Abstract
Background
Atrial fibrillation (AF) is one of the most prevalent causes of cryptogenic stroke. Also, apart from AF itself, structural and remodelling changes in the atria might be an underlying cause of cryptogenic stroke. We aimed to discover circulating proteins and reveal pathways altered in AF and atrial cardiomyopathy, measured by left atrial volume index (LAVI) and peak atrial longitudinal strain (PALS), in patients with cryptogenic stroke.
Methods
An aptamer array (including 1310 proteins) was measured in the blood of 20 cryptogenic stroke patients monitored during 28 days with a Holter device as a case-control study of the Crypto-AF cohort. Protein levels were compared between patients with (n = 10) and without AF (n = 10) after stroke, and the best candidates were tested in 111 patients from the same cohort (44 patients with AF and 67 without AF). In addition, in the first 20 patients, proteins were explored according to PALS and LAVI values.
Results
Forty-six proteins were differentially expressed in AF cases. Of those, four proteins were tested in a larger sample size. Only DPP7, presenting lower levels in AF patients, was further validated. Fifty-seven proteins correlated with LAVI, and 270 correlated with PALS. NT-proBNP was common in all the discovery analyses performed. Interestingly, many proteins and pathways were altered in patients with low PALS.
Conclusions
Multiple proteins and pathways related to AF and atrial cardiomyopathy have been revealed. The role of DPP7 as a biomarker for stroke aetiology should be further explored. Moreover, the present study may be considered hypothesis-generating.
Keywords: Atrial fibrillation, Atrial cardiomyopathy, Biomarkers, Cryptogenic stroke, Atrial function
1. Introduction
Atrial fibrillation (AF) is a prevalent cardiac rhythm disorder underlying up to one-third of all ischemic strokes [1]. Paroxysmal AF remains undetected in a high proportion of patients after stroke and is one of the most prevalent causes of cryptogenic stroke [2].
Also, there is increasing recognition that atrial dysfunction itself is associated with an increased risk of thromboembolism, even in patients without AF [3]. Therefore, atrial substrate or atrial cardiomyopathy has been proposed as an important cause of cryptogenic strokes [4], [5].
Left atria (LA) enlargement and atrial fibrosis are two structural hallmarks of the atrial substrate [3]. LA size has been associated with cardioembolic stroke and AF detection in patients with embolic stroke of undetermined source (ESUS) [6]. Similarly, peak atrial longitudinal strain (PALS), which measures the LA wall deformability and is a surrogate of LA fibrosis, has been associated with AF in cryptogenic stroke patients [7].
Some blood biomarkers (e.g. natriuretic peptides) have been proposed as useful tools to detect paroxysmal AF [8]. In addition, circulating markers might allow noninvasive assessment of atrial cardiomyopathy before AF appears and might guide the selection of patients for more intensive post-stroke monitoring to personalize the secondary prevention treatments [2], [5].
The present study aims to discover circulating proteins and reveal pathways altered in AF and atrial cardiomyopathy, measured by left atrial volume index (LAVI) and PALS, in patients with cryptogenic stroke.
2. Methods
2.1. Study population
The study population represented a subpopulation of the Crypto-AF study [9], [10]. Non-lacunar acute ischemic stroke patients over 55 years of age with cryptogenic stroke after standard evaluation were included in the study by four Spanish public Stroke Centers from January 2015 to July 2017. All patients included had no prior history of AF. Patients were monitored for 28 days with a wearable Holter device (NuuboTM) following a published protocol [9]. The software algorithm classified every episode of irregular ECG rhythm lasting > 120 s as possible AF. An expert cardiologist blinded to clinical data verified the episodes. From this cohort, a total of ten consecutive patients with AF detected during the monitoring period, and ten matched controls (by sex and age) without AF were selected for the present discovery study. In addition, 111 patients with available blood samples were used for the validation study (44 patients with AF and 67 matched controls).
Blood samples were collected into EDTA and serum separator tubes within 72 h after symptoms onset. After centrifugation at 1500 g and 4 °C for 15 min, plasma and serum aliquots were frozen at −80 °C until further analysis.
Left atrial size was measured by biplane transthoracic echocardiography to obtain the left atrial volume adjusted to body surface index (LAVI, ml/m2) following the latest guidelines [11]. Peak atrial longitudinal strain (PALS) was evaluated by speckle tracking software (GE EchoPAC®) following expert recommendations [12].
Written informed consent was obtained from all participants, and the study was approved (PR (AG)49/2014) by the Ethical Committee of Vall d’Hebrón Hospital, Valladolid Hospital, Virgen Macarena Hospital and Virgen del Rocio Hospital, in line with Helsinki guidelines.
2.2. Aptamer array
Protein levels in plasma were assessed using the SOMAscan® platform (SomaLogic Inc., Boulder, CO, USA), which is an aptamer-based proteomic assay that allowed the simultaneous measurement and quantification of 1310 proteins [13]. This approach uses SOMAmers® reagents, which are short single-stranded DNA sequences with protein affinity. The platform transforms the proteins present in the biological sample into a corresponding SOMAmer signal, which then is quantified using the microarrays technology. Three different dilutions (depending on each protein abundance) were used. Normalization and calibration procedures were performed by SomaLogic according to their protocol [14]. All samples passed SomaLogic quality controls. A set of control calibrator samples were used to detect and remove systematic variability between independent assay runs. Seventy-nine proteins were marked as “flags” due to high inter-plate variability and eliminated from the analysis. Data were reported in relative fluorescent units (RFU) after normalization and calibration.
2.3. Elisa
Serum coiled-coil domain-containing protein 80 (CCDC80)(BosterBio), and plasma dipeptidyl peptidase 7 (DPP7)(R&D Systems), bone morphogenetic protein 1 (BMP-1)(Elabscience), and cystatin-D (BosterBio) were determined by ELISA. All assays were performed blinded to clinical information and according to the manufacturer’s instructions. All samples were tested in duplicate, and inter-assay variation was determined by a commercial control (Human Serum, male AB, USA origin from clotted, SIGMA, ref number H16914; Human plasma K2 EDTA, Innovative Research, ref number IPLA-N) tested in duplicate in each plate. When inter-assay variation was > 20%, biomarker levels were standardized by the common control sample. Samples with a CV (coefficient of variation) > 20% between duplicates were eliminated from the analysis.
2.4. Statistics
R software version 3.6.1 and SPSS version 20 were used to conduct statistical analysis. Categorical variables were expressed as numbers and percentages and continuous variables as mean ± SD, or median (interquartile range) for continuous variables, depending on their distribution. Student’s t-test, Mann–Whitney or x2 were used to compare variables between AF cases and controls depending on the type and distribution of each variable.
SOMAscan data were log-transformed as presented a skewed distribution. Differential expression analyses were performed using the “limma” package (Bioconductor) version 3.42.2, optimized for omics studies with large amounts of data and few samples [15].
Spearman correlations were calculated between LAVI or PALS and all the analyzed proteins. The R package “Venndiagram” version 1.6.20 was used to visualize the common proteins between the different analyses. All p-values were adjusted using Benjamini and Hochberg (BH) false discovery rate (FDR). Group matching by sex and age was used to select control samples in the discovery experiment. The validation sample size was estimated based on Somascan results (power of 80%, α = 0.05) (Ene 3.0, GlaxoSmithKline, UK).
The addition of DPP7 to a logistic regression model fitted by age, sex, echocardiographic markers (LAVI and PALS), and NT-proBNP was tested using the Likelihood Ratio Test. Odds ratios (OR) for an increment of one unit of concentration were shown. The classification performance of the models was compared using Reciever Operating Curves. The R package “ggeffects” version 1.1.1 was used to plot the average predicted probability of the model when varying the variable of interest.
2.5. Pathway analysis
Pathway enrichment analysis was conducted following a published protocol [16]. Gene Set Enrichment Analysis (GSEA) software was applied to all the SOMAscan proteins ordered by T-statistic or correlation coefficient against Reactome Pathways and Gene Ontology (biological processes) databases. Gene sets with < 15 genes or > 200 genes were excluded. GSEA calculates a normalized enrichment score (NES) for each gene set. Positive and negative NES values represent enrichment of the corresponding gene set at the top (i.e., upregulated) or bottom (i.e., downregulated) of the ranked list. P-values were computed by gene set permutation for 1000. Then, multiple testing using a false-discovery rate (FDR) was applied to obtain the Q-values. Results were visualized via Cytoscape Enrichment Map with a Jaccard Overlap Combined Coefficient > 0.375. Significant pathways were considered at Q-value < 0.25.
3. Results
The descriptive characteristics of the 20 patients included in the discovery study are provided in Table 1. The median age was 71.5, and 55% were women. Clinical variables were similar between the two groups.
Table 1.
Clinical characteristics of the patients included in the discovery experiment and comparison according to atrial fibrillation detection.
| All (n = 20) | AF (n = 10) | No AF (n = 10) | p-value | |
|---|---|---|---|---|
| Sex (%female) | 11 (55%) | 6 (60%) | 5 (50%) | 0.65& |
| Age (years) | 71.5 (67–80) | 73.5 (69.75–80) | 67.5 (62.25–81.5) | 0.247$ |
| Hypertension | 14 (70%) | 7 (70%) | 7 (70%) | 1.00& |
| Diabetes | 5 (25%) | 3 (30%) | 2 (20%) | 1.00& |
| Vasculopathy | 1 (5%) | 0 (0%) | 1 (10%) | 1.00& |
| Renal failure | 1 (5.3%) | 1 (11.1%) | 0 (0%) | 0.47& |
| COPD | 1 (5.3%) | 1 (11.1%) | 0 (0%) | 0.47& |
| Obesity | 8 (40%) | 5 (50%) | 3 (30%) | 0.65& |
| Heart disease | 2 (10%) | 2 (20%) | 0 (0%) | 0.47& |
| Basal NIHSS | 4 (2–7) | 3 (1–7) | 5 (3–7) | 0.29$ |
| LVEF (%) | 64.74 ± 34.19 | 63 ± 7.84 | 66.30 ± 7.51 | 0.362# |
| PALS (%) | 25.76 ± 12.98 | 29.89 ± 14.06 | 20.59 ± 10.00 | 0.134# |
| LAVI (ml/m2) | 31 (27–37) | 30 (27–34) | 34 (22.5–37.75) | 0.815$ |
| Number of AF episodes | 28 (7–42.5) | |||
| Longest AF episode (min) | 780.48 (121.25–1510.71) |
COPD, chronic obstructive pulmonary disease; NIHSS, National Institutes of Health Stroke Scale; LVEF, left ventricular ejection fraction; PALS, peak atrial longitudinal strain; LAVI, left atrial volume index.
The “heart disease” terminology included any cardiopathy that the investigator considered of interest, including into this category ischemic cardiopathy, and hypertensive cardiopathy, between others. The two patients with heart disease in this cohort had a mild mitral and aortic valvulopathy, and a hypertensive cardiomyopathy respectively.
Student’s t-test.
Mann–Whitney test.
x2 test.
3.1. Differential protein expression and altered pathways in AF
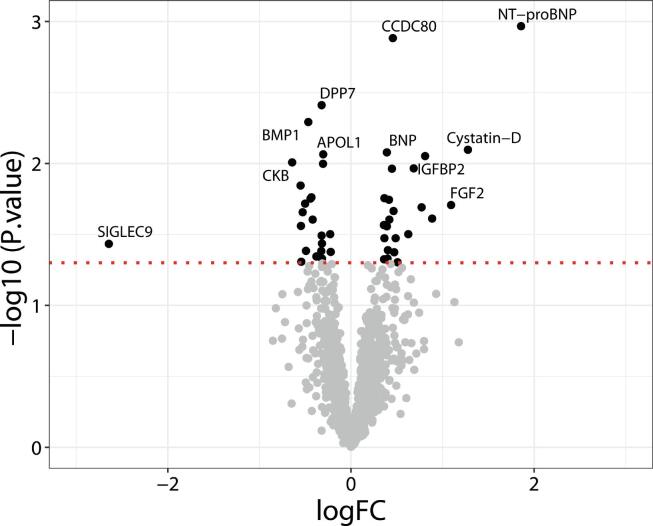
Among the tested proteins, 46 were differentially expressed in AF cases at a nominal p-value of 0.05 (22 down-regulated and 24 up-regulated). Although no protein remained significant after multiple comparison correction, NT-proBNP showed the strongest association (p-value = 0.001, logFC = 1.86) and BNP was ranked sixth (p-value = 0.008, logFC = 0.39). Both natriuretic peptides were well-known biomarkers of AF, already validated in this cohort in a published study [8]. Proteins with p-values between NT-proBNP and BNP were selected to evaluate their usefulness in a larger group of patients: CCDC80 (p-value = 0.0013), DPP7 (p-value = 0.0039), BMP-1 (p-value = 0.0051), and Cystatin-D (p-value = 0.0080) (Fig. 1 and Supplemental Table 1).
Fig. 1.
Volcano plot of differentially expressed proteins between AF cases and no AF. Black dots above the red line indicate significant proteins, while grey dots below the red line indicate non-significant proteins according to nominal p-value < 0.05. Labeled proteins are those with a nominal p-value < 0.01 or nominal p-value < 0.05 and |logFC|>1.Proteins with positive logFC had higher levels in the AF group and vice-versa. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
GSEA analysis revealed five gene sets upregulated in patients with AF (Regulation of cellular response to growth factor stimulus, Lymphocyte chemotaxis, Calcium ion transmembrane transport, Lymphocyte migration, Chemokine receptors bind chemokines), and five gene sets downregulated (Binding and Uptake of Ligands by Scavenger Receptors, Interleukin-12 family signalling, Vesicle-mediated transport, Transport of small molecules and Interleukin-12 signalling). Most altered pathways were related to immune response and intracellular transport (Supplemental Fig. 1).
3.1.1. Biomarker validation
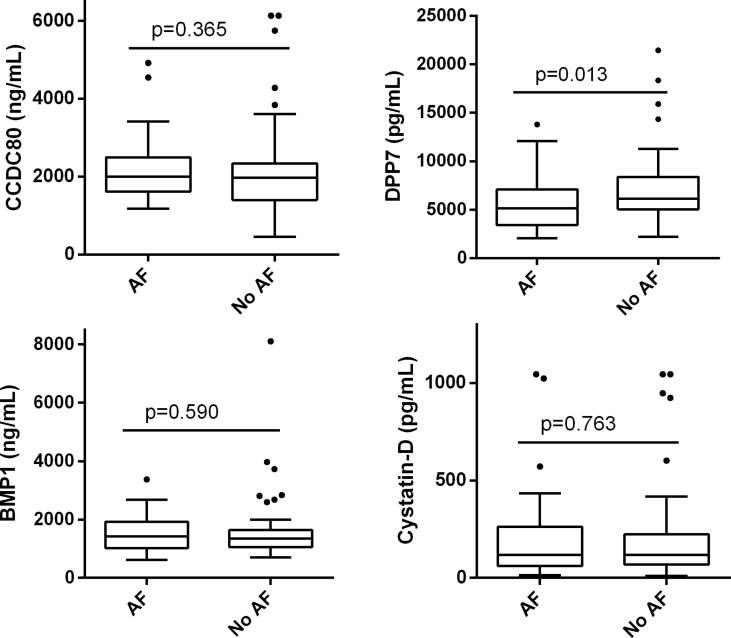
The clinical characteristics of the 111 patients included in the validation study were provided in Supplemental Table 2. This subgroup was older (p = 0.021) and had a lower percentage of obesity (p = 0.041) in comparison to the discovery cohort. Several samples had a CV > 20% between duplicates and were eliminated from the analysis accordingly. Therefore, we had a final sample size of 107 patients for CCDC80, 86 for DPP7, 95 for BMP1, and 97 for Cystatin-D. Levels from the four biomarkers were available from 74 patients. Analysis was performed with the patients available for each biomarker. DPP7 was significantly lower in individuals with AF compared with no AF [5.13 ng/ml (IQR 3.42–7.10) vs 6.16 ng/ml (5.04–8.39), p = 0.013], while the remaining biomarkers were not different between the two groups (Fig. 2 and Supplemental Table 3). Sensitivity analyses were performed only, including the patients with values from all the biomarkers, obtaining similar results.
Fig. 2.
Boxplot distribution of CCDC80, DPP7, BMP1 and Cystatin-D between AF and no AF. P-values indicated correspond to the Mann-Whitney test. P-values were not corrected by Bonferroni, which lowered the significance threshold to p < 0.0125. Boxes extend from the 25th to 75th percentiles. The line in the middle is plotted as the median. Whiskers are drawn according to Tukey methodology (±1.5 IQR), and larger values are plotted as individual points.
DPP7 alone was a predictor of AF (OR = 0.828 per ng/ml [0.695–0.986], p = 0.034). Then, when adding DPP7 to a logistic regression model fitted with age, sex, PALS, LAVI, and NT-proBNP, it remained an independent predictor (OR = 0.729 per ng/ml [0.539–0.988], p = 0.041). This model, including DPP7, showed better fit according to the likelihood ratio test (χ2 = 6.001, p = 0.014), and the AUC increased, although the DeLong test was not significant (0.657 [0.484–0.831] vs 0.756 [0.608–0.904], p = 0.155). The models were built with 51 complete cases with all the variables available (17 AF and 34 no AF) (Supplemental Fig. 2).
3.2. Differential protein expression and altered pathways in atrial myopathy
3.2.1. LAVI
Fifty-seven proteins correlated significantly with LAVI (40 negative correlations and 17 positive correlations). From these, only 17 had a correlation coefficient r>|0.6|. No proteins remained significant after multiple corrections in any of the analyses (Supplemental Table 4 and Supplemental Fig. 3).
The pathway analysis, considering the protein list ordered by the correlation coefficient between protein levels and LAVI, revealed only one gene set upregulated with LAVI with a Q-value < 0.25: Netrin-1 signalling (R-HSA-373752).
3.2.2. PALS
Two-hundred-seventy proteins correlated significantly with PALS (117 negative correlations and 153 positive correlations). From these, only 64 had a correlation coefficient r>|0.6|, and six remained significant after multiple comparison corrections (Supplemental Table 5 and Supplemental Figure 4).
When proteins were ordered by the correlation coefficient between protein levels and PALS, 264 gene sets were enriched in patients with high PALS, and two were enriched in patients with low PALS (R-HSA-1630316, Glycosaminoglycan metabolism, and R-HSA-3781865, Diseases of Glycosylation). Dysregulated pathways were mostly involved in signal transduction, metabolism, immune response, and hemostasis. (Supplemental Table 6 and Supplemental Figure 5).
3.3. Common protein expression in AF and atrial myopathy
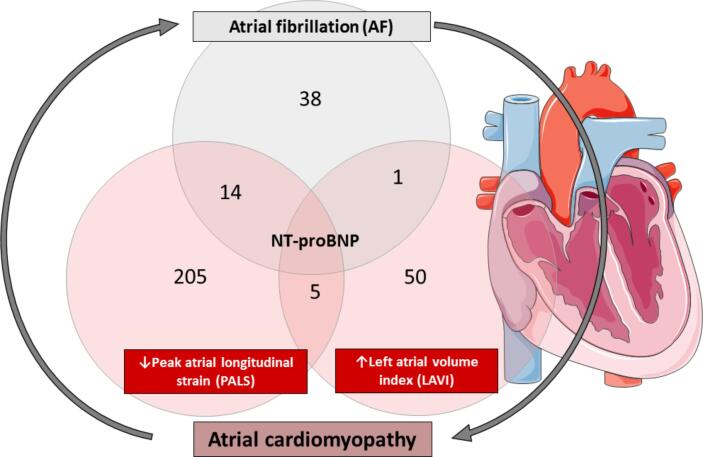
NT-proBNP was the only protein common in all the analyses performed. Five proteins were related to LAVI and PALS (Leukotriene A-4 hydrolase, Alpha-2-macroglobulin, Histone H2A type 3, Secretin, and Fibroblast growth factor 12). One protein was associated with AF and LAVI (Cell adhesion molecule 3). Fourteen proteins were associated with AF and PALS (Urokinase plasminogen activator surface receptor, Lumican, Dermatopontin, Follistatin-related protein 3, C-C motif chemokine 25, Plasminogen, Coagulation factor VII, Natural killer cell receptor 2B4, Angiopoietin-2, Interleukin-23 receptor, Brain natriuretic peptide 32, Erythropoietin, Advanced glycosylation end product-specific receptor, and Macrophage mannose receptor 1) (Fig. 3).
Fig. 3.
Summary of proteins altered in AF and atrial myopathy.The numbers in the circles indicate proteins related to each variable (AF, PALS, and LAVI). Proteins in the overlap circles are those commonly related to two or three variables. The number of proteins related to PALS and LAVI shown in the figure corresponds to those with significant correlations. The number of proteins related to AF corresponds to those significant in the comparison AF vs no AF. In all cases, nominal p-values were considered.
4. Discussion
In the present study, a wide variety of proteins have been explored in a cohort of cryptogenic stroke patients monitored for 1 month to detect AF.
Although several studies had previously explored individual biomarkers of cardioembolic stroke and/or AF in stroke patients [17], few studies had used a discovery approach to identify new candidates related to occult AF. In fact, the majority of AF “discovery” studies enrolled asymptomatic patients [18], [19], [20] and, to our knowledge, only the study of Lambert et al. included stroke patients [21]. This study explored 184 proteins in patients with previously known causes of stroke. In contrast, we evaluated a wider range of proteins in patients with ESUS to ascertain new predictors of AF. Although all the patients included in our study had no prior story of AF, we cannot differentiate between new-onset AF or first-diagnosed AF. However, we aimed to identify AF cases in cryptogenic stroke patients independently of the onset of this AF because, from a clinical perspective, the treatment of the arrhythmia is the same.
Regarding the proteins differentially expressed in our analysis when comparing patients with and without AF, we should highlight that natriuretic peptides, NT-proBNP and BNP, were in the top-ranked positions. Both are well-known surrogates of AF and have already been validated in the present cohort of cryptogenic patients [8]. Therefore, we decided to test other proteins with similar nominal p-values in the comparison. Only DPP7 showed significant differences in the validation from the four proteins selected, with higher levels in the patients without AF. DPP7 (also called DPP2) is a member of the dipeptidyl peptidase family, which has been implicated in many immunologic processes. Although limited data about DPP7 is available compared to other family members, there is a substantial overlap in this family regarding substrate specificity, inhibitors, and functions.
Interestingly, members of the dipeptidyl peptidase family have been linked to atherosclerosis and are considered therapeutic targets for treating this pathology [22]. Therefore, the increase of DPP7 levels in no AF individuals might be linked to an underlying atherosclerotic aetiology in those ESUS cases, but this should be confirmed.
Another hypothesis would be that DPP7 had a role in the pathophysiology of AF, lowering its risk. DPP7 is a lysosomal protein ubiquitously distributed in various tissues and organs, including the heart [23]. Its pathophysiological significance requires additional study, including measurements on atrial tissue and/or biological experiments.
Although DPP7 adds information to current markers (clinical, echocardiographic, and blood biomarkers previously described like NT-proBNP), the logistic regression models presented here were built with reduced sample size. Therefore, the use of DPP7 as a biomarker should be validated in further studies. The fact that the other candidates were not validated indicates that the present discovery approach resulted in several false positives. We cannot discard the possibility that the lack of validation is due to disparity in results between the two techniques used, as previous studies presented weak correlations (r < 0.3) in some proteins between SOMAscan platform and conventional immunoassays [24]. The low reproducibility between techniques may be explained as binding reagents interacting with different epitopes of a specific analyte. That is why discovery studies aiming to find biomarker candidates should include validations with different techniques. More importantly, the difficulty to find new AF biomarkers with “discovery” strategies may be due to the complexity of AF, which makes it challenging to classify the patients in a binary variable. First, we cannot discard there were false-negative cases in the no AF group as cardiac monitoring might not have been prolonged enough to detect some AF cases [25]. Also, AF could be just a bystander, and atrial dysfunction is the real underlying disease that causes stroke susceptibility [26].
Consequently, there is a need to search for biomarkers that identify not only AF but also the underlying atrial substrate. Therefore, and taking advantage of the availability of well-phenotyped patients by echocardiography, we have explored circulating proteins and functional pathways that might be associated with atrial cardiomyopathy. Two echocardiographic variables have been considered to perform this analysis: LAVI and PALS. Although evaluating different characteristics of the left atria, both variables are used to identify atrial myopathy and have been shown useful to predict AF [3]. LAVI measures the enlargement of the atria while PALS provides information on its deformability, but both variables are highly related.
Interestingly, in our analysis, while many proteins and pathways were altered in patients with lower PALS, few were affected in patients with high LAVI. This may reflect that more pathophysiological changes are behind the remodelling that results in atrial distensibility impairment compared to atrial enlargement. Previous studies stated that PALS measurements were more sensitive than volumetric measurements to predict AF and that LA dysfunction may precede and/or be independent of anatomical changes like LA enlargement [27]. This may also explain why few protein candidates are associated with both variables in our analysis.
The bioinformatics analysis revealed some interesting pathways playing a role in AF and atrial cardiomyopathy. The study highlighted the importance of the inflammatory response in AF, especially the role of lymphocytes and chemokine signalling. This confirms the role of inflammation in the initiation and maintenance of AF, which has extensively been described [28]. Also, we found an enrichment of growth factors signalling, which can also be linked to the inflammatory response or the fibrosis occurring in the heart [29]. Finally, calcium handling is essential for the correct electrical functioning of the heart, and similarly of what we found, abnormal calcium handling can lead to cardiac arrhythmias like AF [30].
On the other hand, several pathways were downregulated in the AF group compared to the no AF group, which could be related to other stroke etiologies in the second group. This is the case of interleukin-12 (IL-12) signalling. Interleukin-12 family members have been related to various cardiovascular diseases, including atherosclerosis, hypertension, aortic dissection, and several cardiac pathologies [31]. Also, in patients without AF, we found an upregulation of vesicle-mediated intracellular transport and activity of scavenger receptors, which are membrane-bound receptors that bind a variety of ligands, including low-density-lipoproteins (LDL). These, together with the transport of small molecules, are pathways that might describe increased levels of LDL, involved in atherosclerosis, in patients without AF.
4.1. Study limitations
The present study has several limitations. The main limitation is the small sample size, limiting our statistical power to correct multiple comparisons. Second, the selection of patients was performed according to AF diagnosis, and then a posterior exploratory analysis according to echocardiographic variables was performed. As continuous variables, correlations between the echocardiographic variables and protein levels were assessed, but we should consider the difficulties of obtaining significant correlations with a reduced sample size. The same reason precluded sex- and age-based analysis. Therefore, we cannot eliminate the effect of age on the proteins/pathways revealed, especially in the case of left atrial strain that correlates with age. Nevertheless, we may not be interested in eliminating this effect as fibrosis of the atrium is a cumulative process that may result from pathways altered with age [32].
Similarly, LAVI is larger in women than in men in our cohort, and the association of some proteins’ abundance with LAVI may reflect sex differences in our population. Therefore, these results need to be interpreted as hypotheses-generating. Selection of patients according to extreme PALS and LAVI matched by sex and age and validation of altered markers in a cohort with a longer follow-up would provide further insights. Moreover, we should state that LAVI and PALS might not be specific markers of atrial cardiomyopathy. Other parameters and techniques can be used to characterize the disease in other studies (e.g., left atrial voltage mapping, late gadolinium-enhanced magnetic resonance imaging, and histological analysis).
Also, the lack of a matched set of similar aged non-stroke patients limits insight into biomarkers specifically associated with stroke. Finally, we only analyzed a small portion of the full proteome, which may have conditioned our findings.
5. Conclusions
The present study revealed multiple proteins and pathways that may have a role in the development of atrial fibrillation. In particular, the role of DPP7 as a biomarker for stroke aetiology should be further explored. Also, we have proposed a strategy considering echocardiographic parameters to discover new biomarkers and pathways that may have a role in atrial fibrillation and atrial myopathy. In this regard, the present study may be considered hypothesis-generating.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Instituto de Salud Carlos III, Spain [grant number PI15/02265 and PI18/00804]. The Spanish stroke research network INVICTUS+ (RD16/0019/00021) of the Instituto de Salud Carlos III (co-financed by the European Regional Development Fund, FEDER) is also acknowledged. EP has received a predoctoral grant from Vall D’Hebron Institute of Research. This project is supported by AFFECT-EU, receiving funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N°847770. Sponsors did not have any role in the study design, analysis, interpretation of data, report writing, or the decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.100977.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Freedman B., Potpara T.S., Lip G.Y.H. Stroke prevention in atrial fibrillation. Lancet. 2016;388(10046):806–817. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 2.R.B. Schnabel, K.G. Haeusler, J.S. Healey, et al., Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration, Circulation 140 (2019) 1834–50. doi:10.1161/CIRCULATIONAHA.119.040267. [DOI] [PubMed]
- 3.Goldberger J.J., Arora R., Green D., Greenland P., Lee D.C., Lloyd-Jones D.M., Markl M., Ng J., Shah S.J. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132(4):278–291. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkind M.S.V. Atrial cardiopathy and stroke prevention. Curr. Cardiol. Rep. 2018;20(11) doi: 10.1007/s11886-018-1053-0. [DOI] [PubMed] [Google Scholar]
- 5.Kamel H., Longstreth W.T., Tirschwell D.L., Kronmal R.A., Broderick J.P., Palesch Y.Y., Meinzer C., Dillon C., Ewing I., Spilker J.A., Di Tullio M.R., Hod E.A., Soliman E.Z., Chaturvedi S., Moy C.S., Janis S., Elkind M.SV. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: Rationale and methods. Int. J. Stroke. 2019;14(2):207–214. doi: 10.1177/1747493018799981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan K., Yaghi S., Poppas A., Chang A.D., Mac Grory B., Cutting S., Burton T., Jayaraman M., Tsivgoulis G., Sabeh M.K., Merkler A.E., Kamel H., Elkind M.S.V., Furie K., Song C. Left atrial volume index is associated with cardioembolic stroke and atrial fibrillation detection after embolic stroke of undetermined source. Stroke. 2019;50(8):1997–2001. doi: 10.1161/STROKEAHA.119.025384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagola J., González-Alujas T., Flores A., Muchada M., Rodriguez-Luna D., Seró L., Rubiera M., Boned S., Ribó M., Álvarez-Sabin J., Evangelista A., Molina C.A. Left Atria Strain Is a Surrogate Marker for Detection of Atrial Fibrillation in Cryptogenic Strokes. Stroke. 2014;45(8) doi: 10.1161/STROKEAHA.114.005540. [DOI] [PubMed] [Google Scholar]
- 8.E. Palà, J. Pagola, J. Juega, et al., BNP over NT‐proBNP to predict incident atrial fibrillation after cryptogenic stroke, Eur. J. Neurol. 2020;ene.14579. doi:10.1111/ene.14579. [DOI] [PubMed]
- 9.J. Pagola, J. Juega, J. Francisco-Pascual, et al., Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: Pilot study of Crypto-AF registry, Int. J. Cardiol. 251 (2018) 45–50. doi:10.1016/j.ijcard.2017.10.063. [DOI] [PubMed]
- 10.Pagola J., Juega J., Francisco‐Pascual J., Bustamante A., Penalba A., Pala E., Rodriguez M., De Lera Alfonso M., Arenillas J.F., Cabezas J.A., Moniche F., Torres R., Montaner J., González‐Alujas T., Alvarez‐Sabin J., Molina C.A. Large vessel occlusion is independently associated with atrial fibrillation detection. Eur. J. Neurol. 2020;27(8):1618–1624. doi: 10.1111/ene.14281. [DOI] [PubMed] [Google Scholar]
- 11.Donal E., Lip G.Y.H., Galderisi M., Goette A., Shah D., Marwan M., Lederlin M., Mondillo S., Edvardsen T., Sitges M., Grapsa J., Garbi M., Senior R., Gimelli A., Potpara T.S., Van Gelder I.C., Gorenek B., Mabo P., Lancellotti P., Kuck K.-H., Popescu B.A., Hindricks G., Habib G., Cosyns B., Delgado V., Haugaa K.H., Muraru D., Nieman K., Cohen A. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging. 2016;17(4):355–383. doi: 10.1093/ehjci/jev354. [DOI] [PubMed] [Google Scholar]
- 12.Pathan F., D'Elia N., Nolan M.T., Marwick T.H., Negishi K. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2017;30(1):59–70.e8. doi: 10.1016/j.echo.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 13.L. Gold, D. Ayers, J. Bertino, et al., Aptamer-based multiplexed proteomic technology for biomarker discovery, PLoS One 5 (2010). doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed]
- 14.Candia J., Cheung F., Kotliarov Y., Fantoni G., Sellers B., Griesman T., Huang J., Stuccio S., Zingone A., Ryan B.M., Tsang J.S., Biancotto A. Assessment of variability in the SOMAscan assay. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-14755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.G.K. Smyth, Linear models and empirical bayes methods for assessing differential expression in microarray experiments, Stat. Appl. Genet. Mol. Biol. 3 (2004). doi:10.2202/1544-6115.1027. [DOI] [PubMed]
- 16.Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., Wadi L., Meyer M., Wong J., Xu C., Merico D., Bader G.D. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA Cytoscape and EnrichmentMap. Nat Protoc. 2019;14(2):482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markus A., Valerie S., Mira K. Promising biomarker candidates for cardioembolic stroke etiology. A brief narrative review and current opinion. Front. Neurol. 2021;12:1–10. doi: 10.3389/fneur.2021.624930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko D., Benson M.D., Ngo D., Yang Q., Larson M.G., Wang T.J., Trinquart L., McManus D.D., Lubitz S.A., Ellinor P.T., Vasan R.S., Gerszten R.E., Benjamin E.J., Lin H. Proteomics profiling and risk of new‐onset atrial fibrillation: framingham heart study. JAHA. 2019;8(6) doi: 10.1161/JAHA.118.010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind L., Sundström J., Stenemo M., Hagström E., Ärnlöv J. Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart. 2017;103(5):377–382. doi: 10.1136/heartjnl-2016-309764. [DOI] [PubMed] [Google Scholar]
- 20.W.Y. Zhong, H. Yang, Y.L. Wang, et al., Proteomic profiles of patients with atrial fibrillation provide candidate biomarkers for diagnosis, Int. J. Cardiol. 344 (2021) 205–12. doi:10.1016/j.ijcard.2021.09.047. [DOI] [PubMed]
- 21.Tancin Lambert A., Kong X., Ratajczak-Tretel B., Atar D., Russell D., Skjelland M., Bjerkeli V., Skagen K., Coq M., Schordan E., Firat H., Halvorsen B., Aamodt A. Biomarkers associated with atrial fibrillation in patients with ischemic stroke: a pilot study from the NOR-FIB Study. Cerebrovasc Dis Extra. 2020;10(1):11–20. doi: 10.1159/000504529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waumans Y., Baerts L., Kehoe K., Lambeir A.-M., De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front. Immunol. 2015;6 doi: 10.3389/fimmu.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maes M.-B., Scharpé S., De Meester I. Dipeptidyl peptidase II (DPPII), a review. Clin. Chim. Acta. 2007;380(1-2):31–49. doi: 10.1016/j.cca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 24.L.M. Raffield, H. Dang, K.A. Pratte, et al., Comparison of proteomic assessment methods in multiple cohort studies, Proteomics 20 (2020) 1–34. doi:10.1002/pmic.201900278. [DOI] [PMC free article] [PubMed]
- 25.Sanna T., Diener H.-C., Passman R.S., Di Lazzaro V., Bernstein R.A., Morillo C.A., Rymer M.M., Thijs V., Rogers T., Beckers F., Lindborg K., Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 26.Freedman B., Kamel H., Van Gelder I.C., Schnabel R.B. Atrial fibrillation: Villain or bystander in vascular brain injury. Eur. Hear J.Suppl. 2020;22(Supplement_M):M51–M59. doi: 10.1093/eurheartj/suaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan G.C.H., Ferkh A., Boyd A., Thomas L. Left atrial function: evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018;8(1):29–46. doi: 10.21037/cdt.2017.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y.-F., Chen Y.-J., Lin Y.-J., Chen S.-A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015;12(4):230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol. 2017;3(5):425–435. doi: 10.1016/j.jacep.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Lip G.Y.H., Fauchier L., Freedman S.B., Van Gelder I., Natale A., Gianni C., Nattel S., Potpara T., Rienstra M., Tse H.-F., Lane D.A. Atrial fibrillation. Nat. Rev. Dis. Prim. 2016;2(1) doi: 10.1038/nrdp.2016.16. [DOI] [PubMed] [Google Scholar]
- 31.Ye J., Wang Y., Wang Z., Liu L., Yang Z., Wang M., Xu Y., Ye D.i., Zhang J., Lin Y., Ji Q., Wan J. Roles and mechanisms of Interleukin-12 Family members in cardiovascular diseases: opportunities and challenges. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagola J., Juega J., Francisco-Pascual J., Bustamante A., Penalba A., Pala E., Rodriguez M., De Lera-Alfonso M., Arenillas J.F., Cabezas J.A., Moniche F., de Torres R., Montaner J., González-Alujas T., Alvarez-Sabin J., Molina C.A. Predicting atrial fibrillation with high risk of embolization with atrial strain and NT- proBNP. Transl. Stroke Res. 2021;12(5):735–741. doi: 10.1007/s12975-020-00873-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.