Abstract
Human gut is colonized by numerous microorganisms, in which bacteria present the highest proportion of this colonization that live in a symbiotic relationship with the host. This microbial collection is commonly known as the microbiota. The gut microbiota can mediate gut epithelial and immune cells interaction through vitamins synthesis or metabolic products. The microbiota plays a vital role in growth and development of the main components of human’s adaptive and innate immune system, while the immune system regulates host-microbe symbiosis. On the other hand, negative alteration in gut microbiota composition or gut dysbiosis, can disturb immune responses. This review highlights the gut microbiota-immune system cross-talk in both eubiosis and dysbiosis.
Keywords: Gut microbiota, Immune system, Eubiosis, Dysbiosis
1. Introduction
The human gut is colonized by a huge number of microorganisms, including bacteria, viruses, fungi, and parasites, generally known as microbiota. In humans, more than 1014 microorganisms live in the gastrointestinal tract, mostly in the distal gut (Amada et al., 2013). More than 2000 species and 12 distinct phyla constitute the gut microbiota with microbiome comprising of 150 –500 fold more genes than in the human DNA (Lazar et al., 2018).
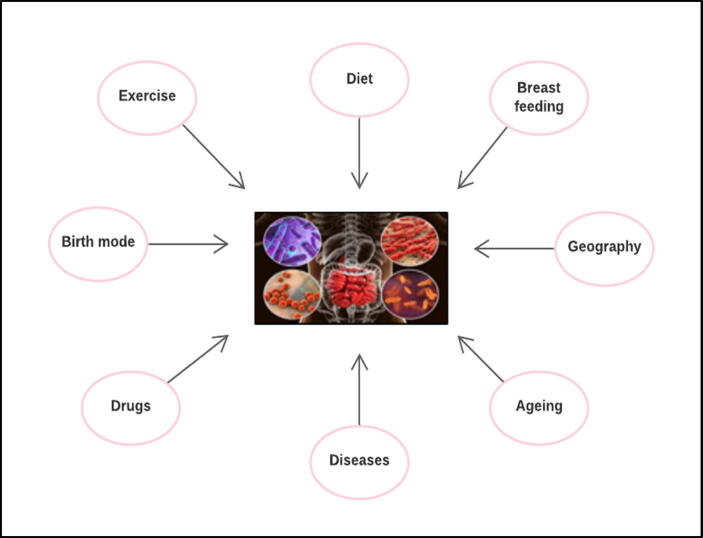
Gut microbiota has a large diversity, and its composition varies in different individuals. Several factors influence the microbial colonization of the gut such as age, gender, host genetics, immune system and health or disease state of the host, use of therapeutic drugs, geographical location, socio-economical factors such as urban/rural and sanitary conditions, diet, and birth mode such as pre- or term-birth and mode of delivery (Fig. 1) (Ardissone et al., 2014). Current metagenomic records confirm that the most of microbiota species do not exist in different individuals at the same time. Moreover, gut microbiota is not only different with regards to distribution along the gut segments but also in the three different transversal layers; epithelial cells at the surface, floating cells in the intestinal lumen, and cells adherent to the mucus layer (Sekirov et al., 2010).
Fig. 1.
Factors affecting gut microbiota composition: Different factors can influence gut microbial colonization such as mode of delivery and pre-/term-birth, geographical location, diseases, use of drugs, type of food or diet, life style such as exercise, ageing and breast feeding.
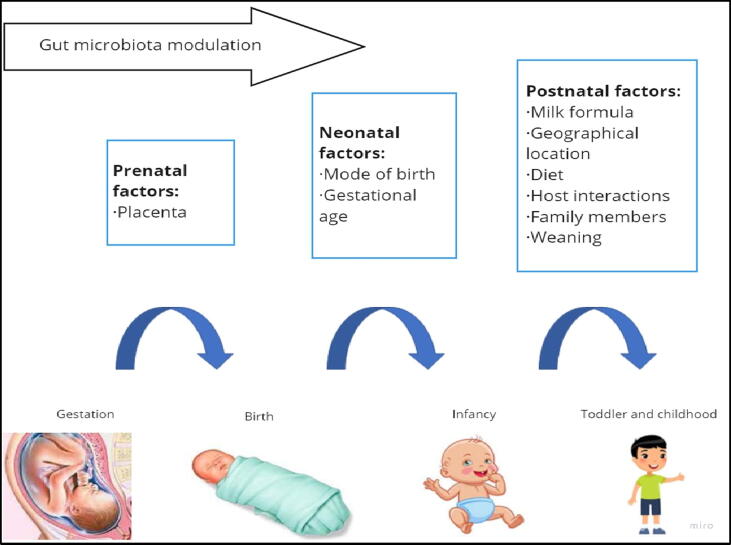
Gut colonization has been reported to occur before birth, and is affected by the placental microbiota profile, which consists of species such as Proteobacteria, Firmicutes, Bacteroidetes, Fusobacteria and Tenericutes, which are similar to the human oral microbiota (Aagaard et al., 2014). Furthermore, the full-term infant meconium is not sterile, containing thirty species that are usually present in vagina, amniotic fluid, and oral cavity (Fig. 2) (Clemente et al., 2012).
Fig. 2.
Window of opportunity for gut microbiota modulation: Several factors modulate the gut microbiota in each stage of human life.
Vaginally born babies have gut microbiota similar to that in their mother’s vagina. In contrast, the microbiota in the caesarean section-delivered infants, mostly resemble the skin microbiota, comprising of Staphylococcus and Propionibacterium spp. (Dominguez-Bello et al, 2010).
Additionally, breast milk has been implicated in gut microbiota and immune system maturity. It contains several immunological components and bioactive substances that control the composition and maturation of newborn intestinal microbiota. Many studies have reported that breast milk protects infants as it contains IgA, lysozyme, lactoferrin, alpha-lactalbumin, complex lipids, free oligosaccharides, and other glycoconjugates (Gordon et al., 2012). Lactoferricin is a powerful antimicrobial agent, clearing up gastrointestinal infection in breast-fed infants, which therefore decreases infant death cases (Ballard and Morrow, 2013). In addition, breast milk contains about 109 bacterial cells/L and prebiotic oligosaccharides (fructans) that induce the multiplication of probiotics such as Bifidobacterium spp. and Lactobacillus spp. (Endesfelder et al., 2014). On the other hand, artificial milk stimulates the proliferation of gut enterobacteria and enterococci (Guaraldi and Salvatori, 2012). With the growth of infants, solid foods increase the microbiota diversity, and transform it to the adult-like state (Lazar et al., 2018).
Recent studies indicate that gut microbiota is not only an inert onlooker, but also dynamically affects different host functions, such as metabolism, nutritional response, circadian rhythmicity and immunity (Lynch and Hsiao, 2019). Gut microbiota controls several physiological processes of the host and, the host in turn, provides niche and nutrients for the survival of microbes (Stecher and Hardt, 2011). The gut microbiota digests and ferments carbohydrates, produces vitamins, develops gut-associated lymphoid tissues (GALTs), polarizes gut immune response and prevents pathogens’ colonization (Renz et al., 2012). In turn, gut immune response, which is provoked by commensals, controls the microbiota composition. Wherefore, a multifarious interaction between host immune system and microbiota is essential for intestinal homeostasis. Nevertheless, when this friendship between microbiota and host is disturbed, the gut microbiota can throw in illness (Honda and Littman, 2012). In other words, eubiosis is a case of an interspecies balance of microbiota community, while a disturbance of eubiosis, is known as dysbiosis, that could cause infectious and non-infectious diseases (Clemente et al., 2012). This review highlights the gut microbiota-immune system interaction in both eubiosis and dysbiosis.
2. Gut microbiota and immunity in eubiosis
2.1. Gut microbiota and innate immunity in eubiosis
Gut-associated lymphoid tissues (GALTs) consist of crypt patches, Peyer’s patches and isolated lymphoid follicles (ILFs). Antigen-presenting cells in GALTs can take up and present antigen and this could stimulate lymphocytes and lead to inflammation or tolerance (Bouskra et al., 2008). In the fetus, the development of Peyer’s patches is encouraged by lymphoid tissue inducer cell (LTi) in nonexistence of inhabiting bacteria. However, Peyer’s patches size in specific-pathogen-free mice are larger than those in germ-free mice (Amada et al., 2013). In contrast to Peyer’s patches, the maturation of the crypt patches and the ILFs needs gut microbiota stimulation (Pabst et al., 2006). The maturation of the immune system requires commensal microorganisms, therefore, it “learns” to differentiate between pathogenic bacteria and commensal microbes (that are analogous to quasi-self antigens and tolerated antigens) (Nakanishi et al., 2015, Thaiss et al., 2016).
Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), in the membrane of epithelial cells and lymphoid cells of the small intestine have been implicated in this differential recognition, and are responsible for the development of normal mucosal immune system of the intestine (Francino, 2014). In addition, TLRs inhibit the inflammatory response and promote immunological tolerance to normal microbial flora. TLRs identify various microbe-associated molecular patterns (MAMPs) including different bacterial antigens (e.g., lipopolysaccharides, capsular polysaccharides, muramic acid, peptidoglycan, unmethylated bacterial DNA CpG motifs, flagellin) and activate the innate intestinal immune response (Shanahan, 2005). After TLRs stimulation, a signaling cascade is triggered, resulting in the release of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), that activates genes encoding cytokines, acute phase proteins, chemokines, and other mediators of the humoral immune response (Thomas and Versalovic, 2010; Belkaid and Hand, 2014). TLR activity declines during the first weeks of life to permit the expansion of gut microbiota community in a steady state. Additionally, TLR stimulation by normal intestinal microbiota antigens signals inhibition of inflammatory reactions, that is important to maintain intestinal homeostasis (Rakoff-Nahoum et al., 2004).
The host discrimination between pathogenic and commensal bacteria is not understood yet. It is thought that the indigenous microflora sequestration by surface epithelium prevents TLR activation by commensals, while pathogenic bacteria harbour virulence factors that helps them to cross the epithelial barrier to be recognized by TLRs expressed on dendritic cells and macrophages (Pickard et al., 2017).
TLRs sense commensal microbiota and preserve tissue integrity (Kubinak et al., 2012). TLR5 expression shapes gut microbiota profile, mainly in the neonates (Mazmanian et al., 2008, Wen et al., 2008, Vijay-Kumar et al., 2010, Carvalho et al., 2012, Ubeda et al., 2012, Price et al., 2018). Capsular polysaccharide A (PSA) of commensal Bacteroides fragilis is a well-studied model of a single MAMP, up-holding gut eubiosis and host immune system learning (Lee et al., 2018, Ramakrishna et al., 2019). PSA can be identified via TLR2 and TLR1 heterodimers together with Dectin, which is C-type lectin PRR. Dectin-1 and TLR1/TLR2 signaling leads to activation of phosphoinositide 3-kinase (PI3K) pathway resulting in glycogen synthase kinase 3β (GSK3β) inactivation, that in turn promotes cAMP response element binding protein (CREB)-associated activation of anti-inflammatory genes. Furthermore, Dectin-1 controls gut immune reaction by alteration of microbiota pattern leading to regulatory T cell differentiation (Brown, 2006, Tang et al., 2015, Erturk-Hasdemir et al., 2019).
Other PRRs that shape gut microbiota symphony are nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). NOD-containing protein 1 (NOD1) senses both innate immune response and adaptive immunity in the lymphoid tissues to maintain gut homeostasis (Zheng et al., 2020). The bacterial sensor, NOD2 switches off gut inflammation by restricting the growth of commensal Bacteroides vulgates (Ramanan et al., 2014). NOD2 stimulation by normal gut microbiota potentiates gut epithelial renewal and epithelial stem cell survival (Nigro et al., 2014).
Some NLRs assemble into multi protein complexes named inflammasomes which are abundant in varied cells, and have pleiotrophic immune functions. Inflammasomes trigger inflammatory caspases that stimulate interleukin (IL)1β and IL18 maturation, and induce a lytic cell death named pyroptosis (Broz and Dixit, 2016). NOD-leucine-rich repeats (LRR) and NOD-pyrin domains-containing 6 (NLRP6) are proteins that assemble into inflammasomes in gut mucosa. NLRP6 inflammasome regulates microbiota composition and maintains gut homeostasis. Microbiota-derived metabolites modulate NLRP6 inflammasome signaling which subsequently regulates antimicrobial peptides (AMP) expression and IL18 release from epithelial cells. In addition, NLRP6 inflammasome regulates intestinal goblet cell mucus secretion, which support body defense from pathogens (Levy et al., 2015). NLRP6 controls gut antiviral innate immune response. Notably, the effect of NLRP6 on microbiota depends upon the existing microbiota composition. Dysbiosis occurs in mice deficient in NLRP6 alone, in the presence of microbiota having pathobionts such as Helicobacter spp. (Galvez et al., 2017). NLRP3 is another important example of NLR assembling inflammasome. Maintennace of intestinal homeostasis requires NRLP3 inflammasome signaling. In ulcerative colitis patients, an abundant anti-commensal IgG interacts with gut macrophages expressing FcγR, resulting in induction of NLRP3-and reactive oxygen species which in turn stimulates the pro-inflammatory cytokine IL1β production. Upon intestinal injury, Proteus mirabilis, a member of gut microbiota stimulates monocytes to induce NLRP3-dependent IL1β production, leading to gut inflammation (Castro-Dopico et al., 2019).
Myeloid differentiation primary response 88 (MyD88) is another innate immune receptor that identifies microbial signals, resulting in induction of effector molecules such as IL1 and IL18 (Janeway and Medzhitov, 2002). Deficiency of MyD88 in mice results in a distorted gut microbiota composition (Vaishnava et al., 2011a, Vaishnava et al., 2011b). The expression of numerous AMPs of epithelial cells is controlled by MyD88. One of these AMPs is RegIIIγ that limits some surface gram-positive bacteria and inactivates adaptive immune response. Also, MyD88 controls T cell differentiation, and stimulates IgA which maintains microbiota homeostasis and controls Th17 cells expansion via inhibition of segmented filamentous bacterial growth in mice (Wang et al., 2015).
One of innate immune cells is innate lymphoid cell (ILC) that has some functional characteristics of T cell (Walker et al., 2013, Sonnenberg and Artis, 2012). ILC originates from common lymphoid precursor cells and differentiates according to the particular transcriptional factor expression. ILCs are classified into three subsets: group 1 ILC (containing ILC1 and NK cell), group 2 ILC (containing ILC2) and group 3 ILC (containing ILC3 and lymphoid tissue-inducer (LTi) cell), depending on their ability to produce type 1, type 2 and TH17 cell-associated cytokines, respectively (Cella et al., 2009, Spits and Di Santo, 2011). The microbiota plays a role in the maturity and functioning of ILCs. Some studies reported the importance of the microbiota for ILC production of IL-2 (Satoh-Takayama et al., 2008, Sanos et al., 2009). However, another study reported that the microbiota can inhibit IL22 production by RORγt + ILCs (Sawa et al., 2011). IL22 induces the production of AMPs from gut epithelium (Zheng et al., 2008). IL22 stimulates, in particular, the expression of C-type lectin AMPs regenerating islet-derived protein IIIβ (REGIIIβ) and REGIIIγ, that control gut microbiota (Vaishnava et al., 2011a, Vaishnava et al., 2011b). Absence of either IL22-producing ILC or IL22 leads to potentially pathogenic bacterial overgrowth and spread, such as Alcaligenes xylosoxidans, predisposing to intestinal damage and systemic inflammation (Sonnenberg et al., 2012). Therefore, gut microbiota modulates gut-specific immune cells and subsequently mediates immune homeostasis, and regulates the equilibrium among useful and other commensals that has the ability to cause diseases, and resists intestinal pathology.
2.2. Gut microbiota and adaptive immune system in euobyosis
Currently it is well known that gut microbiota controls the expansion of specific lymphocyte subsets. T helper 17 (TH17) cell is a specific lineage of CD4+ TH cell. TH17 cell is essential for host defense and is a key mediator in the progression of autoimmune disease through the production of pro-inflammatory cytokines, IL17A, IL 17F and IL-22 (Littman and Rudensky, 2010). In contrast to other subsets of CD4+ TH cell, like TH1 and TH2 cells, TH17 cells accumulate in the gut, further indicating that the TH17 cell maturity is induced by gut-intrinsic mechanism. According to this theory, gut TH17 cells are significantly diminished in antibiotic-treated or germ-free mice (Atarashi et al., 2008). The production of TH17 cells in mice is promoted by Clostridia-related bacteria, which is a segmented filamentous bacteria (SFB) (Ivanov et al., 2008). The attachment of SFB to host epithelium stimulates serum amyloid A protein (SAA) expression, which, subsequently induces CD11 expressing lamina propria dendritic cells (DCs) to release IL 6 and IL 23 that thereafter induce differentiation of TH17 cells (Ivanov et al., 2009). Also, commensal microbiota, but not the pathogenic ones, provides luminal ATP which induces TH17 cell development by a mechanism different from that mediated by SFB (Gaboriau-Routhiau et al., 2009). Gut microbiota promotes IL1β production that is also crucial for TH17 cell differentiation (Shaw et al., 2012). It was found that reconstitution of germ-free mice with microbiota from conventionally raised mice saved the gut TH17 cells (Chung et al., 2012).
Forkhead box P3 (FOXP3)+ regulatory T cell (TReg) represents another CD4+ TH cell subset that accumulates in gut and mediates gut homeostasis. TReg cells diminution results in unusual expansion of CD4+ TH cells expressing commensal bacteria-specific T cell receptors (TCRs) and intestinal inflammation (Powrie et al., 1993). Notably, the gut TReg cells development is partially dependent upon gut microbiota, since T Reg cell number is severely reduced in the gut lamina propria of germ-free mice (Atarashi et al., 2011). TReg cells generation can be induced through particular species of commensal bacteria (Geuking et al., 2011). An increase of gut IL10-producing T Reg cells has been reported to be stimulated by colonization of germ-free mice with one of the following bacterial members: the human commensal bacterium Bacteroides fragilis, 46 Clostridium spp. mixtures, cluster IV and XIVa strains, or altered Schaedler flora (ASF) containing a cocktail of 8 distinct commensal bacteria (Round et al., 2011). One of the mechanisms that commensal bacteria can stimulate the development of TReg cells involves TGF-β activation in epithelial cells that, in turn, can stimulate the generation of TReg from TH cells (Amada et al., 2013).
A strong relationship is also present between gut microbiota and gut-specific B cell response. IgA is the most important immunoglobulin class produced in gut mucosa (Fagarasan et al., 2010). In gut lumen, IgA is secreted as polymeric IgA at high concentrations which is transfered via polymeric immunoglobulin receptor (pIgR) that is expressed on gut epithelium and is released into the lumen as secreted IgA (SIgA) (Strugnell and Wijburg, 2010). SIgA coats soluble antigen or commensal bacteria to restrain their attachment to host epithelium and their dissemination into the lamina propria. Therefore, SIgA mediates intestinal barrier action and upholds host–commensal mutualism (Fagarasan et al., 2010).
Furthermore, IgA is found to regulate gut microbiota composition and function (Macpherson et al., 2012). For example, change in the gut microbiota composition occurs due to IgA dysfunction or deficiency resulting from mutation or deficiency of activation-induced cytidine deaminase (AID), respectively (Fagarasan et al., 2002, Wei et al., 2011). Additionally, binding of commensal Bacteroides thetaiotaomicron to IgA suppresses innate immune response via influencing bacterial gene expression (Peterson et al., 2007). The gut microbiota also controls the number of gut IgA-producing cells and it was reported that IgA-forming cells were considerably reduced in the germ-free mice gut (Fagarasan et al., 2010). There are different mechanisms by which the microbiota can induce the development of IgA-producing cells. Follicular DCs (FDCs) can recognize bacteria through MYD88 which is essential for IgA production (Suzuki et al., 2010). In addition, commensal gut microbiota-derived flagellin stimulates retinoic acid synthesis, which essentially facilitates the IgA-producing B cells differentiation (Mora et al., 2006, Uematsu et al., 2008). Likewise, commensal gut microbiota induces expression of factors such as tumor necrosis factor (TNF), inducible nitric oxide synthase (iNOS; also called NOS2), B cell activating factor (BAFF; also called TNFSF13B) and a proliferation-inducing ligand (APRIL; also called TNFSF13) in lamina propria DCs, that are responsible for induction of IgA producing plasma cells (Tezuka et al., 2007, Tezuka et al., 2011). Additionally, plasma cells of the gut release TNF and iNOS following microbial stimulation, which further stimulates secretory IgA function of B cells (Fritz et al., 2011). Therefore, the microbiota supports lamina propria DCs and FDCs to induce IgA-producing B cells differentiation, and in line, IgA controls the gut microbiota composition and function to keep up symphony between the microbiota and host.
Regarding IgE, gut microbiota has been reported to induce immune regulatory signals to maintain basal levels of IgE and thereby reduce disease intensity in antigen-induced oral anaphylaxis model (Herbst et al., 2011). In germ-free mice, abnormal IgE serum accumulation has been observed (McCoy et al., 2006, Hill et al., 2012). It was hypothesized that sufficient microbial exposure during early life is required for proper induction of immune regulation (Cahenzli et al., 2013).
2.3. Host-made compartmentalization of gut microbiota
Gut mucosa is an exellent interface for microbiota-host interactions. An important feature of the gut immune system is establishment of immunological tolerance towards an array of harmless microbiota in parallel with elicitation of immune response against commensal or pathogenic microbes that invade the sterile body (Mowat, 2018). During normal conditions, the host immune reaction to gut microbiota is restricted to mucosal surface (Konrad et al., 2006). A single layer of epithelial cells segregates the underlying tissues from the intestinal lumen. Different mechanisms are engaged to maintain compartmentalization of microbiota. Gut epithelium is separated from inhabitant microbiota by a thick mucus layer (Belkaid and Naik, 2013). The mucus barrier has a hyper glycosylated mucin, MUC2. MUC2 protects through static shielding, and suppresses the antigenicity of gut microbiota via directing enteric DCs toward anti-inflammatory status (Shan et al, 2013). Tight junctions are other important structures that limit trans-epithelial permeability. Indole, a microbial metabolite signal stimulates strengthening of the epithelial barrier via up-regulation of tight junctions and related cytoskeletal proteins (Bansalet al., 2010). Additionally, SIgA and AMPs maintain the mucosal barrier functions (Peterson et al., 2007). Gut DCs play a vital role in gut microbiota compartmentalization, through sampling of gut microbiota for antigen presentation (Macpherson and Uhr, 2004).
3. Environment-gut microbiota interaction
Many environmental factors together with host genetics shape the gut microbiota. Antibiotics, diet, and westernized lifestyle are examples of the factors affecting microbiota and subsequently inducing inflammatory and autoimmune diseases. Understanding environmental gut microbiota alteration and how it can involve in some diseases is still unclear. The impact of antibiotics and diet on gut microbiota interaction will be discussed in this review.
3.1. Antibiotic-gut microbiota interaction
The use of antibiotics has considerably improved human health. On the other hand, studies have reported that antibiotic usage during childhood is accompanied with a variety of immune-mediated disorders such as inflammatory bowel disease (Yamamoto-Hanada et al., 2017). Antibiotics administration greatly influences the composition and function of gut microbiota, and possibly causes lifelong undesirable effects on the host (Becattiniet al., 2016). Antibiotics-associated gut microbial dysbiosis can affect immune cells. In rats, antibiotic intake suppresses gut mucosal mast cell induction and dietetic lipid absorbtion (Sato et al., 2016). Broad-spectrum antibiotics-associated gut microbial dysbiosis causes depletion of microbiota-derived short chain fatty acids (SCFAs) that leads to gut macrophages hyperactivation, proinflammatory T helper cell expansion and enhance capability to infections (Scott et al.,2018). Moreover, antibiotics allow gut fungi overgrowth, thus stimulating polarization of pulmonary M2 macrophages, which subsequently induces allergic pulmonary inflammation (Kim et al., 2014, Kim et al., 2018). It was found also that disruption of microbiota by antibiotics promotes microbial-specific Th1 cell response with histopathology in a CX3CR1 + MNP-dependent mechanism (Ohnmacht et al., 2015). Also, germ free or antibiotic-treated mice develop extensively reduced RORγt + Treg cells that potentiate Th2 type-associated immune response with severe inflammatory reaction upon helminth infection (Ohnmacht et al., 2015). Depletion of microbiota by broad-spectrum antibiotics in human with pre-existing immune system injury leads to a low antibody response to seasonal influenza vaccine, increased circulatory inflammatory profiles and distorted plasma metabolome profile (Hagan et al., 2019). However, the long-term effects of antibiotic-associated microbiota dysbiosis in humans need further observational researches and clinical trials.
3.2. Diet-gut microbiota interaction
Recent studies have reported association between dietetic microbiota alteration and host immune reaction. Western diets extremely affect gut microbiota composition and negatively influence host immunity (Christ et al., 2019). For instance, the secondary bile acid, taurocholic acid level, increases with intake of a high saturated fat diet, and consequetively promotes Bilophila wadsworthia growth. Bilophila wadsworthia, a pathobiont boosts Th1 type immunity and enhances the susceptibility towards colitis in mice (Devkota et al., 2012, Cheng et al., 2016). High-fat diet can disturb intestinal DCs homeostasis with butyrate and retinoic acid reduction, resulting in exacerbation of chemically induced colitis in mice (Haghikia et al., 2015, He et al., 2017). Dietetic long-chain fatty acids alter gut microbiome and metabolome resulting in worsening of central nervous system autoimmunity (Viennois et al., 2017, Rodriguez-Palacios et al., 2018). In mice, the gut microbiota composition can be changed by intake of dietary carbohydrates, artificial sweeteners, emulsifiers, certain probiotics with consequent immune modulation and inflammation. Serum level of the proinflammatory cytokine IL 6 was found to be reduced with higher fecal levels of Dialister and lower levels of Coriobacteriaceae, in individuals after short period of whole grain consumption (Martinez et al., 2013). In addition, microbiota composition and consecutively, immunity is affected by dietetic amount, type of diet and timing of food intake. Intermittent fasting promotes microbiota-induced balance of IL17 production and regulatory T cells that could decrease disease severity of autoimmune encephalomyelitis in mice and in patients with multiple sclerosis (Cignarella et al., 2018). In murine colitis model, fasting-simulating diet has been reported to exert a protective effect by alteration of gut microbiota including Lactobacillus excess (Rangan et al., 2019). On the contrary, alcohol-associated cancer colon is accelerated by mistimed dietary intake through reduction of butyrate and SCFA-forming microbiota, causing discrepancy in numbers of mucosal Th17/TReg cells (Bishehsari et al., 2020).
4. Microbiota-immunity interaction in dysbiosis
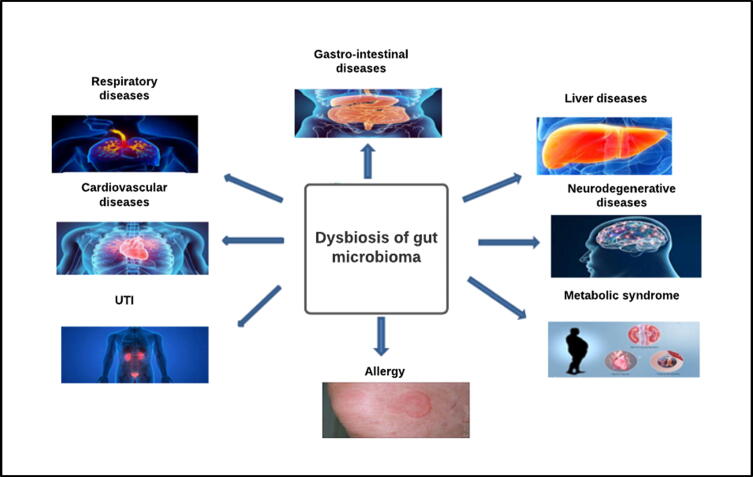
Microbiota-host abnormal immune interaction in genetically prone individuals may predispose to development of complex immunological diseases. Inflammatory bowel diseases, cardio metabolic diseases, systemic autoimmune diseases and cancer are the most widely studied examples of immune mediated disorders. In addition, other multi factorial disorders like neurodegenerative diseases are suggested to be modulated by microbiota-immunity linkage but necessitates additional human studies and remains to be confirmed (Fig. 3).
Fig. 3.
Microbiota-immunity interaction in dysbiosis: Gut dysbiosis can cause immune-mediated disorders in different systems.
4.1. Dysbiosis of gut microbiota and inflammatory bowel disease
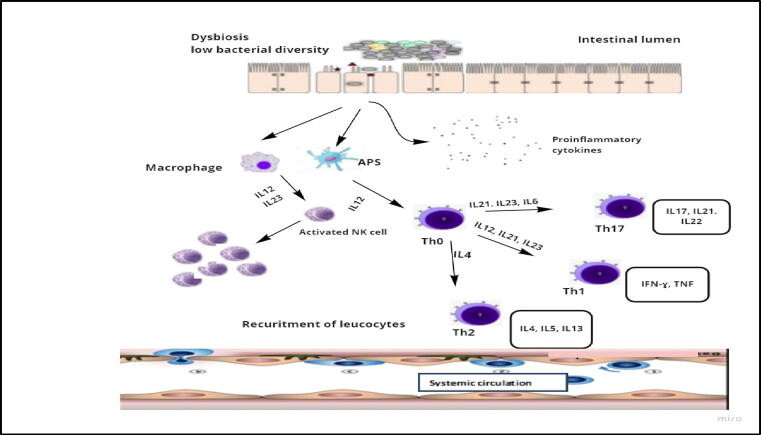
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis, is a chronic recurring gastrointestinal tract inflammation, with an incraesing worldwide prevalence (Kaplan,2015). Gut microbiota dysbiosis has been suggested to have fundamental roles in IBD pathogenesis. These comprise of low microbiota diversity with a distinct shift to distinct bacterial taxa, such as low levels of Lactobacillus, Bacteroides, Firmicutes, Ruminococcaceae, Clostridia and high levels of Gammaproteobacteria and Enterobacteriaceae (Gevers et al., 2014, Kostic et al., 2014), with distorted microbiota associated metabolite patterns (Franzosa et al., 2019, Lloyd-Price et al., 2019). Bacterial symbiont strains predominance in mucosal layer results from rupture of firmly regulated intestinal barrier, stimulating abnormal host immune response and tissue injury (Fig. 4) (de Souza and Fiocchi, 2016). Disruption of gut barrier integrity, including epithelial cell junction, mucus layers, and AMP secretion contributes to IBD pathogenesis (Martini et al., 2017). For example, deficiency of Muc2 in mice might develop spontaneous colitis (Van der Sluis et al., 2006). Early gut dysbiosis results in Muc2 mutation with subsequent mucus layer defects in colitis-susceptible mice (Liso et al., 2020).
Fig. 4.
Dysbiosis of gut microbiota and IBD: An increase in the intestinal epithelial cells permeability in IBD during gut dysbiosis induces the production of pro-inflammatory cytokines and activates T cells, macrophages and NK cells. Furthermore, during inflammation adhesion molecules are produced for leucocyte recruitment.
Genomic studies reported that over than 200 IBD susceptibility loci, most of them encode proteins associated with adaptive and innate immune sensing and response to microbial signal. Amongst them, NOD2 gene mutation was strongly coupled with susceptibility to CD (Hugot et al., 2001, Ogura et al., 2001). NOD2, an intracellular PRR, recognizes microbial peptidoglycan and regulates gut microbiota, through controlling the expression and secretion of AMPs. It can also suppress the growth of some proinflammatory microbial species as Bacteroides vulgates (Petnicki-Ocwieja et al., 2009). Induction of microbiota dysbiosis promotes CD-like inflammation in genetically susceptible germ free mice, thereby supporting the vital role of gut dysbiosis in IBD (Schaubeck et al., 2016). Microbiota transplanted from IBD patients to germ free mice promotes imbalance in intestinal Th17 and RORgt + regulatory T cells (Britton et al., 2019). More noticeably, a single pathobiont, Mucispirillum schaedleri, could stimulate Th1 cells resulting in murine gut inflammation with NOD2 deficiency (Caruso et al., 2019). Likewise, ectopic colonization of oral Klebsiella spp. obtained from IBD patients, promotes murine Th1-type gut inflammatory response (Atarashi et al., 2017). Moreover, microbiota of infant born to IBD-prone mother could transmit abnormal B cell and T cell adaptive immunity to germ free mice (Torres et al. 2020). However, more information about the molecular effects of microbiota and their products on IBD pathogenesis may enable development of future targeted intervention.
4.2. Dysbiosis of gut microbiota and immune-mediated liver diseases
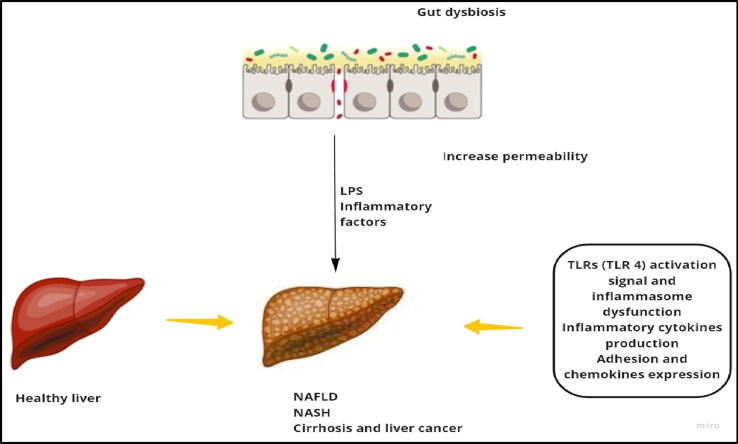
The liver is anatomically joined with the gastro-intestinal tract through portal venous circulation and bile duct system; therefore, it is exposed to gut microbiota and its products (gut-liver axis). Gut microbiota and its products repetitively translocate from gut lumen to liver, and that may affect the hepatic immune response. For instance, hepatic Kupffer cells (KCs) number, maturation and function, a vital element of hepatic innate immunity, are directly influenced by microbial associated molecular patterns (MAMPs) of gut microbiota (Corbitt et al., 2013). Immunological hepatic damage could be aggravated by gut pathogens because of activation of hepatic natural killer T cells (NKTs) and DCs (Chen et al., 2014). Likewise, it was reported that probiotics rich in glycolipid antigen activate hepatic NKT cells in a dose- and strain-dependent way (Liang et al., 2014). Microbial lipopolysaccharide (LPS) promotes TLR4 signaling which in turn stimulates hepatic stellate cell, a major hepatic fibrosis inducing cell line, resulting in expression of several chemokines and adhesion molecules (Fig. 5) (Paik et al., 2003).
Fig. 5.
Association between gut dysbiosis and liver diseases: In gut dysbiosis, bacteria can invade the liver and induce TLR4 by LPS which stimulates different immune responses (proinflammatory cytokines production, adhesion and chemokines expression, etc.) which leads to progression of different diseases (NAFLD).
Moreover, in primary sclerosing cholangitis (PSC), a chronic cholestatic and inflammatory hepatic disorder, it was reported that gut microbiota could affect hepatic inflammation through TLRs sensing of gut microbial products. Klebsiella pneumonia a gut pathobiont, derived from PSC patients was reported to rupture gut epithelial barrier, and stimulate bacterial translocation resulting in Th17 cell response in murine liver (Nakamoto et al., 2019). Recently, in PSC patients, bile microbiota alteration, with reduced biodiversity, abundant pathobiont Enterococcus faecalis, and higher level of toxic secondary bile acid taurolithocholic acid were reported (Liwinski et al., 2020). However, it is not known whether these changes were randomly implicated in PSC or a result of biliary disease (Yoshimoto et al., 2013). Recent studies also established that microbiota-derived small molecules have carcinogenic effects through secondary bile acids that stimulate hepatic NKT cell, deoxycholic acid that modulates inflammatory secretome, lipoteichoic acid that regulates prostaglandin E2 secretion, and TLR4 that signals LPS in liver malignancy (Dapito et al., 2012, Loo et al., 2017).
Again, it was reported that activation of TLRs can develop nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), via an extensively known pathway; LPS-TLR4 signaling (Kolodziejczyk et al., 2019). Together with TLRs, NLRP6 and NLRP3 inflammasomes may protect against NAFLD and NASH throughout gut microbiota alteration (Henao-Mejia, et al., 2012).
4.3. Dysbiosis of gut microbiota and immunometabolic disease
Metabolic disorders, such as diabetes mellitus, obesity and atherosclerosisis are a hallmarkof chronic low-grade inflammation. Immune cells-parenchymal cells cross talk has a decisive role in pathogenesis of metabolic disorders in metabolically highly active organs like the adipose tissue (Hotamisligil, 2017). An increasing evidence suggests that gut microbiota-derived metabolites can enter systemic circulation through gut barrier and stimulate metabolic inflammation (Tilg et al., 2020). It has been indicated that host immune system-gut microbiota interactions implicated in type I diabetes. For instance, germ free non-obese diabetic mice with MyD88 deficient signaling vigorously develop type I diabetes, although the disease could be attenuated by microbial colonization. Akkermansia muciniphila depletion translocates endotoxin-activated CCR2 + monocytes to systemic circulation. This in turn, triggers innate pancreatic B1a cells, followed by decreased insulin sensitivity (Bodogai et al., 2018). Additionally, the crosstalk between microbiota and immunity plays an important role in obesity. In obesity, microbiota-derived tryptophan metabolites induce white adipose tissue inflammation, via miR-181 family of micro RNAs (Virtue et al., 2019). Recently, high fat diet-induced murine obesity has been reported to be decreased by NLRP12 through SCFA derived from members of Lachnospiraceae family (Truax et al., 2018).
Atherosclerosis with its complications is the most dangerous common sequelae of cardio metabolic disorders. In humans, the metabolite trimethyl amine N-oxide (TMAO) derived from gut microbiota is associated with atherosclerotic heart disease (Koeth et al., 2019). Interestingly, atherosclerosis could be enhanced by TMAO through up regulation of macrophages scavenger receptors CD36 and SR-A1, by increasing cholesterol level in macrophages and foam cells development (Wang et al., 2011).
4.4. Dysbiosis of gut microbiota and rheumatoid arthritis
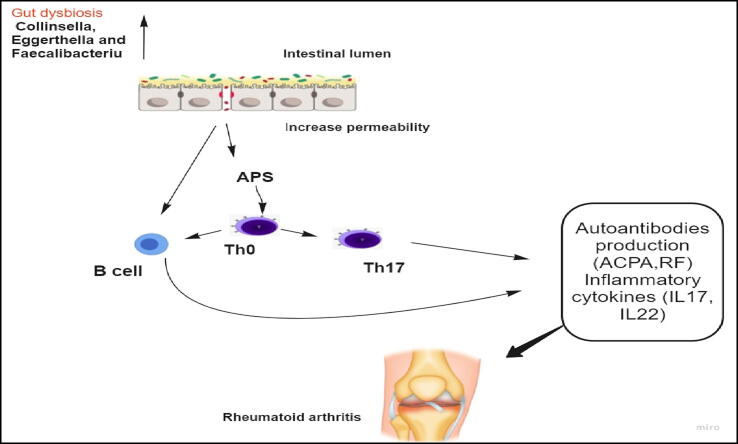
Rheumatoid arthritis (RA) is an autoimmune disease involving synovial joints, characterized by synovial inflammation with concomitant damage of bone cartilage. Its pathogenesis is still ambiguous. Genetic factors (e.g.HLADRB1), environmental factors, and microbiome dysbiosis have been concerned with its pathogenesis (Scher et al., 2013). A plethora of Prevotella copri has been implicated in naive new-onset RA treatment (Maeda et al., 2016) and at in patients with high risk for RA (Alpizar-Rodriguez et al., 2019). Three uncommon genera (Collinsella, Eggerthella and Faecalibacterium) were reported to be strongly linked with RA, amongst which Collinsella induces proinflammatory IL-17A production (Fig. 6) (Chen et al., 2016).
Fig. 6.
Gut dysbiosis can stimulate Rheumatoid arthritis: Gut dysbiosis can induce the production of autoantibodies and inflammatory cytokines that mediate RA.
In a Chinese group, RA patients showed gut, salivary and dental Lactobacillus salivarius overgrowth and low levels of Haemophilus spp. (Zhang et al., 2015). SCFAs formed by gut microbiota interact with multiple immune pathways concerned with RA (Wang and Xu, 2019). Microbial ligands stimulate TLR2 and TLR4 and T cell-induced autoimmune arthritis spontaneously develop in IL1rn–/– mice (Abdollahi-Roodsaz et al., 2008). Moreover, dysbiotic microbiota stimulates gut IL17 production in IL1rn–/– mice (Rogier et al., 2017). Furthermore, colonization of genetically susceptible mice with dysbiotic microbiota from RA patients induces a profound Th17 response. In the same way, germ free mice colonization with SFB is enough to provokeTh17 activation and autoimmune arthritis (Wu et al., 2010). Besides gut microbiota, Porphyromonas gingivalis a periodontal pathobiont, stimulates TLR2- and IL-1-induced Th17 response and thus exacerbates autoimmune arthritis (de Aquino et al., 2014).
4.5. Dysbiosis of gut microbiota and cancer
Gut microbiota-immune system interaction is assumed to affect cancer immune surveillance. The existence of Fusobacterium nucleatum in colon cancer micro environment directly inhibits killing of tumors by NK cell. This is partially due to binding of bacterial Fap2 protein to human TIGIT (T cell immune receptor with Ig and ITIM domains) receptor (Gur et al., 2015). Furthermore, abundance of F. nucleatum in human colorectal carcinoma micro environment could decrease CD3+ T cells, which are associated with a better clinical prognosis (Mima et al., 2015).
In primary or metastatic liver tumors, gut commensal Clostridium species consumes bile acids as messenger to boost the antitumor response of hepatic CXCR6+ NKT cell (Ma et al., 2018). Anticancer immunotherapeutic response is reported to be modulated by microbiota. For example, large numbers of commensals Enterococcus faecium, Bifidobacterium longum and Collinsella aerofaciens fuel T cell-promoted responses to anti-PD-1 therapy in either preclinical model or cases have metastasized melanoma (Gopalakrishnan et al., 2018, Matson et al., 2018, Sivan et al., 2015). Another study revealed an association between excess of fecal Akkermansia muciniphila and PD-1 blockade effectiveness in epithelial tumors patients, due to CCR9+CXCR3+CD4+ T lymphocyte influx and IL-12 expression (Routy et al., 2018). Immune response to other anticancer therapy, such as CTLA-4 blockade (Vetizou et al., 2015) or cyclophosphamide (Viaud et al., 2013) was also associated with different gut microbiota patterns. Interestingly, unfolding the gut microbiota role regarding anticancer immune surveillance and immunotherapy may have promise in enhancement of treatment response in cancer patients, and needs to be extensively studied (Zitvogel et al., 2016).
Besides gut microbiota, a recent study discovered that cancer immunity could be regulated by intra-tumor tissue microbiome. For example, intra-pancreatic adenocarcinoma microbiota in mice and humans stimulates carcinogenesis by inducing a tolerogenic immune pathway, including suppression of monocyte differentiation through selective TLRs and T cell anergy (inactive Tcell) (Pushalkar et al., 2017). Additionally, the existence of Gammaproteobacteria in colon cancer of mice or human pancreatic adenocarcinoma results in resistance to anti-cancer gemcitabine (Geller et al., 2017). Interestingly, in long-term pancreatic adenocarcinoma surviving patients, intra-tumor microbiota shows high microbial heterogeneity, that can stimulate robust immune infiltration and antitumor immune response (Riquelme et al., 2019). These studies highlight the therapeutic role of intratumor tissue microbiota, calling for further mechanistic studies.
4.6. Dysbiosis of gut microbiota and neurological disorders
Balanced neuroimmunity and healthy brain development depends on combination of various internal and environmental factors. Amongst these, molecular signals emanating from gut microbiota may modulate brain cell function (Sharon et al., 2016). Microglia which is a primary innate immune neurological cell, is responsible for brain immune defense and maintains brain development and homeostasis (Butovsky and Weiner, 2018). The microbiota affects microglia homeostasis, via signaling through SCFAs (Erny et al., 2015). Germ free mice were found to have impaired microglial structure and function with signs of impaired CNS innate immune responses (Matcovitch-Natan et al., 2016). Notably, microglial developmental through prenatal stages is affected by maternal microbiota, and absence of microbiota results in microglial defect that is displayed in a sex-dimorphic manner (Thion et al., 2018). Microglial dysfunction resulting from microbial dysbiosis was reported in some neurological disorders, such as neurodegenerative, behavioral and inflammatory disorders (Abdel-Haq et al., 2019). However, microbiota-microglia interaction in pathogenesis of neurological disorders warrants further studies.
Additionally, diet rich in SCFAs have been reported to stimulate TReg cells to down-regulate CNS autoimmune disorders. Moreover, gut microbiota dysbiosis can stimulate meningeal IL-17+ γδ T cells, which have a role in the pathogenesis of ischemic brain injury (Benakis et al., 2016). In spite of recent advances, the study of microbiota-neuroimmunity interaction in eubiosis and dysbiosis is still immature. Some studies have elucidated possible mechanisms driving gut-brain axis in relation to neuroimmunity. For example, antibiotic-mediated gut microbiota depletion could ameliorate murine autoimmune encephalomyelitis, possibly mediated by IL-10-producing Treg cells (Ochoa-Repáraz et al., 2009). Pregnant female mice offsprings have higher risk to develop neurodevelopmental disorders when the resident gut microbiota has the tendency to induce T helper 17 immune response (Kim et al., 2017). On the other hand, IL-17a-mediated inflammatory response was found to improve the social behavior of immune activated pregnant mice offsprings (Reed et al., 2020). Microbiota association in these immune mechanisms requires further prospective studies. Further research in this area may hold a therapeutic promise in unravelling new regulatory mechanisms regarding many degenerative, inflammatory and developmental neurological disorders.
4.7. Dysbiosis of gut microbiota and urinary tract infections
Resembling the gut-brain axis, a bidirectional connection is also present between gut and kidney (Mestrovic et al., 2021). With evidence of gut–kidney axis, gut microbiota dysbiosis has a role in pathogenesis of different renal disorders, such as hypertension and chronic kidney disease and urinary stones (Yang et al., 2018). In addition, a direct association between gut microbiome and urinary tract is obvious in urinary tract infection (UTI) (Lee and Stern, 2019).
Characteristically, the pathogenesis of UTI begins with periurethral space contamination by gut-resident uropathogens, subsequent colonization to the urethra and ascending immigration to the urinary bladder (Flores-Mireles et al., 2015). UTI is most frequently due to uropathogenic Escherichia coli (UPEC), which is the causative agent for more than 80% of community-acquired UTIs. Abundance of UPEC strains in guts of UTI patients indicate their gut origin (Nielsen et al., 2014). UTIs are prevalent in women due to closeness of the female urethra to the anus and it reduced length compared to the male urethra and, facilitating the immigration of gut microorganisms to urinary tract and subsequent colonization (Foxman, 2010).
Abundance of Enterobacter in gut microbial flora of pedriatic UTI patients compared with healthy controls, suggested that gut microbiota is associated with risk of children to UTI (Paalanne et al., 2018). Recently, Magruder et al. (2019) demonstrated gut microbiota–UTI axis. They explained that preponderance of E. coli in the gut was linked with high occurence of E. coli-induced bacteriuria and UTI. Furthermore, in one subject, gut E. coli strains showed major similarity to the urinary E. coli strain, corroborating the assumption that both urinary tract colonization and UTI are related to gut microbiota. Moreover, it was demonstrated that higher abundance of bacterial taxa Faecalibacterium and Romboutsia with relatively scarce flora of Enterobacteriaceae could correspond to the decreased risk for Enterobacteriaceae related bacteriuria and UTI in kidney transplant recipients (Magruder et al., 2020).
4.8. Dysbiosisn of gut microbiota and autoimmune skin disorders
Microbiota is a significant factor involved in many skin disorder related diseases. Surprisingly, not only the alteration of skin microbiota influences skin condition, but change in gut microbiota is also associated with some skin diseases. In addition, regarding the gut–skin relationship in humans, some studies indicate that variation in the skin microbiota can modulate the gut microbiota, and likewise, change in skin physiology could induce change in gut microbiota. Therefore, skin microbiota could control that of the gut and vice-versa (Loś-Rycharska et al., 2021).
Autoimmune skin disorders are increasingly related to dysbiosis of gut microbiota and its metabolites (Ni et al., 2020). It was found that vitiligo, an autoimmune skin disorder, was associated with a particular skin microbiota distribution (Ganju et al., 2016). Skin microbiota is found to be highly variable and controlled by multiple factors such as skin sites and different microenvironments. On the other hand, gut microbiota composition tends to be stable since early childhood, although it could alter with high specificity in autoimmunity (Ni et al., 2020). Furthermore, ampicillin-induced depigmentation was reported to be associated with gut dysbiosis rather than skin dysbiosis (Dellacecca et al., 2020). A case-control study, done using 16S rRNA sequencing, found significantly decreased Bacteroidetes: Firmicutes ratio in vitiligo cases compared to matched healthy controls. The study also found that Corynebacterium, Ruminococcus, Jeotgalibaca and Psychrobacter with elevated serum IL-1β levels correlated significantly with disease duration in vitiligo patients (Ni et al., 2020).
4.9. Dysbiosis of gut microbiota and cardiovascular disorders
Atherosclerosis is an inflammatory illness with an autoimmune backdrop (Hansson and Jonasson, 2009). Infection is a major contributor to inflammation with a risk of atherosclerosis. Many microbes like Helicobacter pylori, Chlamydophila pneumoniae, Porphyromonas gingivalis, Hepatitis C virus, Influenza A virus, Cytomegalovirus, and human immunodeficiency virus are found to be linked to an increased threat of cardiovascular diseases (Rosenfeld and Campbell, 2011). Infection leads to atherosclerosis through two main mechanisms. The first mechanism occurs through direct infection of walls of the blood vessels with subsequent plaque formation. The second indirect mechanism is an immune mediated reaction that occurs through production of proinflammatory mediators as a result of a distant site infection, enhancing plaque growth (Jonsson and Bäckhed, 2017). Furthermore, gut dysbiosis also leads to formation of atherosclerotic metabolites in gut such as trimethylamine N-oxide (TMAO) that can also alter metabolism of bile acids (Bu and Wang, 2018).
Lactobacillales, Eubacterium, Anaeroglobus, Clostridium, and Roseburia genera are principally found in the gut cavity and have been also detected in atherosclerotic plaques (Koren et al., 2011). Apparently, gut microbiota, particularly Clostridium, Bacteroides and Lactobacillales have been considered as diagnostic markers in patients with coronary artery diseases (Emoto et al., 2017).
Microorganisms stimulate production of inflammatory cytokines and acute-phase reactants resulting in atherosclerosis by amplifying chronic inflammation inside the atheromatous plaques (Rosenfeld and Campbell, 2011). Another likely mechanism for augmented inflammation is molecular mimicry or cross-reactivity between self-antigens and bacterial antigens like heat-shock proteins and oxidized low-density lipoproteins (Lamb et al., 2003). Human heat-shock protein 60 (HSP60) is expressed on the surface of arterial endothelial cells in response to stress such as hypercholesterolemia or acute hypertension. In addition, bacterial heat-shock protein 60 is the main bacterial antigenic element during infection. As a result of high degree of sequence homology between bacterial and human HSPs, antibodies produced against the bacterial HSPs could cross-react and target host cells expressing human HSP60 (EL-Ageery et al., 2020).
Alteration of gut microbiota specially, Gram-negative bacteria, results in escalating LPS levels and saturated fatty acids that induce inflammation. This promotes bacterial and endotoxins translocation from the lumen of intestinal to the blood stream as a consequence of an intestinal permeability increase, with consequent activation of TLR4 expressed in most cardiovascular cells (Rocha et al., 2016). Decreased proinflammatory cytokines, plaque lipids and aortic atherosclerosis were reported in TLR4-deficient animal model (Michelsen et al., 2004). Over expression of TLR1, TLR2 and TLR4 in human atherosclerotic plaques, is suggestive of a probable involvement in pathogenesis (Edfeldt et al., 2002).
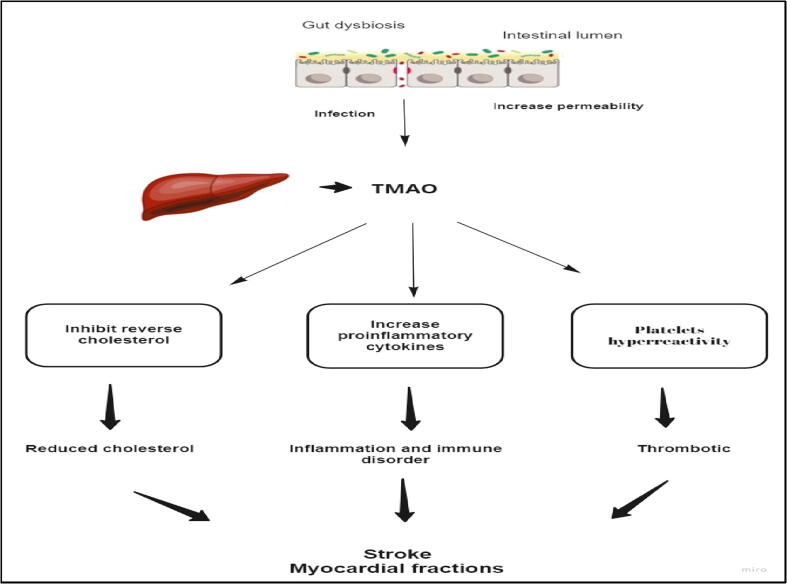
TMAO is one of the gut microbiota metabolites. Different animal-based foodstuffs and energy drinks contain phosphatidylcholine, choline and carnitine, which are subsequently metabolized by gut microbiota to trimethylamine (TMA), that is followed by oxidation by hepatic flavin monooxidases 3 to TMAO (Tang et al., 2013). TMAO causes atherogenesis by several mechanisms. TMAO prevents reverse cholesterol transport resulting in decreased cholesterol elimination from peripheral macrophages; TMAO inhibits high-density lipoprotein-mediated atheroprotective effect hence supporting atherosclerosis (Zhu et al., 2016). In addition, TMAO enhances stimulus-dependent release of Ca2+ from intracellular Ca2+ stores causing increased platelet hyperresponsiveness and hence escalated thrombotic risk (Fig. 7) (Koeth et al., 2013). TMAO was found to promote activation of proinflammatory proteins such as interleukin-6, cyclooxygenase-2, intercellular adhesion molecule-1 and E-cadherin – through the NF-κB signaling pathway in vascular tissues (Seldin et al., 2016). It was found that elevated TMAO level was associated with a higher risk of the major cardiovascular catastrophes such as stroke and myocardial infarction in a 3-year follow-up study of about 4000 patients (Tang et al., 2013). So, the effect of gut microbiota on cardiovascular system is considered as a novel locus that needs to be further explored in animal and human subjects.
Fig. 7.
Gut microbiota and cardiovascular diseases. TMAO causes atherogenesis by several mechanisms such as platelets hyperreactivity, inflammatory cytokines and reverse cholesterol inhibition.
4.10. Dysbiosis of gut microbiota and lung diseases
Previous epidemiological studies have reported that infants who were delivered via caesarean section or whose mothers were frequently given antibiotics prior to birth, had altered gut commensals, coupled with enhanced risk for developing pneumonia. This resulted in a presumption that exposure to commensal bacteria during early life may confer resistance to pneumonia in newborns (Azad et al., 2016). Furthermore, it was reported that alteration of gut microbiota in infancy is related with enhanced vulnerability to inflammatory disorders like allergen-induced airway hyper-reactivity in later life (Russell et al., 2012). Moreover, disruption of postnatal gut microbiota or selective depletion of dendritic cells interrupted the migratory program of lung IL-22+ ILC3 (interleukin 22 producing group 3 innate lymphoid cells) and increased the susceptibility of newborn mice to pneumonia, which was retrograded by adoptive transfer of commensal bacteria soon after birth (Gray et al., 2017).
4.11. Perspectives
Since the last century, gut microbiome has been extensively investigated. Recent findings have explored enormous role of this microbial flora in health status and diverse disease status. Though the gut microbiome is intricate, there is an extremely accurate equilibrium in this population. Any disturbance in this equilibrium results in dysbiosis and, therefore, resistance to pathogen colonization decreases, with preferential growth of pathobionts and pathological immunological response. Though the involvement of dysbiosis in disease pathogenesis is conspicuous, it is still mostly indefinite. Apparently, the microbiota shaping is affected by both genetic and environmental factors. However, the association of these two factors and the mechanism of interaction that results in dysbiosis remains an active area of exploration.
Other questions needed to be additionally determined are whether dysbiosis is specific to particular disease and whether occurrence of dysbiosis throughout the lifetime of host is significant for disease pathogenesis, especially as colonization of microbiota in the early phase of life is important for optimal maturity and functioning of the immune system. Given the significance of gut microbiota and dysbiosis in disease pathogenesis, targeting of microbiota is an important therapeutic goal. Faecal microbiota transplantation (FMT) can successfully treat Clostridium difficile infection, and this signifies the importance of dysbiosis treatment to reduce susceptibility to other diseases, including IBD. In fact, a deeper understanding of the host–microbiota interaction is required to avoid or treat intestinal as well as extra-intestinal disorders.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014;6:237. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Haq R., Schlachetzki J.C.M., Glass C.K., Mazmanian S.K. Microbiomemicroglia connections via the gut-brain axis. J. Exp. Med. 2019;216:41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahi-Roodsaz S., Abdollahi-Roodsaz S., Joosten L.A., Koenders M.I., Devesa I., Roelofs M.F., Radstake T.R., Heuvelmans-Jacobs M., Akira S., Nicklin M.J., Ribeiro-Dias F., van den Berg W.B. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpizar-Rodriguez D., Lesker T.R., Gronow A., Gilbert B., Raemy E., Lamacchia C., Gabay C., Finckh A., Strowig T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019;78(5):590–593. doi: 10.1136/annrheumdis-2018-214514. [DOI] [PubMed] [Google Scholar]
- Amada N., Seo S., Grace Y., Chen G., Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. May. 2013;13(5):321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Ardissone A.N., de la Cruz D.M., Davis-Richardson A.G., Rechcigl K.T., Li N., Drew J.C., Murgas-Torrazza R., Sharma R., Hudak M.L., Triplett E.W., Neu J., Weitkamp J.-H. Meconium microbiome analysis identifies bacteria correlatedwith premature birth. PLoS ONE. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., Kiguchi Y., Yasuma K., Watanabe E., Tanoue T., Thaiss C.A., Sato M., Toyooka K., Said H.S., Yamagami H., Rice S.A., Gevers D., Johnson R.C., Segre J.A., Chen K., Kolls J.K., Elinav E., Morita H., Xavier R.J., Hattori M., Honda K. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358(6361):359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.B., Konya T., Persaud R.R., Guttman D.S., Chari R.S., Field C.J., Sears M.R., Mandhane P.J., Turvey S.E., Subbarao P., Becker A.B., Scott J.A., Kozyrskyj A.L. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. CHILD Study Investigators. 2016;123(6):983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- Ballard O., Morrow A.L. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini S., Taur Y., Pamer E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016;22(6):458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013;14(7):646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y., Hand T. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C., Brea D., Caballero S., Faraco G., Moore J., J., Commensal microbiota affects ischemic stroke outcome by regulating intestinal gamma delta T cells. Nat. Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehsari F., Engen P.A., Voigt R.M., Swanson G., Shaikh M., Wilber S., Naqib A., Green S.J., Shetuni B., Forsyth C.B., Saadalla A., Osman A., Hamaker B.R., Keshavarzian A., Khazaie K. Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2020;9(2):219–237. doi: 10.1016/j.jcmgh.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodogai M., O’Connell J., Kim K., Kim Y., Moritoh K., Chen C., Gusev F., Vaughan K., Shulzhenko N., Mattison J.A., Lee-Chang C., Chen W., Carlson O., Becker K.G., Gurung M., Morgun A., White J., Meade T., Perdue K., Mack M., Ferrucci L., Trinchieri G., de Cabo R., Rogaev E., Egan J., Wu J., Biragyn A. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 2018;10(467) doi: 10.1126/scitranslmed.aat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D., Brézillon C., Bérard M., Werts C., Varona R., Boneca I.G., Eberl Gérard. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Britton G.J., Contijoch E.J., Mogno I., Vennaro O.H., Llewellyn S.R., Ng R., Li Z., Mortha A., Merad M., Das A., Gevers D., McGovern D.P.B., Singh N., Braun J., Jacobs J.P., Clemente J.C., Grinspan A., Sands B.E., Colombel J.-F., Dubinsky M.C., Faith J.J. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and ROR γt+ regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50(1):212–224.e4. doi: 10.1016/j.immuni.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Broz P., Dixit V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- Bu J., Wang Z. Cross-talk between gut microbiota and heart via the routes of metabolite and immunity. Gastroenterol Res Pract. 2018;2018:1–8. doi: 10.1155/2018/6458094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Weiner H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018;19(10):622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J., Köller Y., Wyss M., Geuking M.B., Kathy D., McCoy K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14(5):559–570. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R., Mathes T., Martens E.C., Kamada N., Nusrat A., Inohara N., Núñez G. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 2019;4:eaaw4341. doi: 10.1126/sciimmunol.aaw4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho F.A., Koren O., Goodrich J.K., Johansson M.E.V., Nalbantoglu I., Aitken J.D., Su Y., Chassaing B., Walters W.A., González A., Clemente J.C., Cullender T.C., Barnich N., Darfeuille-Michaud A., Vijay-Kumar M., Knight R., Ley R.E., Gewirtz A.T. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12(2):139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Dopico T., Dennison T.W., Ferdinand J.R., Mathews R.J., Fleming A., Clift D., Stewart B.J., Jing C., Strongili K., Labzin L.I., Monk E.J.M., Saeb-Parsy K., Bryant C.E., Clare S., Parkes M., Clatworthy M.R. Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity. 2019;50(4):1099–1114.e10. doi: 10.1016/j.immuni.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K.M., Doherty J.M., Mills J.C., Colonna M. A human natural killer cell subset provides an innate source of IL–22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Alpizar-Rodriguez D., Lesker T.R., Gronow A., Gilbert B., Raemy E., Lamacchia C., Gabay C., Finckh A., Strowig T. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8:43. doi: 10.1186/s13073-016-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wei Y., He J., Cui G., Zhu Y., Lu C., Ding Y., Xue R., Bai L., Uede T., Li L., Diao H. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota. Sci. Rep. 2014;4:7259. doi: 10.1038/srep07259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Jin H., Qiang Y., Wu S., Yan C., Han M., Xiao T., Yan N., An H., Zhou X., Shao Q., Xia S. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016;40:1–10. doi: 10.1016/j.intimp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Christ A., Lauterbach M., Latz E. Western diet and the immune system: An inflammatory connection. Immunity. 2019;51(5):794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- Chung H., Pamp Sünje.J., Hill J.A., Surana N.K., Edelman S.M., Troy E.B., Reading N.C., Villablanca E.J., Wang S., Mora J.R., Umesaki Y., Mathis D., Benoist C., Relman D.A., Kasper D.L. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella F., Cantoni C., Ghezzi L., Salter A., Dorsett Y., Chen L., Phillips D., Weinstock G.M., Fontana L., Cross A.H., Zhou Y., Piccio L. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27(6):1222–1235.e6. doi: 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbitt N., Kimura S., Isse K., Specht S., Chedwick L., Rosborough B.R., Lunz J.G., Murase N., Yokota S., Demetris A.J. Lunz JG, Murase N, Yokota S, Demetris AJ. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation reperfusion injury after orthotopic liver transplantation. Am. J. Pathol. 2013;182(1):180–191. doi: 10.1016/j.ajpath.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapito D.H., Dapito D.H., Mencin A., Gwak G.Y., Pradere J.P., Jang M.K., Mederacke I., Caviglia J.M., Khiabanian H., Adeyemi A., Bataller R., Lefkowitch J.H., Bower M., Friedman R., Sartor R.B., Rabadan R., Schwabe R.F. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino S.G., Abdollahi-Roodsaz S., Koenders M.I., van de Loo F.A.J., Pruijn G.J.M., Marijnissen R.J., Walgreen B., Helsen M.M., van den Bersselaar L.A., de Molon R.S., Campos M.J.A., Cunha F.Q., Cirelli J.A., van den Berg W.B. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol. 2014;192(9):4103–4111. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- de Souza H.S.P., Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- Dellacecca E.R., Cosgrove C., Mukhatayev Z., Akhtar S., Engelhard V.H., Rademaker A.W., Knight K.L., Le Poole I.C. Antibiotics drive microbial imbalance and vitiligo development in mice. J. Invest. Dermatol. 2020;140(3):676–687.e6. doi: 10.1016/j.jid.2019.08.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edfeldt K., Swedenborg J., Hansson Göran.K., Yan Z.-qun. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105(10):1158–1161. [PubMed] [Google Scholar]
- EL-Ageery, S.M., Gouda, N S., Fawzy, I.M., Bahy-Eldeen, A., Mahmoud, R., Serological evidence of association between Helicobacter pylori infection and coronary artery disease. Afr J Clin Exper Microbiol. 2020;21:88–96. [Google Scholar]
- Emoto T., Yamashita T., Kobayashi T., Sasaki N., Hirota Y., Hayashi T., So A., Kasahara K., Yodoi K., Matsumoto T., Mizoguchi T., Ogawa W., Hirata K.-ichi. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32(1):39–46. doi: 10.1007/s00380-016-0841-y. [DOI] [PubMed] [Google Scholar]
- Endesfelder D., zu Castell W., Ardissone A., Davis-Richardson A.G., Achenbach P., Hagen M., Pflueger M., Gano K.A., Fagen J.R., Drew J.C., Brown C.T., Kolaczkowski B., Atkinson M., Schatz D., Bonifacio E., Triplett E.W., Ziegler A.-G. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. 2014;63(6):2006–2014. doi: 10.2337/db13-1676. [DOI] [PubMed] [Google Scholar]
- Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher Bärbel, Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk-Hasdemir D., Oh S.F., Okan N.A., Stefanetti G., Gazzaniga F.S., Seeberger P.H., Plevy S.E., Kasper D.L. Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. USA. 2019;116(52):26157–26166. doi: 10.1073/pnas.1915978116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S., Kawamoto S., Kanagawa O., Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28(1):243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Muramatsu M., Suzuki K., Nagaoka H., Hiai H., Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Francino M. Early development of the gut microbiota and immune health. Pathogens. 2014;3(3):769–790. doi: 10.3390/pathogens3030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., Vatanen T., Hall A.B., Mallick H., McIver L.J., Sauk J.S., Wilson R.G., Stevens B.W., Scott J.M., Pierce K., Deik A.A., Bullock K., Imhann F., Porter J.A., Zhernakova A., Fu J., Weersma R.K., Wijmenga C., Clish C.B., Vlamakis H., Huttenhower C., Xavier R.J. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019;4(2):293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz Jörg.H., Rojas O.L., Simard N., McCarthy D.D., Hapfelmeier S., Rubino S., Robertson S.J., Larijani M., Gosselin J., Ivanov I.I., Martin A., Casellas R., Philpott D.J., Girardin S.E., McCoy K.D., Macpherson A.J., Paige C.J., Gommerman J.L. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481(7380):199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., Eberl Gérard, Snel J., Kelly D., Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Gálvez E.J.C., Iljazovic A., Gronow A., Flavell R., Strowig T. Shaping of intestinal microbiota in Nlrp6- and Rag2-deficient mice depends on community structure. Cell Rep. 2017;21(13):3914–3926. doi: 10.1016/j.celrep.2017.12.027. [DOI] [PubMed] [Google Scholar]
- Ganju P., Nagpal S., Mohammed MH, Nishal Kumar P., Pandey R., Natarajan V.T., Mande S.S., Gokhale R.S. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016;6(1) doi: 10.1038/srep18761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller L.T., Lia I., Vincent R., Coker C., Castro S., Treuting P.M., Hinchliffe T.E., Arpaia N., Danino T. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking M.B., Cahenzli J., Lawson M.A.E., Ng D.C.K., Slack E., Hapfelmeier S., McCoy K.D., Macpherson A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M., Morgan X.C., Kostic A.D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier R.J. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., Cogdill A.P., Zhao L., Hudgens C.W., Hutchinson D.S., Manzo T., Petaccia de Macedo M., Cotechini T., Kumar T., Chen W.S., Reddy S.M., Szczepaniak Sloane R., Galloway-Pena J., Jiang H., Chen P.L., Shpall E.J., Rezvani K., Alousi A.M., Chemaly R.F., Shelburne S., Vence L.M., Okhuysen P.C., Jensen V.B., Swennes A.G., McAllister F., Marcelo Riquelme Sanchez E., Zhang Y., Le Chatelier E., Zitvogel L., Pons N., Austin-Breneman J.L., Haydu L.E., Burton E.M., Gardner J.M., Sirmans E., Hu J., Lazar A.J., Tsujikawa T., Diab A., Tawbi H., Glitza I.C., Hwu W.J., Patel S.P., Woodman S.E., Amaria R.N., Davies M.A., Gershenwald J.E., Hwu P., Lee J.E., Zhang J., Coussens L.M., Cooper Z.A., Futreal P.A., Daniel C.R., Ajami N.J., Petrosino J.F., Tetzlaff M.T., Sharma P., Allison J.P., Jenq R.R., Wargo J.A. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.I., Dewey K.G., Mills D.A., Medzhitov R.M. The human gut microbiota and undernutrition. Sci. Transl. Med. 2012;6(4):137. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- Gray J., Oehrle K., Worthen G., Alenghat T., Whitsett J., Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. 2017;9:eaaf9412. doi: 10.1126/scitranslmed.aaf9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi F., Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell. Infect. Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur C., Ibrahim Y., Isaacson B., Yamin R., Abed J., Gamliel M., Enk J., Bar-On Y., Stanietsky-Kaynan N., Coppenhagen-Glazer S., Shussman N., Almogy G., Cuapio A., Hofer E., Mevorach D., Tabib A., Ortenberg R., Markel G., Miklić K., Jonjic S., Brennan C.A., Garrett W.S., Bachrach G., Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan T., Cortese M., Rouphael N., Boudreau C., Linde C., Maddur M.S., Das J., Wang H., Guthmiller J., Zheng N.-Y., Huang M., Uphadhyay A.A., Gardinassi L., Petitdemange C., McCullough M.P., Johnson S.J., Gill K., Cervasi B., Zou J., Bretin A., Hahn M., Gewirtz A.T., Bosinger S.E., Wilson P.C., Li S., Alter G., Khurana S., Golding H., Pulendran B. Cervasi Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313–1328.e13. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia A., Jörg S., Duscha A., Berg J., Manzel A., Waschbisch A., Hammer A., Lee D.-H., May C., Wilck N., Balogh A., Ostermann A.I., Schebb N.H., Akkad D.A., Grohme D.A., Kleinewietfeld M., Kempa S., Thöne J., Demir S., Müller D.N., Gold R., Linker R.A. Demir. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Hansson Göran.K., Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29(11):1714–1717. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- He B., Hoang T.K., Wang T., Ferris M., Taylor C.M., Tian X., Luo M., Tran D.Q., Zhou J., Tatevian N., Luo F., Molina J.G., Blackburn M.R., Gomez T.H., Roos S., Rhoads J.M., Liu Y. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J. Exp. Med. 2017;214:107–123. doi: 10.1084/jem.20160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T., Thaiss C.A., Kau A.L., Eisenbarth S.C., Jurczak M.J., Camporez J.-P., Shulman G.I., Gordon J.I., Hoffman H.M., Flavell R.A. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst T., Sichelstiel A., Schär C., Yadava K., Bürki K., Cahenzli J., McCoy K., Marsland B.J., Harris N.L. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- Hill D.A., Siracusa M.C., Abt M.C., Kim B.S., Kobuley D., Kubo M., Kambayashi T., LaRosa D.F., Renner E.D., Orange J.S., Bushman F.D., Artis D. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 2012;18(4):538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Littman D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012;30(1):759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil Gökhan.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- Hugot J.-P., Chamaillard M., Zouali H., Lesage S., Cézard J.-P., Belaiche J., Almer S., Tysk C., O'Morain C.A., Gassull M., Binder V., Finkel Y., Cortot A., Modigliani R., Laurent-Puig P., Gower-Rousseau C., Macry J., Colombel J.-F., Sahbatou M., Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos R.de.L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific microbiota direct the differentiation of IL–17–producing T–helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20(1):197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jonsson A.L., Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14(2):79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- Kim M., Galan C., Hill A.A., Wu W.J., Fehlner-Peach H., Song H.W., Schady D., Bettini M.L., Simpson K.W., Longman R.S., Littman D.R., Diehl G.E. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity. 2018;49:151–163. doi: 10.1016/j.immuni.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., Longman R.S., Honda K., Littman D.R., Choi G.B., Huh J.R. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-G., Udayanga K.G.S., Totsuka N., Weinberg J.B., Núñez G., Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe. 2014;15(1):95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth, R.A., Lam-Galvez, B.R., Kirsop, J., Wang, Z., Levison, B.S., Gu, X., Copeland, M.F., Bartlett, D., Cody, D.B., Dai, H.J., Culley, M.K., Li, X.S., Fu, X., Wu, Y., Li, L., DiDonato, J.A., Tang, W.H.W., Garcia-Garcia, J.C., Hazen, S.L., 2019. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 129, 373–387. [DOI] [PMC free article] [PubMed]
- Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., Smith J.D., DiDonato J.A., Chen J., Li H., Wu G.D., Lewis J.D., Warrier M., Brown J.M., Krauss R.M., Tang W.H.W., Bushman F.D., Lusis A.J., Hazen S.L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A., Cong Y., Duck W., Borlaza R., Elson C.O. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130(7):2050–2059. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Koren O., Spor A., Felin J., Fak F., Stombaugh J., Tremaroli V., Behre C.J., Knight R., Fagerberg B., Ley R.E., Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Supplement_1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinak J.L., Round J.L., Goldman W.E. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8(7):e1002785. doi: 10.1371/journal.ppat.100278510.1371/journal.ppat.1002785.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D.J., El-Sankary W., Ferns G.A.A. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167(2):177–185. doi: 10.1016/s0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Lazar V., Ditu L.-M., Pircalabioru G.G., Gheorghe I., Curutiu C., Holban A.M., Picu A., Petcu L., Chifiriuc M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.A., Stern J.M. Understanding the link between gut microbiome and urinary stone disease. Curr Urol Rep. 2019;20:19. doi: 10.1007/s11934-019-0882-8. [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Mehrabian P., Boyajian S., Wu W.-L., Selicha J., Vonderfecht S., Mazmanian S.K., Oh J. The protective role of Bacteroides fragilis in a murine model of colitis-associated colorectal. 2018;3(6) doi: 10.1128/mSphere.00587-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Thaiss C.A., Zeevi D., Dohnalová L., Zilberman-Schapira G., Mahdi J.A., David E., Savidor A., Korem T., Herzig Y., Pevsner-Fischer M., Shapiro H., Christ A., Harmelin A., Halpern Z., Latz E., Flavell R.A., Amit I., Segal E., Elinav E. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Webb T., Li Z. Probiotic antigens stimulate hepatic natural killer T cells. Immunology. 2014;141(2):203–210. doi: 10.1111/imm.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liso, M. , De Santis, S., Verna, G., Biol,M., Manuela Dicarlo, M., Calasso, M., 2020. A specific mutation in Muc2 determines early dysbiosis in colitisprone Winnie mice. Inflamm. Bowel Dis. 26, 546–556. [DOI] [PMC free article] [PubMed]
- Littman D.R., Rudensky A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Liwinski T., Zenouzi R., John C., Ehlken H., Rühlemann M.C., Bang C., Groth S., Lieb W., Kantowski M., Andersen N., Schachschal G., Karlsen T.H., Hov J.R., Rösch T., Lohse A.W., Heeren J., Franke A., Schramm C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69(4):665–672. doi: 10.1136/gutjnl-2019-318416. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., Andrews E., Ajami N.J., Bonham K.S., Brislawn C.J., Casero D., Courtney H., Gonzalez A., Graeber T.G., Hall A.B., Lake K., Landers C.J., Mallick H., Plichta D.R., Prasad M., Rahnavard G., Sauk J., Shungin D., Vázquez-Baeza Y., White R.A., Braun J., Denson L.A., Jansson J.K., Knight R., Kugathasan S., McGovern D.P.B., Petrosino J.F., Stappenbeck T.S., Winter H.S., Clish C.B., Franzosa E.A., Vlamakis H., Xavier R.J., Huttenhower C. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T.M., Loo T.M., Kamachi F., Watanabe Y., Yoshimoto S., Kanda H., Arai Y., Nakajima-Takagi Y., Iwama A., Koga T., Sugimoto Y., Ozawa T., Nakamura M., Kumagai M., Watashi K., Taketo M.M., Aoki T., Narumiya S., Oshima M., Arita M., Hara E., Ohtani N. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- Loś-Rycharska E., Golębiewski M., Sikora M., Grzybowski T., Gorzkiewicz M., Popielarz M., Gawryjolek J., Krogulska A. A combined analysis of gut and skin microbiota in infants with food allergy and atopic dermatitis: a pilot study. Nutrients. 2021;13(5):1682. doi: 10.3390/nu13051682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.B., Hsiao E.Y. Microbiomes as sources of emergent host phenotypes. Science. 2019;365(6460):1405–1409. doi: 10.1126/science.aay0240. [DOI] [PubMed] [Google Scholar]
- Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M., Agdashian D., Terabe M., Berzofsky J.A., Fako V., Ritz T., Longerich T., Theriot C.M., McCulloch J.A., Roy S., Yuan W., Thovarai V., Sen S.K., Ruchirawat M., Korangy F., Wang X.W., Trinchieri G., Greten T.F. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Geuking M.B., McCoy K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33(4):160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Kurakawa T., Umemoto E., Motooka D., Ito Y., Gotoh K., Hirota K., Matsushita M., Furuta Y., Narazaki M., Sakaguchi N., Kayama H., Nakamura S., Iida T., Saeki Y., Kumanogoh A., Sakaguchi S., Takeda K. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 2016;68(11):2646–2661. doi: 10.1002/art.39783. [DOI] [PubMed] [Google Scholar]
- Magruder M., Edusei E., Zhang L., Albakry S., Satlin M.J., Westblade L.F., Malha L., Sze C., Lubetzky M., Dadhania D.M. Gut commensal microbiota and decreased risk for Enterobacteriaceae bacteriuria and urinary tract infection. Gut Microbes. 2020;12:1805281. doi: 10.1080/19490976.2020.1805281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magruder M., Sholi A.N., Gong C., Zhang L., Edusei E., Huang J., Albakry S., Satlin M.J., Westblade L.F., Crawford C., Dadhania D.M., Lubetzky M., Taur Y., Littman E., Ling L., Burnham P., De Vlaminck I., Pamer E., Suthanthiran M., Lee J.R. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-13467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I., Martínez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G., Louk J.A., Rose D.J., Kyureghian G., Peterson D.A., Haub M.D., Walter J. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017;4(1):33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O., Winter D.R., Giladi A., Vargas Aguilar S., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., Zelada González F., Perrin P., Keren-Shaul H., Gury M., Lara-Astaiso D., Thaiss C.A., Cohen M., Bahar Halpern K., Baruch K., Deczkowska A., Lorenzo-Vivas E., Itzkovitz S., Elinav E., Sieweke M.H., Schwartz M., Amit I. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301) doi: 10.1126/science:aad8670. [DOI] [PubMed] [Google Scholar]
- Matson V., Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McCoy K.D., Harris N.L., Diener P., Hatak S., Odermatt B., Hangartner L., Senn B.M., Marsland B.J., Geuking M.B., Hengartner H., Macpherson A.J.S., Zinkernagel R.M. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24(3):329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Mestrovic T., Matijasic M., Peric M., Cipcic Paljetak H., Baresic A., Verbanac D. the role of gut, vaginal, and urinary microbiome in urinary tract infections: from bench to bedside. Diagnostics (Basel) 2021;11(1):7. doi: 10.3390/diagnostics11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K.S., Wong M.H., Shah P.K., Zhang W., Yano J., Doherty T.M., Akira S., Rajavashisth T.B., Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima K., Sukawa Y., Nishihara R., Qian Z.R., Yamauchi M., Inamura K., Kim S.A., Masuda A., Nowak J.A., Nosho K., Kostic A.D., Giannakis M., Watanabe H., Bullman S., Milner D.A., Harris C.C., Giovannucci E., Garraway L.A., Freeman G.J., Dranoff G., Chan A.T., Garrett W.S., Huttenhower C., Fuchs C.S., Ogino S. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1(5):653. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Eksteen B., Song S.-Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., Rajewsky K., Adams D.H., von Andrian U.H. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mowat A.McI. To respond or not to respond - a personal perspective of intestinal tolerance. Nat. Rev. Immunol. 2018;18(6):405–415. doi: 10.1038/s41577-018-0002-x. [DOI] [PubMed] [Google Scholar]
- Nakamoto N., Nakamoto N., Sasaki N., Aoki R., Miyamoto K., Suda W., Teratani T., Suzuki T., Koda Y., Chu P.S., Taniki N., Yamaguchi A., Kanamori M., Kamada N., Hattori M., Ashida H., Sakamoto M., Atarashi K., Narushima S., Yoshimura A., Honda K., Sato T., Kanai T. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 2019;4:492–503. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Sato T., Ohteki T. Commensal Gram-positive bacteria initiates colitis by inducing monocyte/macrophage mobilization. Mucosal Immunol. 2015;8(1):152–160. doi: 10.1038/mi.2014.53. [DOI] [PubMed] [Google Scholar]
- Ni, Q., Ye, Z., Wang, Y., Chen, J., Zhang, W., Ma, C., Li, K., Liu, Y., Liu, L., Han, Z., Gao, T., Jian, Z., Li, S., Li, C., 2020. Gut microbial dysbiosis and plasma metabolic profile in individuals with vitiligo.Front Microbiol. 11, 592248. [DOI] [PMC free article] [PubMed]
- Nielsen K.L., Dynesen P., Larsen P., Frimodt-Møller N. Faecal Escherichia coli from patients with E. coli urinary tract infection and healthy controls who have never had a urinary tract infection. J Med Microbiol. 2014;63:582–589. doi: 10.1099/jmm.0.068783-0. [DOI] [PubMed] [Google Scholar]
- Nigro G., Rossi R., Commere P.-H., Jay P., Sansonetti P.J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15(6):792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz, J., Mielcarz, D.W., Ditrio, L.E., Burroughs, A.R., Foureau, D.M., Haque-Begum, S., Kasper, L.H., 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009). [DOI] [PubMed]
- Ogura Y., Bonen D.K., Inohara N., Nicolae D.L., Chen F.F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R.H., Achkar J.-P., Brant S.R., Bayless T.M., Kirschner B.S., Hanauer S.B., Nuñez G., Cho J.H. A frame shift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C., Park J.-H., Cording S., Wing J.B., Atarashi K., Obata Y., Gaboriau-Routhiau V., Marques R., Dulauroy S., Fedoseeva M., Busslinger M., Cerf-Bensussan N., Boneca I.G., Voehringer D., Hase K., Honda K., Sakaguchi S., Eberl Gérard. Mucosal immunology. The microbiota regulates type 2 immunity through ROR γt+T cells. Science. 2015;349(6251):989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- Paalanne N, Husso A, Salo J, Pieviläinen O, Tejesvi MV, Koivusaari P, Pirttilä AM, Pokka T, Mattila S, Jyrkäs J, Turpeinen A, Uhari M, Renko M, Tapiainen T. Intestinal microbiome as a risk factor for urinary tract infections in children. Eur J Clin Microbiol Infect Dis. 2018 doi: 10.1007/s10096-018-3322-7. [DOI] [PubMed] [Google Scholar]
- Pabst O., Herbrand H., Friedrichsen M., Velaga S., Dorsch M., Berhardt Günter, Worbs T., Macpherson A.J., Förster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J. Immunol. 2006;177(10):6824–6832. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- Paik Y.H., Schwabe R.F., Bataller R., Russo M.P., Jobin C., Brenner D.A. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- Peterson D.A., McNulty N.P., Guruge J.L., Gordon J.I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T., Hrncir T., Liu Y.-J., Biswas A., Hudcovic T., Tlaskalova-Hogenova H., Kobayashi K.S. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. USA. 2009;106(37):15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: role in pathogen colonization, immune responses and inflammatory disease. Immunol. Rev. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Caddie L.B., Coffman R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Price A.E., Shamardani K., Lugo K.A., Deguine J., Roberts A.W., Lee B.L., Barton G.M. A map of Toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49(3):560–575.e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S., Hundeyin M., Daley D., Zambirinis C.P., Kurz E., Mishra A., Mohan N., Aykut B., Usyk M., Torres L.E., Werba G., Zhang K., Guo Y., Li Q., Akkad N., Lall S., Wadowski B., Gutierrez J., Kochen Rossi J.A., Herzog J.W., Diskin B., Torres-Hernandez A., Leinwand J., Wang W., Taunk P.S., Savadkar S., Janal M., Saxena A., Li X., Cohen D., Sartor R.B., Saxena D., Miller G. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramakrishna C., Kujawski M., Chu H., Li L., Mazmanian S.K., Cantin E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019;10:2153. doi: 10.1038/s41467-019-09884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan D., Tang M.S., Bowcutt R., Loke P’ng, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41(2):311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangan P., Choi I., Wei M., Navarrete G., Guen E., Brandhorst S., Enyati N., Pasia G., Maesincee D., Ocon V., Abdulridha M., Longo V.D. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019;26(10):2704–2719.e6. doi: 10.1016/j.celrep.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M.D., Yim Y.S., Wimmer R.D., Kim H., Ryu C., Welch G.M., Andina M., King H.O., Waisman A., Halassa M.M., Huh J.R., Choi G.B. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577(7789):249–253. doi: 10.1038/s41586-019-1843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Brandtzaeg P., Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature Rev. Immunol. 2012;12(1):9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- Riquelme, E. Zhang, Y., Zhang, L., Montiel, M., Zoltan, M., Dong, W., Quesada, P., Sahin, I., Chandra, V., San Lucas, A., Scheet, P., Xu, H., Hanash, S.M., Feng, L., 2019. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019). [DOI] [PMC free article] [PubMed]
- Rocha D.M., Caldas A.P., Oliveira L.L., Bressan J., Hermsdorff H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–215. doi: 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Palacios, A. Harding, A., Menghini, P., Himmelman, C., Retuerto, M., Nickerson, K.P., Lam, M., 2018. The artificial sweetener splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm. Bowel Dis. 24, 1005–1020. [DOI] [PMC free article] [PubMed]
- Rogier R., Ederveen T.H.A., Boekhorst J., Wopereis H., Scher J.U., Manasson J., Frambach S.J.C.M., Knol J., Garssen J., van der Kraan P.M., Koenders M.I., van den Berg W.B., van Hijum S.A.F.T., Abdollahi-Roodsaz S. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome. 2017;5:63. doi: 10.1186/s40168-017-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Campbell LA. Pathogens and Atherosclerosis: Update on the Potential Contribution of Multiple Infectious Organisms to the Pathogenesis of Atherosclerosis. Thromb Haemost. 2011 doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., Fidelle M., Flament C., Poirier-Colame V., Opolon P., Klein C., Iribarren K., Mondragón L., Jacquelot N., Qu B., Ferrere G., Clémenson Céline, Mezquita L., Masip J.R., Naltet C., Brosseau S., Kaderbhai C., Richard C., Rizvi H., Levenez F., Galleron N., Quinquis B., Pons N., Ryffel B., Minard-Colin Véronique, Gonin P., Soria J.-C., Deutsch E., Loriot Y., Ghiringhelli F., Zalcman Gérard, Goldwasser F., Escudier B., Hellmann M.D., Eggermont A., Raoult D., Albiges L., Kroemer G., Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Russell S.L., Gold M.J., Hartmann M., Willing B.P., Thorson L., Wlodarska M., Gill N., Blanchet M., Mohn W.W., McNagny K.M., Finlay B.B. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos S.L., Bui V.L., Mortha A., Oberle K., Heners C., Johner C., Diefenbach A. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nature Immunol. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Zhang L.S., Martinez K., Chang E.B., Yang Q., Wang F., Howles P.N., Hokari R., Miura S., Tso P. Antibiotics suppress activation of intestinal mucosal mast cells and reduce dietary lipid absorption in Sprague-Dawley rats. Gastroenterology. 2016;151(5):923–932. doi: 10.1053/j.gastro.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A.J., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.-J., Thiam K., Cerf-Bensussan N., Mandelboim O., Eberl G., Di Santo J.P. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sawa S., Lochner M., Satoh-Takayama N., Dulauroy S., Bérard M., Kleinschek M., Cua D., Di Santo J.P., Eberl Gérard. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nature Immunol. 2011;12(4):320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- Schaubeck M., Clavel T., Calasan J., Lagkouvardos I., Haange S.B., Jehmlich N., Basic M., Dupont A., Hornef M., Bergen M.von., Bleich A., Haller D. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65(2):225–237. doi: 10.1136/gutjnl-2015-309333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher, J.U., cher, J.U., Sczesnak, A., Longman, R.S., Segata, N., Ubeda, C., Bielski, C., Rostron, T., Cerundolo, V., Pamer, E.G., Abramson, S.B., Huttenhower, C., Littman, D.R., 2013. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202. [DOI] [PMC free article] [PubMed]
- Scott N.A., Andrusaite A., Andersen P., Lawson M., Alcon-Giner C., Leclaire C., Caim S., Le Gall G., Shaw T., Connolly J.P.R., Roe A.J., Wessel H., Bravo-Blas A., Thomson C.A., Kästele V., Wang P., Peterson D.A., Bancroft A., Li X., Grencis R., Mowat A.McI., Hall L.J., Travis M.A., Milling S.W.F., Mann E.R. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 2018;10(464) doi: 10.1126/scitranslmed.aao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J., Shih D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5(2) doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M., Gentile M., Yeiser J.R., Walland A.C., Bornstein V.U., Chen K., He B., Cassis L., Bigas A., Cols M., Comerma L., Huang B., Blander J.M., Xiong H., Mayer L., Berin C., Augenlicht L.H., Velcich A., Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342(6157):447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F. Physiological basis for novel drug therapies to treat the inflammatory bowel diseases: Pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest. Liver Physiol. 2005;288:417–421. doi: 10.1152/ajpgi.00421.2004. [DOI] [PubMed] [Google Scholar]
- Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M.H., Kamada N., Kim Y.G., Nunez G. Microbiota-induced IL–1β, but not IL–6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Man Lei Y., Jabri B., Alegre M.-L., Chang E.B., Gajewski T.F. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37(4):601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., Tardif Mélanie.R., Sathaliyawala T., Kubota M., Farber D.L., Collman R.G., Shaked A., Fouser L.A., Weiner D.B., Tessier P.A., Friedman J.R., Kiyono H., Bushman F.D., Chang K.-M., Artis D. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Di Santo J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Stecher Bärbel, Hardt W.-D. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 2011;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Strugnell R.A., Wijburg O.L.C. The role of secretory antibodies in infection immunity. Nature Rev. Microbiol. 2010;8(9):656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Maruya M., Kawamoto S., Sitnik K., Kitamura H., Agace W.W., Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33(1):71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Tang C., Kamiya T., Liu Y., Kadoki M., Kakuta S., Oshima K., Hattori M., Takeshita K., Kanai T., Saijo S., Ohno N., Iwakura Y. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18(2):183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Tang W.H.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka H., Abe Y., Iwata M., Takeuchi H., Ishikawa H., Matsushita M., Shiohara T., Akira S., Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448(7156):929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- Tezuka H., Abe Y., Asano J., Sato T., Liu J., Iwata M., Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Thion M.S., Low D., Silvin A., Chen J., Grisel P., Schulte-Schrepping J., Blecher R., Ulas T., Squarzoni P., Hoeffel G., Coulpier F., Siopi E., David F.S., Scholz C., Shihui F., Lum J., Amoyo A.A., Larbi A., Poidinger M., Buttgereit A., Lledo P.-M., Greter M., Chan J.K.Y., Amit I., Beyer M., Schultze J.L., Schlitzer A., Pettersson S., Ginhoux F., Garel S. Microbiome influences prenatal and adult microglia in a sex specific manner. Cell. 2018;172(3):500–516.e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.M., Versalovic J. Probiotics-host communication modulation of signaling pathways in the intestine. Gut Microbe. 2010;1(3):148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20(1):40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- Torres J., Hu J., Seki A., Eisele C., Nair N., Huang R., Tarassishin L., Jharap B., Cote-Daigneault J., Mao Q., Mogno I., Britton G.J., Uzzan M., Chen C.-L., Kornbluth A., George J., Legnani P., Maser E., Loudon H., Stone J., Dubinsky M., Faith J.J., Clemente J.C., Mehandru S., Colombel J.-F., Peter I. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42–51. doi: 10.1136/gutjnl-2018-317855. [DOI] [PubMed] [Google Scholar]
- Truax A.D., Chen L., Tam J.W., Cheng N., Guo H., Koblansky A.A., Chou W.-C., Wilson J.E., Brickey W.J., Petrucelli A., Liu R., Cooper D.E., Koenigsknecht M.J., Young V.B., Netea M.G., Stienstra R., Sartor R.B., Montgomery S.A., Coleman R.A., Ting J.-Y. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis. Cell Host Microbe. 2018;24(3):364–378.e6. doi: 10.1016/j.chom.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda, C., Lipuma, L., Gobourne, A, Viale, A., 2012. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456. [DOI] [PMC free article] [PubMed]
- Uematsu S., Fujimoto K., Jang M.H., Yang B.-G., Jung Y.-J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K.J., Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunol. 2008;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIII gamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B.A.E., De Bruijn A.C.J.M., Velcich A., Meijerink J.P.P., Van Goudoever J.B., Büller H.A., Dekker J., Van Seuningen I., Renes I.B., Einerhand A.W.C. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Vetizou M., Pitt J.M., Daillere R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P.M., Poirier-Colame V., Roux A., Becharef S., Formenti S., Golden E., Cording S., Eberl G., Schlitzer A., Ginhoux F., Mani S., Yamazaki T., Jacquelot N., Enot D.P., Berard M., Nigou J., Opolon P., Eggermont A., Woerther P.L., Chachaty E., Chaput N., Robert C., Mateus C., Kroemer G., Raoult D., Boneca I.G., Carbonnel F., Chamaillard M., Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillère R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., Schlitzer A., Ginhoux F., Apetoh L., Chachaty E., Woerther P.-L., Eberl Gérard, Bérard M., Ecobichon C., Clermont D., Bizet C., Gaboriau-Routhiau V., Cerf-Bensussan N., Opolon P., Yessaad N., Vivier E., Ryffel B., Elson C.O., Doré J., Kroemer G., Lepage P., Boneca I.G., Ghiringhelli F., Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennois E., Merlin D., Gewirtz A.T., Chassaing B. Dietary emulsifier induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77(1):27–40. doi: 10.1158/0008-5472.CAN-16-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M., Aitken J.D., Carvalho F.A., Cullender T.C., Mwangi S., Srinivasan S., Sitaraman S.V., Knight R., Ley R.E., Gewirtz A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue A.T., McCright S.J., Wright J.M., Jimenez M.T., Mowel W.K., Kotzin J.J., Joannas L., Basavappa M.G., Spencer S.P., Clark M.L., Eisennagel S.H., Williams A., Levy M., Manne S., Henrickson S.E., Wherry E.J., Thaiss C.A., Elinav E., Henao-Mejia J. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019;11(496) doi: 10.1126/scitranslmed.aav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.A., Barlow J.L., McKenzie A.N.J. Innate lymphoid cells — how did we miss them? Nature Rev. Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xu R. Data-driven multiple-level analysis of gut-microbiome immune- joint interactions in rheumatoid arthritis. BMC Genom. 2019;20:124. doi: 10.1186/s12864-019-5510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Charbonnier L.-M., Noval Rivas M., Georgiev P., Li N., Gerber G., Bry L., Chatila T.A. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity. 2015;43(2):289–303. doi: 10.1016/j.immuni.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., DuGar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.-M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H.W., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Shinkura R., Doi Y., Maruya M., Fagarasan S., Honjo T. Mice carrying a knock–in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nature Immunol. 2011;12(3):264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- Wen L., Ley R.E., Volchkov P.Y., Stranges P.B., Avanesyan L., Stonebraker A.C., Hu C., Wong F.S., Szot G.L., Bluestone J.A., Gordon J.I., Chervonsky A.V. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hanada K., Yang L., Narita M., Saito H., Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann. Allergy Asthma Immunol. 2017;119(1):54–58. doi: 10.1016/j.anai.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Yang T., Richards E.M., Pepine C.J., Raizada M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S., Iwakura Y., Oshima K., Morita H., Hattori M., Honda K., Ishikawa Y., Hara E., Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang D., Jia H., Feng Q., Wang D., Liang D., Wu X., Li J., Tang L., Li Y., Lan Z., Chen B., Li Y., Zhong H., Xie H., Jie Z., Chen W., Tang S., Xu X., Wang X., Cai X., Liu S., Xia Y., Li J., Qiao X., Al-Aama J.Y., Chen H., Wang L., Wu Q.-jun., Zhang F., Zheng W., Li Y., Zhang M., Luo G., Xue W., Xiao L., Li J., Chen W., Xu X., Yin Y., Yang H., Wang J., Kristiansen K., Liu L., Li T., Huang Q., Li Y., Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. Interleukin–22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Ayyoub M., Routy B., Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., Sartor R.B., McIntyre T.M., Silverstein R.L., Tang W.H.W., DiDonato J.A., Brown J.M., Lusis A.J., Hazen S.L. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]