Abstract
Through the boost of the natural medicinal market, individuals began to use a variety of organic materials in the marketed herbal preparation. Lagerstroemia speciosa (LS) leaves are known as banaba. People have been using a decoction of LS leaves as antidiabetic. The study aimed to investigate the acute and sub-acute oral toxicity of LS in Sprague-Dawley rats. The acute toxicity was determined by a single oral dose of LS (2000 mg/kg). Therein animal behaviour and mortality rate were observed for 14 days. The LS (200 mg/kg) was given for 28 days daily in the sub-acute study. The body weight, organ weight, food, water intake, biochemical, haematological parameters, and histopathology were studied. The findings of this study showed no mortality or morbidity was found in acute and sub-acute toxicity studies in rats.
Additionally, no significant variations were found in the respective weight of organ, haematological and biochemical parameters of treated groups with reference to the control group. Moreover, no visible histological changes were detected in the liver of treated groups with reference to the control. In conclusion, the oral administration of LS did not fabricate any major toxic effect in rats. No toxic consequences were reported during acute and sub-acute toxicity investigations. Overall, LS is a safe, natural bio-actives as studied. Further investigations of cytotoxicity and genotoxicity of the above drug(s) or their combinations may be executed for appreciative safety.
Abbreviations: LS, Lagerstroemia speciosa; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; SOD, Superoxide dismutase; FBG, Fasting blood glucose; MDA, Malondialdehyde; GSH, Reduced glutathione; ROS, Reactive oxygen species
Keywords: Toxicity, Biochemical investigations, Haematology, Histopathology
1. Introduction
Through the boost of the natural medicinal market, individuals began to use a variety of organic materials in the marketed herbal preparation. Many people believe that all plant related products are safe and they do not required to be studied for their safety. There has not been any significant work on the toxicity of such herbal extracts. The toxin is primarily produced from the varied parts of plant, for example, leaves, fruits, and barks (Molyneux and Ralphs, 1992, Wittstock and Gershenzon, 2002). Not just for conventional treatment, but for integrative medicine too, a toxicity test is must to detect any detrimental effects (Farzaei et al., 2020) that are not evident before signs and indications surface after prolonged intake (Aneesh et al., 2009, Bandaranayake, 2006). For so many years, nature has been an important source of therapeutics, and plant-based products persist to show an vital role in the healthcare services of approximately 80–85 percent globally (Thiagarajan et al., 2019). Owing to advances in our understanding of the processes by which medicinal plants undoubtedly affect health and quality of life, medicinal plants have gained growing public and professional acceptance (Gurib-Fakim, 2006, Hoffmann, 2003). Many diseases are treated with medicinal plants, which are generally considered safe (Süntar, 2020); however, certain medicinal plants might be harmful in high doses (Tariq et al., 2021) or interfere with modern drugs (Palombo, 2006, Taylor et al., 2001). As per World Health Organization (WHO), public demand for research to bridge information gaps about the protection of medicinal plants is increasing (Bodeker and Ong, 2005, Kala et al., 2006, Kartal, 2007).
Lagerstroemia speciosa (LS) leaves are known as banaba, and the tree is popular in the Philippines. People have been using a decoction of LS leaves as blood-glucose-lowering aid for several years (Carew and Chin, 1961). It has antioxidant activity in HepG2 cell lines (Thakur and Devaraj, 2020). In vitro experiments on improving glucose transporter by corosolic acid (Murakami et al., 1993). It has anti-inflammatory activity (Rohit Singh and Ezhilarasan, 2021). Currently reginin A, lagerstroemin, and flosin B inhibits the digestive enzyme (Hayashi et al., 2002, Suzuki et al., 2001). In contrast to rooibos tea (Aspalathus linearis), green tea (Camellia sinensis), and tochu tea, an extract from LS (aqueous) was one of the positive samples with a powerful xanthine oxidase inhibitory consequence, according to our preliminary screening report (Eucommiaulmoides) (Unno et al., 2000). Till date no toxicity study has been carried out of this plant extract. Therefore, the present study aimed to investigate LS acute and sub-acute oral toxicity in Sprague-Dawley rats.
2. Materials and methods
2.1. Animals
From the animal facility of PNU (Princess Nourah bint Abdulrahman University), Saudi Arabia, twenty-four male SD rats weighing 150–180 g were obtained and maintained under standard environmental conditions of 25 ± 2 °C with 50 ± 10% RH at 12 h light/dark cycle in polypropylene cages. Studies on animals were done in accordance to the PNU IRB guidelines and approved by PNU, Saudi Arabia - IRB log No. 20–0336.
2.2. Toxicity studies
The toxicity assessments were done in accordance with globally recognized standards (Dutta et al., 2017, Ridgway, 2002).
3. Acute oral toxicity study
It was done as per Organization for Economic Co-operation and Development (OECD) guidelines 423 for acute toxicity studies (Clemente et al., 2019). The rat was administered with a single dose of LS at a dose of 2000 mg/kg, orally. Animals were grouped into two groups, each consisting of six rats (n = 6). The control group (n = 6) received only normal saline. Individual animals were carefully examined at different time periods, after the administration of the LS plant extract (n = 6). Besides, they were twice monitored daily for 14 days after the administration of LS to detect clinical signs such as toxicity, mortality, behavioral pattern, and physical appearance, as well as other adverse effects, for example lethargy, diarrhea, tremors,salivation, etc.
3.1. Subacute oral toxicity study
This subacute toxicity studies was performed as indicated by OECD guideline 407 (Kunimatsu et al., 2004, Park et al., 2013). The male SD rats (weight 150–180 g) were grouped into two groups (n = 6) randomly. The first marked (n = 6) as control and second as treated group (n = 6). The second group was administered with the extract of LS at a dose of 200 mg/kg, every day for 28 days, via the oral route. The behavioral pattern, and physical appearance, in addition to other adverse effects, for example lethargy, diarrhea, tremors, salivation, etc were monitored timely.
All the animals were fasted for 12hrs on the 29th day, after which the rats were anesthetized (xylazine at 10 mg/kg and ketamine at 25 mg/kg, i.p.). The organs were weighed, and the histological investigations of liver tissues were done. Hematological, biochemical, lipid peroxidation, and glutathione assays were done on the blood obtained. The body weight, food, and water intake were recorded.
3.2. Measurement of blood glucose level
The blood glucose level for all groups was measured on 29th day of the experiment. The blood glucose level was measured by Glucometer (Contour Glucometer).
3.3. Measurement of organ weights
The organ weights were observed on 29th day of the experiment. The organs e.g., the heart, liver, brain, lungs, kidney, and spleen were cautiously dissected out and then weighed.
3.4. Haematological estimation
The erythrocytes, leukocytes, blood platelets, hematocrit, and hemoglobin level were estimated using haematological autoanalyzer.
3.5. Determination of biochemical parameters
The biochemical analyses performed were calculation of total bilirubin, direct bilirubin, serum creatinine, and liver function test (AST, ALT, and ALP) using autoanalyzer.
3.6. Antioxidant parameters
The antioxidant parameters (ROS, MDA, GSH, and SOD) were done as per the protocol described earlier (Abid et al., 2016, Syed et al., 2021). The ROS was estimated using Dichlorodihydrofluorescein Diacetate. The MDA was estimated using thiobarbituric acid (TBA) and trichloroacetic acid (TCA) and the absorbance was taken at 532 nm. The GSH was estimated using (5,5-dithio-bis-(2-nitrobenzoic acid) DTNB also known as (Ellman's Reagent) and the absorbance was taken at 412 nm. The SOD was estimated using Tris HCl having pH 8.2, diethylene triamine penta acetic acid and pyrogallol. The activity was measured at absorbance 420 nm.
3.7. Haematoxylin-eosin (H & E) staining of liver tissue
For the purpose of histopathological examinations, the liver tissues were excised from the control group in addition to LS treated group. Then it was processed and stained as per the protocol illustrated earlier (Syed et al., 2021, Syed et al., 2020, Syed et al., 2016a).
3.8. Data analysis
Graph Pad Prism 7.0 software was exploited for statistical analysis and in mean ± SEM, all the data was expressed. The data was analysed employing t-test. Data were supposed to be statistically significant at P < 0.05.
4. Result
4.1. Acute toxicity study
On administration of LS at a dose of 2000 mg/kg revealed no toxicity/mortality or morbidity in the acute toxicity study. No alterations were found in movement pattern, physical and behavioral parameters, general appearance, salivation, and lacrimation in the rats.
4.2. Sub-acute toxicity study
The treatment with LS at a 200 mg/kg dose has not shown any toxicity, mortality, or morbidity. There were no alterations observed in movement, physical and behavioral parameters, general appearance, salivation, and lacrimation in the rats from the day of administration or throughout the study period.
4.3. LS does not affect body weight
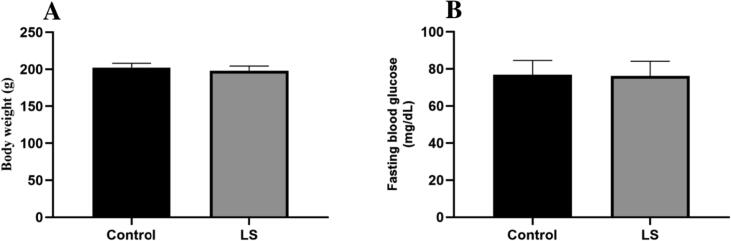
No effect of the extract was found on the body weights of rats all through the sub-acute treatment. Additionally, no major alterations in mean body weight were reported after daily administration of LS (200 mg/kg) for 28 days in comparison to the control (Fig. 1).
Fig. 1.
Effect of LS on (A) body weight, (B) fasting blood glucose level. Data are represented as mean ± SEM.
4.4. LS does not alter the fasting blood glucose (FBG) level
There was no alteration on the FBG level of the LS-treated rats in the sub-acute study. The FBG was nearly equal to the control (Fig. 1).
4.5. LS does not affect the food and water intake
No change was observed in the food and water intake pattern of normal rats and that of treated animals in the sub-acute study (Table 1).
Table 1.
Effect of LS on food and water consumptionin SD rats.
| Parameters | Control | LS | |
|---|---|---|---|
| Sub- toxicity | Food intake (g/day) | 21.08 ± 0.56 | 21.71 ± 0.53 |
| Water intake (ml/day) | 67.47 ± 1.74 | 71.45 ± 2.08 | |
All values were presented as Mean ± SEM.
4.6. Organ weight was not affected by the intake of LS
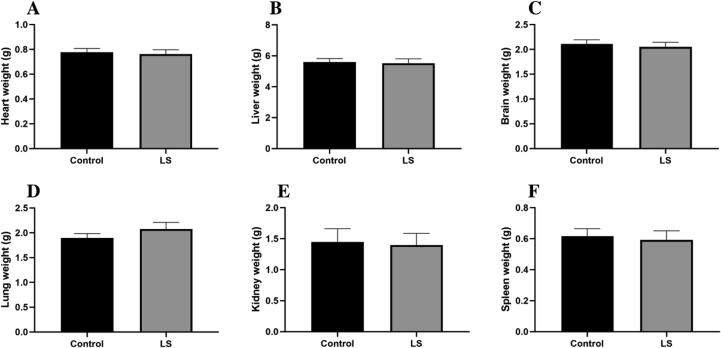
No significant effect was seen on the organ weight of treated and control group rats (Fig. 2).
Fig. 2.
Effect of LS on different organ weights of the body of rat. Data are represented as mean ± SEM.
4.7. LS exerts an antioxidant effect
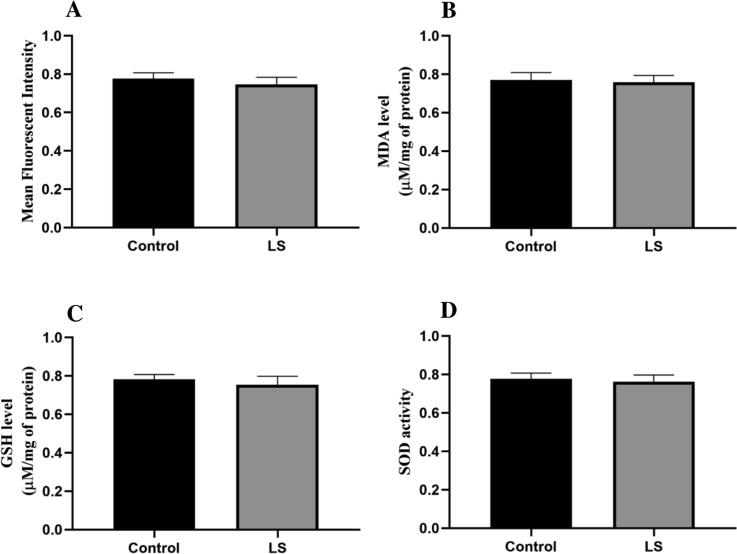
No significant effect was observed on the ROS production of treated and in control group rats. The LS extract has no toxic effect as it has an antioxidant effect (Fig. 3).
Fig. 3.
Effect of LS on (A) ROS, (B) MDA level, (C) GSH level, and (D) SOD activity. Data are represented as mean ± SEM.
4.8. Hematological and biochemical parameters were not affected by the LS treatment
In Table 2, effects of sub-acute administration of LS on hematological parameters are given. Administration of LS for 28 days daily has not cause any important alteration in hematological parameters.
Table 2.
Effect of LS on haematological parameters in SD rats.
| Parameters | Control | LS | |
|---|---|---|---|
| Sub-acute toxicity | Erythrocytes (106/µL) | 6.67 ± 0.10 | 6.46 ± 0.23 |
| Leukocytes (cells/mm3) | 7760 ± 360 | 7840 ± 258.07 | |
| Platelets (Lac cells/mm3) | 2.78 ± 0.21 | 2.79 ± 0.16 | |
| Hematocrit (%) | 37.02 ± 0.79 | 36.92 ± 1.23 | |
| Hemoglobin (g/dL) | 12.78 ± 0.21 | 12.18 ± 0.39 | |
All values were presented as Mean ± SEM.
The effects of sub-acute administration of LS on biochemical parameters are illustrated in Table 3. The administration of LS daily for 28 days induced no alteration in liver and kidney function test of treated as well as control group. Other parameters for example hepatic biomarkers (AST, ALT, and ALP) along with kidney function test markers (total bilirubin, direct bilirubin, and serum creatinine) exhibited normal levels.
Table 3.
Effect of LSonbiochemical parameters in SD rats.
| Parameters | Control | LS | |
|---|---|---|---|
| Sub-acute toxicity | Total bilirubin (mg/dl) | 0.72 ± 0.06 | 0.72 ± 0.05 |
| Bilirubin direct (mg/dl) | 0.28 ± 0.03 | 0.27 ± 0.04 | |
| Serum Cr (mg/dl) | 0.74 ± 0.02 | 0.77 ± 0.06 | |
| AST (IU/L) | 36.26 ± 0.44 | 35.60 ± 0.36 | |
| ALT (IU/L) | 31.93 ± 0.36 | 31.20 ± 0.47 | |
| ALP (IU/L) | 38.94 ± 0.44 | 38.28 ± 0.36 | |
All values were presented as Mean ± SEM.
4.9. LS safeguards the liver tissue in histopathological analysis
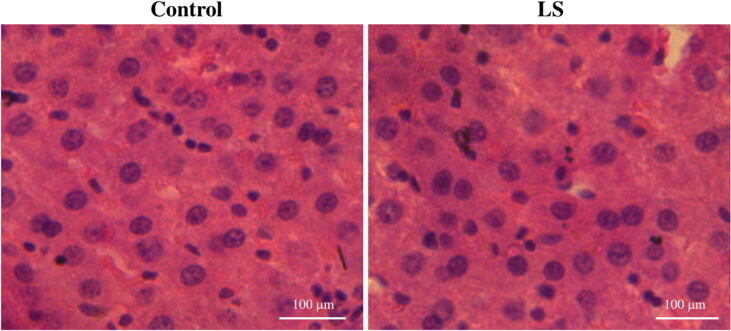
There was no change in the architecture of the liver tissue of the control group and in the LS treated group in the sub-acute study. The was no apoptosis, degradation of tissue or ballooning seen in the tissues (Fig. 4).
Fig. 4.
Histopathological analysis of liver tissue on administration of LS in sub-acute toxicity.
5. Discussion
Mainly at the organism, organ, tissue, and cell stage, various compound substances may cause widespread modes of toxicity (Rubino, 2015). Phytotherapeutic components derived from herbal plants are comprehensively employed mainly in developing countries for disease treatment.
A large number of people have wrongly assumed that the phytoconstituents present in the herbal plants to be harmless simply because they are originated from the nature or procured from the natural source. Nonetheless, these bioactive phytopharmaceutical preparations derived from herbal medicinal plants are commonly used as self-medication as these are contemplated to be safe and have no adverse health effects (Jt and Ja, 2007, Nath and Yadav, 2015, Vaghasiya et al., 2011). Nevertheless, no clinical investigations have been done on the toxicity profile and side effects of these phytopharmaceutical remedies. (Sahoo et al., 2010).
Consequently, advanced acute oral toxicity research is essential to establish the doses that may be exploited in future and discover the potential clinical signs produced by the compounds in question. It might also be used to ascertain the therapeutic potential of drugs (Jordan et al., 2010, Ritter et al., 2014, Sahoo et al., 2010). The toxicological evaluation of several traditional medicines and their effectiveness to test through histopathological analysis and oral acute toxicity tests is known as in vivo toxicity research. Here, we have done the HE staining of the liver tissue, and we found no changes in the LS treated group, and the integrity of the cells was normal equivalent to that of the normal group rats. Considering the basic assumption that pharmacology is essentially toxicology at a lower dosage, oral acute toxicity testing in rodents may be utilized to assess phytopharmaceuticals for various biological effects (Sasidharan et al., 2008, Veeresham, 2012). At a lower non-toxic dosage, a toxic substance can produce interesting pharmacological responses. If medicinal plants are proven to have the potential for development into pharmacological products, animal toxicity findings will be critical in determining their safety (Fu et al., 2018, Moshi et al., 2007).
Though LS is utilized extensively as traditional medicine for the treatment of a variety of diseases, comprising diabetes, and is a good antioxidant (Kakuda et al., 1996, Mishra et al., 1990, Suzuki et al., 2001, Thuppia et al., 2009, Unno et al., 2000, Unno et al., 1997). Traditional medicines are used to treat several diseases and possess less or no toxicity (Chidambaram and Carani Venkatraman, 2010, Khatoon et al., 2018, Syed et al., 2020, Syed et al., 2016b, Syed et al., 2016a). There is little clinical and scientific data on the efficacy as well as the safety of LS extract. Acute and sub-acute toxicity assessments of LS were done. Earlier studies have indicated that LS exhibits a diversity of pharmacological activities like antioxidant antimicrobial, hepatoprotective, anti-fertility, anti-ulcer, antipyretic, anti-inflammatory, and wound healing effects (Abid et al., 2014, Rahmani et al., 2015, Abid et al., 2016). No significant effect was observed on the ROS production of treated and in control group rats. The LS extract has no toxic effect as it has an antioxidant effect as depicted by MDA, GSH and SOD activity.
In the current work, certain parameters were estimated subsequent to in vivo sub-acute and acute administration of LS. Mortality is a crucial condition in toxicological estimations (Asare et al., 2012); both sub-acute and acute administration of the LS did not indicate significant mortality., A single dose administration of LS (2000 mg/kg) have not illustrate any indications of toxicity or mortality through the whole observation time in acute toxicity experimentation. Therefore, LS's approximate lethal dose (LD50) is assessed to be greater than 2000 mg/kg. Determination of weight augmentation or relative organs’ weight is essential to differentiate probable organ damages by means of exposure to the substances toxic nature. The damaged organ weight would change depending on the level of toxicity and body weight ratio (Rosidah et al., 2009). In the subacute toxicity study, LS administered up to 2000 mg/kg, have not/neither caused any alteration in clinical signs, morbidity, nor mortality. Besides, it has not got any harmful effect with respect to relative organs’ weight in compare to the control. The hematopoietic system, one of the major sensitive goals of toxic substances, resides in the bone marrow where the red blood cell is produced (Birbrair and Frenette, 2016). This system is an essential index of pathological as well as physiological and states in both animals and men (JT and JA, 2007). In current work, sub-acute administration of LS did not indicate any significant changes in the hematological profile of rats that have given different doses of LS as compared with the control, signifying that LS is perhaps non-toxic to the blood system.
Evaluation of liver function is crucial in assessing the toxicity of synthetic drugs in addition to plant extracts (Begriche et al., 2011). AST, ALT, bilirubin, total bilirubin and creatinine in serum are the universal clinical biomarkers of liver condition (Al-Mamary et al., 2002). No significant variations were detected in serum levels of AST, ALT, bilirubin, total bilirubin, and creatinine in sub-acute LS fed groups. The current research indicated that LS administration up to a dose of 2000 mg/kg have not shown any adverse effects on the liver. Correspondingly, the histological result of the liver also did not indicate any alterations in the tissue; it confirms that LS administration is comparatively harmless for the liver. The kidneys are believed to be common targets of toxicity (Dekant and Vamvakas, 1996). LS fed groups showed unimportant differences in urea level and creatinine. It designates that LS might not influence the normal renal function following sub-acute exposure with respect to the control. Moreover, no significant alteration was reported in the blood glucose level in sub-acute LS-fed groups. In the same way, histological analysis of the liver indicated no modifications in the tissue architecture of the organ subsequent to sub-acute LS administration.
The regulatory authorities are concerned about the safety of traditional medicines, and their exploitation by ethnopharmacologists, pharmacists, medical practitioners, and other healthcare professionals (Ekor, 2014, Parveen et al., 2015, Rousseaux and Schachter, 2003) for the use of herbal plants/extracts should have least toxic effect so that it does not harm the body.
6. Conclusion
Neither lethality nor adverse effects in the acute and sub-acute toxicity studies were exhibited in rats. Our works also provided accommodating data on the utilization of LS. Since all medicaments used in the study were of natural ones and also utilized in the traditional system of medicines, the oral doses of LS might be deemed safe. It could be trustworthy for its further medicinal uses. Moreover, chronic toxicity, carcinogenicity, and mutagenicity may be performed to better understand the safety profile of all selected drugs and their combinations.
Availability of data and materials
The data generated or analyzed in this article are online publicly available without request.
Authors' contributions
Nada H. Aljarba and Hamzah Algamdy were performed the majority of the animal experiment and laboratory work; Abdullah AlKahtane was acquired and analyzed the data; Md Saquib Hasnain and Saad Alkahtani were involved in the conception and design of the study, data interpretation, and critically revised the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1441-536).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abid R., Mahmood R., Rajesh K.P., Kumara Swamy B.E. Potential in vitro antioxidant and protective effect of Cassia fistula Linn. Fruit extracts against induced oxidative damage in human erythrocytes. Int. J. Pharm. Pharm. Sci. 2014 [Google Scholar]
- Abid R., Mahmood R., Santosh Kumar H.S. Hypolipidemic and antioxidant effects of ethanol extract of Cassia fistula fruit in hyperlipidemic mice. Pharm. Biol. 2016 doi: 10.1080/13880209.2016.1185445. [DOI] [PubMed] [Google Scholar]
- Al-Mamary, M., Al-Habori, M., Al-Aghbari, A.M., Baker, M.M., 2002. Investigation into the toxicological effects of Catha edulis leaves: A short term study in animals. Phyther. Res. https://doi.org/10.1002/ptr.835. [DOI] [PubMed]
- Aneesh, T.P., Hisham, M., Sekhar, S., Madhu, M., Deepa, T. V, 2009. International market scenario of traditional Indian herbal drugs–India declining…. Int. J. Green Pharm. 3.
- Asare G.A., Gyan B., Bugyei K., Adjei S., Mahama R., Addo P., Otu-Nyarko L., Wiredu E.K., Nyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012 doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Bandaranayake W.M. Quality control, screening, toxicity, and regulation of herbal drugs. Mod. Phytomedicine. 2006;1:25–57. [Google Scholar]
- Begriche K., Massart J., Robin M.-A., Borgne-Sanchez A., Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Birbrair A., Frenette P.S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 2016 doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodeker, G., Ong, C.-K., 2005. WHO global atlas of traditional, complementary and alternative medicine. World Health Organization.
- Carew D.P., Chin T.F. Constituents of Lagerstroemia Flos-reginae Retz. Nature. 1961;190:1108–1109. doi: 10.1038/1901108a0. [DOI] [PubMed] [Google Scholar]
- Chidambaram J., Carani Venkatraman A. Cissus quadrangularis stem alleviates insulin resistance, oxidative injury and fatty liver disease in rats fed high fat plus fructose diet. Food Chem. Toxicol. 2010;48:2021–2029. doi: 10.1016/j.fct.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Clemente M., Miguel M.D., Felipe K.B., Gribner C., Moura P.F., Rigoni A.G.R., Fernandes L.C., Carvalho J.L.S., Hartmann I., Piltz M.T. Acute and sub-acute oral toxicity studies of standardized extract of Nasturtium officinale in Wistar rats. Regul. Toxicol. Pharmacol. 2019;108 doi: 10.1016/j.yrtph.2019.104443. [DOI] [PubMed] [Google Scholar]
- Dekant W., Vamvakas S. Biotransformation and membrane transport in nephrotoxicity. Crit. Rev. Toxicol. 1996 doi: 10.3109/10408449609012526. [DOI] [PubMed] [Google Scholar]
- Dutta A.K., Sharma R., Sharma R.K. Acute toxicity Study of Sanjeevani Vati according to OECD Guideline 420. J. Ayurveda. 2017:11. [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaei M.H., Bayrami Z., Farzaei F., Aneva I., Das S.K., Patra J.K., Das G., Abdollahi M. Poisoning by Medical Plants. Arch. Iran. Med. 2020;23:117–127. [PubMed] [Google Scholar]
- Fu B., Wang N., Tan H.-Y., Li S., Cheung F., Feng Y. Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: a review on experimental and clinical evidences. Front. Pharmacol. 2018;9:1394. doi: 10.3389/fphar.2018.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Maruyama H., Kasai R., Hattori K., Takasuga S., Hazeki O., Yamasaki K., Tanaka T. Ellagitannins from Lagerstroemia speciosa as activators of glucose transport in fat cells. Planta Med. 2002;68:173–175. doi: 10.1055/s-2002-20251. [DOI] [PubMed] [Google Scholar]
- Hoffmann D. Simon and Schuster; 2003. Medical herbalism: the science and practice of herbal medicine. [Google Scholar]
- Jordan S.A., Cunningham D.G., Marles R.J. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010;243:198–216. doi: 10.1016/j.taap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- JT, M., JA, S., 2007. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. [DOI] [PubMed]
- Kakuda T., Sakane I., Takihara T., Ozaki Y., Takeuchi H., Kuroyanagi M. Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetic diabetic KK-AY mice. Biosci. Biotechnol. Biochem. 1996;60:204–208. doi: 10.1271/bbb.60.204. [DOI] [PubMed] [Google Scholar]
- Kala C.P., Dhyani P.P., Sajwan B.S. Developing the medicinal plants sector in northern India: challenges and opportunities. J. Ethnobiol. Ethnomed. 2006;2:1–15. [Google Scholar]
- Kartal M. Intellectual property protection in the natural product drug discovery, traditional herbal medicine and herbal medicinal products. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007;21:113–119. doi: 10.1002/ptr.2036. [DOI] [PubMed] [Google Scholar]
- Khatoon A., Khan F., Ahmad N., Shaikh S., Rizvi S.M.D., Shakil S., Al-Qahtani M.H., Abuzenadah A.M., Tabrez S., Ahmed A.B.F. Silver nanoparticles from leaf extract of Mentha piperita: eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci. 2018;209:430–434. doi: 10.1016/j.lfs.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Kunimatsu T., Yamada T., Miyata K., Yabushita S., Seki T., Okuno Y., Matsuo M. Evaluation for reliability and feasibility of the draft protocol for the enhanced rat 28-day subacute study (OECD Guideline 407) using androgen antagonist flutamide. Toxicology. 2004;200:77–89. doi: 10.1016/j.tox.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Mishra Y., Khan M.S.Y., Zafar R., Agarwal S.S. Hypoglycaemic activity of leaves of Lagerstroemia speciosa (L) Pers. Indian J. Pharmacol. 1990;22:174. [Google Scholar]
- Molyneux, R.J., Ralphs, M.H., 1992. Plant toxins and palatability to herbivores.
- Moshi M.J., Van den Beukel C.J., Hamza O.J.M., Mbwambo Z.H., Nondo R.O.S., Masimba P.J., Matee M.I., Kapingu M.C., Mikx F., Verweije P.J. Brine shrimp toxicity evaluation of some Tanzanian plants used traditionally for the treatment of fungal infections. African J. Tradit. Complement. Altern. Med. 2007;4:219–225. doi: 10.4314/ajtcam.v4i2.31211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, C., Myoga, K., Kasai, R., Ohtani, K., Kurokawa, T., Ishibashi, S., DAYRIT, F., PADOLNA, W.G., Yamasaki, K., 1993. Screening of plant constituents for effect on glucose transport activity in Ehrlich ascites tumour cells. Chem. Pharm. Bull. 41, 2129–2131. [DOI] [PubMed]
- Nath P., Yadav A.K. Acute and sub-acute oral toxicity assessment of the methanolic extract from leaves of Hibiscus rosa-sinensis L. in mice. J. Intercult. Ethnopharmacol. 2015;4:70. doi: 10.5455/jice.20141028021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006;20:717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- Park S.-J., Lim K.-H., Noh J.-H., Jeong E.J., Kim Y.-S., Han B.-C., Lee S.-H., Moon K.-S. Subacute oral toxicity study of Korean red ginseng extract in Sprague-Dawley rats. Toxicol. Res. 2013;29:285–292. doi: 10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen A., Parveen B., Parveen R., Ahmad S. Challenges and guidelines for clinical trial of herbal drugs. J. Pharm. Bioallied Sci. 2015;7:329. doi: 10.4103/0975-7406.168035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A.H., Aldebasi Y.H., Srikar S., Khan A.A., Aly S.M. Aloe vera : Potential candidate in health management via modulation of biological activities. Pharmacogn. Rev. 2015 doi: 10.4103/0973-7847.162118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway P. Revised fixed dose procedure: OECD Test Guideline 420. Rapp. ISTISAN. 2002:27–31. [Google Scholar]
- Ritter J.M., Rang H.P., Flower R.J., Henderson G. Rang & Dale’s Pharmacology E-Book: with STUDENT CONSULT Online Access. Elsevier Health Sciences. 2014 [Google Scholar]
- Rohit Singh T., Ezhilarasan D. Lagerstroemia speciosa (L.) Pers., ethanolic leaves extract attenuates dapsone-induced liver inflammation in rats. Drug Chem. 2021;Toxicol:1–10. doi: 10.1080/01480545.2021.1945079. [DOI] [PubMed] [Google Scholar]
- Rosidah, Yam, M.F., Sadikun, A., Ahmad, M., Akowuah, G.A., Asmawi, M.Z., 2009. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. https://doi.org/10.1016/j.jep.2009.03.011. [DOI] [PubMed]
- Rousseaux C.G., Schachter H. Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003;68:505–510. doi: 10.1002/bdrb.10053. [DOI] [PubMed] [Google Scholar]
- Rubino F.M. Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics. 2015;3:20–62. doi: 10.3390/toxics3010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo N., Manchikanti P., Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010;81:462–471. doi: 10.1016/j.fitote.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Sasidharan S., Darah I., Jain K. In Vivo. and In Vitro. Toxicity Study of Gracilaria changii. Pharm. Biol. 2008;46:413–417. [Google Scholar]
- Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem. Rev. 2020;19:1199–1209. [Google Scholar]
- Suzuki Y., Hayashi K., Sakane I., Kakuda T. Effect and mode of action of banaba (Lagerstroemia speciosa L.) leaf extracts on postprandial blood glucose in rats. J. Japanese Soc. Nutr. Food Sci. 2001 [Google Scholar]
- Syed A.A., Lahiri S., Mohan D., Valicherla G.R., Gupta A.P., Kumar S., Maurya R., Bora H.K., Hanif K., Gayen J.R. Cardioprotective effect of ulmus wallichiana planchon in β-adrenergic agonist induced cardiac hypertrophy. Front. Pharmacol. 2016;7:510. doi: 10.3389/fphar.2016.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A.A., Lahiri S., Mohan D., Valicherla G.R., Gupta A.P., Riyazuddin M., Kumar S., Maurya R., Hanif K., Gayen J.R. Evaluation of anti-hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt-and L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2016;193:555–565. doi: 10.1016/j.jep.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Syed, A.A., Reza, M.I., Husain, A., Singh, P., Gayen, J.R., 2021. Inhibition of NOX4 by Cissus quadrangularis extract protects from Type 2 diabetes induced-steatohepatitis. Phytomedicine Plus 100021. https://doi.org/https://doi.org/10.1016/j.phyplu.2021.100021.
- Syed, A.A., Reza, M.I., Shafiq, M., Kumariya, S., Singh, P., Husain, A., Hanif, K., Gayen, J.R., 2020. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 257, 118118. https://doi.org/https://doi.org/10.1016/j.lfs.2020.118118. [DOI] [PubMed]
- Tariq, L., Bhat, B.A., Hamdani, S.S., Mir, R.A., 2021. Phytochemistry, Pharmacology and Toxicity of Medicinal Plants. Med. Aromat. Plants Healthc. Ind. Appl. 217.
- Taylor J.L.S., Rabe T., McGaw L.J., Jäger A.K., Van Staden J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001;34:23–37. [Google Scholar]
- Thakur R.S., Devaraj E. Lagerstroemia speciosa (L.) Pers. triggers oxidative stress mediated apoptosis via intrinsic mitochondrial pathway in HepG2 cells. Environ. Toxicol. 2020;35:1225–1233. doi: 10.1002/tox.22987. [DOI] [PubMed] [Google Scholar]
- Thiagarajan, S.K., Rama Krishnan, K., Ei, T., Husna Shafie, N., Arapoc, D.J., Bahari, H., 2019. Evaluation of the effect of aqueous Momordica charantia Linn. extract on zebrafish embryo model through acute toxicity assay assessment. Evidence-Based Complement. Altern. Med. 2019. [DOI] [PMC free article] [PubMed]
- Thuppia A., Rabintossaporn P., Saenthaweesuk S., Ingkaninan K., Sireeratawong S. The hypoglycemic effect of water extract from leaves of Lagerstroemia speciosa L. in streptozotocin-induced diabetic rats. Songklanakarin. J. Sci. Technol. 2009;31 [Google Scholar]
- Unno T., Sakane I., Kakuda T. Inhibition of xanthine oxidase by an aqueous extract of banaba leaves (Lagerstroemia speciosa). Nippon Shokuhin Kagaku Kogaku Kaishi= J. Japanese Soc. Food Sci. Technol. 2000;47:740–743. [Google Scholar]
- Unno T., Sakane I., Masumizu T., Kohno M., Kakuda T. Antioxidative activity of water extracts of Lagerstroemia speciosa leaves. Biosci. Biotechnol. Biochem. 1997;61:1772–1774. doi: 10.1271/bbb.61.1772. [DOI] [PubMed] [Google Scholar]
- Vaghasiya Y.K., Shukla V.J., Chanda S.V. Acute oral toxicity study of Pluchea arguta boiss extract in mice. J Pharmacol Toxicol. 2011;6:113–123. [Google Scholar]
- Veeresham C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012;3:200. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U., Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002;5:300–307. doi: 10.1016/s1369-5266(02)00264-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed in this article are online publicly available without request.