Abstract
Tectona grandis L.f is a timber plant that is commonly referred to as teak. Its wide use as a medicine in the various indigenous systems makes it a plant of importance. A wide gamut of phytoconstituents like alkaloids, phenolic glycosides, steroids, etc. has been reported. A renewed interest in this plant has resulted in scientific investigations by various researchers towards the isolation and identification of active constituents along with scientific proof of its biological activities. The different parts of the plant have been scientifically evaluated for their antioxidant, antipyretic, analgesic, hypoglycemic, wound healing, cytotoxic, and many more biological activities. Documentation of this scientific knowledge is of importance to have consolidated precise information encompassing the various aspects of this plant, which could provide a base for future studies. This review is a compilation of the salient reports on these investigations concerning phytochemistry, the methods used to identify and quantify the constituents, the evaluation methods of the biological activity, toxicological studies, allergies and the patent/patent applications. This will further help researchers to find an area of the gap for future studies.
Keywords: Tectona grandis L.f, Phytochemical profile, Biological activities, Patents
1. Introduction
Plants are indispensable sources of medicine. Research on products obtained from nature is usually aimed to determine the medicinal values by exploring the available scientific knowledge and traditional uses. The phytochemicals isolated from these plants can be used as templates for further optimization of the lead molecules. It has been reported that in developing countries, 25% of the drugs are based on plants and their derivatives (Ramesh et al., 2013, Nahida et al., 2012). Several plants have been investigated for their phytochemical and pharmacological activities by various groups of researchers. One such plant of interest is Tectona grandis L.f (TG). It belongs to the family Verbenaceae. It is commonly referred to as teak. It is a large deciduous tree and may reach a height of 30–40 m with fluting and buttresses found at the base of older trees. The color of the bark is light grayish-brown. The leaves are large, shiny, opposite, and elliptic. The lower surface of the leaf is gray and covered with glandulous hairs. The flowers are small, white in color, and bisexual, appearing as large panicles. The fruit is a green, hairy, woody, irregularly rounded drupe (Nilesh et al., 2017). The tree can be found in several regions of south Asian countries and its parts such as root, bark, flowers, wood and oil are reported to be an important source of medical properties. The various parts of the plant have been used traditionally and ethnopharmacologically for the treatment of common cold, headache, in wound healing, bronchitis scabies, as a laxative, diuretic, antidiabetic, anti-inflammatory, antioxidant, lipid disorders, constipation, and diuretic (Kruger and Schulz, 2007). These pharmacological activities were found to be augmented when combined with other extracts. The unique combinations of such natural ingredients have been filed for patents. This review intended to compile the phytoconstituents identified along with the part and the solvent used for the extract and methods utilized for quantifying these compounds, listing the biological activities along with the methods applied, the extracts used, a brief account of toxicological evaluation, allergic manifestations and also the list of important information regarding patents/patent applications that have been filed concerning this plant.
1.1. Search strategy, inclusion and exclusion crieteria
The search engines used for retrieving published data include databases that are universally recognized, specially Scopus, PubMed, Science Direct, Web of Science and Google Scholar. The various search terms used as key words were Tectona grandis L.f,phytochemical, biological activities, toxicology, allergy, phytoconstituents, HPLC,UV,IR GC–MS. The related articles were identified and screened for the title and abstract. Data extracted included the title, author(s), journal and year of publiction. Related articles were retrieved in full text and validated for including them in the review. This study focused on all the major aspects of the plant under consideration. Papers that reported the pharmacology, phytoconstituents, allergy, toxicological were included in this study. Dissertations were also included. The studies included in this review were in English language. Inappropriate articles were excluded for the following reasons i.e. unrelated topic, insufficient data, duplication and unavailablity of the abstract or full-text. The qualification of each paper was assessed by reading the full-text. There was no limitation in the search period. In the systematic review, articles were included from the available databases from 1986 to 2021.
2. Phytochemical profile of Tectona grandis
Several instrumental methods are available for identifying and quantifying the phytoconstituents in plants. The literature review describes the use of classical techniques such as high-performance liquid chromatography (HPLC), high-performance thin-layer chromatography (HPTLC), gas chromatography-mass spectrometry [GC–MS], and various other methods in the field of medicinal and aromatic plants (Kruger and Schulz, 2007). Researchers have reported a wide gamut of phytoconstituents. The preliminary investigation of the different parts of the plant, such as bark, wood, leaves, flowers, fruits, etc. has revealed the presence of flavonoids, phenolics, alkaloids, and certain glycosides (Nayeem and Karvekar, 2011a). Several methods have been reported for quantifying the secondary metabolites found in the various parts of TG following the ICH guidelines.
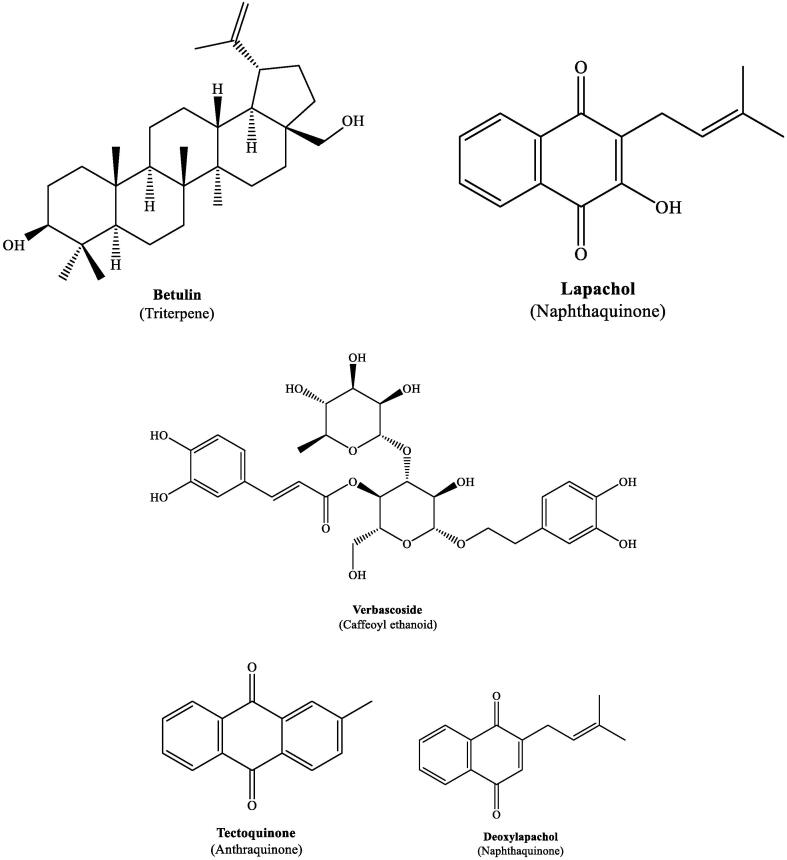
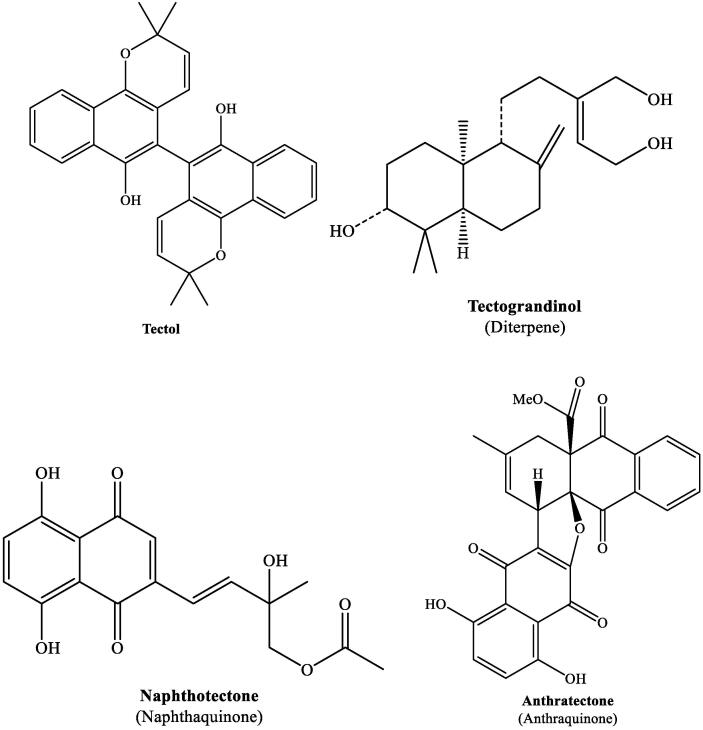
The chemical structures of the different constituents of TG are provided in earlier publications (Neha and Sangeetha, 2013, Vyas et al., 2019, Goswami et al., 2009). The chemical structures of some important constituents of TG are provided below.
Some phytoconstituent, along with their techniques of identification/quantification, are listed in the following Table 1.
Table 1.
Phytochemical profile of TG.
| S. No. | Part (Solvent extract) | Phytoconstituents | Chemical class | Technique | Ref. |
|---|---|---|---|---|---|
| 1 | Stem bark (Methanol) | Betulin | Triterpenoid | HPLC | Singh et al., 2016 |
| 2 | Roots (Methanol) | Tannic acid, Caffeic acid, Gallic acid, Ferulic acid | Phenolic acids | HPLC | Shalini and Srivastava, 2009 |
| 3 | Leaves (Methanol) | Sinapic, gallic, p-hydroxybenzoic, ferulic, p-coumarate, chlorogenic, cinnamic, vanillic acids | Phenolic compounds | RP-HPLC | Murukan and Kumara, 2018 |
| 4 | Seed (Petroleum ether) | Linoleic acid, Octadecenoic acid methyl ester, Palmitic acid, Oleic acid. | Fatty acids | GC & GCMS | Bachheti et al., 2012 |
| 5 | Heartwood, sapwood (Dichloromethane, Ethanol, and Ethanol-Toluene) | Lapachol, 2-Methylanthraquinone, 1,4-Naphthoquinone | Quinones | GCMS and HPLC | Bhat et al., 2010 |
| 6 | Leaves (Aqueous) | Verbascoside | Phenyl ethanoid | LCMS | Emmanuel et al., 2016 |
| 7 | Heart wood (Acetone) | 2,3-Dimethyl-1,4,4a,9a-tetrahydro-9,10-anthracenedione, Acetonyldimethylcarbinol, 4-Tert-butyl-2-phenyl-phenol, 2-Methyl-anthraquinone estriol, Lappaol, Deoxylactam, Squalene, Chloranol, Palmitic acid, 2,3-Dimethyl-1,4,4a,9a-tetrahydro-9,10-nonanedione | Phenols, Quinones, Fatty acids, Triterpene | GCMS | Qui et al., 2019 |
| 8 | Heartwood (Aqueous) | 2-(Hydroxymethyl)anthraquinone, 2-Anthraquinone carboxylic acid, Tectoquinone, 1,4-Naphthoquinone and 4′,5′-Dihydroxy-epiisocatalponol | Quinones | HPLC, NIR | Niamke et al., 2013 |
| 9 | Leaf (Aqueous) | Protocatechuic acid, Quinic acid, and its derivatives, Apigenin 7-O-diglucuronide, Luteolin, Luteolin 7-O-diglucuronide, Luteolin glucuronide, Diglucuronide, Apigenin glucuronide, | Flavonoids, Phenolic acids, Glucuronides | LCMS | Koffi et al., 2015 |
| 10 | Flower (Methanol) | Quercetin, Kaempferol Rutin, Ellagic acid, Gallic acid, Ferulic acid, | Flavonoids, Phenolic acids | HPLC | Ramachandran and Rajasekaran, 2014 |
| 11 | Wood knots (Isopropanol) | Forsytoside B, Isoacteoside | Phenylethanoid, Glycosides | HPLC | Tsvetkov, et al., 2010 |
| 12 | Leaf (Methanol) | Gallic acid, Cinnamic acid, Tannic, Ellagic acid, Rutin Quercetin, Umbelleferone | Phenolic acids, Flavonoids, Coumarin | HPLC | Nayeem and Karvekar, 2010a, Nayeem and Karvekar, 2010b |
| 13 | Seed (Methanol) | Luteolin, Acacetin, Quercetin, Narengin, Hesperdin, Rutin, Rosmarinic, Quercetin, Naringenin, Hespertin, Kaempferol, Apigenin, Rhamnetin | Flavonoids | HPLC | Hesham et al., 2017 |
| 14 | Teak dust (Methanol) | Lapachol, deoxylapachol, Isodeoxylapachol, 4-Naphthoquinone, 2-Methylanthraquinone | Quinones | GC | Carrieri et al., 2014 |
| 15 | Leaves (Not mentioned) | 4-Hydroxy-4-methyl-2-pentanone, Glycerin monoacetate, Glycerin diacetate and 1-Eicosanol, Malvidin-3-o- (6-o-acetyl)-5-o-diglucoside | Aliphatic ketones, esters & alcohol, Anthocyanins | UV–Visible, GCMS, and LCMS | Suryanti et al., 2020 |
| 16 | Wood (n-Hexane, Benzene, Chloroform, Water) | Bis(2-ethylhexyl) phthalate, n-Hexadecanoic acid, Phthalic acid, Di(2-propylpentyl) ester, Di(oct-3-yl) ester | Aromatic acids, Esters | GCMS | Alabi and Oyeku, 2017 |
| 17 | Hardwood sawdust (Hexane, methanol) | Tectol, Hemitectol, Deoxylapachol, Tectoquinone, 2-Hydroxymethylanthraquinone, 3′–OH-deoxyisolapachol | Quinones | Centrifugal partition chromatography | Sumthong et al., 2008 |
| 18 | Sawdust (n-Hexane-methanol–water) | Abeograndinoicacid, 2-Oxokovalenic acid, 9-Hydroxyferruginol | Diterpenes | CC, HPLC | Francisco et al., 2010 |
| 19 | Heartwood (Methanol) | Rhein, Emodin, and Aloe-emodin Resveratrol, Coumestrol, Baicalein, 3-Hydroxyflavone, Rhamnetin Pinocembrin, 2′-Hydroxygenistein, Anhydroglycinol, Hydroxygenkwanin, Tectorigenin, Ginkgolide A, Rhein, Piperine | Phenylpropanoids, Flavonoids, and Anthraquinone | UPLC-ESI-MS/MS | Yang et al., 2020 |
| 20 | Bioactive extracts (Not mentioned) | Naphthotectone and Anthratectone | Quinones | 1D and 2D NMR | Lacret et al., 2011 |
3. Biological activities of Tectona grandis L.f (non-patent literature)
The plant has been used by traditional healers from time immemorial. Some of the mentioned traditional used in the literature are laxative, sedative, in treatment of piles, dysentery, leukoderma, anti-inflammatory, in bronchitis, urinary and liver related troubles, as hair promoter and useful in scabies. It also possesses anthelmintic and expectorant properties (Deepali et al., 2010a, Kruger and Schulz, 2007, Nayeem and Karvekar, 2011a, Nayeem and Karvekar, 2011b). Review reports several in vitro and in vivo biological activities of the plant of interest (Singh et al., 1996, Ramesh and Mahalakshmi, 2014). Extracts isolated from different parts of the plant is used either alone or in combination with other extracts for various diseased conditions. Some of the active constituents identified for the therapeutic activities include; 5-hydroxy-1,4-naphthalenedione (antibacterial), 4-hydroxy lapachol (cytotoxic), naphthaquinone (anti-ulcerogenic), benzene-1-carboxylic acid-2-hexadeconate (antiviral), lapachol (anti-tumor), 4′,5′-dihydroxy epi-isocatalapachol (anti-fungal) and 5,8-dihydroxy-2-methyl anthraquinone (anti-plasmodic) (Vyas et al., 2019, Goswami et al., 2009). Some of the pharmacological activities reported are compiled in Table 2.
Table 2.
Biological activity profile of TG (Non-Patent Literature).
| S. No. | Part (Solvent) | Activity | Animal/Microorganism/Other | Method of evaluation | Ref. |
|---|---|---|---|---|---|
| 1 | Leaf (Hydroalcoholic extract) | Wound healing | Sprague Dawley rat | Burn wound, Excision wound, incision wound, dead space wound | Nayeem and Karvekar, 2011a, Nayeem and Karvekar, 2011b |
| 2 | Bark, fruit (Methanol, Ethanol) | Anti-bacterial | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Escherichia aerogenes | Disc diffusion, Broth micro-dilution method | Neamatallah et al., 2005, Lanka and Parimala, 2017, Kamath and Shabarya, 2020 |
| 3 | Bark (Ethyl acetate, Petroleum, Ethanol, Water) | Anti-asthmatic | Swiss albino mice | Clonidine induced catalepsy, haloperidol-induced catalepsy, milk induced leucocytosis, in vivo animal models like mast cell degranulation and capillary permeability | Goswami et al., 2010a, Goswami et al., 2010b |
| 4 | Heartwood, Stem bark, leaves (Petroleum ether, Methanol) | Anti-tumor | Artemia salina | Brine shrimp assay | Pathak et al., 1988, Ghareeb et al., 2014 |
| 5 | Heartwood, Sawdust (Dichloromethane) | Antifungal | Aspergillus niger, Phanerochaete chrysosporium | Disc diffusion | Florence et al., 2012, Sumthong et al., 2006, Bhat et al., 2010 |
| 6 | Leaves, Fruits (Ethanol) | Anthelmintic | Pheritima posthumaas | Time of paralysis and time of death | Gururaj et al., 2011, Akshay et al., 2019 |
| 7 | Bark (Petroleum ether, Chloroform, Ethanol, Water) | Anticonvulsant | Male Wistar rats | Maximal electroshock induced seizures and pentylenetetrazole induced seizures | Azizah et al., 2017 |
| 8 | Seeds (Methanol) | Hepatoprotective | Rats | CCl4 and Ranitidine induced hepatotoxicity model | Rawal and Patil, 2017, Jangame et al., 2017, Jangme et al., 2017 |
| 9 | Fruit (Chloroform, Acetone, Methanol, Water) | Anti-urolithiatic | Calcium oxalate crystals | In vitro dissolution calcium oxalate crystals | Gudulkar et al., 2016 |
| 10 | Leaves, Flowers (Petroleum ether, Chloroform, Methanol, n-Butanol, Ethanol, Water) | Antidiabetic | Rats | Alloxan-induced diabetes | Pradeep et al., 2012, Ramachandran and Rajasekaran, 2014, Shukla et al., 2010 |
| 11 | Stem, Flowers (Methanol) | Analgesic and anti-inflammatory | Albino rats, mice | Radiant heat method, Writhing test Carrageenan of rat paw, Acetic acid, Hot-plate |
Giri and Varma, 2015, Ramachandran et al., 2011, Nayeem and Karvekar, 2010a, Nayeem and Karvekar, 2010b,Nayeem and Karvekar, 2012 |
| 12 | Roots (Methanol, Water) | Antitussive | Rats | Cough model induced by sulfur dioxide gas | Kaushik et al., 2011 |
| 13 | Plant (Ethanol) | Gastroprotective | Rats | Cold restraint and pyloric ligation induced gastric ulcer models | Singh et al., 2010 |
| 14 | Roots (Not mentioned) | Anti-ulcerogenic | Rats and guinea pigs | Experimentally induced ulcers | Goel et al., 1987 |
| 15 | Stem bark (Ethanol) | Antioxidant | In vitro studies | DPPH, FRAP, H2O2 scavenging assay | Ghaisas et al., 2008, Sahay and Sharma, 2015 |
| 16 | Plant (Aqueous) | Diuretic | Wistar rats | Hydrochlorothiazide induced | Kore et al., 2011 |
| 17 | Roots (Methanol) | Hypoglycemic | Albino rats | Alloxan induced, Dexamethasone Induced |
Mahesh et al., 2009, Pooja et al., 2011 |
| 18 | Leaves (Ethanol) | Anti-hemolytic anemia. | Rats | Induced by intraperitoneal injection of phenylhydrazine | Diallo et al., 2008 |
| 19 | Root, heartwood (Petroleum ether) | Cytotoxic activity | Artemis | Brine shrimps’ assay | Rafullah and Suleiman, 1999 |
| 20 | Seeds (Petroleum ether) | Hair growth activity | Albino mice | Shaved denuded skin of albino mice | Deepali et al., 2010b |
| 21 | Leaves (Methanol) | Antiplasmodial | P. falciparum | In vitro | Osman and Hadiani, 2018 |
| 22 | Leaves (Ethanol) | Anti-hypertensive | Wistar rats | Renal artery occluded hypertensive rats | Ajayi et al., 2011 |
| 23 | Leaves (Methanol) | Antifungal | Arthrinium phaeospermum, Aspergillus fumigatus, Aspergillus flavus | Well diffusion method, Agar slant double dilution tubes method | Astiti and Suprapta, 2012, Kouassi et al., 2016 |
| 24 | Stem extract (Not mentioned) | Uterine relaxant activity | Female albino Wistar rats | Estradiol benzoate injected uterus | Deepali et al., 2010a |
| 25 | Leaves | Hepato protective | Mice | CCl4 induced liver injury | Somayya et al., 2021 |
| 26 | Seeds | Antipyretic activity | Adult Wistar rats | Yeast induced antipyretic model | Jhansi and Lakshmi, 2021 |
4. Toxicological studies
Acute toxicity studies are designed so as to determine the dose that will produce death or serious toxicological manifestations when the dose is given once or over a few administrations. These studies are significant in determining the margin of safety of a drug. Several reports are available for the toxicological screening of the different parts of TG. Review reveals that various parts were evaluated for their toxicity in a dose ranging from 1000 mg/kg to 5000 mg/kg body weight. The solvents used for the preparation of the extracts were water, methanol and ethanol. The extract was found to show no signs of toxicity even at a dose of 5000 mg/kg. However the maximum dose used in most of the studies were limited to 2000 mg/kg. The following table depicts some of the toxicological studies conducted on the plant along with the part, solvent and animal used.
5. Teak allergy
Plants are one of the major causes of contact dermatitis (Verma et al., 2001). Dust from tropical hardwoods such as teak can cause both irritant contact dermatitis and allergic contact dermatitis. Teak is a fairly potent sensitizer it contains primary irritants and is also a common cause of allergic contact dermatitis which have been confirmed by various studies (Rao and Balachandran, 2010, Estlander et al., 2001). The main allergens that have been identified are polyphenols, naphthoquinones, their dimers, lapachol and deoxylapachol. The presence of these constituents explains the allergenic properties of this plant species. Lapachol is less potent than deoxylapachol as sensitizer (Christensen, 2018, Carrieri et al., 2014). The most common reactions are eye, skin, and respiratory irritation and nausea.
6. Patent literature of Tectona grandis L.f
The patents for plants were filed in diversified areas taking into consideration the cultivation, harvesting, drying, extraction, standardization, formulation methods, the devices used, etc (Pennyroyal et al., 2011). The patent literature of TG was collected by performing the Keyword search (Tectona grandis and teak wood) in the Espacenet Patent Search database (https://worldwide.espacenet.com/patent/search). The claims of the obtained patents/patent applications were reviewed. The patents/patent applications mentioning the name of TG or teak wood along with pharmaceutical use were segregated. Authors independently analyzed the language, content and description mentioned in the patents. The important data from the selected patent applications are mentioned in Table 3.
Table 3.
Toxicity studies of TG.
| Sl no | Part | Solvent | Animal used | Lethal dose (DL50) | Reference |
|---|---|---|---|---|---|
| 1 | Leaves | Aqueous | Wistar albino rats | No signs of toxicity, even at a dose of 5000 mg/kg in a single administration. | Kamsu et al., 2021 |
| 2. | Leaves | Ethanol | Wistar rat | No physiological changes or toxicity, even at a dose of 5000 mg/kg | Hamdin et al., 2019 |
| 3 | Seed | Methanol | Albino mice | No mortality upto 1000 mg/kg | Dokuparthi et al., 2017 |
| 4 | Stem bark | Ethanol and water | Wistar rats | No toxicity upto2000 mg/kg | Asif, 2011 |
| 5 | Seeds | Methanol, petroleum ether | Male albino rats | No toxicity upto2000 mg/kg | Jangme et al., 2017 |
| 6. | Root | Methanol | Albino rats | No toxicity upto3000 mg/kg | Pooja et al., 2011 |
| 7. | Seed | Methanol | Mice | No mortality upto 1000 mg/kg | Jhansi and Lakshmi, 2019 |
| 8. | Leaves | Methanol | Male Wistar rats | No mortality upto2000 mg/kg | Nayeem and Karvekar, 2012 |
| 9. | Leaves | Methanol | Sprague Dawley strain | No mortality upto2000 mg/kg | Kushwah et al., 2018 |
It is evident from the data of Table 4 that TG is present as an ingredient in many pharmaceutical compositions, which are claimed to have different therapeutic uses. These uses include treatment/prevention of optic atrophy, pneumonia, synovitis, insomnia, thyroid diseases, otitis media, diabetic retinopathy, bladder cancer, colon cancer, esophageal hiatus hernia, Chikungunya, eczema, blood and heart/skin related diseases, active oxygen scavenger, and tranquilizing the nerves, aiding in sleep. However, this patent literature is silent about the mechanism of action/function of TG in the claimed compositions.
Table 4.
Patent Literature of TG.
| S. No. | Patent / Patent Application Number (Publication Date) | Assignee/Name of the First inventor | Short Description | Ref. |
|---|---|---|---|---|
| 1 | CN108938948A (December 7, 2018) | Wang Dengsheng | It discloses an incense coil containing a specified amount of teak wood, cypress seed, hehuanpi, lavender, lemongrass, Lingzhi, lounge, starch, and CM-cellulose for tranquilizing the nerves and aiding in sleep | Dengsheng, 2018 |
| 2 | CN106822380A (June 13, 2017) | Jinan Haoyu Qingtian Medical Technology Co., Ltd. (China) (JHQMTCL) | It discloses a pharmaceutical composition comprising TG, Trigonella ruthenica, Pedicularis longiflora, maritimetin, and Lindera obtusiloba for the prevention and treatment of optic atrophy | Jinan Medical Technology Company, 2017a |
| 3 | CN106728432A (May 31, 2017) | JHQMTCL | It discloses a pharmaceutical composition comprising TG, Plagiogyria distinctissima, jujuboside B, Lysimachia heterogeneous, and Centaurium pulchellum for treating/preventing pneumonia | Jinan Medical Technology Company, 2017b |
| 4 | CN106728431A (May 31, 2017) | JHQMTCL | A pharmaceutical composition for the treatment of synovitis of the knee comprising TG, Petrocosmea minor, acrifoline, and nerolidol as crude drugs | Jinan Medical Technology Company, 2017c |
| 5 | CN106728433A (May 31, 2017) | JHQMTCL | A pharmaceutical composition for the prevention and treatment of insomnia comprising TG, Doryopteris concolor, Lonicera caerulea, saikosaponin C and Sium suave as crude drugs | Jinan Medical Technology Company, 2017d |
| 6 | CN106668346A (May 17, 2017) | JHQMTCL | A pharmaceutical composition for the prevention and treatment of thyroid diseases comprising TG, Parthenocissus himalayana, Dalbergia hancei, capaurine, and xylopinine as a crude drug | Jinan Medical Technology Company, 2017e |
| 7 | CN106668337A (May 17, 2017) | JHQMTCL | A pharmaceutical composition for the treatment of optic atrophy comprising TG, trifolirhizin, and Lindera obtusiloba as a crude drug | Jinan Medical Technology Company, 2017f |
| 8 | CN106668336A (May 17, 2017) | JHQMTCL | A pharmaceutical composition for the treatment of otitis media comprising TG, Myriophyllum spicatum, asiatic acid, Euonymus myrianthus, and Ulva conglobata as a crude drug | Jinan Medical Technology Company, 2017g |
| 9 | CN106540004A (March 29, 2017) | JHQMTCL | A pharmaceutical composition for the treatment of diabetic retinopathy comprising TG, rose apple, esculentoside B, Parthenocissus himalayana, and globe amaranth as bulk drugs | Jinan Medical Technology Company, 2017h |
| 10 | CN106138463A (November 23, 2016) | JHQMTCL | A pharmaceutical composition for treating advanced bladder cancer comprising TG, Limnaea, β-amyrin acetate, mesembrine, and dryocrassin | Jinan Medical Technology Company, 2016b |
| 11 | CN106138462A (November 23, 2016) | JHQMTCL | A pharmaceutical composition for treating advanced colon cancer comprising TG, Diplazium donianum, and Nothosmyrnium japonicum | Jinan Medical Technology Company, 2016a |
| 12 | CN106074957A (November 9, 2016) | Yantai Ruizhi Biomedical Technology Co., Ltd. (China) | The invention relates to a traditional Chinese medicine composition for treating liver and stomach disharmony type esophageal hiatus hernia comprising TG, Tetrapanax papyrierus, Manglietia yuyuanensis, Citrius medica, Citrius wilsonii, Amomum tsaoko, Lithocarpus polystachyus, Pyropolyporus adamantinus, Kadsura coccinea, Microsorium dilatatum, Scirpus triqueter, Cremanthodium liheare, Quercus fabri, Rosa bracteaea, coriander fruits, Actinidia arguta, and Glycyrrhiza sp. Roots | Yantai Biomedical technology company, 2016 |
| 13 | IN3267/CHE/2014A (February 12, 2016) | Rajarajan Swaminathan | A method for preparing a lyophilized extract from TG for treating the Asian and East Central South African genotype of Chikungunya virus. | Rajarajan et al., 2016 |
| 14 | CN103356878B (November 25, 2015) | Cheng Yueyin | A traditional Chinese medicine powder for treating pediatric eczema comprising TG, Arcangelisia loureirin, pansy, celastrus leaves, Asparagus brachyphylus, pine bark, Carex lanceolata, Vaccinium fragile, Cudrania tricuspidata, and talc | Yueyin, 2015 |
| 15 | WO2006075336A1 (July 20, 2006) | Katkar Rama Dhondiba | Herbal composition for treatment of blood and heart/skin related diseases comprising TG, Murraya Paniculata, Latana camara, Terminalia, Todalia asiatica, and Chawat | Dhondiba, 2006 |
| 16 | JP2013224318A (October 31, 2013) | Kawabata Aya | It claims an active oxygen scavenger comprising the extracts of TG, Anaquiculus pyrethrum, Anacyclus pyrethrum, Oculocarps longifolius, and Aganosma marginata. | Aya and Misao, 2013 |
| 17 | JP2010018545A (January 28, 2010) | Kawabata Aya | A reactive oxygen scavenger comprising extracts of TG, Parkia speciose, Anachromus pyrethrum, Ochrocarpus longifolius, Wrightia tomentosa, Diospyros rhodocalyx, and Burmanica Griff. | Aya and Misao, 2010 |
| 18 | JP2006176445A (July 6, 2006) | Ikeda Naosuke | It relates to a composition comprising about 10 herbal drugs including TG that is effective for health promotion and nutrition. | Naosuke, 2006 |
| 19 | JP2006166803A (June 29, 2006) | Nobashi Kenzou | A shelf life-improving composition comprising an organic acid and an acetone extract of TG. | Kenzou, 2006 |
7. Conclusion
Herbs are widely used for the treatment of various diseases. This review highlights the importance of phytochemistry, biological activity, and the patents of Tectona grandis. The result of the phytochemical study shows that it contains compounds with diverse structures. The different parts of the plant possess various activities like antioxidants, wound healing, analgesic, anti-inflammatory, antipyretic, etc. However, it has come to the notice that very few patents have been fielded concerning this plant, thereby paving the way for more studies and applications of patents in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to AlMaarefa University, Riyadh for providing support to do this review article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ajayi G.O., Olowe J.A., Ajuluchukwu J.N. Tectona grandis Linn. (Verbenaceae) leaf ethanol extract in renal artery occluded hypertensive rats. Planta Med. 2011;77:81–89. [Google Scholar]
- Akshay J., Shaikh H., Sargar M., Survanshi H., Rathod M. In-vitro anti- inflammatory and anthelmintic activity of Tectona grandis leaves extract. Int. J. Herb Med. 2019;7(3):36–40. [Google Scholar]
- Alabi K., Oyeku T. The Chemical Constituents Extractable From Teak Tree (Tectona grandis Linn) Obtained From Fountain University. Osogbo. Nigerian J. Basic Appl. Sci. 2017;25(1):73–80. [Google Scholar]
- Asif M. In vivo analgesic and anti-inflammatory effects of Tectona grandis Linn. stem bark extracts. Mal. J. Pharma. Sci. 2011;9(1):1–11. [Google Scholar]
- Astiti N.P.A., Suprapta D.N. Antifungal activity of teak (Tectona grandis L.F) leaf extract against Arthrinium phaeospermum (corda) M.B. Ellis, the cause of wood decay on Albizia falcataria (L.) Fosberg. J. Int. Soc. South East Agr. Sci. 2012;18(1):62–69. [Google Scholar]
- Aya, K., Misao, Y., 2010. Active oxygen scavenger and skin care preparation for external use, composition for oral cavity and food. Japanese Patent Application Number JP2010018545A, January 28, 2010.
- Aya, K., Misao, Y., 2013. Active oxygen scavenger, skin care preparation, composition for oral cavity and food product. Japanese Patent Application Number JP2013224318A, October 31, 2013.
- Azizah, A., Suselo, Y.H., Muthmainah, M., Indarto, D., 2017. A new candidate of calcium channel blocker in silico from Tectona grandis for treatment of gestational hypertension. In: 1st International Conference on Science, Mathematics, Environ. Edu. 12054, pp. 1–9.
- Bachheti R.K., Sharma A., Rai I., Joshi A., Mamgain R. Fatty acid composition and elemental analysis of seed oil of Tectona grandis collected from Dehradun, Uttarakhand. India. Int. J. Chem. Tech. Res. 2012;4(3):119–1123. [Google Scholar]
- Bhat I.H., Abdul-Khalil H.P.S., Shuib N.S., Noor A.M. Antifungal activity of heartwood extracts and their constituents from cultivated Tectona grandis against phanerochaete chrysosporium. Wood Res. 2010;55(4):59–66. [Google Scholar]
- Carrieri M., Bartolucci G.B., Lee T., Barbero A., Harper M. Chemical Markers of Occupational Exposure to Teak Wood Dust. The Annals Occu Hyg. 2014;58(5):566–578. doi: 10.1093/annhyg/meu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, L.P., 2018.Polyphenols and Polyphenol-Derived Compounds From Plants and Contact Dermatitis. Polyphenols: Prevention and Treatment of Human Disease (Second Edition).Chap 29, vol. 2, pp. 349–384.
- Deepali J., Varma S., Bonde V., Gite A. Effect of Tectona grandis stem extract on estradiol benzoate injected uterus of female albino wistar rats. Asian J. Pharm. Clin. Res. 2010;3(2):123–125. [Google Scholar]
- Deepali J., Varma S., Gagne N., Bonde V., Gite A., Bhosle D. Effect of Tectona grandis Linn. Seeds on hair growth activity of albino mice. Int. J. Ayurveda Res. 2010;1(4):211–215. doi: 10.4103/0974-7788.76783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengsheng, W., 2018. Incense coil with effects of soothing nerves and helping sleep. Chinese Patent Application Number CN108938948A, December 7, 2018.
- Dhondiba, K.R., 2006. An herbal composition for treatment for blood and heart /skin related diseases and process of preparing thereof. PCT Publication Number WO2006075336A1, July 20, 2006.
- Diallo A., Gbeassor M., Vovor A. Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats. Fitoter. 2008;79(5):332–336. doi: 10.1016/j.fitote.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Dokuparthi S.K., Khan A., Anusha A., Mashma B., Shaibaz, Shahajeb, et al. Acute oral toxicity study of Tectona grandis Linn. methanolic seed extract in albino mice. J. Phytopharmacol. 2017;6(3):183–185. [Google Scholar]
- Emmanuel, N.K., Paul, L., Emmanuelle, M., Felix, A.A., 2016. Identification and characterization of polyphenols from aqueous extract of Tectona grandis Linn leaves obtained at pilot plant scale. In: 2nd International congress green chemistry and sustainable engineering. International conference on green chemistry and sustainable engineering, 2016.
- Estlander T., Jolanki R., Alanko K., Kanerva L. Occupational allergic contact dermatitis caused by wood dusts. Con. Derma. 2001;44:213–217. doi: 10.1034/j.1600-0536.2001.044004213.x. [DOI] [PubMed] [Google Scholar]
- Florence B.N., Nadine A., Didier S., Gilles C., Yves L., Adjumane A.K. 4’,5’-Dihydroxyepiisocatalponol, a new naphthoquinone from Tectona grandis L. f. heartwood, and fungicidal, activity. Int. Biodeterioration Biodegradation. 2012;74:93–98. [Google Scholar]
- Francisco A.M., Lacret R., Varela R.M., Nogueiras C. Isolation and Phytotoxicity of Terpenes from Tectona grandis. J. Chem. Ecol. 2010;36:396–404. doi: 10.1007/s10886-010-9769-3. [DOI] [PubMed] [Google Scholar]
- Ghaisas M.M., Navghare V.V., Takawale A.R., Zope V.S., Deshpande A.D. In-vitro antioxidant activity of Tectona grandis linn. Pharmacologyonline. 2008;3:296–305. [Google Scholar]
- Ghareeb M.A., Shoeb H.A., Madkour H.M.F., Refaey L.A.G., Mohamed M.A.M., Saad A.M. Antioxidant and cytotoxic activities of Tectona grandis linn. Leaves. Int. J. Phytopharmacol. 2014;5(2):143–157. [Google Scholar]
- Giri S.P., Varma S.B. Analgesic and anti-inflammatory activity of Tectona grandis Linn. stem extract. J. Basic Clin. Physio. Pharmacol. 2015;26(5):479–484. doi: 10.1515/jbcpp-2014-0043. [DOI] [PubMed] [Google Scholar]
- Goel R.K., Pathak N.K.R., Biswas M., Pandey V.B., Sanyal A.K. Effect of lapachol, a naphthaquinone isolated from Tectona grandis, on experimental peptic ulcer and gastric secretion. J. Pharm. Pharmacol. 1987;39(2):138–140. doi: 10.1111/j.2042-7158.1987.tb06962.x. [DOI] [PubMed] [Google Scholar]
- Goswami D.V., Nirmal S.A., Patil M.J., Dighe N.S., Laware R.B., Pattan S.R. An Overview of Tectona grandis. Chemistry and Pharmacological Profile. Phcog. Rev. 2009;3(5):181–185. [Google Scholar]
- Goswami D.V., Sonawane, Nirmal S.A., Patil M.J. Evaluation of antiasthmatic activity of Tectona grandis linn. Bark. Int. J. Pharm. Sci. Res. 2010;1(1):10–16. [Google Scholar]
- Goswami D.V., Sonu S., Anuj M., Umesh B. Telrandhe, Patil MJ. Effect of Various Extracts of Tectona grandis Linn. Bark on Bronchitis Pharmacol. 2010;1:816–820. [Google Scholar]
- Gudulkar S., Rajbhar K., Dawda H., Mukundan U. Plant system as a tool for validating ethnobotanical claims for kidney stone treatment. World J. Pharm. Res. 2016;5(10):776–785. [Google Scholar]
- Gururaj M.P., Joshi H., Bhat I.K., Satyanarayana D., Shastry C.S. Anthelmentic activity of Tectona grandis Linn. Fruits. Int. Res. J. Pharmacy. 2011;2(1):219–221. [Google Scholar]
- Hamdin Candra Dwipayana, Prasedya Eka Sunarwidhi, Utami Sinta Wahyu, Galanova Dandiko, Saputro Dheny Cahyo, Nurrochmad Arief, Murwanti Retno, Jupri Ahmad, Sunarpi Haji. Acute Toxicity of Indonesian Natural Food Colorant Tectona grandis Leaf Extract in Wistar Rats. J. Med. Sci. 2019;19(2):69–74. [Google Scholar]
- Hesham M.A., Dawoud G.T.M., El-Hela A.A., Emad A. Comparative evaluation of the flavonoids constituents in some verbenaeus species cultivated in Egypt. World J. Pharm. Res. 2017;6(12):118–127. [Google Scholar]
- Jangame C.M., Bais S.K., Shivakumar S., Wadulkar R.D. A study on hepatoprotective activity of methanolic extracts of Tectona grandis seeds. Res. J. Pharm. Bio. Chem. Sci. 2017;8(3):1659–1666. [Google Scholar]
- Jangme C.M., Ladde, Shivakumar S., Wadulkar R.D. Evaluation of hepatoprotective activity of Tectona grandis seeds by using CCl4-induced hepatic injury in rats. Eur. Biomed. Pharm. Sci. 2017;4(5):368–371. [Google Scholar]
- Jhansi G.R., Lakshmi N.B. Phytochemical investigation and Antipyretic activity of Tectona grandis Linn. Res. J. Pharma. Tech. 2021;14(8):4221–4225. [Google Scholar]
- Jhansi G.R., Lakshmi N.B. Acute toxicity studies and evaluation of analgesic property of Tectona grandis methanolic seed extract in swiss albino mice.Asian J Pharm. Clin Res. 2019;12(11):106–108. [Google Scholar]
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2016. Medicinal composition for treating advanced colorectal cancer. Chinese Patent Application Number CN106138462A, November 23, 2016.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Medicine composition for treating knee joint synovitis. Chinese Patent Application Number CN106728431A, May 31, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition capable of preventing and treating optic atrophy and preparation method of pharmaceutical composition. Chinese Patent Application Number CN106822380A, June 13, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition capable of preventing and treating pneumonia and preparation method of pharmaceutical composition. Chinese Patent Application Number CN106728432A, May 31, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition for preventing and treating insomnia and preparation method thereof. Chinese Patent Application Number CN106728433A, May 31, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition capable of preventing and treating thyroid gland diseases. Chinese Patent Application Number CN106668346A, May 17, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition for treating optic atrophy. Chinese Patent Application Number CN106668337A, May 17, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition for treating otitis media. Chinese Patent Application Number CN106668336A, May 17, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2017. Pharmaceutical composition used for treating diabetic retinopathy. Chinese Patent Application Number CN106540004A, March 29, 2017.
- Jinan Haoyu Qingtian Medical Technology Company Limited, 2016. Pharmaceutical composition for treating advanced bladder cancer. Chinese Patent Application Number CN106138463A, November 23, 2016.
- Kamath K.K., Shabarya R.A. Preliminary phytochemical screening and antibacterial activity of frontal leaves of Tectona grandis (family: verbenaceae) World J. Pharm. Pharma. Sci. 2020;5(6):2377–2384. [Google Scholar]
- Kamsu Gabriel Tchuente, Djamen Chuisseu Dieudonné Pascal, Fodouop Chegaing Siméon Pierre, Laure Feudjio Huguette Bocanestine, Ndel Famen Louis-Claire, Kodjio Norbert, Sokoudjou Jean Baptiste, Gatsing Donatien, DeCaprio Anthony. Toxicological Profile of the Aqueous Extract of Tectona grandis L.F. (Verbenaceae) Leaves: A Medicinal Plant Used in the Treatment of Typhoid Fever in Traditional Cameroonian Medicine. Med. J. Toxicol. Article ID. 2021;2021:1–10. doi: 10.1155/2021/6646771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A., Kumari M., Ambesajir A. Studies on antitussive effect of Tectona grandis roots using a cough model induced by sulfur dioxide gas in guinea pigs. Int. J. Phytomed. 2011;3:279–284. [Google Scholar]
- Kenzou, N., 2006. Storable duration improving composition, storable duration improving method for food and germicidal/antibacterial agent. Japanese Patent Application Number JP2006166803A, June 29, 2006.
- Koffi E.N., Meudec E., Adje F.A., Lozanod P.R., Lozanod Y.F., Bekroa Y.A. Effect of reverse osmosis concentration coupled with drying processes on polyphenols and antioxidant activity obtained from Tectona grandis leaf aqueous extracts. J. Appl. Res. Med. Aromat. Plants. 2015;2(2):54–59. [Google Scholar]
- Kore K.J., Jadhav P.J., Shete R.V., Shetty S.C. Diuretic activity of Tectona grandis leaves aqueous extract in wistar rats. Int. J. Pharm. Res. Dev. 2011;3(7):141–146. [Google Scholar]
- Kouassi E.K., Coulibaly I., Gervais M.M., Sitapha O., Koffi M.K.A., Oniga I., Allico J.D. Comparison of antiaspergillar activity of extracts of Tectona grandis Linn according to two antifungal susceptibility testing. J. Phytopharmacol. 2016;5(3):93–99. [Google Scholar]
- Kruger H., Schulz H. Analytical techniques for medicinal and aromatic plants. Postharvest Rev. 2007;3(4):1–12. [Google Scholar]
- Kushwah A.S., Kaur P., Shivanandappa T.B. Effect of methanolic extracts of Tectona grandis linn leaves on diabetic neuropathy in streptozotocin–induced diabetic rats. MOJ Drug Des Develop Ther. 2018;2(4):203–209. [Google Scholar]
- Lacret R., Varela R.M., Molinillo J.M.G., Macias C., Macias F.A. Anthratectone and naphthotectone, two quinones from bioactive extracts of Tectona grandis. J. Chem. Ecol. 2011;37(12):1341–1348. doi: 10.1007/s10886-011-0048-8. [DOI] [PubMed] [Google Scholar]
- Lanka S., Parimala Antimicrobial activities of Tectona grandis leaf and bark extracts. Eur. J. Pharm. Med. Res. 2017;4(12):245–248. [Google Scholar]
- Mahesh G., Vijay N., Abhijit T., Vinit Z., Mukesh T., Avinash D. Effect of Tectona grandis Linn. On dexamethasone-induced insulin resistance in mice. J. Ethnopharmacol. 2009;122:304–307. doi: 10.1016/j.jep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Murukan G., Kumara M. Comparison of phenolic acids and antioxidant activities of young and mature leaves of Tectona grandis. Asian J. Pharm. Res. 2018;11(1):60. [Google Scholar]
- Nahida, Ansari S.H., Siddiqui A.N. Pistacia Lentiscus: A Review on Phytochemistry and Pharmacological Properties. Int. J. Pharm. Pharm Sci. 2012;4(4):16–20. [Google Scholar]
- Naosuke, I., 2006. Composition for internal use. Japanese Patent Application Number JP2006176445A, July 6, 2006.
- Nayeem N., Karvekar M. Analgesic and anti-inflammatory activity of the methanolic extract of the frontal leaves of Tectona grandis. Internet J. Pharmacol. 2010;8(1):138–143. [Google Scholar]
- Nayeem N., Karvekar M. Comparative phytochemical and pharmacological screening of the methanolic extracts of the frontal and mature leaves of Tectona grandis. Int. J. Pharm. Bio. Sci. 2010;1(3):1–6. [Google Scholar]
- Nayeem N., Karvekar M. Antimicrobial and antioxidant properties of the isolated compounds from the methanolic extract from the leaves of Tectona grandis. J. Basic Clin. Pharm. 2011;2(4):163–165. [PMC free article] [PubMed] [Google Scholar]
- Nayeem N., Karvekar M. Stability studies and evaluation of the semisolid dosage form of the rutin, quercitin, ellagic acid, gallic acid and sitosterol isolated from the leaves of Tectona grandis for wound healing activity. Arch. Appl. Sci. Res. 2011;3(1):43–51. [Google Scholar]
- Nayeem N., Karvekar M. Effect of plant stages on the analgesic and anti-inflammatory activity of the leaves of Tectona grandis. Euro. J. Exper. Bio. 2012;2(2):396–399. [Google Scholar]
- Neamatallah A., Yan L., Dewar S.J., Austin B. An extract from teak (Tectona grandis) bark inhibited Listeria monocytogenes and methicillin resistant Staphylococcus aureus. Lett. Appl. Microbio. 2005;41:94–96. doi: 10.1111/j.1472-765X.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- Neha K., Sangeetha B. Phytochemical and pharmacological evaluation of Tectona grandis. Linn. Int. J. Pharm. Pharm. Sci. 2013;5(3):923–927. [Google Scholar]
- Niamke F.B., Amusant N., Kadio A.A., Thevenon M.F., Nourissier S., Adima A.A. Rapid Prediction of Phenolic Compounds as Chemical Markers for the Natural Durability of Teak (Tectona grandis Linn f.). Heartwood by near Infrared Spectroscopy. J. Near Infrared Spectro. 2013;22(1):101–106. [Google Scholar]
- Nilesh B.C., Kailasam K., Sachin X., Nilesh B.C. Tectona grandis linn: A global overview. World J. Pharma. Res. 2017;33(6):427–440. [Google Scholar]
- Osman C.P., Hadiani I.N. Antiplasmodial Anthraquinones from Medicinal Plants: The Chemistry and Possible Mode of Actions. Nat. Prod. Com. 2018;13(12):1591–1597. [Google Scholar]
- Pathak N.K.R., Neogi P., Biswas M., Tripathi Y.C., Pandey V.B. Betulin aldehyde, an antitumour agent from the bark of Tectona grandis. Ind. J. Pharm. Sci. 1988;50(2):124–125. [Google Scholar]
- Pennyroyal G., Dhondup L., Husted C. A Review of Medicinal Plant Patents. Recent Patents Biomed. Eng. 2011;4(2):126–138. [Google Scholar]
- Pooja, Vipin S., Samanta K.C. Hypoglycemic activity of methanolic extract of Tectona grandis Linn. Root in alloxan induceddiabetic rats. J. Appl Pharm. Sci. 2011;1(04):106–109. [Google Scholar]
- Pradeep G., Reddy V.R., Reddy G.N., Narayana T.V., Vijayakumar G., Ramanjaneyulu J. Anti-microbial and antidiabetic activity of Tectona grandis extract against alloxan-induced diabetic rats. Int. J. Res. Pharm. Nano Sci. 2012;1(2):139–146. [Google Scholar]
- Qui H., Liu R., Long L. Analysis of Chemical Composition of Extractives by Acetone and the Chromatic Aberration of Teak (Tectona grandis L.F.) from China. Molecules. 2019;24(10):1989. doi: 10.3390/molecules24101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafullah M.K., Suleiman M.M. 5-Hydroxylapachol: a cytotoxic agent from Tectona grandis. Phytochem. 1999;50:439–442. [Google Scholar]
- Rajarajan, Swaminathan, Kothandan, Sangeetha, 2016. Novel antiviral combination for treating Asian and East Central South African genotypes of chikungunya virus and method for producing the same. Indian Patent Application Number IN3267/CHE/2014A, February 12, 2016.
- Ramachandran S., Rajasekaran A. Blood glucose-lowering effect of Tectona grandis flowers in type 2 diabetic rats: A study on identification of active constituents and mechanisms for antidiabetic. J. Diabetes. 2014;6(5):427–437. doi: 10.1111/1753-0407.12121. [DOI] [PubMed] [Google Scholar]
- Ramachandran S., Rajini kanth B., Rajasekaran A., Manisenthil K. Evaluation of anti–inflammatory and analgesic potential of methanol extract of Tectona grandis flowers. Asian Pac. J. Trop. Biomed. 2011;1(2):S155–S158. [Google Scholar]
- Ramesh B.N., Mahalakshmi A.M. Pharmacology of Tectona grandis Linn. Int. J. Pharmacog. Phytochem. Res. 2014;6(1):86–90. [Google Scholar]
- Ramesh B.N., Mahalakshmi A.M., Mallappa S.H. Towards a Better Understanding of an Updated Ethnopharmacology of Celosia Argentea L. Int. J. Pharm PharmSci. 2013;5(3):54–59. [Google Scholar]
- Rao Raghavendra, Balachandran C. Occupational allergic contact dermatitis due to teak wood. Indian. J. Dermatol. Venereol. Leprol. 2010;76(3):287. doi: 10.4103/0378-6323.62980. [DOI] [PubMed] [Google Scholar]
- Rawal V.P., Patil L.P. Anticonvulsant activity of Tectona grandis linn bark extracts. Pharm. Sci. Monitor. 2017;8(2):174–189. [Google Scholar]
- Sahay M., Sharma R. Antioxidant Activity of Tectona grandis linn Stem Bark Extract. Int. J. Inno. Sci. Eng. Techno. 2015;2(11):906–908. [Google Scholar]
- Shalini, Srivastava R. Antifungal activity screening and HPLC analysis of crude extract from Tectona grandis, Shilajit, Valeriana wallachi. J. Environ. Agri. Food Chem. 2009;8(4):218–229. [Google Scholar]
- Shukla N., Kumar M., Akanksha X., Ahmad G., Rahuja X., Neha S., Amar B. Tectone, a new antihyperglycemic anthraquinone from Tectona grandis leaves. Nat. Prod. Comm. 2010;5(3):427–430. [PubMed] [Google Scholar]
- Singh J., Bhuyan T.C., Ahmed A. Enthnobotanical studies on the Mishing tribes of Assam with special reference to food and medicinal plants. J. Econ. Taxon. Bot. 1996;12:350–356. [Google Scholar]
- Singh N., Shukla N., Singh P., Sharma R., Rajendran S.M., Maurya R. Verbascoside isolated from Tectona grandis mediates gastric protection in rats via inhibiting proton pump activity. Fitoter. 2010;81(7):755–761. doi: 10.1016/j.fitote.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Singh P.A., Brindavanam N.B., Kimothi G.P., Verma R., Aeri V. A validated HPLC method for the determination of Betulin in the stem bark of Tectona Grandis Linn. Int, J. Pharma. Sci. Res. 2016;7(2):1–8. [Google Scholar]
- Somayya T., Brice L.K., Ayesha M., Sidra R., Bushra I., Ahmad A.S. Tectona grandis leaf extract ameliorates hepatic fibrosis: Modulation of TGF- β /Smad signaling pathway and upregulating MMP3/TIMP1 ratio. J. Ethnopharmacol. 2021;23:272. doi: 10.1016/j.jep.2021.113938. [DOI] [PubMed] [Google Scholar]
- Sumthong P., Damveld R.A., Choi Y.H., Arentshorst M., Ram A.F., Van den Hondel C.A., Verpoorte R. Activity of quinones from teak (Tectona grandis) on fungal cell wall stress. Planta Med. 2006;72(10):943–944. doi: 10.1055/s-2006-946676. [DOI] [PubMed] [Google Scholar]
- Sumthong P., Romero-Gonzalez R.R., Verpoorte R. Identification of Anti-Wood Rot Compounds in Teak (Tectona grandis L.f.) Sawdust Extract. J. Wood Chem. Tech. 2008;28(4):247–260. [Google Scholar]
- Suryanti V., Kusumaningsih T., Marliyana S.D., Setyono H.A., Trisnawati E.W. Identification of active compounds and antioxidant activity of teak (Tectona grandis) leaves. J. Biol. Diversity. 2020;21(3):946–952. [Google Scholar]
- Tsvetkov D.E., Dmitrenok A.S., Tsvetkov Y.E., Menshov V.M., Yashunsky D.V., Yashin A.Y. Phenylethanoid Glycosides from Teak Wood Knots and Their Antioxidant Activity. Int. J. Pharm. Bio. Sci. 2010;1(3):124–130. [Google Scholar]
- Verma K.K., Sirka C.S., Ramam M. Contact dermatitis due to plants: Challenges and prospects. Indian. Pract. 2001;54:791–796. [Google Scholar]
- Vyas P., Yadav D.K., Khandelwal P. Tectona grandis (teak) – A review on its phytochemical and therapeutic potential. J. Nat. Prod. Res. 2019;33(16):2338–2354. doi: 10.1080/14786419.2018.1440217. [DOI] [PubMed] [Google Scholar]
- Yang G., Liang K., Zhou Z., Wang X.G. UPLC-ESI-MS/MS-Based Widely Targeted Metabolomics Analysis of Wood Metabolites in Teak (Tectona grandis) Molecules. 2020;259(9):2189. doi: 10.3390/molecules25092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantai Ruizhi Biomedical Technology Company Limited, 2016. Traditional Chinese medicine composition for treating liver-stomach disharmony type hiatus hernia. Chinese Patent Application Number CN106074957A, November 9, 2016.
- Yueyin, C., 2015. Traditional Chinese medicine powder for treating infantile eczema and preparation method thereof. Chinese Patent Number CN103356878B, November 25, 2015.