Abstract
Three cefoxitin-resistant Escherichia coli isolates from stool specimens of a patient with leukemia were either resistant, intermediate, or sensitive to imipenem. Conjugation experiments showed that cefoxitin resistance, but not imipenem resistance, was transferable. All isolates were shown by isoelectric focusing to produce two β-lactamases with isoelectric points of 5.4 (TEM-1, confirmed by sequencing of a PCR product) and >8.5 (consistent with a class C β-lactamase). The gene coding for the unknown β-lactamase was cloned and sequenced and revealed an enzyme which had 99.9% sequence identity with the plasmid-determined class C β-lactamase CMY-2. The cloned β-lactamase gene differed from blaCMY-2 at one nucleotide position that resulted in an amino acid change, tryptophan to arginine at position 221. We propose that this enzyme be designated CMY-4. Both the imipenem-resistant and -intermediate isolates lacked a 38-kDa outer membrane protein (OMP) that was present in the imipenem-sensitive isolate. The lack of an OMP alone did not explain the difference in carbapenem susceptibilities observed. However, measurement of β-lactamase activities (including measurements under conditions where TEM-1 β-lactamase was inhibited) indicated that the imipenem-intermediate isolate expressed six- to eightfold less β-lactamase than did the other isolates. This study illustrates that carbapenem resistance in E. coli can arise from high-level expression of plasmid-mediated class C β-lactamase combined with an OMP deficiency. Furthermore, in the presence of an OMP deficiency, the level of expression of a plasmid-mediated class C β-lactamase is an important factor in determining whether E. coli isolates are fully resistant to carbapenems.
In the laboratory, imipenem-resistant Escherichia coli K-12 strains have been constructed by the introduction of the carbapenem-hydrolyzing enzyme from Aeromonas hydrophila, CphA, into porin-deficient mutants (7). However, there has been only one description of a carbapenem-resistant E. coli strain isolated from a clinical specimen, and the mechanism of resistance was not determined (6). Carbapenem resistance among other members of the family Enterobacteriaceae has previously been found associated with either expression of a carbapenem-hydrolyzing enzyme (18, 20, 22, 28), a penicillin-binding protein alteration (17), or high-level expression of a chromosomally encoded class C β-lactamase combined with reduced outer membrane permeability (11, 15, 21). Recently, there have been described carbapenem-resistant Klebsiella pneumoniae isolates where resistance was attributed to a lack of an outer membrane protein in combination with the expression of the plasmid-mediated class C β-lactamase ACT-1 (4).
In this study, we report the mechanisms responsible for intermediate-level and high-level resistance to carbapenems in clinical isolates of E. coli. We show that in the presence of an outer membrane protein deficiency, the level of expression of a plasmid-mediated class C β-lactamase is an important factor in determining whether E. coli isolates are fully resistant to carbapenems.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Three cefoxitin-resistant E. coli isolates from stool specimens from a patient with leukemia at St. Thomas’ Hospital were studied. The isolates, which were collected within a few days of each other, were identified as E. coli with the API-20E identification system (Analytab Products, Montalier Vercier, France) and by amplification and sequencing of the nadC-ampC region of the E. coli chromosome.
E. coli J62.1 was used as a recipient strain in conjugation studies, while E. coli XL-1 Blue (Stratagene Ltd., Cambridge, United Kingdom) was used as the host strain in DNA cloning and DNA transformation experiments. Citrobacter freundii 31A12, which has derepressed chromosomal β-lactamase synthesis (24), and E. coli TB1(pTZ18U), carrying the blaTEM-1 gene, were used as controls in β-lactamase assays. Plasmid vector pUC18, used in DNA cloning, was obtained from Pharmacia Biotech Ltd. (St. Albans, United Kingdom).
Determination of MICs.
MICs were determined by agar dilution on diagnostic sensitivity test agar (CM261; Oxoid, Basingstoke, United Kingdom) with an inoculum of about 104 organisms per spot as described previously (25). E. coli NCTC 10418 was used as the control strain.
Antibiotics and reagents.
The following companies kindly supplied antibiotic powders of known potency: Bristol-Myers Company (cefepime), Cyanamid (piperacillin), Eli Lilly Company Ltd. (moxalactam), E. R. Squibb & Sons (aztreonam), Glaxo Group Research Ltd. (ceftazidime), Merck Sharp & Dohme (cefoxitin and imipenem), Roussel Laboratories Ltd. (cefotaxime), SmithKline Beecham (ampicillin and clavulanic acid), and Zeneca (meropenem). Restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs (Hitchin, United Kingdom).
Isoelectric focusing.
Cells were harvested from 20-h brain heart infusion (BHI) broth cultures by centrifugation and resuspended in 0.5 ml of phosphate buffer (0.1 M, pH 7), and the β-lactamase was released by sonication. Sonication was performed for 20 s with a W-385 sonicator (Heat Systems-Ultrasonics, Inc.) with the following settings: 5-s cycle time, 50% duty cycle, 1.5 output control setting. Enzymes were identified by isoelectric focusing in agarose-IEF (Pharmacia Biotech) gels containing Pharmalyte (pH range, 3 to 10; Pharmacia Biotech) and subsequent staining with nitrocefin (100 μg/ml). Preparations of strains known to produce TEM-1, OXA-1, SHV-1, or SHV-5 β-lactamase were used as standards.
β-Lactamase studies.
Each strain was grown overnight at 37°C in BHI broth, with shaking (200 rpm). A 1:20 dilution was performed in fresh prewarmed BHI broth, and the culture was shaken for a further 2 h. Cells were harvested by centrifugation and resuspended in 0.5 ml of sterile distilled water, and the β-lactamase was released by sonication (as described above). β-Lactamase activity was measured by monitoring the rate of hydrolysis of nitrocefin (20 μM) at 482 nm in a Biochrom 4060 spectrophotometer (Pharmacia Biotech). Assays were performed in both the presence and the absence of clavulanate at 37°C in 0.1 M phosphate buffer (pH 7.0). Inhibition studies were performed on samples that had been preincubated for 10 min at 37°C in the presence of clavulanate (1 μM) before β-lactamase measurement. Specific β-lactamase activities are expressed as nanomoles of nitrocefin hydrolyzed per minute standardized against the amount of protein in the sample.
Plasmid studies.
Conjugation was performed with overnight cultures of the clinical isolate and E. coli J62.1 which were mixed in a ratio of 1:2 (donor to recipient) in BHI broth and grown for a further 16 h. Transconjugants were selected for by plating the mixture onto MacConkey agar containing rifampin (200 μg/ml) and cefotaxime (2 μg/ml). Plasmid extracts were prepared by the alkaline lysis procedure (23) unless otherwise stated. Plasmid DNA was introduced into competent XL-1 Blue by a heat shock procedure (23).
Nucleic acid techniques.
Total DNA extractions were performed as described by Ausubel et al. (1). DNA cloning of the blaCMY-4 gene was achieved from a Sau3AI partial digest of total DNA ligated into the BamHI site of pUC18. E. coli XL-1 Blue was transformed with the recombinant DNA, and clones carrying the blaCMY-4 gene were selected by plating the transformants onto Luria-Bertani agar plates containing cefotaxime (2 μg/ml). DNA sequencing of both strands of the cloned insert was performed with a primer-walking strategy with fluorescein-labelled sequencing primers custom made by Pharmacia Biotech. Sequencing reactions were performed on QIAprep plasmid DNA preparations (Qiagen, Crawley, United Kingdom) with the reagents contained within a Thermo Sequenase cycle sequencing kit (Amersham International, Amersham, United Kingdom) according to the manufacturer’s instructions. Template preparation and PCR amplification of the blaTEM gene were performed as described previously (26). Amplification of the blaCMY-4 gene was performed with an annealing temperature of 55°C and primers TR8A3U (5′-GATTCCTTGGACTCTTCAG-3′) and TR8A3R (5′-TAAAACCAGGTTCCCAGATAGC-3′) based on the sequence of blaCMY-4 deduced in this study. The blaCMY-4 PCR product was labelled with digoxigenin-11-dUTP by the incorporation of digoxigenin-11-dUTP in the PCR mixture according to the instructions of the manufacturer (Boehringer Mannheim UK, Lewes, England). Southern blotting and DNA hybridization were performed as described by Sambrook et al. (23). Amplification of the ampR gene was performed with an annealing temperature of 55°C and primers PSN006 (5′-ATGACGCGTAGCTATATCCCTCTT-3′) and PSN007 (5′-TTATTTGTGCAGCACCCCGGT-3′) based on the sequence of the ampR gene from C. freundii described by Lindquist et al. (13).
Outer membrane protein studies.
Cell membranes of a 16-h culture were disrupted for 2 min with a W-385 sonicator (Heat Systems-Ultrasonics, Inc.) with the following settings: 5-s cycle time, 50% duty cycle, and 1.5 output control setting. Cell debris was removed by centrifugation at 6,000 × g for 15 min at 4°C, and the supernatant was subjected to ultracentrifugation at 50,000 × g for 45 min to collect the membranes. Cytoplasmic membrane proteins were differentially solubilized for 20 min at room temperature with 1.7% sodium lauryl-sarcosinate in 50 mM Tris, pH 7.6. The suspension was recentrifuged at 50,000 × g for 45 min at 4°C, and the pellet containing the outer membrane proteins was resuspended in 100 μl of sterile distilled water. The outer membrane proteins were separated on a vertical sodium dodecyl sulfate-containing polyacrylamide gel (acrylamide/bisacrylamide ratio of 3:0.27; 5% stacking gel with a 10% separating gel) and were visualized after staining with polyacrylamide gel electrophoresis Blue 83 (BDH, Lutterworth, United Kingdom). Molecular weights of the proteins were determined from a calibration curve derived from the migratory positions of protein standards of known molecular weights run on the same gel. The standard proteins used in this study had the molecular weights 94,000, 67,000, 43,000, 30,000, 20,100, and 14,400 and were provided in a calibration kit supplied by Pharmacia Biotech.
Nucleotide sequence accession number.
The nucleotide sequence reported in this study can be obtained from EMBL with accession no. AJ007826.
RESULTS
Bacterial strains and plasmids.
The three clinical isolates investigated in this study were cultured from stool specimens from a patient with leukemia at St. Thomas’ Hospital. They were identified as E. coli by API-20E substrate profile analysis and shown to have identical XbaI-digested genomic DNA profiles by pulsed-field gel electrophoresis (data not shown). Plasmid profiles revealed the presence of five plasmids in the strains with the following approximate sizes: 3, 5.5, 7, 30, and 45 kb. All plasmids except the smallest one were transferred to the recipient strain E. coli J62.1 after mating with strain 69. Transconjugants of strains 68 and 79 were found to contain the 5.5-, 7-, and 45-kb plasmids.
When plasmid preparations were used to transform E. coli XL-1 Blue, all transformants were found to contain the 7-kb plasmid either alone (strains 68 and 69) or in combination with the 45-kb plasmid (strain 79).
Antimicrobial susceptibilities.
Based on the MIC interpretative standards of the National Committee for Clinical Laboratory Standards (16), the three clinical isolates and the transconjugants of strains 69 and 79 were resistant to ampicillin, piperacillin, aztreonam, cefoxitin, ceftazidime, cefotaxime, and the β-lactam–β-lactamase inhibitor combinations (Table 1). Strain 68 exhibited a lower level of resistance to these compounds than did the other strains but was less susceptible to imipenem, meropenem, and moxalactam than was strain 69 (Table 1). Strain 79 was significantly more resistant to imipenem, meropenem, cefepime, and moxalactam than were the other strains, but like strain 68, neither the transconjugant nor the transformant of the strain was resistant to imipenem, indicating that the carbapenem resistance was nontransferable in both cases. Revertants of strain 79 (79-rev), which were susceptible to imipenem, had a similar resistance phenotype to strain 69 and displayed increased susceptibility to cefepime and moxalactam compared to the parental strain.
TABLE 1.
Plasmid sizes and MICs of β-lactam antimicrobials for E. coli isolates, transconjugants, transformants, and the recipient strains
| Strain | MIC (μg/ml) of drugc:
|
Plasmid size(s) (kb) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMP-CA | PIP | ATM | IPM | MPM | FOX | CAZ | CAZ-CA | CTX | CPM | MOX | ||
| 69 | 4,096 | 4,096 | 1,024 | 256 | 0.5 | 0.25 | 1,024 | 512 | 256 | 512 | 8 | 32 | 3, 5.5, 7, 30, 45 |
| 68 | 1,024 | 512 | 256 | 64 | 4 | 2 | 512 | 64 | 64 | 64 | 2 | 256 | 3, 5.5, 7, 30, 45 |
| 79 | >4,096 | >4,096 | 1,024 | 512 | 64 | 16 | >1,024 | 1,024 | 1,024 | 1,024 | 32 | >1,024 | 3, 5.5, 7, 30, 45 |
| 79-rev | 4,096 | ND | 512 | ND | 0.5 | ND | 256 | 1,024 | ND | 128 | 2 | 16 | 3, 5.5, 7, 30, 45 |
| 69-tca | 2,048 | 2,048 | 512 | 128 | 0.5 | ≤0.06 | 256 | 256 | 256 | 128 | 2 | 16 | 5.5, 7, 30, 45 |
| 68-tca | 256 | 256 | 32 | 16 | 0.5 | ≤0.06 | 32 | 32 | 16 | 8 | 0.25 | 2 | 5.5, 7, 45 |
| 79-tca | 2,048 | 2,048 | 512 | 128 | 0.5 | 0.12 | 256 | 512 | 256 | 128 | 4 | 16 | 5.5, 7, 45 |
| 69-tfb | 64 | ND | 4 | ND | 0.12 | ND | 32 | 8 | ND | 4 | 0.12 | 2 | 7 |
| 68-tfb | 64 | ND | 4 | ND | 0.12 | ND | 32 | 8 | ND | 4 | 0.12 | 2 | 7 |
| 79-tfb | 4,096 | ND | 256 | ND | 0.25 | ND | 128 | 512 | ND | 128 | 2 | 16 | 7, 45 |
| J62.1 | 8 | 8 | 4 | 0.12 | 0.25 | ≤0.06 | 4 | 0.25 | 0.12 | ≤0.06 | ≤0.06 | 0.25 | |
| XL-1 Blue | 4 | ND | ≤1 | ND | 0.12 | ND | 4 | 0.25 | ND | ≤0.06 | ≤0.06 | 0.25 | |
Transconjugant in E. coli J62.1.
Transformant in E. coli XL-1 Blue.
Abbreviations: AMP, ampicillin; AMP-CA, ampicillin-clavulanate (2 μg/ml); PIP, piperacillin; ATM, aztreonam; IPM, imipenem; MPM, meropenem; FOX, cefoxitin; CAZ, ceftazidime; CAZ-CA, ceftazidime-clavulanate (2 μg/ml); CTX, cefotaxime; CPM, cefepime; MOX, moxalactam; ND, not determined.
With the exception of imipenem and meropenem, the transconjugant of strain 68 (68-tc) showed greater susceptibility to the compounds tested than did the other transconjugants. Strain 68-tc was also more sensitive to the majority of the compounds tested than was the donor strain, which was particularly noticeable with moxalactam, where a 128-fold reduction of the MIC was observed.
Transformants 68-tf and 69-tf, which harbored a 7-kb plasmid but not the 45-kb plasmid, were resistant to cefoxitin and displayed reduced susceptibility to ampicillin, piperacillin, ceftazidime, cefotaxime, and moxalactam compared to that of the host strain E. coli XL-1 Blue, which did not carry any plasmids. However, the transformants were more susceptible to these compounds than was either the transformant 79-tf or the respective clinical isolates, all of which harbored both the 7- and 45-kb plasmids.
β-Lactamases.
Isoelectric focusing revealed the presence of two β-lactamases in all three clinical isolates, one with a pI of 5.4, consistent with TEM-1, and one with a pI of >8.5. PCR amplification with primers specific for the blaTEM gene confirmed the presence of this gene in the isolates, and DNA sequencing of the PCR product established that the encoded enzyme was TEM-1. Measurement of the total β-lactamase activities of the isolates revealed that strain 68 produced approximately six- to eightfold less β-lactamase than did the other strains (Table 2). The rate of nitrocefin hydrolysis by the β-lactamases from the strains was reduced by 25 to 40% in the presence of 1 μM clavulanate, indicating that one of the enzymes was not inhibited by clavulanate, an observation consistent with the presence of a class C β-lactamase. Since 1 μM clavulanate was effective in reducing the β-lactamase activity of an E. coli strain hyperproducing TEM-1 β-lactamase to 4% of its activity in the absence of the inhibitor (Table 2), activities measured in the presence of clavulanate should give an indication of the level of class C β-lactamase expressed. When the β-lactamase activities of the isolates, measured in the presence of clavulanate, were compared with those of a C. freundii strain (31A12) that constitutively expressed high levels of class C β-lactamase, we found that strain 68 produced 2.6-fold less β-lactamase than did the C. freundii strain. In contrast, the β-lactamase activities of strains 79 and 69 were 2.7- and 3.5-fold higher, respectively, than that of the C. freundii strain. None of the strains exhibited inducible β-lactamase expression in the presence of imipenem (data not shown).
TABLE 2.
β-lactamase activities for clinical E. coli isolates, C. freundii 31A12, and E. coli TB1 carrying pTZ18U
| Strain | Description | β-Lactamase activitya
|
|
|---|---|---|---|
| Without clavulanate | With 1 μM clavulanate | ||
| E. coli 69 | Imipenem sensitive | 1,182 | 855 |
| E. coli 68 | Imipenem intermediate | 151 | 90 |
| E. coli 79 | Imipenem resistant | 863 | 646 |
| C. freundii 31A12 | High-level AmpC β-lac-tamase producer | 273 | 239 |
| E. coli TB1(pTZ18U) | High-level TEM-1 β-lac-tamase producer | 4,667 | 190 |
Activities are expressed as nanomoles of nitrocefin hydrolyzed per minute per milligram of protein.
Sequencing of the cloned β-lactamase gene.
In order to determine the identity of the unknown β-lactamase, a Sau3AI DNA fragment of the genome of imipenem-resistant strain E. coli 79 was cloned into the BamHI site of pUC18. Sequence determination of the insert by a primer-walking strategy revealed the presence of an open reading frame of 1,146 bp in length. A nucleotide sequence search showed that the open reading frame had 99.9% sequence identity with a plasmid-mediated class C β-lactamase, CMY-2, from K. pneumoniae (2). The β-lactamase gene cloned in this study differed from CMY-2 by one amino acid residue, tryptophan instead of arginine at position 221 in the deduced amino acid sequence, the result of one nucleotide difference, T instead of A, in the gene coding sequence. We proposed that this enzyme be designated CMY-4. The ampR coding gene from C. freundii was not found upstream from the blaCMY-4 gene, nor could the ampR gene be amplified from the isolates. The lack of the ampR gene thus accounts for the inability to induce β-lactamase expression in the isolates. Comparison of the upstream region of blaCMY-4 with the corresponding region of the chromosomal β-lactamase of C. freundii OS60 (13) revealed that the homology ended 14 bases downstream from the ampR initiation codon. Downstream from blaCMY-4 was part of the sequence (119 nucleotides) coding for a C. freundii outer membrane lipoprotein (3).
Location of the blaCMY-4 gene.
PCR amplification of the blaCMY-4 gene followed by direct DNA sequencing of the PCR product revealed that all the isolates had the blaCMY-4 gene. In order to determine the location of the blaCMY-4 gene, a digoxigenin-labelled blaCMY-4 DNA probe was prepared and hybridized to total DNA preparations from each of the strains and their transconjugants. The probe hybridized to give a strong signal with the 7-kb plasmid and a weak signal with the larger plasmid (data not shown). No cross-reaction of the blaCMY-4 probe with the ampC gene in a chromosomal DNA preparation of E. coli J62.1 or with the chromosomal DNA of the clinical isolates was observed.
Outer membrane profiles.
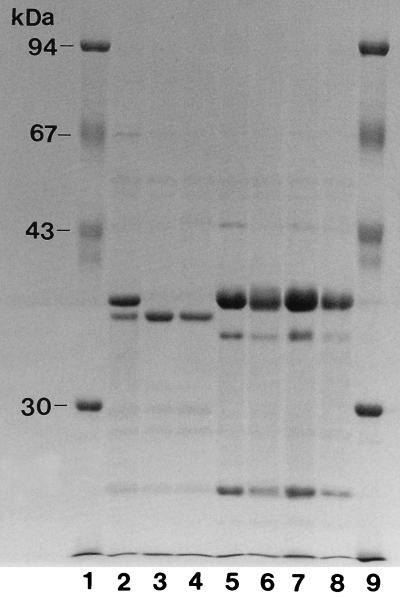
The transconjugant and transformant from the imipenem-resistant isolate were not resistant to imipenem, suggesting that an additional mechanism was acting in concert with CMY-4 production. Examination of the outer membrane profiles of the original isolates revealed that the imipenem-resistant (strain 79) and imipenem-intermediate-resistant (strain 68) isolates lacked a 38-kDa protein (Fig. 1).
FIG. 1.
Outer membrane protein profiles of E. coli clinical isolates and their transconjugants. Lane 1, molecular mass standards; lane 2, strain 69; lane 3, strain 68; lane 4, strain 79; lane 5, strain J62.1 (recipient strain); lane 6, strain 69-tc; lane 7, strain 68-tc; lane 8, strain 79-tc; lane 9, molecular mass standards.
DISCUSSION
The mechanism of carbapenem resistance in clinical isolates of E. coli has not previously been reported. From studies of derepressed mutants of C. freundii and Enterobacter cloacae, it is known that high-level expression of a class C β-lactamase alone is not sufficient to confer carbapenem resistance (14, 25). But resistance can arise if the high level of β-lactamase expression is combined with a decrease in outer membrane permeability (11, 15, 21). Consistent with these studies, the imipenem-resistant E. coli isolate (strain 79) in this investigation expressed high levels of a class C β-lactamase, CMY-4, and lacked an outer membrane protein that was found in an imipenem-susceptible isolate. However, unlike with derepressed C. freundii and E. cloacae strains the high level of β-lactamase expression in the E. coli isolates was plasmid determined and not due to overexpression of the chromosomal β-lactamase. The mechanism was thus similar to that recently reported to explain imipenem resistance in K. pneumoniae isolates where resistance was associated with high-level expression of a plasmid-determined class C β-lactamase, ACT-1, in combination with the loss of a 42-kDa outer membrane protein (4).
The importance of the level of class C β-lactamase expression in conferring carbapenem resistance was illustrated by a comparison of strain 68 with strain 79. Both strains lacked a 38-kDa outer membrane protein and expressed the CMY-4 β-lactamase, so that one might have expected both strains to be resistant to carbapenems. However, strain 68 was not resistant to imipenem and was more susceptible to cefoxitin, extended-spectrum cephalosporins, and the penicillins tested than were the other strains. Mainardi et al. (15) have proposed residual expression of an outer membrane protein to explain intermediate-level carbapenem resistance in a C. freundii strain, but no trace of the missing 38-kDa protein could be detected in this case. Instead, measurement of the total β-lactamase activities of the strains revealed that strain 68 expressed six- to eightfold less β-lactamase than did the other strains. Since the MIC of cefoxitin for the transconjugant of strain 68 was lower than that for the other transconjugants, it is likely that this strain expressed a smaller amount of the CMY-4 β-lactamase. This would explain both the lower level of resistance to extended-spectrum cephalosporins and moxalactam observed and the increased susceptibility to carbapenems exhibited by strain 68 compared to the imipenem-resistant isolate.
The reason why strain 68 produced less β-lactamase than did the other isolates is not known. From isoelectric focusing, PCR, and DNA sequencing, it is known that the three isolates in this study produced two β-lactamases, TEM-1 and CMY-4, in addition to the E. coli chromosomal β-lactamase. DNA hybridization studies revealed that the gene coding for the CMY-4 β-lactamase was located on two different plasmids that had approximate sizes of 7 and 45 kb. Since MICs of cephalosporin for the transconjugant of strain 68 were lower than those for the other transconjugants, we infer that host factors were not responsible for the variation in β-lactamase expression observed. The MICs of β-lactams for E. coli XL-1 Blue carrying the 7-kb plasmid from either the imipenem-sensitive strain or strain 68 were identical, suggesting that this plasmid was not responsible for the observed difference in the levels of β-lactamase expressed. Therefore, by process of elimination, the presence of the 45-kb plasmid was the most likely cause of the phenotype observed. Whether this was due to lower CMY-4 β-lactamase production from the 45-kb plasmid or to the expression of a regulatory factor that led to differential expression of the blaCMY-4 gene on the 7-kb plasmid has yet to be determined.
Based on the deduced amino acid sequence, the class C β-lactamase reported in this study differed from the plasmid-determined β-lactamase, CMY-2, by one residue, an arginine instead of tryptophan at position 221. This position is located far from the active site in the crystal structure of the C. freundii AmpC β-lactamase (19), so that we would expect little difference in catalytic activities between the two enzymes. Both enzymes belong to a small subset of β-lactamases (BIL-1, CMY-2, CMY-3, LAT-1, and LAT-2) thought to originate from C. freundii (2, 5, 8–10, 27). Consistent with these findings was part of the sequence coding for an outer membrane lipoprotein (3), found next to the blaCMY-4 gene. This outer membrane lipoprotein-encoding gene usually lies downstream from ampC in C. freundii, so that this is consistent with the blaCMY-4 gene being of C. freundii chromosomal origin.
Transposable elements responsible for the transmission of ampC-type genes have not been described to date, but the occurrence of β-lactamase genes on both plasmids and the chromosomes of foreign bacterial hosts has led previous workers to imply that they exist. Bradford et al. (4) have suggested that the gene coding for ACT-1 may occur on a transposable element together with two other genes coding for β-lactamases with pIs of 5.4 and 7.0. The blaCMY-4 gene characterized in this study was found on two different plasmids, which suggests that this gene may also form part of a transposable element. However, since the blaTEM-1 gene was not found on the 7-kb plasmid which harbored blaCMY-4 it is less likely that blaCMY-4 is associated with the blaTEM-1 gene on the same transposable element.
Upstream from the ampC gene in C. freundii usually lies the ampR gene, which codes for a transcriptional regulatory protein involved in inducible β-lactamase expression (13). The ampR gene was not present upstream from the blaCMY-4 gene, and this therefore accounts for the inability to induce CMY-4 β-lactamase expression in the E. coli isolates. Studies involving E. coli, which naturally lacks ampR, have shown that introduction of ampC from C. freundii on a plasmid into E. coli results in only a two- to threefold increase in β-lactamase expression over that for E. coli carrying both ampC and ampR (12). Despite this, the imipenem-sensitive and imipenem-resistant isolates in this study expressed high levels of CMY-4 β-lactamase and were resistant to extended-spectrum cephalosporins, cefoxitin, and combinations of clavulanate with either ampicillin or ceftazidime. While one would normally associate this phenotype with derepressed β-lactamase expression, the lack of the ampR gene makes this unlikely. Consequently, it is likely that gene and plasmid copy number play an important role in giving rise to the high level of β-lactamase expression observed, but other factors such as influence of insertion sequences or other regulatory factors cannot be ruled out.
In summary, carbapenem resistance among members of the family Enterobacteriacae is uncommon, and consequently there have been few descriptions of carbapenem-resistant E. coli strains in the literature. This study has investigated the mechanism responsible for carbapenem resistance in clinical isolates of E. coli and shown the resistance to be conferred by high-level expression of a plasmid-mediated class C β-lactamase, in combination with the loss of an outer membrane protein. We have also shown that in the presence of an outer membrane protein deficiency, the level of expression of a plasmid-mediated class C β-lactamase, CMY-4, is an important factor in determining the degree of resistance of E. coli to carbapenems.
ADDENDUM
The CMY-4 β-lactamase has recently been identified in a clinical Proteus mirabilis isolate (27a).
ACKNOWLEDGMENTS
We thank Richard Anthony (St. Thomas’ Hospital) for typing the isolates by pulsed-field gel electrophoresis.
This work was funded by a Project 804 grant from the Special Trustees of St. Thomas’ Hospital.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R F, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 2.Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996;40:221–224. doi: 10.1128/aac.40.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop R E, Penfold S S, Frost L S, Höltje J-V, Weiner J H. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origins of lipocalins. J Biol Chem. 1995;270:23097–23103. doi: 10.1074/jbc.270.39.23097. [DOI] [PubMed] [Google Scholar]
- 4.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bret L, Chanal-Claris C, Sirot D, Chaibi E B, Labia R, Sirot J. Chromosomally encoded AmpC-type β-lactamase in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 1998;42:1110–1114. doi: 10.1128/aac.42.5.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D F J, Farrington M, Warren R E. Imipenem-resistant Escherichia coli. Lancet. 1993;342:177. doi: 10.1016/0140-6736(93)91382-v. [DOI] [PubMed] [Google Scholar]
- 7.Cornaglia G, Guan L, Fontana R, Satta G. Diffusion of meropenem and imipenem through the outer membrane of Escherichia coli K-12 and correlation with their antibacterial activities. Antimicrob Agents Chemother. 1992;36:1902–1908. doi: 10.1128/aac.36.9.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fosberry A P, Payne D J, Lawlor E J, Hodgson J E. Cloning and sequence analysis of blaBIL-1, a plasmid-mediated class C β-lactamase gene in Escherichia coli BS. Antimicrob Agents Chemother. 1994;38:1182–1185. doi: 10.1128/aac.38.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazouli M, Tzouvelekis L S, Prinarakis E, Miriagou V, Tzelepi E. Transferable cefoxitin resistance in enterobacteria from Greek hospitals and characterization of a plasmid-mediated group 1 β-lactamase (LAT-2) Antimicrob Agents Chemother. 1996;40:1736–1740. doi: 10.1128/aac.40.7.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeck J L, Arlet G, Philippon A, Basmaciogullari S, Thien H V, Buisson Y, Cavallo J D. A plasmid-mediated CMY-2 β-lactamase from an Algerian clinical isolate of Salmonella senftenberg. FEMS Microbiol Lett. 1997;152:255–260. doi: 10.1111/j.1574-6968.1997.tb10436.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee E H, Nicolas M H, Kitzis M D, Pialoux G, Collatz E, Gutmann L. Association of two resistance mechanisms in a clinical isolate of Enterobacter cloacae with high-level resistance to imipenem. Antimicrob Agents Chemother. 1991;35:1093–1098. doi: 10.1128/aac.35.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg F, Westman L, Normark S. Regulatory components in Citrobacter freundii AmpC β-lactamase induction. Proc Natl Acad Sci USA. 1985;82:4620–4624. doi: 10.1073/pnas.82.14.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist S, Lindberg F, Normark S. Binding of the Citrobacter freundii AmpR regulator to a single DNA site provides both autoregulation and activation of the inducible ampC β-lactamase gene. J Bacteriol. 1989;171:3746–3753. doi: 10.1128/jb.171.7.3746-3753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore D M. Clinical significance of β-lactamase induction and stable derepression in Gram-negative rods. Eur J Clin Microbiol. 1987;6:439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- 15.Mainardi J-L, Mugnier P, Coutrot A, Buu-Hoï A, Collatz E, Gutmann L. Carbapenem resistance in a clinical isolate of Citrobacter freundii. Antimicrob Agents Chemother. 1997;41:2352–2354. doi: 10.1128/aac.41.11.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Neuwirth C, Siéblor E, Duez J M, Pechinot A, Kazmierczak A. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J Antimicrob Chemother. 1995;36:335–342. doi: 10.1093/jac/36.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas M-H. Biochemical properties of a carbapenem-hydrolyzing β-lactamase from Enterobacter cloacae and cloning into Escherichia coli. Antimicrob Agents Chemother. 1993;37:939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oefner C, Darcy A, Daly J J, Gubernator K, Charnas R L, Heinze I, Hubschwerfen C, Winkler F K. Refined crystal structure of Citrobacter freundii indicates a mechanism for β-lactam hydrolysis. Nature. 1990;343:284–288. doi: 10.1038/343284a0. [DOI] [PubMed] [Google Scholar]
- 20.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raimondi A, Traverso A, Nikaido H. Imipenem- and meropenem-resistant mutants of Enterobacter cloacae and Proteus rettgeri lack porins. Antimicrob Agents Chemother. 1991;35:1174–1180. doi: 10.1128/aac.35.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen B A, Bush K, Keeney D, Yang Y, Hare R, O’Gara C, Medeiros A A. Characterization of IMI-1 β-lactamase, a novel class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob Agents Chemother. 1996;40:2080–2086. doi: 10.1128/aac.40.9.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Stapleton P, Shannon K, Phillips I. DNA sequence differences of ampD mutants of Citrobacter freundii. Antimicrob Agents Chemother. 1995;39:2494–2498. doi: 10.1128/aac.39.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stapleton P, Shannon K, Phillips I. The ability of β-lactam antibiotics to select mutants with derepressed β-lactamase synthesis from Citrobacter freundii. J Antimicrob Chemother. 1995;36:483–496. doi: 10.1093/jac/36.3.483. [DOI] [PubMed] [Google Scholar]
- 26.Stapleton P, Wu P-J, King A, Shannon K, French G, Phillips I. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob Agents Chemother. 1995;39:2478–2483. doi: 10.1128/aac.39.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzouvelekis L S, Tzelepi E, Mentis A F. Nucleotide sequence of a plasmid-mediated cephalosporinase gene (blaLAT-1) found in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1994;38:2207–2209. doi: 10.1128/aac.38.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Verdet C, Arlet G, Ben Redjeb S, Ben Hassen A, Lagrange P H, Philippon A. Characterization of CMY-4, an AmpC-type plasmid-mediated beta-lactamase in a Tunisian clinical isolate of Proteus mirabilis. FEMS Microbiol Lett. 1998;169:235–240. doi: 10.1111/j.1574-6968.1998.tb13323.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Wu P-J, Livermore D M. Biochemical characterization of a β-lactamase that hydrolyzes penem and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother. 1990;34:755–758. doi: 10.1128/aac.34.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]