Abstract
(S)-1-[3-Hydroxy-2-(phosphonylmethoxy)propyl]cytosine (HPMPC) is a nucleoside phosphonate analog which in its active diphosphorylated form is known to inhibit herpesvirus DNA polymerase. In this study, we have demonstrated that, in a dose-dependent manner, this compound irreversibly suppressed proliferation of cells infected with human papillomavirus (HPV), which does not possess a viral DNA polymerase. To elucidate the mechanism of cell growth inhibition, cell cycle indicator-regulator expression, thymidine incorporation, transcript levels of apoptosis factors, and anabolic products of HPMPC following drug treatment were evaluated. HPMPC treatment reduced WAF1 (p21) levels independent of those of p53, while proliferating cell nuclear antigen increased. However, in comparison to controls, HPMPC-treated cells displayed a decrease in thymidine incorporation, indicating an inhibition of host DNA polymerase activity. In normal primary keratinocytes, HPMPC predominantly accumulated in the form of the choline adduct HPMPCp-choline. However, in HPV type 16-transformed keratinocytes, HPMPCpp was the most abundant anabolic product, with little HPMPCp-choline having formed. The data imply that an unrecognized viral factor is modulating the conversion of nucleotides, including HPMPC, to the triphosphorylated form.
Human papillomaviruses (HPVs) are the primary etiological agents in several proliferative diseases of the epithelia. In women, infections with HPV type 16 (HPV-16) are considered to put individuals at high risk for development of cervical carcinoma, the second leading cause of female mortality worldwide (8). A variety of therapeutic compounds (e.g., interferon [IFN], podophyllotoxin, and 5-FU) have been utilized in the treatment of HPV-induced disease, but with only limited success. With conventional therapy, undesirable responses which are manifested as acute inflammatory side effects, an ensuing insensitivity to drug treatments, or a relapse of lesions often occur.
Alternative compounds for the treatment of HPV-induced disease are required, and a class of analogs which have antiproliferative and antiviral activity, the nucleoside phosphonates, may be useful in this role (5, 7). Of these compounds, the cytosine analog (S)-1-[3-hydroxy-2-(phosphonomethoxy)-propyl]cytosine (HPMPC) (cidofovir) (see Fig. 1) is highly effective in inhibiting herpesvirus replication and is approved for treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Inhibition studies with CMV have revealed that replication of the CMV genome was significantly reduced (by ∼31%) when the elongating chain contained an incorporated HPMPC (4, 11). With in vivo studies, the toxicity associated with HPMPC was markedly reduced when administered topically rather than intravenously (3).
FIG. 1.
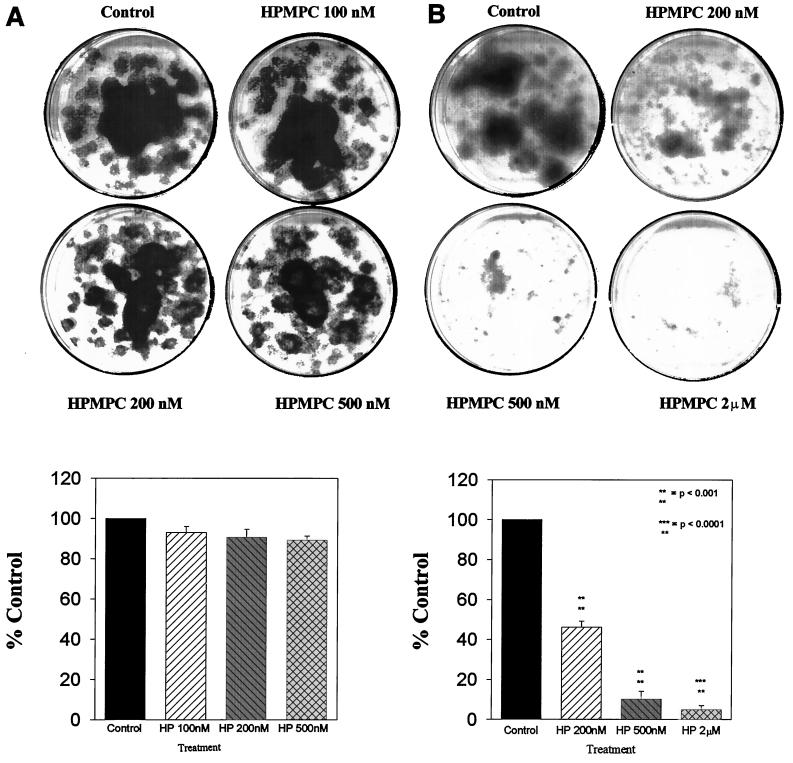
Effect of increasing HPMPC dose on normal human keratinocyte (A) and HPV-transformed keratinocyte (B) proliferation. Triplicate plates were seeded with 100 cells each, which were allowed to adhere for 24 h. Cultures were treated three times per week at the indicated doses for 2 weeks. Cells were fixed and stained with Giemsa and proliferation was determined by densitometric analysis of plate surface areas.
Intracellularly, nucleoside monophosphate kinase phosphorylates HPMPC to HPMPCp in the rate-limiting reaction of drug anabolism (see Fig. 2) (2). HPMPCp is phosphorylated to HPMPCpp principally by pyruvate kinase and to a lesser degree by nucleoside diphosphate kinase. HPMPCpp acts as an analog of dCTP and, likewise, is incorporated into replicating DNA with normal deoxynucleoside triphosphate kinetics. In CMV-infected cells, the activities of pyruvate kinase and particularly that of NDP kinase were observed to increase severalfold (2). This activity enhances the conversion of the compound into its biologically active form and may serve to augment the selective antiproliferative effect in the virus-infected cell.
FIG. 2.
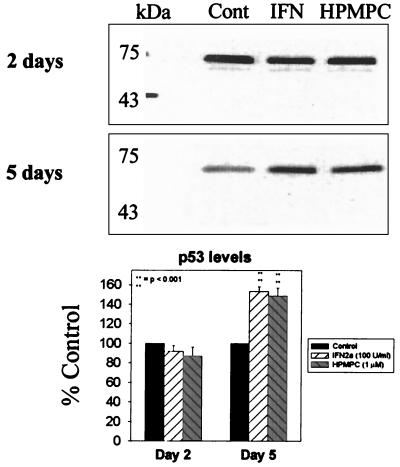
Western analysis of p53 levels following a 2-day treatment with 50 U of IFN-α2a per ml and 1 μM HPMPC. Protein was detected by monoclonal mouse antibody and HRP-conjugated goat anti-mouse secondary antibody. Cont, control.
Recently, our in vitro evaluation and others from clinical investigations (10) have indicated that HPMPC has irreversible antiproliferative activity against HPV-infected cells. These observations have raised interest in the mechanisms of drug action since HPV utilizes the host cell DNA polymerase, and not a virally encoded polymerase, to replicate its genome. Our preliminary examinations suggested that, at the concentrations examined, HPV-transformed cells were selectively affected by the analog while uninfected cells were not subject to the cytotoxic effects. Incorporation of HPMPC into the cellular DNA of the infected cell may sufficiently disrupt the genomic integrity of the host cell and lead to cell necrosis. Alternatively, the genetic insult may induce an apoptotic response as a means of preventing proliferation of the mutated cell.
Our data indicate that HPMPC treatment of HPV-transformed cells entrapped the infected cells in the S phase of the cell cycle. Within HPV-transformed cells, HPMPC anabolism was driven to synthesize the HPMPCpp form of the drug. In noninfected keratinocytes, however, HPMPCpp levels were relatively low and HPMPCp-choline comprised the predominant anabolite. This differential modulation of HPMPC anabolism provides evidence for its selective activity in HPV-transformed cells. Our data indicate the value of further study of HPMPC as a potential clinical agent for the treatment of HPV-induced disease.
MATERIALS AND METHODS
Cell culture.
Normal human keratinocytes were harvested from neonatal foreskin by trypsinization and selective growth in keratinocyte medium. HPV-16-transfected HKc/HPV-16d-2C (d-2C) cells were a kind gift from L. Pirisi (University of South Carolina School of Medicine). Both normal and transformed keratinocyte cultures were maintained in Keratinocyte-SFM (Gibco) medium and 5% CO2 for not more than 10 and 30 passages, respectively.
Antiviral compounds.
HPMPC and [3H]HPMPC (specific activity, 29 Ci/mmol) were obtained from Gilead Sciences. Compounds were diluted in sterile double-diluted H2O. Recombinant alpha IFN (IFN-α) was obtained from Hoffmann-La Roche.
Colony growth inhibition.
Cells were plated at ∼500 cells/60-mm-diameter culture dish in triplicate and were allowed 24 h to adhere. The treatment regimen involved the addition of HPMPC three times per week for 2 weeks. After the 2-week period, plates were washed with phosphate-buffered saline (PBS) and fixed and stained with Giemsa stain. Cell colony density and area were determined by image analysis. The paired t test was used to calculate statistical significance of proliferation inhibition in comparisons of mean colony densities of control and treatment groups.
Cell cycle synchronization.
Cultures were synchronized by the addition of 2 mM thymidine to the normal growth medium for 24 h, after which time the cells were washed three times and replenished with unmodified growth medium (1).
Kinetic uptake of HPMPC.
Synchronized normal and HPV-16-transformed cells were incubated with 200 nM [3H]HPMPC (specific activity, 23 μCi) for 0.5, 1, 2, 4, and 8 h. Following incubation, cells were washed four times with PBS and then harvested. Pellets were lysed in 200 μl of hypertonic lysis solution, and cell debris was cleared by centrifugation for 15 min at 13,000 × g. Drug was further extracted with 500 μl of ice-cold 70% methanol for 1 h. Extracts were again clarified by centrifugation as described above, and the counts per minute of the entire supernatants were measured by scintillation in Scintiverse II (Fisher) liquid scintillation fluid.
Cell cycle analysis.
Synchronized cultures were treated with HPMPC for 2, 5, and 7 days, after which time cells were harvested, washed with PBS, and permeabilized and fixed overnight in ice-cold 70% ethanol. Following fixation, cells were resuspended in PBS, incubated with RNase, and stained with 1 mg of propidium iodide/ml for 1 h. The cell cycle phases were evaluated by flow cytometry on an ASICS single beam (Coulter) and with cell cycle analysis software.
Thymidine incorporation studies.
Synchronized cultures were treated with HPMPC for 2 days, after which plates were pulsed for 2 h with [3H]thymidine (specific activity, 71 Ci/mmol; ICN Pharmaceuticals). After the 2-h incubation, cultures were washed four times with PBS and incubated for 24 h in normal growth medium. Cells were then trypsinized and harvested. Cell pellets were resuspended in a hypertonic lysis solution containing proteinase K and incubated for 30 min at 37°C. Cellular DNA was precipitated in cold 5% trichloroacetic acid (TCA) for 20 min, and the precipitate was applied to 24-mm GF/C filters (Whatman) (6). Filters were washed with cold 2.5% TCA and 70% ethanol, and the counts per minute were read with a Beckman LS 7500 scintillation counter in 8 ml of fluid (0.05% POPOP and 0.5% PPO in toluene).
Western blot analysis.
To evaluate effects on cell cycle factors, HPMPC was compared with IFN-α2a, the preferred compound for treatment of HPV-induced lesions. Protein lysates of treated cultures were generated by four freeze-thaws in WCE buffer and centrifuged at 13,000 × g for 15 min to clear insoluble components. Next, 40 μg of lysate/ml was denatured by boiling for 8 min in loading buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% Tris-glycine polyacrylamide gels along with Kaleidoscope standards (molecular masses, 208, 127, 85, 45, 32.8, 18.1, and 7.4 kDa) and low-range standards (molecular masses, 102, 78, 49.5, 34.2, 28.3, and 19.9 kDa) (Bio-Rad). For proliferating cell nuclear antigen (PCNA) detection, lysates were precipitated with 8% TCA to remove the biologically inactive form and were then neutralized with 2 N NaOH in loading buffer. Proteins were transferred to a 0.2-μm-pore-size natural nitrocellulose membrane for electrophoresis for 50 min at 24 V followed by 50 min at 12 V with a Genie blotter. The membranes were probed with 1.5 μg of monoclonal antibodies to p21 or PCNA (Santa Cruz Biotech)/ml overnight at 4°C. Membranes were incubated in 0.025 μg of horseradish peroxidase (HRP)-conjugated goat anti-mouse/ml for 2 h, and bands were detected by enhanced chemiluminescence (ECL) and exposure to Hyperfilm-ECL (Amersham).
HPLC analysis of HPMPC anabolic products.
Duplicate plates of normal and HPV-16-transformed cultures were treated with 80 μCi of [3H]HPMPC/plate (specific activity, 29 Ci/mmol) for 8 and 16 h. Drug was extracted as described for the kinetic uptake analysis. Modified forms of HPMPC were fractionated by high-performance liquid chromatography (HPLC) using a Partisil 10-SAX column and a 5 to 700 mM ammonium phosphate (pH 3.5) gradient by using cold HPMPC and known elution times as the standard. Entire 3-ml fractions corresponding to HPMPC, HPMPCp, HPMPCpp, and HPMPCp-choline peaks were measured for counts by liquid scintillation in Scintiverse II.
RNase protection assay.
Transcript levels of apoptotic factors were measured with the RiboQuant RNase protection system by using the hApo1c and hApo2 probe sets (Pharmingen). Briefly, riboprobes were [32P]UTP labeled and hybridized against total RNA extracts (RNEasy; Qiagen) from 7-day untreated and HPMPC-treated HPV-16-transformed keratinocytes. Unhybridized RNA was RNase degraded for 45 min as recommended by the Pharmingen kit instructions, and the hybridized segments were column purified (RNEasy). Hybridizations were electrophoresed in 5% acrylamide gels with free probe used as molecular size indicators.
Mitochondrial activity determination.
Cells were treated with 500 nM and 1 μM HPMPC for 7 days, after which time cultures were washed and incubated in the presence of CMX-Ros (Molecular Probes) as recommended by the manufacturer. Integrated red fluorescence for each treatment group versus untreated controls was determined by using 20,000 cells/sample and an EPICS 751 flow cytometer (Coulter) with a 610 band-pass filter. Data analysis was performed with Cyclops Software (Cytomation, Inc.).
RESULTS
Inhibition of HPV-transformed cell colony proliferation by HPMPC.
Culture dishes (diameter, 60 mm) seeded at 500 cells/plate with either normal human keratinocytes or early-passage (passage 15 or earlier) d-2C HPV-transformed keratinocytes were treated for 2 weeks with HPMPC at concentrations of 0.1, 0.2, 0.5, 1, and 2 μM. Concentrations up to 0.5 μM had no effect on normal cell growth (Fig. 1A); however, the same drug concentrations profoundly reduced transformed cell proliferation (Fig. 1B). At 1 μM HPMPC and above, normal cell proliferation was inhibited by >15% versus untreated controls. At 0.2 μM HPMPC, d-2C cell proliferation was reduced to 48% of that of the control, and cell growth was significantly retarded (by ∼90%) following 0.5 μM HPMPC treatments. At 2 μM HPMPC, proliferation of HPV-transformed keratinocytes was only 5% of that of the control. Following a 1-week drug treatment, cultures were incubated in untreated normal growth medium to ascertain whether growth could resume after the removal of drug. There was no recovery of cell growth following the 1-week 1 or 2 μM HPMPC treatments, and fragmentation of cellular DNA was observed (not shown).
Transformed cell growth inhibition on later-passage cells (passages 20 to 30) was markedly less than what was observed with the earlier-passage d-2C. With 1.2 μM HPMPC, growth of passage-29 transformed keratinocytes yielded a nominal (23%) reduction in cell growth (not shown). The loss of sensitivity to drug treatment exhibited by these later passage cells is similar to that observed in in vitro evaluation of antiproliferative activity with established cervical carcinoma lines (CaSki).
Effect of HPMPC treatment on cell cycle proteins.
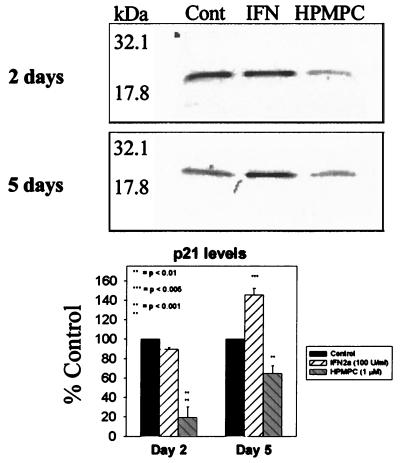
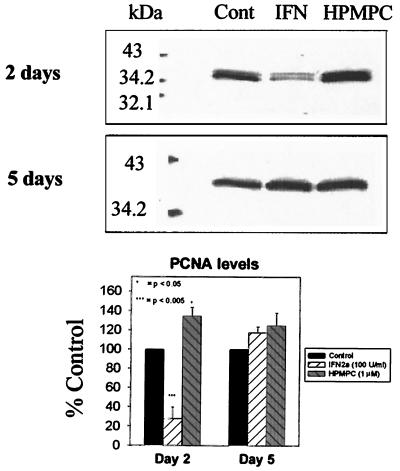
d-2C-transformed cells were treated for 2 days with 1 μM HPMPC. Levels of p21 and PCNA proteins were assayed following treatment to evaluate their potential roles in the observed drug-induced growth inhibition. With Western analysis, 2-day HPMPC treatments did not affect p53 levels (Fig. 2); however, p21 levels were observed to drastically decrease (by 82%) (Fig. 3). Levels of PCNA, an indicator of DNA polymerase activity, increased (34%) following 2 days of treatment with HPMPC (Fig. 4). This pattern of increased PCNA expression with HPMPC was expected given the observed decreases in p21, its inhibitor.
FIG. 3.
Western analysis of p21 regulator factor levels following treatment with 50 U of IFN-α2a per ml and 1 μM HPMPC. Protein was detected by monoclonal mouse antibody and HRP-conjugated goat anti-mouse secondary antibody. Cont, control.
FIG. 4.
Western blot analysis of PCNA processivity factor levels following treatment with 50 U of IFN-α2a/ml and 1 μM HPMPC. Protein was detected by monoclonal mouse antibody and HRP-conjugated goat anti-mouse secondary antibody. Cont, control.
Kinetic uptake of HPMPC in normal and HPV-16-transformed cells.
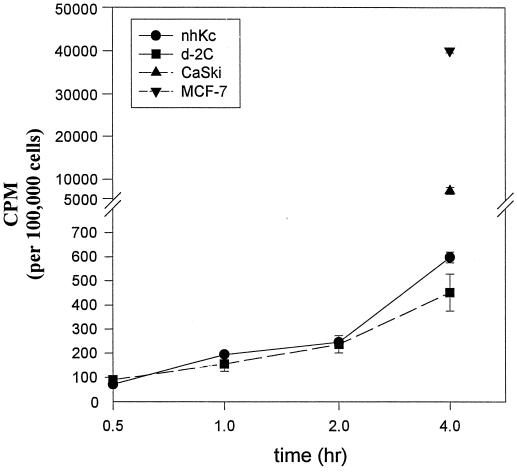
Both normal and d-2C keratinocytes displayed similar unincorporated, intracellular levels of [3H]HPMPC at 0.5, 1, 2, and 4 h of incubation with drug (Fig. 5). The levels in solution account for 60 to 70% of the drug sequestered by the cell. Uptake by nontumorigenic keratinocytes is >10-fold less than cervical and breast carcinoma lines. These data indicate that the sensitivity of HPV-transformed cells to HPMPC cannot be attributed to a more rapid accumulation of drug within these cells. Drug incorporation into host cell DNA is maximal during the S phase of the cell cycle (data not shown).
FIG. 5.
Cellular uptake of [3H]HPMPC in normal human keratinocytes (nhKc), HPV-16-transformed keratinocytes (d-2C), a tumorigenic cervical carcinoma line (CaSki), and a virus negative breast carcinoma line (MCF7). Drug was analyzed from clarified methanol extracts of cell pellets and represents unincorporated drug levels. For CaSki and MCF7, only 4-h intracellular drug levels were measured.
DNA replication activity following HPMPC treatment.
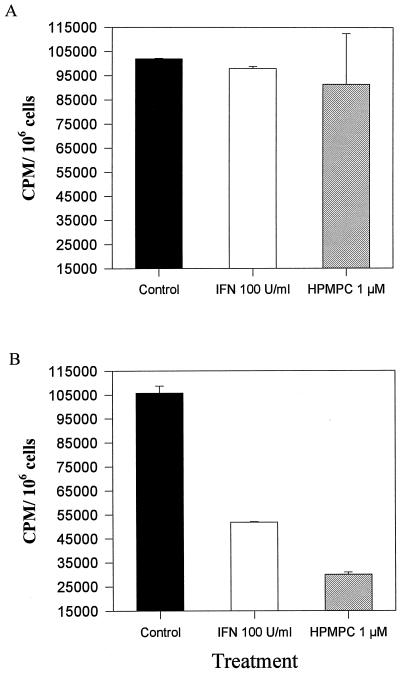
DNA replication was evaluated following treatment with 1 μM HPMPC and 100 U of IFN-α/ml as a control. These drug concentrations did not affect normal keratinocyte DNA replication (Fig. 6A). As expected, IFN caused a reduction (∼50%) in transformed cell DNA polymerase activity (Fig. 6B). Unexpectedly, given the apparent activation of replication machinery following HPMPC treatments, DNA replication in HPMPC-treated cultures occurred at only 30% of the level of untreated controls. This inhibition of DNA synthesis supports the suppression of transformed-cell proliferation observed in the colony reduction assays.
FIG. 6.
[3H]thymidine incorporation following 2-day treatments with 1 μM HPMPC. The data are the means of duplicate treatment groups ± standard deviations. IFN-α2a is used as a positive inhibition control. Results for normal keratinocytes (A) and d-2C cells (B) are shown.
S-phase stimulation by HPMPC.
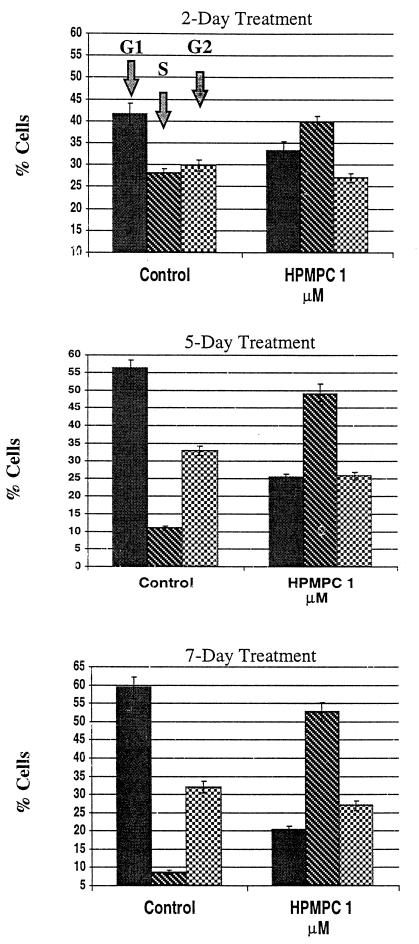
After 2 days of treatment with 1 μM HPMPC, d-2C cells exhibited a shift (12%) in population phase from G1 to S (Fig. 7). This S-phase shift increased to ∼40% after 5 days and increased only slightly more at 7 days of treatment. At all days examined, there was a nominal decrease (<10%) in the G2 population.
FIG. 7.
Graphical representation of flow cytometric analysis. Cell cycle analysis of d-2C following treatment with HPMPC. Cellular DNA was stained with propidium iodide and analyzed by flow cytometry. Note the S-phase stimulation in HPV-16-transformed keratinocytes following HPMPC treatment.
Anabolism of HPMPC in normal cells versus that in HPV-16-transformed cells.
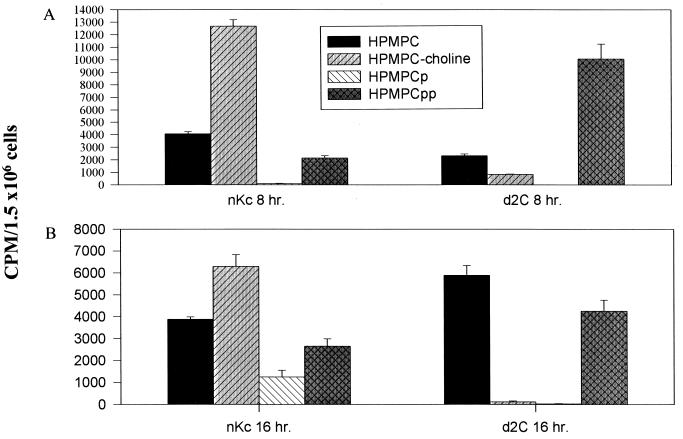
Normal and d-2C keratinocyte drug anabolisms were evaluated by HPLC analysis following 8- and 16-h incubations with 200 nM [3H]HPMPC (Fig. 8). In normal cells, HPMPCp-choline is the predominant drug anabolite detected. Transformed cells, however, produce very little of the choline adduct and an abundance of HPMPCpp, an analog of dCTP. The large pools of HPMPCpp in d-2C increase the likelihood of drug incorporation into replicating DNA.
FIG. 8.
Intracellular conversion of HPMPC in normal (nKc) and HPV-16-transformed keratinocytes. Cells were incubated for 8 h (A) and 16 h (B) in the presence of 200 nM [3H]HPMPC. Quantification was by liquid scintillation counting of HPLC-fractionated cellular extracts. HPMPCp, HPMPC monophosphate; HPMPCpp, HPMPC diphosphate.
Mitochondrial activity and transcription of apoptosis regulatory factor following HPMPC treatment.
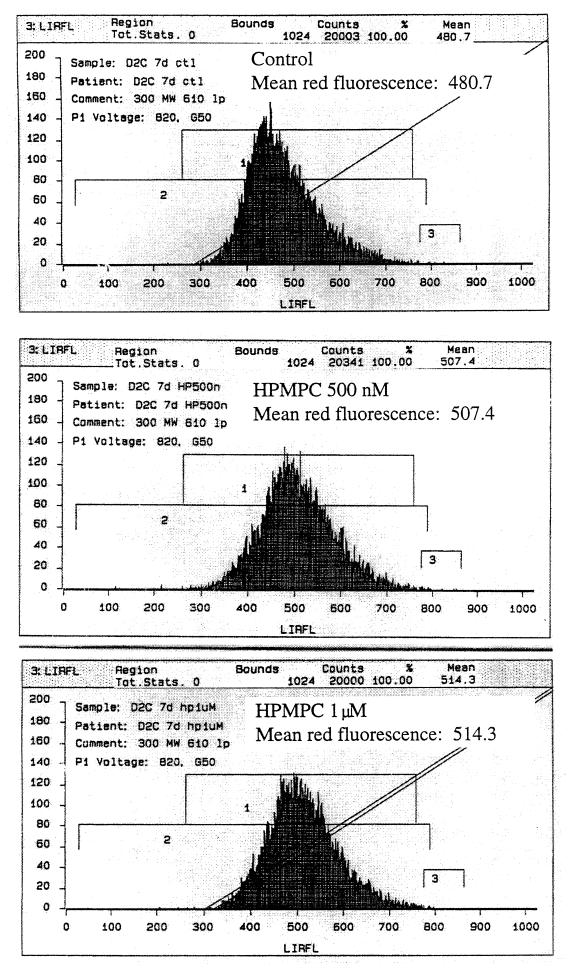
Following 1-week treatments of d-2C with 500 nM and 1 μM HPMPC, cells were stained with the mitochondrial reductase dye CMX-ros. A dose-dependent increase in red fluorescence was observed in transformed cells treated with HPMPC (Fig. 9). When total RNA preparations from the HPMPC-treated transformed cells were compared to untreated controls, there was no difference in the type or amount of apoptosis transcript levels (data not shown).
FIG. 9.
Increased mitochondrial activity following HPMPC treatment. CMX-ros stain for mitochondrial membrane potential activity indicates an increase in energy production. Decreases (left shift) in staining would be indicative of apoptosis.
DISCUSSION
This study describes inhibition of HPV-16-transformed keratinocyte proliferation by the acyclic phosphonate cytosine analog HPMPC. Since HPV does not encode its own polymerase, the expected target of this compound, the mechanism of action in these cells was evaluated. Our analyses revealed the generation of predominantly diphosphorylated HPMPC in HPV-transformed cells while normal cells accumulated HPMPCp-choline, indicating a modulation of cellular kinases in the virus-infected cells. In HPV-16-transformed cells, HPMPC induced DNA fragmentation characteristic of an apoptotic response, indicating that this analog is cytotoxic rather than cytostatic.
Although p53 levels were not affected by HPMPC, a dramatic reduction in the cell cycle inhibitor p21 was observed. The abrogation of a G1 stop coincided with increased PCNA (DNA polymerase delta processivity factor) levels and an accumulation of cells in late S phase. Although PCNA levels were augmented following HPMPC treatment, DNA replication in HPV-transformed cells was reduced. Cell cycle analysis revealed that HPMPC-treated HPV-transformed cells were driven from G1 into the S phase but did not continue on to G2/M. This observation supports the idea of incorporated molecules preventing completion of DNA synthesis, as was observed with CMV replication. Thus, progression of cell cycling into mitosis is inhibited.
The lack of significant HPMPCp-choline levels in the transformed keratinocytes might be a result of a specific loss of choline transferase selectivity for the drug in the virus-infected cells. Alternatively, a choline transferase enzyme other than cytidylyl transferase could be involved in the cholination of nucleoside analogs and is inhibited in HPV-transformed cells. The lack of HPMPC cholination in transformed cells contrasts what is observed in normal keratinocytes, where HPMPCp-choline is the predominant anabolite following HPMPC treatment.
The observed loss of sensitivity to drug treatments in the later-passage d-2C keratinocytes indicates the importance of using primary and early passage cells for in vitro evaluation of potential chemotherapeutic agents. The mechanism by which resistance to treatment is acquired is not understood; however, as observed in high-passage cervical carcinoma lines, it cannot be attributed to a decrease in drug uptake, because high intracellular drug levels are detected (Fig. 5). Additionally, it appears that increased drug uptake is not a virus-driven event, since MCF7 cells accumulate very high levels of the compound. Further evaluation is needed to identify how cells lose sensitivity to these compounds.
In a mouse nasopharyngeal carcinoma model, drug-induced apoptosis was found to be responsible for the tumoricidal activity (9). However, at the concentrations we examined in vitro, we could not substantiate drug-induced apoptosis. We did observe DNA fragmentation in transformed cells following 1 week of HPMPC treatment, but our evaluations of mitochondrial activity and apoptosis factor transcripts could not confirm that apoptosis was the means of cell death. CMX-ros staining for mitochondrial membrane potential would reveal a decrease in red fluorescence during an apoptotic event. We, however, recorded increases in red fluorescence following HPMPC treatments. This may be indicative of a greater cellular energy demand as would be expected in the event of increased kinase activity and constitutive stimulation of DNA replication (S-phase) machinery. These increases in fluorescence were moderately substantial (a ∼35-channel shift) and reproducible.
Our examination has shed more light on the mechanism of HPMPC action on HPV-transformed cells. The data indicate a selective inhibition of HPV-transformed cells at concentrations that do not affect normal cell proliferation. The differential phosphorylation of the nucleoside phosphonate in HPV-infected cells divulges a heretofore undiscovered means of nucleotide modulation by cellular enzymes and raises the possibility of new molecular targets for antiviral therapy. These targets may be particularly relevant to the oncogenic DNA viruses which cause proliferative diseases. Moreover, identification of the virus factor(s) which modulates cellular replication enzymes would provide better insight into virus control mechanisms and may divulge resistance mechanisms which viruses could alternatively utilize to evade antiviral agents.
REFERENCES
- 1.Bootsma D, Budke L, Vos O. Studies on synchronous division of tissue culture cells initiated by excess thymidine. Exp Cell Res. 1964;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- 2.Cihlar T, Chen M S. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol Pharmacol. 1996;50:1502–1510. [PubMed] [Google Scholar]
- 3.Cundy K C, Lynch G, Lee W A. Bioavailability and metabolism of cidofovir following topical administration to rabbits. Antiviral Res. 1997;35:113–122. doi: 10.1016/s0166-3542(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.De Castro L M, Kern E R, De Clercq E, Ghaffar A, Mayer E P, Vogt P E, Gangemi J D. Phosphonylmethoxyalkyl purine and pyrimidine derivatives for treatment of opportunistic cytomegalovirus and herpes simplex virus infections in murine AIDS. Antiviral Res. 1991;16:101–114. doi: 10.1016/0166-3542(91)90062-v. [DOI] [PubMed] [Google Scholar]
- 5.Dvorakova H, Masojidkova M, Holy A, Balzarini J, Andrei G, Snoek R, De Clercq E. Synthesis of 2′-aminomethyl derivatives of N-(2-(phosphonomethoxy)ethyl) nucleotide analogs as potential antiviral agents. J Med Chem. 1996;39:3263–3268. doi: 10.1021/jm9601314. [DOI] [PubMed] [Google Scholar]
- 6.Hilderman R H, Deutscher M P. Macromolecular synthesis in permeable liver cells. Arch Biochem Biophys. 1976;175:534–540. doi: 10.1016/0003-9861(76)90542-7. [DOI] [PubMed] [Google Scholar]
- 7.Holy A, Votruba I, Merta A, Cerny J, Vesely J, Vlach J, Sediva K, Rosenberg I, Otmar M, Hrebabecky H, Travnicek M, Vonka V, Snoek R, De Clercq E. Acyclic nucleotide analogs: synthesis antiviral activity and inhibitory effects on some cellular and virus-encoded enzymes in vitro. Antiviral Res. 1990;13:295–311. doi: 10.1016/0166-3542(90)90014-x. [DOI] [PubMed] [Google Scholar]
- 8.Lörincz A T, Reid R, Jenson A B, Greenberg M D, Lancaster W, Kurman R J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Neyts J, Sadler R, De Clercq E, Raab-Traub N, Pagano J S. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998;58:384–388. [PubMed] [Google Scholar]
- 10.Snoeck R, Wellens W, Desloovere C, Van Ranst M, Naesens L, De Clercq E, Feenstra L. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir. J Med Virol. 1998;54:219–225. doi: 10.1002/(sici)1096-9071(199803)54:3<219::aid-jmv13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Xiong X, Smith J L, Chen M S. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob Agents Chemother. 1997;41:594–599. doi: 10.1128/aac.41.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]