |
| Dreher | 2018 | Germany | Developed | 100 | Cross-sectional study | 68.1 ± 8.6 | Men 67, women 33 | 26.0 ± 4.6 | 4.9 (2.0,10.4) | PSG | – | Lung cancer | 6 |
| Du | 2018 | China | Developing | 10,388 | Cross-sectional study | 46.6 ± 0.4 | Men 5034, women 5354 | - | – | Self-reported | – | Cancers | 6 |
| Martinez-Garcia | 2018 | Spain | Developed | 443 | Cross-sectional study | 55.98 ± 15.3 | Men 224, women 219 | 27.3 ± 4.5 | 8.6 (2.8–20.2) | PSG | – | Melanoma | 6 |
| Gozal | 2016 | United States | Developed | 30.3 million | Cohort study | >30 yr | – | – | – | ICD-9 | 3 yr | Cancers | 6 |
| Lee | 2016 | Korea | Developed | 163 | Case-control study | OSA: 63.0 ± 10.9, non-OSA: 54.9 ± 14.5 | OSA: men 32, women 79; non-OSA: men 32, women 20 | OSA: 25.4 ± 3.3, non-OSA: 24.5 ± 3.3 | – | PSG | – | Colorectal neoplasia | 6 |
| Chang | 2014 | China | Developing | 5076 | Matched case-controlled study | – | All women | – | – | ICD-9 | 5 yr | Breast cancer | 6 |
| Chen | 2014 | China | Developing | 92,220 | Cohort study | OSA: 37.6 ± 8.2, control: 37.6 ± 8.2 | OSA: men 15,401, women 7654; control: men 46,202, women 22,963 | – | – | PSG | 10 yr | Primary central nervous system cancers | 7 |
| Kendzerska | 2014 | Canada | Developed | 10,149 | Cohort study | 48 (39–58) | Men 6284, women 3865 | 28.9 (25.3–33.4) | – | PSG | 7.8 yr | Cancers | 7 |
| Marshall | 2014 | Australia | Developed | 393 | Prospective cohort study | Non-OSA: 52.8 ± 7.6, mild OSA: 54.7 ± 7.3, moderate-severe OSA: 55.1 ± 8.2 | Men 290, women 103 | Non-OSA: 26.2 ± 3.7, mild OSA: 28.0 ± 4.0, moderate-severe OSA: 34.3 ± 7.3 | – | 4-channel portable home-monitoring device | 20 yr | Cancers | 8 |
| Martinez-Garcia | 2014 | Spain | Developed | 5427 | Retrospective cohort study | 53.9 ± 13.1 median (IQR) | – | – | 30 (14–52) | PSG | 4.5 yr | Cancers | 6 |
| Campos-Rodriguez | 2013 | Spain | Developed | 4910 | Retrospective cohort study | – | – | – | – | PSG or validated respiratory polygraphy (RP) | 4.5 (interquartile range, 3.4–5.2) yr | Cancers | 7 |
| Thompson | 2011 | United States | Developed | 1240 | Case-control study | Case group: 57.3 ± 8.0; control: 54.7 ± 8.8 | Case group: men 154, women 184; control: men 293, women 605 | Case group: 29.9 ± 7.2; control: 29.2 ± 6.8 | – | PSQI | – | Colorectal adenoma | 6 |
ICD = International classification of diseases, OSA = obstructive sleep apnea, PSG = polysomnography, PSQI = Pittsburgh sleep quality index.
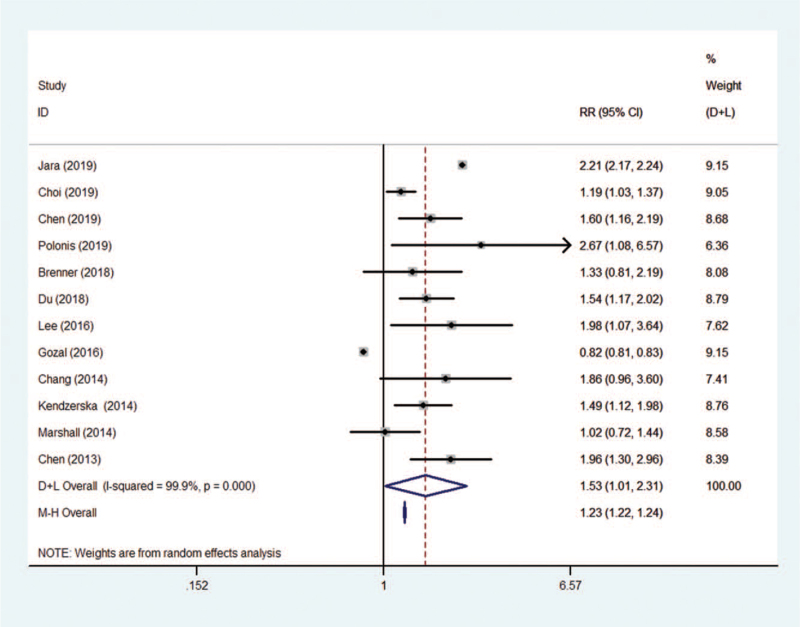
Seven articles reporting data from a total of 2269 cancer patients, and among whom 774 individuals were also suffered OSA. An overall incidence of 46 (95%CI, 27–67)% of OSA positivity in cancer patients was calculated in this meta-analysis, see Figure 1. Meanwhile, the meta-analysis showed the pooled prevalence of cancers in OSA patients reached 1.53 (95%CI, 1.01–2.31) times higher when compared to non-OSA individuals, see Figure 2.
Figure 1.
The overall prevalence of OSA positivity in cancer patients. OSA = obstructive sleep apnea.
Figure 2.
The overall prevalence of cancers in OSA patients vs non-OSA patients. OSA = obstructive sleep apnea.
3.2. Subgroup analysis
Based on the region of each study, the prevalence of cancers in OSA patients was 1.23 (I2 = 99.9%, P < .0001) times higher than the control group in developed countries. However, no statistical significance found in developing countries (Fig. S2, Supplemental Digital Content). And the cancer prevalence was still high in OSA when the patients were divided to three continents (P < .0001), Asia: RR 1.36 (95%CI, 1.22–1.51), America: RR 1.23, (95%CI, 1.22–1.24), and only one article in Oceania with 1.02 (95%CI, 0.27–1.44), see Figure S3, Supplemental Digital Content. In addition, subgroup analysis for cancer incidence according to the severity of OSA is displayed in Figure 3, and cancer incidence for mild OSA was 0.68 (95%CI, 0.52–0.84), 1.68 (95%CI, 1.07–2.30) for moderate to severe OSA, 0.44 (95%CI, 0.40–0.48) for moderate OSA. Severe OSA group has an incidence of 0.49 (95%CI, 0.44–0.54) for cancer.
Figure 3.
The cancer incidence in OSA patients subgroup by severity of OSA. OSA = obstructive sleep apnea.
3.3. Publication bias and sensitivity analysis
Egger test and funnel plot were used to assess the publication bias. Funnel plots of the studies appeared to be symmetrical by visual examination. The data suggested that there was no evidence of publication bias (P > .05; Fig. S5, Supplemental Digital Content and Fig. S6, Supplemental Digital Content). Through omission of each of the included literature studies on prevalence of OSA positivity in cancer patients, results were less changed following sensitivity analysis (Fig. S7A, Supplemental Digital Content). However, sensitivity analysis on studies of cancers’ prevalence in OSA patients demonstrated that prevalence changed to 0.82 (95%CI, 0.81–0.83) and 2.18 (95%CI, 2.15–2.21) by omission the study of Jara et al and Gozal et al, respectively[10,30] (Fig. S7B, Supplemental Digital Content).
4. Discussion
In recent years, there has been reported that OSA played a negative role in cancer incidence. And the high prevalence of OSA epidemiologically linked to the increasing of cancer worldwide. Herein, we performed this study to further explore the overall incidence of cancer and OSA in the corresponding population, and provide some evidence on the association between the two entities. In this up-to-date meta-analysis, we found that more than half of the cancer patients also developed OSA. Additionally, OSA patients are 1.53 times more likely to suffer from cancer when compared to non-OSA patients.
Intermittent hypoxia and sleep fragmentation are highly prevalent conditions and hallmarks of sleep apnea, which have been proposed as the main causes of most of the commodities associated with OSA.[31] It has been demonstrated that intermittent hypoxia can stimulate the production of reactive oxygen species (ROS), which led to oxidative stress and systemic inflammation.[32] Increased activity of the hypoxia-inducible factor-1 (HIF-1) and reduction in antioxidant promoted by intermittent hypoxia could accelerate ROS production, thus contributing to oxidative stress.[6] Increased activity of HIF-1 also contributed to tumor growth by increasing expression of vascular endothelial growth factor (VEGF) and angiogenesis. In the meanwhile, Hakim and colleagues illustrated that sleep fragmentation can accelerate tumor growth and progression through tumor-associated macrophages recruitment and proinflammatory TLR4 signaling pathways in animal models.[33] From the perspective of tumor biology, hypoxia is a frequent phenomenon in solid tumor, and it enhanced the malignant properties in proliferation, invasion, metastasis and angiogenesis of cancer, however, the mechanisms varies from different tumors.[34] Hence, we inferred that OSA could not only increase the incidence of cancer in population, but also enhanced malignancy of cancer. Katarzyna et al investigated the association between telomere length (TL) and risk of cancer in OSA patients. In this study, the authors digged a considerable effect size of TL on the risk of cancer. Moderate-to-severe OSA individuals, who had longer telomeres on average, showed a higher risk of cancer than non-OSA individuals. On the other hand, longer telomeres kept a sustained capacity of cell proliferation to increase the possibility of attaining a critical number of genetic mutations. It speculated that the combination of OSA-mediated telomere elongation and a higher rate of genetic mutation accumulation may represent a survival advantage of precancerous cells, and thus predispose OSA patients to a higher risk of cancer.
Clinical studies did not achieve a unified consensus on the relationship between OSA and the incidence of tumors from earlier literatures.[35] The first clinical cohort investigation on OSA and cancer incidence was performed in Spanish by Campos-Rodriguez et al.[15] A total of 4910 patients from seven teaching hospitals were included and followed up for an average of 4.5 years. Percent nighttime with oxygen saturation < 90% (Tsat90) and AHI were used to evaluate the severity of OSA. The result showed the cancer incidence in Tsat90 > 12% was 2.33 times higher than Tsat90 < 1.2% category. In stratified analyses, together with Tsat90, AHI was associated with cancer incidence in patients younger than 65 years. In the same year, a prospective study from Copenhagen was published by Christensen et al, however, no clear association was observed between the incidence of cancer and the presence of symptoms related to sleep-disordered breathing.[36] This negative result may be attributed to the inclusion of patients who were not diagnosed by polysomnography, but by questionnaires, which led to some non-OSA patients being calculated in this study. For further examine the higher prevalence of OSA among patients with solid tumors, a longitudinal nationwide-based cohort study base on more than 5.6 million individuals was reported. Sleep apnea appeared to increase the risk for only certain types of solid malignancies, including pancreatic and kidney cancer and melanoma, nevertheless, it did not increase the risk of colorectal, breast, and prostate cancers.[30] There was also evidence that the severity of OSA was linked to the aggressiveness of breast cancer and melanoma.[14,27] Because different tumor types have different biological behaviors, heterogeneity still existed in the current published data.
In this study, we found OSA is significantly related to the rising incidence of tumors, and subgroup analysis for severity of OSA disclosed that the incidence of cancers did not linearly increase according to the severity of OSA defined by AHI, from a group to group aspect, moderate to severe group presented a higher incidence than mild group, and severe group was higher than moderate group. Nevertheless, if we only analyzed the studies grouped by mild, moderate, and severe, mild OSA group has the lowest risk rate of 38 (95%CI, 36–41)% for cancer, moderate for 44 (95%CI, 40–48)%, severe for 49 (95%CI, 44–54)% (see Fig. S6, Supplemental Digital Content). This inconsistent may be caused by the different grouping comparison methods of the original literature. However, the high-risk cancer in OSA still not proved OSA directly participant the carcinogenesis, even growing evidences had demonstrated the pathophysiological of OSA related to immune response and biological pathways of cancers. To explore the causality of cancer and OSA, a genome-wide association study (GWAS) was reported in breast cancer to determine the causal effect of risk of OSA on breast cancer.[20] The authors conducted a two-sample study, one from real-world research, another from the Breast Cancer Association Consortium (BCAC), and provided the evidence that rs11588454 and rs11897825 were associated with increased risk of breast cancer. Then, the Mendelian randomization analyses found evidence of a detrimental causal effect of OSA on breast cancer risk was similar to the results of the real-world study. In lung cancer, OSA has also been confirmed to have a higher incidence than healthy control. In addition, the stage of lung cancer in OSA was more advanced, and the mortality, recurrence rate, and metastasis rate increased compared to healthy control in a 1-year follow-up.[24] Besides tumors located in the chest region, OSA also linked to the aggressiveness of cutaneous malignancy. By screening 443 patients who diagnosed with melanoma, 65% of OSA patients were sorted out by respiratory polygraphy, and results found intermittent nighttime oxyhemoglobin desaturation (DI4%) and AHI were independently associated with greater aggressiveness of cutaneous melanoma by detecting some clinical markers.[27]
In sensitivity analysis, we found that the two included studies had some influence on the prevalence of cancer in OSA individuals. Jara et al's study was a retrospective matched cohort study on all veterans diagnosed with OSA from October 1992 to September 2013. The result showed a nearly 2-fold higher hazard of developing any tumor in OSA compared to non-OSA individuals.[10] Such high ratio might related to certain reasons: the subjects included in the study are veterans, rather than the regular community population. As it mentioned in the original text, “the diagnosis of OSA could get disability benefits since 2004, which provided veterans with a financial incentive to receive an OSA diagnosis.”[10] This may be the cause of OSA overdiagnosis in this study. A nationwide population survey based health insurance database conducted by Gozal et al identified that OSA only increased the risk of a very selective number of cancers.[21] Individuals with OSA only increased the risk of pancreatic and kidney cancer and melanoma by adjusted data. The prevalence of cancers in OSA patients reached 1.37 (95%CI, 0.91–20.7) times higher than non-OSA individuals. However, the national insurance data used in this epidemiological survey ignored the uninsured people, who happen to be low-income and relatively poor people. These people lack primary health services and are at higher risk of cancerous diseases. Therefore, the prevalence of tumors with OSA obtained in this study was slightly lower (Supplemental Figure 4).
Some limitations still existed in this meta-analysis. Firstly, in this meta-analysis, there were still two studies diagnosed OSA by questionnaires, which will overdiagnose some healthy individuals, thus, strengthen the association between OSA and cancer. Secondly, in the studies of colorectal cancer, only Chen et al's study included malignant neoplasm. And in Lee et al's study, carcinoma in situ and intramucosal carcinoma were included for incidence statistics of colorectal neoplasia, meanwhile, Thompson et al's study screened colorectal adenoma by colonoscopy. These abnormal growths of glandular tissue in the gastrointestinal tract mentioned above could all attributed to precancerous lesions and included the two studies that might increase the incidence of cancer in this meta-analysis. Thirdly, besides some large epidemiologic studies, a part of the published studies included in our study has a small sample size, which may skew the results. Fourth, not all the studies included for calculating cancer incidence in OSA when compared to the control group were adjusted by age, gender, obesity, smoking, or alcohol intake. This inter-study heterogeneity might have some influence on our results. Lastly, publication bias existed in our study, because only published literatures in English were included.
5. Conclusion
In this study, despite these limitations, we deliberately focused on relationships between cancer and OSA, and currently available data suggested individuals with OSA were more likely to develop tumors, and the incidence was related to the severity of OSA. In the meantime, there was a high prevalence of OSA in cancer patients. Whether OSA potentially promoted the tumor malignancy, and how did it increase tumorigenesis or progression, need further studies to delineate.
Author contributions
Conceptualization: Yuan Cao, Pu Ning, Shuang Wu.
Data curation: Yuan Cao, Pu Ning, Qiao Li.
Formal analysis: Yuan Cao, Pu Ning.
Funding acquisition: Yuan Cao, Pu Ning.
Investigation: Yuan Cao, Qiao Li.
Methodology: Yuan Cao.
Project administration: Yuan Cao, Pu Ning, Qiao Li.
Resources: Yuan Cao.
Software: Yuan Cao.
Supervision: Qiao Li, Shuang Wu.
Validation: Yuan Cao.
Visualization: Yuan Cao.
Writing – original draft: Yuan Cao, Pu Ning, Qiao Li.
Writing – review & editing: Yuan Cao, Pu Ning, Qiao Li, Shuang Wu.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AHI = apnea hypopnea index, BCAC = Breast Cancer Association Consortium, GWAS = genome-wide association study, HIF-1 = hypoxia-inducible factor-1, OSA = obstructive sleep apnea, ROS = reactive oxygen species, Tsat90 = percent nighttime with oxygen saturation < 90%, VEGF = vascular endothelial growth factor.
How to cite this article: Cao Y, Ning P, Li Q, Wu S. Cancer and obstructive sleep apnea: An updated meta-analysis. Medicine 2022;101:10(e28930).
This work was supported by Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2020JQ-543; 2020JQ-927); Fundamental Research Funds for the Central Universities under Grant xzy012019129; Xi’an Science and Technology program SF1510(4); Xi’an Science and Technology program under Grant J20170219II.
Authors declare no conflict of interests.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. [DOI] [PubMed] [Google Scholar]
- [2].Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- [3].Vicente E, Marin JM, Carrizo SJ, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J 2016;48:1108–17. [DOI] [PubMed] [Google Scholar]
- [4].Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses 2006;66:1069–73. [DOI] [PubMed] [Google Scholar]
- [5].Hunyor I, Cook KM. Models of intermittent hypoxia and obstructive sleep apnea: molecular pathways and their contribution to cancer. Am J Physiol Regul Integr Comp Physiol 2018;315:R669–87. [DOI] [PubMed] [Google Scholar]
- [6].Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, Pancholy SB. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med 2015;16:1289–94. [DOI] [PubMed] [Google Scholar]
- [8].Zhang XB, Peng LH, Lyu Z, Jiang XT, Du YP. Obstructive sleep apnoea and the incidence and mortality of cancer: a meta-analysis. Eur J Cancer Care 2017;26: [DOI] [PubMed] [Google Scholar]
- [9].Choi JH, Lee JY, Han KD, Lim YC, Cho JH. Association between obstructive sleep apnoea and breast cancer: the Korean National Health Insurance Service Data 2007–2014. Sci Rep 2019;9:19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jara SM, Phipps AI, Maynard C, Weaver EM. The Association of Sleep Apnea and Cancer in Veterans. Otolaryngol Head Neck Surg 2020;162:581–8. [DOI] [PubMed] [Google Scholar]
- [11].Chen CY, Hu JM, Shen CJ, et al. Increased incidence of colorectal cancer with obstructive sleep apnea: a nationwide population-based cohort study. Sleep Med 2020;66:15–20. [DOI] [PubMed] [Google Scholar]
- [12].Brenner R, Kivity S, Peker M, et al. Increased risk for cancer in young patients with severe obstructive sleep apnea. Respiration 2019;97:15–23. [DOI] [PubMed] [Google Scholar]
- [13].Cabezas E, Perez-Warnisher MT, Troncoso MF, et al. Sleep disordered breathing is highly prevalent in patients with lung cancer: results of the sleep apnea in lung cancer study. Respiration 2019;97:119–24. [DOI] [PubMed] [Google Scholar]
- [14].Campos-Rodriguez F, Cruz-Medina A, Selma MJ, et al. Association between sleep-disordered breathing and breast cancer aggressiveness. PloS One 2018;13:e0207591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013;187:99–105. [DOI] [PubMed] [Google Scholar]
- [16].Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med 2014;15:1016–20. [DOI] [PubMed] [Google Scholar]
- [17].Chen JC, Hwang JH. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med 2014;15:749–54. [DOI] [PubMed] [Google Scholar]
- [18].Dreher M, Krüger S, Schulze-Olden S, et al. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm Med 2018;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Du W, Liu J, Zhou J, Ye D, OuYang Y, Deng Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005-2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis 2018;13:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gao XL, Jia ZM, Zhao FF, et al. Obstructive sleep apnea syndrome and causal relationship with female breast cancer: a Mendelian randomization study. Aging 2020;12:4082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep 2016;39:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kendzerska T, Leung RS, Hawker G, Tomlinson G, Gershon AS. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014;186:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee S, Kim BG, Kim JW, et al. Obstructive sleep apnea is associated with an increased risk of colorectal neoplasia. Gastrointest Endosc 2017;85:568.e1–73.e1. [DOI] [PubMed] [Google Scholar]
- [24].Liu W, Luo M, Fang YY, Wei S, Zhou L, Liu K. Relationship between occurrence and progression of lung cancer and nocturnal intermittent hypoxia, apnea and daytime sleepiness. Current Med Sci 2019;39:568–75. [DOI] [PubMed] [Google Scholar]
- [25].Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med 2014;10:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martínez-García MA, Campos-Rodriguez F, Durán-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med 2014;15:742–8. [DOI] [PubMed] [Google Scholar]
- [27].Martinez-Garcia MA, Campos-Rodriguez F, Nagore E, et al. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: a multicenter observational study in 443 patients. Chest 2018;154:1348–58. [DOI] [PubMed] [Google Scholar]
- [28].Sillah A, Watson NF, Gozal D, Phipps AI. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev Med Rep 2019;15:100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011;117:841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gozal D, Farre R, Nieto FJ. Obstructive sleep apnea and cancer: epidemiologic links and theoretical biological constructs. Sleep Med Rev 2016;27:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Almendros I. Does obstructive sleep apnea confer risk to induce or enhance tumor malignancy? Sleep Med Rev 2016;27:106–7. [DOI] [PubMed] [Google Scholar]
- [32].Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: from hypotheses to evolving evidence. Chest 2015;148:1140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res 2014;74:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Almendros I, Gozal D. Intermittent hypoxia and cancer: undesirable bed partners? Respir Physiol Neurobiol 2018;256:79–86. [DOI] [PubMed] [Google Scholar]
- [35].Martínez-García M, Campos-Rodriguez F, Barbé F. Cancer and OSA: current evidence from human studies. Chest 2016;150:451–63. [DOI] [PubMed] [Google Scholar]
- [36].Christensen AS, Clark A, Salo P, et al. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep 2013;36:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.