Abstract
This study aimed to investigate the prevalence and intensity of external parasites in domestic pigeons in Giza, Egypt, from January 2020 to December 2020. A total of 300 domestic pigeons (25 pigeons per month) were examined. The birds were divided into groups based on their age. The oxidative stress parameters; serum zinc concentration, serum malondialdehyde (MDA), and serum Nitric oxide were evaluated in single and mixed external parasitic infestations. The prevalence of external parasites in examined pigeons was 80.3%. The detected parasites were Pseudolynchia canariensis (P. canariensis), Hippobosca equina (H. equina), Columbicola columbae (C. columbae), Menopon gallinae (M. gallinae), Knemidocoptes species (spp.) and Dermanyssus gallinae (D. gallinae); their incidences were 41.6, 26, 7, 5,0.33 and 0.33%, respectively. The highest infestation was recorded in both spring and summer. . The incidence of disease was higher in squabs and young birds than in adults. The mixed external parasitic infestation was recorded in this study. The infected birds showed decreased serum zinc concentration and elevated MDA and serum Nitric oxide levels. In conclusion, regular monthly treatment with deltamethrin is recommended as an effective drug in the treatment of the infested birds and succeeded in reducing the incidence of externalparasites in the treated birds; in addition, pigeon management measures must be implemented to reduce the risk of external parasites.
Keywords: Dermanyssus gallinae; Deltamethrin, Hippobosca equine; Menopon gallinae; Knemidocoptes spp., Pseudolynchia canariensis
Abbreviations: MDA, serum malondialdehyde; P. canariensis, Pseudolynchia canariensis; H. equine, Hippobosca equine; C. columbae, Columbicola columbae; M. gallinae, Menopon gallinae; D. gallinae, Dermanyssus gallinae; spp., Species
1. Introduction
Pigeons coexist with different animal and poultry species as well as humans all over the world (Alkharigy et al., 2018). In Egypt, domestic pigeons are a valuable source of essential protein for people as they gain more weight quickly than other birds, its meat is palatable, easy to breed, and require slight management (El-Dakhly et al., 2016). Pigeons can be infected with a wide range of pathogens and serve as a reservoir for parasitic diseases. The proximity of pigeons to other domestic birds heightens the risk of parasitic infestation in poultry (Alkharigy et al., 2018). Pigeons harbor both external and/or internal parasites (Attia and Salem 2021).
Poultry ectoparasites are abundant in the tropics, where poor husbandry practices and favorable climatic circumstances allow them to thrive (Imura et al., 2012, Badparva et al., 2015, Salem and Attia, 2021). These parasites induce several direct and indirect effects on the pigeon, including weight loss, irritability, decreased productivity, malnutrition, low growth, decreased egg production and the development of a variety of clinical symptoms as; ruffled broken feathers, feathers loss, itching, pruritis, dermatitis, emaciation, inconvenience, death especially in squabs as well as indirect harm by transmitting other diseases (Radfar et al., 2012, Attia and Salem, 2021). The most common external parasites infesting pigeons are P. canariensis, Argas persicus, D. gallinae, M. gallinae, C. columbae, and Menocanthus stramineus (Atkinson et al.,2008).
Insects can harbor a lot of pathogens and convey them to the host from these pathogens “blood parasites” as Haemoproteus columbae which transmitted by the bite of ectoparasitic hippoboscid flies (Hussein and Abdelrahim, 2016); Plasmodium relictum, which is one of the most common species of avian malaria (Beadell et al., 2006) and Leucocytozoon marchouxi (Swinnerton et al., 2005, Martinsen et al., 2006, Hussein and Abdelrahim, 2016). Deltamethrin (a pyrethroid derivative) has become a popular pesticide in many nations due to its low toxicity and wide safety margin for poultry (Eladl et al., 2018). Deltamethrin affects the nervous system of insects that touch or ingest them, which leads to the rapid paralysis and death of these insects (Elyazar et al., 2011). In the same way, deltamethrin considered as an effective insecticide for the control of insects on pigeons and the surrounding environment (Attia and Salem, 2021). Accordingly, the aim of this study is to understand the biodiversity and determine the prevalence of pigeon external parasitic infestation with evaluation of oxidative stress markers and zinc levels which will aid in the planning of actions to improve the health of these birds in Egypt.
2. Material and methods
2.1 vvv. Ethical approval
This study was designed according to the Ethical Committee, Faculty of Veterinary Medicine regulations. Birds were humanly handled and safely released after clinical investigations.
2.2. Pigeon sampling
During the observation period from January to December 2020, 300 pigeons (25 bird per month) were investigated. Three hundred Birds were collected from different poultry clinics, poultry market and pigeon rearing houses from Giza, Egypt which lies in 29.9870°N 31.2118°E with a hot desert climate. The average ambient temperature in Giza in winter ranged from 21.1 °C (70.0)°F: 24.2 °C (75.6)°F with relative humidity 46:57%, in spring, 28.4 °C (83.1)°F : 34.9 °C (94.8)°F with relative humidity 37:44%, in summer 34.5 °C (94.1)°F : 41 °C (1 0 6)°F with relative humidity 39:53% and in autumn 25 °C (77.7)°F : 32.4 °C (90.3)°F with relative humidity 47: 57%. The examined birds were divided according to age as 80 squabs (day old to one month), 100 young (form one month to 7 months old) and 120 adults (above 7 months of age). Every bird was physically examined to detect the age and presence of external parasites (data were retrieved from the owners).
2.3. History and clinical examination of pigeons
2.3.1. External parasites
The feathers and skin were carefully examined for the presence of any macroscopic external parasites by naked eye then parasites were picked up and/ or skin scraping was adopted to identify the parasites using an Olympus Stereoscopic microscope (SZX16; Japan (Soulsby, 1986, Attia and Salem, 2021).
2.4. Identification of the parasites
All the collected parasites from the birds and their premises were preserved in 70% ethyl alcohol. Then, these parasites were identified using the keys recorded by Soulsby, 1986, Attia and Salem, 2021.
2.5. Blood samples
Blood was collected from jugular vein of externally parasitized birds on plain tubes for sera separation. Sera were kept on −20 °C for further investigations according to Attia et al., (2020).
2.6. Biochemical analysis
Serum zinc concentration were analysed using ionized coupled plasma by mass spectrometry method as described by Page et al., 2018, Attia et al., 2020.
2.7. Evaluation of oxidative stress markers
The level of serum malondialdehyde (MDA) using the reaction of thiobarbituric acid and then separation occurred on HPLC. The detection was performed using UV at 532 nm. Serum Nitric oxide (NO) level were analysed as mentioned by Khazaei and Nematbakhsh, 2012; briefly; colorimetric NO assay kit was used (Calbiochem-Novabiochem Corporation, San Diego, Calif), that measures the total nitrate and nitrite on serum based on Griess reaction and measured using wavelength 450 nm as mentioned by Aktas et al., 2017, Aytekin and Unubol Aypak, 2011.
2.8. Recommendation for external parasites control
Deltamethrin (Butox, 12.5 % solution, 1 mL / 4 L of water for birds & 1 mL /L for surrounding environment), is recommended for monthly spraying. Precautions have been taken during deltamethrin spraying, and the remains have been disposed of in a sanitary manner.
2.9. Statistical analysis
Data were statistically analyzed by using SPSS Version 18.0 software (Inc., Chicago, IL, USA). Blood parameters in infested and control non infested group were compared by independent T-test following the normality of data. A p- value consider significant when (p < 0.05).
3. Results
3.1. History and clinical investigations
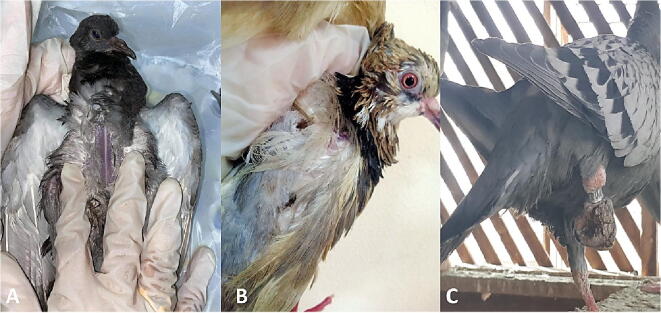
Investigated pigeons were exhibited indications of illness such as general weakness, decreased egg production in layers, un-thriftiness, ruffled feathers, decreased weight gain, emaciation with protrusion of keel bone (Fig. 1A) and in some cases; pigeon flies were observed on birds’ body, under/or on the wings or on tail (Fig. 1B) and Fig. 2.
Fig. 1.
A: Pigeon showing emaciation with ruffled feather; B: Pigeon showing ruffled feather with presence of Pseudolynchia flies on its body; C: Pigeon showing unilateral arthritis with presence of raised grayish color scales with grayish exudates in between scales on pigeon’s shank.
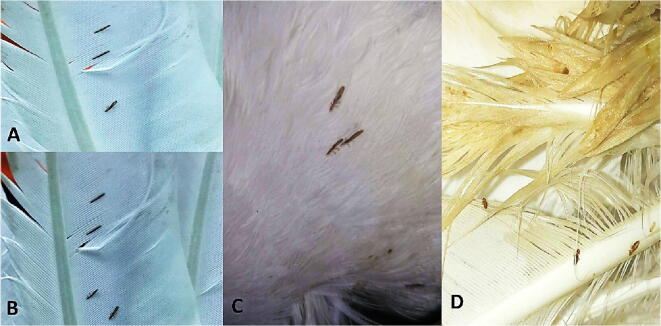
Fig. 2.
A, B & C: Pigeon feathers showing attachment of C. columbae. D: Pigeon feathers showing attachment of M. gallinae.
Most of the examined pigeons were positive with at least with one of external parasitic species. The overall prevalence rate was 80.3%.
3.2. External parasites
External parasites usually observed on living birds. In case of lice infestation, eggs were attached with feathers roots, while in red mite; the red spots were observed surround the bird vent, unilateral arthritis was observed in birds mange with presence of dull raised scales tinged with grayish to brown color exudate on shank of pigeon leg (Fig. 1C).
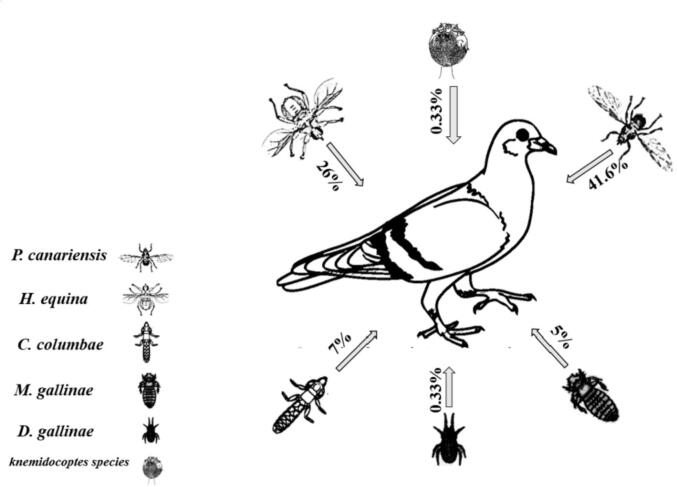
Two hundred and forty-one; out of 300 examined pigeons, were positive for external parasites infestations. Six different types of external parasites were detected as follow: P. canariensis, H. equina, C. columbae, M. gallinae, Knemidocoptes spp. and D. gallinae with prevalence 41.6, 26, 7, 5, 0.33 and 0.33%, respectively. The intensity of external parasites on pigeon’s body were ranged from (1–6), (1–4), (8–25) and (5–20) in case of P. canariensis, H. equina, C. columbae, and M. gallinae, respectively.
The prevalence and intensity of ectoparasites in domestic pigeons based on their age were summarized in (Fig. 3 & Table 1) and Fig. 4.
Fig. 3.
A: Adult P. canariensis. B: Microscopic appearance of D. gallinae.
Table.1.
Prevalence and intensity of ectoparasites in domestic pigeons in Giza, Egypt from January 2020 to December 2020.
| Parasites | Positive cases (%) | Parasite’s intensity |
Age |
||
|---|---|---|---|---|---|
|
Squabs (80) |
Young (1 0 0) |
Adults (1 2 0) |
|||
| P. canariensis | 125 (41.6) | 1–6 | 50 (62.5%) | 65 (65%) | 10 (8.33%) |
| H. equina | 78 (26) | 1–4 | 12 (15%) | 22 (28%) | 44 (36.6%) |
| C. columbae | 21 (7) | 8–25 | 10 (12.5%) | 8 (8%) | 3 (2.5%) |
| M. gallinae | 15 (5) | 5–20 | 6 (0.75%) | 4 (4%) | 5 (4.16%) |
| D. gallinae | 1 (0.33) | – | 0 | 0 | 1 (0.33%) |
| Knemidocoptes spp. | 1(0.33) | – | 0 | 0 | 1 (0.33%) |
| Total | 241 (80.3%) | – | 78/80(97.5%) | 99/100 (99%) | 63/120 (52.5%) |
Fig. 4.
A summarized diagram showing the prevalence of different external parasites of pigeons in Giza, Egypt from January 2020 to December 2020.
Single infestation of pigeons with P. canariensis, H. equina, C. columbae, M. gallinae, Knemidocoptes spp. and D. gallinae were recorded also; mixed external parasites infestation between (P. canariensis and H. equina); (P. canariensis and C. columbae); (C. columbae and M. gallinae) and (P. canariensis; C. columbae and M. gallinae) were observed and summarized in; Table 2.
Table.2.
Prevalence of single and mixed infestation of external parasites in domestic pigeons.
| Parasite | Type of infestation |
Prevalence (Positive cases/300 total examined birds) |
|
|---|---|---|---|
| No. | % | ||
| P. canariensis | Single | 90 | 30 |
| H. equina | 66 | 22 | |
| C. columbae | 9 | 3 | |
| M. gallinae | 3 | 1 | |
| D. gallinae | 1 | 0.33 | |
| Knemidocoptes spp. | 1 | 0.33 | |
| P. canariensis + H. equina | mixed | 22 | 7.33 |
| P. canariensis + C. columbae | 31 | 10.33 | |
| C. columbae + M. gallinae | 12 | 4 | |
| P. canariensis + C. columbae + M. gallinae | 6 | 2 | |
| Total No. of positive pigeons | 241 | 80.3 | |
The highest prevalence of external parasites was detected in spring and summer seasons as seen in (Table 3).
Table.3.
Seasonal prevalence of external and internal parasites in domestic pigeons.
| External | Parasite | Spring |
Summer |
Autumn |
Winter |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Pseudolynchia canariensis | 57 | 76 | 60 | 80 | 5 | 6.66 | 3 | 4 | |
| Hippobosca equina | 18 | 24 | 48 | 66.6 | 12 | 16 | 0 | 0 | |
| Columbicola columbae | 8 | 10.66 | 10 | 13.33 | 2 | 2.66 | 1 | 1.33 | |
| Menopon gallinae | 6 | 8 | 9 | 12 | 0 | 0 | 0 | 0 | |
| Dermanyssus gallinae | 0 | 0 | 1 | 1.33 | 0 | 0 | 0 | 0 | |
| Knemidocoptes spp. | 0 | 0 | 1 | 1.33 | 0 | 0 | 0 | 0 | |
No. = number of positive cases.
%= prevalence (No. of positive birds/ total [75 examined bird in each season]).
Infested pigeon with H. equina showed significantly higher in Nitric oxide (120.56 ± 4.56, ±95% C.I.) while in mixed infestation with P. canariensis it reaches to 145.89 ± 9.43; the Nitric oxide levels was low in M. gallinae reach to 56.89 ± 3.76. Serum MDA reach to 100.30 ± 6.48, 95% C.I.; in mixed infestation with P. canariensis, H. equina, while it was low in C. columbae infestation. zinc levels were decreased with parasitic infestation as it reaches to low levels in P. canariensis, H. equina mixed infestation; all data were compared to control levels of oxidative stress markers and zinc levels (Table 4).
Table.4.
Oxidative stress markers in relation to single and mixed infection of external parasites.
| Parasite |
Oxidative stress markers |
||
|---|---|---|---|
| Nitric oxide | MDA serum | Zinc | |
| P. canariensis | 95.57 ± 3.67 | 78.38 ± 5.17 | 54.04 ± 2.85 |
| H. equina | 120.56 ± 4.56 | 88.38 ± 4.49 | 36.77 ± 1.32 |
| C. columbae | 67.98 ± 7.00 | 30.33 ± 3.35 | 100.24 ± 1.00 |
| M. gallinae | 56.89 ± 3.76 | 33.56 ± 3.46 | 110.52 ± 2.96 |
| D. gallinae | 79.89 ± 5.99 | 50.33 ± 1.03 | 33.57 ± 2.82 |
| Knemidocoptes spp. | 80.14 ± 6.33 | 52.35 ± 1.67 | 34.66 ± 3.62 |
| P. canariensis + H. equina | 145.89 ± 9.43 | 100.30 ± 6.48 | 28.89 ± 1.52 |
| P. canariensis + C. columbae | 110.00 ± 3.59 | 98.33 ± 2.45 | 59.97 ± 3.95 |
| C. columbae + M. gallinae | 74.49 ± 9.32 | 60.38 ± 2.98 | 100.67 ± 2.96 |
| P. canariensis + C. columbae + M. gallinae | 160.84 ± 3.57 | 98.89 ± 2.78 | 34.56 ± 2.96 |
| Control | 49.78 ± 0.95 | 27.89 ± 0.69 | 118.00 ± 0.94 |
Periodical, monthly usage of deltamethrin in the infested flocks revealed a marked decrease in external parasites numbers after two days of treatment with relieve of the clinical signs.
4. Discussion
Pigeons are a point of anxiety since they can spread zoonoses to humans and are a reservoir for numerous parasite diseases affecting poultry (Sari et al., 2008). Most of examined pigeons showed signs of ruffled feathers, severe emaciation. Similar signs were observed in case of parasitic infestation as recorded by Abd El-Rahman et al., 2008, Mohamed et al., 2009, Abdel Rahman et al., 2019.
Ectoparasites live on feathers and skin of the host, using as a shelter and food provider, they also have a considerable negative influence on host health and productivity. From our results, the overall prevalence of external parasites was 80.3% (241/300) either single or mixed infestations, six different arthropods species were identified as follow: P. canariensis (41.6%), H. equina (26%), C. columbae (7%), M. gallinae (5%), D. gallinae (0.33 %), and Knemidocoptes spp (0.33%). Higher prevalence 89% (89/100) was recorded in Tripoli, Libya by Alkharigy et al., (2018) as they found the prevalence of C. columbae (82%), Goniodes gallinae (18%), M. gallinae (3%) and P. canariensis (1%). Also, Radfar et al., (2011) recorded higher prevalence of external parasites in pigeon, C. columbae (79.41%), M. gallinae (44.11%) and P. canariensis (63.72%) in south khorasan; Iran while, in Colombia, Pérez-García et al., (2015) found that the prevalence of C. columbae (64%), P. canariensis (52%), M. gallinae (24%). Borji et al., (2012) reported that the prevalence of C. columbae (42.8%), P. canariensis (16.1%) and M. gallinae (7.1%) in Mashhad city Iran. Also, Ali et al., (2020) recorded two external parasites on (Harami) pigeons, including the shaft louse M. gallinae and P. canariensis with 100 and 88.90% prevalence, respectively in Medina, Saudi Arabia.
In another study, the incidence of H. equina was 26% while Sokół and Michalski, (2015) noticed the low host specificity of H. equina which could attack cattle, dogs, hares, birds, and humans.
The prevalence of C. columbae was 21% however, Jahantigh et al., 2016, Dranzoa et al., 1999, Foronda et al., 2004 recorded higher prevalence of C. columbae 78.40%, 94.1 and 100% in Iran, Uganda, and Tenerife, respectively.
From our findings, Knemidocoptes spp. was observed in one case only with incidence of 0.33% during summer season and its lesion was found as unilateral arthritis and presence of severe dermatitis that appeared as raised dull color scales with presence of grayish color exudate between scales. Similar findings were observed in a parallel study conducted by Abou-Alsoud and Karrouf, (2016) who detected Knemidocoptes Pilae with prevalence of (11.5 %) in Budgerigars in Mansoura public park, Egypt.
The mixed infestation was reported as follow: P. canariensis + H. equina with prevalence 7.33%. P. canariensis + C. columbae with 10.33 % prevalence; C. columbae + M. gallinae (4%) and P. canariensis + C. columbae + M. gallinae (2%). Also, Alkharigy et al., (2018) recorded mixed infestation with external parasites in Libya. The mixed infestation of pigeon with external parasites could be contributed to that ectoparasites can live together without causing any hurtful effects on each other (Radfar et al., 2011).
The occurrence of ectoparasitic infestations in squabs (82.5%) was higher than young (77%) and lowest prevalence was recorded in adults (18%). Same observations were recorded by da Cunha Amaral et al., (2013) in case of P. canariensis infestation but, Msoffe et al., 2010, Radfar et al., 2012 noticed that the prevalence of P. canariensis were higher in adult pigeons than squabs. Adult pigeons are predicted to have a lower incidence of ectoparasites since they have a higher level of parasite immunity (Merila et al.,1995) and adult birds use their claws and the bill to get rid of ectoparasites (Clayton et al., 2010, Waite et al., 2012).
From our results, the intensity (number of external parasites that are found per bird) of external parasites on pigeon’s boy ranged from (1:6), (1:4), (8:25) and (5:20) in case of P. canariensis, H. equina, C. columbae and M. gallinae, respectively. This result is agreed with Attia and Salem (2021) as they noticed that the intensity of P. canariensis flies was 1: 6 (2.00 ± 1.0) on pigeons' body in El- Gharbia, Egypt.
The ambient temperature is the key determinant of the frequency, quantity, and diversity of external parasites allover year (Al-Barwari and Saeed, 2012, Attia et al., 2017). In our study the highest prevalence of external parasites infestations was recorded in both spring and summer. The prevalence of P. canariensis was 76% and 80%, C. columbae was 10.66% and 13.33%, M. gallinae was 8 % and 12% in spring and summer, respectively. D. gallinae only detected in summer season only 1.33%. The lowest prevalence of external parasites infestation in our study was recorded in autumn and winter season, P. canariensis was 6.66% and 4%, C. columbae was 2.66% and 1.33% in autumn and winter, respectively. on the other hand, no positive cases of M. gallinae and D. gallinae were recorded in autumn and winter. The differences in the seasonal prevalence may be contributed to the ambient temperature in Egypt exceed 25 Co in both spring and summer which considered a favorable condition for insects’ population.
Continuous exposure of poultry to ectoparasites that act as intermediate hosts for different parasitic worms increases the potential for the spread of parasitic diseases among birds (Ashenafi and Eshetu, 2004). So, monthly spray of deltamethrin is recommended and provoke acceptable result and succeeded in limitation of external parasites load. This result is agreed with (Attia and Salem, 2021) as they found deltamethrin is effective in P. canariensis control. Deltamethrin has proven its efficiency in controlling insects and we recommend in the future to load the active substance on biological nanoparticles to increase its efficiency. Also, recommend the use of some additives on pigeon food and drink as prebiotics (Abd El-Hack et al., 2021b, Yaqoob et al., 2021) probiotics (Abd El-Hack et al., 2021, Alagawany et al., 2021a, El-Saadony et al., 2021a), bioactive plants compounds (El-Saadony et al., 2021b), bioactive peptides (El-Saadony et al., 2021c, El-Saadony et al., 2021e), green synthesized nanoparticles (El-Saadony et al., 2021d, El-Saadony et al., 2021f), herbal extracts (Reda et al., 2021, Abou-Kassem et al., 2021), phytogenic compounds (Abd El-Hack et al., 2021c, Ashour et al., 2020), and essential oils (Alagawany et al., 2021b, El-Tarabily et al., 2021, Abd El-Hack et al., 2020) to strengthen their immune system and increase disease resistance.
The oxidative stress markers as (Nitric oxide and MDA levels) were raised in parasitic infestation either in single parasites or in mixed infestation due to rapid erythrocyte destruction and secretion of oxygen radicles (Mousa and Soliman, 2016); as recorded by Nazifi et al., 2011, Razavi et al., 2011 found that the antioxidant enzyme activity of superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase, were raised during parasitic infestation.
5. Conclusion
When we spoke with pigeon owners, we discovered that most of them were unaware of dealing with a parasitic infestation in domestic pigeons, and some of them do not remedy them at all. From our findings, pigeons have a significant prevalence of external parasites, with single or mixed infestation. Oxidative stress markers were significantly elevated during either single or mixed external parasites infestations. Pathogenicity studies, regular monitoring, treatment, and control strategies must be put in place to reduce pigeon parasitic infestation considering that these birds are in close contact with other poultry and human. Deltamethrin could be considered as an effective insecticide for controlling of external parasites in the pigeon and would be recommended for regular use hand by hand with biosecurity measures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The current work was funded by Taif University Researchers Supporting Project number (TURSP - 2020/221), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Heba M. Salem, Email: dr.hebasalem@cu.edu.eg.
Ahmed M. El-Shehawi, Email: elshehawi@hotmail.com.
References
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric.in press. 2021 doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.-E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.-E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2020;28(5):4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Rahman M.A.M., Seddiek S.A., Soliman A.S. Some studies on trichomoniasis of pigeons at Qualiobia governorate. Egypt. J. Comp. Pathol. Clin. Pathol. 2008;21(2):123–141. http://erepository.cu.edu.eg/index.php/EJCPCP/article/view/177 [Google Scholar]
- Abdel Rahman M.M.I.A., Tolba H.M.N., Abdel-Ghany H.M. Ultrastructure, morphological differentiation, and pathological changes of Ascaridia species in pigeons. Adv. Anim. Vet. Sci. 2019;7(2):66–72. doi: 10.17582/journal.aavs/2019/7.2.66.72. [DOI] [Google Scholar]
- Abou-Alsoud M.E., Karrouf G. Diagnosis and Management of Knemidocoptes Pilae in Budgerigars (Melopsittacus Undulates): Case Reports in Egypt. M J Vete. 2016;1(1):007. [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Aktas, M.S., Kandemir, F.M., Kirbas, A., Hanedan, B., Aydin, M.A., 2017. Evaluation of oxidative stress in pigeon infected with Psoroptes ovis using total antioxidant capacity, total oxidant status, and malondialdehyde level. Vet. Res. 61(2):197–201.https://doi. 10.1515/jvetres-2017-0025. [DOI] [PMC free article] [PubMed]
- Alagawany M., El-Saadony M., Elnesr S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6) doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Al-Barwari S.H., Saeed I. The Parasitic Communities of the rock pigeon Columba livia from Iraq: Component and Importance. Turkiye Parazitol Derg. 2012;36:232–239. doi: 10.5152/tpd.2012. [DOI] [PubMed] [Google Scholar]
- Ali, M., Ibrahim, R., Alahmadi, S., Elshazly, H., 2020. Ectoparasites and intestinal helminths of pigeons in Medina, Saudi Arabia. J Parasitol. 12, 106(6):721–729. https://doi.10.1645/20-64. DOI: 10.1645/20-64. [DOI] [PubMed]
- Alkharigy, F.A., El Naas, A.S., EL Maghrbi, A.A., 2018. Survey of parasites in domestic pigeons (Columba livia) in Tripoli, Libya. Open Vet. J. 8(4): 360–366. http://dx.doi.org/10.4314/ovj.v8i4. [DOI] [PMC free article] [PubMed]
- Ashenafi, H., Eshutu, Y., 2004. Study on Gastro-intestinal helminths of local chickens in Central Ethiopia. Rev. Med. Vet. 155(10): 504–507. https://www.revmedvet.com › RMV155_504_507.
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Atkinson, C.T., Thomas, N.J., Hunter, D.B., 2008. Parasitic diseases of wild birds. John Wiley & Sons, Inc. ISBN: 978-0-813-82081-1. U.S. Library of Congress. https://doi.10.1002/9780813804620.
- Attia, M.M., El-Gameel, S.M., Ismael, E., 2020. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J. Parasit. Dis.44(2): 332–337.https://doi.org/10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2021 doi: 10.1007/s42690-021-00597-2. [DOI] [Google Scholar]
- Attia M.M., Soliman S.M., Khalf M.A. Hydrophilic nanosilica as a new larvicidal and molluscicidal agent for controlling of major infectious diseases in Egypt. Vet World. 2017;10(9):1046–1051. doi: 10.14202/vetworld.2017.1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin, I., Unubol Aypak, S., 2011. Levels of selected minerals, nitric oxide, and vitamins in aborted Sakis pigeon raised under semitropical conditions. Trop. Anim. Health Prod. 43:511–514.https://doi.10.1007/s11250-010-9724-x. [DOI] [PMC free article] [PubMed]
- Badparva E., Ezatpour B., Azami M., Badparva M. First report of bird's infection by intestinal parasites in Khorramabad, west Iran. J. Parasit. Dis. 2015;39(4):720–724. doi: 10.1007/s12639-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadell J.S., Ishtiaq F., Covas R., Melo M., Warren B.H., Atkinson C.T., Bensch S., Graves G.R., Jhala Y.V., Peirce M.A., Rahmani A.R., Fonseca D.M., Fleischer R.C. Global phylogeographic limits of Hawaii’s avian malaria. Proc R Soc Lond. Series B. 2006;273(2935–2944) doi: 10.1098/rspb.2006.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borji, H., Moghaddas, E., Razmi, G.R., Azad, M., 2012. A survey of ecto- and endo-parasites of domestic pigeons (Columba livia) in Mashhad, Iran. Iran. J. Vet. Sci. Technol. 4(2):37–42. https://doi: 10.4314/ovj.v8i4.2.
- Clayton, D.H., Koo, J., Harbiso, C., Moye, B., Bush, S., 2010. How birds combat ectoparasites. J. Ornithol. 3: 41–71. https//doi: 10.2174/1874453201003010041.
- da Cunha Amarala H., Bergmannb F.B., Silveirac T., Silveira dos Santosd P.R., Krügera R.F. Pseudolynchia canariensis (Diptera: Hippoboscidae): distribution pattern and phoretic association with skin mites and chewing lice of Columba livia (Aves: Columbidae) J. Nat. Hist. 2013;47(47–48):2927–2936. doi: 10.1080/00222933.2013.791939. [DOI] [Google Scholar]
- Dranzoa, C., Ocaido, M., Katete, P., 1999. The ecto,gastro-intestinal and haemoparasites of live pigeons (Columba livia) in Kampala, Uganda. Avian Pathol. 28, 119–124. https://doi: 10.1080/03079459994830. [DOI] [PubMed]
- Eladl A., Hamed H.R., El-Shafei R.A. Prevalence of mites and their impact on laying hen (Gallus gallus domesticus) farm facilities in Egypt, with an analysis of deltamethrin residues in eggs and tissue. Avian Pathol. 2018;47(2):161–171. doi: 10.1080/03079457.2017.1388500. [DOI] [PubMed] [Google Scholar]
- El-Dakhly, K., Mahrous, L.N., Mabrou, G.A., 2016. Distribution pattern of intestinal helminths in domestic pigeons (Columba livia domestica) and turkeys (Meleagris gallopavo) in Beni-Suef province Egypt. J. Vet. Med. Res. 23(1):112–120. https//doi: 10.21608/JVMR.2016.43226.
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Abdel-Latif H.M. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: A review. Food Rev. Int. 2021:1–23. [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan Sa.ad., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.-S.M., Fouda S.E.-S.E.-S., El-Tahan A.M. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi. J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyazar, I. R.F., Hay, S. I., Baird, J. K., 2011. Chapter 2 - Malaria distribution, prevalence, drug resistance and control in Indonesia. ADV PARASIT .74: 41–175. https://doi.org/10.1016/B978-0-12-385897-9.00002-1. [DOI] [PMC free article] [PubMed]
- Foronda, P., Valladares, B., Rivera-Medina, J.A., 2004. Parasites of Columba livia (Aves: Columbiformes) in Tenerife (Canary Islands) and their role in the conservation biology of the Laurel pigeons. Parasite. 1: 311-316. https//doi: 10.1051/parasite/2004113311. [DOI] [PubMed]
- Hussein, N.M., Abdelrahim, E.A., 2016. Haemoproteus Columbae infection and its histopathological effects on pigeons in Qena Governorate, Egypt. IOSR-JPBS. 11(1): 79-90. https//doi: 10.9790/3008-11117990.
- Imura, T., Suzuki, Y., Ejiri, H., Sato, Y., Ishida, K., Sumiyama, D., Murata, K., Yukawa, M., 2012. Prevalence of avian haematozoa in wild birds in ahigh-altitude forest in Japan. Vet. Parasitol. 183: 244–248. https//doi: 10.1016/j.vetpar.2011.07.027. [DOI] [PubMed]
- Jahantigh, M., Esmailzade Dizaji, R., Teymoori. Y., 2016. Prevalence of external parasites of pigeon in Zabol, southeast of Iran. J. Parasit. Dis. 40(4):1548-1551. https//doi: 10.1007/s12639-015-0725-6. [DOI] [PMC free article] [PubMed]
- Khazaei M., Nematbakhsh M. Effect of experimentally induced metabolic acidosis on aortic endothelial permeability and serum nitric oxide concentration in normal and high-cholesterol fed rabbits. Arch. Med. Sci. 2012;8(4):719–723. doi: 10.5114/aoms.2012.30296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen, E.S., Paperna, I. Schall, J., 2006. Morphological versus molecular identification of avian Haemosporidia: an exploration of three species concepts. Parasitol. 13(3):279–288. https//doi: 10.1017/S0031182006000424. [DOI] [PubMed]
- Merila, J., Bjorklund, M., Bennett, G.F., 1995. Geographic and individual variation in haematozoan infections in the greenfinch, Carduelis chloris. Can. J. Zool. 73:1798–1804. https//doi: 10.1139/z95-212.
- Mohamed I.E., El-Sakkar G.H., Moursi M.M. Pathological studies on pigeon trichomoniasis with reference to the associated bacteria. J. Comp. Pathol. Clin. Pathol. 2009;22(2):67–87. http://www.pathologyeg.com/publication2009_2/Publication3.pdf [Google Scholar]
- Mousa S.A., Soliman S.M. Oxidant and Antioxidant Status in Pneumonic Goats with Special Reference to Bacterial Etiology. Int. J. Livestock Res. 2016;6(5):15–23. doi: 10.5455/ijlr.20160417045508. [DOI] [Google Scholar]
- Msoffe P.L.M., Muhairwa A.P., Chiwanga G.H., Kassuku A.A. A study of ecto- and endo-parasites of domestic pigeons in Morogoro unicipality. Tanzania. Afr. J. Agric. Res. 2010;5(3):264–2672. [Google Scholar]
- Nazifi S., Razavi S.M., Kianiamin P., Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation and serum trace elements associated with progressive anemia in ovine malignant theileriosis. Parasitol. Res. 2011;109(275–281) doi: 10.1007/s00436-010-2248-5. [DOI] [PubMed] [Google Scholar]
- Page C.M., Murphy T.W., Van M.L., Emon J.G., Bowman P., Wyffels S.A., Stewart W.C. Blood serum mineral element concentrations of weaned Montana ram lambs and their relationship with water quality characteristics. Prof. Anim. Sci. 2018;34(5):410–420. doi: 10.15232/pas.2018-01747. [DOI] [Google Scholar]
- Pérez-García J., Monsalve-Arcila D., MárquezVillegas C. Presencia de parásitos y enterobacterias en palomas ferales (Columba livia) en áreas urbanas en Envigado Colombia. Rev. Fac. Na.c Salud Pública. 2015;33(3):370–376. [Google Scholar]
- Radfar, M.H, Asl, E.N., Seghinsara, H.R., Dehaghi, M.M., Faith, S., 2012. Biodiversity and prevalence of parasites of domestic pigeons (Columba livia domestica) in a selected semiarid zone of South Khorasan, Iran. TROP. ANIM. HEALTH PRO. 44(2):225–229. https//doi: 10.1007/s11250-011-0002-3. [DOI] [PubMed]
- Radfar, M.H., Fathi, S., Asl, E.N., Dehaghi, M.M., Seghinsara, H.R., 2011. A survey of parasites of domestic pigeons (Columba livia domestica) in South Khorasan, Iran. J. Vet. Res. 4(1):18–23. https//doi: 10.3923/vr.2011.18.23. [DOI] [PubMed]
- Razavi, S., Nazifi, S., Bateni, M., Rakhshandehroo, E., 2011. Alterations of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation and serum trace elements associated with anemia in bovine tropical theileriosis. Vet. Parasitol. 180(3):209–214. https//doi: 10.1016/j.vetpar.2011.03.011. [DOI] [PubMed]
- Reda F., El-Saadony M., El-Rayes T., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100(1) doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M.H., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect. Sci. 2021 doi: 10.1007/s42690-021-00492-w. [DOI] [Google Scholar]
- Sari B., Karatepe B., Karatepe M., Kara M. parasites of domestic (Columba livia domestica) and wild (Columba livia livia) pigeons in niğde, turkey. Bull. Vet. Inst. Pulawy. 2008;52(4):551–554. [Google Scholar]
- Sokół R., Michalski M.M. Occurrence of Hippobosca equina in Polish primitive horses during the grazing season. Ann. Parasitol. 2015;61(2):118–122. [PubMed] [Google Scholar]
- Soulsby E.J.L. 7th ed. Bailliere Tindall; London: 1986. Helminths, Arthropods and Protozoa of Domesticated Animals; pp. 167–174. [Google Scholar]
- Swinnerton, K.J., Peirce, M.A., Greenwood, A., Chapman, R.E., Jones, C.G., 2005. Prevalence of Leucocytozoon marchouxi in the endangered Pink Pigeon Columba mayeri. Ibis. 147(4):725–737. https/doi: 10.1111/j.1474-919X.2005. 00454.x
- Waite, J.L., Henry, A.R., Clayton, D.H., 2012. How effective is preening against mobile ectoparasites? An experimental test with pigeons and hippoboscid flies. Int. J. Parasitol. 42(5):463–467. https/doi: 10.1016/j.ijpara.2012.03.005. [DOI] [PubMed]
- Yaqoob M., Abd El-Hack M., Hassan F., El-Saadony M., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100(7) doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]