Abstract
Abiotic stress causes extensive loss to agricultural yield production worldwide. Salt stress is one of them crucial factor which leads to decreased the agricultural production through detrimental effect on growth and development of crops. In our study, we examined the effect of a defense growth substance, salicylic acid (SA 1 mM) on mature vegetative (60 Days after sowing) and flowering (80 DAS) stage of Pusa Sadabahar (PS) variety of Capsicum annuum L. plants gown under different concentrations of NaCl (25, 50, 75, 100 and 150 mM) and maintained in identical sets in pots during the whole experiment. Physiological studies indicated that increase in root & shoot length, fresh & dry weight, number of branches per plant, and yield (number of fruits per plant) under salt + SA treatment. Biochemical studies, enzymatic antioxidants like CAT, POX, and non-enzymatic antioxidant such as ascorbic acid (AsA content), carotenoids, phenolics, besides other defense compounds like proline, protein, chlorophyll contents were studied at 10 days after treatment at the mature vegetative and flowering stage. The addition of SA led to lowering of in general, all studied parameters in the mature vegetative stage but increased the same during the flowering stage, especially in the presence of NaCl; although the control I (without SA and NaCl) remained lower in value than control II (with SA, without NaCl). Interestingly, total phenolics were higher in control I (without SA or NaCl) whereas chlorophylls were higher in treatments with SA and NaCl. Thus, physiological concentration of SA (1 mM) appears to be significantly effective against salt stress during the flowering stage. In addition, during the mature vegetative stage, however, proline accumulates in SA treated sets, to help in developing NaCl-induced drought stress tolerance.
Keywords: Ascorbate, Catalase (CAT), Peroxidase (POX), Proline, Protein, SA (Salicylic acid)
1. Introduction
Plants encountered with multitude environmental restrains categorized into biotic and abiotic stresses. Among the abiotic stresses, salt stress is the severe environmental cue which cause detrimental effects in plant growth, physiological and molecular processes and productivity of economically important crops (Alam et al., 2019, Ahanger et al., 2020, Kaya et al., 2020a, Amin et al., 2021, Gupta et al., 2021). High salinity in soil may affects plant growth and development due to ionic pressure. As the osmotic potential of soil solution become lower it affects essential nutrients uptake (Dhiman et al., 2021). Moreover, salinity induced osmotic and oxidative stress disturb metabolic balance of the cell. High concentration of salt (sodium chloride) leads to ion accumulation in the cytosol which is harmful to the redox environmental of the cell. Ionic toxicity disrupts the non-covalent interaction between amino acids and also decrease concentrations of cations such as potassium and calcium (Ali et al., 2021, Johnson and Puthur, 2021).
Saline areas account for approximately 800 million hectares worldwide, which is parallel to more than 6% of the world’s total and area. Saline water irrigation is one of the great environmental constituents for salinization of the soil that leads to loss of agricultural yield (Rasheed et al., 2020). The disproportionate environmental harnessing, climate change and global warming have also become major threats, adversely affecting plant productivity (Sadiq et al., 2020).
The abiotic stresses can modify the physio-chemical and molecular activities of plants in almost every stage of the plant causing severe loss to yield of various crop plants (Mantri et al., 2012). High salinity is reported to cause deleterious effects on many aspects of plants maturity and productivity. Salinity induced ionic and osmotic stresses reduce the mass and yield of the plants (Munns, 2002). Moreover, severe salt concentration causes many metabolic disturbances in plants by generating Reactive Oxygen Species (ROS), ionic toxicity, peroxidation of lipids, enzyme inactivation and degradation of proteins which perturbs the cellular redox potential and ultimately leads to cell death (Hasanuzzaman et al., 2020).
Many strategies have been employed by scientists to alleviate the deleterious effect of stresses and make plants tolerant to these conditions. Among them, exogenic application of plant growth substance has gathered attention as minute amount of such growth regulator may be able to save land from other mineral treatments which may have long lasting adverse consequences. Growth regulators could be effective in enhancing the yield of crop plants under varying conditions besides mitigating stress. Salicylic acid acts as signalling molecule showing ameliorative effect against various biotic and abiotic stress conditions (Sakhabutdinova et al., 2003, Vimala and Gupta, 2006, Kazemi et al., 2011).
Salicylic acid is a naturalistic plant phenolic compound, contributes as a non-enzymatic antioxidant and an endogenous signalling molecule inducing stress tolerance against abiotic (drought, heavy metal and salt stress) and biotic (against pathogen) stresses in plants (Ma et al., 2017, Rajeshwari and Bhuvaneshwari, 2017, Janah et al., 2021, Zhou et al., 2021). It is reported to produce osmolytes, such as proline and antioxidants (Gautam and Singh, 2009, Hayat et al., 2010).
Ameliorative effect of salicylic acid against abiotic and biotic stress is limited to low concentration of SA application, higher concentrations are reported to induce oxidative damage decreasing the tolerance ability to abiotic stresses (Miura and Tada, 2014).
The plant material used in the present investigation is Capsicum annuum (chilli), a worldwide cultivated dicotyledonous flowering plant of family Solanaceae, usually grown in temperate areas and withstands most of the environmental fluctuations. Chilli is annual as well as perennial shrub distributed in tropical areas. Chilli contains various secondary metabolites including steam-volatile oil, vitamins, fibre, proteins, carotenoids, capsaicinoids, mineral elements and fatty oils (Bosland and Votava, 2000, Krishna De, 2003). Chilli also contains important constituents of nutritional value, aroma, texture, flavour, and colour for its own defense. Vitamin C is especially found in ripe fruits (Marín et al., 2004) besides, pro-vitamin A, B1, B2, B3, B6, E and antioxidants which prevent oxidative damage of human body and prevent it from cardiovascular diseases and cancer (Oboh and Rocha, 2007, Chuah et al., 2008). The pungent component of chillies is also used as spice and a weapon.
Plants complete their life cycle in different stages (germination, vegetative, flowering and post-harvest) undergoing various abiotic and biotic stresses. Drought, salinity and heavy metal ions, high intensity of light, UV radiation etc., abiotic stressors along with attack of predators, parasites, pathogens and allelopaths as biotic stressors, which highly threaten the plant growth and development. Our study is based on growth and developmental stage (vegetative/ flowering) dependent effective demonstration of salinity and strategic role of salicylic acid as a growth regulator-cum-signalling molecule on the Capsicum annuum L. plant. Hence, the main objective of our study was to find the effective stage of Capsicum annuum L undergoing salinity stress which can be ameliorated by treatment with physiological concentration of salicylic acid.
2. Materials and methods
2.1. Experimental design and treatments
The seeds of chilli (Capsicum annuum L. cv. Pusa Sadabahar) were procured from IARI, Pusa Delhi. The plastic pots were filled with 3 kg soil and single sterilized seed was sown in each pot. Pots were organized in a completely randomized block design and were maintained in greenhouse of the Department of Botany, Chaudhary Charan Singh University, Meerut, Campus. For inducing salinity stress at mature vegetative (60 DAS; before initiation of flowering) and flowering stage (80 DAS) pots were irrigated with 500 ml NaCl solution with or without SA (1 mM). The different concentrations of NaCl used were 0, 25, 50, 75, 100, and 150 mM. SA was given through root along with NaCl, however control plants were fed with either water or 1 mM SA. Samples were collected 10 days after salinity and SA treatment i.e., 70 DAS and 90 DAS for each stage. Different physiological and biochemical analysis carried are described below.
2.2. Measurement of growth parameters
Root and shoot length was measured manually using tape. For the measurement of fresh weight were weighed immediately after uprooting while as dry weight was recorded after oven drying the samples at 70 °C for 48 h, In addition number of branches per plant, root shoot ratio and yield (number of fruits per plant) were also recorded.
2.3. Measurement of chlorophyll content
For chlorophyll estimation fresh leaf tissue was extracted in 80 % acetone (v/v) and supernatant was centrifuged at 5000 rpm. Absorbance of supernatant was recorded at 663, 645 and 436 nm using Shimadzu UV-2600 spectrophotometer (Arnon 1949).
2.4. Antioxidant activity
For extraction of enzymes fresh 100 mg leaf tissue was homogenized in 10 ml chilled extraction buffer i.e.,0.2 M Tris-Maleate Buffer, pH 7.2 (TMB) and centrifuged at 10,000 rpm for 15 min at 4 °C (Vimala, 1984). Supernatant was collected and used for enzyme assay.
Peroxidase activity was assayed according to Maehly and Chance (1954) and change in absorbance was read at 475 nm. Assay mixture contained enzyme extract, 1% benzidine solution and 7.5% hydrogen peroxide (H2O2). The total activity of peroxidase was expressed as ΔA475min-1gfw-1. Assay of catalase was carried in in an assay mixture containing 2.5 ml 50 mM sodium phosphate buffer (pH 7.0), 0.2 ml enzyme extract and 0.3 ml of 3% H2O2. Absorbance was measured at 240 nm for 2 min (Aebi, 1984).
2.5. Estimation of ascorbate content
200 mg fresh plant leaf tissue was extracted in 5 % (mass/volume) metaphosphoric acid followed by centrifugation at 3000 rpm for 20 min at 4 °C. One ml aliquot was mixed with 2.5 ml freshly prepared double diluted phenol reagent and mixture was allowed to stand at room temperature for 40 min. Absorbance was read at 730 nm using spectrophotometer Shimadzu UV-2600 (Shrivas et al., 2005).
2.6. Determination of protein content
Protein content was estimated by homogenising 200 mg fresh leaf tissue in 5.0 ml of TrisHCl buffer (pH 7.0) and centrifuged at 10,000 rpm for 10 min. To 1.0 ml of sample extract, 5.0 ml of Coomassie brilliant blue dye was added and absorbance was recorded at 595 nm (Bradford, 1976).
2.7. Estimation of free proline content
Fresh 200 g leaf was homogenized in 10 ml of 3% sulfosalicylic acid and then centrifuged at 5000 rpm for 10 min. Supernatant was collected and reacted with 2.0 ml acetic acid glacial and 2.0 ml freshly prepared acid ninhydrin solution in water bath for 1 h at 100 °C. Reaction was terminated in ice bath and proline was separated using toluene. Absorbance was recorded spectrophotometrically (Shimadzu UV-2600) at 520 nm (Bates et al., 1973). Standard curve of proline was used for calculation.
2.8. Estimation of phenolics
Phenolics were estimated according to method of Bray and Thorpe (1954). 50 mg plant tissue was homogenized in mortar and pestle with 5 ml of 80% ethanol and centrifuged at 5000 rpm. Supernatant was collected and pellet was reextracted with 2.5 ml 80% ethanol. Pooled supernatants were evaporated to dryness and residue was dissolved in 5 ml of distilled H2O. Aliquot was reacted with 0.5 ml of phenol regent followed by addition of 2.0 ml 20 % Na2CO3. Absorbance was recorded at 650 nm and calibration curve of gallic acid was used for calculation.
2.9. Statistical analysis
The obtained data were analysed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. Mean (±SD) was calculated from three replicates per treatment. Using Duncan’s Multiple Range Test (DMRT) at p ≥ 0.05 significant difference in mean compared to control is displayed using bars with different letters.
3. Results
The observations in experiments on tolerance expression upon treatments with salt (0, 25, 50, 75, 100 and 150 mM NaCl) and SA (1.0 mM) with respect to stage (vegetative vs. flowering) in Capsicum annuum cv. PS was studied using basic physiological and biochemical parameters. Shoot & root length, fresh & dry weight number of branches, root: shoot ratio and yield (number of fruits per plant), antioxidant enzyme activity (CAT, POX) and metabolite status of leaf (protein, proline, phenolics, ascorbate, total chlorophylls and carotenoids) as biomarker. All means of the observations were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test at p ≤ 0.05 level of significance.
3.1. Growth and physiological parameters
3.1.1. Shoot and root length, fresh and dry weight, root: shoot ratio and yield (number of fruits per plant) in 90 DAS old plants
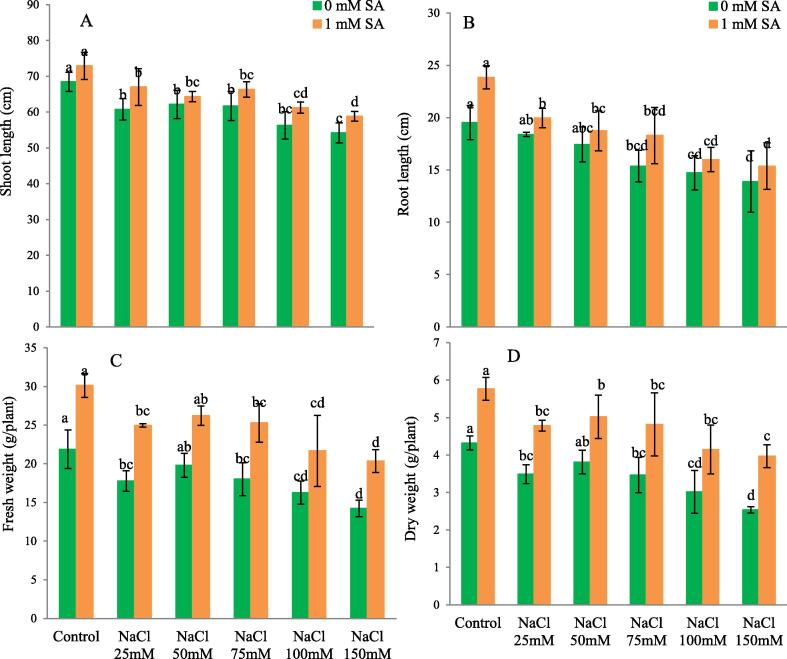
The growth characteristic was assessed at 90 DAS after sowing. Shoot & root length significantly decrease with increasing concentration of NaCl treatment. There is the maximum reduction in shoot & root length was observed in treated plants 26.27 and 40.52% respectively under 150 mM NaCl compared to untreated plants. Similar results also observed in fresh & dry weight of 90 DAS old capsicum plant. Fresh & dry weight markedly decrease 53.76% and 70.53% respectively under150 mM treatment of NaCl compared to control. With the application SA shoot & root length, fresh & dry weight significantly increased treated and untreated plants because of the SA mitigate the deleterious effect of NaCl (Fig. 1A, B and C, D).
Fig. 1.
(A) Shoot length (cm) without and with SA, (B) Root length (cm) without and with SA, (C) Fresh weight (gm) of 90 DAS old capsicum plant without and with SA, (D) Dry weight (g) of 90 DAS old capsicum plant without and with SA. The obtained data were analysed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. Mean (±SD) was calculated from three replicates per treatment. Using Duncan's Multiple Range Test (DMRT) at p < 0.05 significant difference in means compared to control, is displayed using bars with different letters.
Among physiological parameters shoot & root length, fresh & dry weight of plants significantly increased in SA treated plants. However, the shoot to root ratio was observed to be maximum under at 75 mM NaCl without SA. Addition of SA showed highest ratio of shoot to root under 100 and 150 mM NaCl treated plants. Interestingly, irrigation with SA (rhizospheric application) resulted in higher shoot to root ratio, with higher biomass compared to respective controls. It may also be pertinent to report that branching was higher at 50 and 150 mM NaCl in the absence of salicylic acid but in the presence of salicylic acid, 150 mM NaCl showed maximum number of branches. The fruits appear maximally on 90 DAS in 50 mM NaCl with SA treated plants, whereas in control (with SA) fruiting process appears to be slow as on 90 DAS only flowers were there, which could account for 4.33 fruits per plant (Plate 1, Table 1).
Plate 1.
showing the effect of NaCl without salicylic acid (A, 60 days) & (B, 90 days) while picture (C, 60 days) & (D, 90 days) showing the effect of NaCl with salicylic acid on pot grown plants of chilli.
Table 1.
The number of branches, number of fruits per plant and root: shoot ratio without and with SA on 90 DAS old pot grown plant of capsicum.
| Parameters (-SA and + SA) | Treatments of NaCl |
|||||
|---|---|---|---|---|---|---|
| Control | 25 mM | 50 mM | 75 mM | 100 mM | 150 mM | |
| No of branches (-SA) | 1.00 ± 00 | 1 ± 00 | 2.66 ± 1.52 | 2 ± 1.00 | 2 ± 1.00 | 2.33 ± 0.57 |
| No of branches (+SA) | 4 ± 1.00 | 1.66 ± 0.57 | 3 ± 1.00 | 3 ± 1.00 | 1.33 ± 0.57 | 4.33 ± 1.52 |
| No of flowers per plant (-SA) | 3.66 ± 1.52 | 4.66 ± 1.52 | 3.33 ± 2.51 | 4 ± 2.00 | 2 ± 2.64 | 1.33 ± 1.52 |
| No of flowers per plant (+SA) | 4.33 ± 1.52 | 6 ± 3.00 | 5.33 ± 2.51 | 4.66 ± 1.15 | 4.66 ± 5.03 | 3.66 ± 1.15 |
| Root: shoot ratio (-SA) | 3.50 ± 0.3 | 3.30 ± 0.01 | 3.56 ± 0.16 | 4.01 ± 0.53 | 3.81 ± 0.51 | 3.90 ± 0.53 |
| Root: shoot ratio (+SA) | 2.88 ± 0.38 | 3.35 ± 0.27 | 3.43 ± 0.38 | 3.62 ± 0.32 | 3.82 ± 0.21 | 3.82 ± 0.81 |
The data showing the mean (±SD) was calculated from three replicates per treatment.
3.1.2. Total chlorophyll
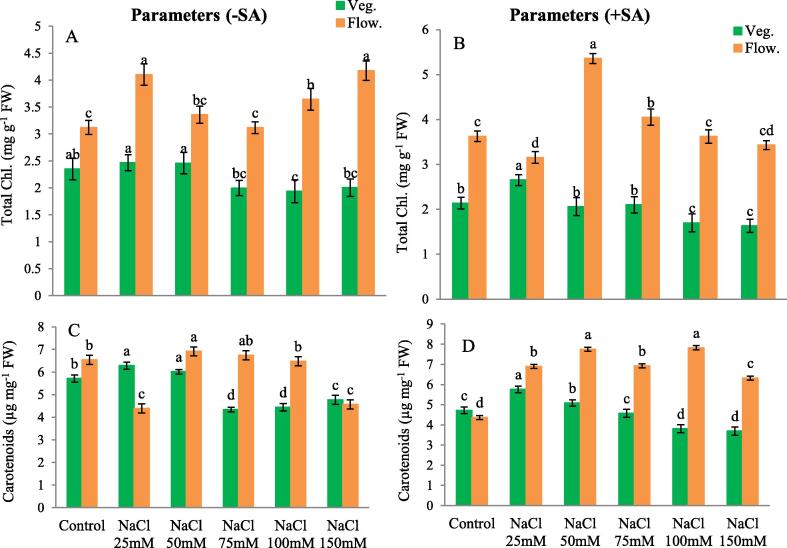
In vegetative stage only in 100 mM NaCl treatment significant decrease (17.58 %) in chlorophyll content was noted. However, in flowering stage there was significant increase (31.50 %) in 25, and (33.83 %) in 150 mM NaCl compared to control which remained high after application of SA in both vegetative as well as flowering stage highly increased (47.72 %) in 50 mM of NaCl + SA compared to salt free control (with SA) (Fig. 2 A-B).
Fig. 2.
(A) Total Chlorophyll without SA, (B) Total Chlorophyll with SA, (C) Carotenoids without SA (D) Carotenoids with SA. The obtained data were analysed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. Mean (±SD) was calculated from three replicates per treatment. Using Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05 significant difference in means compared to control, is displayed using bars with different letters.
3.1.3. Carotenoids
In vegetative stage 25 and 50 mM NaCl significantly increased (9.87 and 5.20 %) carotenoid content whereas in flowering stage 50 mM NaCl significantly increased (5.69 %) carotenoids but 150 mM NaCl treatment highly decreased (30.10 %). 25 and 50 mM NaCl (21.89 and 7.67 %) with SA led to increase in carotenoid content whereas all the other treatments led to decrease in carotenoids during vegetative stage. Interestingly carotenoids significantly increased in all the treatments especially (78.91 %) in 100 mM and (77.44 %) in 50 mM NaCl with SA compared to controls in flowering stage (Fig. 2 C&D).
3.2. Biochemical studies
3.2.1. Peroxidase
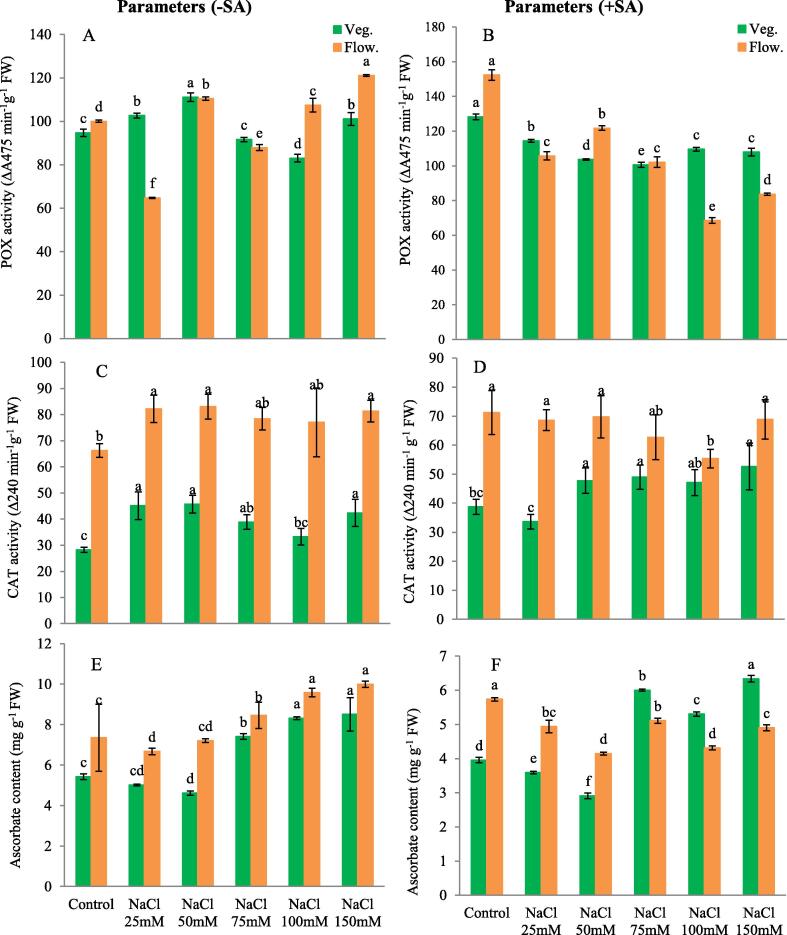
In vegetative stage 50 mM NaCl did not significantly increase (17.58 %) or decrease (12.29 %) POX activity in 100 mM NaCl whereas in flowering stage 150 mM NaCl significantly increased (21.01 %) the POX activity as compared to control as analysed according to DMRT at 0.05 level of significance. However, application of SA in vegetative stage led to significant decrease in POX activity in all concentrations of NaCl, 75 mM NaCl showing the (21.58 %) decreased compared to control. Similar results were also found in flowering stage where peroxidase activity highly decreased (55.00 %) in 100 mM NaCl in the presence of SA compared to control without salt but with SA (Fig. 3 A- B).
Fig. 3.
(A) Peroxidase without SA, (B) Peroxidase with SA, (C) Catalase without SA, (D) Catalase with SA, (E) Ascorbate without SA, (F) Ascorbate with SA. The obtained data were analysed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. Mean (±SD) was calculated from three replicates per treatment. Using Duncan's Multiple Range Test (DMRT) at p < 0.05 significant difference in means compared to control, is displayed using bars with different letters
3.2.2. Catalase
There was significant increase in catalase activity in all concentrations of NaCl. Catalase activity highly increased (61.94 %) 50 mM treated plant compared to untreated plant in vegetative stage, whereas in flowering stage only 25, 50 and 150 mM NaCl treatments significantly increased approximately (22.26 %) catalase activity as compared to control. Application of SA in vegetative stage led to significant increase (35.80 %) in catalase activity at 150 mM NaCl treatment, whereas in flowering stage catalase activity remained unaffected at 150 mM NaCl treatment. CAT significantly decreased (22.28 %) in 100 mM NaCl and SA treatment compared to salt free control with SA (Fig. 3 C - D).
3.2.3. Ascorbate
In vegetative stage under 100 and 150 mM salt treatment, ascorbate (AsA) content significantly increased (53.30 & 56.71 %) respectively, while in flowering stage, it significantly increased (30.29 and 35.96 %) under 100 and 150 mM NaCl treatment as compared to control. Addition of SA to salt treatment led to further significant increase in AsA content in almost all treatments, 150 mM NaCl + SA showing the highest (60.34 %) value while decrease (26.43 %) in 50 mM NaCl + salicylic acid in vegetative stage as compared to salt free control with SA. In flowering stage application of salicylic acid led to significant decrease in ascorbate content in all the treatments of NaCl and SA as compared to salt free control with SA (Fig. 3 E - F).
3.2.4. Protein
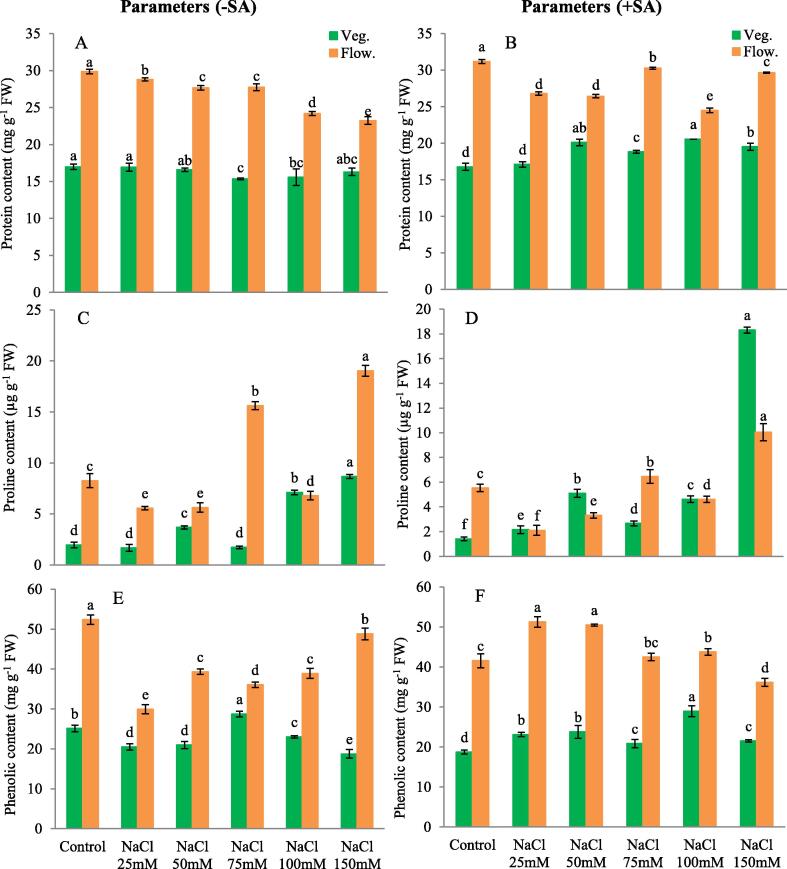
In vegetative stage only under 75 mM (9.54 %) and (8.24 %) 100 mM NaCl treatment and in flowering stage in all the salt treatments leaf protein of C. annuum cv. PS significantly decreased, compared to control and highly decreased (22.22 %) in 150 mM NaCl. Application of SA in vegetative stage led to significant protein content increase in all the treatments except under 25 mM NaCl treatment and contrastingly increased (22.64 %) in 100 mM concentration of NaCl + 1 mM SA compared to salt free control with SA. Flowering stage protein content significantly decreased in all the treatments, highest decrease being in 75 and 100 mM NaCl + SA treatment compared to control with SA (Fig. 4 A - B).
Fig. 4.
(A) Protein without SA, (B) Protein with SA, (C) Proline without SA, (D) Proline with SA, (E) Phenolics without SA, (F) Phenolics with SA. The obtained data were analysed by one-way analysis of variance (ANOVA) using SPSS 16.0 software. Mean (±SD) was calculated from three replicates per treatment. Using Duncan's Multiple Range Test (DMRT) at p < 0.05 significant difference is means compared to control, is displayed using bars with different letters.
3.2.5. Proline
In contrast to leaf protein content, proline content did not significantly increase or decrease (11.60 %) during vegetative stage at 75 mM NaCl though at 100 and 150 mM NaCl there was significant increase (263.13 and 342.42 %) in proline content in both vegetative and flowering stage compared tocontrol. Application of salicylic acid led to further increase in proline content significantly (1202.8 %), under 150 mM NaCl + salicylic acid treatment, proline content being the highest in vegetative stage as well as in flowering stage compared to salt free control with SA (Fig. 4 C - D).
3.2.6. Phenolics
At vegetative stage 50 mM onwards all concentrations of salt significantly increased (14.42 %) phenolics, 75 mM NaCl being the most significant. In contrast during flowering stage all treatments decreased phenolic content as compared to control,150 mM (6.42 %) showing the least decrease and 25 mM (42.67%) NaCl showing the maximum decrease compared to control. Addition of SA, increased phenolics significantly in vegetative stage highest being (54.64 %) in 100 mM NaCl + SA compared to salt free control with SA. Similar results were recorded in flowering stage but with highly value (23.39 %) in 25 mM NaCl and SA but slightly decrease in 150 mM NaCl and SA compared to control (Fig. 4 E - F).
4. Discussion
Soil salinity is a world-wide problem for agricultural land, imparting negative influence on plant growth and development and extensively affecting the yield productivity of majopr food crops including rice, maize, wheat and Brassica juncea (Khan et al., 2012, Anjum et al., 2014, Taïbi et al., 2016, Ahanger and Agarwal, 2017, Siddiqui et al., 2017, Ahanger et al., 2019, Singh et al., 2021). Several studies have suggested that various approaches for mitigation of the adverse effect of salinity, including exogenously applied of plant growth regulators in different crop plants such as tobacco (Nicotiana tobacum), wheat, rice and soybean (Glycine max), and capsicum (Hoque et al., 2007, Ali and Athar, H.-u.-R., Ashraf, M., 2008, Qiu et al., 2014, Akram et al., 2017, Mostofa et al., 2017, Kaya et al., 2020b). Among the phytohormones SA has been reported to regulate growth and prevent the adverse affects of stresses including salinity (Khan et al., 2012, Ahanger et al., 2020). Salinity induces ion toxicity which results in oxidative and osmotic stress. Salicylic acid one of the key plant growth regulators for mitigating the salinity stress. Present study investigates the role of exogenous SA treatment on the alleviation of salinity induced growth damage in capsicum. Salinity stress markedly decreased the plant growth and development resulting reduction in plant root and shoot length under salinity stress. Plant growth decreased with the increasing concentration of salt in the present study and the results corroborates with the findings of Ahanger et al. (2020) who showed decreased plant growth in Vigna radiata under salt stress. Reduced growth in wheat, soybean, Acacia auriculifornus, sugar beet and maize (Wu et al., 2013, Akram et al., 2017, Rahman et al., 2017, Siddiqui et al., 2017, Kaya et al., 2020a, Kaya et al., 2020b) has been reported.
On the other hand application of salicylic acid alleviated the decline in plant growth caused due to salinity. Growth enhancement due to supplementation of SA has been also reported by (Kohli et al., 2019) in mustard and (Kaya et al., 2020a) in maize, (Saddiq et al., 2021) in winter and spring wheat. SA protects the different plant species against toxic effect of biotic and abiotic stress including salinity (Ahmad et al., 2017, Nie et al., 2018, Kaya et al., 2020b), root length increase in capsicum annum (Otálora et al., 2020), soybean (Gutiérrez-Coronado et al., 1998). Besides this the fresh and dry weight of Capsicum annuum in present study significantly decreased with increasing concentration of NaCl. Fresh and dry weight is mostly depending up on relatively water content (RWC) of the plant. RWC decreased especially under biotic and abiotic stresses, such as drought, metal, and salinity (Li et al., 2017, Nguyen et al., 2018), and in maize (Tahjib-Ul-Arif et al., 2018). Salinity decreased in H2O availability in various parts of the plant. However, with the application of salicylic acid RWC improved in salt stressed plants (salt + SA) as well as control (Control + SA) plants. Similar to our results increased RWC due to SA application has been reported in salt stressed Solanum lycopersicum (Görgényi Miklósné Tari et al., 2015), pea (Ahmad et al., 2017), citrus sinensis (Khoshbakht and Asgharei, 2015), rice (Jini and Joseph, 2017), maize (Tahjib-Ul-Arif et al., 2018) and capsicum (Kaya et al., 2020b). (Stevens et al., 2006) who showed that application of SA alleviate the harmful effect of salinity stress on fresh and dry weight of plants (Bastam et al., 2013) in pistachio.
Usage of 1 mM SA as physiological concentration was reported by Jini and Joseph (2017) in rice which has been adopted in our experiments, too. Besides, Souri and Tohidloo (2019) have reported foliar application of similar concentration of SA (100 mg L-1) to increase shoot: root ratio and biomass whereas they also mentioned poor shoot length and yield by rhizospheric application of SA in tomato.
In our case, the contradiction to their finding rhizospheric application of SA successfully improved the yield of NaCl treated plants compared to controls with or without SA. Even at 150 mM NaCl treatment SA could improve the fruit yield to as much as in controls (without salt and SA). Addition of salt led to increase in branching compared to control probably to increase the number of apices bearing flowers and fruits and hence to conserve progeny before the plant succumbs to increasing stress.
Addition of SA added to the number of branches in control but in salt treated sets maximum branches appeared in highest concentration of salt (150 mM NaCl) treatment, lowest number of fruits per plant could be recorded as against 25 mM NaCl treated with highest number of fruits. Thus, NaCl in small concentration (25 mM) supports early fruiting which is further enhanced by SA.
Photosynthesis is the photo-chemical reaction in which light energy is get converted in to chemical energy and it is the main part of plant life cycle which, is directly related to yield of the plant (Chaves et al., 2009) under stress condition decreasing the yield (Ahmad et al., 2017).
Chlorophyll is more sensitive to salinity, drought, and metal stress. Chlorophyll content decreased with increasing concentration of NaCl in capsicum annuum cv. PS. Our results are agreement with other findings. Salinity stress adversely affect most of the step of photo-chemical reaction and reduced the rate of photosynthesis (Ashraf and Harris, 2013, Sehar et al., 2019, Jahan et al., 2020, Rasheed et al., 2020). Photosynthetic rate is decreased under salt stress due to osmotic, nutrient and disbalancing in ionic homeostasis in rice, wheat, soybean (Jini and Joseph, 2017, Li et al., 2017, Shahbaz et al., 2017, Maswada et al., 2018).
However, salicylic acid mitigates the toxic NaCl by decreasing the oxidative damage and clearly enhanced the photosynthetic rate in capsicum annuum. (Fariduddin et al., 2003) in Brassica juncea, (Nazar et al., 2011) in mung bean, and (Li et al., 2013) in wheat under salt stress. Salicylic acid assisted photosynthesis by protecting chlorophyll pigments from the phytotoxic effect of ions and oxidative protection of chloroplast (Foyer and Shigeoka, 2011).
(Nazar et al., 2015) salicylic acid enhances the chlorophyll content in mustard. Several studies suggested chl. content is a biochemical marker of salt tolerance in plants (Taïbi et al., 2016, Ishikawa and Shabala, 2019).
Carotenoids an important non enzymatic lipid soluble antioxidant found in plants as well microorganisms. Carotenoids play a multiple function in plant metabolism including oxidative stress tolerance they protect the chloroplast form 1O2 and other harmful ROS (Collins, 2001). Carotenoids decreased with increasing concentration of salt stress in veg. stage of capsicum (Sehar et al., 2019, Rasheed et al., 2020). Whereas, in flowering stage carotenoids climatically increased under salt stress. Carotenoid content significantly increased in the presence of salicylic acid. Hussain et al. (2021) also reported that SA protects the chlorophyll content in mungbean (Nazar et al., 2015) in mustard. To fight against salinity induced osmotic stress / oxidative stress and ionic imbalancing plants up-regulate the indigenously existing immune system that protects the plants from toxic effect of reactive oxygen species (ROS). The excess production of free radicals causes deleterious effects on lipid, protein and other macromolecules leading to cellular metabolism arrest or cell death (Bhuyan et al., 2020, Jahan et al., 2020). For alleviation of ROS effects, plants enhance the ROS scavenging antioxidant enzyme activity including CAT, POX and APX etc. and content of non-enzymatic antioxidants (Ahmad et al., 2018, Ahanger et al., 2019). ROS include O2•- OH•, H2O2 and 1O2 which impart damaging effects on the key macromolecules hereby hamper the normal plant functioning (Ahanger et al., 2017). Hydrogen peroxide is reduced into H2O by the action of catalase mainly in peroxisomes whereas, APX is the first enzyme of the AsA-GSH cycle catalysing the dismutation of H2O2 by utilizing AsA as an electron donor in different organelles including mitochondria, chloroplast and cytosol (Garg and Manchanda, 2009). Peroxidase activity increased in both stages of capsicum plant. POX performs an important role in the auxin metabolism, heavy metal and salt stress tolerance, lignification and senescence, etc. (Passardi et al., 2005). Hence, POD has often worked as a parameter of metabolism action during growth modifications and environmental stress conditions. POX and CAT activity increased with increasing concentration of NaCl in Jatropha curcas (Gao et al., 2008).
Peroxidase activity increased with increased concentration of NaCl at vegetative as well as flowering stage of capsicum annuum cv. SA application alleviates the toxic effect of NaCl stress by enhancing the antioxidant activity of plants (El-Tayeb, 2005, Syeed et al., 2011, Ahanger et al., 2020). Increased activities of antioxidant enzymes and the increased synthesis of non-enzymatic antioxidants provides strength to plants to withstand the stress mediated growth restrictions by protecting major cellular functioning like photosynthesis (Ahanger et al., 2019, Ahanger et al., 2020). Presents study indicates that SA in general, increases the antioxidant potential of Capsicum annuum plants at both vegetative as well as flowering stage in terms of enzyme activity (increase of POX and CAT). Similar results have also been observed by other others (Mehak et al., 2021, Punia et al., 2021). Catalase activity increased with increased concentration of NaCl in capsicum annuum PS (Jini and Joseph, 2017) in rice, (Tahjib-Ul-Arif et al., 2018) in maize.
Flowering stage is described as plant’s way of preparation for stress through securing the progeny. C. annuum leaves appear to show the impact of stress through decline in protein specifically, whereas tolerance through increase in proline, maintaining the ionic homeostasis and water retention capacity and enhancement of antioxidant activity through increased CAT activity. Our finding similar to another study CAT activity increased in Oenanthe javanica (Kumar et al., 2021a, Mehak et al., 2021).
Increased the CAT activity was also reported in tomato (Rodrıguez-Rosales et al., 1999), in rice (Jini and Joseph, 2017), and Faghih et al., 2017, Kim et al., 2017, Ahmad et al., 2018, Pirasteh-Anosheh and Emam, 2018, Tahjib-Ul-Arif et al., 2018, Punia et al., 2021 clearly suggested that positive role of SA in response to salt stress.
Ascorbate content (AsA) is a low molecular weight, water soluble non-enzymatic antioxidant (Ahanger et al., 2017, Zaid and Wani, 2019). AsA most abundant antioxidant present in most of the cell organelles e.g., chloroplast, peroxisomes, mitochondria and cytosol, which function in coordination to control the redox signaling cascades to scavenge ROS for protecting cell from oxidative damage (Hasanuzzaman et al., 2020, Hussain et al., 2021). In our case AsA content increased with the increasing concentration of NaCl in capsicum annuum cv. PS. Our results are agreement with study of Hossain et al. (2021) in rice. Interestingly in the presence of salicylic acid AsA content increased up to 50 mM to 150 mM of NaCl in vegetative stage of capsicum. In contrast AsA content decreased with increasing concentration of NaCl in flowering stage of PS.
The increase in AsA in 150 mM salt treated plants during vegetative stage might account for reduced AsA, POX activity though in flowering stage SA addition leads to probable degradation of AsA under increased activity of CAT and reduced activity of POX. Such antioxidant defence modulation has been reported by Sehar et al. (2019) in wheat and Jahan et al. (2020) in mustard, too.
In the study of capsicum annuum protein content may be unaffected in vegetative stage. However, similar pattern also showing in flowering stage with slightly decline in high concentration of NaCl Interestingly, carotenoids, proteins and phenolics were recorded to accumulate at 70 DAS (vegetative) in chilli plant leaves at 100 mM NaCl in the presence of SA, but at flowering stage both protein and phenolics in the presence of NaCl with or without SA declined. Protein also accumulated in vegetative stage upto addition of 150 mM salt + 1 mM SA and at flowering stage upto 150 mM salt but without SA upto 75 mM NaCl only. Total chlorophyll content increased up to 25 mM NaCl with SA at every stage. Total phenolics were highest in controls without SA or NaCl and chlorophylls were maximum with SA and NaCl. This suggests that accumulation of phenolics in general, is not in response to salt stress as it is higher in controls rather than a result of treatment with salt or SA, besides higher chlorophylls under salt, salt and SA together refer to stress on photosynthesis being mitigated by SA.
Accumulation of proline content under salinity stress contributes to osmoprotectant and a scavenger of OH•, 1O2, inhibiter of lipid peroxidation, metal chelator, protein stabilizer (Ashraf and Foolad, 2007, Trovato et al., 2008). Proline synthesis protects the redox cycling which is most important in antioxidant defence processes in plant under biotic and abiotic stress (Babiychuk et al., 1995). Proline content increased with increasing concentration of NaCl in capsicum annuum which maintain the water retention in call and protect the plant under salinity stress and play the role in stress tolerance. Our studies are similar to other findings (Jiménez-Bremont et al., 2006) in bean, (El-Shabrawi et al., 2010) in Pokkali rice, (Kumar et al., 2021b) in tomato. Excessive accumulation of proline in vegetative stage under 150 mM NaCl and 1 mM SA treatment with marginally significant increase in proline indicates increased water retention to sustain membrane integrity. Overall, addition of SA has indicated non-accumulation of phenolics during climacteric rise in respiration or oxidation losses, and accumulation of proline (Shahid et al., 2020) under flowering stage. Meaning thereby, the mechanism of SA action is through proline accumulation in flowering stage, too (Lotfi et al., 2020) in mungbean, (Souana et al., 2020) in Vicia faba.
Phenolics are aromatic ring containing organic compounds which are secondary metabolites derived from phenylpropanoid acetate biosynthetic pathway. They play an important role in defence mechanism in higher plants with variable toxicity they target cellular functions at multiple sites (Haig, 2008, Begum et al., 2021).
Phenolic content increased with the increasing concentration of NaCl in capsicum annuum cv. PS in flowering stege. An increase in the total phenolic content was observed in plants exposed to SA along with salt treatment. Salinity phenolic content increased with increasing concentration NaCl (Sogoni et al., 2021) phenolic content increased in Tetragonia decumbens.
SA also enhanced phenolic content at vegetative as well as flowering stage of the plant, reiterating amelioration of salinity induced damage by SA. However, the highest phenolic content was observed at 100 mM NaCl. Similarly, Kazemi et al. (2020) have reported increased phenolics in blueberries improving their tolerance to salinity stress. Hence, salicylic acid is an important stimulator of antioxidant enzyme activity in plants under salinity stress and can be effective for growth and development capsicum annum cv. Pusa Sadabahar under high concentration of NaCl.
5. Conclusion
Salinity proved damaging to the growth and biomass production of Capsicum annum cv. Pusa Sadabahar. Exogenous treatment of SA protected the capsiscum plants from the damaging effects of salinity by strengthening the tolerance mechanisms. SA treatment alleviated the oxidative damage by up-regulating the antioxidant system and increasing the synthesis of key metabolites including proline, phenolics and ascorbate. Hence the SA mediated growth and stress alleviation seems to be associated with the strengthening of tolerance mechanisms against the increased salinity.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Availability of data and material
All data and materials as well as software application or custom code support our claims and comply with field standards. All data generated or analyzed during this study are included in this published article.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
SK conceived the original research plan. VY conducted the analyses. VY wrote the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to the Head, Department of Botany, Chaudhary Charan Singh University, Meerut campus for support and necessary facility of research work. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/168), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahanger M.A., Agarwal R. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Ahanger M.A., Aziz U., Alsahli A.A., Alyemeni M.N., Ahmad P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules. 2020;10:42. doi: 10.3390/biom10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger, M.A., Qin, C., Maodong, Q., Dong, X.X., Ahmad, P., Abd_Allah, E.F., Zhang, L., 2019. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 144, 1–13. [DOI] [PubMed]
- Ahanger M.A., Tomar N.S., Tittal M., Argal S., Agarwal R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants. 2017;23:731–744. doi: 10.1007/s12298-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F., Singh A., Kamal A. Ameliorative effect of salicylic acid in salinity stressed Pisum sativum by improving growth parameters, activating photosynthesis and enhancing antioxidant defense system. Biosci. Biotech. Res. Commun. 2017;10:481–489. [Google Scholar]
- Ahmad P., Alyemeni M., Ahanger M., Egamberdieva D., Wijaya L., Alam P. Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russian. J. Plant Physiol. 2018;65 [Google Scholar]
- Akram N.A., Shafiq F., Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P., Albalawi T.H., Altalayan F.H., Bakht M.A., Ahanger M.A., Raja V., Ashraf M., Ahmad P. 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 2019;9:640. doi: 10.3390/biom9110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Afzal S., Parveen A., Kamran M., Javed M.R., Abbasi G.H., Malik Z., Riaz M., Ahmad S., Chattha M.S. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021;158:208–218. doi: 10.1016/j.plaphy.2020.10.040. [DOI] [PubMed] [Google Scholar]
- Ali, Q., Athar, H.-u.-R., Ashraf, M., 2008. Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul. 56, 107–116.
- Amin I., Rasool S., Mir M.A., Wani W., Masoodi K.Z., Ahmad P. Ion homeostasis for salinity tolerance in plants: a molecular approach. Physiol. Plant. 2021;171:578–594. doi: 10.1111/ppl.13185. [DOI] [PubMed] [Google Scholar]
- Anjum, N.A., Gill, S.S., Gill, R., 2014. Plant adaptation to environmental change: significance of amino acids and their derivatives, CABI.
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Foolad M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59:206–216. [Google Scholar]
- Ashraf M., Harris P.J. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51:163–190. [Google Scholar]
- Babiychuk E., Kushnir S., Belles-Boix E., Van Montagu M., Inzé D. Arabidopsis thaliana NADPH Oxidoreductase Homologs Confer Tolerance of Yeasts toward the Thiol-oxidizing Drug Diamide (∗) J. Biol. Chem. 1995;270:26224–26231. doi: 10.1074/jbc.270.44.26224. [DOI] [PubMed] [Google Scholar]
- Bastam N., Baninasab B., Ghobadi C. Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regul. 2013;69:275–284. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Begum N., Akhtar K., Ahanger M.A., Iqbal M., Wang P., Mustafa N.S., Zhang L. Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environmental Science and Pollution Research. 2021:1–20. doi: 10.1007/s11356-021-13755-3. [DOI] [PubMed] [Google Scholar]
- Bhuyan M.B., Hasanuzzaman M., Parvin K., Mohsin S.M., Al Mahmud J., Nahar K., Fujita M. Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020;90:409–424. [Google Scholar]
- Bosland, P., Votava, E., 2000. Peppers: vegetable and spice capsicums. crop production science in horticulture. Peppers: Vegetable And Spice Capsicums. CAB International Publishing, Wallingford, CT. 204.
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bray H., Thorpe W. Analysis of phenolic compounds of interest in metabolism. Methods Biochem. Anal. 1954:27–52. doi: 10.1002/9780470110171.ch2. [DOI] [PubMed] [Google Scholar]
- Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah A.M., Lee Y.-C., Yamaguchi T., Takamura H., Yin L.-J., Matoba T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008;111:20–28. [Google Scholar]
- Collins A.R. Carotenoids and genomic stability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001;475:21–28. doi: 10.1016/s0027-5107(01)00071-9. [DOI] [PubMed] [Google Scholar]
- Dhiman P., Rajora N., Bhardwaj S., Sudhakaran S.S., Kumar A., Raturi G., Chakraborty K., Gupta O.P., Devanna B., Tripathi D.K. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021 doi: 10.1016/j.plaphy.2021.02.023. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H., Kumar B., Kaul T., Reddy M.K., Singla-Pareek S.L., Sopory S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245:85–96. doi: 10.1007/s00709-010-0144-6. [DOI] [PubMed] [Google Scholar]
- El-Tayeb M. Response of barley grains to the interactive e. ect of salinity and salicylic acid. Plant Growth Regul. 2005;45:215–224. [Google Scholar]
- Faghih S., Ghobadi C., Zarei A. Response of strawberry plant cv. ‘Camarosa’to salicylic acid and methyl jasmonate application under salt stress condition. J. Plant Growth Regul. 2017;36:651–659. [Google Scholar]
- Fariduddin Q., Hayat S., Ahmad A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica. 2003;41:281–284. [Google Scholar]
- Foyer C.H., Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Ouyang C., Wang S., Xu Y., Tang L., Chen F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008;54:374–381. [Google Scholar]
- Garg N., Manchanda G. ROS generation in plants: boon or bane? Plant Biosyst. 2009;143:81–96. [Google Scholar]
- Gautam S., Singh P.K. Salicylic acid-induced salinity tolerance in corn grown under NaCl stress. Acta Physiol. Plant. 2009;31:1185–1190. [Google Scholar]
- Görgényi Miklósné Tari, I., Csiszár, J., Horváth, E., Poór, P., Takács, Z., Szepesi, Á., 2015. The alleviation of the adverse effects of salt stress in the tomato plant by salicylic acid shows a time-and organ-specific antioxidant response. Acta Biol. Cracovien. Series Bot. 57, 21–30.
- Gupta S., Schillaci M., Walker R., Smith P.M., Watt M., Roessner U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: current knowledge, perspectives and future directions. Plant Soil. 2021;461:219–244. [Google Scholar]
- Gutiérrez-Coronado M.A., Trejo-López C., Larqué-Saavedra A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998;36:563–565. [Google Scholar]
- Haig, T., 2008. Allelochemicals in plants. In: Allelopathy in sustainable agriculture and forestry. Springer. pp: 63–104.
- Hasanuzzaman M., Bhuyan M., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat Q., Hayat S., Irfan M., Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ. Exp. Bot. 2010;68:14–25. [Google Scholar]
- Hoque M.A., Okuma E., Banu M.N.A., Nakamura Y., Shimoishi Y., Murata Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007;164:553–561. doi: 10.1016/j.jplph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Hoque T.S., Zaid A., Wani S.H., Mostofa M.G., Henry R. Targeting the Ascorbate-Glutathione Pathway and the Glyoxalase Pathway for Genetic Engineering of Abiotic Stress-Tolerance in Rice. Mol. Breed. Rice Abiotic Stress Tolerance Nutrit. Qual. 2021:398–427. [Google Scholar]
- Hussain S.J., Khan N.A., Anjum N.A., Masood A., Khan M.I.R. Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J. Plant Growth Regul. 2021;40:1000–1016. [Google Scholar]
- Ishikawa T., Shabala S. Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiol. Plant. 2019;165:619–631. doi: 10.1111/ppl.12758. [DOI] [PubMed] [Google Scholar]
- Jahan B., AlAjmi M.F., Rehman M.T., Khan N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020;168:490–510. doi: 10.1111/ppl.13056. [DOI] [PubMed] [Google Scholar]
- Janah I., Elhasnaoui A., Ali O.I., Lamnai K., Aissam S., Loutfi K. Physiochemical Responses of Stevia rebaudiana Bertoni Subjected to Sodium Chloride (NaCl) Salinity and Exogenous Salicylic Acid Application. Gesunde Pflanzen. 2021:1–12. [Google Scholar]
- Jiménez-Bremont J., Becerra-Flora A., Hernández-Lucero E., Rodríguez-Kessler M., Acosta-Gallegos J.A., Ramírez-Pimentel J. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol. Plant. 2006;50:763–766. [Google Scholar]
- Jini D., Joseph B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017;24:97–108. [Google Scholar]
- Johnson R., Puthur J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021;162:247–257. doi: 10.1016/j.plaphy.2021.02.034. [DOI] [PubMed] [Google Scholar]
- Kaya C., Ashraf M., Alyemeni M.N., Corpas F.J., Ahmad P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020;399 doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- Kaya C., Higgs D., Ashraf M., Alyemeni M.N., Ahmad P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020;168:256–277. doi: 10.1111/ppl.12976. [DOI] [PubMed] [Google Scholar]
- Kazemi E.M., Kolahi M., Yazdi M., Goldson-Barnaby A. Anatomic features, tolerance index, secondary metabolites and protein content of chickpea (Cicer arietinum) seedlings under cadmium induction and identification of PCS and FC genes. Physiol. Mol. Biol. Plants. 2020;26:1551–1568. doi: 10.1007/s12298-020-00804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M., Hadavi E., Hekmati J. Role of Salicylic Acid in Decreases of Membrane Senescence in Cut. Am. J. Plant Physiol. 2011;6:106–112. [Google Scholar]
- Khan N.A., Nazar R., Iqbal N., Anjum N.A. Springer Science & Business Media; 2012. Phytohormones and abiotic stress tolerance in plants. [Google Scholar]
- Khoshbakht D., Asgharei M. Influence of foliar-applied salicylic acid on growth, gas-exchange characteristics, and chlorophyll fluorescence in citrus under saline conditions. Photosynthetica. 2015;53:410–418. [Google Scholar]
- Kim Y., Kim S., Shim I.-S. Exogenous salicylic acid alleviates salt-stress damage in cucumber under moderate nitrogen conditions by controlling endogenous salicylic acid levels. Hortic. Environ. Biotechnol. 2017;58:247–253. [Google Scholar]
- Kohli, S.K., Bali, S., Tejpal, R., Bhalla, V., Verma, V., Bhardwaj, R., Alqarawi, A., Abd_Allah, E.F., Ahmad, P., 2019. In-situ localization and biochemical analysis of bio-molecules reveals Pb-stress amelioration in Brassica juncea L. by co-application of 24-Epibrassinolide and Salicylic Acid. Sci. Rep. 9, 1–15. [DOI] [PMC free article] [PubMed]
- Krishna De A. Taylor & Francis:; London: 2003. Capsicum: The Genus Capsicum. Medicinal and aromatic plants–Industrial profiles; p. 275. [Google Scholar]
- Kumar, S., Li, G., Yang, J., Huang, X., Ji, Q., Liu, Z., Ke, W., Hou, H., 2021a. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 12. [DOI] [PMC free article] [PubMed]
- Kumar, S., Singh, T.B., Agnihotri, R.K., Chaturvedi, P., 2021b. Comparative Effect of NaCl and PEG on Physiological and Biochemical Attributes during Vegetative Stage of Tomato.
- Li G., Peng X., Wei L., Kang G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene. 2013;529:321–325. doi: 10.1016/j.gene.2013.07.093. [DOI] [PubMed] [Google Scholar]
- Li Y., Li H., Li Y., Zhang S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017;5:231–239. [Google Scholar]
- Lotfi R., Ghassemi-Golezani K., Pessarakli M. Salicylic acid regulates photosynthetic electron transfer and stomatal conductance of mung bean (Vigna radiata L.) under salinity stress. Biocatalysis and Agricultural. Biotechnology. 2020;26 [Google Scholar]
- Ma X., Zheng J., Zhang X., Hu Q., Qian R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017;8:600. doi: 10.3389/fpls.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehly, A., Chance, B., 1954. The Assay of catalases and peroxidases. In: Methods of Biochemical Analysis. Interscience Inc., New York, pp. 357–424. [DOI] [PubMed]
- Mantri, N., Patade, V., Penna, S., Ford, R., Pang, E., 2012. Abiotic stress responses in plants: present and future. In: Abiotic stress responses in plants. Springer, pp. 1–19.
- Marín A., Ferreres F., Tomás-Barberán F.A., Gil M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.) J. Agric. Food. Chem. 2004;52:3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- Maswada H.F., Djanaguiraman M., Prasad P. Response of photosynthetic performance, water relations and osmotic adjustment to salinity acclimation in two wheat cultivars. Acta Physiol. Plant. 2018;40:1–15. [Google Scholar]
- Mehak G., Akram N.A., Ashraf M., Kaushik P., El-Sheikh M.A., Ahmad P. Methionine-induced regulation of growth, secondary metabolites and oxidative defense system in sunflower (Helianthus annuus L.) plants subjected to water deficit stress. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0259585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014;5:4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M.G., Hossain M.A., Siddiqui M.N., Fujita M., Tran L.-S.-P. Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere. 2017;178:212–223. doi: 10.1016/j.chemosphere.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Nazar R., Iqbal N., Syeed S., Khan N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011;168:807–815. doi: 10.1016/j.jplph.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Nazar R., Umar S., Khan N., Sareer O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015;98:84–94. [Google Scholar]
- Nguyen K.H., Mostofa M.G., Li W., Van Ha C., Watanabe Y., Le D.T., Thao N.P., Tran L.-S.-P. The soybean transcription factor GmNAC085 enhances drought tolerance in Arabidopsis. Environ. Exp. Bot. 2018;151:12–20. [Google Scholar]
- Nie W., Gong B., Chen Y., Wang J., Wei M., Shi Q. Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hortic. 2018;235:413–423. [Google Scholar]
- Oboh G., Rocha J. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens) J. Food Biochem. 2007;31:456–473. [Google Scholar]
- Otálora G., Piñero M.C., Collado-González J., López-Marín J., Del Amor F.M. Exogenous salicylic acid modulates the response to combined salinity-temperature stress in pepper plants (Capsicum annuum l. var. tamarin) Plants. 2020;9:1790. doi: 10.3390/plants9121790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F., Cosio C., Penel C., Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Pirasteh-Anosheh H., Emam Y. Modulation of oxidative damage due to salt stress using salicylic acid in Hordeum vulgare. Arch. Agron. Soil Sci. 2018;64:1268–1277. [Google Scholar]
- Punia H., Tokas J., Mor V.S., Bhuker A., Malik A., Singh N., Alsahli A.A., Hefft D.I. Deciphering reserve mobilization, antioxidant potential, and expression analysis of starch synthesis in sorghum seedlings under salt stress. Plants. 2021;10:2463. doi: 10.3390/plants10112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z., Guo J., Zhu A., Zhang L., Zhang M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014;104:202–208. doi: 10.1016/j.ecoenv.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Rahman M.M., Rahman M.A., Miah M.G., Saha S.R., Karim M., Mostofa M.G. Mechanistic insight into salt tolerance of Acacia auriculiformis: the importance of ion selectivity, osmoprotection, tissue tolerance, and Na+ exclusion. Front. Plant Sci. 2017;8:155. doi: 10.3389/fpls.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshwari V., Bhuvaneshwari V. Salicylic acid induced salt stress tolerance in plants. Int. J. Plant Biol. Res. 2017;5:1067–1073. [Google Scholar]
- Rasheed F., Anjum N.A., Masood A., Sofo A., Khan N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020:1–14. [Google Scholar]
- Rodrıguez-Rosales M., Kerkeb L., Bueno P., Donaire J. Changes induced by NaCl in lipid content and composition, lipoxygenase, plasma membrane H+-ATPase and antioxidant enzyme activities of tomato (Lycopersicon esculentum. Mill) calli. Plant Sci. 1999;143:143–150. [Google Scholar]
- Saddiq M.S., Iqbal S., Hafeez M.B., Ibrahim A.M., Raza A., Fatima E.M., Baloch H., Woodrow P., Ciarmiello L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy. 2021;11:1193. [Google Scholar]
- Sadiq, Y., Zaid, A., Khan, M.M.A., 2020. Adaptive physiological responses of plants under abiotic stresses: Role of phytohormones. In: Plant ecophysiology and adaptation under climate change: Mechanisms and perspectives I. Springer, pp. 797–824.
- Sakhabutdinova A., Fatkhutdinova D., Bezrukova M., Shakirova F. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant Physiol. 2003;21:314–319. [Google Scholar]
- Sehar Z., Masood A., Khan N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019;161:277–289. [Google Scholar]
- Shahbaz M., Abid A., Masood A., Waraich E.A. Foliar-applied trehalose modulates growth, mineral nutrition, photosynthetic ability, and oxidative defense system of rice (Oryza sativa L.) under saline stress. J. Plant Nutr. 2017;40:584–599. [Google Scholar]
- Shahid M.A., Sarkhosh A., Khan N., Balal R.M., Ali S., Rossi L., Gómez C., Mattson N., Nasim W., Garcia-Sanchez F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy. 2020;10:938. [Google Scholar]
- Shrivas K., Agrawal K., Patel D.K. A Spectrophotometric Determination of Ascorbic Acid. J. Chin. Chem. Soc. 2005;52:503–506. doi: 10.1002/jccs.200500072. [DOI] [Google Scholar]
- Siddiqui M.N., Mostofa M.G., Akter M.M., Srivastava A.K., Sayed M.A., Hasan M.S., Tran L.-S.-P. Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere. 2017;187:385–394. doi: 10.1016/j.chemosphere.2017.08.078. [DOI] [PubMed] [Google Scholar]
- Singh, J., Singh, V., Dutt, V., Walia, N., Kumawat, G., Jakhar, M.L., Yadava, D.K., ChanderSharma, P., 2021. Insights into Salt tolerance of Mustard (Brassica juncea L. Czern&Coss): A metabolomics perspective. Environmental and Experimental Botany, 104760
- Sogoni A., Jimoh M.O., Kambizi L., Laubscher C.P. The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa. Horticulturae. 2021;7:140. [Google Scholar]
- Souana K., Taïbi K., Abderrahim L.A., Amirat M., Achir M., Boussaid M., Mulet J.M. Salt-tolerance in Vicia faba L. is mitigated by the capacity of salicylic acid to improve photosynthesis and antioxidant response. Sci. Hortic. 2020;273 [Google Scholar]
- Souri M.K., Tohidloo G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019;6:1–7. [Google Scholar]
- Stevens J., Senaratna T., Sivasithamparam K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006;49:77–83. [Google Scholar]
- Syeed S., Anjum N.A., Nazar R., Iqbal N., Masood A., Khan N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011;33:877–886. [Google Scholar]
- Tahjib-Ul-Arif M., Siddiqui M.N., Sohag A.A.M., Sakil M.A., Rahman M.M., Polash M.A.S., Mostofa M.G., Tran L.-S.-P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018;37:1318–1330. [Google Scholar]
- Taïbi K., Taïbi F., Abderrahim L.A., Ennajah A., Belkhodja M., Mulet J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016;105:306–312. [Google Scholar]
- Trovato M., Mattioli R., Costantino P. Multiple roles of proline in plant stress tolerance and development. Rendiconti Lincei. 2008;19:325–346. [Google Scholar]
- Vimala, Y., 1984. Changes in certain enzymes accompanying natural and induced loss of seed viability.
- Vimala, Y., Gupta, M., 2006. A physiochemical approach towards understanding of salicylic acid-mediated modification in salinity response of Brassica campestris. In: ICPEP-2, Plant Response to Environmental Stress. International Book Distributing Co, Lucknow, pp: 295–302.
- Wu G.-Q., Liang N., Feng R.-J., Zhang J.-J. Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol. Plant. 2013;35:2665–2674. [Google Scholar]
- Zaid, A., Wani, S.H., 2019. Reactive oxygen species generation, scavenging and signaling in plant defense responses. In: Bioactive molecules in plant defense. Springer, pp. 111–132.
- Zhou F., Last R.L., Pichersky E. Degradation of salicylic acid to catechol in Solanaceae by SA 1-hydroxylase. Plant Physiol. 2021;185:876–891. doi: 10.1093/plphys/kiaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials as well as software application or custom code support our claims and comply with field standards. All data generated or analyzed during this study are included in this published article.