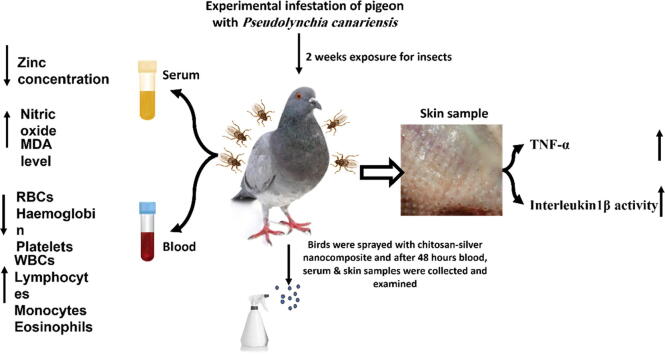
Graphical abstract
Keywords: Blood parameters, Chitosan-silver nanocomposites, Deltamethrin; Pseudolynchia canariensis; Tumor necrosis factor alpha (TNF-α); Interleukin1β (IL1-β); Pigeon fly; Oxidative stress in pigeon
Abbreviations: RBCs, Red blood cells; WBCs, white blood cells; MCHC, mean corpuscular hemoglobin concentration; TNF-α, Tumor necrosis factor alpha; IL-1β, interleukin-1β; MDA, malondialdehyde; P. canariensis, Pseudolynchia canariensis; CMI, cellular-mediated immunity; IgY, immunoglobulins Y; EDTA, ethylene diamine tetra acetic acid; Ag NPs, Silver nanoparticles; NaOH, Sodium hydroxide; LC50, Lethal concentration 50; h, Hour; PCV, packed cell volume; GPX, glutathione peroxidase; SOD, Superoxide dismutase; LDL, Low-density lipoproteins; HDL, High-density lipoproteins
Abstract
This study aimed to evaluate the efficacy of chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis (P. canariensis) with evaluation of different immunological parameters before and after treatment. Therefore, fourteen birds were divided into 2 groups; group1(infested group including 12 birds) which subdivided into 6 sub-groups experimentally infested pigeons 2 pigeons each, and five group of them were treated with chitosan-silver nanocomposites and sub-group number 6 was treated with deltamethrin while, group 2 including two pigeons were kept as control negative ones. P. canariensis flies distributed under the wing and /or under the tail in infested group and these pigeons showed significantly lower RBCs and higher WBCs than that in non-infested pigeons. The cell mediated immune response against experimentally infested pigeons with P. canariensis was studied. P. canariensis infestation in pigeons have a negative impact on pigeon’s blood parameters, increase TNF-α and IL-1β cytokines levels. This study cleared out the role of P. canariensis in the induction of a case of oxidative stress indicated by high level of nitric oxide and malondialdehyde (MDA) with low antioxidant capacity in shape of reduced zinc concentration in the sera of experimentally infested pigeon. Chitosan-silver nanocomposite has a promising effect in the elimination of P. canariensis infestation in pigeons.
1. Introduction
Poultry farming is an effective way to obtain animal protein in the shortest time. Therefore, poultry producers aim to use pigeon and quail meat as another source of animal protein (Basit et al., 2006, Salem and Attia, 2021). There are many different diseases that cause huge economic losses in the animal and poultry production sector (Abd El-Hack et al., 2020, Swelum et al., 2020) including parasitic diseases. The immune system of pigeons includes two main components, the innate and the acquired (cellular mediated or humoral immunity) (Fairbrother et al., 2004). Heterophils are the most abundant leucocytes of the innate immunity, while cellular-mediated immunity (CMI) involves T-lymphocyte proliferation, which is enhanced by release of cytokines as they respond rapidly to the entry of pathogens (Genovese et al., 2013). Mature pigeons feed their squabs with (crop milk) which is a lipid-rich material formed in their crop (Johnston and Janiga, 1995). Crop milk consists of nutrients, minerals, growth factors, immune active compounds such as carotenoids and immunoglobulins which play a role in strengthening of squab's immunity (Shetty et al., 1992, Engberg et al., 1992, Fairbrother et al., 2004, Eraud et al., 2008). Maternal antibodies can be conveyed for squabs through two main ways; before the birth (prenatal antibody transmission) immunoglobulins Y (IgY) in pigeon egg yolk or after the birth (postnatal antibody transmission) in crop milk immunoglobulins (Chucri et al., 2010). Pigeons can be infested by different types of external parasites that lives on their bodies or in the places they reared as, ticks, fleas, mites, lice, and pigeon flies. The ectoparasites bites, irritates, distracts hosts, often triggering defense behavior and sucks in blood containing different microorganisms and can transmit many pathogens through their saliva between different hosts (Koop et al., 2012, Bahrami et al., 2013, Attia and Salem, 2021). Insect bite triggers the birds’ behavioral defenses mechanisms against external parasites through head shaking, foot stamping, self-preening, scratching, dusting, nuthatch sunning and water bathing (Bush and Clayton, 2018). The Behavioral defenses have indirect benefits as it reduces insect fitness, lowering its populations and limiting the rate of pathogens transmission (Waite et al., 2014). There are many preparations that used as antiparasitic agents as herbal extracts (Abou-Kassem et al., 2021); plant extracts (El-Saadony et al., 2021a), Essential oil (El-Tarabily et al., 2021, Alagawany et al., 2021a). Nanotechnology is a promising research point, and it has many applications in poultry industry (Abdel-Ghany et al., 2021, Abd El-Hack et al., 2021a, Reda et al., 2021a, Reda et al., 2020a) and many research aimed to evaluate green synthetized nanoparticles as alternatives for chemical drugs (El-Saadony et al., 2021b, El-Saadony et al., 2021c). Insects transfer salivary compounds with each bite, changing the local physiological parameters at the site of bite that stimulates the immune system to interact with the various components of the insect saliva (Gillespie et al., 2000). Insects’ saliva stimulates acquisition of host blood by impeding hemostasis, producing vasodilation, and lowering inflammation (Champagne, 2004). Also, insect saliva contains serious antigens that stimulate the host immune system to interact and response against insect bite (Owen et al., 2010). Indeed, maternal antibodies can shield the young from parasites for a short time and thus have a beneficial impact on offspring survival as it has a significant impact on the immune ontogeny of squabs by influencing the strength of the humoral response and immune system ontogeny (Heeb et al., 1998, Grindstaff et al., 2006, Reid et al., 2006). Parasitic infestation in pigeon is usually associated with increased phagocytosis, presence of large number of mononuclear leucocytes, increase immunoglobulin synthesis, macrophage activation and presence of basophilic erythrocytes (Ahmed and Mohammed, 1978). Pigeons acquired a particular antibody response to hippoboscidae flies after being exposed to them (Waite et al., 2014). Populations of hippoboscidae flies on pigeons that have antibodies against these flies, resulted in reduced insects’ fitness with shorter lifespan and smaller offspring mass (Waite et al., 2014). Nanotechnology is a promising research point, and it has many applications in poultry industry (Abdel-Ghany et al., 2021). Silver nanoparticles have a constant set of flexible features as well as antibacterial effect that make them suitable for a wide range of biomedical and related applications, but its residual effect limits its use (Stefania et al., 2015, Salem et al., 2021). Chitosan is a non-toxic biopolymer with antibacterial and antifungal properties against a wide spectrum of bacteria and fungi; chitosan nanoparticles and their derivatives have been recommended by many researchers as one of the best nanomaterials for imparting antibacterial and antiparasitic activity (Chandrasekaran et al., 2020). Regarding to silver-based nanocomposites, chitosan-silver nanocomposite found to be an emerging group of bio-nanostructured hybrid materials due to their biocompatibility and biodegradability as well as, they are considered as an effective antibacterial, antiparasitic agents and it have many promising results in elimination of parasitism in veterinary field (Gaafar et al., 2014, Sherif et al., 2017, Abu-Elala et al., 2018, AbdElKader et al., 2021). So, the current study aimed to evaluate the cell mediate immune response and blood parameters of pigeons after their experimental exposure to P. canariensis flies as well as, evaluation the antiparasitic effect of chitosan-silver nanocomposite on P. canariensis flies.
2. Material and methods
2.1. Experimental design
2.1.1. Insects’ collection
Adult P. canariensis flies were picked up from naturally infested pigeons and kept in glass jar and then transported to the laboratory for further investigations (Attia and Salem, 2021).
2.1.2. Preparation of the pigeons for the experimental infestation
Fourteen adult males of domestic pigeons (Columba livia domestica) were purchased from a private pigeon house in which pigeons raised in captivity under controlled hygienic conditions to exclude the previous exposure of pigeons' flies or any blood parasites. Pigeons dropping were examined carefully for presence of any internal parasitic eggs or oocyst (Soulsby, 1986). Fourteen birds were divided into 2 groups; group1(infested group including 12 birds) were subdivided into 6 replicates with 2 pigeons each and group 2 including two pigeons kept as control negative non infested birds. Group1 (experimentally infested) including 6 sub-groups, five of them were treated with nanocomposites and the sixth group was treated with deltamethrin spray (Butox, 12.5 % solution, 1 ml / 4 L of water) for birds as chemical control treatment.
Each cage in infested group 1, includes two pigeons exposed to ten P. canariensis flies; the second group including two birds kept as control non infested one as seen in (Fig. 1).
Fig. 1.
Showing cages that designed for experimental infection of pigeons with P. canariensis flies; each cage includes two adult pigeons.
Every two pigeons were kept in wooden cages with wire nets with dimensions of 90 cm width, 90 cm length and 90 cm height. Birds kept under daily observation for 15 days under normal ambient temperature and normal relative humidity. Pigeons were fed on balanced seed mixtures and clean water ad libitum. After observation period (two weeks infestation period), birds in treated groups were sprayed and treated then after 48 h from treatment birds were humanly slaughtered by severing of jugular vein and blood was collected from each bird in two tubes: one tube containing anticoagulant 0.5 mg/ml ethylene diamine tetra acetic acid (EDTA) and other plain tube for serum collection. The clotted blood was centrifuged at 3500 rpm for 15 min, and the sera were then separated and preserved at −20 °C until used. Skin of birds were dissected and preserved at −20 °C for further work (Saad and Attia, 2021).
All the collected samples (whole blood, sera, dropping, and skin) were rapidly transferred on ice box to Faculty of Veterinary Medicine, Cairo University for further analysis.
All pigeon handling procedures were following the ethical rules of Research Ethics Committee of faculty of Veterinary Medicine Cairo University.
2.2. Parasitological examination
Blood samples were collected on EDTA and used to make a thin blood film that was stained with Giemsa stain and inspected for the presence of any blood parasites. Dropping samples were collected for detection of any enteric parasites using direct microscopical examination and concentration techniques as mentioned by Soulsby, 1986, Attia et al., 2018.
2.3. Preparation of chitosan-silver nanocomposites
Chitosan was prepared from shrimp shells with deacetylation (85%; Sigma-Aldrich; Germany). Silver nanoparticles (Ag NPs) with particle size ranged from 10 to 20 nm (NanoTech; Egypt).
The chitosan-silver nanocomposites were prepared from combination of (1 wt% chitosan solution in 2% acetic acid and Ag NPs in ethanol) which subjected to sonication for 20–25 min then the chitosan nanoparticles were added slowly with continuous stirring (Abu-Elala et al., 2018). Then continuous stirring and sonicated as recorded in Attia et al., (2017). Precipitation of the mixture using NaOH solution; filtration occurred with washing with deionized water till obtaining of colorless solution; then dry it 60 °C.
2.3.1. Characterization of the chitosan-silver nanocomposites using transmission electron microscope study
The characterized of the nanocomposite of chitosan-silver was done using transmission electron microscopy. The sample of nanocomposites was sonicated in ethanol, which deposited onto a copper-coated carbon grid, then allowed to evaporate Imaging was analyzed using a JEM-2100 (JEOL, Japan) operating at 80 kV (Attia et al., 2017, Abu-Elala et al., 2018).
2.4. Determination of the lethal concentration 50 (LC50) of the prepared chitosan-silver nanocomposite on P. Canariensis
Two-fold serial dilutions of several concentration of the prepared chitosan-silver nanocomposite in phosphate buffered saline (PBS,Himedia) as 25; 12.5; 6.25; 3; 2 ppm. A total of 50 adult highly active P. canariensis were collected and divided as ten P. canariensis per dilution in one replicate. observation of the movement of the fly during 15 min; 1 h till 2 h exposure (ingestion assay) to the different concentration of the prepared chitosan-silver nanocomposite. The adult fly was examined carefully under stereoscopic microscope for any movement and the mortality rate was recorded.
2.5. In vivo insecticidal efficacy of the prepared chitosan-silver nanocomposite
Ten experimentally infested pigeon were exposed to 12.5 ppm of the chitosan-silver nanocomposite as a spray on the most site; under wings; on the tail. The pigeons were monitored for 48 h. Then, the pigeon was slaughtered; blood and sera were collected as well as, its skin (1 cm) was cut from the most site of the insect bite; and kept on in −20 °C until used (Attia and Salem, 2021).
2.6. Parasitological examination
Blood samples were collected on EDTA and used to make a thin blood film that was stained with Giemsa stain and inspected for the presence of any blood parasites. Dropping samples were collected for detection of any enteric parasites using direct microscopical examination and concentration techniques as mentioned by Attia et al., 2019, Attia and Salem, 2021.
2.7. Biochemical analysis
Zinc concentration in serum were estimated using ionized coupled plasma through mass spectrometry method as described by Page et al., (2018).
2.8. Evaluation of oxidative stress markers
The level of MDA was estimated in the collected sera as mentioned by Attia et al. 2020. Nitric oxide level was detected in the collected sera as described by Aytekin and Unubol Aypak (2011).
2.9. Assessment of tumor necrosis factor alpha (TNF-α) and Interleukin1β activity
Skin of pigeons were dissected from experimentally infected groups and control non infected ones and all samples were aseptically preserved in −20 °C for further investigations.
2.10. RNA extraction
Extraction of mRNA from 100 mg of pigeon skin were performed by total RNA kit (Ambion, Applied Biosystems), according to the manufacturer's instructions.
The skin was homogenized and applied in Lysing Matrix D tubes (MP Biomedicals) using a FastPrep-24 homogenizer (MP Biomedicals, 2 cycles of 30 s at 6 m/s). Nanodrop (Thermo Scientific) were used to determine the RNA quantity and purity. A 500 ng of RNA were obtained with DNaseI amplification grade (Invitrogen) according to the manufacturer's instructions. The reverse transcription of treated RNA was obtained by High-Capacity cDNA Archive Kit (Applied Biosystems) following Liu et al., (2014) &Younis et al., (2020).
2.11. Quantitive real-time PCR protocol (qRT-PCR)
PCR primers specific for tumor necrosis factor alpha (TNF-α) and Interleukin-1β specific for pigeon were designed and based on the sequences submitted in the GenBankby Liu et al., (2014). (IL1β(DQ393270); Forward: CACCCGCTCCCAGTCCT; Reverse: TGGGTGACTCCAGCACGAATNFα(AY765397) Forward: AGTTGCCCTTCCTGTAACCAG; Reverse: TCCACATCTTTAGAGCATCA). βactin(L08165); Forward:CACCACAGCCGAGAGAGAAAT; Reverse: TGACCATCAGGGAGTTCATAGC was used as a reference gene and for sample normalization. The genes expression included in this study was tested on a separate pool of cDNA, generated from five un infested pigeon previously examined for presence of any parasites.
2.12. PCR cycling conditions.
Amplification was performed for 40 cycles as: denaturation for 30 s at 94 °C, annealing for 30 s at 60 °C and extension for 45 s at 72 °C. Real-time PCR protocol following Liu et al. (2014). The samples were repeated three times.
2.13. Statistical analysis
Data were statistically analyzed by using SPSS Version 18.0 software (Inc., Chicago, IL, USA). Blood parameters in infested; treated and control non infested group were compared by independent T-test following the normality of data. A P- value consider significant when p < 0.05. The lethal concentration (LC50) with 95% confidence intervals was statistically analyzed using probit analysis (Finney, 1971).
3. Results
3.1. Characterization of the chitosan- silver nanocomposites
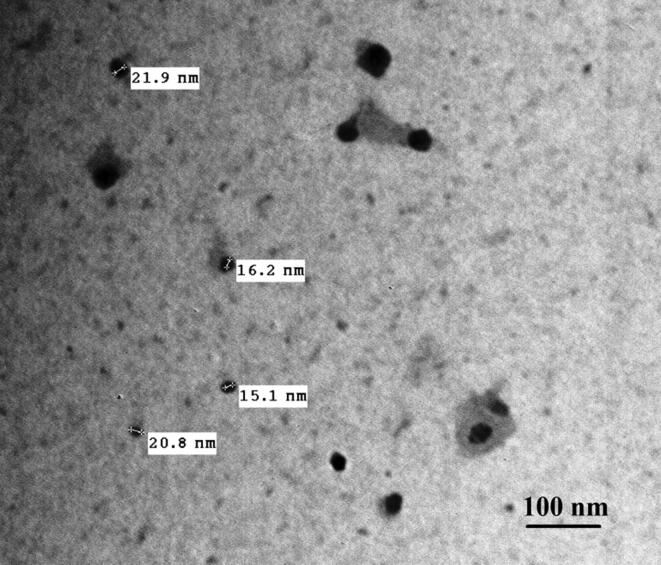
The transmission electron microscopic image analysis of the prepared nanocomposites, revealing that the Ag NPs ranging from 15 to 22 nm which was spherical with the chitosan around it as seen in (Fig. 2).
Fig. 2.
Transmission electron microscope of chitosan- silver nanocomposites showing the rounded shape of the nanoparticles with its diameter ranged from 16 −21.9 nm.
3.2. Parasitological examination
There is a direct relation between the concentration and mortality rate of the insect when the concentration increases the mortality increase. Concerning the preliminary study (Table 1), the concentrations<2 ppm did not cause any mortalities in the treated insect.
Table 1.
Insecticidal efficacy of chitosan-sliver nanocomposite (primary screening).
| Tested concentration |
P. canariensis adult flies |
||
|---|---|---|---|
|
M.M. % ± S.E | |||
| 15 min | 1 h | 2 h | |
| 5 ppm | 50.5 ± 0.34 | 36 ± 0.24 | 00 ± 0.00 |
| 12.5 ppm | 30 ± 0.5 | 71.42 ± 0.54 | 100 ± 0.00 |
| 6.25 ppm | 20 ± 0.45 | 50 ± 0.46 | 50 ± 0.47 |
| 3 ppm | 00 ± 0.00 | 50.5 ± 0.56 | 60 ± 0.54 |
| 2 ppm | 00 ± 0.00 | 00 ± 0.00 | 50.5 ± 0.58 |
| Control treatment with Deltamethrin | 100 + 0.00 | 00 | 00 |
| Control group in cups | 00 | 00 | 00 |
*M.M % ±S. E = mean mortality ± standard deviation.
* No mortalities in corresponding groups during the same exposure periods.
There is a direct relation between the concentration and mortality rate of the insect when the concentration increases the mortality increase. Concerning the preliminary study (Table 1), the concentrations<2 ppm did not cause any mortalities in the treated insect.
Mortalities in P. canariensis adult start as 50.5 ± 0.58 % after exposure to 2 ppm / 2hr. This effect increases with increase concentration as it reached to 50.5 ± 0.56 % after 1 h exposure period with 3 ppm. With increasing the concentration to 6.25 & 12.5 ppm, their insecticidal effects increased from 30 ± 0.5 % & 71.42 ± 0.54% after 15 min and 1 hr to 100 ± 0.00% after 2 h exposure. The concentration of 5 ppm this effect increased to 50.8 ± 0.34% and 36 ± 0.24% after 15 min and 1hr exposure then reached to 100% after 2 hr exposure time (Table 1). No mortality was recorded in control group in screw capped cup; mortality with 100% at 15 min with Deltamethrin.
Experimentally infested pigeon showed the presence of P. canariensis under the wing and /or under the tail.
3.3. Determination of the lethal concentration 50 (LC50) of the prepared chitosan-silver nanocomposite on P. Canariensis
Infested pigeon with P. canariensis showed significantly lower RBCs/cmm (6.45 × 106 ± 0.35 × 106) when compared with both nano-treated and control non-infested birds (11.22 ± 0.236 × 106 & 11.57 × 106 ± 0.88 × 106), respectively. Also, Hemoglobin level (g/dl) were (7.63 ± 0.41) followed by treated group (10.2 ± 0.082), and (10.57 ± 0.09) in control negative one. Platelets/cmm count were (366 × 103 ± 53 × 103, 806.66 × 103 ± 54.36 × 103& 850 × 103 ± 29 × 103) in infested, nano-treated & non-infested groups, respectively. Segmented cells% appeared in lower count in infested group (5.00 ± 0.58) while, were (14.00 ± 0.408&14.00 ± 2.08) in nano-treated & non-infested groups, respectively. Eosinophils% was significantly increased in infested pigeons (15.00 ± 1.73) than both nano-treated (1.64 ± 0.029) and non-infested group (1.67 ± 0.33). All differences in blood parameters between experimental groups were summarized in (Table 2). Stress markers levels were significantly increased in experimentally infested group as level of Nitric oxide was (95.57 ± 3.67) and MDA level was (30.33 ± 2.03) but serum zinc level was marked decreased (55.67 ± 2.96) when compared by both treated and non-infested groups as shown in (Table 3).
Table 2.
Blood parameters of infested pigeons with P. canariensis (Means ± SE).
| Experimentally infested group | Nanoparticles treated group | Control | p - value | |
|---|---|---|---|---|
| RBCs/cmm | 6.45 × 106 ± 0.35 × 106 | 11.22 ± 0.236 × 106 | 11.57 × 106 ± 0.88 × 106 | 0.012* |
| Heamoglobin (g/dl) | 7.63 ± 0.41 | 10.2 ± 0.082 | 10.57 ± 0.09 | 0.002* |
| Platelets/cmm | 366 × 103 ± 53 × 103 | 806.66 × 103 ± 54.36 × 103 | 850 × 103 ± 29 × 103 | 0.001* |
| Haematocrit% | 29.42 ± 1.99 | 27.933 ± 0.33 | 28.33 ± 0.88 | 0.827 |
| WBCs/cmm | 54680.00 ± 2915.94 | 4932 ± 1.41 | 4933.33 ± 233.33 | <0.0001* |
| Basophils% | 1.00 ± 1.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.317 |
| Eosinophils% | 15.00 ± 1.73 | 1.64 ± 0.029 | 1.67 ± 0.33 | 0.002* |
| Staff% | 4.33 ± 0.88 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.008* |
| Segmented% | 5.00 ± 0.58 | 14.00 ± 0.408 | 14.00 ± 2.08 | 0.014* |
| Lymphocytes% | 75.67 ± 2.60 | 44.00 ± 0.816 | 45.00 ± 2.89 | 0.001* |
| Monocytes% | 16.00 ± 2.08 | 3.9 ± 0.294 | 4.00 ± 0.58 | 0.005* |
| MCV (fl) | 33.33 ± 0.33 | 33.31 ± 0.125 | 33.33 ± 2.60 | 0.817 |
| MCH (pg) | 10.03 ± 0.15 | 8.233 ± 0.205 | 8.53 ± 0.19 | 0.003* |
| MCHC (g%) | 30.13 ± 0.19 | 32.232 ± 0.262 | 32.67 ± 0.33 | 0.003* |
*: Indicate significant difference at p-value < 0.05; SE: Standard error.
Table 3.
Stress factor of infested pigeon with P. canariensis (Means ± SE).
| Tested parameters | Experimentally infested group | Nanoparticles treated group | Control | p - value |
|---|---|---|---|---|
| Nitric oxide level | 95.57 ± 3.67 | 47.533 ± 0.205 | 47.37 ± 0.54 | 0.017* |
| MDA level | 30.33 ± 2.03 | 16.5 ± 0.163 | 16.38 ± 0.57 | 0.001* |
| Zinc concentration | 55.67 ± 2.96 | 115.667 ± 3.3 | 120.00 ± 0.88 | <0.0001* |
*: Indicate significant difference at p-value < 0.05; SE: Standard error.
Gene expression for IL1β& TNFα in experimentally infested group was significant elevated (24.33 ± 5.71&19.00 ± 5.51), then decreased after treatment with chitosan silver nanocomposites when compared with control non infested group, respectively as summarized in (Table 4).
Table 4.
Immunological evaluation of IL1β and TNFα in pigeon infested with P. canariensis versus control pigeon (Means ± SE).
| Tested parameters | Experimentally infested group | Nanoparticles treated group | Control | p - value |
|---|---|---|---|---|
| IL1β | 24.33 ± 5.71 | 5. 67 ± 0.262 | 4.67 ± 0.48 | 0.007* |
| TNFα | 19.00 ± 5.51 | 7.73 ± 0.205 | 6.50 ± 0.19 | 0.005* |
*: Indicate significant difference at p-value < 0.05; SE: Standard error.
Results of experimentally infested pigeons with P. canariensis flies were summarized in (Fig. 3).
Fig. 3.
A summarized diagram showing that during experimental infection of pigeons with P. canariensis flies; serum samples resulted in decrease in serum zinc concentration and increase in serum nitric oxide and MDA level; blood samples revealed decrease in RBCs, Haemoglobin & platelets count and increase in WBCs, lymphocytes, monocytes & eosinophils, while skin tissue examination revealed increase in both TNF-α & Interleukin1β activity.
4. Discussion
P. canariensis are abundant worldwide annoying flies which have a direct life cycle as shown in Fig. 4.
Fig. 4.
Life cycle of P. canariensis fly; The adult female only produces one larva at a time and keeps it in her body until it is ready to pupate. The larva absorbs the secretions of a “milk gland” in its mother's uterus. Female gives birth to the white pre-pupa after three larval instars, when the larva has reached its maximum size, and it begins to darken and form the puparium or pupal shell. P. canariensis pupa resembles a dark brown pupa in the host nest or surrounding environment. The adult P. canariensis fly leaves the puparium and flies around looking for a new host.
From our findings, experimentally infested pigeon with P. canariensis revealed significantly decrease in RBCs, Hemoglobin, platelets, MCHC and Segmented cells in comparison with control negative birds. In parallel study conducted by Razavi et al., (2016) they noticed that pigeons infected with Haemoproteus columbae showed anemia appeared in shape of significant decrease in erythrocytes, hemoglobin, and the hematocrit (PCV) values.
Experimentally infested pigeons showed decrease in serum zinc concentration and increase in serum nitric oxide and MDA level. In parallel study conducted by Samani et al., (2018) they found a significant increase in Ferric Reducing Ability of Plasma, uric acids concentration, blood lipid peroxidation, and catalases activity in pigeons infected with Haemoproteus columbae. Also, Nazifi et al., 2011, Razavi et al., 2011 found that the antioxidant enzyme activity, including as superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase, may be modulated by parasitic infestation, resulting in rapid erythrocyte clearance by phagocytic cells. Also, Samani et al., (2018) confirmed that the parasitic infestation in pigeons have an impact on the body's dynamic equilibrium between the creation and removal of reactive free radicals (oxidative status). On the other hand, on a parallel study, Razavi et al., (2016) noticed that pigeons infected with Haemoproteus columbae showed no significant differences in serum level of lipid contents (triglyceride, cholesterol, LDL and HDL), antioxidant trace elements (manganese, copper, iron, zinc, and selenium) and antioxidant vitamins (E, C and A) in infected birds.
Infested pigeons showed increased gene expression for TNF-α & Interleukin1β activity. Also, Garcia-Longoria et al., (2020) concluded that gene expression of Plasmodium homocircumflexum (lineage pCOLL4) was increased after experimental infection of two different bird species with Plasmodium homocircumflexum.
Many stressors in pigeon can cause alterations to the humoral and cellular immune system Blakley et al., 1999. The cytokines are important in the cellular immune response for assessment of the change in the body during the infection. Pro-inflammatory cytokines as Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), are very important in the immune reaction against stressors as toxins and any pathogens causing inflammation; Kim et al., 2004. Therefore, TNF-α and IL-1β can be used as markers for noticing the immune function.
Cytokines have pleiotropic or dismissed functions, and the level of one cytokine is firmly regulated by the other cytokines. The increase in T- helper-2 (Th2) cytokines can result decreased in Th1 cytokines Fleshner et al., (1998).
The increase levels of the pro-inflammatory cytokines as (IL-1β and TNF-α) promotes the organisms to respond to any infectious and induce a series of effects, leading to inflammation.
From our results, chitosan-silver nanocomposite showed a significant antiparasitic effect against P. canariensis flies' infestation in pigeons as after insect exposure for chitosan-silver nanocomposite, swelling, cracks and shrinkage of insect body were observed. This finding may contribute to the characterization of the chitosan- silver nanocomposites in this study were very small ranged from 16 to 22 nm that can penetrate the cuticle of the arthropod or even any microbial cell wall; which in turn increase the permeability of the cuticle leading to rupture of the insect causing its mortality (Attia et al., 2017, Abu-Elala et al., 2018).
In other parallel studies, chitosan-silver nanocomposite, revealed an effective antiparasitic effect as following; Abu-Elala et al., (2018) found that chitosan-silver nanocomposite was effective in treatment of Lernaea cyprinacea infection in goldfish aquaria. Also, Gaafar et al., (2014) confirmed that chitosan-silver nanocomposite has a significant anti-toxoplasma effect. In addition to, many researchers confirmed that chitosan-silver nanocomposite has a potent broad spectrum antibacterial destructive effect against Escherichia coli and Salmonella typhimurium (Badawy et al., 2019).
From our observations, on comparison with infected group, all blood parameters in chitosan-silver nanocomposite treated group were nearly like control non-infected group. In addition to, the treated group with chitosan-silver-nanocomposite showed marked decrease in the stress factor to levels of normal or control negative group, that may be due to healing of the inflamed tissues; this process caused by exposure of the pigeon skin to the prepared composites (Robert and Bolluck, 1976, Abu-Elala et al., 2018, AbdElKader et al., 2021). Also, silver and chitosan nanoparticles improve healing process and prevent the bacterial growth through its roles in wound fibrogenesis which accelerate its healing. The chitosan act as good wound healing as it resembles a hyaluronic acid and it improves the roles of several immune and inflammatory cells, as (neutrophils and macrophages) (Muzzarelli, 1999, Suzuki et al., 2000, Şenel and McClure, 2004). In a parallel study adopted by AbdElKader et al., (2021), they found that chitosan-silver nanocomposite succeeded in correction of blood parameters and stress markers levels and effectively treat infected donkeys with Gasterophilus intestinalis. Finally, we recommend the use of some additives on pigeon food and drink as prebiotics (Abd El-Hack et al., 2021b, Yaqoob et al., 2021), probiotics (Alagawany et al., 2021b, El-Saadony et al., 2021d), symbiotics, green synthesized nanoparticles (El-Saadony et al., 2021e, Saad et al., 2021a), herbal extracts (Ashour et al., 2020, Saad et al., 2021b), bioactive peptides (El-Saadony et al., 2021f, El-Saadony et al., 2021g), Bioactive plants and plant products (Abd El‐Hack et al., 2021c, Reda et al., 2021b), safety natural pigments (Abdelnour et al., 2020a, Abdelnour et al., 2020b) to strengthen the general health status of the birds and increase their resistance to diseases, side by side with applying the biosecurity measures in poultry farms for the advancement of the poultry industry.
5. Conclusion
In this study, the nanotechnology opens new facilities in control of the insect which transmits several diseases to pigeon which selected as a model for insect fly because it is easier to use in experimental infection in the laboratory. Spraying of the pigeon with a chitosan-silver nanocomposite with low concentrations is a more viable role to control of most flies in veterinary field which act as potent biocidal. Application as spraying of chitosan-silver nanocomposites in cages for 15 min gave a promising result as antiparasitic agent and antimicrobial as well as healing of the wound caused by insect bite.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/09), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, Zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El‐Hack M.E., El‐Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., et al. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101(14):5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.-E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2020;28(5):4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: An updated review. World Poult. Sci. J. 2021;77(4) doi: 10.1080/00439339.2021.1960235. [DOI] [Google Scholar]
- AbdElKader N.A., Sheta E., AbuBakr H.O., El-Shamy O.A.A., Oryan A., Attia M.M. Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: Gasterophilidae) larvae in donkeys (Equus asinus) Int J Trop Insect Sci. 2021;41(1):43–54. doi: 10.1007/s42690-020-00171-2. [DOI] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240:104220. doi: 10.1016/j.livsci.2020.104220. [DOI] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19(1):1046–1056. [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20(1):896–910. [Google Scholar]
- Abu-Elala N.M., Attia M.M., Abd-Elsalam R.M. Chitosan-silver nanocomposites in goldfish aquaria: A new perspective in Lernaea cyprinacea control. Int J Bio Macromol. 2018;111:614–622. doi: 10.1016/j.ijbiomac.2017.12.133. [DOI] [PubMed] [Google Scholar]
- Ahmed F.E., Mohammed A.-H. Haemoproteus columbae: Course of Infection, Relapse and Immunity to Reinfection in the Pigeon. Z. Parasitenkd. 1978;57(3):229–236. doi: 10.1007/BF00928036. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6):101172. doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276:114920. doi: 10.1016/j.anifeedsci.2021.114920. [DOI] [Google Scholar]
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10(10):457. doi: 10.3390/agriculture10100457. [DOI] [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int J Trop Insect Sci. 2021 doi: 10.1007/s42690-021-00597-2. [DOI] [Google Scholar]
- Attia M.M., El-Gameel S.M., Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J Parasit. Dis. 2020;44(2):332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Ismael E., Saleh N.M.K. A sensitive serodiagnostic tool for the detection of active infection of zoonotic visceral and nasopharyngeal linguatulosis. Vet. World. 2019;12(6):883–889. doi: 10.14202/vetworld.2019.883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Khalifa M.M., Atwa M.T. The prevalence and intensity of external and internal parasites in working donkeys (Equus asinus) in Egypt. Vet. World. 2018;11(9):1298–1306. doi: 10.14202/vetworld.2018.1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Soliman S.M., Khalf M.A. Hydrophilic nanosilica as a new larvicidal and molluscicidal agent for controlling of major infectious diseases in Egypt. Vet. World. 2017;10(9):1046–1051. doi: 10.14202/vetworld.2017.1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin I., Unubol Aypak S. Levels of selected minerals, nitric oxide, and vitamins in aborted Sakis pigeon raised under semitropical conditions. Trop Anim Health Prod. 2011;43:511–514. doi: 10.1007/s11250-010-9724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy M.E.I., Lotfy T.M.R., Shawir S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull Natl Res Cent. 2019;43(1) doi: 10.1186/s42269-019-0124-8. [DOI] [Google Scholar]
- Bahrami A.M., Hosseini E., Razmjo M. Important Parasite in Pigeon, its Hematological Parameter and Pathology of Intestine. World App. Sci. J. 2013;21(9):1361–1365. doi: 10.5829/idosi.wasj.2013.21.9.71210. [DOI] [Google Scholar]
- Basit, M.T., Pervez, K., Avais, M., Rabbani, I., 2006. Prevalence and chemotherapy of nematodes Shamsi, 2012. Pathological study on parasitism in infestation in wild and domestic pigeon and its racing pigeon. J. Anim. PI. Community Health. Advance in Environmental Sci. 16(1-2).
- Blakley B., Brousseau P., Fournier M., Voccia I. Immunotoxicity of pesticides: a review. Toxicol. Ind. Health. 1999;15(1-2):119–132. doi: 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- Bush S.E., Clayton D.H. Anti-parasite behavior of birds. Phil. Trans. R. Soc. B. 2018;373:20170196. doi: 10.1098/rstb.2017.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D.E. Antihemostatic strategies of blood-feeding arthropods. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:375–396. doi: 10.2174/1568006043335862. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran M., Kim K.D., Chun S.C. Antibacterial Activity of Chitosan Nanoparticles. A Review. Processes. 2020;8:1173. doi: 10.3390/pr8091173. [DOI] [Google Scholar]
- Chucri T.M., Monteiro J.M., Lima A.R., Salvadori M.L.B., Junior J.R.K., Miglino M.A. A review of immune transfer by the placenta. J Reprod Immunol. 2010;87(1-2):14–20. doi: 10.1016/j.jri.2010.08.062. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A.O., Dhama K., et al. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M. T., Saad, A. M., Taha, T. F., Najjar, A. A., Zabermawi, N. M., Nader, M. M., et al., 2021e. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. in press. [DOI] [PMC free article] [PubMed]
- El-Saadony, M. T., Zabermawi, N. M., Zabermawi, N. M., Burollus, M. A., Shafi, M. E., Alagawany, M., et al., 2021a. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: A review. Food Rev. Int. 1-23.
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. [Google Scholar]
- El-Saadony, M.T., Alkhatib, F.M., Alzahrani, S.O., Shafi, M.E., Abdel-Hamid, S.E., Taha, T.F., Aboelenin, S.M., Soliman, M.M., Ahmed, N.H., 2021b. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 28 (8), 4592-4604. [DOI] [PMC free article] [PubMed]
- El-Saadony, M.T., Khalil, O.S., Osman, A., Alshilawi, M.S., Taha, A.E., Aboelenin, S.M., Shukry, M., Saad, A.M., 2021g. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 28 (8), 4581-4591. [DOI] [PMC free article] [PubMed]
- El-Saadony, M.T., Saad, A.M., Najjar, A.A., Alzahrani, S.O., Alkhatib, F.M., Shafi, M.E., Selem, E., Desoky, E.-S.M., Fouda, S.E.-S.E.-S., El-Tahan, A.M., 2021c. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 28 (8), 4461- 4471. [DOI] [PMC free article] [PubMed]
- El-Tarabily, K.A., El-Saadony, M.T., Alagawany, M., Arif, M., Batiha, G.E., Khafaga, A.F., Elwan, H.A., Elnesr, S.S., Abd El-Hack, M.E., 2021. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 28 (9), 5145-5156. [DOI] [PMC free article] [PubMed]
- Engberg R.M., Kaspers B., Schranner I., Kösters J., Lösch U. Quantification of the immunoglobulin classes IgG and IgA in the young and adult pigeon (Columba livia) Avian Pathol. 1992;21(3):409–420. doi: 10.1080/03079459208418859. [DOI] [PubMed] [Google Scholar]
- Eraud C., Dorie A., Jacquet A., Faivre B. The crop milk: a new route for carotenoid-mediated effects. J Avian Biol. 2008;39:247–251. [Google Scholar]
- Fairbrother A., Smits J., Grasman K.A. Avian immunotoxicology. J. Toxicol. Environ. Health B. 2004;7(2):105–137. doi: 10.1080/10937400490258873. [DOI] [PubMed] [Google Scholar]
- Finney D.J. Cambridge University Press; Cambridge: 1971. Probit Analysis; p. p333. [Google Scholar]
- Fleshner M., Nguyen K.T., Cotter C.S., Watkins L.R., Maier S.F. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am. J. Physiol. Regul., Integr. Comp. Physiol. 1998;275(3):R870–R878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- Gaafar M.R., Mady R.F., Diab R.G., Shalaby T.I. Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp. Parasitol. 2014;143:30–38. doi: 10.1016/j.exppara.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Longoria L., Palinauskas V., Ilgūnas M., Valkiūnas G., Hellgren O. Differential gene expression of Plasmodium homocircumflexum (lineage pCOLL4) across two experimentally infected passerine bird species. Genomics. 2020;112(4):2857–2865. doi: 10.1016/j.ygeno.2020.03.025. [DOI] [PubMed] [Google Scholar]
- Genovese K.J., He H., Swaggerty C.L., Kogut M.H. The avian heterophil. Dev. Comp. Immunol. 2013;41(3):334–340. doi: 10.1016/j.dci.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Gillespie R.D., Mbow M.L., Titus R.G. The immunomodulatory factors of blood feeding arthropod saliva. Parasite Immunol. 2000;22:319–331. doi: 10.1046/j.1365-3024.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- Grindstaff J.L., Hasselquist D., Nilsson J.-Å., Sandell M., Smith H.G., Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proceedings of the Royal Society B. 2006;273(1600):2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb P., Werner I., Kölliker M., Richner H. Benefits of induced host responses against an ectoparasite. Proceedings of the Royal Society B. 1998;265(1390):51–56. [Google Scholar]
- Johnston R.F., Janiga M. Oxford University Press; Oxford: 1995. Feral Pigeons. [Google Scholar]
- Kim M.-S., Yi J.-M., Kim S.H., Hong S.-H., Kim H.M. Madimadi, Korean folk medicine, blocks TNF-α, IL-1β, and IL-8 production by activated human immune cells. Cytokine. 2004;25(4):179–186. doi: 10.1016/j.cyto.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Koop J.A.H., Huber S.K., Clayton D.H. Does sunlight enhance the effectiveness of avian preening for ectoparasite control? J Parasitol. 2012;98(1):46–48. doi: 10.1645/GE-2889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li M., Cao Y., Qu J.P., Zhang Z.W., Xu S.W., Li S. Effects of avermectin on immune function and oxidative stress in the pigeon spleen. Chem Biol Interact. 2014;5(210):43–50. doi: 10.1016/j.cbi.2013.12.015. Epub 2014 Jan 8 PMID: 24412236. [DOI] [PubMed] [Google Scholar]
- Muzzarelli, R. A. A., 1999. Mattioli-Belmonte, M. Pugnaloni, A. Biagini, G. Biochemistry, histology and clinical uses of chitinsand chitosans in wound healing, in: P. Jolles, R.A.A. Muzzarelli (Eds.), Chitin and Chitinases, BirkhauserVerlag, Basel, Switzerland, 251– 264. [DOI] [PubMed]
- Nazifi S., Razavi S.M., Kianiamin P., Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation and serum trace elements associated with progressive anemia in ovine malignant theileriosis. Parasitol. Res. 2011;109(2):275–281. doi: 10.1007/s00436-010-2248-5. [DOI] [PubMed] [Google Scholar]
- Owen J.P., Nelson A.C., Clayton D.H. Ecological immunology of bird-ectoparasite systems. Trends Parasitol. 2010;26(11):530–539. doi: 10.1016/j.pt.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Page C.M., Murphy T.W., Van Emon M.L., Bowman J.G.P., Wyffels S.A., Stewart W.C. Blood serum mineral element concentrations of weaned Montana ram lambs and their relationship with water quality characteristics. Prof Anim Sci. 2018;34(5):410–420. [Google Scholar]
- Razavi S.M., Nazifi S., Afsar M., Yazdanpanah Z., Rakhshandehroo E. Evaluation of the blood oxidant-antioxidant interactions in pigeons naturally infected with Haemoproteus columbae. VETERINARSKI ARHIV. 2016;86(3):395–405. [Google Scholar]
- Razavi S.M., Nazifi S., Bateni M., Rakhshandehroo E. Alterations of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation and serum trace elements associated with anemia in bovine tropical theileriosis. Vet. Parasitol. 2011;180(3-4):209–214. doi: 10.1016/j.vetpar.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A.A., Ahmed S.Y.A., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20(1):324–335. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M., Arcese P., Keller L.F., Hasselquist D. Long-term effect on offspring immune response in song sparrows Melospiza melodia. Biol. Lett. 2006;2:573–576. doi: 10.1098/rsbl.2006.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert R.J., Bolluck A.M. The dermatology of marine teleost fish. II. Dermatopathology of integument. Oceanography and Marine Biology Animal Review. 1976;14:227–246. [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A.M., Taha A.E., Taha T.F., et al. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate Sustainable Silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Saad M.F., Attia M.M. Milk as a new diagnostic tool for rapid detection of Fascioliasis in dairy goats using excretory/secretory antigen. Acta Parasitol. 2021;66(2):336–345. doi: 10.1007/s11686-020-00286-z. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int J Trop. Insect Sci. 2021 doi: 10.1007/s42690-021-00492-w. [DOI] [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the Effects of Silver Nanoparticles Against Experimentally Induced Necrotic Enteritis in Broiler Chickens. Int J Nanomedicine. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani A.D., Kheirabadi K.P., Mohebbi A., Ghahfarokhi S.M., Samani A.D. Effect of blood parasites on biomarkers of oxidative status in pigeons. Journal of Dairy Veterinary and Animal Research. 2018;7(1):00186. doi: 10.15406/jdvar.2018.07.00186. [DOI] [Google Scholar]
- Şenel S., McClure S.J. Potential applications of chitosan in veterinary medicine. Adv. drug delivery rev. 2004;56(10):1467–1480. doi: 10.1016/j.addr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Sherif H.H.A., Khalil S.K.H., Hegazi A.G., Khalil W.A., Moharram M.A. Factors affecting the antibacterial activity of chitosan-silver nanocomposite. IET Nanobiotechnol. 2017;11(6):731–737. [Google Scholar]
- Shetty S., Bharathi L., Shenoy K.B., Hedge S.N. Biochemical properties of pigeon milk and its effects on growth. J Comp Physiol B. 1992;162:632–636. [Google Scholar]
- Soulsby E.J.L. 7th Edition. Bailliere Tindall; London: 1986. Helminths, Arthropods & Protozoa of Domesticated Animals. [Google Scholar]
- Stefania, M., George, M.V., Roxana, E.T., Ioana, R.B., Madalina, L., Maria, M.M., Alexandru, M.G., 2015. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr Top Med Chem.15 (16): 1596-1604 (9). [DOI] [PubMed]
- Suzuki Y., Okamoto Y., Morimoto M., Sashiwa H., Saimoto H., Tanioka S., Shigemasa Y., Minami S. Influence of physico-chemical properties of chitin and chitosan on complement activation. Carbohydr. Polym. 2000;42:307–310. [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., et al. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J.L., Henry A.R., Owen J.P., Clayton D.H. An experimental test of the effects of behavioral and immunological defenses against vectors: do they interact to protect birds from blood parasites? Parasit Vectors. 2014;7:104. doi: 10.1186/1756-3305-7-104. http://www.parasitesandvectors.com/content/7/1/104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob, M., Abd El-Hack, M., Hassan, F., El-Saadony, M., Khafaga, A., Batiha, G., Yehia, N., Elnesr, S., Alagawany, M., El-Tarabily, K., 2021. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 100(7):101143. [DOI] [PMC free article] [PubMed]
- Younis N.A., Laban S.E., Al-Mokaddem A.K., Attia M.M. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquac Int. 2020;28(6):2247–2262. doi: 10.1007/s10499-020-00589-y. [DOI] [Google Scholar]