Abstract
Objectives:
The efficacy of repetitive transcranial magnetic stimulation (rTMS) in clinically-relevant neuroplasticity research depends on the degree to which stimulation induces robust, reliable effects. The high degree of inter- and intra-individual variability observed in response to rTMS protocols, such as continuous theta burst stimulation (cTBS), therefore represents an obstacle to its utilization as treatment for neurological disorders. Brain-derived neurotrophic factor (BDNF) is a protein involved in human synaptic and neural plasticity, and a common polymorphism in the BDNF gene (Val66Met) may influence the capacity for neuroplastic changes that underlie the effects of cTBS and other rTMS protocols. While evidence from healthy individuals suggests that Val66Met polymorphism carriers may show diminished or facilitative effects of rTMS compared to their homozygous Val66Val counterparts, this has yet to be demonstrated in the patient populations where neuromodulatory therapies are most relevant.
Materials and Methods:
We examined the effects of BDNF Val66Met polymorphism on cTBS aftereffects in stroke patients. We compared approximately 30 log-transformed motor-evoked potentials (LnMEPs) obtained per time point: at baseline and at 0, 10, 20, and 30 minutes after cTBS-600, from 18 patients with chronic stroke using single TMS pulses. We used linear mixed-effects regression with trial-level data nested by subject for higher statistical power.
Results:
We found a significant interaction between BDNF genotype and pre-/post-cTBS LnMEPs. Val66Val carriers showed decrease in cortical excitability, whereas Val66Met carriers exhibited a modest increase in cortical excitability for 20 minutes post-stimulation, followed by inhibition 30 minutes after cTBS-600.
Conclusions:
Our findings strongly suggest that BDNF genotype differentially affects neuroplastic responses to TMS in individuals with chronic stroke. This provides novel insight into potential sources of variability in cTBS response in patients, which has important implications for optimizing the utility of this neuromodulation approach. Incorporating BDNF polymorphism genetic screening to stratify patients prior to use of cTBS as a neuromodulatory technique in therapy or research may optimize response rates.
Keywords: Continuous Theta-Burst Stimulation, Brain-derived neurotrophic factor, Chronic Stroke, Inter-individual variability, Repetitive Transcranial Magnetic Stimulation
INTRODUCTION
Despite its having been introduced over 30 years ago, clinical and research applications of repetitive transcranial magnetic stimulation (rTMS) continue to grow, owing to its ability to both probe and modulate cortical activity. Since its approval by the FDA in 2008, rTMS has become widely used as a treatment for major depressive disorder1, and is also being used as an intervention in obsessive-compulsive disorder2 and several other neurological and psychiatric disorders3. It also remains a critical research tool for elucidating the structure-function relationships in the brain related to a wide range of motor, perceptual, and cognitive abilities4–6, and for characterizing the physiologic mechanisms that underlie cortical excitability7 and neuroplasticity8.
Theta burst stimulation (TBS), a modified form of rTMS, is understood to produce robust effects on cortical excitability in a fraction of the time of other rTMS protocols, making it an attractive approach for research and clinical applications. TBS consists of 50 Hz bursts of stimulation pulses delivered in triplets at 5 Hz. An intermittent pattern of TBS (iTBS) has been shown to exhibit excitatory aftereffects on cortical activity, while continuous TBS (cTBS) has been associated with inhibitory aftereffects9. These aftereffects last from 30 minutes for 20s cTBS (300 pulses) to a 60 minutes for 40s cTBS (600 pulses), which is longer lasting than 1Hz rTMS9. Evidence suggests that the persistent effects of TBS may be due to N-methyl-D-aspartate (NMDA)-mediated changes at synapses and may be mediated by mechanisms similar to long-term potentiation (LTP) and long-term depression (LTD) effects observed in animal studies10–12. Because synaptic plasticity has been associated with recovery of functions after stroke and other forms of brain injury, TBS may be a promising approach in neurorehabilitation. TBS also affects motor task performance for up to 30 minutes after stimulation in healthy subjects9, and improves reaction times (and increases corticospinal excitability) in patients with chronic stroke13. The advantages of TBS over other non-invasive brain stimulation strategies are its low intensity, short duration of application and long-lasting effects.
Efforts to extend the application of TBS to studies of motor, language or cognitive recovery following stroke have been pursued13–17. However, despite the promising features of rTMS and of TBS specifically, a few recent large-scale randomized clinical trials have failed to show significant effects of stimulation on motor recovery after stroke18,19. Evidence suggests that physiologic responses to TBS can exhibit a high degree of inter- and intra-individual variability14,15. Thus, one potential explanation for null results from studies employing TBS may be inter-individual variability in susceptibility to the modulatory effects of rTMS on cortical excitability20 and/or motor network connectivity21. Identifying factors that contribute to this variability and stratifying patients and research participants accordingly could be crucial to successfully advancing TBS treatments for neurological disorders, and for optimizing research protocols involving rTMS more broadly. In healthy individuals, a variety of factors that impact cortical excitability and synaptic plasticity have been shown to impact response to rTMS. For example, preceding motor activity22, subject age23, time of day9, and phase of menstrual cycle24 have all been associated with inter- and intra-individual variability in neurophysiological response to rTMS protocols.
One factor that has also been shown to be associated with variable responses to rTMS among healthy individuals is the naturally occurring difference in the gene encoding brain-derived neurotrophic factor (BDNF)25. BDNF, a protein produced by the BDNF gene, is a neurotrophin critical to neural repair and plasticity, which exhibits activity-dependent release at synapses26. It has been shown to modulate LTP11 and LTD12 processes and plays a role in neural development12. In humans, the BDNF gene has a single nucleotide polymorphism (SNP) Val66Met, which is associated with a decrease in activity-dependent release of BDNF27 and diminished synaptic plasticity in animal models28. This polymorphism has been associated with disruptions to learning and memory in humans29,30. Furthermore, the Val66Met allele correlates with poor motor function after stroke31, stronger inter-hemispheric imbalance with greater excitability over the unaffected hemisphere32, worse outcomes for subcortical stroke33, and possibly different mechanisms of neural recovery34. Whether BDNF genotype status impacts response to neuroplasticity-inducing brain stimulation protocols, including TBS, remains to be clarified, as the evidence to date obtained in healthy individuals is mixed25, 27, 35–37. Some studies have suggested influence of BDNF genotype on the responses to rTMS in stroke patients38–40. However, to our knowledge this question had yet to be explored for TBS in chronic stroke patients.
Given that the persistent effects of rTMS—including TBS—are believed to be mediated by LTP- or LTD-like effects on synaptic plasticity, individuals with the Val66Met allele may be less responsive to rTMS interventions that aim to spur neuroplastic changes in patient populations. The Val66Met allele, ranging from 0% to 72% in various global populations, is relatively common41 (~25% Val66Met in the European population; ~5% in Sub-Saharan and Northern African populations; ~40% in Asian populations), making any impact that this polymorphism may have on rTMS potentially relevant to a wide range of therapeutic studies25. Furthermore, the association of Val66Met polymorphism with poor stroke recovery indicates that the frequency of this allele may be significantly higher in the chronic stroke patient populations for whom TBS is being tested as a therapy. We therefore examined the effects of BDNF polymorphism on the response to cTBS in a cohort of patients with chronic stroke. We hypothesized that BDNF genotype would be a reliable source of variation in response to cTBS in these patients, and that this could potentially inform whether and how individuals may be more efficiently stratified to TBS therapies.
METHODS
Experimental Design
This experiment comprised a single session, which began by determining each patient’s resting motor threshold (rMT). Then, in order to obtain baseline MEPs of 1mV peak-to-peak amplitudes, stimulation intensity was increased appropriately above rMT and noted for each subject. Approximately thirty MEPs were obtained before cTBS (baseline) and immediately following stimulation at 0, 10, 20 and 30 minutes (refer to Figure 1), using single TMS pulses at the stimulation intensity that had elicited 1mV baseline MEPs for each subject. The active motor threshold (aMT), used to determine cTBS intensity, was determined after baseline MEP collection, approximately 10–15 minutes prior to administration of cTBS.
Figure 1. Schematic of experimental design.
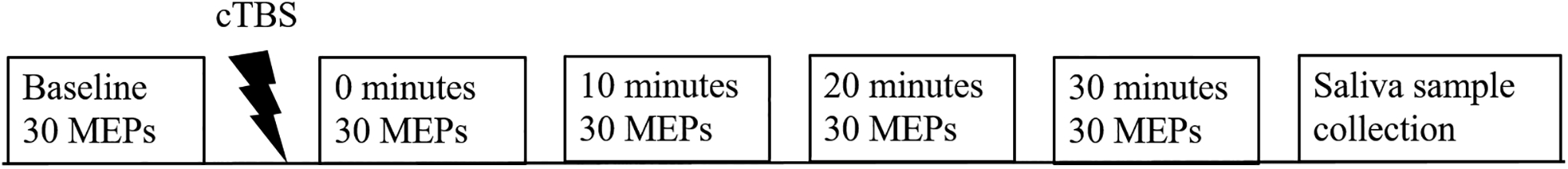
cTBS was administered for 40 s (600 pulses). 30 MEPs each were recorded at times pre-cTBS and at 0, 10, 20, and 30 minutes after cTBS. Saliva sample was collected after all stimulation.
Subject Recruitment
Nineteen individuals (16 males) aged 29–79 (mean (M) ± standard deviation (SD) = 57.3 ± 13.6 years) with a single, left hemisphere ischemic stroke (lesion volume M ± SD = 87.0 ± 70.4 cc) that occurred at least 6 months prior to the study (time since stroke onset M ± SD = 51.6 ± 57.6 months) participated in this study. Refer to Table 1 for demographic and neurophysiological information. Informed consent was obtained from all participants in accordance with the guidelines if the Institutional Review Board at the University of Pennsylvania.
Table 1.
Participant Demographics, Neurophysiological Characteristics, and Stimulation Parameters
| Sub. ID | Age | Yrs Ed | Gender | Race | MPO | Stroke Vol (cc) | Lesion Location | SI1mV | rMT | aMT | SI % rMT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Val66Val | |||||||||||
| 1 | 51 | 12 | M | Caucasian | 26 | 89.597 | L frontal (BA 44 & 45), insula, M1, S1, IPL & pSTG | 89 | 80 | 82 | 111.25 |
| 2 | 68 | 19 | M | Caucasian | 173 | 282.640 | L frontotemporoparietal | 63 | 60 | 61 | 105.00 |
| 3 | 29 | 14 | M | Caucasian | 43 | 421.001 | L frontotemporoparietal | 69 | 53 | 58 | 130.19 |
| 4 | 58 | 18 | M | African American | 24 | 7.242 | L insula & putamen (BA 8) | 55 | 48 | 68 | 114.58 |
| 5 | 47 | 16 | F | Caucasian | 156 | 182.123 | L mid. frontal, inf. frontal (BA 44, 45, & 47), insula, M1, S1, & aMTG & aSTG | 70 | 51 | 41 | 137.25 |
| 6 | 48 | 12 | M | African American | 11 | 27.004 | L mid. frontal & M1 | 61 | 47 | 58 | 129.79 |
| 7 | 66 | 10 | F | African American | 11 | 56.156 | L inf. frontal (BA 44), IPL, M1, & S1 | 49 | 41 | 43 | 119.51 |
| 8 | 71 | 18 | M | Caucasian | 7 | 122.203 | L frontotemporal | 44 | 43 | 45 | 102.33 |
| Mean | 54.8 | 14.9 | 56.4 | 148.5 | 62.5 | 52.9 | 57 | 118.74 | |||
| SD | 13.9 | 3.4 | 67.9 | 141.8 | 14.0 | 12.5 | 13.9 | 12.7 | |||
| Val66Met | |||||||||||
| 9 | 51 | 14 | M | African American | 63 | 61.364 | L frontal, (BA 44, 45, & 47), insula, & M1 | 55 | 43 | 56 | 127.91 |
| 10 | 79 | 21 | M | Caucasian | 89 | 51.961 | L parietal, pMTG, & pSTG | 46 | 42 | 52 | 109.52 |
| 11 | 57 | 14 | M | Caucasian | 111 | 172.288 | L frontal (BA 44, 45, & 47), insula, M1, S1, IPL, & pSTG | 53 | 51 | 63 | 103.92 |
| 12 | 37 | 14 | M | Caucasian | 6 | 87.999 | L frontal (BA 44), insula, & temporal | 52 | 51 | 77 | 101.96 |
| 13 | 56 | 16 | M | Caucasian | 8 | 22.447 | L frontal (BA 44 & 45) & insula | 75 | 66 | 72 | 113.64 |
| 14 | 49 | 16 | M | Caucasian | 146 | 28.025 | L inf. frontal (BA 44), insula, M1 & sup. Temporal | 64 | 59 | 70 | 108.47 |
| 15 | 70 | 18 | M | Caucasian | 25 | 57.466 | L pos. inf. parietal, sup. temporal, & occipital | 49 | 45 | 61 | 108.89 |
| 16 | 47 | 14 | M | Caucasian | 6 | 112.157 | L mid. frontal, inf. frontal (BA 44, 45, & 47), insula, M1, S1, IPL & sup. temporal | 33 | 31 | 42 | 106.45 |
| 17 | 55 | 16 | M | Caucasian | 10 | 64.764 | L inf. frontal (BA 44), insula, M1, aMTG & aSTG | 56 | 53 | 59 | 105.66 |
| 18 | 79 | 14 | M | Caucasian | 13 | 54.234 | L mid. frontal, IPL, pSTG, mid & sup. occipital | 58 | 44 | 54 | 131.82 |
| Mean | 58 | 15.7 | 47.7 | 71.3 | 54.1 | 48.5 | 60.6 | 111.8 | |||
| SD | 13.9 | 2.3 | 51.4 | 44.0 | 11.0 | 9.8 | 10.4 | 10.1 | |||
Abbreviations: Yrs Ed = years of formal education; MPO = months post stroke onset; Vol = volume; cc = cubic centimeters; SI1mV = stimulation intensity to elicit 1mV MEP; rMT = resting motor threshold; aMT = active motor threshold; SI % rMT = stimulation intensity expressed as a percentage of resting motor threshold stimulator output; L = left; BA = Brodmann area; M1 = primary motor cortex; S1 = primary somatosensory cortex; pMTG = posterior middle temporal gyrus; pSTG = posterior superior temporal gyrus; aMTG = anterior middle temporal gyrus; aSTG = anterior superior temporal gyrus; ITG = inferior temporal gyrus; IPL = inferior parietal lobule; IOG = inferior occipital gyrus
BDNF Genotyping
Genomic DNA from human saliva samples was collected in Oragene® DNA collection kits and then isolated using the prepIT.L2Preagent (cat # PT-L2P-5, DNA Genotek Inc, Canada) and precipitated with ethanol according to manufacturer’s instructions. The DNA samples were genotyped for BDNF (the single nucleotide polymorphism rs6265) using the TaqMan SNP Genotyping Assay (C_11592758_10) designed by Thermo Fisher Scientific. Primers and probes were mixed with TaqMan® Universal PCR Master Mix (Thermo Fisher Scientific). 4.5 μL of genomic DNA (2.5 ng/ μL) was transferred in triplicate to a 384-well plate, with each well containing 5.5 μL of the PCR mixture. PCR reaction was performed following a protocol provided by ABI. The allele was discriminated by post-PCR plate reading on the ViiA™ 7 System. Data were processed using the ViiA™ 7 Software (Thermo Fisher Scientific).
Transcranial Magnetic Stimulation
Single pulse TMS with a monophasic waveform was administered to the primary motor cortex of the (intact) right hemisphere using a Magstim 2002 Stimulator with a 70mm figure-eight coil (Magstim Co., Whitland, Dyfield, UK). Participants’ T1-weighted MRI scans were uploaded to the Brainsight® Neuronavigation system (Rogue Research, Montreal) and were used to identify the optimal scalp position within the right primary motor cortex for eliciting a MEP from the left first dorsal interosseous (FDI) muscle. In line with widely employed methods, the intensity of stimulation was adjusted such that resulting baseline MEPs for each subject had an average amplitude of ~1mV9. The MEPs were acquired as participants were seated in a chair with their arms resting on their lap or a pillow. In line with accepted methods, rMT was defined as the minimum pulse intensity required to elicit MEPs with peak-to-peak amplitudes of at least 50 μV in 5 of ten consecutive trials with the FDI at rest42,43. The starting point for the stimulation intensity to acquire MEPs was at 110% of rMT, after which the intensity was steadily increased by 1–2% until 10–12 consecutive MEPs were close to 1 mV in peak-to-peak amplitude. The coil position was maintained at the optimal scalp location and orientation during the acquisition of MEPs using the neuronavigation system. The single TMS pulses were delivered with an inter-stimulus interval of 6s with a random jitter of 6%. Refer to Table 1 for individual subjects’ stimulation parameters.
Continuous Theta Burst Stimulation
CTBS-600 was administered with a biphasic waveform using a Magstim SuperRapid2 Stimulator (Magstim Co., Whitland, Dyfield, UK). CTBS-600 consisted of a continuous delivery of 50 Hz triplets of TMS pulses at 5 Hz for a total of 600 pulses (~40s). Intensity of cTBS-600 was set to 80% of active motor threshold (aMT), defined as the minimum pulse intensity required to produce MEPs with peak-to-peak amplitudes of at least 200 μV in 5 of ten consecutive pulses while participants contracted the FDI muscle at 20% of the maximum voluntary contraction. Setup used to ensure 20% contraction of the FDI included recording EMG of the study subject contracting at maximal force, and then the person practicing to push at strength to fill 20% of the maximal EMG bounds. The same biphasic stimulator was used to determine aMT and administer cTBS.
Electromyography
Electromyographic (EMG) activity was recorded using surface electrodes spanning the belly of the first dorsal interosseous (FDI) muscle of each patient’s left hand, with ground electrode placed along the wrist. Signals were amplified and band-pass-filtered between 20 and 2000 Hz, digitized (sample-rate 5 kHz), and stored for off-line analysis using SIGNAL software (Cambridge Electronic Devices, Cambridge, UK).
Statistical Analysis
We used linear mixed effects regression implemented in the lme4 package of R version 3.6.044 with models containing trial-level MEP data as the expected outcome. The use of linear mixed effects modelling is increasingly common in complex biological data45 with more than one source of variability, as it allows one to assess the relationship between a particular independent variable and the expected outcome after adjusting for relationships of other variables with the outcome. We used linear mixed effects modelling as it allows to make use of trial-level data nested within subjects, which allows for the inclusion of random effects such as the by-participant intercept adopted in our models here. The by-participant random effect structure also takes into account individual differences prior to the intervention (i.e., MEP differences between subjects at baseline). We were also able to assess the effect of a single variable and the interaction between two variables. Analyses were conducted on 2865 MEPs, which were natural log-transformed to ensure a normal distribution (hereafter, LnMEP).
We adopted a forward-fitting model comparison approach to determine whether the addition of factors significantly accounted for more of the variability in cTBS-induced changes in LnMEPs. We first fit a base model to account for the influence of variables not of theoretical interest here (hereafter, covariates) on LnMEP. Our initially chosen covariate was Age, following which we sequentially added covariates (Education, MSO, Race, MPO, Stroke Volume, rMT and aMT) to the base model, looking for covariates that significantly improved model fit. Our final base model was compared to models that included factors of theoretical interest. To the base model, we sequentially added fixed effects of interest, which included Time (baseline vs. 0, 10, 20, and 30 minutes post-cTBS), BDNF (Val66Val vs. Val66Met), and the Time*BDNF interaction. Model comparisons assessed whether the inclusion of additional variables (covariates and fixed effects) significantly improved model fit by a chi-squared log-likelihood test, and also computed effect size46. Covariates and fixed effects that did not improve model fit were excluded from subsequent models. All models included by-participant random intercepts to capture the inherent correlation among multiple measurements within a participant. Post-hoc pairwise comparison tests were conducted, to compute the within-group comparisons of changes in LnMEP from baseline, as well as between-group comparisons in order to assess differences in LnMEPs between the two BDNF genotype groups at each time point. This was done using the estimated marginal means implemented in the emmeans package47 in R version 3.6.044 computed from the final optimal model with a Tukey adjustment for multiple comparisons.
RESULTS
Among the 19 individuals, 8 were BDNF Val66Val carriers, 10 were Val66Met allele carriers while 1 was a Met66Met carrier. The Met homozygote was excluded due to an insufficient number of such subjects for analysis. All subjects tolerated the cTBS with no adverse effects.
In linear mixed modeling, only Age was included as a covariate in the base model because age is known to affect plasticity48 and the addition of other covariates considered for inclusion did not significantly improve model fit (p’s > 0.11, refer to Supplementary Table 1). Fixed and random effects structures and model comparison results are reported in Table 2. Adding Time significantly improved the fit of the model. Adding BDNF alone did not significantly improve the fit of the model. However, adding the interaction between BDNF Status and Time did significantly improve model fit compared to the model that included Time and the covariate. This interaction between BDNF Status and Time is significant at 0, 10, and 20 minutes after cTBS, but not 30 minutes after cTBS (refer to Table 3). The estimates for each predictor and their significance are plotted in Figure 2. We found that relative to baseline, Val66Val carriers exhibited a decrease in mean LnMEP from 0–30 minutes after cTBS. Whereas, relative to baseline, Val66Met carriers had no significant change in mean LnMEP from 0–20 minutes after cTBS before decreasing relative to baseline at 30 minutes after cTBS (see Figure 2). The difference in mean LnMEP at each time post-cTBS from baseline is negative for Val66Val carriers, but is positive up to and including 10 minutes after cTBS for Val66Met carriers (refer to Table 4). Please refer to supplementary Table 2 for participant-level LnMEP data.
Table 2.
Model Comparison Results
| Model | logLik | Deviance | χ2 | df | p-value |
|---|---|---|---|---|---|
| LnMEP ~ Age + (1 | Subj_ID) | −3728.9 | 7457.7 | |||
| LnMEP ~ Age + Time + (1 | Subj_ID) | −3682.5 | 7365.1 | 92.62 | 4 | < .001 |
| LnMEP ~ Age + Time + BDNF + (1 | Subj_ID) | −3682.3 | 7364.6 | 0.51 | 1 | 0.47 |
| LnMEP ~ Age + Time*BDNF + (1 | Subj_ID) | −3619.7 | 7239.5 | 125.6 | 5 | < .001 |
Note. The first row represents the base model, which includes covariates only. Subsequent rows illustrate the model comparison results after adding the fixed effect of interest highlighted in bold. Subsequent models are compared to the last significant model.
Abbreviations: logLik = log-likelihood test; χ2 = chi-squared test statistic; df = degrees of freedom; LnMEP = log-transformed motor-evoked potential; Time = time point (baseline vs. 0, 10, 20, and 30 minutes post-cTBS); BDNF = brain-derived neurotropic factor (Val66Val vs. Val66Met); (1 | Subj_ID) = random effects structure representing the inclusion of a by-participant random intercept.
Table 3.
Linear Mixed Effects Model Coefficients and Associated Test Statistics
| Estimate | SE | t-value | p-value | d | |
|---|---|---|---|---|---|
| Fixed Effects | |||||
| Age | −0.01 | 0.01 | −1.09 | 0.29 | −0.01191 |
| Time 0 | −0.67 | 0.07 | −9.51 | < .001 | −0.6321 |
| Time 10 | −0.84 | 0.07 | −11.73 | < .001 | −0.79069 |
| Time 20 | −0.56 | 0.07 | −7.59 | < .001 | −0.53166 |
| Time 30 | −0.44 | 0.07 | −5.96 | < .001 | −0.41661 |
| BDNF | −0.19 | 0.32 | −0.60 | 0.56 | −0.17664 |
| Time 0 × BDNF | 0.72 | 0.10 | 7.29 | < .001 | 0.677257 |
| Time 10 × BDNF | 0.90 | 0.10 | 9.09 | < .001 | 0.847445 |
| Time 20 × BDNF | 0.51 | 0.10 | 5.09 | < .001 | 0.482617 |
| Time 30 × BDNF | 0.04 | 0.10 | 0.37 | 0.71 | 0.034079 |
| s2 | Std. Deviation | ||||
| Random Effects | |||||
| Subject ID | 0.3408 | 0.5838 |
Note: Reference level is baseline for “Time” and Val66Val for “BDNF”. Significant fixed effects and interactions are highlighted in bold. SE = standard error; d = Effect Size, calculated as the ratio of Estimate to square root of the sum of random effects variances; BDNF = brain-derived neurotropic factor (reference level = Val66Val); Time = time point (reference level = Baseline); s2 = random effect variance; Std. Deviation = standard deviation.
Figure 2. cTBS effects on LnMEPs by BDNF Genotype.
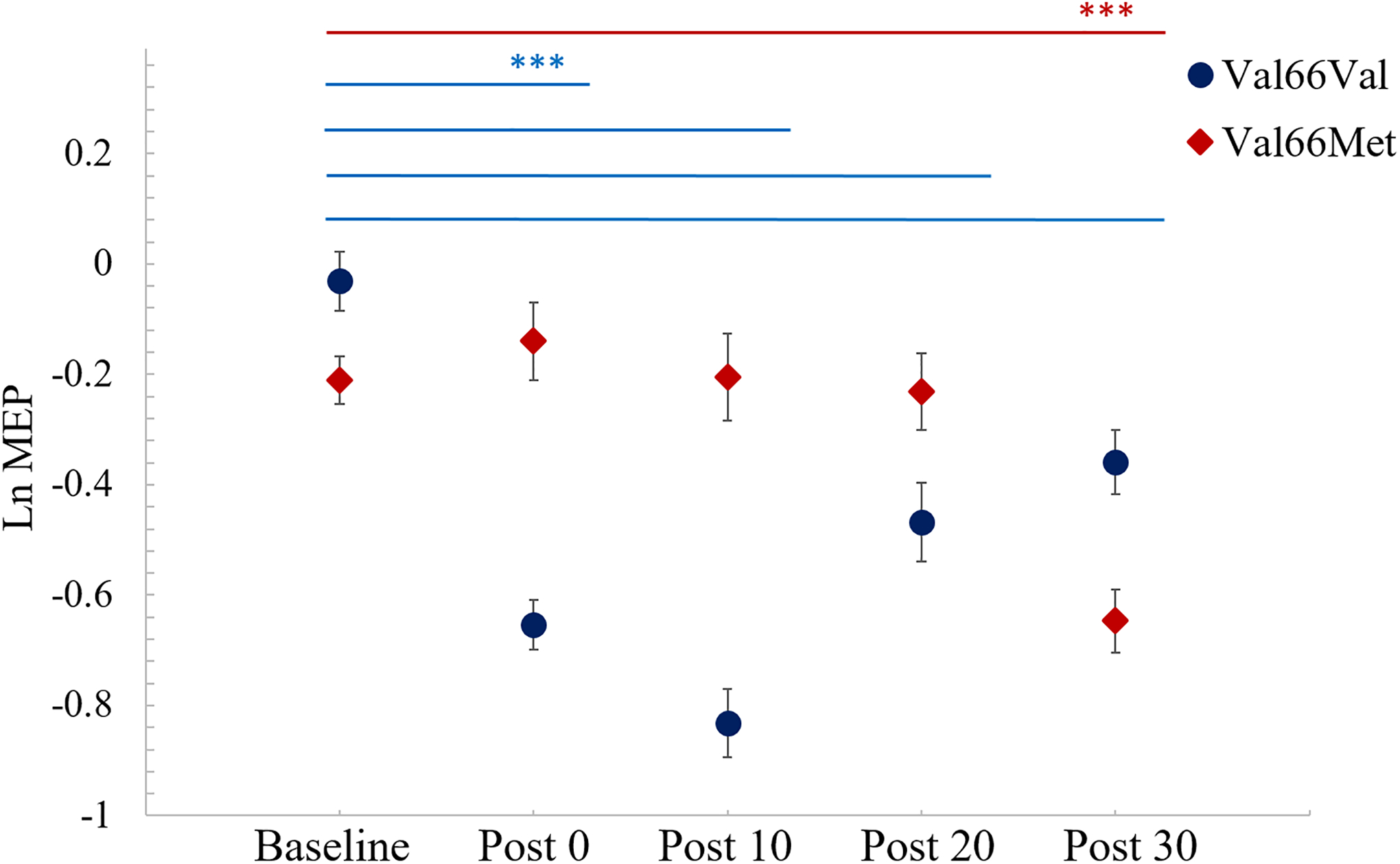
Mean LnMEPs at baseline and 0, 10, 20, and 30 minutes post-cTBS for BDNF Val66Val (blue) and Val66Met (red) carriers. Logarithmic scale relates to MEPs in mV. Error bars reflect Standard Error. Significance shown based on post-hoc pairwise comparisons tests of LnMEPs at different times for either Val66Val (blue bars) or Val66Met (red bars) group using estimated marginal means computed from model with a Tukey adjustment. *** indicates p <0.001.
Table 4.
Estimated Marginal Means Post-hoc Comparisons
| Comparison | Estimate | SE | df | t-value | p-value |
|---|---|---|---|---|---|
| Within-group Val66Val | |||||
| Val66Val Baseline - Val66Val Post0 | 0.6711 | 0.0706 | 2855.3 | 9.499 | <.0001 |
| Val66Val Baseline - Val66Val Post10 | 0.8394 | 0.0717 | 2855.1 | 11.714 | <.0001 |
| Val66Val Baseline - Val66Val Post20 | 0.5644 | 0.0744 | 2856.4 | 7.582 | <.0001 |
| Val66Val Baseline - Val66Val Post30 | 0.4423 | 0.0743 | 2856.5 | 5.953 | <.0001 |
| Within-group Val66Met | |||||
| Val66Met Baseline - Val66Met Post0 | −0.0479 | 0.0690 | 2858.8 | −0.695 | 0.9995 |
| Val66Met Baseline - Val66Met Post10 | −0.0603 | 0.0685 | 2858.8 | −0.880 | 0.9970 |
| Val66Met Baseline - Val66Met Post20 | 0.0521 | 0.0679 | 2858.8 | 0.767 | 0.9990 |
| Val66Met Baseline - Val66Met Post30 | 0.4061 | 0.0650 | 2855.2 | 6.249 | <.0001 |
| Cross-group at same times | |||||
| Val66Val Baseline - Val66Met Baseline | 0.1875 | 0.3442 | 23.2 | 0.545 | 0.5911 |
| Val66Val Post0 - Val66Met Post0 | −0.5315 | 0.3451 | 23.5 | −1.540 | 0.1370 |
| Val66Val Post10 - Val66Met Post10 | −0.7122 | 0.3453 | 23.5 | −2.062 | 0.0504 |
| Val66Val Post20 - Val66Met Post20 | −0.3248 | 0.3459 | 23.7 | −0.939 | 0.3571 |
| Val66Met Post30 - Val66Met Post30 | 0.1514 | 0.3453 | 23.5 | 0.438 | 0.6651 |
Note: Significant differences in MEP amplitudes for Val66Val and Val66Met carriers are highlighted in bold. Estimate = difference in model-estimated MEPs from Baseline to Post-cTBS time points within each BDNF genotype group (Val66Val and Val66Met); SE = standard error of the estimate; df = degrees of freedom. Statistical test results represent Tukey-adjusted values correcting for multiple comparisons within the family of estimates compared.
Post-hoc tests of estimated marginal means for within-group comparisons show significant differences for Val66Val in the pairwise tests of LnMEPs at Baseline vs Post 0, Baseline vs Post 10, Baseline vs Post 20, and Baseline vs Post 30 (p’s <0.001). These same pairwise tests of the Val66Met group show a significant difference, of decreasing LnMEP, only between Baseline and Post 30 (p <0.001) but not at other time points (p’s >0.99). Further, comparisons between two groups at each time point revealed marginally significant difference at Post 10 (p =0.0504) but not at Baseline, Post 0, Post 20 or Post 30. See Table 4 for full details of the pairwise comparisons using estimated marginal means. This indicates that cTBS has an inhibitory effect on the mean LnMEP in Val66Val patients that lasts at least 30 minutes post-stimulation; however, for Val66Met patients, this inhibitory effect of cTBS does not emerge until 30 minutes after stimulation (see Figure 2).
DISCUSSION
The current study demonstrates that BDNF genotype has a significant impact on response to cTBS within stroke patients, which is crucial for understanding how genetic factors may impact response to cTBS in this patient population. Most studies in healthy individuals have shown that homozygous Val66Val BDNF carriers exhibit the expected response to TBS protocols25, 27, 35–37. Our data extend this result to stroke patients, revealing that Val66Val carriers exhibit the expected inhibitory response due to cTBS, i.e. MEP suppression. However, there have been mixed evidence for Met allele carriers in the healthy population, with studies indicating either a facilitative response to cTBS35 or no difference in cTBS responses as a function of BDNF genotype status25, 36. Thus, the effects of cTBS observed here expand on those reported in previous studies, confirming that BDNF impacts cTBS aftereffects in the stroke patient population while also revealing that the initial inhibitory effect of cTBS for stroke patients with the Val66Val genotype is not seen for stroke patients with the Val66Met polymorphism.
This finding has several implications on pairing TMS with other behavioral therapies. Current neurorehabilitation studies involving TMS (including studies specifically using cTBS) are pairing this therapy with physical/behavioral therapies administered after stimulation49–53. That is, cTBS can be used in motor rehabilitation to inhibit activity of the contralesional hemisphere. This notion is based on the interhemispheric inhibition hypothesis54, which postulates that the intact contralesional hemisphere exerts a deleterious inhibitory influence on the lesioned hemisphere. By this account, inhibiting the contralesional hemisphere with cTBS during periods of physical therapy may better allow for the functional recovery in perilesional areas55. Hence, repeated applications of cTBS over multiple sessions is increasingly being investigated as a tool to enhance the rehabilitative effects when paired with repeated physical therapy sessions. The underlying assumption of this approach is that the delivery of repetitive protocols such as cTBS may have facilitative effects on cortical excitability and enhance use-dependent learning to enhance responsiveness to concurrent behavioral therapies. That patients with the Val66Met genotype in the current study do not have an inhibitory response for the first 20 minutes after stimulation has important implications for studies pairing neuromodulation approaches with physical or behavioral therapies, as this timeframe coincides with when therapeutic interventions would typically be performed10. Relatedly, differences in post-stimulation behavioral response based on BDNF genotype have also been observed in patient populations in studies involving transcranial direct current stimulation (tDCS), another noninvasive neuromodulation approach. In prior studies, BDNF Val66Met patients did not show improvement in aphasia following tDCS56 and motor learning in patients with BDNF Val66Met allele was slower57.
The response of Val66Met patients in our study changes to the expected inhibitory effect at 30 minutes after stimulation, which may also suggest a delayed response to cTBS. This finding should prompt caution and spur further investigation of whether the same protocols that may have beneficial effects in persons of the more common genotype are either ineffective or potentially even deleterious in persons with the Val66Met genotype. The differences in the time course of cTBS response based on BDNF may relate to differences in cortical resilience58, in that a quicker response to and recovery from cTBS may suggest greater resilience in Val66Val patients compared to Val66Met patients. The short period of data collection is a limitation of this study. Future investigations should follow the timeline further out after cTBS (e.g. 60–80 minutes), in order to better elucidate whether or not Val66Met carriers experience a delayed response to stimulation or something more complex. Therapeutic implications may naturally follow from such an analysis. As the patients with BDNF Val66Met polymorphism are more likely to be in the chronic population in need of such interventions31–34, the unexpected response among Val66Met carriers found here is especially crucial when considering how best to utilize brain stimulation to enhance recovery in patient populations. Accounting for the diminished, delayed, or possibly facilitative responses of Val66Met carriers, who may represent a significant percentage of the stroke population that will receive rTMS therapies, may promote the development of personalized stimulation paradigms based on genotype.
These results further suggest a potential for the use of cTBS as a biomarker for stroke recovery potential. Studies have implicated the BDNF Val66Met genotype in poor stroke recovery25,26,31. Therefore, the relation of BDNF genotype to cTBS response as well, may suggest an underlying common mechanism that yields altered neuroplasticity effects in these individuals. There is a need for developing further biomarkers of stroke recovery that may enhance future clinical trials59. The predictive value of BDNF polymorphism, a known predictor of stroke recovery31–33, as a modulator of cTBS responses in the current study may point toward cTBS responses as another biomarker for predicting stroke recovery outcomes. In the future, BDNF genotype may serve not only as a stratifier of who will respond to brain stimulation, but also as a broader biomarker of brain plasticity to help predict which individuals are most likely to respond to an intervention. This may be an important clinical measure to personalize therapy for stroke recovery.
Here, we analyzed the neurophysiological responses to cTBS instead of considering a particular motor or language recovery outcome measure. Our focus on motor physiology may be a limitation for directly applying these results to all studies of cTBS in neurorehabilitation, or for cTBS therapeutic applications for other cognitive functions and their neuroanatomical bases besides the motor physiology. However, neurophysiological response is somewhat generalizable to the individual’s overall brain response to cTBS8,9. This represents a starting point for future studies that may wish to characterize BDNF-mediated variability in cTBS applied to other regions of the brain for the enhancement and/or rehabilitation of other cognitive domains. Our results showed an unexpected inhibitory response of Val66Met carriers at 30 minutes post-cTBS. The BDNF Val66Met allele is also postulated to have duration-dependent effects on cortical plasticity, e.g. late modulation of neural plasticity60, which may explain this phenomenon. Future studies could also build on the current results by combining measures of contralesional motor excitability post-cTBS with measures of motor excitability in the lesioned hemisphere, which are also likely to be influenced by BDNF genotype32. Another limitation is the small sample size of our study. However, to our knowledge, this is the first study to examine effects of BDNF genotype on cTBS responses in stroke patients; future confirmatory studies would benefit from testing larger cohorts. Unlike prior studies where Val66Met carriers were a small sub-group, our sample sizes with each BDNF genotype are comparable to each other. Importantly, our use of a mixed effects model approach is relatively robust to smaller sample sizes due to use of trial-level data61. Finally, a general methodologic limitation of our approach is that there was no sham stimulation condition, such that both subjects and experimenters were aware that subjects were receiving stimulation. However, we do not believe that this awareness alone is likely to account for the differential response to brain stimulation as a function of BDNF genotype that we observed in this study.
CONCLUSION
This study provides novel insight into the potential sources of variability in cTBS response in patients, which has important implications for optimizing the utility of this neuromodulation approach in clinical settings. Incorporating BDNF polymorphism genetic screening to stratify patients prior to use of cTBS as a neuromodulatory technique in therapy or research may optimize response rates. This may help to decrease, or provide an explanation for, heterogeneity in responses to cTBS. Future studies may investigate BDNF as a mediator of variability in patient responses to a variety of NIBS protocols including other rTMS approaches and tDCS. Further research on the differential effects of BDNF genotype on synaptic and neural plasticity in humans may help elucidate various mechanisms of stroke rehabilitation. We suggest that studies and clinical trials currently utilizing TMS for treating neurological disorders take into account BDNF polymorphisms to further understand how this factor may be used to optimally stratify patients in future work and in turn improve the efficacy of this therapeutic approach.
Supplementary Material
Sources of Financial Support:
This work was supported by the National Institutes of Health/ NIDCD [grant number R01 DC012780].
Footnotes
Conflicts of Interest: None
REFERENCES
- [1].Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567–73. [DOI] [PubMed] [Google Scholar]
- [2].Voelker R Brain stimulation approved for obsessive-compulsive disorder. JAMA. 2018;320(11):1098. [DOI] [PubMed] [Google Scholar]
- [3].Schlaepfer TE, George MS, Helen Mayberg on behalf of the WFSBP Task Force on Brain Stimulation. WFSBP guidelines on brain stimulation treatments in psychiatry. The World Journal of Biological Psychiatry. 2010;11(1):2–18. [DOI] [PubMed] [Google Scholar]
- [4].Sack AT. Transcranial magnetic stimulation, causal structure–function mapping and networks of functional relevance. Current Opinion in Neurobiology. 2006;16(5):593–9. [DOI] [PubMed] [Google Scholar]
- [5].Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130(3):610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Medaglia JD, Harvey DY, White N, Kelkar A, Zimmerman J, Bassett DS, Hamilton RH. Network controllability in the inferior frontal gyrus relates to controlled language variability and susceptibility to TMS. Journal of Neuroscience. 2018;38(28):6399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology. 1998;15(4):333–43. [DOI] [PubMed] [Google Scholar]
- [8].Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of Neuroengineering and Rehabilitation. 2009;6(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6. [DOI] [PubMed] [Google Scholar]
- [10].Müller‐Dahlhaus JF, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex–a pharmacological TMS study. The Journal of Physiology. 2008;586(2):495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–9. [DOI] [PubMed] [Google Scholar]
- [12].Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature Reviews Neuroscience. 2005;6(8):603–14. [DOI] [PubMed] [Google Scholar]
- [13].Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clinical Neurophysiology. 2007;118(2):333–42. [DOI] [PubMed] [Google Scholar]
- [14].Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA. Effects of rTMS on grip force control following subcortical stroke. Experimental Neurology. 2008;211(2):407–12. [DOI] [PubMed] [Google Scholar]
- [15].Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. Journal of Rehabilitation Medicine. 2010;42(8):758–64. [DOI] [PubMed] [Google Scholar]
- [16].Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O’Sullivan JD, Coulthard A, Wong A. Improved language performance subsequent to low‐frequency rTMS in patients with chronic non‐fluent aphasia post‐stroke. European Journal of Neurology. 2011;18(7):935–43. [DOI] [PubMed] [Google Scholar]
- [17].Bowden M The use of rTMS to augment walking recovery after stroke. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2019;12(2):450–1. [Google Scholar]
- [18].Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, VandenBos M, Hammerbeck U, Barratt K, Gillini C, Musumeci G. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabilitation and Neural Repair. 2012;26(8):976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harvey RL, Edwards D, Dunning K, Fregni F, Stein J, Laine J, Rogers LM, Vox F, Durand-Sanchez A, Bockbrader M, Goldstein LB. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke: the NICHE trial. Stroke. 2018;49(9):2138–46. [DOI] [PubMed] [Google Scholar]
- [20].Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Experimental Brain Research. 2000;133(4):425–30. [DOI] [PubMed] [Google Scholar]
- [21].Nettekoven C, Volz LJ, Leimbach M, Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage. 2015;118:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goldsworthy MR, Müller-Dahlhaus F, Ridding MC, Ziemann U. Inter-subject variability of LTD-like plasticity in human motor cortex: a matter of preceding motor activation. Brain Stimulation. 2014;7(6):864–70. [DOI] [PubMed] [Google Scholar]
- [23].Müller-Dahlhaus JF, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Experimental Brain Research. 2008;187(3):467–75. [DOI] [PubMed] [Google Scholar]
- [24].Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clinical Neurophysiology. 2004;115(5):1063–8. [DOI] [PubMed] [Google Scholar]
- [25].Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain‐derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology. 2008;586(23):5717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu B BDNF and activity-dependent synaptic modulation. Learning & memory. 2003;10(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Crespo LM, Procaccio V, Cramer SC. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex. 2010;20(5):1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. Journal of Neuroscience. 2010;30(26):8866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(1):79–85. [DOI] [PubMed] [Google Scholar]
- [30].Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim DY, Quinlan EB, Gramer R, Cramer SC. BDNF Val66Met polymorphism is related to motor system function after stroke. Physical Therapy. 2016;96(4):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, Paolucci M, Bucossi S, Ventriglia MC, Brown P, Capone F. Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimulation. 2015. Jan 1;8(1):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim WS, Lim JY, Shin JH, Park HK, Tan SA, Park KU, Paik NJ. Effect of the Presence of Brain-Derived Neurotrophic Factor Val66Met Polymorphism on the Recovery in Patients With Acute Subcortical Stroke. Annals of Rehabilitation Medicine. 2013;37(3):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Di Pino G, Pellegrino G, Capone F, Assenza G, Florio L, Falato E, Lotti F, Di Lazzaro V. Val66Met BDNF polymorphism implies a different way to recover from stroke rather than a worse overall recoverability. Neurorehabilitation and Neural Repair. 2016. Jan;30(1):3–8. [DOI] [PubMed] [Google Scholar]
- [35].Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clinical Neurophysiology. 2017;128(11):2268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mastroeni C, Bergmann TO, Rizzo V, Ritter C, Klein C, Pohlmann I, Brueggemann N, Quartarone A, Siebner HR. Brain-derived neurotrophic factor–a major player in stimulation-induced homeostatic metaplasticity of human motor cortex?. PloS one. 2013;8(2):e57957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vallence AM, Goldsworthy MR, Hodyl NA, Semmler JG, Pitcher JB, Ridding MC. Inter-and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience. 2015;304:266–78. [DOI] [PubMed] [Google Scholar]
- [38].Chang WH, Uhm KE, Shin YI, Pascual-Leone A, Kim YH. Factors influencing the response to high-frequency repetitive transcranial magnetic stimulation in patients with subacute stroke. Restor Neurol Neurosci. 2016. Sep 21;34(5):747–55. [DOI] [PubMed] [Google Scholar]
- [39].Chang WH, Bang OY, Shin YI, Lee A, Pascual-Leone A, Kim YH. BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain Stimulation. 2014. Jul-Aug;7(4):553–8. [DOI] [PubMed] [Google Scholar]
- [40].Uhm KE, Kim YH, Yoon KJ, Hwang JM, Chang WH. BDNF genotype influence the efficacy of rTMS in stroke patients. Neuroscience Letters. 2015. May 6;594:117–21. [DOI] [PubMed] [Google Scholar]
- [41].Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Molecular Psychiatry. 2010;15(8):810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W, Pauri F. Applications of magnetic cortical stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:171–85. [PubMed] [Google Scholar]
- [43].Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- [44].Team RC. R: A language and environment for statistical computing. https://www.R-project.org/.
- [45].Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CE, Robinson BS, Hodgson DJ, Inger R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 2018;6:e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brysbaert M, Stevens M. Power analysis and effect size in mixed effects models: A tutorial. Journal of Cognition. 2018;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lenth R emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.2–1 2020. https://CRAN.R-project.org/package=emmeans
- [48].Freitas C, Perez J, Knobel M, Tormos JM, Oberman LM, Eldaief M, Bashir S, Vernet M, Peña-Gómez C, Pascual-Leone A. Changes in cortical plasticity across the lifespan. Frontiers in Aging Neuroscience. 2011;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Di Lazzaro V, Capone F, Di Pino G, Pellegrino G, Florio L, Zollo L, Simonetti D, Ranieri F, Brunelli N, Corbetto M, Miccinilli S. Combining robotic training and non-invasive brain stimulation in severe upper limb-impaired chronic stroke patients. Frontiers in Neuroscience. 2016;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Galvão SC, Dos Santos RB, Dos Santos PB, Cabral ME, Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2014;95(2):222–9. [DOI] [PubMed] [Google Scholar]
- [51].Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology. 2012;78(4):256–64. [DOI] [PubMed] [Google Scholar]
- [52].Di Lazzaro V, Rothwell JC, Talelli P, Capone F, Ranieri F, Wallace AC, Musumeci G, Dileone M. Inhibitory theta burst stimulation of affected hemisphere in chronic stroke: a proof of principle, sham-controlled study. Neuroscience letters. 2013;553:148–52. [DOI] [PubMed] [Google Scholar]
- [53].Koch G, Bonni S, Giacobbe V, Bucchi G, Basile B, Lupo F, Versace V, Bozzali M, Caltagirone C. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78(1):24–30. [DOI] [PubMed] [Google Scholar]
- [54].Daskalakis Z, Christensen B, Fitzgerald P, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. The Journal of Physiology. 2002;543(1):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fiori F, Chiappini E, Candidi M, Romei V, Borgomaneri S, Avenanti A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: a dual site TMS study. Scientific Reports. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fridriksson J, Elm J, Stark BC, Basilakos A, Rorden C, Sen S, George MS, Gottfried M, Bonilha L. BDNF genotype and tDCS interaction in aphasia treatment. Brain stimulation. 2018;11(6):1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van der Vliet R, Ribbers GM, Vandermeeren Y, Frens MA, Selles RW. BDNF Val66Met but not transcranial direct current stimulation affects motor learning after stroke. Brain Stimulation. 2017;10(5):882–92. [DOI] [PubMed] [Google Scholar]
- [58].Hall PA, Erickson KI, Lowe CJ, Sakib MN. Quantifying Cortical Resilience in Experimental, Clinical, and Epidemiological Studies: A Conceptually Grounded Method Using Noninvasive Brain Stimulation. Psychosomatic Medicine. 2020. Apr 1;82(3):281–6. [DOI] [PubMed] [Google Scholar]
- [59].Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, Carter AR, Leff AP, Copland DA, Carey LM, Cohen LG. Biomarkers of stroke recovery: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. International Journal of Stroke. 2017;12(5):480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Teo JT, Bentley G, Lawrence P, Soltesz F, Miller S, Willé D, McHugh S, Dodds C, Lu B, Croft RJ, Bullmore ET. Late cortical plasticity in motor and auditory cortex: role of met-allele in BDNF Val66Met polymorphism. International Journal of Neuropsychopharmacology. 2014;17(5):705–13. [DOI] [PubMed] [Google Scholar]
- [61].Che X, Xu S. Generalized linear mixed models for mapping multiple quantitative trait loci. Heredity. 2012;109(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


