Abstract
The efficacy and safety of penciclovir (PCV) for the treatment of herpes simplex virus (HSV) infections in immunocompromised (IC) patients were studied in a double-blind, acyclovir (ACV)-controlled, multicenter study. A total of 342 patients with mucocutaneous HSV infections received 5 mg of PCV per kg every 12 or 8 h (q12h or q8h) or 5 mg of ACV per kg q8h, beginning within 72 h of lesion onset and continuing for up to 7 days. The mean age of the patients was 49 years; 94% were white and 52% were female. The main reasons for their IC states were hematologic disorder (63%) and transplant plus hematologic disorder (16%). Clinical and virological assessments were performed daily during the 7-day treatment and then every other day until lesion healing. The primary efficacy parameter addressed new lesion formation. Secondary end points focused on viral shedding, healing, and pain. Approximately 20% of patients in each treatment group developed new lesions during therapy; thus, equivalence with ACV (defined prospectively) was demonstrated for both q12h and q8h PCV regimens. For all three treatment groups, the median time to the cessation of viral shedding was 4 days and the median time to complete healing was 8 days; there were no statistically significant differences in the rates of complete healing or the cessation of viral shedding when the results for PCV q12h and q8h were compared with those for ACV q8h. In addition, there was no statistically significant difference between PCV q12h or q8h, compared with ACV q8h, for the resolution of pain. PCV was well tolerated, with an adverse event profile comparable to that of ACV. In conclusion, PCV q12h is a well-tolerated and effective therapy for mucocutaneous HSV infection in IC patients and offers a reduced frequency of dosing compared with ACV q8h.
Herpes simplex virus (HSV) infections in immunocompetent patients are of relatively short duration and are generally self-limiting (15). HSV infections in an immunocompromised host, however, may be severe and prolonged and can spread without treatment, causing severe morbidity or mortality (9). The reactivation rate among seropositive transplant patients has been reported to be between 60 and 80% for patients with solid organ transplants and over 80% after allogenic bone marrow transplantation (6, 10, 12, 20). Intravenous treatment with acyclovir (5 mg/kg every 8 h [q8h] for 7 days), effective for the treatment of immunocompromised patients with HSV infection, is the most commonly used therapy (7, 8, 20).
Penciclovir, a novel acyclic nucleoside analog, has demonstrated efficacy in cell culture against HSV types 1 and 2 as well as against varicella-zoster virus (2). The intracellular triphosphate of penciclovir is considerably more stable than acyclovir triphosphate (in vitro half-life of 10 to 20 h in HSV-infected cells compared to 0.7 to 1 h for acyclovir), a potential pharmacological advantage for penciclovir (19). Also, penciclovir has been shown to be effective against a small percentage of acyclovir-resistant HSV strains in vitro (2). This activity may translate into a potential benefit in a subgroup of patients in whom the virus has become resistant to acyclovir, an important consideration for an immunocompromised patient population. A topical formulation of penciclovir currently is marketed for the treatment of recurrent herpes labialis in immunocompetent patients. Famciclovir, the orally bioavailable prodrug, is approved in the United States and other countries for the treatment of acute herpes-zoster virus infection and the treatment and suppression of genital herpes (14, 16, 17).
The results of an open, dose-escalation study of intravenously administered penciclovir in immunocompromised patients with mucocutaneous HSV infections indicated that intravenously administered penciclovir was effective for the treatment of mucocutaneous HSV infection in immunocompromised patients (18). The optimum intravenous dose of penciclovir for the treatment of HSV disease in such patients was 5 mg/kg q8h or every 12 h (q12h). The present report describes a randomized, double-blind, multicenter study comparing these two doses of intravenous penciclovir with acyclovir (5 mg/kg q8h for 7 days) for the treatment of mucocutaneous HSV infections in immunocompromised patients.
(The results of this trial were presented, in part, at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997 [5a].)
MATERIALS AND METHODS
Study medication.
Penciclovir and acyclovir were provided as vials of freeze-dried powder for reconstitution. Penciclovir vials contained 250 mg of active drug per vial. Each vial of acyclovir contained either 250 or 500 mg, depending upon licensure in the participating countries.
Treatment groups.
Patients who met the entry criteria were randomly assigned in a double-blind fashion to receive a 7-day course of treatment with 5 mg of penciclovir per kg q12h, 5 mg of penciclovir per kg q8h, or 5 mg of acyclovir per kg q8h.
A computer-generated randomization code with restricted access was used to allocate patients to the three treatment groups. An unblinded pharmacist at each center identified the treatment regimen assigned to each patient by opening randomization envelopes in sequence. The pharmacist reconstituted the study medication designated in the randomization envelope as instructed in a detailed pharmacy manual, withdrew a quantity of drug sufficient for a 5-mg/kg dose, and diluted the withdrawn quantity with an appropriate infusion solution. Placebo infusions consisting of infusion solution alone also were prepared. Each infusion (active and placebo) then was labeled in a blinded fashion and dispensed. Each patient received four infusions per day (i.e., either three active infusions and one placebo or two active infusions and two placebos) in order to maintain the blind for the q12h and q8h treatment regimens.
Patient population.
Eligible patients were at least 14 years of age with clinical evidence of mucocutaneous HSV infection who were immunocompromised due to treatment for cancer or leukemia, an organ or bone marrow transplant, or a chronic rheumatologic condition. Therapy with a study drug had to be initiated within 72 h of lesion onset. Patients were excluded from the study if they were pregnant or had a positive serum human chorionic gonadotropin test, were known to be human immunodeficiency virus positive, had disseminated HSV infection, or evidence of renal (i.e., calculated creatinine clearance of <50 ml/min) or hepatic (i.e., serum bilirubin levels of >10 mg/dl or aspartate aminotransferase or alanine aminotransferase levels more than five times the upper limit of normal) dysfunction. All patients gave written informed consent and were screened by the study staff for dermatological conditions that would interfere with the assessment of lesions or viral shedding. Antiviral therapy other than study medication was prohibited within 14 days of study entry and during the study. The application of topical products to the lesion area(s) also was prohibited. However, the use of oral anesthetics and antifungals (e.g., nystatin) was permitted.
Study design and procedures.
This trial was a multicenter, randomized, double-blind, acyclovir-controlled study which was approved by an institutional review board or ethics committee for each institution. All patients gave written informed consent. At inclusion, patients were assessed for baseline herpetic lesions and lesion-associated pain and symptoms. Lesion data was recorded separately for each anatomical location (i.e., orolabial, anogenital, and others). Swabs were obtained from each location with lesions for baseline viral culture results. Assessments continued daily during the 7-day treatment period and every other day thereafter until the lesions had healed completely. The presence or absence of new lesions was determined at each subsequent evaluation. Blood and urine samples were obtained for the assessment of safety at baseline and again at the end of therapy (day 8) and 1 week posttherapy (day 15). In addition, details of any adverse experiences and use of concomitant medications were recorded at each study visit. Patients with a persistence of lesions after day 7 or a rapid return of lesions could be withdrawn and treated at the discretion of the investigator; however, study-specific lesion assessments did not continue after withdrawal from the study.
Virus isolation.
Swabs from lesions were placed in virus transport media for subsequent viral isolation in tissue culture. Susceptibility testing against penciclovir was performed by a plaque reduction assay method in MRC-5 cells according to the method of Boyd et al., with minor modifications. Briefly, testing was performed in triplicate in MRC-5 cells by using a series of penciclovir concentrations to provide at least two data points on either side of the 50% inhibitory concentration (IC50). After virus adsorption, the drug, 2× media, and 0.8% agarose were mixed and added to the infected monolayer. After 3 days at 37°C, the plates were fixed with 1.0 ml of a 10% formaldehyde solution for 1 h at room temperature. Cell monolayers were stained with crystal violet after removal of the agarose plugs. The IC50 was defined as the antiviral concentration that reduced the plaque numbers to 50% of those in the control wells containing virus alone (1).
Known acyclovir-resistant HSV strains for which penciclovir and acyclovir IC50 were >3.0 μg/ml were included in the assay as controls. A testing laboratory approved by the College of American Pathologists defined criteria for resistance based on parallel assays of sensitive and resistant control virus strains. For an isolate to be labeled resistant, it must have an IC50 of ≥2.0 μg/ml or have an IC50 ≥10-fold above the IC50 for the sensitive control virus within that particular assay. The IC50 for the sensitive control virus ranged from 0.07 to 0.21 μg of penciclovir per ml, with a mean of 0.145 ± 0.038 μg of penciclovir per ml.
Efficacy end points.
The cessation of new lesion formation was viewed as the best clinical indication of effective antiviral therapy, because new lesion formation is a clinical manifestation of active virus replication and contributes to the morbidity of HSV disease in immunocompromised patients. Therefore, the proportion of patients with new lesion formation during therapy was chosen as the primary efficacy parameter. Secondary end points included the time to lesion healing, the time to the cessation of viral shedding, the proportion of patients who ceased shedding of virus by day 7, the proportion of patients withdrawn for treatment failure, and the time to the resolution of pain.
In this study, herpetic lesions comprised papules, vesicles or pustules, ulcers, and crusts on mucocutaneous membranes which were the result of HSV infection. Complete healing was defined as the first visit at which the patient reported no papules, vesicles or pustules, ulcers, or crusts and did not report any of these at a subsequent visit. The number of lesions was recorded as 0, 1, 2 to 5, or >5. Anatomical diagrams were provided as an aid for monitoring lesion progression and the appearance of new lesions. The cessation of viral shedding was defined as the first negative culture with no subsequent positive cultures. Approximately 60% of patients were expected to be HSV positive and thus eligible for viral shedding end points based on observations from a previous study (18) of similar design (i.e., patients were enrolled based on clinical suspicion of HSV infection rather than virological confirmation). Patients could be withdrawn from the study as a treatment failure if they had dissemination of HSV infection, experienced new lesion formation beyond day 7 of the study (i.e., after the cessation of study medication), or had a clinical or virological failure requiring further anti-HSV therapy. Pain (e.g., tenderness, burning, or other pain) was recorded on a scale of none, mild, moderate, and severe according to the patient’s response to direct questioning.
Statistical analysis.
All analyses were performed on an intent-to-treat population consisting of patients who received at least one dose of study medication. Sample size requirements were calculated by using a confidence interval approach. The study was designed to demonstrate the equivalence between penciclovir and acyclovir for the primary end point. Equivalence was defined as the upper limit of the two-sided 97.5% confidence interval for the penciclovir-minus-acyclovir difference being less than 20%. The Bonferroni approach was used to adjust for multiple treatment comparisons. As the overall significance level was 5%, this resulted in a 97.5% confidence interval. One hundred evaluable patients per treatment group were targeted to demonstrate that penciclovir was at least as good as acyclovir at preventing new lesion formation. Equivalence was assessed only with respect to the primary efficacy variable. Two-tailed significance testing was applied to the secondary parameters. Time-to-event variables were measured in days from the start date of the first infusion until the resolution of the condition. Only patients with the condition of interest (e.g., viral shedding) were included in the analysis of the time to loss of the condition. Patients who continued to experience the condition at their last assessment were censored at that time point. Differences between treatments were analyzed with the Cox proportional hazards regression model and were summarized by Kaplan-Meier plots (4). The treatment difference was considered to be statistically significant if the 97.5% confidence interval for the hazard ratio lay entirely above or below 1.0. Proportion end points were analyzed by using confidence intervals for the difference in proportions (penciclovir minus acyclovir). The treatment difference was considered to be statistically significant if the 97.5% confidence interval for the difference lay entirely above or below 0.
RESULTS
Characteristics of the study patients.
A total of 342 patients from 40 centers in nine countries were randomized and received at least one dose of study medication. Ten patients were withdrawn from the study during the treatment period, and a further 76 patients were withdrawn after the cessation of treatment. The most common reasons for withdrawal were concurrent disease or adverse experience, treatment failure, loss to follow-up, and protocol violation (Table 1). With the exception of protocol violation (higher for the acyclovir group), the numbers of patients withdrawn for each reason were similar across treatment groups.
TABLE 1.
Withdrawal of patients from study
| Treatment (no. of patients) | No. (%) of patients withdrawn during:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug administration
|
Follow-up
|
Total | |||||||||
| Other reasona | Lost to follow-up | AE or concurrent diseaseb | Treatment failure | AE or concurrent diseaseb | Treatment failure | Protocol violation | Lost to follow-up | Other reasona | Lack of compliance | ||
| Penciclovir q12h (115) | 2 (2) | 1 (1) | 2 (2) | 0 | 6 (5) | 7 (6) | 4 (4) | 3 (3) | 2 (2) | 1 (1) | 28 (24) |
| Penciclovir q8h (114) | 0 | 1 (1) | 0 | 2 (2) | 7 (6) | 5 (4) | 3 (3) | 5 (4) | 2 (2) | 1 (1) | 26 (23) |
| Acyclovir q8h (113) | 1 (1) | 1 (1) | 0 | 0 | 6 (5) | 6 (5) | 8 (7) | 7 (6) | 2 (2) | 1 (1) | 32 (28) |
Includes withdrawal of consent, continuation of antiviral therapy posthealing, and early discharge from the hospital.
Includes exacerbations of underlying diseases and adverse experiences (AE).
The intent-to-treat population, which includes all 342 patients, is the basis for the analysis. Patients were allocated equally to the three treatment groups (115 received penciclovir q12h, 114 received penciclovir q8h, and 113 received acyclovir q8h). A comparison of patient demographic characteristics for the intent-to-treat population is shown in Table 2. In general, the demographic characteristics were similar across the three treatment groups. The majority of patients were Caucasian (94%), and the overall population was almost equally divided with respect to gender. The mean age ranged from 48 to 50 years.
TABLE 2.
Characteristics of patient population by treatment group
| Category | Treatment group
|
||
|---|---|---|---|
| Penciclovir q12h (n = 115) | Penciclovir q8h (n = 114) | Acyclovir q8h (n = 113) | |
| No. (%) of | |||
| Males | 51 (44) | 58 (51) | 56 (50) |
| Females | 64 (56) | 56 (49) | 57 (50) |
| Whites | 107 (93) | 107 (94) | 106 (94) |
| Blacks | 8 (7) | 5 (4) | 5 (4) |
| Orientals | 0 | 2 (2) | 2 (2) |
| Age (yr) | |||
| Mean (SD) | 50 (15) | 50 (16) | 48 (17) |
| Median | 49 | 52 | 52 |
| Range | 18–82 | 18–88 | 18–81 |
Most patients (58 to 67%) were immunocompromised due to a hematologic disorder, which included leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome, and aplastic anemia (Table 3). Approximately 25% of the population had had either a bone marrow or solid organ transplant. More than 90% of patients had received chemotherapeutic or immunomodulatory treatments for underlying conditions (e.g., cytarabine, cyclophosphamide, etoposide, dexamethasone, or hydrocortisone) either within 30 days of study entry or during the study. Twenty-four percent of patients reported a history of having taken acyclovir at a time in the past for a medical condition. Of note, a large percentage of patients received strong narcotic analgesics during the study (e.g., at least 20% of patients in each treatment group received morphine in various salt forms), which complicated the assessment of lesion pain as perceived by the patient.
TABLE 3.
Summary of reasons for immunocompromised state
| Diagnosis | No. (%) of patients in treatment group
|
||
|---|---|---|---|
| Penciclovir q12h (n = 115) | Penciclovir q8h (n = 114) | Acyclovir q8h (n = 113) | |
| Hematologic disordera | 77 (67) | 66 (58) | 73 (65) |
| Transplantb | 28 (24) | 27 (24) | 24 (21) |
| Cancer | 8 (7) | 12 (11) | 8 (7) |
| Otherc | 2 (2) | 9 (8) | 8 (7) |
Includes leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome, and aplastic anemia.
Includes solid organ and bone marrow transplants.
Includes combinations of the above diagnoses in addition to chronic rheumatologic conditions.
Most patients were able to initiate therapy within 48 h of the onset of lesions. Lesion appearance at baseline was similar across treatment groups. Lesions were predominantly orolabial (>90% of patients), and most patients presented with ulcers (Table 4). Over half of the population had no prior history of orolabial mucocutaneous HSV infection. Approximately 80% of patients in each treatment group reported pain at baseline, with a slightly higher percentage of patients in the penciclovir groups reporting severe pain (19 to 21% for penciclovir versus 12% for acyclovir).
TABLE 4.
Baseline lesion assessment
| Lesiona | No. (%) of patients in treatment group
|
||
|---|---|---|---|
| Penciclovir q12h (n = 115) | Penciclovir q8h (n = 114) | Acyclovir q8h (n = 113) | |
| Location | |||
| Orolabial | 109 (95) | 108 (95) | 105 (93) |
| Anogenital | 5 (4) | 6 (5) | 7 (6) |
| Other | 2 (2) | 5 (4) | 4 (4) |
| Stage | |||
| Papules | 25 (22) | 31 (27) | 29 (26) |
| Vesicles or pustules | 45 (39) | 51 (45) | 44 (39) |
| Ulcers | 77 (67) | 61 (54) | 69 (61) |
| Crusts | 14 (12) | 19 (17) | 15 (13) |
Patients may have had lesions in more than one location or at more than one stage at baseline.
The percentage of patients shown to have a positive culture at baseline was 60% and 62% for the penciclovir q12h and q8h groups, respectively, compared with 54% of patients in the acyclovir q8h group. More than 90% of patients who had a positive culture were positive for HSV type 1, with the values comparable between treatment groups.
Lesion end points.
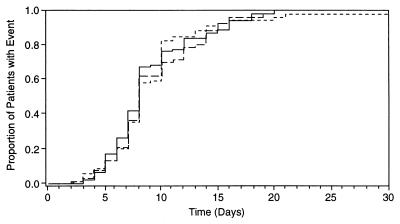
During the course of the study, approximately 20% of patients developed new lesions during therapy (19% in the penciclovir q12h and acyclovir q8h groups and 21% in the penciclovir q8h group). Both penciclovir treatments were shown to be equivalent to acyclovir therapy for this primary end point; i.e., the upper limits of the 97.5% confidence intervals, 12% for penciclovir q12h and 14% for penciclovir q8h, were below the prespecified equivalence level of 20%. As assessments were made once daily, the time to healing is expressed as an integer. The median time to the healing of all lesions was 8 days across all three treatment groups, and comparisons of penciclovir to acyclovir were not statistically significant for the time to healing (Fig. 1 and Table 5).
FIG. 1.
Kaplan-Meier plot of time to healing of lesions for penciclovir q12h (long-dashed line), penciclovir q8h (short-dashed line), and acyclovir q8h (solid line).
TABLE 5.
Lesion end points
| Treatment (no. of patients) | % of patients with new lesions during therapy (97.5% confidence interval)a | Time to healing (days)
|
||
|---|---|---|---|---|
| Median | Hazard ratio | 97.5% confidence intervalb | ||
| Penciclovir q12h (115) | 19 (−11, 12) | 8 | 0.9 | 0.6, 1.3 |
| Penciclovir q8h (114) | 21 (−9, 14) | 8 | 0.9 | 0.6, 1.3 |
| Acyclovir q8h (113) | 19 | 8 | ||
Treatments are statistically equivalent because the upper limit of the 97.5% confidence interval is less than 20%.
There is no significant difference between treatments because the 97.5% confidence interval spans 1.
Viral shedding end points.
The median time to the cessation of viral shedding from all lesions was 4 days for all three treatment groups, and comparisons of penciclovir to acyclovir were not statistically significant (i.e., 97.5% confidence intervals for comparisons of penciclovir with acyclovir spanned 1). The percentage of patients who ceased shedding virus by day 7 was 85 to 87% in each treatment group. The confidence intervals for this proportion analysis spanned 0, indicating that there was no statistical evidence of any treatment difference between the penciclovir and acyclovir groups.
Other clinical end points.
No patients were withdrawn because of the dissemination of HSV. Twenty patients (seven in each penciclovir group and six in the acyclovir group) were withdrawn for the other treatment failure reasons (i.e., clinical or virological failure requiring further anti-HSV therapy or continued new lesion formation beyond day 7). The comparisons of penciclovir to acyclovir were not statistically significant. Most of the patients with treatment failures received acyclovir as an additional antiviral therapy even though acyclovir was one of the blinded treatment arms. As these patients were withdrawn from the study when further antiviral therapy was initiated, the impact of additional antiviral therapy on lesion healing is unknown.
As with the other efficacy parameters, no significant difference was found between the penciclovir and acyclovir treatments for pain resolution.
Viral resistance.
Antiviral-resistant strains of HSV have become a concern for immunocompromised patients who receive multiple treatment courses or suppressive antiviral therapy for recurrent HSV episodes. In the present study, no penciclovir-resistant HSV isolates were identified. The testing of susceptibility to penciclovir was performed on 419 HSV samples, with 306 isolates from 125 patients treated with penciclovir and 113 isolates from 48 patients treated with acyclovir. A trend analysis on data for paired isolates (pretreatment and posttreatment HSV isolates) tested for penciclovir susceptibility indicates that there are no statistically significant differences in IC50 between these isolates from either penciclovir-treated or acyclovir-treated patient populations (P = 0.121 [analysis of covariance]). Moreover, for all isolates tested IC50 were below 0.7 μg of penciclovir per ml. The testing of all isolates for resistance to acyclovir is ongoing, and the complete results of resistance testing will be presented in a separate report.
Adverse events.
The incidence of adverse events was generally comparable between the penciclovir and acyclovir groups. The most frequently reported adverse events were fever (reported by 11 to 15% of patients) and nausea (reported by 7 to 12% of patients). Less than 4% of patients in any treatment group experienced fever or nausea that was considered likely or possibly related to therapy. A total of 5 to 9% of patients in each treatment group reported serious, nonfatal adverse events during therapy, with hypotension being the only event which was reported by more than one patient in a treatment group (two acyclovir-treated patients). The majority (90%) of serious adverse events were considered by the investigators to be either unrelated or probably unrelated to treatment. The percentage of patients who were withdrawn from the study due to adverse events during therapy was low (4 to 6%) and comparable between the penciclovir and acyclovir treatment groups. None of the patients in the study reported hemolytic uremic syndrome or thrombotic thrombocytopenic purpura. Abnormal laboratory profiles for hematology and clinical chemistry tests reflected the immunocompromised population and were comparable across treatment groups.
DISCUSSION
HSV is a common opportunistic infection in immunocompromised patients. Due to the potential severity of HSV infection in these patients, effective therapies have been sought and the disease has been shown to be treatable (6–8, 18, 20). The results of this study demonstrate that penciclovir given either q8h or q12h is safe and as effective as acyclovir for the treatment of mucocutaneous HSV infection in immunocompromised patients.
The success of the penciclovir q12h regimen is particularly noteworthy because the reduced frequency of administration translates into possible patient convenience and the potential for reduced administration and nursing time compared with the q8h acyclovir regimen. In both groups 19% of patients experienced new lesion formation during therapy. Median values for the time to healing and the time to the cessation of viral shedding were also the same. The percentages of patients who had ceased viral shedding by day 7 were similar in the penciclovir and acyclovir groups (87 and 86%, respectively). In addition, the percentages of patients withdrawn for treatment failure were also similar between the penciclovir q12h and acyclovir q8h regimens (6 and 5%, respectively), and no statistically significant differences between groups were shown for the resolution of pain.
The majority of patients included in this study had underlying diseases which were severe enough to require hospitalization for at least the 7-day treatment period and, therefore, were treated with intravenous rather than oral therapy. Fever and nausea were expected to be two frequently reported adverse events because of the degree of immunosuppression and the number and types of concomitant medications administered. The low incidence of these events (<4% in any group) and of serious adverse events which were considered to be related or possibly related to treatment demonstrates that the overall safety profile reflects the immunocompromised state of the study participants. Both penciclovir and acyclovir were well tolerated by immunocompromised patients with HSV infection.
The lack of penciclovir resistance among the isolates tested appears to be unusual for an immunocompromised patient population, where resistance rates up to 9% have been noted (3, 5, 11, 13). The patient populations in studies which report high percentages of resistant viruses are typically bone marrow transplant recipients or patients in the late stages of AIDS who have serious or long-standing HSV infections. In the present study, only 25% of the patients had received a solid organ or bone marrow transplant. Most were immunocompromised due to immunosuppressive chemotherapeutic regimens for hematologic or other malignancies. Patients with disseminated HSV infection or those known to be infected with human immunodeficiency virus at study entry were excluded from the study. More importantly, though, more than half of the patients did not report having had a previous HSV episode at study entry, and only 24% had received acyclovir prior to participation in the study. Therefore, the absence of resistant virus in this population is not unexpected.
In conclusion, penciclovir administered either q8h or q12h is a safe and effective treatment for mucocutaneous HSV in these patients. In addition, penciclovir q12h offers a reduced frequency of dosing compared with current recommendations for acyclovir in this indication.
ACKNOWLEDGMENTS
This study was funded, in part, by a grant from SmithKline Beecham Pharmaceuticals.
We thank Robert Sarisky and Jeffry Leary for providing virus resistance data.
Appendix
The Penciclovir Immunocompromised Study Group comprises E. Anaissie, Houston, Tex.; C. Andre, Liège, Belgium; F. Andrien, Liège, Belgium; E. Archimbaut, Lyon, France; M. Baccarani, Udine, Italy; S. Ballester, Tampa, Fla.; C. Bernasconi, Pavia, Italy; W. Blau, Idar-Oberstein, Germany; J. Blumer, Cleveland, Ohio; D. Bodensteiner, Kansas City, Kans.; R. Boon, Harlow, United Kingdom; J. Bourhis, Villejuif, France; Y. Bousquet, Paris, France; P. Bramlett, Kansas City, Kans.; K. Briscoe, Fountain Valley, Calif.; J. Cahn, Besançon, France; C. Chabas, Besançon, France; P. Chervenick, Tampa, Fla.; C. DeCervans, Nantes, France; E. Deconinck, Besançon, France; R. DeConti, Tampa, Fla.; J. Desens, Paris, France; W. Dinwoodie, Tampa, Fla.; G. Doolittle, Kansas City, Kans.; J. Dutcher, Bronx, N.Y.; A. Einstein, Tampa, Fla.; A. Einzig, Bronx, N.Y.; G. Elfenbein, Tampa, Fla., C. Fabian, Kansas City, Kans.; A. Fauser, Idar-Oberstein, Germany; K. Fields, Tampa, Fla.; T. File, Jr., Akron, Ohio; M. Gobbi, Genova, Italy; S. Goldstein, Tampa, Fla.; J. Greene, Tampa, Fla.; E. Greenwald, Bronx, N.Y.; S. Grehn, Berlin, Germany; J. Grote-Kiehn, Duisburg, Germany; R. Gucalp, Bronx, N.Y.; J. Harrousseau, Nantes, France; J. Hiemenz, Tampa, Fla.; S. Hiemenz, Tampa, Fla.; M. Hoffmann, Augsburg, Germany; J. Horton, Tampa, Fla.; A. Indorf, Akron, Ohio; P. Jessamine, Ottawa, Ontario, Canada; H. Jhangiani, Fountain Valley, Calif.; G. Justice, Fountain Valley, Calif.; W. Kaiser, Essen, Germany; J. Koenig, Akron, Ohio; J. Lacha, Prague, Czech Republic; A. Leaf, Bronx, N.Y.; A. Lin, Regina, Saskatchewan, Canada; H. Link, Hannover, Germany; P. Ljungman, Huddinge, Sweden; G. Lyman, Tampa, Fla.; S. Lynch, Harlow, United Kingdom; R. MacDonald, Harlow, United Kingdom; J. Magnette, Besançon, France; M. Magnette, Besançon, France; U. Malik, Bronx, N.Y.; R. McKittrick, Kansas City, Kans.; J. Mendelson, Montreal, Quebec, Canada; N. Milpied, Nantes, France; W. Moriconi, St. Louis, Mo.; R. Navari, Birmingham, Ala.; F. Nobile, Reggio Calabria, Italy; M. Nowrousian, Essen, Germany; F. Oberling, Strasbourg, France; C. Pailler, Villejuif, France; G. Perrine, Birmingham, Ala.; I. Pierri, Genova, Italy; C. Pipan, Udine, Italy; A. Prahst, Hannover, Germany; S. Rai, Harlow, United Kingdom; M. Reed, Cleveland, Ohio; S. Renger, Hannover, Germany; F. Rodeghiero, Vicenza, Italy; C. Ross, Akron, Ohio; J. Ruckdeschel, Tampa, Fla.; H. Saba, Tampa, Fla.; R. Sackstein, Tampa, Fla.; R. Sadasivan, Kansas City, Kans.; R. Saltzman, Collegeville, Pa.; D. Schapira, Tampa, Fla.; G. Schiller, Los Angeles, Calif.; G. Schlimok, Augsburg, Germany; S. Schwartz, Berlin, Germany; A. Serr, Idar-Oberstein, Germany; S. Shafran, Edmonton, Alberta, Canada; D. Signs, Akron, Ohio; J. Sparano, Bronx, N.Y.; T. Stein, Kansas City, Kans.; R. Stephens, Kansas City, Kans.; L. Sutton, Paris, France; J. Tan, Akron, Ohio; S. Taylor, Kansas City, Kans.; E. Thiel, Berlin, Germany; B. Toth, Houston, Tex.; M. Trneny, Prague, Czech Republic; R. Trochelman; Akron, Ohio; B. Tucker, Birmingham, Ala.; A. Uden, Stockholm, Sweden; S. Vartivarian, Houston, Tex.; U. Venkatraj, Bronx, N.Y.; J. Vorlicek, Brno, Czech Republic; S. Wadler, Bronx, N.Y.; M. Wegner, Augsburg, Germany; M. Westerhausen, Duisburg, Germany; P. Wijermans, The Hague, The Netherlands; C. Williams, Tampa, Fla.; S. Williamson, Kansas City, Kans.; P. Zorsky, Tampa, Fla.; and K. Zuckerman, Tampa, Fla.
REFERENCES
- 1.Boyd M R, Bacon T H, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987;31:1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd M R, Safrin S, Kern E R. Penciclovir: a review of the spectrum of activity, selectivity, and cross resistance pattern. Antivir Chem Chemother. 1993;4(Suppl. 1):3–11. [Google Scholar]
- 3.Collins P, Ellis N M. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J Med Virol. 1993;1993(Suppl. 1):58–66. doi: 10.1002/jmv.1890410512. [DOI] [PubMed] [Google Scholar]
- 4.Cox D R. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 5.Englund J A, Zimmerman M E, Sweirkosz E M, Goodman J L, Scholl D R, Balfour H H. Herpes simplex virus resistant to acyclovir: a study in a tertiary care center. Ann Intern Med. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 5a.Lazarus H, Belanger R, Candoni A, Aoun M, Jurewicz R, Lynch S, Boon R, Marks L the Penciclovir Immunocompromised Study Group. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Efficacy and safety of penciclovir (PCV) for the treatment of HSV infections in immunocompromised (IC) patients, abstr. H-72; p. 226. [Google Scholar]
- 6.Meyers J D, Flournay N, Thomas E D. Infection with herpes simplex virus and cell-mediated immunity after marrow transplant. J Infect Dis. 1980;142:338–346. doi: 10.1093/infdis/142.3.338. [DOI] [PubMed] [Google Scholar]
- 7.Meyers J D, Wade J C, Mitchell C D, Saral R, Lietman P S, Durack D T, Levin M J, Segreti A C, Balfour H H. Multicenter collaborative trial of intravenous acyclovir for the treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982;73:229–235. doi: 10.1016/0002-9343(82)90097-3. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell C D, Bean B, Gentry S R, Groth K E, Boen J R, Balfour H H. Acyclovir therapy for mucocutaneous herpes simplex infections in immunocompromised patients. Lancet. 1981;i:1389–1392. doi: 10.1016/s0140-6736(81)92569-1. [DOI] [PubMed] [Google Scholar]
- 9.Montgomerie J Z, Becroft D M, Croxson M C, Doak P B, North J D K. Herpes simplex virus infection after renal transplantation. Lancet. 1969;ii:867–871. doi: 10.1016/s0140-6736(69)92327-7. [DOI] [PubMed] [Google Scholar]
- 10.Naraqi S, Jackson G G, Jonasson O, Yamashiroya H M. Prospective study of the prevalence, incidence and source of herpesvirus infections in patients with renal allografts. J Infect Dis. 1977;136:531–540. doi: 10.1093/infdis/136.4.531. [DOI] [PubMed] [Google Scholar]
- 11.Nugier F, Colin J N, Aymard M, Langlois M. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J Med Virol. 1992;36:1–12. doi: 10.1002/jmv.1890360102. [DOI] [PubMed] [Google Scholar]
- 12.Pass R F, Whitley R J, Whelchel J D, Diethelm A G, Reynolds D W, Alford C A. Identification of patients with increased risk of infection with herpes simplex virus after renal transplantation. J Infect Dis. 1979;140:487–492. doi: 10.1093/infdis/140.4.487. [DOI] [PubMed] [Google Scholar]
- 13.Pottage J C, Kessler A., II Herpes simplex virus resistance to acyclovir: clinical relevance. Infect Agents Dis. 1995;4:115–124. [PubMed] [Google Scholar]
- 14.Sacks S L, Aoke F Y, Diaz-Mitoma F, Sellors J, Shafran S. Patient-initiated, twice-daily oral famciclovir for early recurrent genital herpes: a randomized, double-blind multicenter trial. JAMA. 1996;276:44–49. [PubMed] [Google Scholar]
- 15.Spruance S L, Overall J C, Kern E R, Krueger G G, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis; implications for antiviral therapy. N Engl J Med. 1977;297:69–75. doi: 10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]
- 16.Spruance S L, Rea T L, Thoming C, Tucker R, Saltzman R, Boon R. Penciclovir cream for the treatment of herpes simplex labialis. JAMA. 1997;277:1374–1379. [PubMed] [Google Scholar]
- 17.Tyring S, Barbarash R A, Nahlik J E, Cunningham A, Marley J, Heng M, Jones T, Rea T, Boon R, Saltzman R the Collaborative Famciclovir Herpes Zoster Study Group. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and post herpetic neuralgia. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Vartivarian S, Toth B, Anaissie E J, Eron L. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Intravenous penciclovir for the treatment of mucocutaneous herpes simplex infection in immunocompromised patients, abstr. H109; p. 199. [Google Scholar]
- 19.Vere Hodge R A. Famciclovir and penciclovir: the mode of action of famciclovir including its conversion to penciclovir. Antivir Chem Chemother. 1993;4:67–84. [Google Scholar]
- 20.Wade J C, Newton B, McLaren C, Flournoy N, Keeney R E, Meyers J D. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double-blind trial. Ann Intern Med. 1982;96:265–269. doi: 10.7326/0003-4819-96-3-265. [DOI] [PubMed] [Google Scholar]