Abstract
Given the progressive nature of type 2 diabetes (T2D), most individuals with the disease will ultimately undergo treatment intensification. This usually involves the stepwise addition of a new glucose-lowering agent or switching to a more complex insulin regimen. However, complex treatment regimens can result in an increased risk of hypoglycaemia and high treatment burden, which may impact negatively on both therapeutic adherence and overall quality of life. Individuals with good glycaemic control may also be overtreated with unnecessarily complex regimens. Treatment simplification aims to reduce individual treatment burden, without compromising therapeutic effectiveness or safety. Despite data showing that simplifying therapy can achieve good glycaemic control without negatively impacting on treatment efficacy or safety, it is not always implemented in clinical practice. Current clinical guidelines focus on treatment intensification, rather than simplification. Where simplification is recommended, clear guidance is lacking and mostly focused on treatment of the elderly. An expert, multidisciplinary panel evaluated the current treatment landscape with respect to guidance, published evidence, recommendations and approaches regarding simplification of complex insulin regimens. This article outlines the benefits of treatment simplification and provides practical recommendations on simplifying complex insulin treatment strategies in people with T2D using illustrative cases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01222-2.
Keywords: Antidiabetic drug, Glycaemic control, Insulin therapy, Primary care, Type 2 diabetes
Key Summary Points
| Most people with type 2 diabetes (T2D) will eventually undergo treatment intensification, often resulting in a more complex regimen that can negatively impact on both quality of life (QoL) and adherence. |
| Simplifying T2D therapy strategies, when suitable and without compromising treatment efficacy and safety, offers the opportunity to ease both disease and treatment burden, but is not always implemented in clinical practice. |
| Current treatment guidelines have a greater focus on intensification rather than on simplification, are mostly focused on the elderly, and lack clear guidance and examples on how simplification can be achieved. |
| Triggers for simplification should include a broad range of people, rather than just the elderly or the frail. |
| Where possible, simplification should be considered and regularly re-assessed for each individual with T2D receiving a complex insulin therapy regimen, with the aim of improving clinical outcomes, such as hypoglycaemic risk and QoL. |
Introduction
Given the chronic and progressive nature of type 2 diabetes (T2D), most people with T2D undergo treatment intensification with the aim of ensuring adequate glycaemic control and preventing or delaying long-term complications [1]. As per American Diabetes Association (ADA) guidelines, lifestyle modifications should be implemented first, followed by oral antidiabetic drugs (OADs) [2]. Insulin therapies are often added in a stepwise manner when glycaemic control has failed with other agents, starting with basal insulin in combination with OADs, with or without a glucagon-like peptide 1 receptor agonist (GLP-1 RA) [2]. If glycaemic targets are still not achieved, treatment is further intensified through the use of a premixed insulin injection, fixed-ratio combinations (FRCs) of basal insulin and a GLP-1 RA or a basal-prandial or basal-bolus insulin regimen [2–4]. In some countries, the last of these is often considered the final treatment option [5]. In this article, the use of ‘fixed-ratio combination’ or ‘FRC’ refers only to a combined formulation of basal insulin and a GLP-1 RA.
T2D is highly heterogenous in nature, with a number of subgroups with predominance of insulin deficiency having been identified, accounting for 20–25% of newly diagnosed cases in adults [6]. This highlights that, alongside forming a part of multimodal glucose-lowering treatment regimens, insulin continues to be relevant as a primary therapy [7]. Measuring insulin secretory capacity also represents a potential tool for tailoring treatment strategies, particularly in older individuals (65 years of age or more) [8, 9].
Achieving and maintaining glycaemic targets is associated with a reduction in the risk of developing long-term micro- and macrovascular complications of T2D [10, 11]. However, many individuals fail to achieve adequate lowering of glycated haemoglobin (HbA1c), even when using complex insulin regimens [11–14]. Real-world data show that approximately 25% of people do not achieve their target glycaemic goals when treated with basal-bolus insulin [15].
Challenges with Complex Insulin Regimens
Insulin therapy may involve multiple daily injections (MDI) and premixed insulin strategies. These can represent complex treatment plans and be challenging for some individuals to implement, particularly older people, the frail or those who struggle with self-management [16]. Frequent blood glucose monitoring, adapting multiple insulin doses to food intake and other daily activities can all contribute to regimen complexity. The increased treatment burden of such regimens negatively impacts on treatment adherence and overall quality of life (QoL) [17, 18]. Both an increase in healthcare costs and poor glycaemic control are associated with low persistence to MDI, suggesting that increased adherence can lead to improved outcomes [5, 19]. Moreover, low adherence can be secondary to a fear of hypoglycaemia [20]. Higher numbers of hypoglycaemic episodes, in any form, have been reported in individuals with T2D receiving complex regimens involving premix or prandial insulin, compared to those receiving a simpler basal insulin strategy [21]. In addition, those experiencing hypoglycaemia in the first 6 months of basal insulin initiation are at greater risk of hospitalisation and discontinuing insulin therapy compared with individuals who do not have hypoglycaemic events [22].
Much clinical focus is given to treatment intensification in order to provide good glycaemic control and limit hyperglycaemic-related complications. As a result of the progressive nature of T2D, the risk of long-term micro- and macrovascular complications, such as chronic kidney disease (CKD) or cardiovascular disease (CVD), increases with age. However, in older individuals, where the risk of hypoglycaemia and its consequences occurring is high, the costs of aggressive lowering of HbA1c may outweigh the benefits of tight glycaemic control on these complications and can increase the risk of hypoglycaemia, weight gain and treatment burden [23, 24]. Hypoglycaemia is not only associated with difficulties in adherence and reduced QoL but can also incur significant healthcare system expenditure [25–27].
Absence of Guidance on Treatment Simplification in Clinical Guidelines
Guidelines recommend achieving individual glycaemic goals through personalising care, using shared decision-making, which should include regular assessment of the person’s individual circumstances [3, 28]. There is a need to find a balance between the relative risks of relaxing glucose targets by simplifying glucose-lowering therapy and keeping a complex regimen which could increase treatment burden [29]. This is particularly relevant when considering age-related comorbidities, or those who struggle with adherence. It is also crucial that healthcare practitioners (HCPs) engage in open discussions with individuals when evaluating this personalised care, to ensure their preferences are respected and to increase the likelihood of treatment being adequately incorporated into the person’s everyday life [30]. Indeed, diabetes treatment should not only fit the point-of-care but also the individual’s current personal situation [31].
The aim of this article is to provide HCPs with practical recommendations on simplifying complex insulin treatment regimens in people with T2D, based on the conclusions from a consensus group meeting and to highlight some of the issues with illustrative cases.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Multidisciplinary Consensus Method for Developing Recommendations
An expert multidisciplinary group convened to identify and address the unmet need for simplifying T2D treatment. Using a similar approach to that employed by the American College of Cardiology [32], we conducted a review of the published literature on complex insulin treatment simplification. Practical recommendations to simplify unnecessarily complex insulin regimens were also considered, with the aim of improving clinical outcomes for people with T2D. These outcomes include achieving individualised glycaemic goals, a reduced risk/frequency of hypoglycaemia, weight gain prevention and an improvement in QoL (Table 1). The experts took part in a 2-day consensus group discussion, in which the terminology used around simplification, the current landscape and existing literature on simplification, personalisation of T2D care and psychological aspects were evaluated.
Table 1.
Relevant outcome measures of antidiabetic therapies
| Measure | Importance of improving outcome measure |
|---|---|
| HbA1c | A key indicator of both long-term glycaemic control [68] and a useful predictor of lipid profile [69]. Decreasing HbA1c is associated with both a reduction in diabetes-related death and all-cause mortality [70] |
| Frequency of hypoglycaemic events | Hypoglycaemia can cause dysrhythmia, unstable haemodynamics and an increased number of CV events [71] |
| Weight/BMI | Weight gain prevention is associated with a significant decrease in HbA1c and an improvement in CV risk factors [72] |
| Psychological well-being and overall QoL | Improvement is associated with good self-care management and increased treatment adherence [73, 74] |
| Micro- and macrovascular complications | Reducing the likelihood of such complications prevents development of other serious disease, such as sight loss and heart failure [75] |
BMI body mass index, CV cardiovascular, QoL quality of life
A pre-meeting survey was also shared with all experts to understand their views on treatment simplification and to aid discussions during the meeting. The consensus group were in agreement that basal-bolus insulin therapy and premixed insulins (administered more than once-daily) were the treatments most commonly considered as complex insulin regimens. When using long-acting basal insulins, the experts agreed with the recommendation in clinical guidelines that a second-generation analogue (e.g. insulin glargine 300 U/mL and insulin degludec 100 or 200 U/mL) is preferred owing to an improved safety profile compared to first-generation analogues (e.g. insulin glargine 100 U/mL and insulin detemir), when used in combination with oral agents [2]. They were also aligned with the ADA guidance to initiate early insulin therapy in those individuals with HbA1c > 10% who experience weight loss, or present with severe hyperglycaemia, and that treatment can be simplified and/or changed to using an oral agent once glucose toxicity is resolved [2].
Terminology
Confusingly, a number of terms are used interchangeably in the published literature with respect to reducing treatment complexity [33]. It is important to differentiate between these terms in order to aid HCP understanding and to enable clear peer-to-peer communication and conversations with the person receiving treatment. The group considered common terminology used in the literature to define simplification (Table 2). It was agreed that simplification can be viewed as an attempt to decrease the complexity and thereby the burden of treatment for the individual.
Table 2.
Common terminology used in the context of reducing type 2 diabetes treatment complexity and burden
| Term | Definition |
|---|---|
| Treatment complexity | The level of complexity determined by the number of medications prescribed, administration route and the frequency of dosing and glucose monitoring [76]. Adapting drug doses to food intake and other daily activities are also considered |
| Burden of treatment | The workload of a treatment strategy and its impact on an individual’s function and well-being [77] |
| Simplification | An attempt to decrease treatment complexity and burden of treatment, particularly insulin therapy |
| De-escalation | Changing from more intensive to less intensive insulin regimens |
| Deprescribing | Reducing medication without compromising safety |
| Deintensification |
Medication is simplified, reduced or completely withdrawn in an effort to prevent the risk of polypharmacy and its associated adverse events [37] or As complete withdrawal, discontinuation, reducing dosage, conversion, or substitution of at least one medication [35] |
| Liberalisation | Relaxing of glycaemic goals for people who are unlikely to benefit from their current glycaemic targets [33] |
Adapted from Munshi and Neumiller [33]
Recommendation: Simplification is considered the most appropriate terminology to describe reducing the number of insulin injections (including discontinuation) and adapting the treatment strategy to take into account each person’s circumstances. HCPs should make efforts to use this terminology consistently in conversations with individuals with T2D and/or their carers, as well as other HCPs, to avoid confusion.
Current Evidence and Treatment Landscape
A literature review was undertaken to analyse the treatment landscape with respect to articles addressing simplification of complex insulin regimens. The results suggested that the need to simplify treatment is mostly recognised in older individuals and those with comorbidities [24, 34–39]. Data from older populations suggest simplification of treatment can be achieved in these people with good glycaemic control. In a real-world, prospective, single-arm clinical trial of older individuals with T2D, treatment simplification reduced the risk of hypoglycaemia and diabetes-related stress, without compromising glycaemic control [36]. However, little guidance and scarce data are available regarding non-elderly groups [24, 34–39].
Evaluation of the literature also highlighted that a substantial number of people with T2D may be subject to overtreatment (i.e. where the same clinical result could be achieved by reducing the number of injections) [23, 29]. A population-based, retrospective cohort study using data from a claims database demonstrated that for frail individuals, or those with multiple comorbidities, over three quarters with HbA1c < 6.5% did not have their therapy simplified (defined as discontinuation of at least one glucose-lowering medication) [38]. In addition, data highlight that older individuals managed with intensive glycaemic control are more likely to experience hypoglycaemic events [40, 41].
A number of advantages of simplification were also identified. A systematic review conducted by Seidu et al. [35] demonstrated that the benefits of deintensification (defined as ‘complete withdrawal, discontinuation, reducing dosage, conversion, or substitution of at least one medication’) outweighed the harm in older people with T2D, with or without comorbidities. The authors also showed that, following deintensification, there were no significant differences observed between comparison groups in the majority of studies, with regard to adverse events, mortality or HbA1c.
Overtreatment can occur following implementation of complex regimens in response to a transient or acute illness resulting in hospitalisation. This is particularly relevant for those with T2D treated for COVID-19, who may have had their treatment intensified to prevent hyperglycaemia. Treatment should be re-evaluated at discharge and simplified, if appropriate. Other individuals may have a long history of T2D and still be following a historical treatment strategy which was created when fewer therapeutic options were available. These people may benefit from simplification of insulin regimens and a decrease in diabetes-related impairment in QoL, stress and complications arising from hypoglycaemia [36].
Current treatment guidelines have a greater focus on intensification, rather than on simplification [42, 43]. Where guidelines do refer to simplification, guidance is often vague or unclear, and the detrimental effects of hypoglycaemia resulting from overtreatment are rarely discussed.
When individuals require an injectable therapy, ADA guidelines recommend the use of an FRC of basal insulin and a GLP-1 RA, in addition to OADs. A second-generation insulin analogue is preferred because of an improved safety profile compared to first-generation analogues. FRCs offer the opportunity for simplifying treatment, since their application requires fewer injections compared with basal-prandial or basal-bolus insulin regimens. Two options for basal insulin/GLP-1 RA FRCs are available in Europe and the USA: iGlarLixi (insulin glargine 100 U/mL plus lixisenatide) [44] and iDegLira (insulin degludec 100 U/mL plus liraglutide) [45].
Similarly, premixed insulins can provide an avenue for simplification. These fixed preparations of short-acting insulins with intermediate- or long-acting insulins offer a comparable safety and efficacy profile to that of basal-bolus regimens, but with fewer injections and therefore reduced complexity [46, 47]. However, a high proportion of people with suboptimal controlled T2D display low rates of achieving acceptable glycaemic control whilst receiving these insulin preparations [46, 47]. Premixed insulins also carry an increased risk of weight gain and hypoglycaemia compared with other strategies, such as an FRC [48], and individuals often do not experience any additional clinical benefit when switched to premixed preparations from other insulin regimens [49–51].
Simplification can also be achieved by use of OADs, as shown in the BEYOND trial [52]. The trial evaluated the feasibility of switching individuals with inadequate glycaemic control from a basal-bolus regimen to an FRC of basal insulin with a GLP-1 RA, or a sodium-glucose co-transporter 2 inhibitor (SGLT2i). Similar improvements in HbA1c were reported in both those who were switched to an FRC or to basal insulin with an SGLT2i compared to those managed with a basal-bolus regimen, but with the added benefit of fewer daily injections and significantly less hypoglycaemia and no weight gain.
Recommendation: Current clinical guidelines should be developed further to provide clear and specific guidance on simplifying treatment. They should aid in identifying clinical situations where simplification would be appropriate, and if possible, how it can be achieved.
Personalisation of Care and Psychosocial Aspects Are the Key Drivers
Individualised treatment targets are often not met in a large proportion of people with T2D, despite the emphasis in clinical guidelines on the importance of personalisation of care [53]. When creating management plans, guidelines recommend actively involving the individual with the use of shared decision-making and highlight the need for regular assessment of the individual’s personal circumstances and their adherence to treatment [3, 54]. Personalised care should consider the impact on the individual’s QoL, which is frequently compromised by complex treatment regimens, especially when insulin regimens with MDI or premixed formulations are introduced. It is also important to consider the psychological impact of complex therapy. Individuals can experience diabetes distress as a consequence of their fear of hypoglycaemia and other complications or the potential negative impact of therapy on work or relationships, and anxiety around daily activities being restricted by their treatment regimen [36, 55]. However, current guidelines do not offer explicit guidance or tools on how to give individuals a better understanding of their condition and its management [56]. Subsequently, HCPs often fail to address individual preferences because of a lack of time and resources (tools to aid shared decision-making, etc.) and uncertainties with respect to the impact of agreed treatment decisions.
HCPs need to engage in open discussions with individuals when personalising care, taking into account the person’s cognitive capabilities and emotional well-being as well as the individual’s treatment goals [30]. It is also important that caregivers are consulted where the individual receiving treatment is unable to be involved in the shared decision-making process, as a result of impaired cognitive function or inability to care for themselves. Decision aids, available in paper or digital forms, empower the individual with T2D to make informed treatment choices, thereby assisting the decision-making process [57, 58]. Patient-reported outcome measures (PROMs) are another useful tool in this regard, allowing insights into a person’s experience of treatment and can be used to promote discussions about potential therapeutic options [56].
Recommendation: HCPs should consider simplification as a means of personalising therapeutic choices using a shared decision-making approach, decision aids and open communication with individuals and/or caregivers. Cognitive capabilities and emotional well-being, alongside overall QoL, treatment burden and satisfaction with the current treatment plan should also be evaluated. PROMs may also provide a useful tool for considering a person’s experience of T2D therapy and estimating treatment burden. Once treatment has commenced, re-assessment and communication should be conducted on an ongoing basis. These evaluations and discussions should be individualised and occur no less than twice a year. The individual with T2D and their caregivers should be involved in all decision-making and setting of therapeutic goals.
Candidates for Treatment Simplification
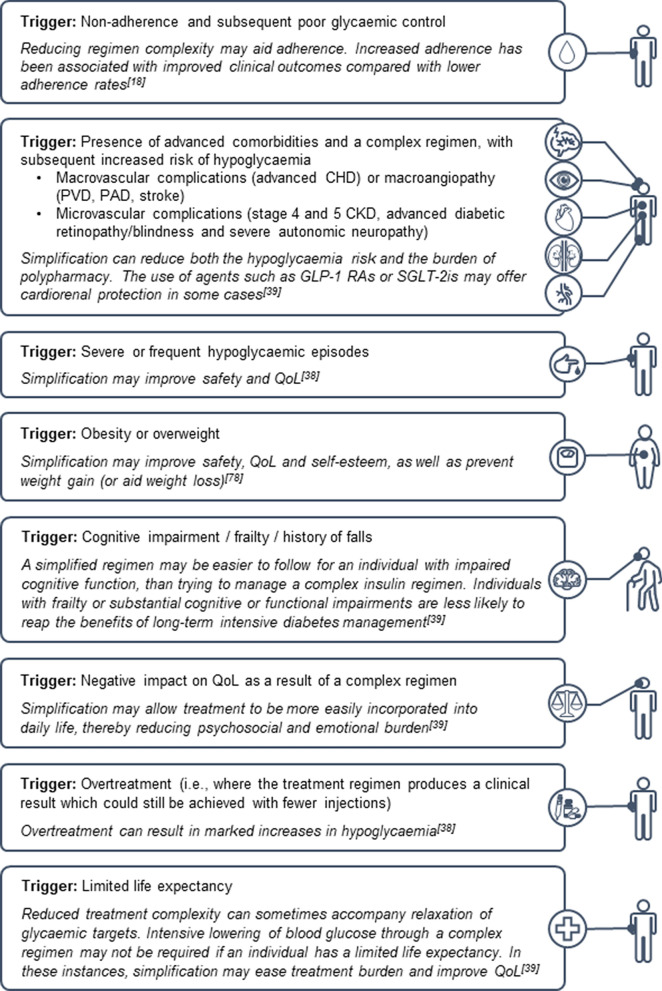
The recommendations presented in this manuscript are supported by the personal clinical experiences of the expert panel members. Where it is possible to improve health outcomes or QoL, treatment simplification should be considered for all people with T2D. A broader range of individuals should be assessed for treatment simplification, rather than just the frail or older individuals. Those already receiving multiple medication regimens for comorbidities such as CVD and CKD should be considered, in an effort to reduce their polypharmacy. People experiencing psychosocial and emotional distress related to T2D, obesity and individuals with impaired or diminished cognitive function can also benefit from simplification. Those who consistently do not achieve their target HbA1c targets should also have their treatment plans assessed for simplification, in order to ensure they are receiving the most appropriate and adequate treatment. A list of triggers for considering simplification are presented in Fig. 1.
Fig. 1.
Triggers for considering simplification. CHD coronary heart disease, CKD chronic kidney disease, GLP-1 RA glucagon-like peptide 1 receptor agonist, PAD peripheral arterial disease, PVD peripheral vascular disease, QoL quality of life, SGLT-2i sodium-glucose co-transporter 2 inhibitor
Examples of individuals for whom treatment simplification may be appropriate and how this could be applied to their regimens are provided in Supplementary Fig. 1. It is important to note that the potential treatment options for each example are not exclusive to those suggested in this publication. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Illustrative Profile 1
This 71-year-old man, with a 9-year history of T2D, also has arterial hypertension and hypercholesterolaemia. He was initially prescribed OADs, before being switched to basal insulin because of inadequate diabetes control and raised HbA1c. Treatment was further intensified 3 years later to twice daily premixed insulin. He frequently experiences hypoglycaemic episodes and is struggling to manage his disease and the number of medications he is on. Simplification of his current treatment plan from premixed insulin to an FRC would reduce the number of daily injections and the likelihood of hypoglycaemia, thereby improving overall QoL.
Illustrative Profile 2
This 56-year-old man was diagnosed with T2D 14 years ago. His initial OAD treatment was substituted with basal-bolus insulin after 4 years. He is overweight, has mild cognitive impairment and was diagnosed with depression and generalised anxiety disorder 7 years ago. He finds it difficult to adhere to his recommended diet and cannot undertake exercise because of mobility issues. The individual struggles with managing the disease and experiences low self-esteem due to weight gain. He does not have any immediate family for support. Changing to an FRC, or a free combination of basal insulin and a GLP-1 RA, may prevent further weight gain and potentially aid weight loss in addition to improving the individual’s emotional well-being and QoL.
Illustrative Profile 3
Besides being diagnosed with T2D 10 years ago, this 83-year-old woman also has micro- and macrovascular complications, a history of falls, limited mobility and metastasised melanoma. A nurse visits her daily to assist with her care. She has irregular food intake, putting her at risk of hypoglycaemia. Her current therapy regimen involves multiple premixed insulin injections. Simplification could be achieved through modification to a single dose of basal insulin. Furthermore, her advanced comorbidities mean she is less likely to reap the benefits of long-term intensive diabetes treatment and a reduction in regimen complexity would improve her overall QoL.
Illustrative Profile 4
Diagnosed with diabetes 13 years ago, this 63-year-old female supermarket worker was prescribed OADs for 6 years, before treatment was intensified during an acute viral infection to include basal-bolus insulin. Given her hectic work schedule, she cannot always administer her injections conveniently during her working hours. Switching from basal-bolus insulin to OADs with a basal analogue would simplify her therapy and make it easier to incorporate into her daily routine. This could also be achieved by replacing basal-bolus insulin with a GLP-1 RA and basal insulin, as an FRC or a free combination. Regular assessment would be needed to monitor that her glycaemic control is maintained as she continues long-term therapy.
Recommendation: Where possible, treatment simplification should be considered for all individuals with T2D receiving a complex insulin therapy regimen. Triggers for considering simplification should include people who are experiencing poor glycaemic control, difficulties with adherence and self-management, severe or frequent hypoglycaemic episodes or substantial weight gain. Individuals with comorbidities, those with cognitive impairments, the frail, those with limited life expectancies, those with a history of falls and instances where current treatment plans negatively impact on QoL should also be considered for simplification.
Practical Recommendations for Simplification of Complex Insulin Regimens
A key consideration when simplifying regimens is safely balancing the risk of developing long-term complications, symptom burden or severe hyperglycaemia with overburdening the individual, QoL and convenience with respect to incorporating the treatment plan into their daily routine.
Although current ADA guidelines advise a stepwise approach when assessing treatment plans [2], most candidates for treatment simplification are still likely to require an injectable therapy. Clinical guidelines recommend a GLP-1 RA or SGLT2i for people with T2D at high cardiovascular (CV) risk and should be considered as an integral part of most simplified treatment models or added to the model if not used earlier [2, 59].
GLP-1 RAs hold some advantages over insulin, such as reduced likelihood of major CV events in individuals considered at high CV risk [43]. They also offer effective initial HbA1c reduction, without increased risk of hypoglycaemia or weight gain [43]. Importantly for simplification, they can provide equal or greater reductions in HbA1c compared with basal insulin, but with fewer injections [60]. Alongside injectable forms, an oral GLP-1 RA is now also commercially available [61]. It should be noted, however, that GLP-1 RAs are associated with gastrointestinal-related adverse effects and appetite loss, which presents challenges for frail individuals with T2D, in whom weight loss may be detrimental.
For those in whom a GLP-1 RA or basal insulin alone is insufficient to reach the treatment goal, guidelines recommend the use of FRCs [3]. FRCs are considered to have a better safety profile, with a lower risk of hypoglycaemia and weight gain when compared with basal-bolus insulin [62]. They also involve a single daily injection, contributing to regimen simplification and a reduction of treatment burden [51, 63]. Improved efficacy and safety of FRCs compared to basal-bolus insulin have been reported [64], suggesting an FRC is an effective substitute for basal-bolus regimens. A recent post hoc analysis used propensity score matching to compare outcomes for individuals with uncontrolled T2D who were treated with an FRC versus basal-bolus. Those receiving an FRC reported greater decreases in HbA1c and weight, and lower rates of hypoglycaemic events compared to the basal-bolus group [62]. The addition of OADs such as SGLT2i, dipeptidyl peptidase 4 inhibitors and in some circumstances sulfonylureas (despite the risk of hypoglycaemia) can be considered in the treatment model. These, together with a GLP-1 RA and/or basal insulin (either as an FRC or free combination), may help to reduce the number of insulin injections and/or dose.
Recommendation: In line with ADA recommendations, treatment modifications should be conducted in a stepwise manner. GLP-1 RAs should be considered an integral part of simplified treatment models. If OADs, a GLP-1 RA or basal insulin alone have been insufficient in providing adequate glycaemic control, a GLP-1 RA and basal insulin combination should be administered in an FRC or as a free combination [3].
Clinical Situations Where Simplification May Be Difficult to Implement
Simplifying regimens may be difficult in some circumstances. OADs and non-insulin-based injectable therapies can be contraindicated in certain people, or an individual may be intolerant to them. System-related barriers could also impact on the feasibility of simplification. For example, the treatments which allow regimens to be simplified may be unaffordable for the individual with T2D. Similarly, reimbursement may not be available (or only partially available).
Sometimes simplification accompanies the relaxation of glycaemic control targets. This may not be suitable in instances where the person is at higher risk of micro- or macrovascular complications. This may also be the case for hospitalised individuals or in those where it has not been possible to reach glycaemic targets with other glucose-lowering therapies. Additionally, simplifying a regimen is not appropriate for those with severe insulin deficiency.
Recommendation: All individuals should be assessed periodically for suitability for treatment simplification. However, in some instances complex insulin regimens may still be the most appropriate form of treatment. For such individuals, complex treatment plans need to be followed in line with current clinical guidance and supported by appropriate follow-up. These individuals should still be regularly reconsidered for simplification and guidelines should be referred to frequently to determine if simplification has become possible for an individual. Additionally, educational support programmes and telehealth services can be utilised to ease the burden of complicated therapy strategies by providing information about self-management.
Discussion
Several areas for simplification were identified through the literature review and group discussions. Firstly, there is often confusion surrounding the terminology used for regimen simplification. Analysis of the current literature also demonstrates that clear, explicit guidance around simplification is still lacking and mostly focused on treatment of older people. Additionally, it was evident that with the multitude of therapeutic options available, treatment plans, particularly those involving insulin, can be unnecessarily complex and difficult to navigate for both HCPs and individuals with T2D. With respect to reaching glycaemic goals, ADA guidelines highlight the need for personalisation of care using shared decision-making and the importance of optimising QoL [3, 28]. More studies are needed to evaluate the benefits of simplification in clinically appropriate situations.
There is a need to raise awareness around simplification for HCPs, people with T2D and others involved in these dialogues. Additionally, it is important to change the ‘mindset’ of not modifying treatment plans or considering complex insulin treatments as the best, final option.
With this in mind, tools and solutions to help HCPs address the unmet need of simplifying complex regimens are needed. Support can be offered to patients at home via telemedicine and virtual consultations. This is particularly relevant during challenging times, as seen with the COVID-19 pandemic, where face-to-face consultations may be reduced or unavailable. Individuals may feel the need for additional medical and mental health support to help manage psychological distress as a result of the pandemic [65].
Other tools which may aid simplification include continuous glucose monitoring, which could be used to identify those receiving unnecessarily intensive regimens. Such tools also have the advantage of collecting objective and physiological data that complement HCP clinical experience [66]. In addition, they present an opportunity to highlight unnecessarily complex strategies in those who may not recognise signs of a hypoglycaemic episode, such as those with cognitive impairments. For these vulnerable individuals, a personalised system of support should also be available for their caregivers.
The use of digital applications and patient support programmes may provide further means of assistance and empower individuals with T2D to engage in improved self-management [67]. Whenever possible, nurses and general practitioners should offer additional follow-up support as needed where treatment has been simplified or changed. This assistance can be provided through thorough explanation of the regimen change and its benefits. Liaison with diabetes specialists in circumstances where the individual may be at risk of deterioration of glycaemic control would also be helpful. Practical recommendations as identified by the group for simplifying T2D treatment are summarised in Table 3.
Table 3.
Summary of recommendations following the discussions of the consensus group
| Recommendations |
|---|
| Simplification is considered the most appropriate terminology to describe reducing the number of insulin injections (including discontinuation) and individualising treatment. HCPs should make efforts to use this terminology consistently in conversations with individuals with T2D and/or their carers, as well as other HCPs |
| Current clinical guidelines should be developed further to provide clear and specific guidance on simplifying treatment, identifying clinical situations where it would be appropriate and if possible, how it can be achieved |
| HCPs should consider simplification to personalise therapeutic choices, using a shared decision approach, decision aids and open communication with individuals and/or caregivers. Cognitive capabilities, emotional well-being, overall QoL, treatment burden and satisfaction with the current treatment need to be taken into account. PROMs may provide a useful tool for evaluating experience of T2D therapy. Once treatment has commenced, re-assessment and communication should be ongoing, with the individual with T2D and their caregivers involved in all decision-making |
| Where possible, treatment simplification should be considered for all individuals with T2D receiving a complex insulin therapy regimen. Triggers for considering simplification should include a broader range of people, rather than just older people or the frail |
| Treatment modifications should be conducted in a stepwise manner. GLP-1 RAs should be considered as first-line treatment. If OADs, a GLP-1 RA or basal insulin alone have been insufficient for adequate glycaemic control, a GLP-1 RA and basal insulin FRC should be administered [3] |
| In some instances complex regimens may still be the most appropriate form of treatment and should be implemented in line with current clinical guidance, supported by appropriate follow-up. These individuals should be regularly assessed for simplification with frequent referral to guidelines to determine if simplification is possible |
FRC fixed-ratio combination, GLP-1 RA glucagon-like peptide 1 receptor agonist, HCP healthcare practitioner, PROM patient-reported outcome measures, OADs oral antidiabetics, QoL quality of life, T2D type 2 diabetes
Conclusion
Simplifying T2D therapy strategies, when suitable and without compromising treatment efficacy and safety, offers the opportunity to ease disease burden. Simplification should be considered and regularly re-assessed for each individual with T2D, and effective implementation can be achieved with clear guidance, a personalised approach and open communication between HCPs, individuals with T2D and caregivers.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Editorial assistance, organisation of the 2-day consensus group meeting and the journal’s Rapid Service Fee were funded by Sanofi. The authors did not receive payment for their contribution to the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Edward Jude, Maciej Malecki, Ricardo Gomez Huelgas, Martin Prazny, Frank Snoek, Tsvetalina Tankova, Dario Giugliano and Kamlesh Khunti all contributed to the study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by Edward Jude, Maciej Malecki, Ricardo Gomez Huelgas, Martin Prazny, Frank Snoek, Tsvetalina Tankova, Dario Giugliano and Kamlesh Khunti, in addition to commenting on previous versions and approving the final manuscript. Medical writing assistance in the preparation of this article was provided by Katie Musialowski, PhD, Aneela Majid, PhD, and Alex Goonesinghe, PhD, from Lucid Group Communications Ltd, First Floor, Jubilee House, Third Avenue, Globe Park, Marlow, Buckinghamshire, SL7 1EY, UK.
Disclosures
Dario Giugliano has received honoraria for speaking at meetings from Novartis, Sanofi, Eli Lilly and Company, AstraZeneca, and Novo Nordisk. Edward Jude has served on advisory boards and as speaker or received grants from AstraZeneca, Boehringer Ingelheim, Menarini, Novo Nordisk, Roche and Sanofi. Frank Snoek has served on advisory boards and as speaker or received grants from Sanofi, Abbott, Eli Lilly, Novo Nordisk and Roche. Kamlesh Khunti has acted as a consultant, speaker or received grants for investigator-initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and MSD, Boehringer Ingelheim, Bayer, Berlin-Chemie AG/Menarini Group, Janssen, and Napp. Kamlesh Khunti is also supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). Martin Prazny served as a consultant and speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lilly, Sanofi, Medtronic, and Teva. Maciej Malecki has acted as a consultant or speaker for AstraZeneca, Bayer, Novo Nordisk, Sanofi-Aventis, Lilly, Merck, Boehringer Ingelheim, Mundipharma, Abbott, Dexcom, and Medtronic. Maciej Malecki also participates in the National Center for Research and Development CRACoV-HHS project (contract number—SZPITALE-JEDNOIMIENNE/18/2020). Ricardo Gomez Huelgas has served on advisory boards and acted as a consultant or speaker or received grants for AstraZeneca, Boehringer-Lilly, Janssen, MSD, Novo Nordisk, Novartis and Sanofi. Tsvetalina Tankova has served on advisory boards and as a speaker for Sanofi, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novo Nordisk, Novartis, MSD and Servier. The opinions and patient profiles represent the opinions of the experts and not Sanofi.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–S156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association, 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S111. [DOI] [PubMed]
- 3.Davies MJ, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meece J. Basal insulin intensification in patients with type 2 diabetes: a review. Diabetes Ther. 2018;9(3):877–890. doi: 10.1007/s13300-018-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giugliano D, et al. Beyond basal-bolus insulin regimen: is it still the ultimate chance for therapy in diabetes? Diabetes Res Clin Pract. 2019;157:107922. doi: 10.1016/j.diabres.2019.107922. [DOI] [PubMed] [Google Scholar]
- 6.Ahlqvist E, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35(6):1364. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritsche A, et al. Considering insulin secretory capacity as measured by a fasting C-peptide/glucose ratio in selecting glucose-lowering medications. Exp Clin Endocrinol Diabetes. 2020 doi: 10.1055/a-1242-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munshi MN, et al. Use of serum c-peptide level to simplify diabetes treatment regimens in older adults. Am J Med. 2009;122(4):395–397. doi: 10.1016/j.amjmed.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Buse JB, et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali MK, et al. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 12.Khunti K, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409. doi: 10.1111/dom.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipska KJ, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–475. doi: 10.2337/dc16-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jude EB, et al. Effectiveness of premixed insulin to achieve glycaemic control in type 2 diabetes: a retrospective UK cohort study. Diabetes Obes Metab. 2021;23(4):929–37. doi: 10.1111/dom.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantalone KM, et al. The probability of A1C goal attainment in patients with uncontrolled type 2 diabetes in a large integrated delivery system: a prediction model. Diabetes Care. 2020;43(8):1910–9. doi: 10.2337/dc19-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MJ, Fritschi C. Relationships between cognitive impairment and self-management in older adults with type 2 diabetes: an integrative review. Res Gerontol Nurs. 2021;14(2):104–12. doi: 10.3928/19404921-20201117-01. [DOI] [PubMed] [Google Scholar]
- 17.Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):145–155. doi: 10.1007/s12160-013-9585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am Psychol. 2016;71(7):539–551. doi: 10.1037/a0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelman SV, et al. Persistence with basal-bolus insulin therapy in patients with type 2 diabetes mellitus and effect on clinical and economic outcomes: a retrospective claims database study. J Manag Care Spec Pharm. 2019;25(12):1420–1431. doi: 10.18553/jmcp.2019.19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–1307. doi: 10.2147/PPA.S106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman RR, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 22.Dalal MR, Kazemi MR, Ye F. Hypoglycemia in patients with type 2 diabetes newly initiated on basal insulin in the US in a community setting: impact on treatment discontinuation and hospitalization. Curr Med Res Opin. 2017;33(2):209–214. doi: 10.1080/03007995.2016.1248911. [DOI] [PubMed] [Google Scholar]
- 23.Lipska KJ, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taybani Z, et al. Simplifying complex insulin regimens while preserving good glycemic control in type 2 diabetes. Diabetes Ther. 2019;10(5):1869–1878. doi: 10.1007/s13300-019-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen-Bjergaard U, et al. Comparison of the HAT study, the largest global hypoglycaemia study to date, with similar large real-world studies. Diabetes Obes Metab. 2019;21(4):844–853. doi: 10.1111/dom.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khunti K, et al. Impact of hypoglycaemia on patient-reported outcomes from a global, 24-country study of 27,585 people with type 1 and insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2017;130:121–129. doi: 10.1016/j.diabres.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Aronson R, et al. Direct and indirect health economic impact of hypoglycaemia in a global population of patients with insulin-treated diabetes. Diabetes Res Clin Pract. 2018;138:35–43. doi: 10.1016/j.diabres.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association, 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021. 44(Supplement 1):S73. [DOI] [PubMed]
- 29.Khunti K, Davies MJ. Clinical inertia versus overtreatment in glycaemic management. Lancet Diabetes Endocrinol. 2018;6(4):266–268. doi: 10.1016/S2213-8587(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 30.Serrano V, et al. Shared decision-making in the care of individuals with diabetes. Diabet Med. 2016;33(6):742–751. doi: 10.1111/dme.13143. [DOI] [PubMed] [Google Scholar]
- 31.Ruissen MM, et al. Making diabetes care fit—are we making progress? Front Clin Diabetes Healthc. 2021 doi: 10.3389/fcdhc.2021.658817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Januzzi JL, Jr, et al. 2019 Methodology for creating expert consensus decision pathways: a report of the American College of cardiology. J Am Coll Cardiol. 2019;74(8):1138–1150. doi: 10.1016/j.jacc.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Munshi M, Neumiller JJ. Liberalisation, deintensification, and simplification in diabetes management: words matter. Lancet Diabetes Endocrinol. 2020;8(2):95–97. doi: 10.1016/S2213-8587(19)30379-1. [DOI] [PubMed] [Google Scholar]
- 34.Oktora MP, et al. Rates, determinants and success of implementing deprescribing in people with type 2 diabetes: a scoping review. Diabet Med. 2021;38(2):e14408. doi: 10.1111/dme.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidu S, et al. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab. 2019;21(7):1668–1679. doi: 10.1111/dom.13724. [DOI] [PubMed] [Google Scholar]
- 36.Munshi MN, et al. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med. 2016;176(7):1023–1025. doi: 10.1001/jamainternmed.2016.2288. [DOI] [PubMed] [Google Scholar]
- 37.Abdelhafiz AH, Sinclair AJ. Deintensification of hypoglycaemic medications-use of a systematic review approach to highlight safety concerns in older people with type 2 diabetes. J Diabetes Complicat. 2018;32(4):444–450. doi: 10.1016/j.jdiacomp.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 38.McAlister FA, Youngson E, Eurich DT. Treatment deintensification is uncommon in adults with type 2 diabetes mellitus: a retrospective cohort study. Circ Cardiovasc Qual Outcomes. 2017;10(4):e003514. doi: 10.1161/CIRCOUTCOMES.116.003514. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association, 12. Older adults: standards of medical care in diabetes–2020. Diabetes Care. 2020;43(Suppl 1):S152–S162. [DOI] [PubMed]
- 40.Gómez-Huelgas R, et al. Management of type 2 diabetes in very old patients according to glycemic control and health status. Pol Arch Intern Med. 2019;129(7–8):567–570. doi: 10.20452/pamw.14867. [DOI] [PubMed] [Google Scholar]
- 41.Gómez-Huelgas R, et al. Management of elderly patients with type 2 diabetes in long-term care and skilled nursing facilities. Pol Arch Intern Med. 2019;129(2):137–140. doi: 10.20452/pamw.4410. [DOI] [PubMed] [Google Scholar]
- 42.Markovitz AA, et al. An examination of deintensification recommendations in clinical practice guidelines: stepping up or scaling back? JAMA Intern Med. 2018;178(3):414–416. doi: 10.1001/jamainternmed.2017.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Diabetes Association, 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. [DOI] [PubMed]
- 44.Sanofi. Suliqua 100 units/ml + 50 micrograms/ml solution for injection in a pre-filled pen. 2020. https://www.medicines.org.uk/emc/product/9870/smpc#gref. Accessed 16 Feb 2021.
- 45.Novo Nordisk. Xultophy 100 units/ml insulin degludec + 3.6 mg/mL liraglutide solution for injection in a pre-filled pen. 2020. https://www.medicines.org.uk/emc/product/3469. Accessed 16 Feb 2021.
- 46.Kalra S, et al. Expert opinion: patient selection for premixed insulin formulations in diabetes care. Diabetes Ther. 2018;9(6):2185–2199. doi: 10.1007/s13300-018-0521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giugliano D, et al. Intensification of insulin therapy with basal-bolus or premixed insulin regimens in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Endocrine. 2016;51(3):417–428. doi: 10.1007/s12020-015-0718-3. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Peralta F, et al. Titratable fixed-ratio combination of basal insulin plus a glucagon-like peptide-1 receptor agonist: a novel, simplified alternative to premix insulin for type 2 diabetes. Diabetes Obes Metab. 2021;23(7):1445–52. doi: 10.1111/dom.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta R, et al. Practical use of insulin degludec/insulin aspart in a multinational setting: beyond the guidelines. Diabetes Obes Metab. 2020;22(11):1961–1975. doi: 10.1111/dom.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Men P, et al. Comparison of lixisenatide in combination with basal insulin vs other insulin regimens for the treatment of patients with type 2 diabetes inadequately controlled by basal insulin: systematic review, network meta-analysis and cost-effectiveness analysis. Diabetes Obes Metab. 2020;22(1):107–115. doi: 10.1111/dom.13871. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstock J, et al. Advancing therapy in suboptimally controlled basal insulin-treated type 2 diabetes: clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial. Diabetes Care. 2021;44(10):2361–70. doi: 10.2337/dc21-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giugliano D, et al. Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a GLP-1RA or to basal insulin plus an SGLT2 inhibitor: BEYOND, a randomized, pragmatic trial. Diabetes Care. 2021;44(6):1353–1360. doi: 10.2337/dc20-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meneghini LF, et al. The diabetes unmet need with basal insulin evaluation (DUNE) study in type 2 diabetes: achieving HbA1c targets with basal insulin in a real-world setting. Diabetes Obes Metab. 2019;21(6):1429–1436. doi: 10.1111/dom.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Diabetes Association, 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes–2020. Diabetes Care. 2020. 43(Suppl 1):S37–S47. [DOI] [PubMed]
- 55.Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312(7):691–692. doi: 10.1001/jama.2014.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damman OC, et al. The use of PROMs and shared decision-making in medical encounters with patients: an opportunity to deliver value-based health care to patients. J Eval Clin Pract. 2020;26(2):524–540. doi: 10.1111/jep.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wieringa TH, et al. Decision aids that facilitate elements of shared decision making in chronic illnesses: a systematic review. Syst Rev. 2019;8(1):121. doi: 10.1186/s13643-019-1034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karagiannis T, et al. Decision aids for people with type 2 diabetes mellitus: an effectiveness rapid review and meta-analysis. Diabet Med. 2019;36(5):557–568. doi: 10.1111/dme.13939. [DOI] [PubMed] [Google Scholar]
- 59.Cosentino F, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Eur Heart J. 2019;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pratley R, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 62.Tabák ÁG, et al. Efficacy and safety of iGlarLixi, fixed-ratio combination of insulin glargine and lixisenatide, compared with basal-bolus regimen in patients with type 2 diabetes: propensity score matched analysis. Diabetes Ther. 2020;11(1):305–318. doi: 10.1007/s13300-019-00735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran E. Fixed-ratio combinations. Clin Diabetes. 2017;35(4):242–246. doi: 10.2337/cd17-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billings LK, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009–1016. doi: 10.2337/dc17-1114. [DOI] [PubMed] [Google Scholar]
- 65.Alessi J, et al. Mental health in the era of COVID-19: prevalence of psychiatric disorders in a cohort of patients with type 1 and type 2 diabetes during the social distancing. Diabetol Metab Syndr. 2020;12:76. doi: 10.1186/s13098-020-00584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Danne T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giugliano D, et al. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J Endocrinol Invest. 2019;42(5):495–503. doi: 10.1007/s40618-018-0951-8. [DOI] [PubMed] [Google Scholar]
- 68.Kurukulasuriya LR, Sowers JR. Therapies for type 2 diabetes: lowering HbA1c and associated cardiovascular risk factors. Cardiovasc Diabetol. 2010;9:45. doi: 10.1186/1475-2840-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherwani SI, et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stratton IM, et al. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49(8):1761–1769. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- 71.Cheng CN, et al. Clinical outcomes of basal insulin and oral antidiabetic agents as an add-on to dual therapy in patients with type 2 diabetes mellitus. Sci Rep. 2020;10(1):5746. doi: 10.1038/s41598-020-62646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68(6):682–691. doi: 10.1111/ijcp.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massey CN, et al. Psychological well-being and type 2 diabetes. Curr Res Diabetes Obes J. 2017;4(4):555641. doi: 10.19080/crdoj.2017.04.555641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tran BX, et al. Global mapping of interventions to improve quality of life of people with diabetes in 1990–2018. Int J Environ Res Public Health. 2020;17(5):1597. doi: 10.3390/ijerph17051597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nano J, et al. A standard set of person-centred outcomes for diabetes mellitus: results of an international and unified approach. Diabet Med. 2020;37(12):2009–2018. doi: 10.1111/dme.14286. [DOI] [PubMed] [Google Scholar]
- 76.Ayele AA, et al. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an Ethiopian general hospital. BMJ Open Diabetes Res Care. 2019;7(1):e000685. doi: 10.1136/bmjdrc-2019-000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demain S, et al. Living with, managing and minimising treatment burden in long term conditions: a systematic review of qualitative research. PLoS One. 2015;10(5):e0125457. doi: 10.1371/journal.pone.0125457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perreault L, et al. Optimizing fixed-ratio combination therapy in type 2 diabetes. Adv Ther. 2019;36(2):265–277. doi: 10.1007/s12325-018-0868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.