Abstract
In resource-rich countries, almost all severe hemophilia patients receive prophylactic replacement therapy with factor concentrates to prevent spontaneous bleeding in joints and muscles to decrease the development of arthropathy and risk of long-term disability. Pharmacokinetic (PK)-guided dosing can be applied to individualize factor replacement therapy, as interindividual differences in PK parameters influence factor VIII (FVIII) and FIX activity levels. PK-guided dosing may therefore lead to more optimal safeguarding of FVIII/FIX levels during prophylaxis and on demand treatment. The OPTI-CLOT TARGET study is a multicenter, nonrandomized, prospective cohort study that aims to investigate the reliability and feasibility of PK-guided prophylactic dosing of factor concentrates in hemophilia-A and -B patients in daily clinical practice. At least 50 patients of all ages on prophylactic treatment using standard half-life (SHL) and extended half-life (EHL) factor concentrates will be included during 9 months and will receive PK-guided treatment. As primary endpoint, a minimum of four FVIII/FIX levels will be compared with FVIII/FIX levels as predicted by Bayesian forecasting. Secondary endpoints are the association of FVIII and FIX levels with bleeding episodes and physical activity, expectations and experiences, economic analyses, and optimization of population PK models. This study will lead to more insight in the reliability and feasibility of PK-guided dosing in hemophilia patients. Moreover, it will contribute to personalization of treatment by greater knowledge of dosing regimens needed to prevent and treat bleeding in the individual patient and provide evidence to more clearly associate factor activity levels with bleeding risk.
Keywords: hemophilia, pharmacokinetics, factor VIII, factor IX, prophylaxis
Introduction
Hemophilia A and hemophilia B are X-linked recessive bleeding disorders caused by a deficiency or dysfunction of coagulation factor VIII (FVIII) or FIX, respectively. Severe patients (FVIII/FIX < 0.01 IU/mL) and some moderate-to-severe patients (FVIII/FIX: 0.01–0.05 IU/mL) suffer from spontaneous bleeding or bleeding after minimal trauma. Prophylactic treatment by intravenous administration of factor concentrates aims to prevent (spontaneous) bleedings in joint and muscles and subsequent arthropathy with potential long-term disability. 1 2
During prophylaxis theoretically, FVIII/FIX trough levels is targeted to >0.01 IU/mL. This principle is based on observations by Ahlberg as early as in 1965 that bleeding phenotype and joint status are strikingly different between severe and moderate-to-severe hemophilia patients with only minimal baseline FVIII level differences (<0.01 vs. 0.01–0.05 IU/mL). 3 To achieve these FVIII/FIX trough levels during prophylaxis, FVIII/FIX concentrates are mostly prescribed according to body weight. 2 Remarkably, it is still not usual clinical practice to standardly measure and monitor trough FVIII/FIX levels when no bleeding occurs. To personalize dosing, information on trough FVIII/FIX levels is of value to establish if prophylaxis is adequate for each individual patient, during follow–up, and in varying circumstances and when dosing on demand. In addition, the lack of knowledge of achieved FVIII/FIX levels impedes proper switching to novel long-acting factor concentrates due to uncertainties which trough FVIII/FIX target levels should be targeted to prolongate earlier effective prophylactic treatment to prevent bleeding, especially in relation to physical activity or sports.
Pharmacokinetic-Guided Dosing
Large interindividual variability exists in the pharmacokinetics (PKs) of FVIII/FIX concentrates as demonstrated by Björkman et al among others. 4 5 6 To understand and predict the consequences of the interindividual variability of factor concentrates in individuals, population PK models have been constructed for prophylaxis 4 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 and perioperative treatment 33 with standard half-life (SHL) and extended half-life (EHL) FVIII and FIX concentrates for hemophilia-A and -B patients, respectively. With these population PK models, Bayesian forecasting can be performed. Herewith, individual PK parameters are estimated which are subsequently used to calculate the adequate dose for an individual patient to achieve FVIII/FIX target levels, both trough and peak. The availability of population PK models has made limited sampling possible, making prior frequent blood sampling (>10 blood samples) 34 and a wash-out period redundant. PK-guided dosing has also been reported to not only be able to predict dosing requirements to attain certain target FVIII/FIX levels but also to decrease the amount of factor concentrates with concomitant reduction of costs. 33 Carlsson et al was the first to report a dose and cost reduction of 30% of FVIII concentrate without an increase in bleeding events, when PK-guided prophylactic dosing was compared with standard prophylactic dosing in a small patient sample. 35 However, a recent randomized controlled perioperative trial was not able to show a decrease in FVIII concentrate consumption, although achievement of FVIII target ranges was clearly more optimal. 36
We hypothesize PK-guided dosing leads to individualization of prophylaxis which is in accordance with the recommendations of the subcommittee on FVIII, FIX, and rare bleeding disorders of the International Society on Thrombosis and Haemostasis (ISTH). 37 PK-guided dosing may help achieve higher trough levels more efficiently when clinically indicated, as well as provide guidance when patients switch to alternative replacement factor concentrates, while taking cost and benefit of treatment into account. In addition, PK-guided dosing may lead to increased insight into the association between FVIII/FIX levels, bleeding (risk), and physical activity levels in individual patients as factor levels can be predicted at any time point and related to bleeding and activity. Therefore, we aim to prove that FVIII/FIX trough and peak levels as set by treating physician can be predicted and achieved reliably by application of PK-guided prophylaxis and that this intervention is feasible for patients and treatment teams.
Objective
Investigate the reliability and feasibility of PK-guided prophylactic dosing of factor concentrates in hemophilia-A and -B patients in daily clinical practice.
Methods
Study Design
The OPTICLOT TARGET study is a multicenter, nonrandomized, prospective cohort study.
Study Population
Patients will be recruited from the two Dutch Hemophilia treatment Centers; follows Erasmus MC, University Medical Center Rotterdam and Amsterdam University Medical Centers.
Inclusion Criteria
Inclusion criteria are as follows:
Hemophilia-A and -B patients of all ages on prophylaxis.
Prophylaxis with SHL or EHL factor concentrates.
Written (parental) informed consent, according to local law and regulations.
Exclusion Criteria
Exclusion criteria are listed below:
Patients with other severe congenital or acquired hemostatic abnormalities.
General medical conditions which may interfere with participation in the study.
Inability to adhere to prophylaxis and/or inability to keep detailed logs on infusion and bleeding episodes.
Withdrawal of (parental) informed consent.
Presence of FVIII/FIX inhibitor, leading to alternative treatment with bypassing products, immune toleration induction, and/or other immune modulating treatment.
Outcome Measures
Primary Endpoints
Observed FVIII and FIX levels in comparison to FVIII and FIX levels are predicted by Bayesian forecasting. The predictive performance is deemed acceptable when at least 80% of the actual FVIII/FIX levels are within ± 25% of the predicted (target) values as stated by treating professional.
Secondary Endpoints
The four secondary endpoints are briefed below:
Association of (real world or predicted) FVIII/FIX levels with bleeding episodes and daily activities. Additionally, bleeds will be categorized according to the following subclassifications: total number of bleeds over time, number of spontaneous bleeds, number of traumatic bleeds, number of joint bleeds, number of target joint bleeds, and bleed severity.
Expectations, feasibility, and experience with PK-guided dosing with the different factor concentrates (SHL vs. EHL) as reported by patient/caretakers and physician will be measured using a visual analogue scale (VAS) questionnaire at the start and end of the study.
Economic analysis in which costs and benefits of standard prophylactic treatment and PK-guided prophylaxis are compared.
Analysis of described modifiers effecting PK parameters of FVIII/FIX concentrate to further optimize population PK models. Modifiers include demographics (such as lean body mass) and laboratory measurements (such as the von Willebrand Factor levels).
Interventions
Study interventions are depicted in the flowchart ( Fig. 1 ). Patients will be categorized into strata according to type of hemophilia and type of factor concentrate (SHL or EHL). For all patients, the following patient characteristics and demographics will be collected: type of hemophilia, endogenous factor level, DNA mutation, age, height (cm), weight (kg), body mass index (BMI; kg/cm 2 ), lean body mass (kg), blood group, current other medication, and activity patterns.
Fig. 1.
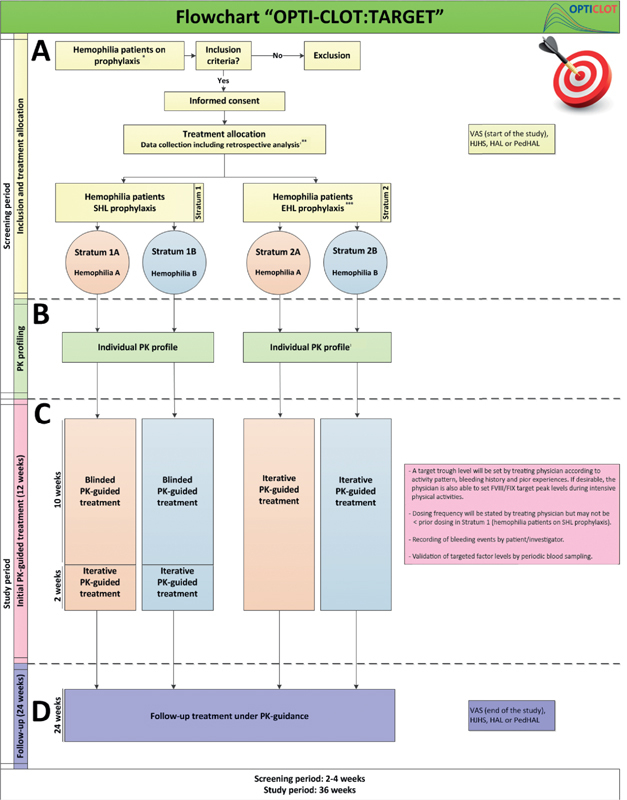
Flow chart. EHL, extended half-life; HAL, Hemophilia Activity List; HJHS, Hemophilia Joint Score; PedHAL, pediatric HAL; PK, pharmacokinetic(s); SHL, standard half-life; VAS, visual analogue scale *Non severe hemophilia patients will be analyzed separately. **In parallel with the prospective study, retrospective data analysis will be performed over a 12 month period prior to inclusion (if no PK profiling has been performed) or from PK profiling prior to inclusion. ***Patients in stratum 2 could undergo PK profiling during SHL prophylaxis as well as during EHL prophylaxis.
Patients/caretakers will fill in the Hemophilia Activities List (HAL) and/or pediatric HAL (PedHAL) before initiation. Moreover, the Hemophilia Joint Health Score (HJHS) will be performed or must have been performed <12 months prior inclusion.
The validated PedHAL/HAL questionnaire and the HJHS are included as clinical parameters to systematically establish baseline values of functional outcome from the patient's perspective and to be informed of joint status, respectively. These clinical parameters may help to evaluate outcomes after implementation of PK guidance.
Furthermore, both patients/caretakers and the treating physician will fill in a specifically developed questionnaire using VAS scales, before the implementation of PK-guided dosing, considering the expectations with PK-guided dosing of prophylaxis. More specifically, in the questionnaire, questions are asked about satisfaction, being informed of factor levels, and expected burden of PK guidance. Moreover, when patients switch to an EHL factor concentrate, the reasons for switching are also asked.
An individual PK profile will be constructed after a factor concentrate dose of 35 to 50 IU/kg, depending on hemophilia type and age of the patient. The frequency and timing of blood sampling during PK profiling is depending on type of hemophilia and type of factor concentrate ( Fig. 2 ). No wash out period is required if three prior infusions and time points of infusion are documented. During sampling of the PK profile, laboratory tests will be performed according to Tables 1 and 2 .
Fig. 2.
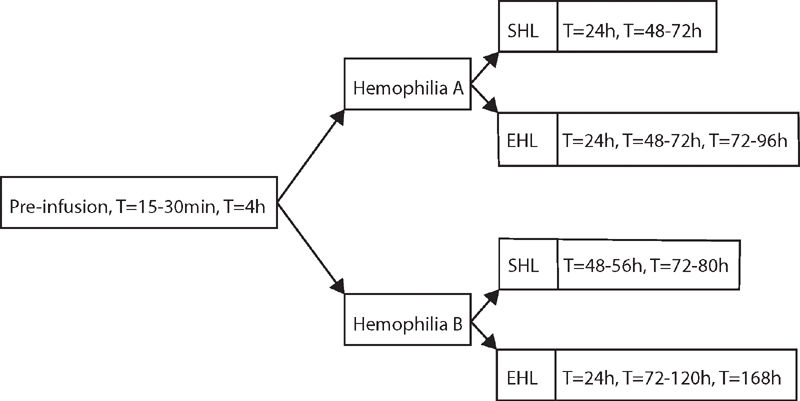
Time points (T) of laboratory tests during individual PK-profiling. A preinfusion, t = 15–30 minutes and t = 4 hours sample (left) are performed in all patients. The other time points (right) depend on hemophilia type and brand of factor concentrate. EHL, extended half-life; PK, pharmacokinetic; SHL, standard half-life.
Table 1. Laboratory tests during individual PK profiling for patients with hemophilia A.
| On FVIII-SHL | Preinfusion | T = 15–30 minute | T = 4 hours | T = 24 hours | T = 48–72 hours | |
|---|---|---|---|---|---|---|
| On FVIII-EHL | Preinfusion | T = 15–30 minute | T = 4 hours | T = 24 hours | T = 48 hours | T = 72–96 hours a |
| ASAT | X | |||||
| ALAT | X | |||||
| GGT | X | |||||
| LDH | X | |||||
| AF | X | |||||
| Albumin | X | |||||
| Urea | X | |||||
| Creatinine | X | |||||
| Hemoglobin | X | |||||
| Hematocrit | X | |||||
| Thrombocytes | X | |||||
| Blood group (if unknown) | X | |||||
| Factor VIII | X | X | X | X | X | X |
| VWF:Ag | X | X | X | X | X | X |
| VWF:Act | X | X | X | X | X | X |
| VWF:CB | X | X | X | X | X | X |
| VWFpp | X | X | X | X | X | X |
| Inhibitor FVIII (only ≥ 18 years) | X | |||||
| Bethesda FVIII | X | |||||
| Buffycoat | X | |||||
| APTT | X | X | X | X | X | X |
| PT/INR | X | |||||
| Factor V | X | |||||
| Fibrinogen | X | |||||
| Max of 10-mL citrate plasma | X | X | X | X | X | X |
Abbreviations: AF, alkaline phosphatase; ALAT, alanine aminotransaminase; APTT, activated partial thromboplastin time; ASAT, aspartate aminotransferase; EHL, extended half-life; FIX, factor IX; FV, factor V; FVIII, factor VIII; GGT, gamma-glutamyltransferase; INR, international normalized ratio; LDH, lactate dehydrogenase; PFA, platelet function assay; PK, pharmacokinetic; PT, prothrombin time; SHL, standard half-life; T, time point; VWF:Act, von Willebrand's factor activity; VWF:Ag, von Willebrand factor antigen; VWF:CB, von Willebrand factor collagen binding; VWFpp, von Willebrand factor propeptide.
Only in case of an EHL concentrate.
Table 2. Laboratory tests during individual PK profiling for patients with hemophilia B.
| On FIX-SHL | Preinfusion | T = 15–30 minutes | T = 4 hours | T = 48–56 hours | T = 72–80 hours | |
|---|---|---|---|---|---|---|
| On FIX-EHL | Preinfusion | T = 15–30 minutes | T = 4 hours | T = 24 hours | T = 72–120 hours | T = 168 hours a |
| ASAT | X | |||||
| ALAT | X | |||||
| GGT | X | |||||
| LDH | X | |||||
| AF | X | |||||
| Albumin | X | |||||
| Urea | X | |||||
| Creatinine | X | |||||
| Hemoglobin | X | |||||
| Hematocrit | X | |||||
| Thrombocytes | X | |||||
| Blood group (if unknown) | X | |||||
| Factor IX | X | X | X | X | X | X |
| Inhibitor FIX (only ≥ 18 years) | X | |||||
| Bethesda FIX | X | |||||
| Factor VIII | X | |||||
| VWF:Ag | X | |||||
| VWF:Act | X | |||||
| VWF:CB | X | |||||
| VWFpp | X | |||||
| Buffy coat | X | |||||
| APTT | X | X | X | X | X | X |
| PT + INR | X | |||||
| Factor V | X | |||||
| Fibrinogen | X | |||||
| Max of 10 mL citrate plasma | X | X | X | X | X | X |
Abbreviations: AF, alkaline phosphatase; ALAT, alanine aminotransaminase; APTT, activated partial thromboplastin time; ASAT, aspartate aminotransferase; EHL, extended half-life; FIX, factor IX; FV, factor V; FVIII, factor VIII; GGT, gamma-glutamyltransferase; INR, international normalized ratio; LDH, lactate dehydrogenase; PFA, platelet function assay; PK, pharmacokinetic; PT, prothrombin time; SHL, standard half-life; T, time point; VWF:Act, von Willebrand factor activity; VWF:Ag, von Willebrand factor antigen; VWF:CB, von Willebrand factor collagen binding; VWFpp, von Willebrand factor propeptide.
Only in case of an EHL concentrate.
Dosing will be advised by clinical pharmacologist on the basis of FVIII/FIX target trough levels as set by treating physician, in accordance to patient characteristics, previous trough levels (if a patient switches between factor concentrates), bleeding history, activity pattern, and in consultation with patient/caretakers. If desirable, physicians are also able to set FVIII/FIX target peak levels during intensive physical activities. In this way, treatment is truly customized and tailored to the needs and lifestyle of each individual as personalization is meant to be. Retrospective data of a patient, such as previous trough levels and factor levels at onset of a bleed or during sport activities, can be informative to the physician to set target levels.
Thereafter, patients will initially be on PK-guided treatment for 12 weeks. During these 12 weeks, a minimum of three factor levels will be measured and compared with predicted FVIII/FIX values to validate predicted dosing regimen ( Table 3 ). Patients on EHL will be on iterative PK-guided treatment with dose adjustment if needed based on both factor levels and bleedings. Iterative treatment is desirable in these patients as most patients initiate treatment with EHL factor concentrates after being on prophylaxis with SHL factor concentrates. Because of the lack of knowledge of most optimal (frequency and dose of) EHL factor concentrate, this period has a dose finding perspective.
Table 3. Laboratory tests during initial PK-guided treatment and follow-up.
| Initial PK-guided treatment (12 weeks) | Follow-up treatment under PK guidance (24 weeks) | Bleeds (only when blood sampling is clinically indicated, during total study period) | |||
|---|---|---|---|---|---|
| Visit | 1 | 2 | 3 | 1 | additional |
| Hemophilia A | |||||
| FVIII | X | X | X | X | X |
| FIX | X | ||||
| VWF:Ag | X | ||||
| VWF:Act | X | ||||
| VWF:CB | X | ||||
| VWFpp | X | ||||
| APTT | X | X | X | X | X |
| Inhibitor FVIII | X | X | (X) | ||
| Max of 10-mL citrate plasma | X | X | X | X | X |
| Hemophilia B | |||||
| FIX | X | X | X | X | X |
| FVIII | X | ||||
| VWF:Ag | X | ||||
| VWF:Act | X | ||||
| VWF:CB | X | ||||
| VWFpp | X | ||||
| APTT | X | X | X | X | X |
| Inhibitor FIX | X | X | (X) | ||
| Max of 10-mL citrate plasma | X | X | X | X | X |
Abbreviations: APTT, activated partial thromboplastin time; FIX, factor IX; FVIII, factor VIII; PK, pharmacokinetic; VWF:Act, von Willebrand factor activity; VWF:Ag, von Willebrand factor antigen; VWF:CB, von Willebrand factor collagen binding; VWFpp, von Willebrand factor propeptide.
For patients on SHL predicted FVIII/FIX values will be blinded to the treating physician and dosages will not be adjusted during the first 10 weeks. Thereafter, dose adjustment can be made.
A subsequent follow-up period of 24 weeks on PK-guided treatment is necessary to further collect data to establish the associations between FVIII/FIX levels and bleeding events. Only if clinically indicated, FVIII/FIX levels will be measured during bleeds ( Table 3 ). At the end of this follow-up period, one final blood sample will be taken to compare the factor level with the predicted value ( Table 3 ). Patients/caretakers will again fill in the HAL or PedHAL questionnaire and the physiotherapist will perform the HJHS to evaluate outcomes after implementation of PK-guidance.
Finally, at the end of the study, both patients/caretakers and the treating physician will fill in the VAS questionnaire, considering the experience with PK-guided dosing and EHL factor concentrate when patients have switched to an EHL factor concentrate.
Importantly, hemophilia patients, who have undergone individual PK-profiling prior to study inclusion or who have already received PK-guided treatment on SHL or EHL concentrate, are also able to participate in the study. PK profiling is required to be performed with a maximum of 1 year prior to study inclusion when <12 years of age and a maximum of 3 years prior to study inclusion when 12 years and older. Patients who already received PK guidance prior to study inclusion, will only complete the VAS questionnaire at the end of the study period, since asking the patient questions with regard to expectations on PK-guided dosing at the beginning of the study would lead to recall bias. Also, the HJHS will not be performed in these patients and PedHAL will not be completed in this subgroup as results after body weight (and bleeding) –based prophylaxis and PK-guided therapy cannot be compared.
In parallel with the prospective study, retrospective data analysis will be performed over a 12-month period prior to inclusion (if no PK profiling has been performed) or from PK profiling prior to inclusion. These data will be utilized as “real-world data” to construct and enrich available population PK models. Moreover, if patients kept a detailed patient log-on infusion dates and timing and bleeding episodes, annualized (joint) bleeding rate (A(J)BR) FVIII/FIX trough levels and FVIII/FIX levels during physical activities and at the onset of a bleed can be calculated.
Factor activity levels will be measured by local laboratories, as this reflects the real-world setting of standard clinical practice. Plasma samples are stored in case centralized measurements are deemed necessary. Laboratory specifications (assay, reagents, deficient plasma, and analyzer) applied in local laboratory will be recorded precisely. Preferably, the local laboratory assays match with the assays as used during population PK model construction.
Bayesian Forecasting
Bayesian forecasting will be performed with the NONMEM software (Icon, Dublin, Ireland); individual PK parameters will be assessed with a limited number of blood samples. Available population PK models in literature and new models that will become available will be used. Based on the estimated individual PK parameters and the FVIII/FIX target trough and peak values as set by the physician, dosing schedules will be calculated.
Sample Size Calculation
In this prospective study, we aim to evaluate the predictive performance of PK-guided dosing in hemophilia patients. It is not common practice to calculate a sample size for prognostic models, and, to the best of our knowledge, it is not possible to calculate a sample size for the determination of predictive performance. What we do know is that as characteristics, such as age, body weight, activity pattern, and bleeding phenotype, are not part of the inclusion or exclusion criteria, the study population will be a reflection of the real-world and thus a heterogeneous hemophilia population. However, we aim to enroll a minimum of 50 patients in all strata together to explore the predictive performance of PK-guided dosing in real life.
Statistical Analysis
Continuous data will be expressed as mean and standard deviation when normally distributed or median and interquartile range when not normally distributed. Categorical data will be expressed as frequency and percentage.
As described in the primary study endpoint, the predictive performance of PK-guided dosing is deemed acceptable when at least 80% of the actual FVIII/FIX levels are within ± 25% of the predicted (target) values as stated by treating professional. Both the mean error between the predicted and observed factor level and the mean absolute difference of the predicted level will be calculated. No significant bias presented as zero is included in the 95% confidence interval (CI) of the mean error. Moreover, differences between the predictive performance of different factor concentrates and age groups will be investigated and described.
Association of factor levels with bleedings will be described according to sub classifications. Comparisons of the ABR and joint status before and during PK-guidance will be analyzed using a paired t -test or Wilcoxon's test, depending on the distribution.
R (version 4.0.3) will be used for statistical analysis.
Ethical Considerations
The study protocol was approved by the Medical Ethics Board of the Erasmus MC, University Medical Center Rotterdam, the Netherlands, and approved by all boards of all participating hospitals.
Registration
The trial is registered at the Dutch Trial register with trial number: NTR7523 ( www.trialregister.nl ).
Conclusion
The proposed study aims to investigate the reliability and feasibility of PK-guided prophylactic dosing of factor concentrates in hemophilia-A and -B patients in daily clinical practice. Moreover, the collected real-world data will lead to enrichment of current population PK models and an increased insight in the association of FVIII/FIX levels with bleeding episodes and daily activities.
Acknowledgments
All authors would like to thank the OPTI-CLOT study group as a whole for their support and Iris van Moort and Jessica Heijdra for their involvement and participation.
The SYMPHONY consortium which aims to orchestrate personalized treatment in patients with bleeding disorders, is a unique collaboration between patients, health care professionals, and translational and fundamental researchers specialized in inherited bleeding disorders, as well as experts from multiple disciplines. It aims to identify the best treatment choice for each individual based on bleeding phenotype. To achieve this goal, workpackages (WP) have been organized according to three themes, for example, diagnostics (WPs 3&4), treatment (WPs 5–9), and fundamental Research (WPs 10–12). This research received funding from the Netherlands Organization for Scientific Research (NWO) in the framework of the NWA-ORC Call grant agreement NWA.1160.18.038. Principal investigator: M.H.C. Project manager: Dr. S.H. Reitsma.
Beneficiaries of the SYMPHONY consortium: Erasmus MC and Erasmus MC Sophia Children's Hospital, University Medical Center Rotterdam, project leadership and coordination; Sanquin Diagnostics; Sanquin Research; Amsterdam University Medical Centers; University Medical Center Groningen; University Medical Center Utrecht; Leiden University Medical Center; Radboud University Medical Center; Netherlands Society of Hemophilia Patients (NVHP); Netherlands Society for Thrombosis and Hemostasis (NVTH); and Bayer B.V., CSL Behring B.V., Swedish Orphan Biovitrum (Belgium) BVBA/SPRL.
Funding Statement
Funding This study was funded by Innovatiefonds Zorgverzekeraars, grant number: Project 3216; and Nederlandse Organisatie voor Wetenschappelijk Onderzoek, grant number: NWA.1160.18.038.
Conflict of Interest M.H.C has received grants from governmental and societal research institutes, for example, NWO-ZonMW, NWO-NWA, Innovation fund, from private funds, institutional grants and unrestricted investigator research grants/educational, and travel funding from the following companies over the years: Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, Nordic Pharma and Roche, and has served as a member on steering boards of Roche and Bayer. All grants, awards and fees go to the Erasmus MC as an institution.
R.A.A.M. has received governmental and societal research institutes such as NWO, ZonMW, and Innovation Fund and unrestricted investigator research grants from Baxter/Baxalta/Shire/Takeda, Bayer, CSL Behring, and Sobi. He has served as an advisor for Bayer, CSL Behring, Merck Sharp & Dohme, and Baxter/Baxalta/Shire/Takeda. All grants and fees were paid to the institution.
H.J.C.J.E. received research support from CSL Behring outside the scope of this project and fees for educational activities from Roche and Celgene which fees go to the institution.
F.W.G.L. received unrestricted research grants from CSL Behring, Shire/Takeda, SOBI, and UniQure. He is a consultant for CSL Behring, Takeda, Biomarin and UniQure of which the fees go to the Erasmus MC as an institution. He served as DSMB member of a study sponsored by Roche.
K.M. reports speaker fees from Bayer and Alexion, participation in trial steering committee for Bayer, consulting fees from UniQure, participation in data monitoring and endpoint adjudication committee for Octapharma. All fees are paid to the institution.
Note
This study is part of the OPTI-CLOT Research Programme (Patient tailOred PharmacokineTIc-guided dosing of CLOTting factor concentrates and desmopressin in bleeding disorders),” an (inter)national multicenter study aiming to implement pharmacokinetic (PK)-guided dosing of replacement therapy and desmopressin by initiating studies to demonstrate its implications and feasibility, improve PK models, and to increase knowledge of patient-tailored PK-guided dosing which is currently part of the SYMPHONY consortium that aims to unravel the origins of the interindividual variation in bleeding phenotype in order to install personalized treatment in all inherited bleeding disorders.
Shared first and last authorships.
References
- 1.Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia Srivastava A, Brewer A K, Mauser-Bunschoten E P.Guidelines for the management of hemophilia Haemophlia 20131901e1–e47.PubMed [DOI] [PubMed] [Google Scholar]
- 2.Leebeek F WG, Mauser-Bunschoten E P. Utrecht: Van Zuiden Communications BV; 2009. Richtlijn diagnostiek en behandeling van hemofilie en aanverwante hemostase stoornissen. [Google Scholar]
- 3.Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand Suppl. 1965;77 77:3–132. doi: 10.3109/ort.1965.36.suppl-77.01. [DOI] [PubMed] [Google Scholar]
- 4.Björkman S, Oh M, Spotts G. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119(02):612–618. doi: 10.1182/blood-2011-07-360594. [DOI] [PubMed] [Google Scholar]
- 5.Björkman S.Prophylactic dosing of factor VIII and factor IX from a clinical pharmacokinetic perspective Haemophilia 2003901101–108., discussion 109–110 [DOI] [PubMed] [Google Scholar]
- 6.Björkman S, Shapiro A D, Berntorp E. Pharmacokinetics of recombinant factor IX in relation to age of the patient: implications for dosing in prophylaxis. Haemophilia. 2001;7(02):133–139. doi: 10.1046/j.1365-2516.2001.00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Chelle P, Yeung C HT, Croteau S E. Development and validation of a population-pharmacokinetic model for Rurioctacog Alfa Pegol (Adynovate): a report on behalf of the WAPPS-Hemo Investigators Ad Hoc subgroup. Clin Pharmacokinet. 2020;59(02):245–256. doi: 10.1007/s40262-019-00809-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Roberts J, Tortorici M. Population pharmacokinetics of recombinant coagulation factor VIII-SingleChain in patients with severe hemophilia A. J Thromb Haemost. 2017;15(06):1106–1114. doi: 10.1111/jth.13662. [DOI] [PubMed] [Google Scholar]
- 9.Allard Q, Djerada Z, Pouplard C. Real life population pharmacokinetics modelling of eight factors VIII in patients with severe haemophilia A: is it always relevant to switch to an extended half-life? Pharmaceutics. 2020;12(04):E380. doi: 10.3390/pharmaceutics12040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEneny-King A, Chelle P, Foster G, Keepanasseril A, Iorio A, Edginton A N. Development and evaluation of a generic population pharmacokinetic model for standard half-life factor VIII for use in dose individualization. J Pharmacokinet Pharmacodyn. 2019;46(05):411–426. doi: 10.1007/s10928-019-09634-7. [DOI] [PubMed] [Google Scholar]
- 11.Brekkan A, Berntorp E, Jensen K, Nielsen E I, Jönsson S. Population pharmacokinetics of plasma-derived factor IX: procedures for dose individualization. J Thromb Haemost. 2016;14(04):724–732. doi: 10.1111/jth.13271. [DOI] [PubMed] [Google Scholar]
- 12.Diao L, Li S, Ludden T, Gobburu J, Nestorov I, Jiang H. Population pharmacokinetic modelling of recombinant factor IX Fc fusion protein (rFIXFc) in patients with haemophilia B. Clin Pharmacokinet. 2014;53(05):467–477. doi: 10.1007/s40262-013-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Björkman S. Population pharmacokinetics of recombinant factor IX: implications for dose tailoring. Haemophilia. 2013;19(05):753–757. doi: 10.1111/hae.12188. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Tomono Y, Korth-Bradley J M. Population pharmacokinetic modelling of factor IX activity after administration of recombinant factor IX in patients with haemophilia B. Haemophilia. 2016;22(05):e359–e366. doi: 10.1111/hae.12969. [DOI] [PubMed] [Google Scholar]
- 15.Nestorov I, Neelakantan S, Ludden T M, Li S, Jiang H, Rogge M. Population pharmacokinetics of recombinant factor VIII Fc fusion protein. Clin Pharmacol Drug Dev. 2015;4(03):163–174. doi: 10.1002/cpdd.167. [DOI] [PubMed] [Google Scholar]
- 16.for UK-EHL Outcomes Registry OPTI-CLOT Collaboration . Bukkems L H, Heijdra J M, Mathias M. A novel, enriched population pharmacokinetic model for recombinant factor VIII-Fc fusion protein concentrate in hemophilia A patients. Thromb Haemost. 2020;120(05):747–757. doi: 10.1055/s-0040-1709522. [DOI] [PubMed] [Google Scholar]
- 17.Shah A, Solms A, Wiegmann S. Direct comparison of two extended-half-life recombinant FVIII products: a randomized, crossover pharmacokinetic study in patients with severe hemophilia A. Ann Hematol. 2019;98(09):2035–2044. doi: 10.1007/s00277-019-03747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Roberts J, Bensen-Kennedy D. Population pharmacokinetics of a new long-acting recombinant coagulation factor IX albumin fusion protein for patients with severe hemophilia B. J Thromb Haemost. 2016;14(11):2132–2140. doi: 10.1111/jth.13444. [DOI] [PubMed] [Google Scholar]
- 19.Solms A, Iorio A, Ahsman M J. Favorable pharmacokinetic characteristics of extended-half-life recombinant factor VIII BAY 94-9027 enable robust individual profiling using a population pharmacokinetic approach. Clin Pharmacokinet. 2020;59(05):605–616. doi: 10.1007/s40262-019-00832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solms A, Shah A, Berntorp E. Direct comparison of two extended half-life PEGylated recombinant FVIII products: a randomized, crossover pharmacokinetic study in patients with severe hemophilia A. Ann Hematol. 2020;99(11):2689–2698. doi: 10.1007/s00277-020-04280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Björkman S, Folkesson A, Jönsson S. Pharmacokinetics and dose requirements of factor VIII over the age range 3-74 years: a population analysis based on 50 patients with long-term prophylactic treatment for haemophilia A. Eur J Clin Pharmacol. 2009;65(10):989–998. doi: 10.1007/s00228-009-0676-x. [DOI] [PubMed] [Google Scholar]
- 22.Stass H. Determination of minimal sampling time points for reliable pharmacokinetic evaluation of recombinant factor VIII - an exploratory population pharmacokinetic analysis in paediatric patients suffering from severe haemophilia. Haemophilia. 2006;12:50–55. [Google Scholar]
- 23.Garmann D, McLeay S, Shah A, Vis P, Maas Enriquez M, Ploeger B A. Population pharmacokinetic characterization of BAY 81-8973, a full-length recombinant factor VIII: lessons learned - importance of including samples with factor VIII levels below the quantitation limit. Haemophilia. 2017;23(04):528–537. doi: 10.1111/hae.13192. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, Solms A, Garmann D. Improved pharmacokinetics with BAY 81-8973 versus antihemophilic factor (recombinant) plasma/albumin-free method: a randomized pharmacokinetic study in patients with severe hemophilia A. Clin Pharmacokinet. 2017;56(09):1045–1055. doi: 10.1007/s40262-016-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Björkman S, Ahlén V. Population pharmacokinetics of plasma-derived factor IX in adult patients with haemophilia B: implications for dosing in prophylaxis. Eur J Clin Pharmacol. 2012;68(06):969–977. doi: 10.1007/s00228-012-1211-z. [DOI] [PubMed] [Google Scholar]
- 26.Bolon-Larger M, Chamouard V, Bressolle F, Boulieu R. A limited sampling strategy for estimating individual pharmacokinetic parameters of coagulation factor VIII in patients with hemophilia A. Ther Drug Monit. 2007;29(01):20–26. doi: 10.1097/FTD.0b013e3180311384. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez-Yuste V, Lejniece S, Klamroth R. The pharmacokinetics of a B-domain truncated recombinant factor VIII, turoctocog alfa (NovoEight), in patients with hemophilia A. J Thromb Haemost. 2015;13(03):370–379. doi: 10.1111/jth.12816. [DOI] [PubMed] [Google Scholar]
- 28.Tiede A, Abdul Karim F, Jiménez-Yuste V. Factor VIII activity and bleeding risk during prophylaxis for severe hemophilia A: a population pharmacokinetic model. Haematologica. 2021;106(07):1902–1909. doi: 10.3324/haematol.2019.241554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delavenne X, Dargaud Y, Ollier E, Négrier C. Dose tailoring of human cell line-derived recombinant factor VIII simoctocog alfa: Using a limited sampling strategy in patients with severe haemophilia A. Br J Clin Pharmacol. 2019;85(04):771–781. doi: 10.1111/bcp.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karafoulidou A, Suarez E, Anastasopoulou I. Population pharmacokinetics of recombinant factor VIII:C (ReFacto) in adult HIV-negative and HIV-positive haemophilia patients. Eur J Clin Pharmacol. 2009;65(11):1121–1130. doi: 10.1007/s00228-009-0699-3. [DOI] [PubMed] [Google Scholar]
- 31.Collins P W, Møss J, Knobe K, Groth A, Colberg T, Watson E. Population pharmacokinetic modeling for dose setting of nonacog beta pegol (N9-GP), a glycoPEGylated recombinant factor IX. J Thromb Haemost. 2012;10(11):2305–2312. doi: 10.1111/jth.12000. [DOI] [PubMed] [Google Scholar]
- 32.Abrantes J A, Nielsen E I, Korth-Bradley J, Harnisch L, Jönsson S. Elucidation of factor VIII activity pharmacokinetics: a pooled population analysis in patients with hemophilia a treated with Moroctocog alfa. Clin Pharmacol Ther. 2017;102(06):977–988. doi: 10.1002/cpt.716. [DOI] [PubMed] [Google Scholar]
- 33.Hazendonk H C, Lock J, Mathôt R A. Perioperative treatment of hemophilia A patients: blood group O patients are at risk of bleeding complications. J Thromb Haemost. 2016;14(03):468–478. doi: 10.1111/jth.13242. [DOI] [PubMed] [Google Scholar]
- 34.Björkman S. Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia. 2010;16(04):597–605. doi: 10.1111/j.1365-2516.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson M, Berntorp E, Björkman S, Lethagen S, Ljung R. Improved cost-effectiveness by pharmacokinetic dosing of factor VIII in prophylactic treatment of haemophilia A. Haemophilia. 1997;3(02):96–101. doi: 10.1046/j.1365-2516.1997.00091.x. [DOI] [PubMed] [Google Scholar]
- 36.OPTI-CLOT study group . van Moort I, Preijers T, Bukkems L H. Perioperative pharmacokinetic-guided factor VIII concentrate dosing in haemophilia (OPTI-CLOT trial): an open-label, multicentre, randomised, controlled trial. Lancet Haematol. 2021;8(07):e492–e502. doi: 10.1016/S2352-3026(21)00135-6. [DOI] [PubMed] [Google Scholar]
- 37.Subcommittee on Factor VIII, Factor IX, and Rare Bleeding Disorders . Ragni M V, Croteau S E, Morfini M, Cnossen M H, Iorio A. Pharmacokinetics and the transition to extended half-life factor concentrates: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16(07):1437–1441. doi: 10.1111/jth.14153. [DOI] [PubMed] [Google Scholar]


