Abstract
A wide range of bacteria possess virulence factors such as aminoacyl-tRNA transferases (ATTs) that are capable of rerouting aminoacyl-transfer RNAs away from protein synthesis to conjugate amino acids onto glycerolipids. We recently showed that, although these pathways were thought to be restricted to bacteria, higher fungi also possess ergosteryl-3β-O-L-aspartate synthases (ErdSs), which transfer the L-Asp moiety of aspartyl-tRNAAsp onto the 3β-OH group of ergosterol (Erg), yielding ergosteryl-3β-O-L-aspartate (Erg-Asp). Here, we report the discovery, in fungi, of a second type of fungal sterol-specific ATTs, namely, ergosteryl-3β-O-glycine (Erg-Gly) synthase (ErgS). ErgS consists of a freestanding DUF2156 domain encoded by a gene distinct from and paralogous to that of ErdS. We show that the enzyme only uses Gly-tRNAGly produced by an independent glycyl-tRNA synthetase (GlyRS) to transfer glycine onto the 3β-OH of Erg, producing Erg-Gly. Phylogenomics analysis also show that the Erg-Gly synthesis pathway exists only in Ascomycota, including species of biotechnological interest, and more importantly, in human pathogens, such as Aspergillus fumigatus. The discovery of a second type of Erg-aa not only expands the repertoire of this particular class of fungal lipids but suggests that Erg-aa synthases might constitute a genuine subfamily of lipid-modifying ATTs.
Keywords: aminoacyl-tRNA, ergosterol, ergosteryl-3β-O-glycine, ergosteryl-3β-O-L-aspartate, fungi, DUF2156, sterol aminoacylation
Abbreviations: aa-tRNA, aminoacyl-transfer RNA; aaGL, aminoacylated glycerolipid; aaGLS, aminoacyl-glycerolipid synthase; aaRS, aminoacyl-tRNA synthetase; Afm, Aspergillus fumigatus; Aor, Aspergillus oryzae; Asp, L-aspartate; AspRS, aspartyl-tRNA synthetase; ATT, aminoacyl-tRNA transferase; Bba, Beauveria bassiana; Cho, cholesterol; DUF2156, Domain of Unknown Function 2156; ErdS, ergosteryl-3β-O-L-aspartate synthase; Erg, ergosterol; Erg-Asp, ergosteryl-3β-O-L-aspartate; Erg-Gly, ergosteryl-3β-O-glycine; ErgS, ergosteryl-3β-O-glycine synthase; FC, flash column chromatography; fDUF, freestanding DUF2156; GL, glycerolipid; Gly∼AMP, glycyl-adenylate; GlyRS, glycyl-tRNA synthetase; GNAT, Gcn5-N-acetyltransferase domain; HPTLC, high-performance TLC; MBP, maltose-binding protein; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; Sce, Saccharomyces cerevisiae; Yli, Yarrowia lipolytica; YPD, yeast extract peptone dextrose
Brown and Goldstein stated in 1985 that cholesterol (Cho) “is the most highly decorated small molecule in biology” (https://www.nobelprize.org/prizes/medicine/1985/goldstein/lecture/) (1). Although this statement was meant to describe Cho biosynthesis (2), this can be extended to sterols modifications on the 3β-hydroxyl position, since 3β-O-sulfated (3), acetylated (4), glycosylated (5, 6, 7), and fatty acylated sterols (8) have been detected in multiple eukaryotes. Ergosterol (Erg) is one of the most essential lipids found within fungal membranes, whose function mirrors that of Cho in animals (9). Recently, we discovered that numerous species within the Dikarya subkingdom, i.e., higher fungi, such as Aspergillus fumigatus (Afm), Aspergillus oryzae (Aor), and Neurospora crassa (Ncr), produce an aminoacylated sterol that has not been detected in other eukaryotes: ergosteryl-3β-O-L-aspartate (Erg-Asp) (10). It corresponds to Erg onto which aspartic acid (Asp) is esterified on the 3β-hydroxyl through its α-carboxyl (10, 11). Other types of O-acylated sterols found in fungi, such as ergosteryl-acetate (Erg-Ac) (4) or ergosteryl-fatty acids (Erg-FA) (8), are synthesized by acyl-transferases that use acyl-coenzyme A (CoA) as donors of activated acyls (12). Erg-Asp is synthesized by an Erg-Asp synthase, which is a member of the aminoacyl-tRNA transferases (ATTs) family of enzymes (13, 14), that uses aspartyl-transfer RNAs (Asp-tRNAAsp) as a source of activated Asp.
Fungal Erg-Asp synthases (ErdS) (KEGG orthology: K24278) are bifunctional. The N-terminal domain is an aspartyl-tRNA synthetase (AspRS) paralog that, like a regular AspRS (13, 14), uses Asp, ATP, and tRNAAsp to produce Asp-tRNAAsp. The latter is channeled to the C-terminal ATT domain, where Asp is transferred onto the 3β-hydroxyl of Erg, yielding Erg-Asp (10). This domain belongs to the DUF2156 family (or LPG_synthase, PF09924 in the Protein FAMily database (15)), in which other types of ATT are classified (16). For example, aminoacyl-glycerolipid synthases (aaGLSs, with aa denoting the amino acid specificity) are bacterial enzymes that esterify amino acids (aa) in a tRNA-dependent manner onto glycerolipids (GLs) and yield aminoacylated glycerolipids (aaGLs) (17, 18). We also demonstrated that an Erg-Asp hydrolase (ErdH, KEGG orthology: K24279) exists in numerous fungi that removes the Asp moiety of Erg-Asp (10). In bacteria, extracytoplasmic hydrolases of this type deacylate aaGLs and have been shown to exist in gram-positive (19, 20) and gram-negative (21, 22) bacteria.
To date, cellular functions of Erg-Asp in the physiology of Dikarya remain unknown and this compound is the first and single example of a 3β-O-L-aminoacyl derivative of sterols (10). In contrast, bacterial aaGLSs carrying distinct substrate specificities produce a variety of aaGLSs (23, 24) using various GLs, such as phosphatidylglycerol (PG), cardiolipin, diacylglycerol, as well as various aa-tRNAs (17, 18). Several paralogs of aaPGSs sometimes coexist in bacterial species (20), such as in Clostridium perfringens, where a LysPGS and an AlaPGS produce LysPG and AlaPG, respectively, whereas in Enterococcus spp., one single aaGLS of broad aa-tRNA specificity (23) synthesizes Ala-, Lys- and ArgPG (24). Given this bacterial diversity, we considered the possibility that Erg-Asp could be one instance of a larger family of aminoacylated sterols in fungi.
Here we report the discovery in Ascomycota, one of the two divisions of Dikarya, of a novel type of DUF2156 proteins. They are all free-standing DUF2156 domains (fDUF2156) phylogenetically related and paralogous to ErdS that, contrary to the latter, are not fused to an aminoacyl-tRNA synthetase (aaRS). Using Afm, Aor, and Yarrowia lipolytica (Yli) as models, we demonstrate that fungal fDUF2156 proteins have an enzymatic activity distinct from that of ErdS: they reroute glycyl-tRNAGly (Gly-tRNAGly) produced by a glycyl-tRNA synthetase (GlyRS) and transfer Gly onto the 3β-hydroxyl of Erg, yielding ergosteryl-3β-O-glycine (Erg-Gly), another and previously undetected type of sterol conjugate of the 3β-O-aminoacyl-type. We therefore name those enzymes Erg-Gly synthases (ErgS, Er: ergosterol, g: glycine, S: synthase). ErgSs expand the repertoire of the newly discovered class of ergosteryl-amino acid synthases. We conclude that ErgS and ErdS represent two members of a new subfamily of AATs, namely, tRNA-dependent ergosteryl-3β-O-amino acid synthases, or ErxS (x denoting one of the 20 canonical protein amino acids).
Results
A second type of DUF2156 proteins in Ascomycota
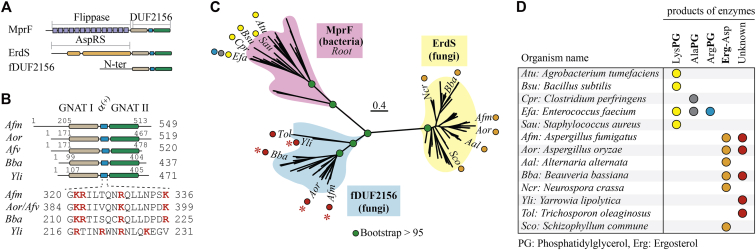
After the discovery of ErdS in Dikarya (10), we noticed that Afm and Aor possess an additional protein that presents distant homology (∼20% identity) to the ATT domain of ErdS and that is recognized as a DUF2156 protein by the PFAM search tool (15). Both proteins, encoded by the AFUA_8g01260 and AO090011000521 genes in Afm and Aor, respectively, are free-standing DUF2156 domains (fDUF2156, from now on shortened fDUF), meaning that they are fused neither to an aaRS domain nor to an N-terminal integral membrane aaGLSs flippase domain, unlike fungal ErdS (10) or bacterial MprFs (17), respectively (Fig. 1, A and B). Sequence searches revealed that fDUF proteins were also present in Beauveria bassiana (Bba, Sordariomycetes, gene: BBA_06338 in the ARSEF 2860 strain) and Y. lipolytica (Yli, Saccharomycetes, gene: YALI0E00330g). Sequence analyses and structure predictions of Afm, Bba, and Yli fDUFs (Fig. S1) show that, like for the ATT domain of ErdS (10) or bacterial aaGLSs, these proteins are all likely constituted of two Gcn5-like N-acetyltransferase (GNAT) domains separated by a positively charged α helix (α(+) helix), a fold characteristic of ATTs of the DUF2156 family (17, 25, 26) (Figs. 1A and S1). Depending on their host organism, fDUF proteins have N-terminal extensions of varying sizes (Fig. 1B) that share neither homology to known proteins nor obvious subcellular localization signals (PSORT II, https://psort.hgc.jp/form2.html). In the case of Aor, however, the fDUF2156 protein seems much shorter than its closest homologs in Afm or in Aspergillus flavus (Afv), with which it shares close ancestry (27, 28). The GNAT II domain appears dramatically truncated (Fig. S1), to the point that most of the putative ATT active site would be absent, suggesting that the enzyme would very likely be inactive (Fig. 1B and see below).
Figure 1.
In silico characterization of fungal fDUF2156 proteins.A, architecture of bacterial aaGLSs (MprFs), with the N-terminal aaGLs flippase (14 transmembrane helices) and the DUF2156 domain. Fungal ErdS (AspRS-DUF2156) are represented as well as fungal fDUF2156 proteins. B, fDUF proteins from Afm, Aor, Afv, Bba, and Yli. The two Gcn5-N-acetyltransferase (GNAT) domains separated by the α(+) helix, which constitute the DUF2156 fold, are detailed (domain coordinates indicated, length of proteins on the right). K (Lys) and R (Arg) residues are shown in red. C, maximum likelihood phylogenetic reconstruction of bacterial (MprF) and fungal (ErdS, fDUF) DUF2156 domains. Three clades are visible that match bacterial MprF- (purple background), ErdS- (yellow background), and fDUF2156- (blue background) specific groups. Positions of some proteins are indicated with the abbreviated name of the organism in which they are found together with (D) the aminoacylated products that they synthesize (if known). Red asterisks indicate the proteins studied in this work.
Because fDUF proteins are not fused to an aaRS, and because the substrate specificities of DUF2156 proteins cannot be predicted based on consensus sequences (17), in silico determination of the aa-tRNA or acceptor lipid substrates were impossible. However, phylogenetic reconstructions (Fig. 1C) showed that fungal ErdS and fDUF are related to the DUF2156 domains of bacterial aaGLSs (MprFs). The latter are known to produce only aaGLs (Fig. 1D) (17, 20), whereas all ErdSs tested yield Erg-Asp (10), making these lipid modifications clade specific. Of interest, ErdS and fDUFs form two distinct and robust clades (Fig. 1C), related to aaGLSs, suggesting that they might represent two types of enzymes, possibly with different activities and/or specificities. ErdS and fDUFs coexist in Afm, Aor, Bba, and many other Ascomycota (Fig. 1D and see below), which reinforces the idea that they might have different and nonredundant activities and/or specificities, like paralogous aaPGSs in bacteria (17, 18). These evolutionary relationships of fDUFs with either ErdSs or aaGLSs, as well as their structural features, supported the hypothesis that they could modify lipids—glycerolipids or Erg—by a tRNA-dependent mechanism similar to that of ErdS (10, 17). We therefore inferred that fDUFs might be able to bind an aa-tRNA of unknown identity and produced by an unknown aaRS to use it for the tRNA-dependent modification of an unknown lipid.
Freestanding DUF2156 proteins modify lipids
In order to determine whether fDUF proteins are involved in the tRNA-dependent aminoacylation of a lipid, we tried to construct ΔfDUF mutants in Afm to be able to identify the lipid species that would disappear in total lipids from deletion strains, when analyzed by thin layer chromatography (TLC) compared with the WT lipid content. Despite multiple attempts in Afm using electroporation (10, 29) to introduce the deletion cassette, we failed to isolate ΔfDUF mutants. However, our in silico analyses show that the yeast Saccharomyces cerevisiae (Sce) lacks not only ErdS (10) but also fDUF proteins (see below). The use of Sce previously greatly facilitated the identification of Erg-Asp through heterologous expression of ErdS, followed by TLC lipid profiling (10). We therefore applied the same strategy to fDUF proteins from diverse representative species within the fDUF clade (Afm, Aor, Bba, and Yli) (Fig. 1C). Genes from Afm, Bba, and Yli presented no introns, so they were PCR-amplified from genomic DNA and cloned into expression vectors for Sce. In the case of Aor, the shortest form described above (Fig. 1B and S1) was unexpected. Closer examination of sequences showed that this was an artifact likely originating from misannotation of an intron (see Fig. S2 and Supporting Methods for details). We amplified the longest form that was used for heterologous expression in Sce.
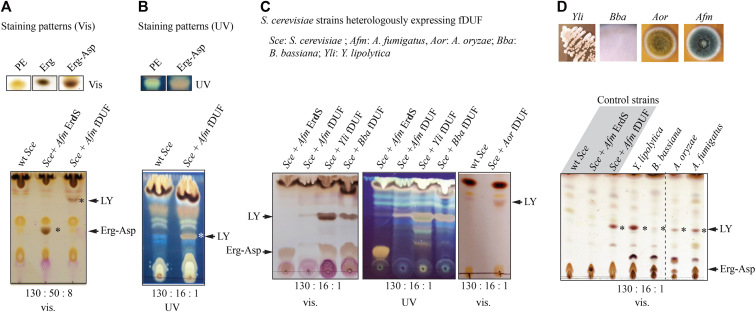
We first extracted total lipids from WT and Afm fDUF-expressing Sce strains (Sce+Afm fDUF) and separated them by TLC, using a solvent system that was appropriate to visualize Erg-Asp (10), i.e., CHCl3:CH3OH:H2O (130:50:8, v:v:v). Comparison of lipid profiles from the two strains revealed that a novel lipid termed lipid Y (LY) and absent from the WT strain (Sce) was produced by the Sce+Afm fDUF strain (Fig. 2A). LY displayed different migration properties than the Erg-Asp produced by Afm ErdS (Sce+Afm ErdS), denoting that the two enzymes have distinct enzymatic activities and synthesize distinct products. LY migrated close to the solvent front, indicating that it was more hydrophobic than Erg-Asp. Like Erg-Asp, it stained with a distinctive brownish color with a MnCl2/H2SO4 solution (30), suggesting that it could also be an Erg or sterol derivative (Fig. 2A) (10). LY weakly but positively stained with ninhydrin and was degraded under alkaline conditions (Fig. S3), which supported the hypothesis that it was likely an aminoacylated sterol in which the amino acid and sterol are linked by an ester (alkaline-sensitive) bond, like in Erg-Asp (10). In order to better visualize LY on TLC, we used the CHCl3:CH3OH:H2O (130:16:1, v:v:v) mobile phase (Fig. 2B). Under these better resolving conditions, we visualized that Afm, Aor, Bba, and Yli fDUF proteins led to the production of the exact same LY when expressed in Sce, e.g., shared the same migration and staining properties (Fig. 2C). Finally, we analyzed total lipid extracts from WT strains of Afm, Aor, Bba, and Yli and confirmed in each case that a lipid band with similar migration and staining properties as LY was present (Fig. 2D), with the exception of Bba, which, under the growth conditions used, produced only barely detectable levels of LY. In conclusion, upon heterologous expression in Sce, all tested fDUFs produce the same LY with identical migration and staining properties. This identical LY is also naturally present in Afm, Aor, Bba, and Yli, regardless of their positions within the fDUF-specific clade (Fig. 1D).
Figure 2.
Ascomycetes possess an fDUF, whose heterologous expression in S. cerevisiae triggers production of a novel lipid.A, heterologous expression of Afm ErdS (Sce+Afm ErdS) and fDUF in Sce (Sce+Afm fDUF). Total lipids were separated with the CHCl3:CH3OH:H2O (130:50:8, v:v:v) solvent, stained with MnCl2/H2SO4 and visualized under white (vis.) light. Control lipid bands (upper panel) were used to illustrate the staining of sterols under visible or UV light. Asterisks indicate Erg-Asp and LY bands. B, TLC analysis of total lipids extracted from a wt and Afm fDUF Sce-expressing strains with the CHCl3:CH3OH:H2O (130:16:1, v:v:v) solvent. TLC was visualized under UV light. C, TLC analysis of total lipids extracted from Sce strains expressing the indicated proteins: Sce+ Afm ErdS (Erg-Asp production), and Sce+ Afm-, or Bba-, or Yli-, or Aor fDUF (LY production). D, separation on HPTLC plates of total lipids from the indicated fungal strains (illustrated in the upper panel) compared with control strains: Sce WT, Sce + Afm ErdS, and Sce + Afm fDUF; the CHCl3:CH3OH:H2O ratios (v:v:v) are indicated. Erg, ergosterol; LY, lipid Y; PE, phosphatidylethanolamine.
fDUF2156 proteins produce an ergosteryl-3β-O-amino acid
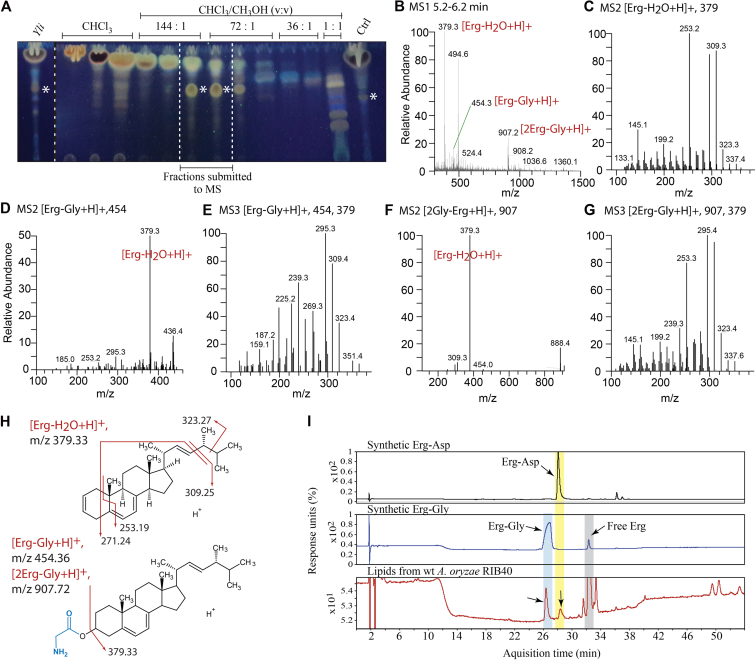
We next used total lipids from Yli that naturally expresses fDUF and produces the highest yields of LY (Figs. 1C and 2) and purified LY using flash column chromatography (FC) (Figs. 3A and S3) (10, 20). LC-MSn analyses of the resulting fractions revealed the presence of protonated ions of dehydrated ergosterol ([Erg-H2O + H]+, m/z = 379, see (10)), as well as of a compound at an m/z of 907. MSn analysis of this latter compound revealed a fragmented ergosterol ion at m/z = 379, suggesting that the parent ion (m/z = 907) maybe a protonated dimeric ion of glycylated ergosterol ([2ErgGly+H]+, m/z = 907), which would imply that LY would be, like Erg-Asp, an aminoacylated sterol, most likely an ergosteryl-3β-O-glycine (Erg-Gly), at least in Yli (Fig. 3). To test this hypothesis, we prepared (11) synthetic Erg-Gly (synErg-Gly) and analyzed it by the same methods. synErg-Gly and an FC-purified fraction both exhibited a peak at m/z = 907 and identical fragmented ion products upon MSn analysis. Tuning the instrument to the m/z peak at 907 using synErg-Gly allowed detection of the monomeric protonated ion [ErgGly+H]+ at m/z = 454 both in the synthetic Erg-Gly preparation and in the FC-purified sample (Figs. 3 and S4).
Figure 3.
Purification and LC-MSnanalysis of LY from Y. lipolytica and characterization of LY in A. oryzae.A, LY was purified from Yli total lipids by flash chromatography on silica gel 60 column. The TLC shows fractions from the purification. Apolar lipids eluted with CHCl3 and LY with CHCl3:CH3OH (v:v) solvents of increasing polarity. Yli, total lipids; Ctrl, total lipids from the Sce+ Yli fDUF strain. Asterisks indicate LY. B, MS1 spectrum between 5.2 to 6.2 min, (C) MS2 spectrum of ergosterol [Erg-H2O + H]+ at m/z = 379, (D) MS2 spectrum of ergosteryl-3β-O-glycine (Erg-Gly) [Erg-Gly+H]+ at m/z = 454, and (E) its MS3 spectrum at m/z = 379 (454→379). F, MS2 spectrum of the ion complex Erg-Gly [2Gly-Erg+H]+ at m/z = 907, and (G) its MS3 spectrum at m/z = 379 (907→379). H, structure of the ions of ergosterol [Erg-H2O + H]+, Erg-Gly [Erg-Gly+H]+ and MS2 fragmentations. MSn data are shown for peaks annotated in red. MS2 fragmentation of ergosterol is identical to that previously described (e.g., (10)). I, HPLC separation of total lipids extracted from wt Aor RIB40 that naturally expresses both ErdS and ErgS. Eluted compounds were monitored at 282 nm and peaks of interest were submitted to an Accurate-Mass Q-TOF LC-MS to confirm their identity. Synthetic Erg-Gly and Erg-Asp were used as a standard to identify, in total lipids, Erg-Gly and Erg-Asp.
To test whether other fDUF protein candidates also synthesize Erg-Gly, LC-MSn analyses were carried out on total lipid extracts originating from the Sce+ Afm fDUF strain, alongside the wt Sce strain (Figs. S3 and S4). Erg-Gly was detected in the dimeric ion form ([2GlyErg+H]+, m/z = 907) in the lipids extracted from the Sce+ Afm fDUF strain but was absent from wt lipids (Fig. S4). Similarly, HPLC separation of total lipids from the Sce+ Aor fDUF strain showed that an additional lipid identical to synErg-Gly, absent from a wt or an empty plasmid-carrying strain, could be detected (Fig. S5). Finally, we analyzed total lipids extracted from wt Aor RIB40 by HPLC. Comparison of profiles with standard profiles obtained with the synthetic Erg-Gly and Erg-Asp that we prepared (11) confirmed that Erg-Gly was present, together with Erg-Asp (Fig. 3I), in agreement with the fact that this fungus possesses a functional ErdS (10) and fDUF (Fig. 1 and see below).
In conclusion, Erg-Gly could be formally detected not only in Yli and Aor total lipids but also in total lipids extracted from Sce strains heterologously expressing Afm and Aor fDUF, demonstrating that the three enzymes (from Afm, Aor, and Yli) produce the same aminoacylated sterol in situ (in Aor and Yli) as well as when heterologously expressed (in Sce). Bba fDUF, although not characterized by MS, produces the same aminoacylated lipid, since its properties on TLC (Fig. 2, C and D) are equivalent to those of Erg-Gly produced by Afm, Aor, and Yli enzymes. Therefore, because fDUF is a homolog of ErdS (Fig. 1), all these results advocate for the hypothesis that fDUF proteins aminoacylate Erg using Gly-tRNAGly as a Gly donor, making them putative ergosteryl-3β-O-glycine synthases, or ErgS (Er: ergosterol, g: glycine, S: synthase).
Mutations in the α(+) helix suggest that ErgS is aa-tRNA dependent in vivo
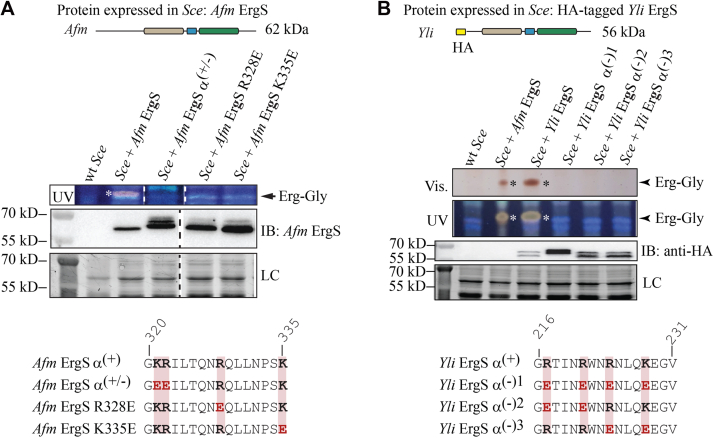
Erg aspartylation by ErdS (10) and addition of Lys, Arg, or Ala onto glycerolipids by aaGLSs (17) are all strictly aa-tRNA dependent. This is also the case for other transferases that modify other types of macromolecules (16, 31). In the case of aaPGSs, the α(+) helix found between GNAT domains I (lipid binding) and II (aa-tRNA binding) (Fig. 1) is crucial to the aa-tRNA substrate utilization, likely because this positively charged helix helps binding the tRNA acceptor arm through charge interaction with the ribose-phosphate backbone of tRNA (26). In the case of ErgS, utilization of Gly-tRNAGly was expected (Fig. 3). In order to verify the tRNA dependency of the reaction, we first used an in vivo approach and heterologously expressed in Sce either wt Afm ErgS or variants of the enzyme mutated in the α(+) helix (Fig. 1B) in order to determine whether mutations impaired Erg-Gly synthesis. K321, R322, R328, and K336 were mutated into glutamate residues in three different combinations (Fig. 4A), yielding helices with negatively charged side chains that, according to the tRNA binding hypothesis, should repel tRNA and decrease Erg-Gly accumulation. Afm ErgSα(+/-), in which K321 and R322 were mutated to E (R328 and K335 remained unchanged), ErgS R328E, and ErgS K335E (in which only one residue at a time was mutated) were used (Fig. 4A). Results showed that, as hypothesized, wt Afm ErgS produces Erg-Gly in Sce, whereas all three mutants failed to synthesize detectable amounts of the Erg-Gly. Anti-Afm ErgS antibodies were used to confirm that the enzyme was expressed in all cases. Note that, when expressed in Sce and analyzed by Western blot, bands of Afm ErgS mutants, but not of the wt enzyme, appear as doublets, which likely accounts for putative mobility changes due to the mutations introduced or possible posttranslational modifications.
Figure 4.
Mutation of the α(+)helix suggests that Erg-Gly synthesis is tRNA dependent in vivo.A, Afm ErgS and three mutants of the α(+) helix (described at the bottom) or (B) Yli HA-tagged ErgS and three mutants of the α(+) helix (described at the bottom) were expressed in Sce. TLCs were developed in the CHCl3:CH3OH:H2O (130:16:1, v:v:v) solvent, stained with MnCl2/H2SO4 and visualized under white (vis.) or UV light. Asterisks indicate the position of Erg-Gly. Immunoblots were also performed on whole extracts to detect expression of Afm ErgS (and mutants) using anti-Afm ErgS antibodies, or anti-HA antibodies to detect Yli HA-ErgS (and mutants). LC, loading control (strain free).
As already observed, Erg-Gly levels were very low in the Sce+Afm fDUF (ErgS) strain, when compared with those of the Sce+Yli fDUF strain (Fig. 2C). We therefore generated Sce strains expressing three Yli fDUF variants mutated in the α(+) helix (residues 216–231) (Fig. 4B).We named these mutants Yli ErgS α(-)1 when R217, R221, R224, and K228 were all mutated to E; Yli ErgS α(-)2 when R217 and R221 were mutated to E; and Yli ErgS α(-)3, when mutation of R224 and K228 to E were present (Fig. 4B). Similar to Afm ErgS, all Yli ErgS mutants were defective in Erg-Gly synthesis, which again supported the hypothesis that the α(+) helix is essential. Since our anti-Afm ErgS antibodies did not cross-react with Yli ErgS (Fig. S6), we expressed the wt and mutant Yli enzymes as N-terminally HA-tagged versions. In all cases, the enzymes were expressed, but no Erg-Gly synthesis was detected for the HA-tagged mutant versions, in contrast to the HA-tagged wt enzyme. Taken together, and given the role attributed to the α(+) helix (26), our results strongly support that Afm and Yli ErgS are, as hypothesized, aa-tRNA dependent and, more specifically, Gly-tRNAGly dependent, a hypothesis that we decided to analyze in more detail in vitro.
Erg-Gly synthesis strictly depends on Gly-tRNAs
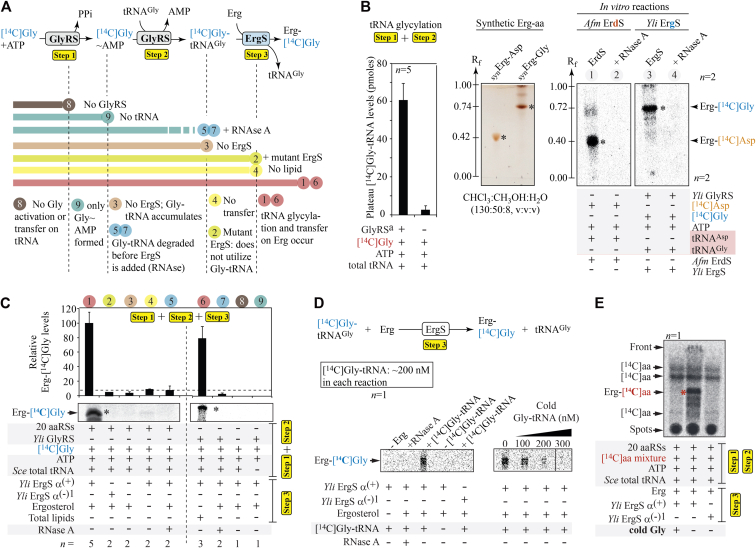
Erg-Gly, like Erg-Asp, is a 3β-O-ester of sterol and formation of ester bonds is nonspontaneous under physiological conditions (32), implying that ErgS must use an activated form of Gly. As for all similar ATTs (10, 31), it must be an aa-tRNA, here Gly-tRNAGly. Gly-tRNAGly is produced by GlyRS, reason for which we expressed and purified recombinant Yli His6-GlyRS. GlyRS sequentially produces two forms of activated Gly: glycyl-adenylate (Gly∼AMP) and Gly-tRNAGly itself (Fig. 5A) that could be utilized by ErgS. In order to decipher Erg glycylation by ErgS in vitro and to understand each step of the reaction (Fig. 5A), we expressed Afm and Yli ErgS in Escherichia coli as maltose-binding protein (MBP) fusions to improve solubility of the recombinant enzymes and purified them by affinity chromatography. With those enzymes, we performed a series of experiments in order to determine which of the GlyRS products was used by ErgS.
Figure 5.
In vitro investigation of Erg-Gly synthesis by ErgS.A, Erg-Gly synthesis reaction scheme (coupled reaction, when GlyRS and ErgS are present at the same time). Step 1: Gly activation into Gly∼AMP by GlyRS and step 2: transfer onto tRNAGly; step 3: Erg-Gly synthesis from Gly-tRNAGly. Colored bands below the scheme indicate up to which step the coupled reaction proceeds, when the indicated component (right side) is omitted (−) or added (+). Numbers refer to the lanes presenting the in vitro results in (C). B, left panel, [14C]Gly-tRNAGly synthesis by YliHis6-GlyRS or total aaRS extracts (containing GlyRS) from wt Sce in a typical coupled reaction. Results are identical for both sources of GlyRS (denoted by GlyRSa). Middle panel, control TLC showing mobility of synthetic Erg-Asp and Erg-Gly in the CHCl3:CH3OH:H2O (130:50:8, v:v:v) mobile phase. Right panel, control reactions with purified Afm ErdS (produces Erg-[14C]Asp, indicated with an asterisk, and a second band (∗∗) discussed below) and purified Yli MBP-ErgS with the indicated components. ErgS produces a compound (∗) with properties identical to synErg-Gly, showing that it synthesizes Erg-Gly. C, Erg-[14C]Gly synthesis experiments under the indicated conditions (+, presence; −, absence of the indicated components). Yli MBP-ErgS (or mutated ErgS) was added to [14C]Gly-tRNAGly produced by Sce GlyRS (in a total protein extract) or YliHis6-GlyRS in the presence/absence of various substrates and/or RNase A. Asterisks indicate position of Erg-[14C]Gly. D, Erg-[14C]Gly synthesis (step 3 only) with added [14C]Gly-tRNA (∼200 nM) and increasing amounts of cold Gly-tRNA (100–300 nM). E, isotopic competition of cold Gly for Erg-[14C]Gly synthesis. 15 [14C]aa-tRNAs were synthesized with 15 aaRSs (Sce total proteins), from 15 [14C]aa and total Sce tRNA, to which Erg, ErgS, the ErgSα(-)1 mutant, or RNase A was added. [14C]aa indicate bands attributed to free radiolabeled amino acids present in the reaction and separated by TLC. The red asterisk indicates Erg-[14C]Gly. In all cases, bars indicate standard deviation and the number of replicates is indicated.
In Figure 5A, we present the overall reactions catalyzed by GlyRS and ErgS and, below, the different steps to which reactions can proceed under the various experimental conditions that we tested to understand which substrates were required for activity. In the presence of [14C]Gly, ATP, and either total tRNA or partially fractionated tRNAGly, Yli His6-GlyRS synthesized [14C]Gly-tRNAGly (Fig. S7). [14C]Gly-tRNAGly could also be produced using GlyRS from wt Sce crude protein extract (Fig. 5B, left panel). [14C]Gly-tRNAGly plateau typically reached ∼60 pmol per reaction. Adding Yli MBP-ErgS to a mixture containing Yli His6-GlyRS, partially fractionated Sce tRNAGly, ATP, [14C]Gly, and Erg confirmed that a radiolabeled band (Fig. 5B, right panel, lane 3) with migration properties similar to our synErg-Gly (10, 11) (Fig. 5B, middle panel) was observed, which we therefore attributed to Erg-[14C]Gly. In parallel, we used purified Afm ErdS (10), in the presence of [14C]Asp, pure Sce tRNAAsp, ATP, and Erg and verified that it produced Erg-[14C]Asp (Fig. 5B, right panel, lane 1), which had very distinct migration properties as compared with Erg-[14C]Gly under the same TLC conditions. None of the enzymes was active when RNase A, which degrades tRNAs, was added. This confirmed the tRNA dependency of the reaction. Since Erg-Gly synthesis occurred in vitro, we further investigated ErgS activity. For yet unknown reasons, purified recombinant Afm MBP-ErgS was inactive in vitro, suggesting that it lost its activity upon purification from E. coli or that the MBP tag hindered activity (Fig. S7 and see below). Furthermore, Erg-Gly accumulation in vivo in the Sce+Afm ErgS strain was also much lower than in the Sce+Yli ErgS strain (Fig. 2C), which prompted us to focus on recombinant Yli MBP-ErgS for further in vitro investigations.
TLC analyses of reaction products under various conditions (Fig. 5, A and C) revealed that, upon [14C]Gly-tRNA production, addition of Yli MBP-ErgS enabled the synthesis of Erg-[14C]Gly when either pure Erg or total lipids from Sce (containing Erg) were used as acceptors (Fig. 5C, lanes 1 and 6). In contrast, the Yli MBP-ErgSα(-)1 mutant (described in Fig. 4B) was inactive (Fig. 5C, lane 2), thereby confirming the essentiality of the α(+) helix for activity. As expected, in the absence of ErgS (Fig. 5C, lane 3), total lipids, or Erg (Fig. 5C, lane 4), no Erg-[14C]Gly could be detected, meaning that the reaction depended on the presence of ErgS and on Erg, either pure or in total lipids. As already described above, addition of RNase A (Fig. 5C, lanes 5 and 7) resulted in the absence of Erg-[14C]Gly production. In agreement, when Yli His6-GlyRS was present, but tRNA absent (Fig. 5C, lane 9), Yli MBP-ErgS could not use glycyl-adenylate (Gly∼AMP) produced under these conditions by GlyRS to synthesize Erg-[14C]Gly. This confirmed that ErgS uses only Gly-tRNA and not Gly∼AMP as a source of activated Gly. In the absence of GlyRS (Fig. 5C, lane 8), ErgS was, as expected, not able to synthesize Erg-[14C]Gly, demonstrating that free and nonactivated Gly is not a substrate of ErgS. Note that, sometimes, Erg-[14C]Gly or Erg-[14C]Asp bands may appear as doublets, which denotes the fact that Erg may be oxidized, yielding an aspartylated oxidized sterol with slightly different migration properties than Erg-Gly or Erg-Asp, respectively.
In line with ErgS’s specificity for Gly-tRNAGly, when Yli MBP-ErgS was added to a reaction mixture containing only Erg and no GlyRS, ATP, free tRNA, or free [14C]Gly, i.e., making [14C]Gly-tRNAGly synthesis impossible, Erg-[14C]Gly synthesis could be initiated only with addition of preformed [14C]Gly-tRNAGly (∼200 nM), unless RNase A was added (Fig. 5D). When increasing concentrations of cold Gly-tRNAGly (100–300 nM) were used to compete free [14C]Gly-tRNAGly, the intensity of Erg-[14C]Gly bands correlatively decreased on TLC, indicating strong and specific isotopic competition (Fig. 5D). In parallel, to probe ErgS’s aa-tRNA specificity, we used a Sce total protein extract, containing all aaRSs, a mix containing 15 [14C]aa (lacking Asn, Gln, Cys, Met, and Trp), total Sce tRNA, and ATP to produce the 15 corresponding [14C]aa-tRNAs (∼50,000 cpm per reaction when plateaus were reached, Fig. S7). To these 15 [14C]aa-tRNAs, we added Yli MBP-ErgS and observed that a band corresponding to Erg-[14C]Gly was present, which completely disappeared on TLC when excess unlabeled cold Gly was added (Fig. 5E), showing that, of the 15 [14C]aa used, only Gly-tRNAGly, formed by GlyRS, is used by ErgS. Again, no Erg-[14C]Gly was observed when the Yli MBP-ErgS α(-)1 mutant was used.
Specificity of ErgS and ErdS
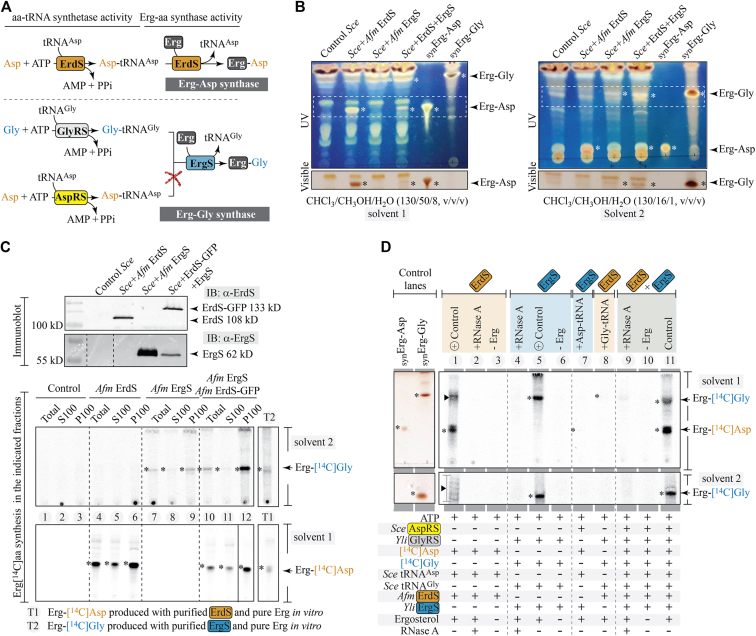
ErdS produces its own Asp-tRNAAsp from ATP, Asp, and tRNAAsp, and the cis-acting transferase (DUF2156) domain uses it to transfer Asp onto Erg. In contrast, ErgS uses a Gly-tRNAGly substrate produced in trans by an independent GlyRS (Figs. 5 and 6A). Because ErdS and ErgS are homologous (Fig. 1B), we wondered whether these enzymes could or not display functional promiscuity both in vitro and in vivo. ErgS is not expected to use Asp-tRNAAsp (Fig. 6A). We first verified it in vivo using Sce strains (Fig. 6B) expressing ErdS alone (Sce+Afm ErdS), ErgS alone (Sce+Afm ErgS), or both enzymes at the same time (Sce+Afm ErdS+Afm ErgS). Heterologous expression was confirmed by Western blot (Fig. 6C, upper panel and Fig. S8) using anti-Afm ErdS and anti-Afm ErgS antibodies, before extracting total lipids to analyze them by TLC. An Sce strain expressing an ErdS version deleted from its Erg-Asp synthase/DUF2156 domain (ErdSΔDUF) was used as a negative control. Results show that only Erg-Asp accumulates when ErdS is expressed, with no detectable Erg-Gly. In contrast, Erg-Gly is detected when ErgS is expressed, with no Erg-Asp visible on TLC. Coexpression of ErdS (in the form of ErdS-GFP fusion) and ErgS resulted in the accumulation of both Erg-Asp and Erg-Gly.
Figure 6.
Specificity of ErgS and ErdS.A, Erg-Asp synthase (ErdS) and Erg-Gly synthase (ErgS) reaction schemes. ErgS is not expected to use Asp-tRNAAsp produced by AspRS. B, TLC profiling of total lipids from Sce strains expressing Afm ErdSΔDUF (AspRS activity-only, no production of Erg-Asp), considered as the control strain, or Afm ErdS alone, Afm ErgS alone, or both Afm ErgS and Afm ErdS fused to a C-terminal Green Fluorescent Protein (GFP) tag. Migration has been conducted with two solvents as to visualize Erg-Asp and Erg-Gly simultaneously (solvent 1) or Erg-Gly alone (solvent 2). TLCs were pictured under UV light (254 nm) upon sulfuric acid/MnCl2 staining (upper TLC) and regions delimited by dashed boxes are provided as visualized under white light (visible) below each panel. Asterisks indicate the presence of bands attributed to Erg-Asp and Erg-Gly as compared with migration profiles of synthetic (syn) Erg-Asp and Erg-Gly standards. C, Western blots showing expressions of Afm ErgS and ErdS (or ErdS-GFP) in the four tested strains (full Western blot and expression controls are provided in Fig. S8). For each of the four strains, we tested the Erg-[14C]aa synthesis activity in total proteins (total), in 100,000g supernatants containing soluble proteins (S100) or in the corresponding 100,000g pellet corresponding to total membrane-associated proteins. The upper TLC presents results obtained in vitro in the presence of purified Yli GlyRS, ATP, total tRNA, [14C]Gly to produce [14C]Gly-tRNA, to which Erg has been added (migration using solvent 1). T1 presents a control reaction under the same conditions, to which purified Afm ErdS was added to produce a reference Erg-[14C]Asp. The lower TLC presents results obtained in vitro in the presence of ATP, purified tRNAAsp, [14C]Asp, from which ErdS produces [14C]Asp-tRNA, to which Erg has been added (migration using solvent 2). T2 presents a control reaction under the same conditions, to which purified Yli MBP-ErgS was added to produce a reference Erg-[14C]Gly. (Details on the composition of reactions are provided in Fig. S8.) D, in vitro Erg[14C]aa synthesis experiments using purified Yli MBP-ErgS and/or Afm ErdS in various combinations in the presence of different combinations of tRNA and/or [14C]aa substrates. Results confirm that both enzymes are tRNA dependent and require Erg and that ErdS is specific for Asp-tRNA and ErgS for Gly-tRNA.
In parallel, we tested Erg-[14C]Asp and Erg-[14C]Gly synthesis activities in protein extracts from all strains in vitro (Figs. 6C and S8). To this end, we extracted total proteins from each strain and separated soluble (S100) and membrane-bound proteins (P100) by centrifugation. As expected, we detected Erg-[14C]Asp synthesis in total, S100, and P100 extracts from the Sce+Afm ErdS (Fig. 6C, lower panel, lanes 4–6) and Sce+Afm ErdS+Afm ErgS (lanes 10–12) strains, while Erg-[14C]Gly only occurred in Sce+Afm ErgS (lanes 7–9) and Sce+Afm ErdS+Afm ErgS strains (lanes 10–12). No activity was detected in the control Sce strain (lanes 1–3). Activity of ErdS was distributed in both the P100 and S100 fractions, with a slight enrichment in the P100 (membrane) fraction, which was much more pronounced for ErgS activity. This was also observed in extracts from wt Afm (Fig. S7E). No detectable Erg-[14C]Asp synthesis activity was associated with ErgS expression in the absence of ErdS, and no significant Erg-[14C]Gly synthesis activity could be detected with expression of ErdS in the absence of ErgS. Only coexpression resulted in the detection of both Erg-[14C]aa products (Fig. 6C, lanes 10–12), advocating for nonoverlapping specificities.
We verified the same results using purified Afm MBP-ErdS and Yli-MBP-ErgS (Fig. 6D). ErdS alone could synthesize Erg-[14C]Asp from [14C]Asp, ATP, and pure Sce tRNAAsp (lane 1), whereas Yli ErgS produced Erg-[14C]Gly in the presence of Yli His6-GlyRS, partially fractionated Sce tRNAGly, [14C]Gly, and ATP (lane 5). None of the enzymes functioned in the presence of RNase A (lanes 2, 4) or in the absence of Erg (lanes 3, 6). Both Erg-[14C]Asp and Erg-[14C]Gly were synthesized when all the required substrates and enzymes were mixed jointly with ErdS and ErgS (lane 11), unless RNase A was added (lane 9) or Erg omitted (lane 10). Lane 1 shows that ErdS produced a second product (as noted in Fig. 5C) with migration properties similar to Erg-Gly (lane 5), which could not correspond to Erg-[14C]Gly, since no [14C]Gly, tRNAGly, or GlyRS was present in the assay. In addition, TLC analyses using the CHCl3:CH3OH:H2O (130:16:1, v:v:v) mobile phase (lower panel, solvent 2) showed that it contained various unidentified [14C]Asp-labeled products that distributed differently from control synErg-Gly and synErg-Asp, or Erg-[14C]Gly (lane 5), likely making these chemical species by-products of the reaction, or unspecific products resulting from the use of low-amount lipid contaminants in commercial Erg. Purified Sce AspRS, tRNAAsp, ATP, and [14C]Asp were used to produce [14C]Asp-tRNAAsp (lane 7) (and Fig. S7), and addition of Yli ErgS led to only barely detectable Erg-[14C]Asp levels, showing that ErgS is highly specific toward Gly-tRNAGly. Similarly, when Yli His6-GlyRS, fractionated tRNAGly, [14C]Gly, and ATP were used to produce [14C]Gly-tRNAGly (lane 8), addition of Afm ErdS only resulted in the synthesis of very low Erg-[14C]Gly levels, showing that the transferase domain of ErdS is also highly specific for Asp-tRNAAsp.
Together, these results demonstrate that the ErgS enzymes that we tested are all Gly-tRNAGly-dependent transferases that transfer Gly onto the 3β-hydroxyl of Erg, yielding Erg-Gly and that ErdS is specific for Erg-Asp production from Asp-tRNAAsp. ErgS and ErdS appear to possess nonoverlapping enzymatic specificities in vivo.
Distribution of ErgS across Dikarya
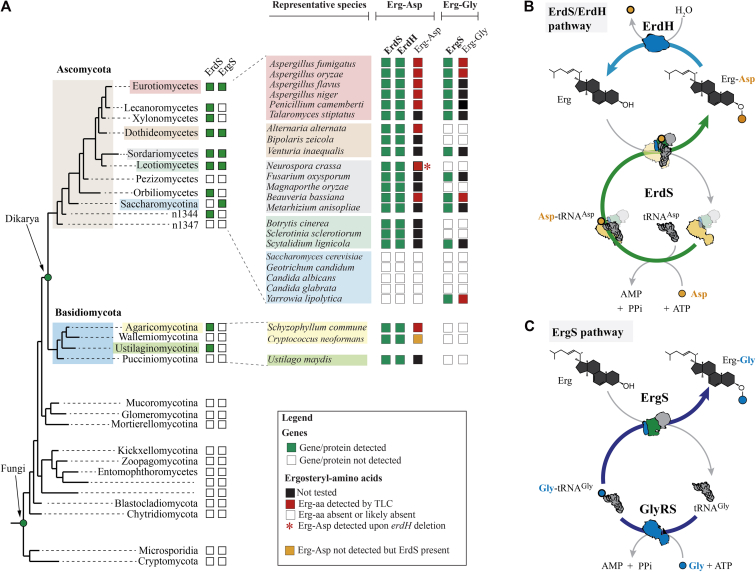
Phylogenomics analyses show that, in contrast to ErdS, which was detected in numerous Dikarya (Ascomycota and Basidiomycota), ErgS proteins seem absent in Basidiomycota, with the exception of one species, Trichosporon oleaginosus (Tol, Fig. 1C). This suggests that ErgS is likely specific of Ascomycota. This includes most of Eurotiomycetes (Aspergillus, Penicillium spp., etc.), a large number of Sordariomycetes (Bba, for example), and several Dothideo- and Leotiomycetes (Fig. 7A). Of interest, ErdS was completely absent in Saccharomycotina (10), whereas we detected one ErgS in Yli (Fig. 1B) and another Yarrowia spp. Of note, in Eurotiomycetes, Sordariomycetes, Dothideomycetes, and Leotiomycetes, when ErgS is detected, it is always found in combination with ErdS, implying that these species almost always possess both paralogs and that they likely produce Erg-Gly and Erg-Asp (Figs. 3I and 7). This is the case, for example, of all Aspergillus and Penicillium spp. studied (Eurotiomycetes). In N. crassa (Sordariomycetes), in which ErdS was detected and studied (10), ErgS is absent. More generally, with the exception of Yarrowia spp., no stand-alone ErgS was detected in all fungal species that we investigated.
Figure 7.
Distribution of ergosteryl-amino acid synthases in fungi, and overview of Erg-Gly and Erg-Asp synthesis mechanisms and factors. ErgS (Erg-Gly synthase) and ErdS (Erg-Asp synthase) sequences were searched across eukaryotes and, most specifically, in the fungal kingdom, and the presence (green squares) or absence (white squares) of the corresponding proteins was mapped onto a simplified version of the phylogenetic tree of fungi (A), as provided by the JGI Genome Portal (see Experimental procedures), in main fungal lineages. In Eurotiomycetes (red), Dothideomycetes (light brown), Sordariomycetes (gray), Leotiomycetes (light green), and Saccharomycotina (blue), the presence/absence of ErgS and ErdS was indicated in several representative species (as well as in three basidiomycetes in Agaricomycotina and Ustilaginomycotina). Erg-aa synthases are detected only in Dikarya and ErgS only in Ascomycota. B, Erg-Asp biosynthesis and degradation pathway. ErdS uses free L-Asp, ATP, and tRNAAsp and produces Asp-tRNAAsp in its AspRS domain (yellow), which is transferred to the appended DUF2156/ATT domain where it serves as the Asp donor for Erg-Asp synthesis. ErdH is an Erg-Asp specific hydrolase (whose distribution is indicated in (A)). C, Erg-Gly synthesis pathway. GlyRS uses Gly, ATP, and tRNAGly to produce Gly-tRNAGly, which is then released, captured by ErgS, and used as the Gly donor for Erg-Gly synthesis.
Discussion
In bacteria, aaGLSs tRNA dependently modify glycerolipids (GLs) with amino acids and modulate membrane properties, increasing pathogenicity and antimicrobial resistance (17, 18). Phylogenetic analyses showed that bacterial aaGLSs are all phylogenetically related and that they can be separated into seven sequence groups (20). Most of aaPGSs possess an N-terminal transmembrane domain, in addition to the ATT/DUF2156 domain (20, 23), that is an aaGL flippase (33, 34) transferring modified lipids from the inner leaflet of the plasma membrane, where they are produced, to the outer leaflet (17, 18). No correlation has been detected between aaPGS clades and aa-tRNA and/or lipid specificities. In addition, in several bacteria, several aaGLSs coexist as paralogous enzymes and increase the diversity of modified GLs (20).
Although aaGLSs and modified GLs could be detected in numerous bacteria and several archaea, no proof on the existence of homologous eukaryotic proteins or similar aminoacylated lipids had been reported until recently (17). In Dikarya, ErdS uses L-Asp, ATP, and tRNAAsp and produces Asp-tRNAAsp in its so-called AspRS domain, and Asp is then transferred from its tRNA by the appended ATT/DUF2156 domain onto Erg to synthesize Erg-Asp (10) (Fig. 7B). Some bacteria possess several paralogs of aaGLSs. In Dikarya, we now demonstrated that Ascomycota possess a second, paralogous enzyme that we named ErgS and that uses the Gly-tRNAGly produced by an independent GlyRS, to form Erg-Gly (Fig. 7C). This observation shows that ergosteryl-3β-O-amino acids (Erg-aa) are diverse. ErgSs seem absent in Basidiomycota and are always found in combination with ErdS, as, for example, in all Eurotiomycetes examined, in a subset of Sordariomycetes, and in several Dothideo- and Leotiomycetes (Fig. 7A) that, like in the case of Aor (Fig. 3I), produce both Erg-Asp and Erg-Gly. Yli, which belongs to Saccharomycotina, is an exception since it only possesses ErgS and consequently only produces Erg-Gly.
The ATT (DUF2156) domain of ErgS and ErdS form two distinct phylogenetic clades (Fig. 1C). Heterologous expression of four ergS genes in Sce, as well as MS detection and characterization of Erg-Gly in Yli and Aor (Figs. 2 and 3), shows that those four ErgS proteins of interest, although scattered throughout the ErgS clade (Fig. 1C), have the same activity, which confirms that there is a good correlation between the DUF2156 clade to which a fungal ATT belongs and its aa-tRNA specificity. ErdSs produce and are specific for Asp-tRNAAsp, whereas ErgSs use Gly-tRNAGly (Figs. 5, 6 and S7) with no overlapping activities in vivo. Both enzymes aminoacylate Erg, suggesting that ErdS and ErgS may be paralogs that descended from an ancestral enzyme that already was sterol specific, i.e., an Erg-aa synthase (ErxS, Er: Erg; x: amino acid in the one letter code; S: synthase). Following a putative duplication event, the two separate enzymes may have therefore undergone functional specialization for Asp-tRNAAsp (ErdS) and Gly-tRNAGly (ErgS). Since ErdS is present in all Dikarya (Ascomycota and Basidiomycota), whereas ErgS only in Ascomycota, we could speculate that the duplication, if it occurred, took place only in this clade to give rise to ErgS, but this requires further and more detailed phylogenetic investigations. Both enzymes are also related to bacterial aaPGSs. Since aaPGSs are widespread in bacteria (17, 20), but apparently absent in eukaryotes (10, 20), we can infer that ErxSs were acquired by fungi, likely through horizontal gene transfer from bacteria (35) to the last common ancestor of Dikarya. Since no known bacterial aaGLSs are sterol specific, the sterol specificity of ErxS might be a fungi-specific derived trait, but this cannot be determined at present. Precise phylogenetic analyses should therefore now be performed to (i) decipher the evolutionary history of both ErxS classes, (ii) understand the origin of their sterol and aa-tRNA specificities, (iii) determine the origin of the AspRS-DUF2156 (ErdS) fusion, and (iv) explain the asymmetric distribution of ErgS in comparison with that of ErdS. It should be emphasized that sequence identity between ErxSs and, more generally, between GNAT proteins, including DUF2156 proteins, is generally low (25). In consequence, other types of cryptic and/or highly diverged ATTs might exist in Dikarya or, alternatively, in other eukaryotes.
Erg is the main sterol lipid present within fungal membranes (9). Like Cho in mammalian cells, it influences the activity of membrane proteins, including V-ATPases that participate in antifungal drugs resistance (36, 37), participates in the formation of membrane microdomains (38, 39), and, together with sphingolipids, regulates membrane trafficking and polarized cell growth in filamentous fungi (40, 41). Even the mating process, during sexual reproduction, is dependent on the integrity of Erg for membranes fusion (42, 43) or for pheromone sensing (44). Erg also plays multiple roles in pathogenicity and antifungal resistance (9, 45, 46). In Sce, Erg acetylation on the 3β-hydroxyl by the Atf2 acetyltransferase and deacetylation by the Say1 esterase participates in a process that detoxifies Erg intermediates and increases resistance against membrane-disrupting agents such as the antifungal eugenol (4, 47). In addition, 3β-O-fatty acylation of Erg by fatty acyl-CoA transferases directs this sterol to lipid droplets where it is stored until environmental cues trigger deacylation of the fatty acid moieties by dedicated esterases for its remobilization (8, 48). Erg 3β-O-glycosylation by the Atg26 protein participates in the regulation of autophagy in several fungal species, including Aspergillus spp. (49, 50, 51), and also strongly influences virulence in pathogens (reviewed in (7)). To these physiologically important modifications of the 3β-hydroxyl of sterols, we now add aspartylation (Erg-Asp) (10) and glycylation (Erg-Gly; this study).
The biological functions of fungal Erg-aa still remain to be deciphered; however, several hypotheses can be proposed. For example, Erg-aa being modified sterols with novel chemical properties, they could be involved in sterols trafficking, like are Erg-FA (8, 48). They could also affect permeability and fluidity of fungal plasma membranes, as is the case for aminoacylated GLs in bacteria (17, 18), or in addition, could modify the properties of membrane microdomains (38). Since microdomains, enriched in Erg and sphingolipids, are crucial to the localization of drug efflux pumps (52), Erg-aa could affect drug efflux or transporters and/or membrane-localized sensing machineries that depend on Erg integrity (36, 42, 44). In other words, Erg-aa might contribute to the overall fitness of fungi under challenging environmental conditions, such as in the presence of membrane-targeting antifungals or antimicrobial peptides, as is the case for Erg-Ac in Sce (4, 47). In addition, Erg-Gly and Erg-Asp could directly participate in the resistance against polyenes that interact with Erg through its free 3β-OH group (53), because ErgS and ErdS add amino acids that mask this free hydroxyl group, which might change polyenes/Erg interactions and change drug susceptibility.
Lipid-derived molecules also often participate in lipid signaling in eukaryotes (41, 54), like, for example, in the case of phosphoinositides and sphingolipids (55) or sulfated sterols (3). As mentioned, Erg-Glc, another sterol conjugate, produced by Erg glycosylation, is nonessential but known to actively participate in the regulation of autophagy in several yeasts and in Aor, likely through the recruitment of protein partners on membrane structures (50). It is therefore possible that Erg-aa could also intervene in the recruitment of protein factors on membranes and influence regulatory or trafficking pathways.
With the discovery of a second Erg-aa synthase, we uncovered an additional subfamily of enzymes within the AATs. Further phylogenomic mining, coupled with biochemical characterization, will be needed to know whether other subfamilies of RNA-dependent sterol-aminoacylating enzymes exist. Sequence-based alignments failed to provide detailed information as to which residues of the GNAT I or GNAT II domains are responsible for lipid recognition or aa-tRNA specificity, respectively. In addition, 3D structures of ErdS and ErgS have not yet been established. Our previous work on ErdS already showed that GNAT I has a certain degree of plasticity for the recognition of the sterol substrate, since it also aspartylates Cho (10). These enzymes could therefore also aminoacylate ergosterol intermediates or derivatives having a free3β-OH position to accept the amino acid. Likewise, it is conceivable that ErgS-like enzymes from fungal species we did not yet study might have different aa-tRNA specificities and could thus further expand the steryl-amino acid repertoire synthesized by fungi.
Experimental procedures
Reagents
All plasmids were purified using the Nucleospin Mini kit (Macherey-Nagel, 740588.50) or the NucleoBond Xtra Midi Plus kit (Macherey-Nagel, 740412.50). All enzymes for molecular cloning and Gibson Isothermal assembly were from New England Biolabs or Thermo Fisher. Thin layer chromatography (TLC, Silica gel 60 aluminum foils) plates were from Sigma-Aldrich (56524-25EA) and high-performance TLC (HPTLC) (HPTLC Silica gel 60) from Merck (1.05547.0001). Silica gel 60 for lipid purification by chromatography was from Sigma-Aldrich (Merck, ref: 227196). Commercial ergosterol was from Sigma-Aldrich (45480-10G-F), as cholesterol (C8667). All reagents and solvents were of high-grade purity. The radiolabeled amino acid mixture was from PerkinElmer (NEC850E050UC), and [14C]Glycine (98 cpm/pmol, 600 μM) and [14C]Asp (280 cpm/pmol) were from Amersham Life Sciences. Anti-Afm ErgS antibodies were developed using purified Afm ErgS (fDUF) with the Covalab company (France). Secondary goat anti-rabbit antibodies were from Bio-Rad (170.6516). Anti-HA antibodies were purchased from Roche (1583816) and secondary rabbit anti-mouse antibodies from Jackson Immuno Research (11035003). Synthetic Erg-Asp and synthetic Erg-Gly were prepared as described in (10, 11), respectively.
Biological resources
Strains used in this study are listed in Table S1. The A. fumigatus CEA17 ΔakuBKU80 (56) (kind gift from Prof. J. P. Latgé, Institute Pasteur, Paris, France) and A. oryzae RIB40 (57) (gift from Dr H. Nakajima) strains were used. Y. lipolytica (Yli) CLIB122 was a gift from Dr C. Bleykasten (IPCB, Strasbourg, France) and the wt B. bassiana 80.2 (Bba) strain was kindly provided by Dr D. Ferrandon (IBMC, Strasbourg, France). The S. cerevisiae (Sce) BY4742 strain (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) (Euroscarf) was used. The E. coli XL-1 Blue or DH5α strain was used as recipient strain for molecular cloning and the E. coli Rosetta-2 strain was used for recombinant protein expression. Primers and plasmids used in this study are reported in Table S2. For routine growth and maintenance, strains were used as indicated in the Supplementary Material and Methods section.
Total lipids extraction
Wt Yli and Sce were grown in yeast extract peptone dextrose (YPD) medium, whereas the Sce strains expressing Yli, Afm, Aor, or Bba ErgS were grown in Synthetic Complete media deprived of leucine (SC-Leu). Cultures were diluted to an A600 = 0.1 and grown overnight (220 rpm), and cells were harvested by centrifugation at 5000g for 15 min at 4 °C, when A600 was 0.6 to 1, depending on the experiment. Aspergillus spp. conidia were harvested as described (10) and inoculated in glass flasks (50 ml) in the YPD2 medium (Glucose 4% w/v, Peptone 1% w/v, and Yeast extract 2% w/v) at a concentration of 106 conidia/ml, shaken at 37 °C (Afm) or 30 °C (Aor) during 18 h. Mycelia were collected by filtration through sterile gauze and extensively washed with sterile distilled water, dried on paper towels and used for lipid extraction. Bba spores were collected as for Afm, inoculated in the YPD2 medium (50 ml) at a concentration of 106 spores/ml and grown for 18 h at 25 °C, and mycelia were collected by filtration through sterile gauze, washed, and dried before use.
Total lipid extraction was performed as described (10). Briefly, 50 A600 of Sce or Yli cells were resuspended in 0.5 ml of Na-Acetate 120 mM, pH 4.5. Then, 3.75 volumes of CHCl3:CH3OH (2: 1) and 1 ml of glass beads (ø 0.25–0.5 mm, Roth) were added and cells were disrupted through mechanical lysis using a FastPrep Instrument (MP Biomedicals, Serial N° 10020698) at 1 min 5.5 m/s repeated 6 times. Cell lysates were incubated 3 h on a rotating wheel at 4 °C. Then, 1.25 volumes of CHCl3 and 1.25 volumes of 120 mM Na-Acetate pH 4.5 were added and the samples vortexed 1 min. Phases were separated by centrifugation (9000g; 30 min; 4 °C), and the lower organic phase containing lipids was transferred into a clean glass tube and dried under vacuum (SpeedVac vacuum concentrator). Drying was finalized under an argon flow. Lipids were stored at −20 °C or resuspended in 50 to 100 μl of CHCl3:CH3OH (1:1) for analysis on TLC or HPTLC. Finally, in the case of Afm, Aor, Bba, and other filamentous species, mechanical cell disruption was performed as described in (10). Briefly, 2 g of fresh mycelia was dried on paper towel and ground in a mortar with a pestle in the presence of liquid nitrogen and the resulting fine powder was resuspended in 1 ml (1 volume) of 120 mM Na-Acetate pH 4.5 and treated as described above.
Preparation of total, soluble, and membrane protein fractions
Sce strains carrying an empty plasmid or plasmids expressing Afm ErdS, Afm ErgS, or both enzymes were grown in liquid SC-LEU until they reached an A600 of 1.5. Cells were harvested by centrifugation (3000g, 5 min, 4 °C), washed with phosphate buffered saline (PBS), and frozen at −20 °C until use. Cells were resuspended in a solution containing 100 mM Na-Hepes buffer, pH 7.0, 30 mM KCl, 150 mM NaCl, 5 mM β-mercaptoethanol, 0.5 mM Na2-EDTA, 1% (w/v) Triton X-100, 0.3% (w/v) NP40, 5 mM β-mercaptoethanol, and protease inhibitors tablet (Roche). One volume of glass beads (Ø 0.25–0.5 mm, Roth) was added, and cell lysis was performed with a FastPrep-24 apparatus (6 × 1 min at 6.5 m/s, with 1 min on ice between each cycle). Cell debris was removed by centrifugation at 500g for 10 min at 4 °C, and the supernatant centrifuged at 100,000g for 1 h. The resulting soluble fraction (supernatant, S100) was recovered, as well as the pellet (P100) containing total membranes and membrane-bound proteins. Membrane-bound proteins were resuspended by sonication in a solution containing 100 mM Na-Hepes buffer, pH 7.0, 30 mM KCl, 150 mM NaCl. Protein concentrations were determined using the Bradford method.
Lipid Y (Erg-Gly) purification by silica column chromatography
Total lipids were obtained from 5 g of Yli or Sce cells, with the procedure described above. Volumes were scaled up according to the initial 15 ml volume of 120 mM Na-Acetate, pH 4.5. In a glass column (1 cm × 20 cm), 8 ml of Silica Gel 60 (Sigma) was poured and washed with 4 column volumes (CV) of CHCl3. Total lipids were resuspended in 500 μl of CHCl3, loaded onto the column, and washed with 4 CV CHCl3. Lipids were sequentially eluted using 3 CV CHCl3:CH3OH (144:1); 3 CV CHCl3:CH3OH (72:1), and 3 CV CHCl3:CH3OH (36:1). Lipid fractions were dried, resuspended in 200 μl CHCl3:CH3OH (1:1), and analyzed by TLC.
Lipid analysis by thin layer chromatography
TLC (Silica gel 60 aluminum foils, Sigma-Aldrich, 10 × 10 cm) or HPTLC (Merck, HPTLC Silica gel 60, 1.05547.0001) plates were used. Lipids in the CHCl3:CH3OH (1:1, v:v) solvent were spotted on TLC, typically, 10 to 20 μl of total lipids or 25 μl of radiolabeled lipids extracted from in vitro reactions. TLCs were developed with the CHCl3:CH3OH:H2O mobile phase (130:50:8 or 130:16:1 (v:v:v)) for 10 min and air dried. TLCs were stained with a sulfuric acid/MnCl2 solution (30) (concentrated sulfuric acid 9 ml, MnCl2.4H2O 0.8 g, CH3OH 120 ml, H2O 120 ml) or with ninhydrin (Sigma-Aldrich, 0.4% w/v in EtOH 100%, v/v) and heated at 100 °C, 15 min. Plates were imaged under white light or at 254 nm. Radiolabeled lipid species were revealed by exposing TLC plates onto a Fuji Imaging Plate and analyzed with a Typhoon TRIO, Variable mode imager (GE Healthcare).
Identification of ergosteryl-3β-O-glycine by mass spectroscopy
Liquid chromatography-mass spectrometry (LC-MS) analyses of total lipid extracts were performed on a liquid chromatograph Surveyor Plus with autosampler connected to an LTQ-XL ion trap analyzer mounted with an Ion Max electrospray ionization probe (Thermo Finnigan). Lipid samples were resuspended in CHCl3:CH3OH 2:1 and diluted 3-fold with injection solvent (isopropanol:acetonitrile:water, IPA:ACN:water, 6.5:3:0.5, v:v:v). A volume of 10 μL was injected on an Ascentis Express C18 (10 cm × 2.1 mm, 2.7 μm, Sigma-Aldrich) HPLC column at a temperature of 45 °C as described in (58) with minor modifications. Elution was performed at a flow rate of 260 μl/min with a binary gradient where A was 60:40 water:ACN and B was 90:10 IPA:ACN. Both solutions contained 0.1% formic acid and 10 mM aqueous ammonium formate. Elution was performed with a 16-min gradient; from 0 to 1 min B was maintained at 32%, from 1 to 2.5 min B was increased to 62%, from 2.5 to 14 min to 99%, and B was maintained at 99% for 2 min. Before identification of Erg-Gly, the instrument was tuned with cholesterol in solvent B ([M-H2O + H]+, m/z = 369). The LC-MS experiment shown in this article and in the supplemental section were acquired with the instrument tuned with synthetic Erg-Gly (11) in solvent B ([2M +H]+, m/z = 907). The drying gas flow rate was 20 units, and temperature of the electrospray ionization was 380 °C. Full-scan spectra were collected in the 110- to 2000-m/z range in positive mode, and data-dependent MS2 spectra were acquired on the 15 most intense MS1 peaks. MS3 spectra were acquired on the MS2 peak at m/z = 379. MSn data were acquired with a collision-induced dissociation energy set at 35. Data were analyzed with Xcalibur Qual Browser.
Lipids extracted from wt Aor, wt Sce, and Sce + Aor fDUF were fractionated on a TSKgel ODS80tmQA (C18) column using a continuous gradient from 100% CH3OH:H2O (4:1, v:v) to 100% CH3OH:CH2Cl2 (3:1, v:v). Eluted compounds were monitored at 282 nm, and peaks of interest were submitted to an Accurate-Mass Q-TOF LC-MS to confirm their identity. Synthetic Erg-Gly and Erg-Asp were used as standards to identify the same lipids.
tRNA aminoacylation reactions
Aminoacylation reactions were performed in 100 mM Na-Hepes buffer pH 7.2, containing 30 mM KCl, 12 mM MgCl2, 10 mM ATP, 40 μM [14C]Gly (98 cpm/pmol, Amersham Life Sciences) or 40 μM [14C]Asp (280 cpm/pmol, Amersham Life Sciences), 0.1 mg/ml bovine serum albumin, 160 μM Sce total tRNA, 5 μM purified Sce tRNAAsp or partially fractionated tRNAGly, and 0.05 to 0.5 μM purified YliHis6-GlyRS or Sce AspRS or 6 μg of total Sce proteins (as a source of aaRSs) at 30 °C. Aliquots, 10 μl, were withdrawn for each time point, and [14C]aa-tRNA was quantified as described (59). Pure Sce tRNAAsp was obtained as described (10) and partially fractionated Sce tRNAGly was part of counter-current fractions obtained with the same procedure. Sce AspRS was purified as described (10).
Preparation of [14C]-radiolabeled and cold Gly-tRNAs
Aminoacylation of total tRNA to prepare [14C]Gly-tRNA or cold Gly-tRNA substrates for ErgS was performed essentially as described above and in (60) at 30 °C in a final volume of 100 μl in the tRNA aminoacylation mix containing 40 μM [14C]Gly (98 cpm/pmol, Amersham Life Sciences) or 1 mM cold Gly and 160 μM total Sce tRNA. Reactions were initiated by adding 0.5 to 1.0 μM Yli His6-GlyRS or 6 μg Sce total proteins, and plateaus were reached after 15 to 20 min. Reactions were then stopped by adding 0.1 vol. 3 M Na-acetate pH 4.5 (in order to preserve the ester linkage in aa-tRNAs) and 1 volume phenol. Tubes were then vortexed for 20 s, centrifuged 1 min at 10,000g at 4 °C, and the aqueous phase was transferred to a new tube. Then, 1 volume chloroform was added, the mixture was vortexed and centrifuged 1 min at 10,000g at 4 °C, and the aqueous phase was transferred to a clean tube before adding isopropanol to a final concentration of 60% (v/v). Total aminoacylated tRNAs were precipitated at -20 °C (2 h), pelleted by centrifugation (13,000g at 4 °C), washed with 500 μl 70% ethanol, centrifuged again (13,000g at 4 °C), and the pellet was dried at room temperature for 20 min. Dried aa-tRNAs were kept at -20 °C until use. As determined with aminoacylation plateaus, total Sce tRNA (160 μM) contained 0.636 ± 0.014% Gly-accepting tRNAs (n = 6), i.e., 1.018 ± 0.025 μM of Gly-accepting tRNA.
Lipid transfer assays
Erg and total lipids preparation
Commercial Erg was prepared in CHCl3 at a concentration of 10 mg/ml. An aliquot was transferred to a clean glass tube and dried under vacuum to obtain a lipid film. Dried Erg was then resuspended by sonication (4–6 30-s pulses at maximal power, Elma S30H, Elmasonic) in a 100 mM Na-Hepes pH 7.2 buffer, supplemented with 30 mM KCl, 12 mM MgCl2 to a concentration of 4 mg/ml and used immediately. Total lipids from Sce were prepared similarly at a final concentration of 10 mg/ml.
On-line lipid transfer assay
In order to test ErgS (Erg-Gly synthase) activity, the tRNA aminoacylation reaction was first performed as described above in a final volume of 50 μl, using 0.5 μM Yli His6-GlyRS, 40 μM [14C]Gly (98 cpm/pmol), and 160 μM total Sce tRNA or 5 μM fractionated Sce tRNAGly, to produce [14C]Gly-tRNA. After 20 min, the tRNA glycylation plateau was reached (∼50–60 pmol [14C]Gly-tRNA/reaction). Then Erg, suspended in a 100 mM Na-Hepes pH 7.2 buffer, supplemented with 30 mM KCl, 12 mM MgCl2, was added to a final concentration of 0.5 mg/ml (total lipids to 2 mg/ml), followed by addition of purified MBP-ErgS (0.5 μM). In the case of ErdS (Erg-Asp synthase) activity, the same protocol was used with 5 μM Sce tRNAAsp and 40 μM [14C]Asp (280 cpm/pmol) to produce [14C]Asp-tRNA. After 20 min, the aspartylation plateau was reached (∼50–60 pmol [14C]Gly-tRNA/reaction). Erg (0.5 mg/ml) and ErdS (1 μM) were then sequentially added to initiate the transfer reaction. When protein extracts from Sce were used, 10 μg total proteins was added to initiate the reaction. The RNA dependency of reactions was tested in all cases using 0.3 μg RNase A incubated for 5 min in the reaction mix after the [14C]aa-tRNA plateau was reached, before adding 1 μM of either ErgS or ErdS, depending on the experiment considered. To test the dependency of the presence of other substrates, these were omitted from the reaction. The mixture was then incubated 30 to 45 min at 30 °C. Reactions were stopped by adding 500 μl CHCl3:CH3OH:Na-Acetate (120 mM, pH 4.5) (1:2:0.8, v:v:v), vortexed for 1 min before addition of 130 μl CHCl3 and 130 μl 120 mM Na-Acetate pH 4.5 to obtain a two-phase mixture. Lipids were recovered and analyzed by TLC as described above.
Isotopic competitions between free [14C]Gly and cold Gly
In the case of isotopic competitions, the exact same protocol was used, using a mixture of 15 [14C]aa (PerkinElmer, NEC850E050UC) in the presence of 6 μg and 160 μM total Sce tRNA, as to reach ∼ 50,000 cpm of [14C]aa-tRNA per vial at the plateau. For isotopic competition, 5 mM cold Gly was added, before Yli GlyRS was used to initiate the tRNA aminoacylation reaction, in order to compete specifically with [14C]Gly and [14C]Gly-tRNA synthesis. Then, at the plateau, 1 μM MBP-Yli ErgS served to initiate the transfer reaction.
Separated LTA and competition of cold Gly-tRNA with [14C]Gly-tRNA
The 50-μl reaction contained a 100 mM Na-Hepes buffer pH 7.2 supplemented with 30 mM KCl, 12 mM MgCl2, and 0.5 mg/ml Erg. Dried [14C]Gly-tRNAs were resuspended in water containing 1 mM MgCl2 and added (200 nM per reaction) to the reaction mix. Then, 0, 100, 200, and 300 nM of purified cold Gly-tRNAs (in 1 mM MgCl2) were added for isotopic competitions. The reaction was initiated by adding 1 μM MBP-ErgS. Competing cold Gly-tRNAs concentrations were evaluated knowing that Gly-accepting tRNAs represented 0.636 ± 0.014% of total tRNAs (see above). In all cases, reactions were stopped as described above.
Other methods
Strains and growth conditions for recombinant protein expression as well as purification protocols of recombinant proteins are described in Supplementary Material and Methods. Preparation of total protein extracts, conditions for Western blotting, and related protocols are described in Supplementary Material and Methods. Software, web servers, and sequences used for bioinformatical analyses are indicated in the Supplementary Material and Methods section.
Data availability
All available data are already included in the article. All materials and strains are freely available (contact: frfischer@unistra.fr; h.becker@unistra.fr; nmahmoudikaidi@unistra.fr).
Supporting information
This article contains supporting information (10, 15, 20, 26, 27, 28, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Pr. J.-P. Latgé (Institut Pasteur, Paris, France) for providing Aspergillus fumigatus strains, Dr D. Ferrandon (Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France) for the B. bassiana 80.2 strain, and Dr C. Bleykasten (Institut de Physiologie et ChimieBiologique, Strasbourg, France, UMR7156) for the Yarrowia lipolytica strain. We thank Prof. H. Nakajima (Meiji University, Japan) for the Aspergillus oryzae RIB40 strain. In addition, we thank Dr Sylvie Friant for critical review, experimental suggestions and careful reading of the manuscript. This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021 to 2028 program of the University of Strasbourg, CNRS and Inserm, was supported by IdEx Unistra (ANR-10-IDEX-0002) and by SFRI-STRAT’US project (ANR 20-SFRI-0012) and EUR IMCBio (ANR-17-EURE-0023) under the framework of the French Investments for the Future Program.
Author contributions
N. Y., N. M., H. D. B., and F. F. conceptualization; N. Y., N. M., G. G., D. Y., Y. S., T. K., H. R., H. S., and F. F. formal analysis; N. M., T. K., H. R., H. S., B. S., H. D. B., and F. F. funding acquisition; N. Y., N. M., T. K., H. R., H. S., B. S., H. D. B., and F. F. methodology; N. M., H. D. B., and F. F. project administration; N. M., T. K., H. D. B., and F. F. resources; Y. S., G. G., and F. F. software; T. K., H. R., B. S., H. D. B., and F. F. supervision; N. Y., N. M., G. G., T. K., H. R., H. S., H. D. B., and F. F. validation; N. Y., G. G., D. Y., Y. S., T. K., H. D. B., and F. F. visualization; N. Y., N. M., T. K., H. D. B., and F. F. writing–original draft; N. Y., N. M., T. K., B. S., H. D. B., and F. F. writing–review and editing.
Funding and additional information
This work was supported as the “N- FLAMS” project by the Agence Nationale de la Recherche (ANR-20-CE44-0002), by the Fondation pour la Recherche Médicale (FRM, DBF20160635713), by the ‘‘MitoCross’’ Laboratory of Excellence funding (Labex, ANR-10-IDEX-0002–02), by the University of Strasbourg and by the CNRS (H. D. B., F. F., N. M., N. Y., G. G., B. S., L. H., H. S.). N. Y. was supported by a fellowship from the French Ministère de l’Enseignement Supérieur et de la Recherche, N. M. by a postdoctoral fellowship from the FRM (DBF20160635713) and ANR (ANR-20-CE44-0002). Funding was also provided by Meiji University, as well as Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (Grant Number 21K05406 to T. K.). H. R. and D. W. were supported by a National Institutes of Health Grant: 1R21AI144481-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies

Nathaniel Yakobov has defended his PhD in 2020 at the University of Strasbourg (GMGM, UMR7156) in the team Dynamics and Plasticity of Synthetases, where he discovered the RNA-dependent ergosteryl-3β-O-amino acids synthesis and degradation pathways in fungi. He is currently in a post-doc position at the University of Geneva (Unige), Switzerland, in Robbie Loewith’s laboratory.

Nassira Mahmoudi has a postdoc position at the University of Strasbourg (GMGM UMR7156). She has worked on infectious diseases and host–pathogens interactions. Since 2018, she has studied the ergosterol aminoacylation pathways in Aspergillus fumigatus in the team Dynamics and Plasticity of Synthetases. In particular, she is interested in deciphering the role of aminoacylated ergosterol in fungal physiopathology, virulence, and drug resistance.
Edited by Karin Musier-Forsyth
Contributor Information
Nassira Mahmoudi, Email: nmahmoudikaidi@unistra.fr.
Hubert D. Becker, Email: h.becker@unistra.fr.
Frédéric Fischer, Email: frfischer@unistra.fr.
Supporting information
References
- 1.Brown M.S., Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Nes W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller J.W., Gilligan L.C., Idkowiak J., Arlt W., Foster P.A. The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 2015;36:526–563. doi: 10.1210/er.2015-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari R., Koffel R., Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J. 2007;26:5109–5119. doi: 10.1038/sj.emboj.7601924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grille S., Zaslawski A., Thiele S., Plat J., Warnecke D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog. Lipid Res. 2010;49:262–288. doi: 10.1016/j.plipres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Shimamura M. Structure, metabolism and biological functions of steryl glycosides in mammals. Biochem. J. 2020;477:4243–4261. doi: 10.1042/BCJ20200532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Normile T.G., McEvoy K., Del Poeta M. Steryl glycosides in fungal pathogenesis: An understudied immunomodulatory adjuvant. J. Fungi (Basel) 2020;6:25. doi: 10.3390/jof6010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquier N., Schneiter R. Mechanisms of sterol uptake and transport in yeast. J. Steroid Biochem. Mol. Biol. 2012;129:70–78. doi: 10.1016/j.jsbmb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues M.L. The multifunctional fungal ergosterol. mBio. 2018;9 doi: 10.1128/mBio.01755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakobov N., Fischer F., Mahmoudi N., Saga Y., Grube C.D., Roy H., Senger B., Grob G., Tatematsu S., Yokokawa D., Mouyna I., Latge J.P., Nakajima H., Kushiro T., Becker H.D. RNA-dependent sterol aspartylation in fungi. Proc. Natl. Acad. Sci. U. S. A. 2020;117:14948–14957. doi: 10.1073/pnas.2003266117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokokawa D., Tatematsu S., Takagi R., Saga Y., Roy H., Fischer F., Becker H.D., Kushiro T. Synthesis of aminoacylated ergosterols: A new lipid component of fungi. Steroids. 2021;169:108823. doi: 10.1016/j.steroids.2021.108823. [DOI] [PubMed] [Google Scholar]
- 12.Korber M., Klein I., Daum G. Steryl ester synthesis, storage and hydrolysis: A contribution to sterol homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1534–1545. doi: 10.1016/j.bbalip.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J.C., Moras D. Class II aminoacyl transfer RNA synthetases: Crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp) Science. 1991;252:1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- 14.Eriani G., Cavarelli J., Martin F., Ador L., Rees B., Thierry J.C., Gangloff J., Moras D. The class II aminoacyl-tRNA synthetases and their active site: Evolutionary conservation of an ATP binding site. J. Mol. Evol. 1995;40:499–508. doi: 10.1007/BF00166618. [DOI] [PubMed] [Google Scholar]
- 15.El-Gebali S., Mistry J., Bateman A., Eddy S.R., Luciani A., Potter S.C., Qureshi M., Richardson L.J., Salazar G.A., Smart A., Sonnhammer E.L.L., Hirsh L., Paladin L., Piovesan D., Tosatto S.C.E., et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moutiez M., Belin P., Gondry M. Aminoacyl-tRNA-utilizing enzymes in natural product biosynthesis. Chem. Rev. 2017;117:5578–5618. doi: 10.1021/acs.chemrev.6b00523. [DOI] [PubMed] [Google Scholar]
- 17.Fields R.N., Roy H. Deciphering the tRNA-dependent lipid aminoacylation systems in bacteria: Novel components and structural advances. RNA Biol. 2018;15:480–491. doi: 10.1080/15476286.2017.1356980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slavetinsky C., Kuhn S., Peschel A. Bacterial aminoacyl phospholipids - biosynthesis and role in basic cellular processes and pathogenicity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1310–1318. doi: 10.1016/j.bbalip.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Smith A.M., Harrison J.S., Sprague K.M., Roy H. A conserved hydrolase responsible for the cleavage of aminoacylphosphatidylglycerol in the membrane of Enterococcus faecium. J. Biol. Chem. 2013;288:22768–22776. doi: 10.1074/jbc.M113.484402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A.M., Harrison J.S., Grube C.D., Sheppe A.E., Sahara N., Ishii R., Nureki O., Roy H. tRNA-dependent alanylation of diacylglycerol and phosphatidylglycerol in Corynebacterium glutamicum. Mol. Microbiol. 2015;98:681–693. doi: 10.1111/mmi.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arendt W., Groenewold M.K., Hebecker S., Dickschat J.S., Moser J. Identification and characterization of a periplasmic aminoacyl-phosphatidylglycerol hydrolase responsible for Pseudomonas aeruginosa lipid homeostasis. J. Biol. Chem. 2013;288:24717–24730. doi: 10.1074/jbc.M113.482935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenewold M.K., Hebecker S., Fritz C., Czolkoss S., Wiesselmann M., Heinz D.W., Jahn D., Narberhaus F., Aktas M., Moser J. Virulence of Agrobacterium tumefaciens requires lipid homeostasis mediated by the lysyl-phosphatidylglycerol hydrolase AcvB. Mol. Microbiol. 2019;111:269–286. doi: 10.1111/mmi.14154. [DOI] [PubMed] [Google Scholar]
- 23.Roy H., Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy H., Ibba M. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J. Biol. Chem. 2009;284:29677–29683. doi: 10.1074/jbc.M109.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favrot L., Blanchard J.S., Vergnolle O. Bacterial GCN5-related N-acetyltransferases: From resistance to regulation. Biochemistry. 2016;55:989–1002. doi: 10.1021/acs.biochem.5b01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebecker S., Krausze J., Hasenkampf T., Schneider J., Groenewold M., Reichelt J., Jahn D., Heinz D.W., Moser J. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10691–10696. doi: 10.1073/pnas.1511167112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang P.K., Ehrlich K.C. What does genetic diversity of Aspergillus flavus tell us about Aspergillus oryzae? Int. J. Food Microbiol. 2010;138:189–199. doi: 10.1016/j.ijfoodmicro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Amaike S., Keller N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011;49:107–133. doi: 10.1146/annurev-phyto-072910-095221. [DOI] [PubMed] [Google Scholar]
- 29.Lambou K., Lamarre C., Beau R., Dufour N., Latge J.P. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 2010;75:910–923. doi: 10.1111/j.1365-2958.2009.07024.x. [DOI] [PubMed] [Google Scholar]
- 30.Goswami S.K., Frey C.F. Manganous chloride spray reagent for cholesterol and bile acids on thin-layer chromatograms. J. Chromatogr. 1970;53:389–390. doi: 10.1016/s0021-9673(01)98486-9. [DOI] [PubMed] [Google Scholar]
- 31.Hemmerle M., Wendenbaum M., Grob G., Yakobov N., Mahmoudi N., Senger B., Debard S., Fischer F., Becker H. Noncanonical inputs and outputs of tRNA aminoacylation. Enzymes. 2020;48:117–147. doi: 10.1016/bs.enz.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Tsakos M., Schaffert E.S., Clement L.L., Villadsen N.L., Poulsen T.B. Ester coupling reactions--an enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 2015;32:605–632. doi: 10.1039/c4np00106k. [DOI] [PubMed] [Google Scholar]
- 33.Ernst C.M., Staubitz P., Mishra N.N., Yang S.J., Hornig G., Kalbacher H., Bayer A.S., Kraus D., Peschel A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernst C.M., Peschel A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011;80:290–299. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick D.A. Horizontal gene transfer in fungi. FEMS Microbiol. Lett. 2012;329:1–8. doi: 10.1111/j.1574-6968.2011.02465.x. [DOI] [PubMed] [Google Scholar]
- 36.Morioka S., Shigemori T., Hara K., Morisaka H., Kuroda K., Ueda M. Effect of sterol composition on the activity of the yeast G-protein-coupled receptor Ste2. Appl. Microbiol. Biotechnol. 2013;97:4013–4020. doi: 10.1007/s00253-012-4470-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y.Q., Gamarra S., Garcia-Effron G., Park S., Perlin D.S., Rao R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farnoud A.M., Toledo A.M., Konopka J.B., Del Poeta M., London E. Raft-like membrane domains in pathogenic microorganisms. Curr. Top. Membr. 2015;75:233–268. doi: 10.1016/bs.ctm.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas L.M., Konopka J.B. Fungal membrane organization: The eisosome concept. Annu. Rev. Microbiol. 2014;68:377–393. doi: 10.1146/annurev-micro-091313-103507. [DOI] [PubMed] [Google Scholar]
- 40.Fischer R., Zekert N., Takeshita N. Polarized growth in fungi--interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 2008;68:813–826. doi: 10.1111/j.1365-2958.2008.06193.x. [DOI] [PubMed] [Google Scholar]
- 41.Rella A., Farnoud A.M., Del Poeta M. Plasma membrane lipids and their role in fungal virulence. Prog. Lipid Res. 2016;61:63–72. doi: 10.1016/j.plipres.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weichert M., Lichius A., Priegnitz B.E., Brandt U., Gottschalk J., Nawrath T., Groenhagen U., Read N.D., Schulz S., Fleissner A. Accumulation of specific sterol precursors targets a MAP kinase cascade mediating cell-cell recognition and fusion. Proc. Natl. Acad. Sci. U. S. A. 2016;113:11877–11882. doi: 10.1073/pnas.1610527113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weichert M., Herzog S., Robson S.A., Brandt R., Priegnitz B.E., Brandt U., Schulz S., Fleissner A. Plasma membrane fusion is specifically impacted by the molecular structure of membrane sterols during vegetative development of Neurospora crassa. Genetics. 2020;216:1103–1116. doi: 10.1534/genetics.120.303623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin H., McCaffery J.M., Grote E. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell Biol. 2008;180:813–826. doi: 10.1083/jcb.200705076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alcazar-Fuoli L., Mellado E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2012;3:439. doi: 10.3389/fmicb.2012.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcazar-Fuoli L., Mellado E. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 2014;166:471–484. doi: 10.1111/bjh.12896. [DOI] [PubMed] [Google Scholar]
- 47.Choudhary V., Darwiche R., Gfeller D., Zoete V., Michielin O., Schneiter R. The caveolin-binding motif of the pathogen-related yeast protein Pry1, a member of the CAP protein superfamily, is required for in vivo export of cholesteryl acetate. J. Lipid Res. 2014;55:883–894. doi: 10.1194/jlr.M047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorda T., Puig S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes. 2020;11:795. doi: 10.3390/genes11070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazarko T.Y., Farre J.C., Polupanov A.S., Sibirny A.A., Subramani S. Autophagy-related pathways and specific role of sterol glucoside in yeasts. Autophagy. 2007;3:263–265. doi: 10.4161/auto.3907. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita S., Oku M., Sakai Y. Functions of PI4P and sterol glucoside are necessary for the synthesis of a nascent membrane structure during pexophagy. Autophagy. 2007;3:35–37. doi: 10.4161/auto.3311. [DOI] [PubMed] [Google Scholar]
- 51.Kikuma T., Tadokoro T., Maruyama J.I., Kitamoto K. AoAtg26, a putative sterol glucosyltransferase, is required for autophagic degradation of peroxisomes, mitochondria, and nuclei in the filamentous fungus Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2017;81:384–395. doi: 10.1080/09168451.2016.1240603. [DOI] [PubMed] [Google Scholar]
- 52.Panwar S.L., Pasrija R., Prasad R. Membrane homoeostasis and multidrug resistance in yeast. Biosci. Rep. 2008;28:217–228. doi: 10.1042/BSR20080071. [DOI] [PubMed] [Google Scholar]
- 53.Kaminski D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014;43:453–467. doi: 10.1007/s00249-014-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A., Del Poeta M. Lipid signalling in pathogenic fungi. Cell Microbiol. 2011;13:177–185. doi: 10.1111/j.1462-5822.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Craene J.O., Bertazzi D.L., Bar S., Friant S. Phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. Int. J. Mol. Sci. 2017;18:634. doi: 10.3390/ijms18030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.da Silva Ferreira M.E., Kress M.R., Savoldi M., Goldman M.H., Hartl A., Heinekamp T., Brakhage A.A., Goldman G.H. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot. Cell. 2006;5:207–211. doi: 10.1128/EC.5.1.207-211.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G., Kusumoto K., Arima T., Akita O., Kashiwagi Y., Abe K., Gomi K., Horiuchi H., Kitamoto K., Kobayashi T., et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 58.Bird S.S., Marur V.R., Sniatynski M.J., Greenberg H.K., Kristal B.S. Serum lipidomics profiling using LC-MS and high-energy collisional dissociation fragmentation: Focus on triglyceride detection and characterization. Anal. Chem. 2011;83:6648–6657. doi: 10.1021/ac201195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kern D., Lapointe J. The twenty aminoacyl-tRNA synthetases from Escherichia coli. General separation procedure, and comparison of the influence of pH and divalent cations on their catalytic activities. Biochimie. 1979;61:1257–1272. doi: 10.1016/s0300-9084(80)80285-9. [DOI] [PubMed] [Google Scholar]
- 60.Bailly M., Blaise M., Roy H., Deniziak M., Lorber B., Birck C., Becker H.D., Kern D. tRNA-dependent asparagine formation in prokaryotes: Characterization, isolation and structural and functional analysis of a ribonucleoprotein particle generating Asn-tRNA(Asn) Methods. 2008;44:146–163. doi: 10.1016/j.ymeth.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Busso D., Delagoutte-Busso B., Moras D. Construction of a set Gateway-based destination vectors for high-throughput cloning and expression screening in Escherichia coli. Anal. Biochem. 2005;343:313–321. doi: 10.1016/j.ab.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., 3rd, Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell L.A., Cai Y., Taylor M., Noronha A.M., Chuang J., Dai L., Boeke J.D. Multichange isothermal mutagenesis: a new strategy for multiple site-directed mutations in plasmid DNA. ACS Synth. Biol. 2013;2:473–477. doi: 10.1021/sb300131w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer F., Huot J.L., Lorber B., Diss G., Hendrickson T.L., Becker H.D., Lapointe J., Kern D. The asparagine-transamidosome from Helicobacter pylori: a dual-kinetic mode in non-discriminating aspartyl-tRNA synthetase safeguards the genetic code. Nucleic Acids Res. 2012;40:4965–4976. doi: 10.1093/nar/gks167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edgar R.C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Criscuolo A., Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010;10:210. doi: 10.1186/1471-2148-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grigoriev I.V., Nikitin R., Haridas S., Kuo A., Ohm R., Otillar R., Riley R., Salamov A., Zhao X., Korzeniewski F., Smirnova T., Nordberg H., Dubchak I., Shabalov I. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014;42:D699–D704. doi: 10.1093/nar/gkt1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuroki Y., Juvvadi P.R., Arioka M., Nakajima H., Kitamoto K. Cloning and characterization of vmaA, the gene encoding a 69-kDa catalytic subunit of the vacuolar H+- ATPase during alkaline pH mediated growth of Aspergillus oryzae. FEMS Microbiol. Lett. 2002;209:277–282. doi: 10.1111/j.1574-6968.2002.tb11144.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data are already included in the article. All materials and strains are freely available (contact: frfischer@unistra.fr; h.becker@unistra.fr; nmahmoudikaidi@unistra.fr).