Abstract
The purpose of this work was to investigate the protective effect of five essential oils (EOs); Rosmarinus officinalis, Thymus vulgaris, Origanum compactum Benth., Eucalyptus globulus Labill. and Ocimum basilicum L.; against oxidative stress induced by hydrogen peroxide in Saccharomyces cerevisiae. The chemical composition of the EOs was analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC/MS). The in vitro antioxidant activity was evaluated and the protective effect of EOs was investigated. Yeast cells were pretreated with different concentrations of EOs (6.25–25 µg/ml) for an hour then incubated with H2O2 (2 mM) for an additional hour. Cell viability, antioxidants (Catalase, Superoxide dismutase and Glutathione reductase) and metabolic (Succinate dehydrogenase) enzymes, as well as the level of lipid peroxidation (LPO) and protein carbonyl content (PCO) were evaluated. The chemical composition of EOs has shown the difference qualitatively and quantitatively. Indeed, O. compactum mainly contained Carvacrol, O. basilicum was mainly composed of Linalool, T. vulgaris was rich in thymol, R. officinalis had high α-Pinene amount and for E. globulus, eucalyptol was the major compound. The EOs of basil, oregano and thyme were found to possess the highest amount of total phenolic compounds. Moreover, they have shown the best protective effect on yeast cells against oxidative stress induced by H2O2. In addition, in a dose dependent manner of EOs in yeast medium, treated cells had lower levels of LPO, lower antioxidant and metabolic enzymes activity than cells exposed to H2O2 only. The cell viability was also improved. It seems that the studied EOs are efficient natural antioxidants, which can be exploited to protect against damages and serious diseases related to oxidative stress.
Keywords: Essential oils, Yeast, S.cerevisiae, Oxidative stress, Hydrogen peroxide, Antioxidants
Abbreviations: ANOVA, Analysis of variance; ABTS, 2,2-azino-bis(3-etilbenzotiazolin)-6-sulfonic acid; BHA, Butylated hydroxyanisole; BHT, Butylated hydroxytoluene; BSA, bovine serum albumin; CAT, catalase; DCIP, 6-Dichlorophenolindophenol; DNPH, 2,4- dinitro-phenylhydrazine reagent; DPPH, 2,2-diphenyl-1-picrylhydrazyl; EDTA, Ethylene diamine tetra acetic acid; EOs, essential oils; FID, flame ionization detector; GC, gas chromatography; GC/MS, gas chromatography-mass spectrometry; GR, Glutathione reductase; GSH, Reduced glutathione; GSSG, Oxidized glutathione; H2O2, Hydrogen peroxide; LPO, Lipid peroxidation; MDA, Malondialdehyde; MgCl2, Magnesium chloride; NaAc, Sodium acetate; NaCl, Sodium chloride; NADH, Nicotinamide adenine dinucleotide; NADHP, Nicotinamide adenine dinucleotide phosphate; OH, Hydroxyl radical; PBS, Phosphate buffer saline; PCO, Protein carbonylation; PMSF, Phenylmethylsulfonylfluoride; ROS, Reactive oxygen species; RP, reducing power; S.c, S. cerevisiae; SD, standard deviation; SDH, Succinate dehydrogenase; SDS, Sodium dodecyl sulphate; SEM, Standard error of the means; SOD, Superoxide dismutase; TBA, Thiobarbituric acid; TBARS, Thiobarbituric acid reactive substances; TCA, Trichloroacetic acid; YPG, yeast-extract-peptone-glucose
1. Introduction
Oxygen is a highly reactive molecule. It can be somewhat reduced to reactive oxygen species (ROS) that are defined as small and highly chemically-reactive agents (Jamieson, 1998). They include free radicals such as; superoxide anion (O2•−) and hydroxyl radicals (•OH) as well as non-radical oxygen species for instance; hydrogen peroxide (H2O2) and singlet oxygen(1O2) (Kurutas, 2015, Sato et al., 2013). The production of ROS by biological systems, mitochondria mainly, is very important as they take part in many physiological processes such as apoptosis, immunity, differentiation and activation of many transcriptional factors… (Rajendran et al., 2014). Nevertheless, the surplus of ROS, that can be caused by environmental factors (e.g. UV, ionizing radiations, pollutants, and heavy metals, smoking…), generates a phenomenon known as oxidative stress which is an imbalance in the generation of pro-oxidants and the ability of antioxidants to neutralize them (Aouacheri et al., 2015, Kurutas, 2015, Pizzino et al., 2017). This will eventually cause damages in the cellular components like membranes, proteins, lipids, nucleic acid (Dröge, 2002) leading to several diseases that have been proven to be associated with oxidative stress such as diabetes (Pizzino et al., 2017), kidney disease (Galle, 2001), cancer (Valko et al., 2004), cardiovascular diseases (Bahoran et al., 2007), neurological diseases (Allan Butterfield, 2002) and respiratory diseases (Caramori, 2004). Antioxidants compounds defined as a defense system, including enzymatic and non-enzymatic compounds, are developed by the organism in order to prevent oxidative stress after consumption (Halliwell, 2008, Pizzino et al., 2017). Hence, the interest in the search for natural antioxidants sources that would be efficient to scavenge the surplus of free radicals has increased over the past few years. Moreover, the studies on essential oils (EOs) and extracts of medicinal and aromatic plants have increased as well as their use as alternatives for treatment of diseases owing to the presence of compounds such as polyphenols, terpenes and flavonoids that were shown to have many beneficial effects including antioxidant activity and have been used to treat many diseases attributable to their ability of free radicals scavenging (Adams, 2007; Nait Irahal et al., 2020, Nait Irahal et al., 2021, Santos-Buelga and Scalbert, 2000). It has been reported that Thymus vulgaris (El-Nekeety et al., 2011), Ocimum basilicum extracts (Kaurinovic et al., 2011) and Origanum compactum (Bouyahya and Jamal, 2016) have shown antioxidant properties. Also, previous studies reported that Rosmarinus officinalis oil has shown many biological effects including antibacterial and antifungal activities (Satyal et al., 2017). In addition, the phytochemical studies, the antibacterial and antimicrobial activities of Rosmarinus, Thymus, Origanum., Eucalyptus and Ocimum have been extensively investigated and were focused mainly on the hexane, chloroform, ethylacetate, acetone, methanol, ethanol and aqueous extracts. However, very few reports are available on the use of the essential oils as a protective potential against the oxidative stress in yeast cells. Therefore, the objective of this study is to present a screening and a comparative study on the antioxidant activity of five different industrial essential oils Rosmarinus officinalis, Ocimum basilicum L., Origanum compactum Benth., Eucalyptus globulus Labill. and Thymus vulgaris. Moreover, Saccharomyces cerevisiae was chosen as a model organism since that the mechanisms of defense against oxidative stress in S. cerevisiae were shown to be similar to humans (Meng et al., 2017).
The different essential oils were analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC/MS) and were investigated for their effect when it comes to the protection against oxidative stress induced by H2O2 in the yeast Saccharomyces cerevisiae by determining antioxidant and metabolic enzymes activity and level of lipid peroxidation and protein carbonylation.
2. Materials and methods
2.1. Chemicals and reagents
All the following chemicals used in this study were of high quality. Yeast extract, Agar and peptone were received from BIOKAR Diagnostics. Tris (hydroxymethyl) aminomethane, Hydrogen peroxide (H2O2), Glycerol, Phenylmethylsulfonyluoride (PMSF), Guanidine hydrochloride and 2-β-mercaptoethanol were purchased from Fluka. Potassium persulfate (K2S2O8) from SOCHID. Vitamin C and Trichloracetic acid (TCA) were bought from Scharlau (Spain). Bovine serum albumin (BSA) was purchased from Janssen Chimica. Potassium dihydrogen phosphate, Potassium cyanide (KCN), Ethylenediaminetetraacetic acid (EDTA), Sodium dodecyl sulphate (SDS) and Sodium carbonate were bought from Riedel-de Haën. Ethanol was received from Biosmart. Acetic acid, 2,6- Dichlorophenolindophenol (DCIP), Succinate, oxidized glutathione (GSSG), Thiobarbituric acid (TBA), Sodium chloride (NaCl), Nicotinamide adenine dinucleotide (NADH), Nicotinamide adenine dinucleotide phosphate (NADPH), 2,4- dinitrophenylhydrazine (DNPH) reagent and Magnesium chloride (MgCl2) were purchased from Sigma-Aldrich.
2.2. Essential oils
In this study five different EOs were used: rosemary (Rosmarinus officinalis), basil (Ocimum basilicum L.), oregano (Origanum compactum Benth.), eucalyptus (Eucalyptus globulus Labill.), thyme (Thymus vulgaris) all received in pure concentration from Naturactive Laboratoires Pierre Fabre France. The geographical origin and batch number of the EOs are shown in Table 1.
Table 1.
Geographic origin and batch numbers of essential oils.
| Essential oil | Part used | Batch number | Collection region | Chemotype |
|---|---|---|---|---|
| O. basilicum L. | Aerial part | 3,401,597,745,942 | Asia / North Africa | Linalool |
| R.officinalis | Leaves | 3,401,566,088,148 | North Africa | 1,8-cineole |
| O. compactum Benth. | Flowering top | 3,401,597,747,083 | North Africa | Carvacrol/ thymol |
| E. globulus Labill. | Leaves and twigs | 3,401,597,746,543 | Europe | 1,8-cineole/ α-pinene |
| T. vulgaris | Aerial part | 3,665,606,000,181 | Europe | Thymol |
2.3. Yeast strain and growth conditions
All the experiments were done using a wild type strain of S. cerevisiae YMES2, isolated from traditional Moroccan bread dough, kindly provided by Professor Faouzi Errachidi from Faculty of Sciences and Technologies of Fes (FST). Yeast strain was grown in liquid YPG medium (1% yeast extract, 1% peptone, 2% glucose) with an orbital shaker at 160 rpm, for 24 h at 30 °C with the ratio of flask volume/medium of 5/1.
2.4. Phytochemical analysis
2.4.1. Determination of total phenols
This assay was carried out using the Folin-Ciocalteau reagent and Gallic acid as standard by adding 0.5 mL of each essential oil and 2 mL of sodium carbonate (75 g. L-1) to 2.5 mL of 10% (v/v) Folin-Ciocalteau as reported by (Slinkard and Singleton, 1977). The mixture was left at room temperature for 30 min, then proceeded to the absorbance measurement using a wavelength of 765 nm. Tests were carried out in triplicate.
2.4.2. Gas chromatographic (GC)
Gas chromatographic analyses were performed using a Shimadzu GC-2010 Plus gas chromatograph equipped with a flame ionization detector (FID) and a BP-5 capillary column (30 m x0.25 mm i.d., film thickness 0.25 μm SGE Ltd). The oven temperature was programmed, 60–200 °C, at 3 °C.min-1, and then held isothermal for 5 min; injector and detector temperatures, 280 °C and 300 °C, respectively; carrier gas, nitrogen, adjusted to a linear velocity of 30 cm.s−1. The samples were injected using split sampling technique, ratio 1:50. The volume of injection was 0.2 μL of a pentane-volatiles solution (1:1)
2.4.3. GC–MS assay
A gas chromatography (GC) and gas chromatography-mass spectrometry (GC/MS) were used to analyze the essential oils. The GC–MS unit consisted on a Shimadzu GC-2010 gas chromatograph, equipped with BP-5 capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm; SGE, Ltd.), and interfaced with a Shimadzu QP2010 Plus mass spectrometer (software version 2.50 SU1). The oven temperature was programmed as described for GC analysis; transfer line temperature, 300 °C; ion source temperature, 200 °C; carrier gas, helium, adjusted to a linear velocity of 36.5 cm.s−1; split ratio, 1:40; ionization energy, 70 eV; scan range, 40 400 u; scan time, 1 s. Component identification was carried out by comparison of their retention indices relative to C9-C20 n alkanes on the BP-5 column (Adams, 2007), confirmed by comparison of recorded mass spectra with those of a computer library (Shimadzu corporation library and NIST05 database/ ChemStation data system) and from a home-made library, constructed based on the analyses of reference oils, laboratory-synthesized components and commercial available standards and other literature data.
2.5. Antioxidant activity
2.5.1. DPPH free radical scavenging assay
This assay was performed according to (Wu et al., 2019) and with some modifications. First of all, 50 μL of each EO at different concentrations (0,6–10 mg/mL) was added to 2 mL DPPH ethanol solution (60 μM). After that, the mixture was left in the dark at room temperature for 30 min then the absorbance was measured at 517 nm. The following equation was used to calculate the activity:
is the absorbance of the control after 30 min; is the absorbance of each EO after 30 min. Ascorbic acid was used as positive control. Tests were carried out in triplicate.
2.5.2. ABTS+ free radical scavenging assay
This test was performed in triplicate and as described by (Dorman et al., 2004). Thus, following to the reaction, of K2S2O8 (2.45 mM) with ABTS (7 mM) aqueous solution, in the dark and at room temperature during 16 h, the radical ABTS•+ solution was generated. Ethanol was added to the prepared solution to adjust the absorbance to 0.7. Samples at different concentrations were added to 9 mL of ABTS•+. The absorbance was measured at 734 nm at time 0 (A0) and after 6 min (A1). Ascorbic acid was used as positive control.
2.5.3. Reducing power
According to the method reported by (Oyaizu, 1986), the reductive power of the EOs samples was defined. The mixture contained: 0.2 M phosphate buffer (pH 6.6), 1% (w/v) potassium ferricyanide, 10% TCA and 0.1% ferric chloride. The absorbance was measured at 700 nm and the ascorbic acid was used as positive control. The assay was carried out in triplicate.
2.6. Cytotoxicity of EOs
The sensitivity of S. cerevisiae to all five EOs was carried out according to Tran and Green (2019) with minor modifications. EOs were used at different concentrations (6.25, 12.5, 25, 50 and 100 μg/mL) was determined after exposing yeast cells to EOs for 2 h then plated in agar for an additional 72 h. Under the same conditions a control was performed but without adding any EOs.
2.7. Oxidative stress induction
In this study, oxidative stress in S. cerevisiae was induced using Hydrogen peroxide (H2O2). Yeast cells at the first exponential phase growing in liquid YPG medium with an initial OD600nm = 0.26 were directly or pretreated with different concentrations of EOs (6,25–100 μg/ mL) for an hour, then incubated with H2O2 (2 mM) during 1 h at 30 °C/160 rpm (de Sá et al. 2013).
2.8. Tolerance determination
The determination of cell viability was determined in normal condition and after inducing oxidative stress, on cells treated or not with EOs. The analysis was performed in triplicate, by plating cells on solidified YPG medium (1% yeast extract, 1% peptone, 2% glucose and 2% agar) after a (10000 x) dilution (Castro et al., 2007, Dani et al., 2008). An incubation at 30 °C/72 h was done then colonies were counted. Survival was expressed as percentage.
2.9. Biochemical assays
2.9.1. Preparation of cell-free extract
After the treatment with H2O2 for 1 h at 30 °C/160 rpm, a centrifugation at 6000g for 5 min at 4 °C was done in order to harvest the yeast cells which were then washed three times with 20 mM Tris-HCl buffer (pH 7.5) and put back into suspension in the lysis buffer that contains 50 mM Tris-HCl buffer (pH 7.5), 1 mM ethylenediamine tetraacetic acid (EDTA), 10 mM 2-β- mercaptoethanol, 1 mM phenylmethylsulfony fluoride (PMSF), and 1% (v/v) glycerol at a ratio of 3 mL/g (wet weight). While disturbing the cells with cold, A Bandelin Sonopuls Sonifier (90%, 20 s, 12 × ) was then used, followed by a centrifugation (15,000g, 45 min at 4 °C) using a Sigma 2–16 K refrigerated centrifuge. The obtained supernatant was later on used for all enzyme activity assays. According to the Bradford procedure, the protein content was determined using bovine serum albumin (BSA) as standard (Bradford, 1976).
2.9.2. Detection of superoxide dismutase (SOD) activity
The superoxide dismutase (SOD) activity measurement was based on the determination of the ability of enzyme extract to inhibit the oxidation of NADH caused by superoxide radicals that produced in a chemical system according to (Paoletti et al., 1986), using 5 mM EDTA, 2.5 mM MgCl2, 3.9 mM 2-mercaptoethanol, 0.27 mM NADH in 50 mM potassium phosphate buffer (pH 7) and 50 μL enzyme extract. The reaction kinetics was measured at a wavelength of 340 nm. The SOD activity unit is the amount of enzyme required to inhibit the oxidation of the initial rate of NADH by 50%, expressed in U/mg of protein.
2.9.3. Determination of catalase (CAT) activity
The CAT activity was determined by following the consumption of H2O2. The reaction mixture contained 7.5 mM H2O2 in 50 mM sodium phosphate buffer (pH 7.0) and 50 μL of the enzyme extract. The kinetics was measured at 240 nm (Aebi, 1984). The molar extinction coefficient of H2O2 (0.0394 mM−1cm−1) was used to calculate the CAT activity. It is defined as μmol H2O2 consumption/min/mg of protein.
2.9.4. Determination of glutathione reductase (GR) activity
The GR activity was determined based on monitoring at 340 nm the decrease in absorbance due to the oxidation of NADPH as described by (Di Ilio et al., 1983). For this purpose, a reaction mixture was used containing: 1 mM EDTA, 0.5 mM GSSG, 50 mM potassium phosphate buffer (pH 7.4) and 50 μL of enzyme extract, all incubated for 2 min at 37 °C, then 100 μL of NADPH (0.1 mM) was added. The kinetics of the activity was measured at a wavelength of 340 nm. The unit of the activity was expressed as nmol NADPH oxidized per min per mg of protein.
2.9.5. Determination of lipid peroxidation
The determination of lipid peroxidation was based on the ability of the extracts to inhibit the formation of malondialdehyde using thiobarbituric acid reactive substances (TBARS) as reported elsewhere (Samokyszyn and Marnett, 1990). The method consisted of transferring 1 mL of extract into 1 mL solution of 0.375% thiobarbituric acid and 15% trichloracetic acid in 0.25 M hydrochloric acid. The mixture was heated to 100 °C for 15 min, then quickly cooled using ice in order to stop the reaction. Centrifugation is then carried out at 1,000 g for 10 min. The absorbance of supernatant was measured at 535 nm and the results were defined as nmoles MDA equivalents per mg protein.
2.9.6. Determination of protein carbonyl content (PCO)
The level of carbonyl group was determined according to Levine’s method (Levine, 2002). Concisely, 100 µL of the simple was mixed with 400 µL of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.5 M HCl. After incubation for 60 min at room temperature, proteins were then precipitated using 500 µL of 20% (w/v) trichloroacetic acid (TCA), left on ice for 5 min, and centrifuged at 10,000g for 10 min at 4 °C. The protein pellet was washed 3 times by 500 µL of 1:1 (v/v) ethanol: ethyl acetate solution. The final protein pellet was resuspended in 250 µL of 6 M guanidine hydrochloride. The absorbance was read at 370 nm. The protein carbonyl group of each sample was calculated by using absorption coefficient (ɛ = 22,000 M−1cm−1). The protein carbonyl content was expressed as nmol/mg of protein.
2.9.7. Succinate dehydrogenase (SDH) activity assay
The measurement of the SDH activity was done according to (King, 1967), based on the reduction of Dichlorophenolindophenol (DCIP) which is known as a chemical compound used as redox dye by the change of its blue color. The activity was measured at a wavelength of 625 nm, using 0.053 mM DCIP, 0.3 mM EDTA in 100 mM potassium phosphate buffer (pH 7.4) and 50 μL enzyme extract. The reaction mixture was incubated at 25 °C for 10 min, after that 50 μL of KCN-Succinate (3.25 mg/mL of KCN in 0.5 M succinate) was added. The unit (μmol DCIP reduced/min/mg protein) was determined using molar extinction coefficient of DCIP (19,100 M−1 cm−1).
2.10. Statistical analysis
The data are represented as the mean ± standard deviation (SD) of at least three independent experiments. Statistical analyses were made using one-way analysis of variance (ANOVA) and Tukey's post-hoc test using the Prism 7 software for Windows (GraphPad Software Inc., San Diego, CA, USA). The probability value of p < 0.05 was considered to denote a statistical significance difference.
3. Results
3.1. Chemical composition of EOs
The GC/MS analysis has allowed the determination of the chemical composition of EOs. Results in Table 2 below present the percentage composition of the compounds of all the essential oils used, showing the identification of a total of 16 components for eucalyptus (E. globulus), 19 for rosemary (R. officinalis) and both of them had cineole (eucalyptol) (46.84%, 66.32%) and α-Pinene (12.58%, 13.95%) respectively as main components. For the other oils, 22 components were determined for thyme (T. vulgaris), 33 for oregano (O. compactum) and 40 for basil (O. basilicum). The major components were linalool (63.95%) in O. basilicum, carvacrol (48.16%) in O. compactum and thymol (76.66%) in T. vulgaris (Table 3).
Table 2.
Chemical composition of five essential oils.
| Compounds | Kovàts Index |
O.Basilicum (Area %) |
O.compactum (Area%) |
T. vulgaris (Area%) |
R. officinalis (Area%) |
E. globulus (Area%) |
|---|---|---|---|---|---|---|
| α-Pinene (-)-β- Pinene β-Myrcene 3-Octanone Octodrine Octane 1-Octen-3-ol o-Cymene Cymen-8-ol trans-1,2-bis-(1-methylethenyl) cyclobutane 1,8-Cineole Tricyclene β-cis-Ocimene γ-Terpinene δ-2-Carene Linalool α-Phellandrene Camphene Camphor Borneol Menthol d-Alaninol Terpinen-4-ol α-Terpineol Anisole, p-allyl- 2,4-Dimethylheptane (R)-Citronellol cis-Geraniol α-Citral 2,5-Dimethylstyrene Isothymol methyl ether Thymol Bornyl, acetate 3-Allylguaiacol Neryl acetate l-Verbenone β-Bourbonene β-Elemene Caryophyllene Trans-α-Bergamotene Carvacrol α-Guaiene α-Humulene trans-Muurola-4(14),5-diene trans-Pinocarveol D-Germacrene β-Sesquiphellandrene γ-Elemene Caryophyllene oxide α-Bulnesene γ-Cadinene δ-Cadinene Nerolidol α-Pinocarvone Diethyl phthalate Cubenol γ -Muurolene Ageratochromene α-Thujene Neryl propanoate 3-Carene (+)-4-Carene (+)-Sylvestrene cis-Sabinene hydrate 4a-Methyl 1,2,3,4,4a,5,6,7octahydro naphthalene 2,6-Dimethyl-6-heptafluorobutyryloxyoctane α-Terpinyl acetate Spathulenol Viridiflorol Bisphenol indane |
0932 0974 0988 0979 0800 0974 1022 1176 1026 0921 1017 1054 1001 1095 1002 0946 1141 1165 1167 1174 1186 1223 1249 1264 1099 1232 1289 1284 1359 1204 1387 1389 1417 1432 1298 1437 1452 1493 1135 1484 1521 1434 1582 1509 1513 1522 1561 1160 1590 1645 1478 1658 0924 1452 1008 1025 1065 1346 1577 1592 |
0.31 0.42 0.32 0.30 0.39 4.68 0.64 0.15 0.19 63.95 0.38 0.30 0.57 1.65 0.52 0.33 0.17 4.44 0.20 1.14 3.15 0.63 0.19 1.04 0.38 3.53 0.33 0.44 0.28 1.84 0.17 0.20 0.50 1.81 0.39 0.22 1.28 0.32 2.03 0.22 |
0.60 1.14 0.07 0.08 0.03 0.03 12.21 0.08 0.07 0.03 14.34 1.22 0.14 0.09 0.20 0.57 0.18 0.11 0.17 15.68 1.19 48.22 0.44 0.05 0.69 0.10 0.16 0.07 1.38 0.38 0.05 0.09 |
0.53 1.07 0.27 5.51 0.09 2.03 2.19 0.14 0.09 0.33 0.81 0.09 0.42 4.07 76.66 0.15 0.58 0.94 0.36 0.28 |
12.58 2.62 0.43 0.19 2.17 2.00 46.84 0.15 0.90 4.24 16.30 2.73 0.39 1.74 0.53 0.26 1.25 0.14 1.28 |
13.95 1.28 3.13 66.32 0.74 0.37 0.55 1.70 0.62 5.17 1.24 1.03 1.51 0.87 0.42 |
Table 3.
Chemical structure of main compounds identified in the EOs studied.
| Plants | Main compound | Structure |
|---|---|---|
| O. compactum | Carvacrol | 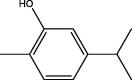 |
|
E. globulus R. officinalis |
Cineole | 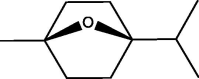 |
| α-Pinene | 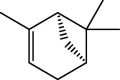 |
|
| O. basilicum | Linalool | |
| T. vulgaris | Thymol | 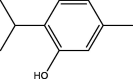 |
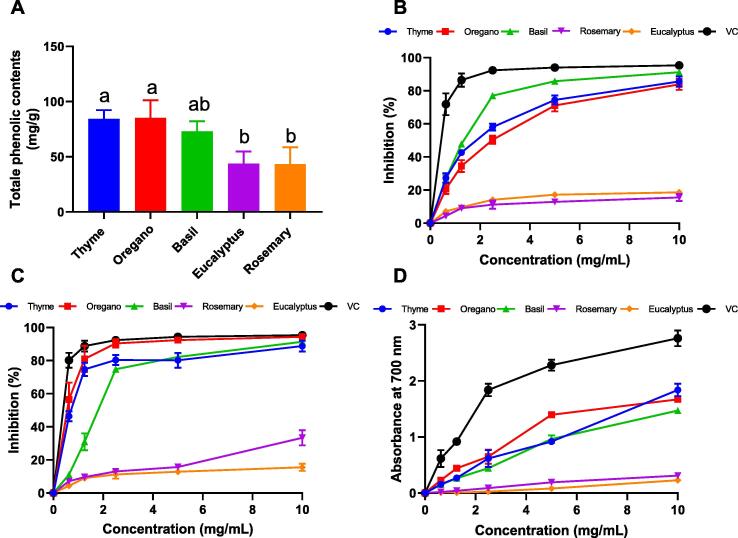
The quantification of the total phenolic content of each EO using the Folin-Ciocalteau method presented in Fig. 1A showed that O. compactum had the highest amount of total phenols (85.19 mg GAE/g) followed by T. vulgaris with a total phenolic content of (84.36 mg GAE/g) and O. basilicum with an amount of (73.03 mg GAE/g). The results obtained are in accordance with the chemical composition of the oils since carvacrol and thymol are the main phenolic constituents according to their chemical structures. However, the essential oils of E. globulus and R. officinalis had lower amounts of total phenols 43.64 and 43.28 mg GAE/g respectively.
Fig.1.
(A) Total phenol content (mg/g) and in vitro antioxidant activity of the EOs by (B) DPPH free radicals scavenging (C) ABTS free radicals scavenging and (D) Reducing power. Values are means ± SD of three independent experiments performed in triplicate. a-b Means without a common superscript letter differ (p < 0.05), as analyzed by one-way ANOVA.
3.2. In vitro antioxidant activity of EOs
In this study, the antioxidant activity of the EOs was carried out using three tests; ABTS, DPPH free radical scavenging and reducing power. Results showed that basil, thyme and oregano had a high antioxidant activity against ABTS•+ and DPPH• radicals and were also characterized by a high reducing power. However, rosemary and eucalyptus showed a low antioxidant activity and very low RP compared to the antioxidant activity of ascorbic acid used as control (Fig. 1). When it comes to DPPH free radicals scavenging, basil had the highest antioxidant activity in a dose–dependent manner with a percentage of inhibition of free radicals near values of ascorbic acid at (10 mg/mL) followed by thyme then oregano (Fig. 1B). As for ABTS free radicals scavenging (Fig. 1C), oregano presented a very high activity similar to the activity of ascorbic acid at (10 mg/mL) followed by basil and thyme.
3.3. Sensitivity of S. Cerevisiae to EOs
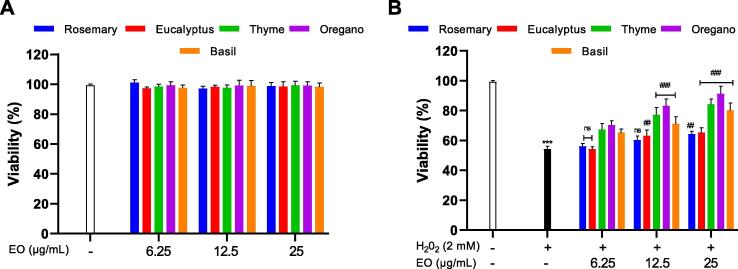
To evaluate the cytotoxicity of the EOs on S. cerevisiae, a range of concentrations of all EOs (6,25–100 μg/mL) was tested. Results showed toxicity at 50 and 100 μg/mL (data not shown), as for concentrations 6.25–25 μg/mL EOs did not show any toxicity effect (Fig. 2A). However, the nontoxic concentrations 6.25–25 μg/mL were chosen to be used later on for the determination of the protective effect of EOs.
Fig. 2.
(A) Dose response effect of the EOs on the viability of S. cerevisiae cells after 72 h of incubation; (B) Effect of EOs treatment on cellular survival after exposure to H2O2. S. cerevisiae cells were pretreated with the essential oils at different concentrations (6.25–100 μg/mL) for 1 h, followed by incubation with H2O2 for another hour. Data for 50 and 100 ug/ml were not shown. The data represent the mean of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with untreated control cells, #P < 0.05, ##P < 0.01 and ###P < 0.001, compared with cells treated with H2O2 only.
3.4. Effect of EOs on cytotoxicity induced by H2O2
In order to investigate the effect of EOs on cytotoxicity induced by H2O2, a pretreatment of the cells with EOs was carried out. Results presented in (Fig. 2B) showed compared to the control cells, a significant decrease of viability to 54%± 2.01 (P < 0.001) when exposured to H2O2. The viability has been restored remarkably after pretreatment with the following EOs: basil (O. basilicum), thyme (T. vulgaris) and oregano (O. compactum) at different concentrations (6.25–25 μg/mL) by 11–26%, 13–30%, 16–47% respectively. As for rosemary (R. officinalis) and eucalyptus (E. globulus) the viability was slightly ameliorated at (25 μg/mL) by 10–11% compared to H2O2 alone treated cells.
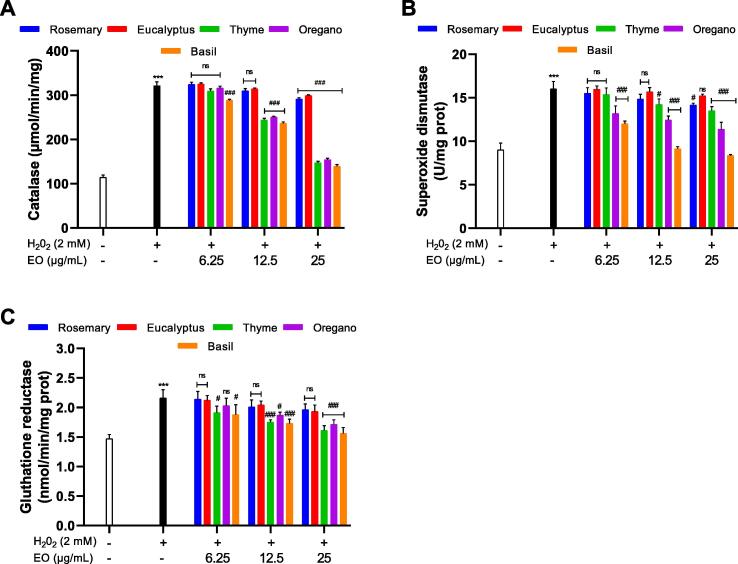
3.5. Antioxidant enzymes activity
The investigation of the effect of H2O2 and the EOs on the activity of antioxidant enzymes in S. cerevisiae showed (Fig. 3) a significant increase in the activity after the exposure of S. cerevisiae cells to H2O2 compared to the control sample. Thus, the activity of CAT, SOD and GR were higher by ∼ 281%, ∼166% and ∼ 146%, (P < 0.001) respectively. After the pretreatment with the EOs (6.25–25 μg/mL); thyme (T. vulgaris), oregano (O. compactum) and basil (O. basilicum) and the exposure to H2O2, the activity of the antioxidant enzymes CAT, SOD and GR decreased to be comparable to levels of control sample with the increase in dose of the essential oils. However, it was observed that the decrease of the activity after the treatment with rosemary (R. officinalis) and eucalyptus (E. globulus) was not significant.
Fig.3.
The effect of the essential oils on the activity of the antioxidant enzymes CAT (A), SOD (B) and GR (C) in S. cerevisiae. Yeast cells were pretreated with different concentrations of EOs (6.25–25 μg/ml) for 1 h, followed by incubation with H2O2 for another 1 h. The data represent the mean of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with untreated control cells, #P < 0.05, ##P < 0.01 and ###P < 0.001, compared with cells treated with H2O2 only.
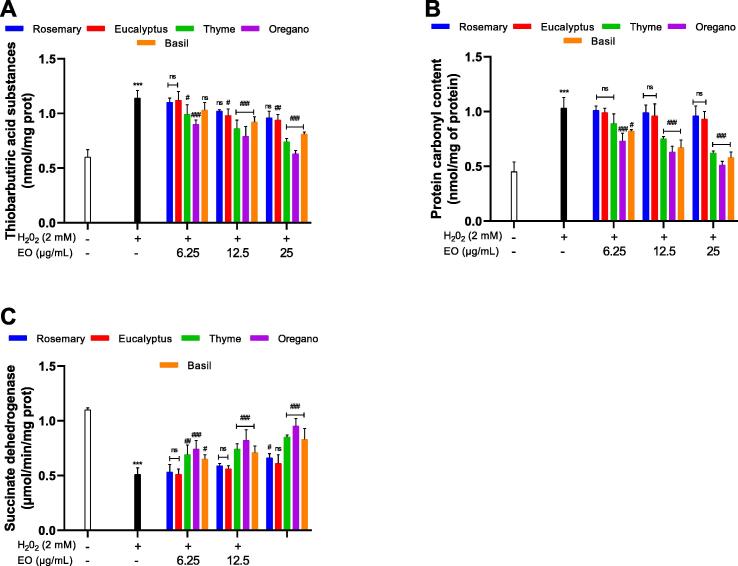
3.6. Lipid peroxidation
One of the consequences of oxidative stress is the damage of the intracellular components such as lipids. In this study, in order to investigate this intracellular damage, level of lipid peroxidation (LPO) was used as a marker (Fig. 4A). Thus, LPO was determined using the level of TBA‐MDA adduct that showed a level of 0.61 ± 0.070 nmol/mg of protein in the control sample. However, When the cells were exposed to H2O2 This level increased significantly to 1.14 ± 0.075 nmol/mg of protein (P < 0.001), that to say 1.86-fold. As for the pretreatment with EOs (6.25–25 μg/mL) showed that rosemary and eucalyptus decreased slightly at (25 μg/mL) the TBARS level by 1.18-fold (0.96 ± 0.066 nmol/mg of protein), and 1.21-fold (0.94 ± 0.050 nmol/mg of protein) respectively. Although, the treatment with the other EOs at (6.25–25 μg/mL); thyme, oregano and basil decreased significantly the TBARS level by 1.15–1.54-fold (0.99 ± 0.091–0.74 ± 0.031 nmol/mg of protein, P < 0.001), 1.26–1.80-fold (0.90 ± 0.04 – 0.63 ± 0.032 nmol/mg of protein, P < 0.001) and by 1.10–1.40-fold (1.03 ± 0.07 – 0.81 ± 0.02 nmol/mg of protein, P < 0.001) respectively, compared to H2O2 treated cells.
Fig.4.
The effect of EOs on lipid peroxidation(A), PCO content (B) and (C) SDH activity in yeast cells treated with H2O2.The data represent the mean of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with untreated control cells, #P < 0.05, ##P < 0.01 and ###P < 0.001, compared with cells treated with H2O2 only.
3.7. Protein carbonyl content (PCO)
Results showed that the concentration of protein carbonyls was significantly higher by 2.3 fold (1.03 ± 0.1 nmol/mg of protein; P < 0. 001) in H2O2 treated cells compared to the control sample (0.452 ± 0.09 nmol/mg of protein). Levels of protein carbonyls decreased significantly in pretreated cells with EOs of oregano, basil and thyme at (6.25–25 μg/mL) in a dose dependent manner by 1.41–2.01fold (0.73 ± 0.072 – 0.51 ± 0.035 nmol/mg of protein), 1.25–1.77 fold (0.82 ± 0.015 – 0.58 ± 0.05 nmol/mg of protein) and by 1.15–1.66 fold (0.89 ± 0.084 – 0.62 ± 0.021 nmol/mg of protein) respectively, compared to H2O2 treated cells. Whilst, the pretreatment with EOs of rosemary and eucalyptus decreased protein carbonyls levels slightly at (25 μg/mL) by 1.07-fold (0.96 ± 0.09 nmol/mg of protein) and 1.10-fold (0.93 ± 0.07 nmol/ mg of protein) respectively, compared to H2O2 treated cells (Fig. 4B).
3.8. Metabolic enzyme activity (SDH)
In this work, the effect of H2O2 induced oxidative stress and EOs on the activity of succinate dehydrogenase (SDH) which is considered as a metabolic enzyme was also investigated (Fig. 4C). It was observed, compared to the control sample, that the activity decreased remarkably to 46.36% (P < 0.01) after the exposure to H2O2. The treatment with the EOs rosemary and eucalyptus showed a very slight increase in the activity of SDH at (12.5–25 μg/mL) by 1.04–1.29-fold (0.53 ± 0.07–0.66 ± 0.04 µmol/min/mg of protein) and 1–1.19-fold (0.51–0.61 µmol/min/mg of protein) respectively. However, the treatment with thyme, oregano and basil increased significantly the activity of SDH in a dose dependent manner to reach a value comparable to the control sample at (25 μg/mL).
4. Discussion
Excessive production of reactive oxygen species (ROS) can exceed the ability of the natural antioxidant defense system of the cells and, in long term, lead to the development of various oxidative stress-associated diseases such as cardiovascular diseases, diabetes, cancer, Alzheimer’s disease (Huang et al., 2006, Martins et al., 2015, Valko et al., 2007). Therefore, antioxidants supplementation can help to maintain an optimal biological system by removing excessive concentrations of free radicals (Deetae et al., 2012, Guenaou et al., 2021b). In recent years, there has been a great interest in the use of phenolic compounds including phenolic acids and flavonoids derived from plants which are naturals, safe and powerful antioxidant molecules that exert their action through a variety of mechanisms including increasing antioxidant enzymes activity, chelating ions, eliminating ROS and inhibiting lipid peroxidation (Ganesan and Baojun, 2017, Nakagawa et al., 2002). Hence, the aim of this study was to investigate the efficacy of five different essential oils in protecting against H2O2-induced oxidative stress in S. cerevisiae cells used as a cellular model.
Firstly, the results of GC/MS showed a chemical variability within tested EOs. It has been determined that the major chemical components of rosemary and eucalyptus were Cineole/ Eucalyptol and α-Pinene respectively. Linalool was the main component in basil, Carvacrol was the most present component in oregano as well as thymol, cymene and terpinene. As for thyme, the main components were thymol and caryophyllene. All of these components are behind many biological activities of EOs including antioxidant activity (Bhavaniramya et al., 2019, Dhifi et al., 2016). Also, we suggest that the higher content of phenolic molecules contribute greatly to the antioxidant activity observed, particularly, the main constituents such as carvacrol and thymol. In addition, it is admitted that the OH group found in linalool, thymol and carvacrol exhibited and showed very high antioxidant activity (Friedman, 2014). The investigation of the total phenolic content quantified using the Folin-Ciocalteau method showed high amounts were present in Oregano, basil and thyme. While for rosemary and eucalyptus, low amounts of phenols have been observed. It has been reported in previous studies that phenolic compounds were shown to have various biological effects such as antioxidant activity (Sanchez-Moreno, 2002). Furthermore, in vitro assays (ABTS, DPPH and reducing power) carried out to evaluate the antioxidant activity specifically free radicals scavenging ability of the EOs also indicated a significant and important antioxidant activity against ABTS•+ and DPPH• radicals and a high reducing power in thyme, basil and oregano. Whereas, rosemary and eucalyptus were showed to be less capable of scavenging ABTS•+and DPPH• free radicals and a low reducing power comparing to ascorbic acid used as standard sample. Therefore, these obtained results are in line with results reported in many previous studies in which cells were treated adding phenolic compounds and using different pro-oxidants, all showing a lower production of ROS than cells treated with pro-oxidants only (Flora et al., 2013, Meng et al., 2017). Phenolic compounds were reported to be involved in in neutralizing free radicals (Sanchez-Moreno, 2002).
A preliminary study on the cytotoxic effect of all the EOs on S. cerevisiae showed no cytotoxic effect at a range of concentrations (6.25–25 μg/mL), but at higher concentrations EOs were toxic (50 μg/mL and above). This may be explained, as a reversed effect of phenolic compounds that goes from being beneficial to lethal for cells due to excessive doses (McGaw et al., 2014). Also, the damages of H2O2 induced stress such as cell death have been prevented after the pretreatment with EOs showing a clearly restored cell viability after the use of EOs compared to cells treated with H2O2 only.
In order to control the ROS generation and protect cells from their oxidative damage, an enzymatic defense system where antioxidant enzymes; catalase (CAT), superoxide dismutase (SOD) and glutathione reductase (GR) work all together in a cooperative way. Hence, SOD catalyzes the conversion of superoxide anions to hydrogen peroxide (H2O2). CAT, efficient in scavenging H2O2, will later on catalyze the conversion of H2O2 to H2O and O2. As for GR, It is involved in the scavenging of ROS by providing consistently intermediate metabolites for instance reduced glutathione (GSH) that when present, GPx accelerates the reduction of hydrogen peroxide and hydroperoxides (Burke, 2010, Schieber and Chandel, 2014, Tongul and Tarhan, 2016). In our study, a significant increase in the activity of the antioxidant enzymes activity was observed when cells were exposed to H2O2 only compared to control. Interestingly, the pretreatment of yeast cells with of T. vulgaris, O. compactum and O. basilicum decreased in a dose dependent manner the activity of CAT, SOD and GR back to their normal values as compared control. This can be attributed to the phytochemical composition of EOs rich in phenolic compounds that are known to scavenge ROS (Sanchez-Moreno, 2002).
Lipid peroxidation which is caused by an excess of ROS that react with polyunsaturated fatty acids in cell membranes, leading to the production of malondialdehyde (MDA) that can eventually be used to determine the level of LPO in biological models (Jia et al., 2019, Parthasarathy et al., 1999). Enhanced MDA formation was previously observed in S. cerevisiae following oxidative stress induction (Meng et al., 2017, Piechowiak and Balawejder, 2019). The present study showed that the level of MDA increased significantly after H2O2 exposure compared to the control. The pretreatment with rosemary and eucalyptus had a very slight and non-significant effect on the level of TBA‐MDA except at (25 μg/mL). Contrariwise, the pretreatment with the remaining EOs (oregano, basil and thyme) significantly reduced the level of LPO (p < 0.001) in a dose dependent manner near their normal level. This effect of EOs can be explained by the presence of phenolic compounds. It has been shown, that phenolic compounds are able to inhibit LPO in cells by producing stable phenoxy radical species that are not capable of any other radical reaction (Meng et al., 2017, Yan et al., 2020). They are able to inhibit LPO at all stages of the cycle, hence the reduction of the degree of the oxidative damage caused in cells constituents (Piechowiak and Balawejder, 2019). Also, this effect was noticed in treated yeast with propolis and quecertin in H2O2 induced stress (de Sá et al. 2013).
Protein carbonylation is associated with oxidative stress related damages. It is irreversible and is considered as one of the most damaging oxidative protein modifications. Protein carbonyl can be formed after oxidative modifications on arginine, proline, histidine and lysine residues, as well as by oxidative cleavage of the peptide chain. The measurement of protein carbonyl content is used to evaluate the degree of the cellular damages related to oxidative stress (Fedorova et al., 2014, Pirinccioglu et al., 2010). In our study, PCO level was remarkably higher in H2O2-treated cells compared to the control sample. Pretreatment with EOs (oregano, basil and thyme) showed a significant protective effect against oxidative damage and stabilizes the protein against oxidation. This can be explained by the antioxidant potential of phenolic compounds found in the EOs to suppress protein carbonylation (Kızıl et al., 2011).
Succinate dehydrogenase (SDH), is an enzyme of the respiratory chain present in the mitochondria of yeast cells. It catalyzes reactions leading to the oxidation of succinate to fumarate in Krebs cycle and the reduction of ubiquinone using the cofactor FAD (Robinson and Lemire, 1996, Rustin, 2002, Sadowska-Bartosz et al., 2013). SDH is considered as a metabolic marker used to evaluate oxidative stress (Mountassif et al., 2007). The expression of SDH is important to produce energy in cells (Guenaou et al., 2021a, Rustin et al., 2002). Moreover, It has been reported in previous studies that H2O2 can inactivate and disable enzymes that contribute to the production of cellular energy (Tretter and Adam-Vizi, 2000). In the present study, a remarkable decrease in the SDH activity was observed in H2O2 stressed cells. The supplementation with EOs especially (oregano, basil and thyme) restored the activity of SDH which displays their antioxidant activity. This results suggest the contribution again of phenolic compounds present in the essential oils in this antioxidant defense since as It has been shown, phenolic compounds are involved in scavenging free radicals and decomposing peroxides… (Dai and Mumper, 2010, Meng et al., 2017, Piechowiak and Balawejder, 2019, Sanchez-Moreno, 2002).
5. Conclusion
We can conclude that the oxidative stress induced in yeast cells by using H2O2 has caused so many damages which was manifested in a high antioxidant enzymes activity in addition of lipid peroxidation and damages of cellular components. Yet the use of the essential oils of T. vulgaris, O. compactum and O. basilicum as a natural source of antioxydants displayed a protective potential against the oxidative stress in yeast cells. Eventually, after the pretreatment with EOs, the activity of antioxidant and metabolic enzymes was brought back to normal values as well as a reduced lipid peroxidation and protein carbonylation. To sum up, further research could be conducted on these efficient oils that can be of great use to protect organisms against the serious damages and diseases associated with oxidative stress.
CRediT authorship contribution statement
Khadija Ridaoui: Conceptualization, Writing – original draft. Ismail Guenaou: Conceptualization, Formal analysis. Ikram Taouam: Conceptualization. Mounia Cherki: Conceptualization. Noureddine Bourhim: Conceptualization. Abdelaziz Elamrani: Methodology, Investigation. Mostafa Kabine: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors are very grateful for the Naturactive Laboratoires Pierre Fabre France, for the made available the precious essential oils. The support from the National Center for Scientific and Technical Research (CNRST) within the Research Excellence Scholarship Program is gratefully acknowledged.
Consent to Publish We confirm that the manuscript has been read and approved and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams R.P. 4th ed. Allured Pub. Corp; Carol Stream, Ill: 2007. Identification of essential oil components by gas chromatography/mass spectorscopy. [Google Scholar]
- Aebi, H., 1984. Catalase in vitro, in: Methods in Enzymology. Elsevier, pp. 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3. [DOI] [PubMed]
- Allan Butterfield D. Amyloid β-peptide (1–42)-induced Oxidative Stress and Neurotoxicity: Implications for Neurodegeneration in Alzheimer’s Disease Brain. A Review. Free Radical Res. 2002;36(12):1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Aouacheri O., Saka S., Krim M., Messaadia A., Maidi I. The Investigation of the Oxidative Stress-Related Parameters in Type 2 Diabetes Mellitus. Can. J. Diabetes. 2015;39:44–49. doi: 10.1016/j.jcjd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Bahoran T., Soobrattee M.A., Luximon-Ramma V., Aruoma O.I. Free Radicals and Antioxidants in Cardiovascular Health and Disease. Internet J. Med. Update – J. 2007;1 doi: 10.4314/ijmu.v1i2.39839. [DOI] [Google Scholar]
- Bhavaniramya S., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019;2(2):49–55. doi: 10.1016/j.gaost.2019.03.001. [DOI] [Google Scholar]
- Bouyahya A., Jamal A. Origanum compactum Benth: A Review on Phytochemistry and Pharmacological Properties. Med. Aromatic Plants. 2016;05(04) doi: 10.4172/2167-041210.4172/2167-0412.1000252. [DOI] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke K.E. Photoaging: the role of oxidative stress. G Ital Dermatol Venereol. 2010;145:445–459. [PubMed] [Google Scholar]
- Caramori G. Oxidants and asthma. Thorax. 2004;59(2):170–173. doi: 10.1136/thorax.2002.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, F.A.V., Herdeiro, R.S., Panek, A.D., Eleutherio, E.C.A., Pereira, M.D., 2007. Menadione stress in Saccharomyces cerevisiae strains deficient in the glutathione transferases. Biochimica et Biophysica Acta (BBA) - General Subjects 1770, 213–220. https://doi.org/10.1016/j.bbagen.2006.10.013. [DOI] [PubMed]
- Dai, J., Mumper, R.J., 2010. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 15, 7313–7352. https://doi.org/10.3390/molecules15107313. [DOI] [PMC free article] [PubMed]
- Dani C., Bonatto D., Salvador M., Pereira M.D., Henriques J.A.P., Eleutherio E. Antioxidant Protection of Resveratrol and Catechin in Saccharomyces cerevisiae. J. Agric. Food. Chem. 2008;56(11):4268–4272. doi: 10.1021/jf800752s. [DOI] [PubMed] [Google Scholar]
- Deetae P., Parichanon P., Trakunleewatthana P., Chanseetis C., Lertsiri S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem. 2012;133(3):953–959. doi: 10.1016/j.foodchem.2012.02.012. [DOI] [Google Scholar]
- Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Polidoro G., Arduini A., Muccini A., Federici G. Glutathione peroxidase, glutathione reductase, glutathione S-transferase, and γ-glutamyltranspeptidase activities in the human early pregnancy placenta. Biochem. Med. 1983;29(2):143–148. doi: 10.1016/0006-2944(83)90034-0. [DOI] [PubMed] [Google Scholar]
- Dorman H.J.D., Bachmayer O., Kosar M., Hiltunen R. Antioxidant Properties of Aqueous Extracts from Selected Lamiaceae Species Grown in Turkey. J. Agric. Food. Chem. 2004;52(4):762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- El-Nekeety A.A., Mohamed S.R., Hathout A.S., Hassan N.S., Aly S.E., Abdel-Wahhab M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon. 2011;57(7-8):984–991. doi: 10.1016/j.toxicon.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Fedorova M., Bollineni R.C., Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies: PROTEIN CARBONYLATION: AN ANALYTICAL UPDATE. Mass Spec. Rev. 2014;33:79–97. doi: 10.1002/mas.21381. [DOI] [PubMed] [Google Scholar]
- Flora S.J.S., Shrivastava R., Mittal M. Chemistry and Pharmacological Properties of Some Natural and Synthetic Antioxidants for Heavy Metal Toxicity. Curr. Med. Chem. 2013;20:4540–4574. doi: 10.2174/09298673113209990146. [DOI] [PubMed] [Google Scholar]
- Friedman M. Chemistry and Multibeneficial Bioactivities of Carvacrol (4-Isopropyl-2-methylphenol), a Component of Essential Oils Produced by Aromatic Plants and Spices. J. Agric. Food. Chem. 2014;62(31):7652–7670. doi: 10.1021/jf5023862. [DOI] [PubMed] [Google Scholar]
- Galle J. Oxidative stress in chronic renal failure. Nephrol. Dial. Transplant. 2001;16:2135–2137. doi: 10.1093/ndt/16.11.2135. [DOI] [PubMed] [Google Scholar]
- Guenaou I., Hmimid F., Lahlou F.A., Errami A., Irahal I.N., Fahde S., Ouafik ’., Bourhim N. Cytoprotective effect of ethyl acetate fraction from Ephedra fragilis on H2O2-induced oxidative damage in Tetrahymena pyriformis. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2021;239:108899. doi: 10.1016/j.cbpc.2020.108899. [DOI] [PubMed] [Google Scholar]
- Guenaou I., Nait Irahal I., Errami A., Lahlou F.A., Hmimid F., Bourhim N. Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules. 2021;26:5998. doi: 10.3390/molecules26195998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys., Highlight Issue: Polyphenol Health. 2008;476(2):107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Huang Y.-H., Shih C.-M., Huang C.-J., Lin C.-M., Chou C.-M., Tsai M.-L., Liu T.P., Chiu J.-F., Chen C.-T. Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J. Cell. Biochem. 2006;98(3):577–589. doi: 10.1002/(ISSN)1097-464410.1002/jcb.v98:310.1002/jcb.20772. [DOI] [PubMed] [Google Scholar]
- Jamieson D.J. Oxidative stress responses of the yeastSaccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jia R., Li Y., Cao L., Du J., Zheng T., Qian H., Gu Z., Jeney G., Xu P., Yin G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus) Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2019;215:56–66. doi: 10.1016/j.cbpc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Kaurinovic B., Popovic M., Vlaisavljevic S., Trivic S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules. 2011;16:7401–7414. doi: 10.3390/molecules16097401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T.E. Preparations of succinate—cytochrome c reductase and the cytochrome b-c1 particle, and reconstitution of succinate-cytochrome c reductase. Methods Enzymol. Elsevier. 1967:216–225. [Google Scholar]
- Kızıl G., Kızıl M., Çeken B., Yavuz M., Demir H. Protective Ability of Ethanol Extracts of Hypericum Scabrum L. and Hypericum Retusum Aucher Against the Protein Oxidation and DNA Damage. Int. J. Food Prop. 2011;14(4):926–940. doi: 10.1080/10942910903491181. [DOI] [Google Scholar]
- Ganesan K., Baojun X.u. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients. 2017;9:455. doi: 10.3390/nu9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2015;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.L. Carbonyl modified proteins in cellular regulation, aging, and disease2,3 2Guest Editor: Earl Stadtman 3This article is part of a series of reviews on “Oxidatively Modified Proteins in Aging and Disease”. The full list of papers may be found on the homepage of the journal. Free Radical Biol. Med. 2002;32(9):790–796. doi: 10.1016/S0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Martins N., Barros L., Santos-Buelga C., Silva S., Henriques M., Ferreira I.C.F.R. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015;167:131–137. doi: 10.1016/j.foodchem.2014.06.094. [DOI] [PubMed] [Google Scholar]
- McGaw L.J., Elgorashi E.E., Eloff J.N. Toxicological Survey of African Medicinal Plants. Elsevier; 2014. Cytotoxicity of African medicinal plants against normal animal and human cells; pp. 181–233. [DOI] [Google Scholar]
- Meng D., Zhang P., Li S., Ho C.-T., Zhao H. Antioxidant activity evaluation of dietary phytochemicals using Saccharomyces cerevisiae as a model. J. Funct. Foods. 2017;38:36–44. doi: 10.1016/j.jff.2017.08.041. [DOI] [Google Scholar]
- Mountassif D., Kabine M., Manar R., Bourhim N., Zaroual Z., Latruffe N., El Kebbaj M.S. Physiological, morphological and metabolic changes in Tetrahymena pyriformis for the in vivo cytotoxicity assessment of metallic pollution: Impact on d-β-hydroxybutyrate dehydrogenase. Ecol. Ind. 2007;7(4):882–894. [Google Scholar]
- Nait Irahal I., azzahra Lahlou F., Hmimid F., Errami A., Guenaou I., Diawara I., Kettani‐Halabi M., Fahde S., Ouafik L., Bourhim N. Identification of the chemical composition of six essential oils with mass spectroscopy and evaluation of their antibacterial and antioxidant potential. Flavour Fragr J. 2021;36(4):465–476. doi: 10.1002/ffj.v36.410.1002/ffj.3657. [DOI] [Google Scholar]
- Nait Irahal I., Hmimid F., Lahlou F., Errami A., Guenaou I., Diawara I., Kettani‐Halabi M., Fahd S., Ouafik L., Bourhim N. Chemical composition, antibacterial and antioxidant activities of some essential oils against multidrug resistant bacteria. Eur. J. Integrat. Med. 2020;35:101074. doi: 10.1016/j.eujim.2020.101074. [DOI] [Google Scholar]
- Nakagawa T., Yokozawa T., Terasawa K., Shu S., Juneja L.R. Protective Activity of Green Tea against Free Radical- and Glucose-Mediated Protein Damage. J. Agric. Food. Chem. 2002;50(8):2418–2422. doi: 10.1021/jf011339n. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japan. J. Nutr. Dietetics. 1986;44(6):307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Paoletti F., Aldinucci D., Mocali A., Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 1986;154(2):536–541. doi: 10.1016/0003-2697(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Santanam N., Ramachandran S., Meilhac O. Oxidants and antioxidants in atherogenesis: an appraisal. J. Lipid Res. 1999;40(12):2143–2157. doi: 10.1016/S0022-2275(20)32089-7. [DOI] [PubMed] [Google Scholar]
- Piechowiak T., Balawejder M. Onion skin extract as a protective agent against oxidative stress in Saccharomyces cerevisiae induced by cadmium. J. Food Biochem. 2019;43(7) doi: 10.1111/jfbc.2019.43.issue-710.1111/jfbc.12872. [DOI] [PubMed] [Google Scholar]
- Pirinccioglu A.G., Gökalp D., Pirinccioglu M., Kizil G., Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010;43(15):1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longevity. 2017;2017:1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Gopas J., Nishigaki I. Antioxidants and human diseases. Clin. Chim. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Robinson K.M., Lemire B.D. Covalent Attachment of FAD to the Yeast Succinate Dehydrogenase Flavoprotein Requires Import into Mitochondria, Presequence Removal, and Folding. J. Biol. Chem. 1996;271(8):4055–4060. doi: 10.1074/jbc.271.8.4055. [DOI] [PubMed] [Google Scholar]
- Rustin P. Mitochondria, from cell death to proliferation. Nat. Genet. 2002;30(4):352–353. doi: 10.1038/ng0402-352. [DOI] [PubMed] [Google Scholar]
- Rustin P., Munnich A., Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur. J. Hum. Genet. 2002;10(5):289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- de Sá R.A., de Castro F.A.V., Eleutherio E.C.A., de Souza R.M., da Silva J.F.M., Pereira M.D. Brazilian propolis protects Saccharomyces cerevisiae cells against oxidative stress. Braz. J. Microbiol. 2013;44:993–1000. doi: 10.1590/S1517-83822013005000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska-Bartosz I., Pączka A., Mołoń M., Bartosz G. Dimethyl sulfoxide induces oxidative stress in the yeast “Saccharomyces cerevisiae”. FEMS Yeast Res. 2013;13(8):820–830. doi: 10.1111/fyr.2013.13.issue-810.1111/1567-1364.12091. [DOI] [PubMed] [Google Scholar]
- SAMOKYSZYN V., MARNETT L. Inhibition of liver microsomal lipid peroxidation by 13-cis-retinoic acid. Free Radic. Biol. Med. 1990;8(5):491–496. doi: 10.1016/0891-5849(90)90063-o. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002;8(3):121–137. doi: 10.1106/108201302026770. [DOI] [Google Scholar]
- Santos-Buelga C., Scalbert A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000;80:1094–1117. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1. [DOI] [Google Scholar]
- Sato H., Shibata M., Shimizu T., Shibata S., Toriumi H., Ebine T., Kuroi T., Iwashita T., Funakubo M., Kayama Y., Akazawa C., Wajima K., Nakagawa T., Okano H., Suzuki N. Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. Neuroscience. 2013;248:345–358. doi: 10.1016/j.neuroscience.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Satyal P., Jones T., Lopez E., McFeeters R., Ali N., Mansi I., Al-kaf A., Setzer W. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods. 2017;6:20. doi: 10.3390/foods6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., Chandel N. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Viticulture. 1977;28:49–55. [Google Scholar]
- Tongul B., Tarhan L. Oxidant and antioxidant status in Saccharomyces cerevisiae exposed to antifungal ketoconazole. Process Biochem. 2016;51(12):1984–1991. doi: 10.1016/j.procbio.2016.08.016. [DOI] [Google Scholar]
- Tran, K., Green, E., 2019. Assessing Yeast Cell Survival Following Hydrogen Peroxide Exposure. BIO-PROTOCOL 9. https://doi.org/10.21769/BioProtoc.3149. [DOI] [PMC free article] [PubMed]
- Tretter L., Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of α-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20(24):8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Izakovic M., Mazur M., Rhodes C.J., Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004;266(1/2):37–56. doi: 10.1023/B:MCBI.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wu Z., Tan B., Liu Y., Dunn J., Martorell Guerola P., Tortajada M., Cao Z., Ji P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Zhong Y., Duan Y., Chen Q., Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020;6(2):115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]