Abstract
Background
Although a full course of coronavirus disease 2019 (COVID-19) vaccine is effective in cancer patients, the duration of the protection and the efficacy of a booster dose against the new variants remain unknown. We prospectively evaluated the immunogenicity of the third dose of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) BNT162b2 messenger RNA vaccine in cancer patients undergoing active treatment.
Patients and methods
Patients with solid cancer, vaccinated with a booster dose during active treatment, were enrolled in this study. Patients were classified into SARS-CoV-2 naïve (without previous COVID-19 infection) and SARS-CoV-2 experienced (with previous COVID-19 infection). Neutralizing antibody (NT Ab) titer and total anti-Spike immunoglobulin G (IgG) concentration were quantified in serum. Heparinized whole blood samples were used for SARS-CoV-2 Interferon Gamma Release Assay (IGRA). The primary endpoint was to assess the increase of IgG antibody level between baseline and 3 weeks after the booster.
Results
One hundred and forty-two consecutive patients were recruited. In SARS-CoV-2-naïve subjects, the median level of IgG was 157 BAU/ml [interquartile range (IQR) 62-423 BAU/ml] at T0 and reached a median of 2080 BAU/ml (IQR 2080-2080 BAU/ml) at 3 weeks after booster administration (T1; P < 0.0001). A median 16-fold increase of SARS-CoV-2 NT Ab titer (IQR 4-32) was observed in naïve subjects (from median 20, IQR 10-40, to median 640, IQR 160-640; P < 0.0001). Median interferon-γ level at T1 was significantly higher than that measured at T0 in SARS-CoV-2-naïve subjects (P = 0.0049) but not in SARS-CoV-2-experienced patients. The median level of SARS-CoV-2 NT Abs was 32-fold lower against Omicron compared to the wild-type strain (P = 0.0004) and 12-fold lower compared to the Delta strain (P = 0.0110).
Conclusions
The third dose is able to trigger both the humoral and the cell-mediated immune response in cancer patients on active treatment. Our preliminary data about the neutralization of the SARS-CoV-2 vaccine against variants of concern seem to confirm the lower vaccine activity.
Key words: BNT162b2 anti-SARS-CoV-2 vaccine, cancer, neutralizing antibody, third dose, VOCs
Highlights
-
•
All subjects but one reached detectable IgG levels 3 weeks after the third dose of the SARS-CoV-2 BNT162b2 messenger RNA vaccine.
-
•
A median 16-fold increase of SARS-CoV-2 NT Ab titer was observed in patients without previous COVID-19.
-
•
The cell-mediated immune response was significantly higher in SARS-CoV-2-naïve subjects after the booster.
-
•
Median level of SARS-CoV-2 NT Abs was 32-fold lower against Omicron compared to the wild-type strain.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic caused over 359 million cases and >5 million deaths worldwide, including 10 212 621 cases and 144 343 deaths in Italy as on 26 January 2022.1 Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, a strong effort has been made for the development of protective vaccines. The BNT162b2 vaccine was the first authorized for active immunization with a 95% protection against SARS-CoV-2 infection in a phase II/III trial.2 The efficacy of this vaccine was demonstrated as early as 11 days after the first dose in 91.7% of cases and it remained 91.3% [95% confidence interval (CI) 89.0% to 93.2%] with a 6-month follow-up.3
Patients with cancer have an increased risk of infection and COVID-19-related morbidity and mortality compared with the non-cancer population.4 A recent meta-analysis has demonstrated that patients with cancer had a suboptimal seroconversion rate after COVID-19 vaccination when compared with non-cancer patients, but a notable increase in humoral response in the group with complete COVID-19 immunization schedule was already observed.5 These findings have been confirmed also by our previously published data. The rate of patients with cancer in immunotherapy with or without chemotherapy, developing positive antibody level measured by S1/S2 assay, was high following two vaccine doses (95%), but only one-third of our patients developed a positive antibody response after the first dose. Furthermore, over 90% of patients developed a sustained Spike-specific T-cell response after the full course of BNT162b2 anti-SARS-CoV-2 vaccine.6
The ongoing discovery of new variants of SARS-CoV-2 has brought major challenges to the effectiveness of vaccines. Studies have shown that the serum neutralizing antibody (NT Ab) titers against the Delta variant after the inoculation with the BNT162b2 SARS-CoV-2 vaccine are 1.41-11.30 times lower than the original strain WT/D614G.7 The SARS-CoV-2 Omicron variant (BA.1/B.1.1.529) has 34 mutations in the Spike protein, which is the main target of NT Abs8 with the consequent potential ability to escape vaccine-induced immunity.
Based on available data, the Centers for Disease Control (CDC) authorized a third dose of COVID-19 vaccine (‘booster’ shots) for immunosuppressed patients.9 On 30 July 2021, the Israeli Ministry of Health approved the use of the booster with BNT162b2 anti-SARS-CoV-2 vaccine, and Arbel and colleagues demonstrated that subjects who had received a booster had 90% lower mortality due to COVID-19 than those who had not received the third dose (95% CI 0.07-0.14; P < 0.001).10
All the above-mentioned data have many practical implications also in patients with cancer on active treatments. While the full course of COVID-19 vaccine is proved to be effective in patients with cancer, the question remains how long the protection lasts, whether it is necessary to give everyone a third dose, how to monitor this population over time and if a booster dose may enhance the breadth of protection against the new variants.
The aim of the present study was to evaluate the immunogenicity and safety of the third dose of the SARS-CoV-2 BNT162b2 messenger RNA (mRNA) vaccine in patients with cancer undergoing active treatment.
Patients and methods
Study design and participants
We conducted a prospective, observational cohort study in order to assess the humoral and cell-mediated response to the third dose of BNT162b2 SARS-CoV-2 vaccine (Pfizer-BioNTech, Germany) in patients with cancer.
Patients undergoing active anticancer treatment (chemotherapy or immunotherapy or a combination of these types of therapies) treated at the Medical Oncology Unit of Fondazione IRCCS Policlinico San Matteo Pavia and AUSL Ospedale Guglielmo Da Saliceto, Piacenza were enrolled. All the patients had received two doses of BNT162b2 SARS-CoV-2 vaccine. A previous infection with SARS-CoV-2 was not an exclusion criterion.
According to our previous paper,11 we have defined ‘SARS-CoV-2 experienced’ as those patients with a documented past positive SARS-CoV-2 RNA in nasopharyngeal swab and/or positive anti-Spike immunoglobulin G (IgG) before the first dose of vaccination; otherwise, they were classified as ‘SARS-CoV-2 naïve’.
The primary endpoint was to assess the increase of IgG antibody level between the baseline and 3 weeks after the third dose of BNT162b2 SARS-CoV-2 vaccine.
The secondary endpoints were:
-
•
the evaluation of the increase of SARS-CoV-2 NT Ab titer between baseline and 3 weeks after the third dose of BNT162b2 SARS-CoV-2 vaccine;
-
•
the evaluation of the increase of Spike-specific T-cell response between baseline and 3 weeks after the third dose of BNT162b2 SARS-CoV-2 vaccine;
-
•
the evaluation of the incidence of adverse reactions to the COVID-19 vaccine, local and systemic, solicited and unsolicited, within the period of 3 weeks after the vaccination.
This study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology Statement for reporting observational studies.12 It was approved by the local ethics committee (Comitato Etico Area Pavia) and institutional review board (P-0103665/21). All the subjects signed a written informed consent before enrollment.
The subjects were monitored before BNT162b2 booster (T0) and after 21 days (T1). Blood samples for humoral and cell-mediated immune response evaluation were obtained at each time point.
Serological assays
Chemiluminescent assay (Liaison SARS-CoV-2 trimeric, Diasorin, Saluggia, Italy) was used for Spike IgG quantification. Results >33.8 BAU/ml were given as positive. SARS-CoV-2 NT Ab titer was determined as previously reported.6 Briefly, 50 μl of serum in serial fourfold dilution was added to two wells of a flat bottom tissue culture microtiter plate (COSTAR, Corning Incorporated, Corning, NY). The same volume of 100 TCID50 of SARS-CoV-2 strain was added and plates were incubated at 33°C in 5% CO2, according to our local protocol.11 After 1-h incubation at 33°C in 5% CO2, VERO E6 cells were added to each well. After 72 h of incubation at 33°C in 5% CO2 under the same conditions, plates were stained with Gram’s crystal violet solution (Merck KGaA, Darmstadt, Germany) plus 5% formaldehyde 40% m/v (Carlo ErbaSpA, Arese, Italy) for 30 min. Microtiter plates were then washed under running water. Wells were scored to evaluate the degree of cytopathic effect (CPE) compared to the virus control. Blue staining of wells indicated the presence of NT Abs. Neutralizing titer was the maximum dilution with the reduction of 90% of CPE. All the experiments were carried out in BSL3 facility. Results ≥1 : 10 serum titer were considered positive, according to our protocol.13
SARS-CoV-2 Interferon Gamma Release Assay (IGRA)
Heparinized whole blood samples were used for SARS-CoV-2 IGRA, according to the manufacturer’s instructions (Euroimmun, Lübeck, Germany). Briefly, 500 μl of sample was added to stimulator tube coated with Spike antigen and to negative and positive control tubes. All the tubes were incubated overnight at 37°C, 5% CO2 and, then, the samples were centrifuged and the plasma supernatant was then collected and stored at −80°C until testing. Interferon-γ (IFN-γ) was detected automatically in the supernatants by an enzyme-linked immunosorbent assay (ELISA, Euroimmun, Lübeck, Germany) using the Euroimmun Analyzer I according to the manufacturer’s instructions. IFN-γ response was defined as Spike-stimulated minus unstimulated. Results >200 IU/ml were given as positive, while results ranging from 100 to 200 IU/ml were defined as borderline. In case of inadequate response to the positive control, the result was given as ‘indeterminate’.
Safety
Adverse events (AEs) were obtained with a questionnaire with specific yes/no questions regarding local reactions (i.e. pain at the injection site) and systemic reactions (i.e. fever >38°C).
Statistical analysis
Data were described with the median and interquartile range (IQR) if continuous and as counts and percentage if categorical. Comparison between two groups was carried out using the Mann–Whitney (unpaired samples) or Wilcoxon (paired samples) test. When the comparison between three or more groups was carried out, the Kruskal–Wallis test with post hoc Dunn’s comparisons was used. Spearman’s test was used for the correlation analysis. Fisher’s exact test was used for comparison of categorical variables. GraphPad Prism 8.3.0 (GraphPad Software, La Jolla, CA) was used for statistical analyses. A two-sided P value <0.05 is considered statistically significant.
Results
Patients’ characteristics
From 17 September to 17 November 2021, 142 consecutive patients with solid cancer, vaccinated with a booster dose during active treatment, were recruited in this study (56 females and 86 males; median age 66 years; range 26-88 years); among them, 11 (7.7%) reported a SARS-CoV-2 infection before the first dose of vaccination (SARS-CoV-2 experienced).
The detailed demographic and clinical characteristics are reported in Table 1.
Table 1.
Demographic and clinical characteristics of enrolled patients
| Patients, n | % | |
|---|---|---|
| Sex | ||
| Female/male | 56/86 | 39/61 |
| Comorbidities | ||
| Cardiovascular | 34 | 24 |
| COPD asthma | 7 | 5 |
| Coronary heart disease | 8 | 6 |
| Diabetes mellitus | 20 | 14 |
| Autoimmune disorders | 6 | 4 |
| HCV | 6 | 4 |
| HBV | 6 | 4 |
| Influenza vaccine | ||
| Yes/no | 89/53 | 63/37 |
| Previous COVID-19 disease | ||
| Yes/no | 9/133 | 6/94 |
| Type of tumor | ||
| Lung cancer | 58 | 41 |
| Breast cancer | 25 | 18 |
| Melanoma | 17 | 12 |
| Kidney cancer | 10 | 7 |
| Head and neck cancer | 6 | 4 |
| Gastrointestinal cancer | 20 | 14 |
| Others | 6 | 4 |
| Type of oncological treatment | ||
| ICIs + chemotherapy | 18 | 12,7 |
| ICIs | 63 | 44,4 |
| Chemotherapy | 61 | 42,9 |
| Number of days between the second dose of vaccine and the booster | ||
| Before 5 months | 45 | 31,7 |
| After 5 months | 97 | 68,3 |
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HBV, hepatitis B virus; HCV, hepatitis C virus; ICIs, immune checkpoint inhibitors.
SARS-CoV-2 humoral response after booster administration
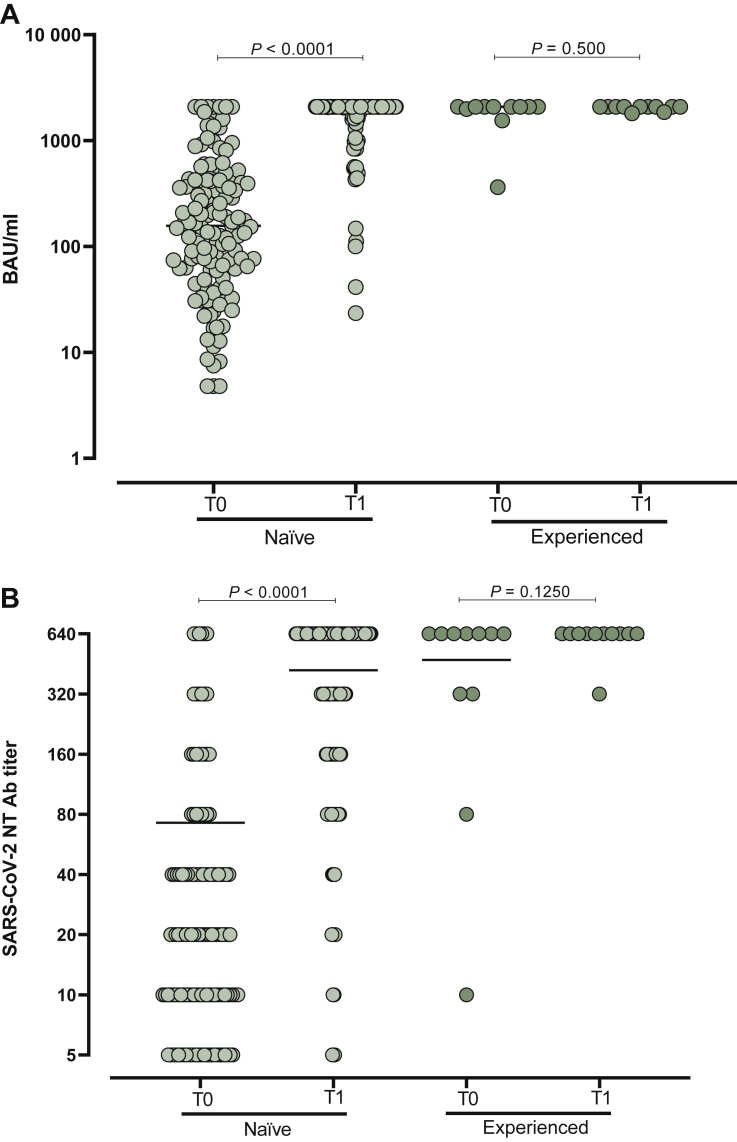
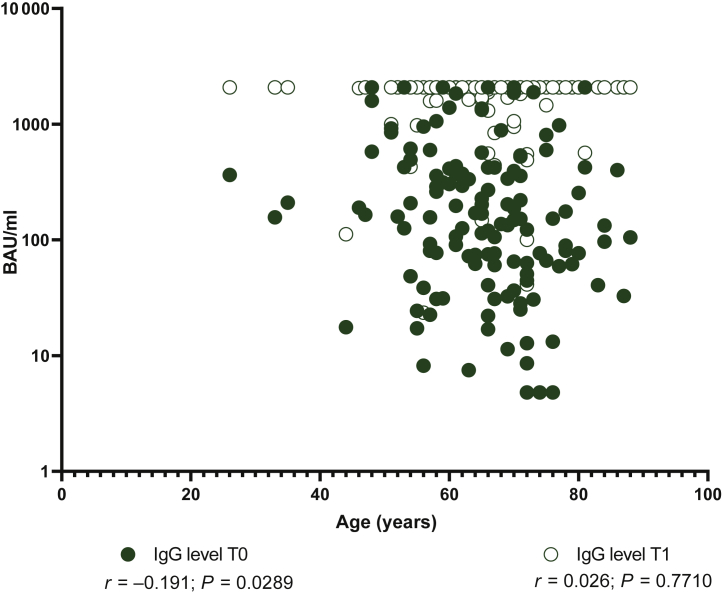
At the time of the booster dose administration, 23/142 (16.2%) subjects showed negative IgG level (<33.8 BAU/ml). At the same time point, 26/142 (18.3%) were negative for SARS-CoV-2 NT Ab titer. All of them were naïve for SARS-CoV-2 infection at the time of the first vaccination. Interestingly, the median age of the non-responder subjects at the time of the booster dose was significantly higher than that observed in patients with positive IgG level and SARS-CoV-2 NT Abs at the time of the booster administration (72, IQR 61.7-74 versus 65, IQR 58-71; P = 0.0359). Overall, in SARS-CoV-2-naïve subjects, the median level of IgG was 157 BAU/ml (IQR 62-423 BAU/ml) at T0 and reached a median of 2080 BAU/ml (IQR 2080-2080 BAU/ml) 3 weeks after the booster administration (T1; P < 0.0001). All the subjects except one reached detectable IgG levels at T1. No further increase was observed in the 11 SARS-CoV-2-experienced subjects (P > 0.999) (Figure 1A). Looking at the SARS-CoV-2 NT Ab titer, a median 16-fold increase (IQR 4-32) was observed in naïve subjects (from median 20, IQR 10-40 to median 640, IQR 160-640; P < 0.0001). No significant increase was observed in SARS-CoV-2-experienced subjects after the booster dose administration (P < 0.999) (Figure 1B). Age inversely correlated with total IgG level at baseline (T0) but not at the time of follow-up (T1) (Figure 2). No difference was observed in terms of median response between patients treated with immunotherapy and chemotherapy (P > 0.05). A stronger correlation between SARS-CoV-2 NT Abs and total IgG level was observed at T0 (r = 0.76; P < 0.0001) compared to T1 (r = 0.27; P = 0.0081). No correlation as regards the number of days was observed from the first to the third vaccination and SARS-CoV-2 NT Abs/total IgG.
Figure 1.
SARS-CoV-2 IgG levels and NT Abs titre.
Total immunoglobulin G (IgG) (A) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibodies (NT Abs) (B) were measured at the time of the booster dose administration (T0) and after 3 weeks (T1) in SARS-CoV-2-naïve solid organ cancer patients and SARS-CoV-2-experienced solid organ cancer patients.
Median levels of response are shown in the graph. P values are given for each comparison.
Figure 2.
Correlation between immunoglobulin G (IgG) level measured at T0 or T1 and age was measured.
P value and r were calculated using Spearman’s test and given in the graph.
T-cell response after booster administration
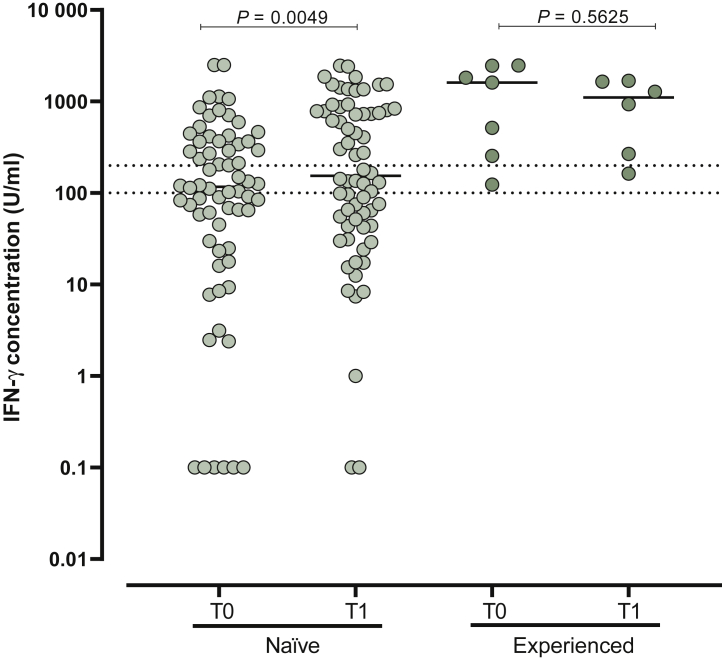
T-cell response elicited by vaccination was measured in 72 paired samples (T0 and T1); 66 subjects were SARS-CoV-2 naïve. Median IFN-γ level at T1 (median 154.1 U/ml, IQR 43.8-787.6 U/ml) was significantly higher than that measured at T0 (median 117.1 U/ml, IQR 28.6-366.4 U/ml) in SARS-CoV-2-naïve subjects (P = 0.0049) but not in SARS-CoV-2-experienced patients (median 1606 U/ml, IQR 255-2457 U/ml versus 1107 U/ml, IQR 240.9-1658 U/ml; P = 0.5625) (Figure 3).
Figure 3.
Spike-specific T-cell response measured as interferon-γ (IFN-γ) concentration was measured at the time of booster dose administration (T0) and 3 weeks after (T1) in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naïve solid organ cancer patients and SARS-CoV-2-experienced solid organ cancer patients.
Median levels of response are shown in the graph. P values are given for each comparison.
Overall, at T1, 41 SARS-CoV-2 were positive for IFN-γ (T-cell responders), 10 were still negative or borderline (T-cell non-responders) and 21 tested indeterminate. Median age of T-cell non-responders was significantly higher than that reported in responder patients (72 years, range 67-76 years versus 61.5 years, range 52.7-70 years; P = 0.0027). Overall, the median IFN-γ level at T1, but not at T0, was significantly higher in immunotherapy only-treated patients than the other patients (median 731.7, IQR 85.7-1389 versus 49, IQR 36.6-724.1; P = 0.0202). No differences were reported when tumor stage and sex were correlated with IFN-γ level. A correlation between SARS-CoV-2 NT Abs and IFN-γ level was observed at T0 (r = 0.53; P < 0.0001) but not at T1 (r = 0.16; P = 0.1489). No correlation as regards the number of days was observed from the first to the third vaccination and IFN-γ level.
SARS-CoV-2 neutralizing response to variants of concern (VOCs)
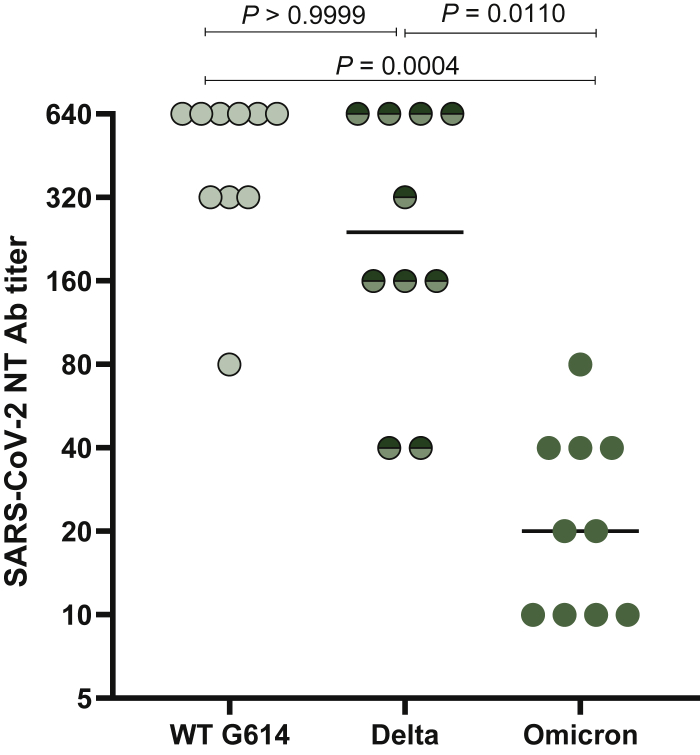
In a small cohort of patients (n = 10), SARS-CoV-2 NT Ab responses against the circulating Delta (B.1.617.2) and Omicron (B.1.1.529) variants were measured and compared to NT Ab titer against D614G wild-type strain. Overall, the median level of SARS-CoV-2 NT Abs was 32-fold lower against Omicron compared to the wild-type strain (P = 0.0004) and 12-fold lower compared to the Delta strain (P = 0.0110) (Figure 4).
Figure 4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibodies (NT Abs) against D614G wild-type strain, Delta and Omicron variants were measured in 10 SARS-CoV-2-naïve solid organ cancer patients.
P values are given for each comparison.
Side-effects
The most common side-effects observed after the vaccine were pain at the injection site (64.8%, 92/142) and fever (24.6%, 35/142). No hypersensitivity AEs, thrombosis or vaccine-related anaphylaxis were reported. Side-effects were typically resolved within the first 24 h after vaccination (Table 2).
Table 2.
Side-effects after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine
| Side-effects | n (%) |
|---|---|
| Pain at the injection site | 92 (64.8) |
| Fever | 35 (24.6) |
| Arthralgia | 25 (17.6) |
| Headache | 17 (12) |
| Myalgia | 13 (9.2) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
In this paper, we have presented the results of a prospective observational study investigating the immunogenicity of the booster with BNT162b2 anti-SARS-CoV-2 vaccine in patients with cancer on active treatment.
Two doses of mRNA anti-SARS-CoV-2 vaccines are effective in preventing a symptomatic SARS-CoV-2 infection,2 but a decrease of both IgG concentration and NT Ab titer has been demonstrated 6 months after the vaccination, especially among the immunocompromised subjects.13 Moreover, the emergence of the Delta variant in June 2021 has highlighted the immune evasion with the waning of vaccine-elicited immunity.14 Caniels et al. observed a substantial reduction in binding and neutralization potency of antibodies against three SARS-CoV-2 VOCs (B.1.1.7, B.1.351 and P.1) in convalescent or immunized sera after a full course of BNT162b2 SARS-CoV-2 vaccine.15 The emergence of SARS-CoV-2 variants and the reduction of NT Ab titer in vaccinated or previously infected subjects have justified the approval of the third dose of COVID-19 vaccine by CDC.9,16
To date, few studies have reported the immunogenicity of the third dose in patients with cancer. Rottenberg and colleagues demonstrated higher antibody levels after the booster in a cohort of 37 patients with cancer receiving active systemic therapy.17 Shapiro et al. confirmed the reinforcement of anti-COVID-19 immunity among the total 88 patients with cancer enrolled, with a statistically significant seroconverting anti-S IgG levels after booster vaccination (P = 0.000062).18 Debie and colleagues reported that the booster induced significantly higher anti-receptor-binding domain IgG levels 28 days post-third dose than 28 days post-second dose in 141 cancer patients.19 Finally, the data from a large cohort of patients with hemato-oncological tumors have confirmed the increased humoral response after the third dose of BNT162b2 SARS-CoV-2 vaccine.20
According to these studies, our data have demonstrated the seroconversion in all patients except one. The patient who failed to successfully seroconvert at T1 was under daily corticosteroid treatment during the vaccination schedule for concomitant rheumatoid arthritis. This negative association between steroid treatment and IgG levels has been described in many papers.21, 22, 23
We have also evaluated the NT Ab titer. In our cohort, the neutralizing activity was significantly higher in seroconverted patients, with a positive correlation between IgG levels and NT Ab titer. This correlation has also been reported in other papers: Di Noia and colleagues found a strong correlation between the levels of anti-S IgG and the titer of NT Abs.23 Also, Levin et al. reported a strong correlation between IgG and NT Abs for the whole 6 months after the full course of vaccine (Spearman’s rank correlation between 0.68 and 0.75), with a time-dependent regression relationship.14
No differences were reported when the type of anticancer treatment (chemotherapy or immunotherapy) was correlated with the SARS-CoV-2 humoral response, while age was inversely correlated with total IgG level and NT Ab titer at baseline (T0) but not after the booster (T1).
This finding confirms that age is an unfavorable prognostic factor for long-lasting humoral response after the two doses of SARS-CoV-2 vaccine, but the booster is immunogenic also in older subjects, as highlighted by Mwimanzi and colleagues too.24
Furthermore, we have also investigated the development of Spike-specific cell-mediated immune response using the SARS-CoV-2 IGRA in a sample of subjects.
In SARS-CoV-2-naïve subjects after the booster, the median IFN-γ level 3 weeks after the booster was significantly higher than that measured before, while this difference was not reported in SARS-CoV-2-experienced subjects. This evidence confirms the hypothesis that the triple exposure to SARS-CoV-2 antigens is able to induce a higher cell-mediated immune response.
Moreover, the third SARS-CoV-2 vaccine dose seems to increase the activity of neutralization against VOCs also in the immunocompromised patients.8,25 Garcia-Beltran and colleagues demonstrated that the mRNA-based COVID-19 vaccine boosters induce a significantly greater humoral immune response against all the variants.8 Also, Zeng et al. reported that the third dose of the mRNA COVID-19 vaccine causes a significantly greater neutralizing capacity against the Omicron variant in comparison to two doses.26
We have analyzed a small sample (n = 10) with the evidence of the median level of SARS-CoV-2 NT Abs 32-fold lower against Omicron compared to the wild-type strain (P = 0.0004) and 12-fold lower compared to the Delta strain (P = 0.0110).
No severe AEs were reported.
Strengths and limitations
The strength of our data consists in the simultaneous detection of both humoral and cellular immune response. One limit is the low numerosity (n = 142), even though this cohort of the patients with cancer is one of the largest available at the moment.17, 18, 19, 20,26 Another limit is the lack of a control group that enables us to extend our conclusions in a definitive way. Finally, at the time of writing, the post-third dose data about the activity of neutralization against VOCs are available for only a small subset of participants.
Conclusion
Our data support the immunogenicity of the booster dose in patients with cancer. The third dose is able to trigger both the humoral and the cell-mediated immune response in patients with cancer on active treatment, independently of the type of therapy. Our preliminary data about the neutralization of the SARS-CoV-2 vaccine against VOCs seem to confirm the lower vaccine activity, but further studies would be designed to address this issue.
Acknowledgments
Funding
This work was supported by Ricerca Corrente [grant no 08067620]; Fondazione IRCCS Policlinico San Matteo, Ricerca Finalizzata [grant BIAS no. 2020-12371760]; and from European Commission—Horizon 2020 (EU project 101003650—ATAC).
Disclosure
The authors have declared no conflicts of interest.
References
- 1.1. Worldometers COVID-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus Available at.
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas S.J., Moreira E.D., Jr., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasagna A., Agustoni F., Percivalle E., et al. A snapshot of the immunogenity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open. 2021;6(5):100272. doi: 10.1016/j.esmoop.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Evidence to Recommendation Framework: Pfizer-BioNTech COVID-19 Booster Dose. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-9-23/03-COVIDOliver.pdf Available at.
- 10.Arbel R., Hammerman A., Sergienko R., et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasagna A., Lilleri D., Agustoni F., et al. Analysis of the humoral and cellular immune response after of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors with or without chemotherapy: an update after six months of follow up. ESMO Open. 2021;7(1):100359. doi: 10.1016/j.esmoop.2021.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(suppl 1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percivalle E., Cambiè G., Cassaniti I., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25(24):2001031. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caniels T.G., Bontjer I., van der Straten K., et al. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci Adv. 2021;7(36) doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rottenberg Y., Grinshpun A., Ben-Dov I.Z., Oiknine Djian E., Wolf D.G., Kadouri L. Assessment of response to a third dose of the SARS-CoV-2 BNT162b2 mRNA vaccine in patients with solid tumors undergoing active treatment. JAMA Oncol. 2021;8(2):300–301. doi: 10.1001/jamaoncol.2021.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro L.C., Thakkar A., Campbell S.T., et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2021;40(1):3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debie Y., Vandamme T., Goossens M.E., van Dam P.A., Peeters M. Antibody titres before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in patients with cancer. Eur J Cancer. 2022;163:177–179. doi: 10.1016/j.ejca.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mair M.J., Berger J.M., Mitterer M., et al. Third dose of SARS-CoV-2 vaccination in hemato-oncological patients and health care workers: immune response and adverse events – a retrospective cohort study. Eur J Cancer. 2022;165:184–194. doi: 10.1016/j.ejca.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 22.Massarweh A., Tschernichovsky R., Stemmer A., et al. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients with primary brain tumors: a prospective cohort study. J Neurooncol. 2022;156(3):483–489. doi: 10.1007/s11060-021-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Noia V., Pimpinelli F., Renna D., et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021;27(24):6815–6823. doi: 10.1158/1078-0432.CCR-21-2439. [DOI] [PubMed] [Google Scholar]
- 24.Mwimanzi F.M., Lapointe H.R., Cheung P.K., et al. Older adults mount less durable humoral responses to a two-dose COVID-19 mRNA vaccine regimen, but strong initial responses to a third dose. medRxiv. 2022 doi: 10.1093/infdis/jiac199. 2022.01.06.22268745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22(4):1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng C., Evans J.P., Chakravarthy K., Qu P., Reisinger S., Song N.J., et al. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell. 2021;40(2):117–119. doi: 10.1016/j.ccell.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]