Abstract
There is a swing in research developments concerning the utilization of natural products as effective pharmacotherapeutic agents due to their comparatively lower toxicities than synthetic compounds. Among natural products, mangiferin is a natural C-glucosyl xanthonoid polyphenol with remarkable pharmacological activities. Emerging evidence indicates the therapeutic benefits of mangiferin against various kidney disorders, including renal injury, diabetic nephropathy, renal fibrosis, hyperuricemic nephropathy, and lupus nephritis, in experimental animal models. The mangiferin induced antioxidant response resulting in vital functions, such as protection against renal inflammation, inhibits renal cell apoptosis, activates autophagy, causes immunomodulation, regulates renal urate transporters and modulates cell signalling pathways. The purpose of this review provide a brief overview of the in vitro/in vivo reno-protective effect of mangiferin and the underlying mechanism(s) in protecting against kidney disorders. Understanding the pharmacological actions of mangiferin is prominence due to its excellent therapeutic potential in managing kidney disorders. Thus, in addition to this review, in-silico molecular docking is performed against nuclear factor kappa B (NF-κB) and soluble epoxide hydrolase (sEH) to study the mechanism of action of mangiferin. It is believed that mangiferin is a safe reno-protective molecule. The observed positive effects are attributed to the inhibition of inflammation caused by NF-κB and sEH upregulation and oxidative stress activation. Studies on the efficacy and safety of mangiferin in clinical trials are further warranted to confirm its medicinal potential as therapeutic agent for kidney disorders in humans.
Keywords: Mangiferin, Mangifera indica, Reno-protective, Drug delivery, In-silico, Kidney disorders
1. Introduction
Mangiferin (Fig. 1), a yellow crystalline natural glucoxilxanthone, is a molecule of interest, which has been extensively investigated for its biological and therapeutic potentials (Lum et al., 2020). It is widely present in 96 species, 28 genera and 19 families of angiospermic plants (Jyotshna and P., Shanker, K., , 2016, Matkowski et al., 2013, Sekar, 2015, Walia et al., 2020). However, Mangifera indica (Mango), which belongs to the Anacardiaceae family, is the primitive and chief source of mangiferin. Almost every part of Mangifera indica, including the fruits (kernels and peels), twigs, leaves and stem bark, is rich in mangiferin (Jyotshna and P., Shanker, K., , 2016, Saha et al., 2016a).
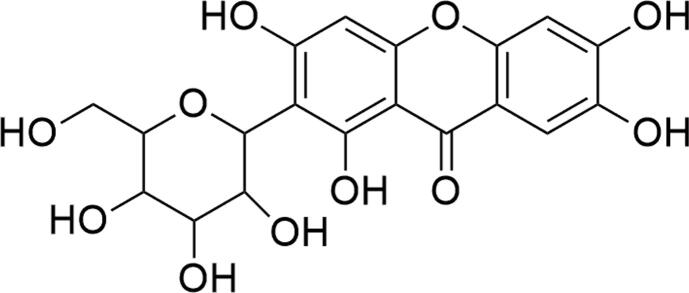
Fig. 1.
Structure of mangiferin.
The xanthonoid structural arrangement is critical to facilitate the interaction of mangiferin with specific drug receptors (Jyotshna et al., 2016). According to Lipinski’s rules, mangiferin exhibits drug-like properties, including its molecular weight, catechol moiety and calculated partition coefficient (C Log P) value, which enhancing its bioavailability (Saha et al., 2016a, Walia et al., 2020). For instance, intestinal permeability is enhanced when mangiferin forms complexes with phospholipids (Pal et al., 2015). Mangiferin also forms a complex structure with β-cyclodextrin, causing an increase in water solubility and thermal stability. Besides, the stable C-glucosyl linkage with multiple aromatic –OH groups in mangiferin contributes to its free radical scavenging potential accompanied by iron chelation against reactive oxygen species (ROS) (Walia et al., 2020). It can inhibit ROS generation in different organs, including the lungs, heart, liver, and kidneys (Mei et al., 2021). In view of this fact, mounting evidence has indicated that mangiferin possesses strong antioxidant properties along with multifactorial pharmacological activities including anti-inflammatory, analgesic, anti-apoptotic, antibacterial, antiviral, antimicrobial, immunomodulatory, antidiabetic, anticancer, cardioprotective, hepatoprotective, reno-protective and neuroprotective effects (Jangra et al., 2021, Lum et al., 2021, Mei et al., 2021, Saha et al., 2016a, Walia et al., 2020). Thus, emerging research suggests that mangiferin is an important bioactive leading compound or is a novel drug for the complementary and alternative approaches of a wide range of oxidative stress-mediated pathophysiology.
Nephropathy or kidney dysfunction is one of the major health problems closely related to nephrotoxicity, mainly due to increased intracellular oxidative stress and inflammation (Adeola et al., 2021, Rahman et al., 2021). To date, considerable studies have been initiated to evaluate the protective potential of mangiferin against kidney disorders. Indeed, the findings indicate mangiferin possesses therapeutic benefits for various kidney disorders under in vitro and in vivo models. In this review, we include the recent information gathering the reno-protective effects of mangiferin against renal disorders based on preclinical models. The pathophysiology of various kidney disorders is outlined. The possible protective mechanisms along with targeted signalling pathways conferred by mangiferin are also briefly described. In addition to this review, to study the mechanism of action of mangiferin, in-silico molecular docking is performed against NF-κB and sEH. This attempt reduces the gap of knowledge on its potential application as an alternative therapeutic agent for kidney disorders.
2. Kidneys structure and function
Kidneys are the renal system's principal functioning organ. They are necessary for homeostatic processes such as (1) filtering blood plasma, returning valuable materials to the blood, separating and eliminating waste from the body, (2) regulating blood volume and osmolarity, (3) producing hormones like calcitriol, erythropoietin, and renin enzyme, (4) maintaining body fluid acid-base equilibrium, and (5) detoxifying superoxides, free radicals, and drugs (Tanner et al., 2017).
The kidneys are paired bean-shaped organs which weight consist of about 125 g to 150 g each. The kidneys' anterior surface and abdominal organs are characterized by the peritoneal reflections intervening in the overlying organ contact with the kidneys. The right kidney’s anterior surface contact structures include the suprarenal gland, duodenum, liver, transverse colon, and jejunum while the left kidney is in contact with the suprarenal gland, stomach, spleen, pancreas jejunum and left colic flexure. The kidney's medial surface is concave, with a hilum. The outer cortex and inner medulla of the renal parenchyma are separated. The medulla is divided into 6–10 renal pyramids, and each is conical, with a blunt end facing the sinus which known as the papilla. The papilla is nestled in a small calyx, which collects the urine leaving from the papilla. A major calyx is formed by two or three joined minor calyces. The renal pelvis is formed by the merging of the main calyces with more narrowed ureter (Tanner et al., 2017). The cortex consists of a lighter outer layer, characterized by granular looking tissue which also seen to dip as renal columns towards the pelvis of the kidney. It is separating the conical renal pyramids of the medulla. In the cortex, the glomeruli and convoluted tubules are found. Meanwhile, the renal pyramids contain loop of Henle and collecting tubes.
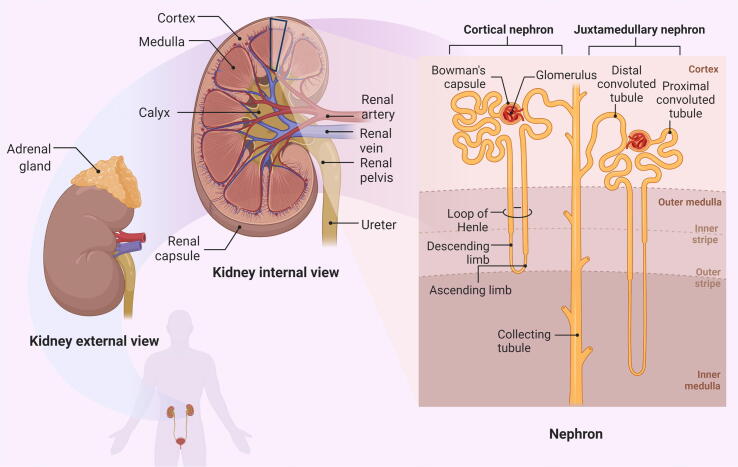
The kidney has 1.2 million nephrons, which are the kidney's functioning units. A nephron consists of blood vessels and renal tubules, as shown in Fig. 2. Nephron filters the blood, resorbs what is needed, and excretes the rest as urine. Its function is essential for homeostasis of blood volume, blood pressure, and plasma osmolarity (Tanner et al., 2017).
Fig. 2.
Anatomy of the kidney. The renal cortex and renal medulla are visible in the frontal sections of the kidney. Renal columns made of connective tissue separate 5–8 renal pyramids in the medulla. Each pyramid produces urine and eventually leads to a renal papilla. Each renal papilla drains into a minor calyx, which unites to form a major calyx. All major calyces connect to the one renal pelvis, which links to the ureter. The figure was created with the support of BioRender.com under a paid subscription.
3. Kidney disorders and pathophysiology
Renal dysfunction is a predominant factor contributing to the occurrence of acute kidney injury (AKI), followed by the progression of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (Wei et al., 2020). AKI involves a sudden decline in glomerular filtration rate (GFR), creatinine accumulation, reduction in urine output, disturbances of electrolyte, and uremia (Hultström et al., 2018, Wei et al., 2020). Clinically, incomplete recovery of renal function along with a persistent decrease in GFR and renal tissue damage may lead to the development of CKD, one of the main contributors to the morbidity and mortality of non-communicable diseases (NCDs) (Wei et al., 2020). In this context, understanding the pathogenesis of kidney disorders is of great significance in developing the novel therapeutic approaches. Table 1 summarizes an overview of kidney disorders and their pathogenesis.
Table 1.
Overview of kidney disorders and their pathogenesis.
| Disorder/disease | Causative factor | Pathophysiology |
|---|---|---|
| Diabetic nephropathy | Diabetes (High blood glucose) | An increase of extracellular matrix leads to a thickened glomerular basement membrane and an enlarged mesangium. The portion of the kidneys that filters blood is damaged. Destroyed filters become 'leaky', leading to protein released into the urine. |
| Diabetics insipidus | Inadequate secretions of anti-diuretics hormones | Impaired arginine vasopressin (AVP) secretions from the posterior pituitary gland lead to an imbalance of plasma osmolality and arterial blood volume, resulting in dilute urine, frequent urination and excessive thirst (Kalra et al., 2016). |
| Hyperuricemia nephropathy | Increased serum uric acid concentration | Reduced excretion (underexcretions) or/and increased production (overproducers) of uric acid. |
| Glomerulonephritis | Immune mediated and inflammatory response | The antigen–antibody reaction and cell-mediated immune response lead to inflammatory reactions. The pro-inflammatory cytokines and complement products, in turn, proliferate the glomerular cells (Trachtman et al., 2019). |
| Kidney stones | Supersaturations of minerals constituent in kidney | The imbalance level between the promoter of crystallizations and urinary inhibitors leads to kidney stones. The ion transformation from a liquid into a solid is influenced by the pH and concentration of excess minerals (Alelign and Petros, 2018). |
| Lupus nephritis | Autoimmune disorders | Tolerance to nuclear autoantigens is lost, and autoreactive T and B cells are activated, resulting in the generation of pathogenic autoantibodies and tissue damage. |
| Polycystic kidney disease (PKD) | Inheritance genetic mutations | Decreased amount of PKD protein results in a disturbance of cell homeostasis and signalling pathways. The vascular endothelial growth factor and hippo signalling is impaired (Bastos and Onuchic, 2011). |
| Renal cell carcinoma (RCC) | The cytotoxic immune system failed to distinguish the cancer cell | Genetic alterations of Von Hippel-Lindau (VHL) gene and protein polybromo-1 gene (PBRM-1) leads to renal carcinoma. The cytotoxic immune system unable to recognize, and eliminate and kill the cancer cells (Petejova and Martinek, 2016). |
| Renal fibrosis | Excessive accumulation and deposition of extracellular matrix components | Failure of tissues wound-healing process due to a persistent and prolonged insult to the kidney tissues. |
| Renal injury | Ischemia, nephrotoxicity or hypoxia | Reduction of renal blood flow followed by the decrease in glomerular filtration rate (GFR). |
| Urinary tract infections (UTI) | Bacterial infections | Bacteria spreading and colonizing on the lower or upper urinary tract. The symptoms include fever and pain during urination (Pulipati et al., 2017). |
4. Effect of mangiferin against kidney disorders
The research investigating the reno-protective potential of mangiferin in combating oxidative stress and modulating inflammation is significant. Collectively, mangiferin is effective in conferring reno-protection against a variety of renal disorders, including kidney injury, diabetic nephropathy, renal fibrosis, hyperuricemic nephropathy and lupus nephritis, mainly via the antioxidant system. Comprehensive detail of their reno-protective efficacy against kidney disorders under in vitro/in vivo models are summarized in Table 2.
Table 2.
Protective effect of mangiferin against kidney disorders based on the reported in vitro and in vivo studies.
| Study models | Cell lines/ animals, sex | Mangiferin concentration/ dose, route | Duration | Outcomes | Mode of action | References |
|---|---|---|---|---|---|---|
| Cadmium-induced human renal endothelial damage | HRGE cells | 75 µM | 24 h | Cell viability ↑; ROS ↓; LPO ↓; SOD activity ↑, GPx activity ↑, DNA damage ↓; alteration of Bax and Bcl-2 inhibited; NF-κB ↓; IL-6 ↓, IL-8 ↓ | Antioxidant; anti-inflammatory; anti-apoptosis | Rajendran et al. (2016) |
| Cisplatin-induced AKI | NKE cells | 2, 5, 10, 15, 20, 25, 30, 40, 50 µM | 24 h | ROS ↓; mitochondrial activity restored; caspase-3 expression ↓ | Antioxidant; anti-apoptosis | Sadhukhan et al. (2018) |
| Swiss albino mice, male | 10, 20 a, 40 mg/kg, po | 21 days | serum BUN ↓, SCr ↓; ROS ↓; CAT activity ↑, GR activity ↑, GPx activity ↑, GST activity ↑, SOD activity ↑; GSH activity ↑; MPO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓; renal histological changes ↓; mean tumour volume ↓; mean tumour mass ↓ | Antioxidant; anti-inflammatory; anticancer | Sadhukhan et al. (2018) | |
| Albino Wistar rats, male | 10, 20 a, 40 a mg/kg, ip | 10 days | Serum BUN ↓, SCr ↓; MDA ↓, CAT ↑, GSH ↑, SOD ↑; TNF-α ↓, IL-6 ↓; Bax expression ↓, Bcl-2 expression ↑, caspase 3 expression ↓; NF-κBp65 ↓, ERK1/2 ↓, JNK ↓, p38 ↓ | Antioxidant; anti-inflammatory; anti-apoptosis | Sahu et al. (2019) | |
| DGal-induced nephrotoxicity | Wistar rats, male | 15 mg/kg, ip | 14 days | Serum BUN ↓, SCr ↓, protein carbonyl ↓, MDA ↓, ROS ↓, NO ↓; TNF-α ↓; CAT activity ↑, GR activity ↑, GPx activity ↑, GST activity ↑, SOD activity ↑; GSH activity ↑, total thiols ↑; caspase 3 and 9 expressions ↓; iNOS ↓, nuclear NF-κB ↓, IκBα ↑; histological changes ↓ | Antioxidant | Ghosh et al. (2012) |
| Hyperuricemia (induced by hypoxathine and oteracil potassium) | ICR mice, male | 50 mg/kg, po | 17 days | Serum BUN ↓, SCr ↓, serum uric acid ↓; inflammatory changes ↓; NLRP3 expression ↓; fibronectin expression ↓; PKCβ expression ↓; urinary uric acid excretion ↑, AQP2 expression ↓, XO activity ↓ | Antioxidant; anti-inflammatory; anti-apoptosis | Li et al. (2020) |
| Hyperuricemia (induced by uric acid and potassium oxonate) | Sprague- Dawley rats | 1.5–24 mg/kg b, ig | Serum urate ↓, urinary urate ↑, FEUA ↑; expression of rURAT1, rGLUT9 and OAT10 ↓ | Renal urate transporters downregulation | Yang et al. (2015) | |
| Lupus nephritis | FasL-deficient B6/gld mice | 20, 40 mg/kg, po | 84 days | SCr ↓; urine protein ↓; α-SMA expression ↓; deposition of IgG ↓; TNF-α ↓, IL-6 ↓, IFN-γ ↓; Tregs proliferation ↑ | Anti-inflammatory; immunomodulatory effects | Liang et al. (2018) |
| NaF-induced nephrotoxicity | NKE cells | 25, 50, 75, 100 µg/ml b | 24, 48, 72 h | Cell viability ↑; ROS ↓; GSH ↑, GSSH ↓ | Antioxidant | Samadarsi and Dutta (2020) |
| Renal ischemia reperfusion injury | C57/BL6 mice, male | 10, 30, 100 a mg/kg, po | 7 days | Serum BUN ↓, SCr ↓; caspase-3 expression ↓; TNF-α ↓, IL-1β ↓; NO ↓; adenosine production ↑; CD-73 expression ↓ | Anti-inflammatory; anti-apoptosis | Wang et al. (2015) |
| Sepsis-induced AKI/LPS-induced AKI | C57BL/6 mice, male | 20, 50, 100 mg/kg b, po | 7 days | Serum BUN ↓, SCr ↓; occludin expression restored; MMP-9 expression ↓; ROS ↓, MDA ↓; NF-κB expression ↓, HMGB1 expression ↓; kidney pathological damage ↓ | Antioxidant; anti-inflammatory | Zhang et al. (2020) |
| RPTC cells | 10, 50, 100µm | 1 h | Increase of annexin V-positive cells inhibited; caspase 3 and 9 expressions ↓; NLRP3 activity inhibited, Nrf2 activity restored | Anti-apoptosis | He et al. (2014) | |
| C57BL/6 mice, male | 15 mg/kg, ig | Serum BUN ↓, SCr ↓, IL-1β ↓, IL-18 ↓; Nrf2 activity ↑, NLRP3 protein ↓ | Antioxidant; anti-inflammatory | He et al. (2014) | ||
| STZ-induced diabetic neuropathy | Sprague- Dawley rats, male | 12.5, 25, 50 mg/kg b, po | 84 days | Urine protein ↓, urea nitrogen ↓, SCr ↓; nephrin expression ↑; p62 expression ↓; LC3-II/LC3-I ratio ↑; p-ULK1 ↑, AMPK and mTOR phosphorylation ↑; renal lesions improved | Autophagy | Wang et al. (2018) |
| MES cells | 10 µM | 48 h | ROS ↓ | Antioxidant | Xu et al. (2017) | |
| ICR mice, male | 50 mg/kg, po | 112 days | Serum BUN ↓, SCr ↓, urine protein ↓; Nox4 expression ↓, caspase-3 expression ↓; histological alteration ↓ | Antioxidant; anti-apoptosis | Xu et al. (2017) | |
| Wistar rats, male | 40 mg/kg, po | 40 days | Serum BUN ↓, SCr ↓, serum uric acid ↓, urinary albumin ↓; plasma glucose ↓; renal injury ↓; LPO ↓, protein carbonyl ↓, GSH ↑; ROS ↓; CAT activity ↑, GR activity ↑, GPx activity ↑, SOD activity ↑; AGE formation suppressed, hydroxyproline ↓; PKCα, PKCβ and PKCɛ expression ↓; p38, JNK and ERK1/2 MAPKs activation inhibited; NF-κB ↓, IKKα ↓, IκBα ↑; TGF-1β ↓; caspase 8 and t-Bid expression ↓; alteration of Bax and Bcl-2 inhibited | Antioxidant; anti-apoptosis | Pal et al. (2014) | |
| Sprague- Dawley rats, male | 15, 30, 60 mg/kg b, po | 63 days | Urine protein ↓, serum BUN ↓, GSH ↑, MDA ↓; Glo-1 mRNA ↑, Glo-1 activity ↑; mesangial matrix expansion ↓; AGEs ↓ | Antioxidant; up-regulation of Glo-1 | Liu et al. (2013) | |
| Wistar rats, male | 40 mg/kg, po | 30 days | Blood glucose ↓, plasma insulin ↓; activities of HK, FBP, G6P, G6PD, LDH and PK restored | Anti-hyperglycaemic; carbohydrate metabolic enzyme activities mediation | Sellamuthu et al. (2012) | |
| STZ-induced diabetic renal fibrosis | C57BL/6 mice, male | 15, 30, 60 mg/kg b, po | 28 days | Serum BUN ↓, SCr ↓, urine protein ↓; TG ↓, TC ↓; TNF-α ↓, IL-1β ↓, IL-6 ↓; ROS ↓; CAT activity ↑, GPx activity ↑, SOD activity ↑; MDA ↓; FN ↓, Col I ↓, α-SMA ↓; TGF-1β ↓ | Antioxidant; anti-inflammatory | Song et al. (2020) |
| Sprague- Dawley rats, male | 15, 30, 60 mg/kg, po | 63 days | Col IV ↓, α-SMA ↓; OPN ↓; IL-1β ↓ | Anti-inflammatory | Zhu et al. (2015) | |
| tBHP-induced renal injury | NKE cells | 10–200 µM | 24 h | ROS ↓; GSH activity ↑; Bax/Bcl-2 ratio ↓; caspase 8 and t-Bid expression ↓; Nrf2 ↓; morphological abnormalities ↓ | Antioxidant; anti-apoptosis | Saha et al. (2016b) |
| Swiss albino mice, male | 75 mg/kg, po | 14 days | Urine protein ↓; Serum BUN ↓, SCr ↓, urea ↓, uric acid ↓; urinary albumin ↓; ROS ↓; CAT activity ↑, GR activity ↑, GPx activity ↑, GST activity ↑, SOD activity ↑; GSH activity ↑; iNOS ↓, NO ↓; TNF-α ↓, IL-1β ↓, IL-6 ↓, MCP-1 ↓, ICAM-1 ↓, VCAM-1 ↓; morphological abnormalities ↓ | Antioxidant; anti-inflammatory; anti-apoptosis | Saha et al. (2019) | |
| Combination therapy of mangiferin and metformin | ||||||
| STZ-induced diabetic neuropathy | Sprague- Dawley rats, male | 40 mg/kg, po | 28 days | Glucose ↓; BUN ↓, creatinine ↓, albumin ↓; LPO ↓; CAT ↑, GPx ↑, GST ↑, SOD ↑; GSH ↑; expression of NF-κB, PKC, TGF-1β and VEGF ↓ | Antioxidant | Sekar et al. (2020) |
Abbreviations: AGEs, Advanced glycation end products; AKI, Acute renal injury; AMPK, AMP-activated protein kinase; AQP2, Aquaporin 2; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; BUN, Blood urea nitrogen; CAT, Catalase; Col I, type-I collagen; Col IV, type-IV collagen; DGal, D(+) galactosamine; ERK1/2, Extracellular signal-regulated kinases 1/2; FBP, Fructose-1,6-bisphosphatase; FEUA, Fractional excretion of uric acid; FN, Fibronectin; G6P, Glucose-6-phosphatase; G6PD, Glucose-6-phosphatase dehydrogenase; Glo-1, glyoxalase; GPx, Glutathione peroxidase; GR, Glutathione reductase; GSH, Reduced glutathione; GSSH, Oxidized glutathione; GST, Glutathione s-transferase; HK, Hexokinase; HMGB1, High mobility group box protein 1; HRGE, Human renal glomerulus endothelial cells; ICAM-1, Intercellular Adhesion Molecule 1; IFN-γ, Interferon γ; ig, Intragastric gavage; IgG, Immunoglobulin G; IL-1β, Interleukin-1β; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-18, Interleukin-18; iNOS, inducible nitric oxide synthase; ip, Intraperitoneal injection; IκBα, Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; JNK, c-Jun N-terminal kinase; LC3-I, cytosolic form of LC3; LC3-II, LC3-phosphatidylethanolamine conjugate; LDH, Lactate dehydrogenase; LPS, Lipopolysaccharide; MAPKs, mitogen-activated protein kinases; MCP-1, Monocyte Chemoattractant Protein-1; MDA, Malondialdehyde; MES, Mouse mesangial; MMP-9, Matrix metallopeptidase 9; MPO, Myeloperoxidase; mTOR, mammalian target of rapamycin; OAT10, renal organic anion transporter 10; OPN, Osteopontin; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; NKE, Normal kidney epithelial; NLRP3, Nod-like receptor protein 3; NO, Nitric oxide; Nox4, NADPH oxidase 4; Nrf2, Nuclear factor erythroid 2-related factor 2; PK, Pyruvate kinase; PKC, Protein kinase C; po, per os, oral administration; p-ULK1, Phosphorylated Unc51-like kinase 1; rGLUT9, renal glucose transporter 9; ROS, Reactive oxygen species; RPTC, Rat kidney proximal tubular cell; rURAT1, renal urate-anion transporter 1; SCr, Serum creatinine; SOD, Superoxide dismutase; STZ, Streptozotocin; tBHP, tert-Butylhydroperoxide; t-Bid, truncated Bid; TC, Total cholesterol; TG, Triglyceride; TGF-1β, Transforming growth factor beta 1; TNF-α, T necrosis factor α; Tregs, Regulatory T cells; VCAM-1, Vascular cell adhesion molecule 1; VEGF, Vascular endothelial growth factor; α-SMA, Smooth muscle alpha-actin; XO, Xanthine oxidase
Most effective concentration/dose. b Concentration/dose dependent
4.1. Mangiferin and renal injury
Renal dysfunction or injury due to nephrotoxicity is an oxidative stress-mediated pathological condition induced by various exogenous or endogenous toxins such as heavy metals and drugs (Kabir et al., 2021). Several studies have indicated that certain environmental toxins including cadmium (Rajendran et al., 2016), D(+) galactosamine (DGal) (Ghosh et al., 2012), sodium fluoride (NaF) (Samadarsi and Dutta, 2020) and tert-butyl hydroperoxide (tBHP) (Saha et al., 2019) induce renal damage in both in vitro and in vivo models. AKI is a frequent and severe complication of sepsis (Zhang et al., 2020), renal ischemia–reperfusion (Wang et al., 2015), shock and due to drug toxicity such as from cisplatin (Sahu et al., 2019). In the development of AKI, a sudden reduction of GFR along with an increase in serum creatinine, uremia, electrolyte imbalance and rapid decline in renal function commonly occurs, leading to a direct AKI-induced cytotoxicity that is accompanied by an extensive or persistent-cell and tubular damage (Succar et al., 2017, Wei et al., 2020). In this case, incomplete recovery of AKI may lead to onset or further development of CKD.
In trying to identify natural product-based therapeutic agents, accumulating reports have suggested mangiferin’s potent reno-protective effects. Interestingly, a report by Ghosh et al. (2012) confirmed the therapeutic benefits of mangiferin (15 mg/kg, ip) against D(+) galactosamine (DGal)-induced nephrotoxicity and renal damage in rats. The protection is largely attributed to the antioxidant activities of mangiferin occurring via the mediation of inducible nitric oxide synthase (iNOS) and NF-KB pathways in renal tissue. A recent study by Samadarsi and Dutta (2020) also supported the antioxidant efficacy of mangiferin against NaF-induced nephrotoxicity in normal kidney epithelial (NKE) cells. The anti-inflammatory activities of mangiferin have been demonstrated in an ischemia–reperfusion renal injury model indicating that mangiferin (100 mg/kg, po) administration alleviated renal injury by suppressing tubular apoptosis and pro-inflammatory response via the adenosine-CD73 signalling pathway. The suppressive effect of mangiferin is also reported in an in vitro study performed on cadmium-treated human renal endothelial cells. In the study, co-treatment with mangiferin (75 μM) inhibits the expression of interleukin-6 (IL-6) and interleukin 8 (IL-8) by deactivating the mitogen-activated protein kinases (MAPKs) and NF-KB signaling pathways (Rajendran et al., 2016).
To gain insight into the reno-protective mechanism, a similar study was conducted by Saha et al. (2016b) on tBHP-induced renal damage on NKE cells. Apparently, the intracellular antioxidant status was maintained by mangiferin corresponding to an enhanced expression of cyclin D1, heme oxygenase-1 (HO-1), superoxide dismutase 2 (SOD-2) and NF-KB via activation of phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (Akt) pathways. Similar mechanisms were also elucidated by Sadhukhan et al. (2018) in both cisplatin-induced AKI in vitro and in vivo models. Mangiferin ameliorated the nephrotoxicity by upregulating the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) cascade via PI3K signalling pathway. Recently, Saha et al. (2019), who further explored the molecular mechanism of mangiferin on tBHP-treated animals in vivo, found that mangiferin (75 mg/kg, po) exhibited an anti-apoptotic activity acting via downregulation of pro-apoptotic cascade as mediated by c-Jun N-terminal kinase (JNK) and p38MAPK pathways. Similarly, Sahu et al. (2019) also demonstrated the attenuation of cisplatin-induced renal injury and apoptotic cascade by mangiferin pre-treatment (20 and 40 mg/kg, po) occurring via modulation of the MAPK pathway.
Additionally, He et al. (2014) confirmed the reno-protective effect of mangiferin by virtue of its antioxidant, anti-inflammatory and anti-apoptotic properties in both sepsis-induced AKI in vitro and in vivo models, with increased renal Nrf2 expression and inhibition of renal Nod-like receptor protein 3 (NLRP3) activation. Supporting the previous studies, the study by Zhang et al. (2020) revealed that mangiferin (20, 50, and 100 mg/kg, po) dose-dependently reduced the renal injury due to sepsis, suggesting the protection to occur via the suppression of high mobility group 1 inflammatory (HMGB1) and NF-KB signalling pathways.
4.2. Mangiferin and diabetic nephropathy
Diabetic nephropathy is the most serious diabetic microvascular complication commonly seen in type I and II diabetes mellitus worldwide (Dagar et al., 2021). It is one of the notorious leading causes of CKD and ESRD (Rossing, 2006). It is typically characterized by persistent proteinuria, glomerular hyperfiltration followed by accumulation of glomerular extracellular matrix proteins (ECM), hypertrophy, basement membrane thickening, expansion of mesangial matrix, injury of podocytes (by autophagy impairment), consequently leading to glomerular sclerosis as well as tubulointerstitial fibrosis (Nogueira et al., 2017, Soetikno et al., 2011). Pathologically, diabetic nephropathy is primarily caused by chronic hyperglycaemia as a result of a long-term impairment in insulin production and resistance (Hajiaghaalipour et al., 2015, Sekar et al., 2020). Under diabetic conditions, there are an increased generation of ROS and production of the inflammatory response due to hyperglycaemia, indicating that oxidative stress and chronic inflammation play a crucial role in the development and progression of diabetic nephropathy (Rivero et al., 2009, Soetikno et al., 2011). Therefore, it has been suggested that metabolic control is the key therapeutic approach in maintaining a balanced state of renal functions (Sekar et al., 2020).
To investigate the reno-protective effects of naturally occurring bioactive compounds, streptozotocin (STZ) has been widely used to induce diabetes in preclinical models. Interestingly, mangiferin administration (40 mg/kg, po) significantly reduces blood glucose levels while restoring renal endogenous antioxidant enzymes in STZ-induced diabetic nephropathy rats, thus suggesting its anti-hyperglycemic effects occurring following mediation of kidney carbohydrate metabolic enzyme activities (Sellamuthu et al., 2012). Another study by Liu et al. (2013) reported that chronic administration of mangiferin (15, 30, and 60 mg/kg, po) dose-dependently alleviate diabetic nephropathy in STZ-treated rats by suppressing the oxidative stress damage and advanced glycation end products (AGEs) formation along with increased glyoxalase (Glo-1) expression in the renal cortex of rats.
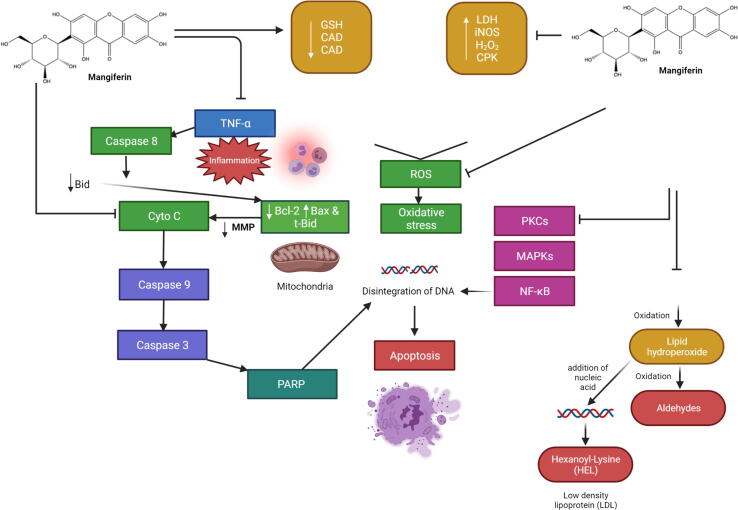
The mechanism underlying the prophylactic effect of mangiferin was further investigated by Pal et al. (2014). Mangiferin treatment (40 mg/kg, po) regulated different signalling molecules including NF-KB, transforming growth factor-beta 1 (TGF-1β), MAPKs and protein kinase C (PKC) isoforms along with the caspase 3 and 9 apoptotic pathways, thereby ameliorating the altered levels of renal biomarkers in STZ-intoxicated diabetic nephropathy rats (Fig. 3). Additionally, mangiferin reduces the collapsed mitochondrial membrane potential (ΔΨ) and prevents oxidative stress damage via inhibition of mitochondrial bound hexokinase II dissociation and NADPH oxidase 4 (Nox4) expression in both STZ-treated mouse mesangial (MES) cells and mice models (Xu et al., 2017).
Fig. 3.
Effect of mangiferin in diabetic nephropathy. The pathophysiology of diabetic nephropathy is multifaceted, involving metabolic and hemodynamic alterations, chronic inflammation, activation of the renin-angiotensin system, and oxidative stress. The activation of AGEs, autoxidation of glucose, and xanthine oxidase activity are some of the most likely main sources of ROS production. PKC isoforms, TGF-β1 pathways, and NF-kB were found to be implicated in diabetic nephropathy's oxidative stress-mediated signaling cascades. In the hyperglycemic state, TNF-α was produced, which activated caspase 8, cleaved Bid to tBid, and finally activated the mitochondria-dependent apoptotic pathway. Following hyperglycemia, mangiferin therapy successfully suppressed all of these alterations and preserved the cells from apoptosis. Several novel markers for early disease detection have emerged from the pathologic processes of underlying renal dysfunction and damage. In normoalbuminuric patients with diabetes mellitus type 1, poor glycemic control is an independent predictor of progression to proteinuria (albuminuria). Abbreviations: AGEs, Advanced glycation end products; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Bid, BH3-interacting domain death agonist; Cyto C, Cytochrome; cMAPKs, mitogen-activated protein kinases; MMP, Mitochondrial membrane potential; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; PKC, Protein kinase C; ROS, Reactive oxygen species; tBid, Truncated form of Bid; TGF-1β, Transforming growth factor beta 1; TNF-α, T necrosis factor α.
Furthermore, Wang et al. (2018) suggested that mangiferin (12.5, 25.0, and 50.0 mg/kg, po) potentially exerted podocyte protection as shown by the restoration of nephrin expression in a dose-dependent manner. In addition, mangiferin mediates a series of autophagy processes, including augmentation of the expression of LC3-phosphatidylethanolamine conjugate (LC3-II), AMP-activated protein kinase (AMPK) phosphorylation and phosphorylated Unc51-like kinase 1 (p-ULK1) levels, as well as the inhibition of p62 expression and mammalian target of rapamycin (mTOR) phosphorylation (Bonam et al., 2020, Wang et al., 2018). Such finding indicates that mangiferin can prevent renal damage due to diabetic nephropathy from occurring via autophagy induction (Wang et al., 2018).
Since renal tubular fibrosis is one of the clinical manifestations of diabetic nephropathy, the therapeutic potency of mangiferin was also evaluated against diabetic renal fibrosis in experimental models. Mangiferin administration (15, 30, and 60 mg/kg, po) effectively suppressed osteopontin (OPN) production, type-IV collagen (Cov IV) and α-smooth muscle actin (α-SMA) levels, which in turn reduce renal inflammation, thereby ameliorating renal interstitial fibrosis (Zhu et al., 2015). Similarly, another recent study also indicates that mangiferin (15, 30, and 60 mg/kg, po) dose-dependently prevented renal fibrosis by decreasing the levels of α-SMA, type-I collagen (Col I) and fibronectin (FN) via downregulation of TGF-1β and vascular endothelial growth factor (VEGF) along with the upregulation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) (Song et al., 2020). A further investigation by Sekar et al. (2020) confirmed the strong synergistic interaction of mangiferin with metformin in attenuating diabetic nephropathy in rats indicating that the combined therapy of mangiferin and the conventional oral hypoglycaemic drug is a suitable alternative therapeutic approach.
4.3. Mangiferin and hyperuricemic nephropathy
Urate or uric acid, the final product of purines, is a breakdown of the liver’s oxidation of xanthine and hypoxanthine. Serum urate level is mainly regulated and is excreted by the kidney (Yang et al., 2015). Hyperuricemia is characterized by an abnormally high circulating urate level as well as accumulation in blood and crystallization in tissues (Li et al., 2020). The development of the abnormal condition is predominantly caused by the excessive production or/and less excretion of urate. However, insufficient renal excretion of urate is the preliminary cause of hyperuricemia among approximately 90% of individuals (Perez-Ruiz et al., 2015). Hyperuricemia induces oxidative stress, renal inflammation, endothelial dysfunction subsequent to renal fibrosis, ultimately leading to hyperuricemic nephropathy (Jalal et al., 2013, Rock et al., 2013). A growing body of evidence has reported that hyperuricemia associated pathological complications involve urolithiasis, gouty arthritis, obstructive uropathy, renal dysfunction, nephritis, metabolic syndrome, diabetes, hypertension, and cardiovascular diseases (Ciarla et al., 2014, Jalal et al., 2013, Zoccali and Mallamaci, 2013) making a higher focus towards research that lean towards the identification of natural products as an alternative to anti-hyperuricemic agent.
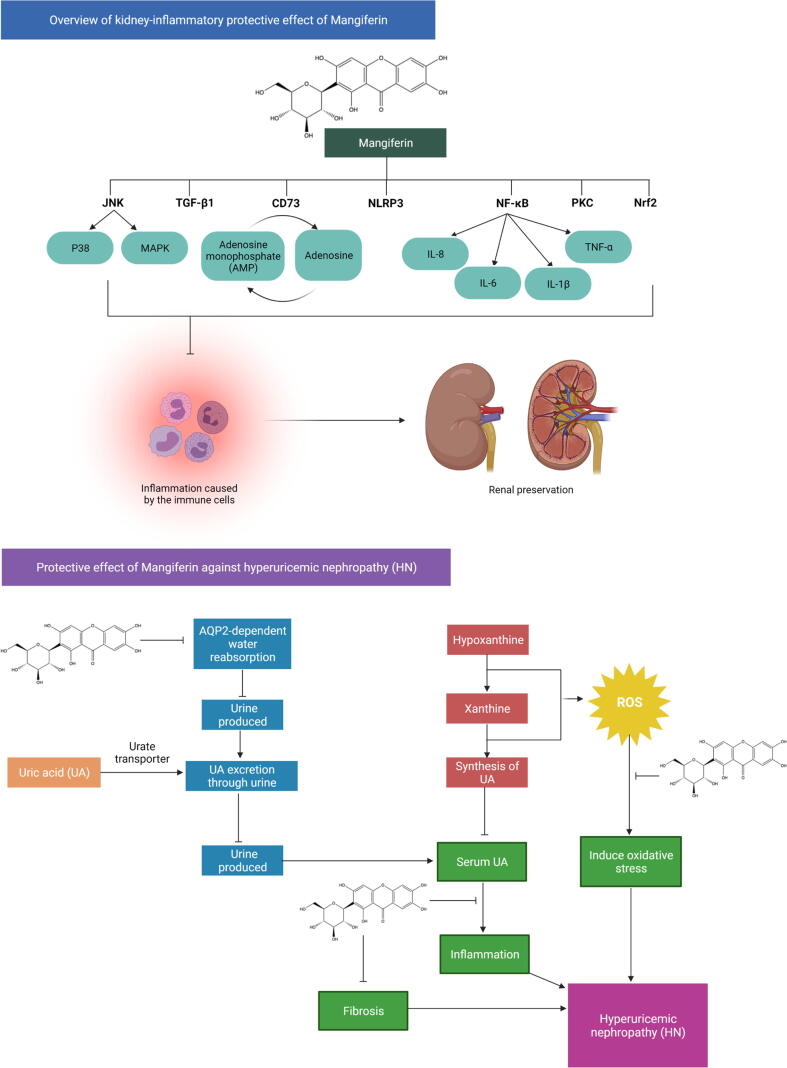
Motivated by the above-mentioned concerns, the uricosuric properties of mangiferin were evaluated in uric acid-induced hyperuricemia experimental models by Yang et al. (2015). Mangiferin treatment (1.5–24.0 mg/kg, ig) effectively decreased serum urate levels and augmented urate excretion in a dose-dependent manner by downregulating the renal urate transporters, including the renal glucose transporter 9 (rGLUT9), urate-anion transporter 1 (rURAT1) and organic anion transporter 10 (rOAT10). On the contrary, the recent study by Li et al. (2020) revealed that instead of reducing the expression of renal urate transporters, mangiferin (50 mg/kg, po) enhances urate excretion mainly by downregulating the expression of renal aquaporin-2 (AQP2) in a hyperuricemic mice model. The decreased expression of AQP2 evidences the findings. Nevertheless, the expression of renal urate transporters was not affected by mangiferin administration. Taken together, these findings suggested that the uricosuric action of mangiferin occurs by prevention of hyperuricemic nephropathy (Fig. 4) via the modulation of oxidative, inflammatory and fibrotic pathways (Li et al., 2020).
Fig. 4.
Protective effect of mangiferin against kidney-inflammation and HN. XO is a fundamental pharmacological target in anti-hyperuricemic treatment since it is the major enzyme in catalyzing uric acid synthesis. The C-glucosyl linkage and polyhydroxy groups in mangiferin's structure contribute mostly to its free radical-scavenging activity, allowing it to suppress the generation of ROS as a consequence, resulting in a reduction in oxidative stress. Thirdly, its ability to modulate the expression of different proinflammatory signaling intermediates like TNF-α, as well as inhibiting the pathogenesis and inflammation of the kidney by modulating JNK, TGF-β1, NF-B, CD73, Nrf2, NLRP3, and their downstream signaling molecules, contribute to its protective mechanism. Mangiferin effectively lowers uric acid levels via enhancing AQP2-related uric acid excretion and decreasing XO-mediated uric acid synthesis. By acting as an antioxidant, anti-fibrotic and anti-inflammatory agent decreased uric acid or resulted in reducing the risk of HN. Abbreviations: AMPK, AMP-activated protein kinase; AQP2, Aquaporin 2; HN, Hyperuricemic nephropathy; IL-1β, Interleukin-1β; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-18, Interleukin-18; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; MAPKs, mitogen-activated protein kinases; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; NLRP3, Nod-like receptor protein 3; Nrf2, Nuclear factor erythroid 2-related factor 2; PKC, Protein kinase C; TGF-1β, Transforming growth factor beta 1; TNF-α, T necrosis factor α; XO, Xanthine oxidase.
4.4. Mangiferin and lupus nephritis
Systemic lupus erythematosus (SLE), an autoimmune disease, is also a chronic inflammatory disease that can trigger renal inflammation, secondary to lupus nephritis (Almaani et al., 2017, Bonam et al., 2018). Lupus nephritis, which is also known as autoimmune glomerular nephritis that causes high morbidity and mortality, is histologically evident in approximately 50% of SLE patients (Lewis and Jawad, 2017). A series of pathological mechanisms, including dysregulation of T-regulatory cells (Tregs) due to the hyperactivity of T- and B- lymphocytes, aberrant autoimmune antibodies generation, immune complexes deposition and pro-inflammatory responses activation which ultimately attack kidney tissues are involved in lupus nephritis-related kidney damage (Almaani et al., 2017, Hoover and Costenbader, 2016).
To date, current therapeutic strategies are aimed at targeting renal immunopathology notably by normalizing Tregs function. Nevertheless, significant advancement in immunosuppressive drugs, various severe side effects are inevitable. Thus, research is dedicated towards investigating natural compounds as an alternative application in treating a wide range of autoimmune diseases. The immunomodulatory activity of mangiferin has been investigated in a FasL-deficient B6/gld mice in vivo model (Liang et al., 2018). Mangiferin (20 and 40 mg/kg, po) conferred reno-protection by attenuating lupus nephritis in mice via immunoregulatory and anti-inflammatory processes. The immunomodulatory effects were exerted by inhibiting T-cells proliferation, inducing the expression of Tregs (CD4+CD25+CD127-FoxP3+) via the suppression of mTOR signalling pathways (Liang et al., 2018).
5. Molecular docking analysis highlights the role of mangiferin in controlling kidney disorders
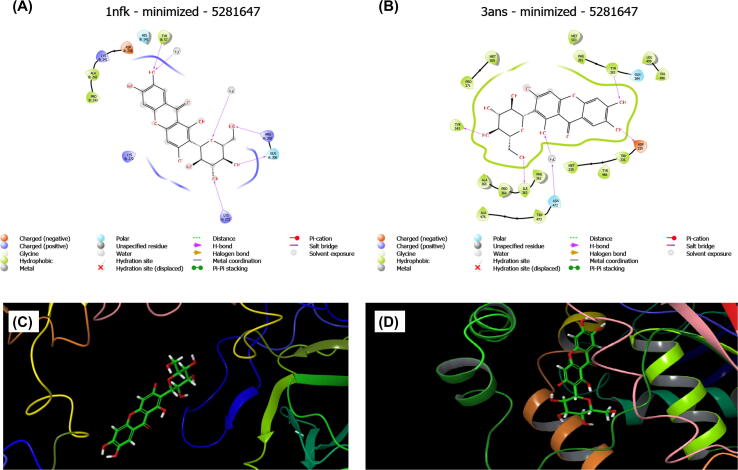
Molecular docking studies demonstrating the association of mangiferin with molecular targets implicated in the development of kidney disorders that are still to be revealed. To circumvent this constraint, molecular docking investigations were conducted using a flexible docking approach (Ghosh et al., 1995) using the Maestro 12.5 package (Schrodinger 2020–3). Protein Data Bank (PDB) was used to obtain the target proteins NF-κB-DNA (PDB ID: 1NFK) (Amilkanthawar et al., 2020) p50 homodimer with a resolution of 2.30 Å and human soluble epoxide hydrolase (sEH; PDB ID: 3ANS) (Tanaka et al., 2011) in complex with synthetic inhibitor with a resolution of 1.98 Å.
Diverse pathophysiological progressions in renal cells stimulate NF-κB, which is responsible for inflammation in kidney disorders (Sanz et al., 2010, Zhang and Sun, 2015). Chronic inflammation and oxidative stress, which link to NF-κB activation, play a vital role in the progress of CKD. sEH is found in a variety of cells and tissues (Khan et al., 2013). Several investigations have indicated that sEH inhibitors have anti-inflammatory and nephroprotective properties (Khan et al., 2013, Liu et al., 2016, Zhang et al., 2017). The aforesaid factors motivated us to look into mangiferin's molecular docking against NF-κB and sEH, which are responsible for the majority of kidney diseases.
Mangiferin showed a docking score of −4.583 kcal/mol against NF-κB and −10.058 kcal/mol against sEH. The hydrogen-bonding interactions of mangiferin with the target 1NFK & 3ANS are depicted in Fig. 5A and 5B, respectively. Also, the docking poses of protein–ligand interaction are depicted in Fig. 5C for NF-κB and Fig. 5D for sEH. Overall, the in-silico results showed that mangiferin potentially inhibits NF-κB and sEH, implying that mangiferin may protect kidneys from injury through anti-inflammatory and antioxidant mechanisms.
Fig. 5.
Stereo-view of mangiferin docked into the DNA binding region of (A) NF-κB (1NFK) and (B) active site of sEH (3ANS). The best docked postions of protein–ligand interaction of mangiferin with (C) NF-κB and (D) sEH.
6. Challenges and opportunities when developing drug delivery system of mangiferin for kidney disorders
Mangiferin is mainly obtained from mango and is reported to have low lipophilicity and hydrophilicity (Imran et al., 2017). It has potent anti-inflammatory, antioxidant and other properties, making it a great candidate for inclusion in the treatment of kidney disorders. Nevertheless, a considerable number of clinical trials are needed to demonstrate its effectiveness in kidney disorders. Mangiferin has a C Log P of −0.26, the molecular weight of 422.3, the rotating bond of 2, hydrogen bonding acceptors of 8 and hydrogen bonding donors of 11. According to the “Lipinski rule of five” (Lipinski et al., 2001), all the above results predicts that mangiferin is less likely to be poorly absorbed.
According to Jiamboonsri et al. (2015), intestinal permeability is a vital aspect that influences the bioavailability of compounds. Although mangiferin may exert its effects by altering intestinal permeability (Vazquez‐Olivo et al., 2019), its efficacy can be enhanced if it can antagonize inflammatory mediators in the bloodstream. In a study by Liu et al. (2011), mangiferin was subjected to substantial phases I and II metabolisms following an oral administration. The first-pass effect was attributed to the low oral bioavailability which constitutes a key impediment for mangiferin. Bhattacharyya et al. (2014) reported that the bioavailability of mangiferin in rats increased by 9.75-fold with the administration of mangiferin complex as opposed to its pure form. Furthermore, a higher bioavailability of a mangiferin-soya phospholipid complex resulted in a better hepatoprotection in rats (Bhattacharyya et al., 2014), indicating the potential of complex or combinations with mangiferin.
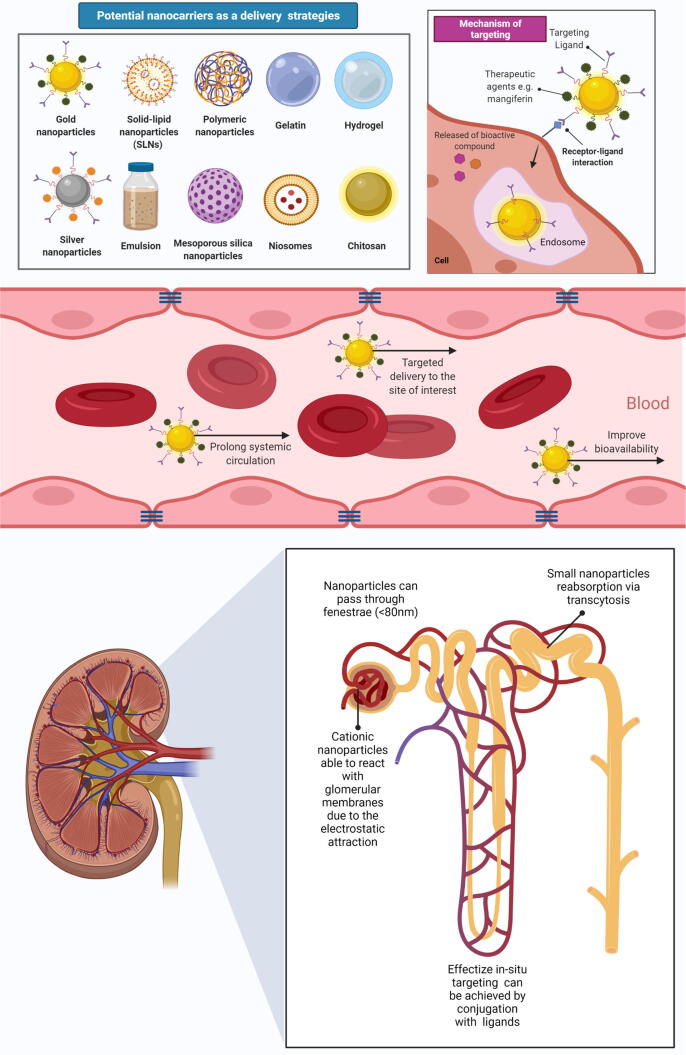
Therefore, the effective kidney-targeted drug delivery systems (KDDSs) is an intriguing method and an appealing option in the quest of improving the therapeutic and pharmacokinetic profiles of mangiferin against kidney disorders (Fig. 6). Drug distribution to the proximal tubules can enhance the treatment of kidney diseases while reducing the side effects on other organs and tissues. KDDSs is of great significance in shock, kidney transplantation, ureteral blockage, diabetes, proteinuria, and other disorders such as Fanconi and Bartter's syndromes which require alterations in the function of the renal tubules (Zhou et al., 2014). Macromolecular carriers, such as lysozyme, low molecular weight chitosan and proteins, anionized polyvinylpyrrolidone, poly-(vinylpyrrolidone-co-dimethylmaleic acid) as well as galectin-3 carbohydrate recognition domain (G3-C12) are highly useful methods for targeting drugs to the kidney. Furthermore, sugar-, amino acid-, and folate-modified prodrugs can effectively deliver drugs to the kidney (Sarko and Georges, 2016, Zhou et al., 2014). Additionally, nanotechnology and nanocarrier-based methods in the delivery of mangiferin include (1) liposomal nanoencapsulation, (2) polymeric micelles (3) cyclodextrins, (4) nanosuspensions and (5) nanoemulsions, may aid in resolving pharmacokinetics-related issues, as well as improving therapeutic responses and efficacy against kidney disorders.
Fig. 6.
Possible drug delivery system of mangiferin for kidney disorders. Kidney-targeted drug delivery systems (KDDSs) serve as an interesting approach and favorable options for optimizing the pharmacokinetic profile of mangiferin and minimizing the undesired side effects. Following oral delivery, mangiferin was subjected to the first-pass effect, which is a significant barrier for mangiferin. Therefore, Liposomal encapsulation, polymeric nanoparticles, and emulsions are being used to regulate the distribution of phytochemicals and to address some of the shortcomings related to free compounds, such as poor bioavailability. The glomerular vascular fenestrations, which normally have a width of 70–130 nm, provide direct access to the mesangial region thus nanocarriers with dimensions of 70–130 nm, can thereby extravasate via the glomerular vasculature for in-situ targeting in the mesangial region.
7. Conclusion
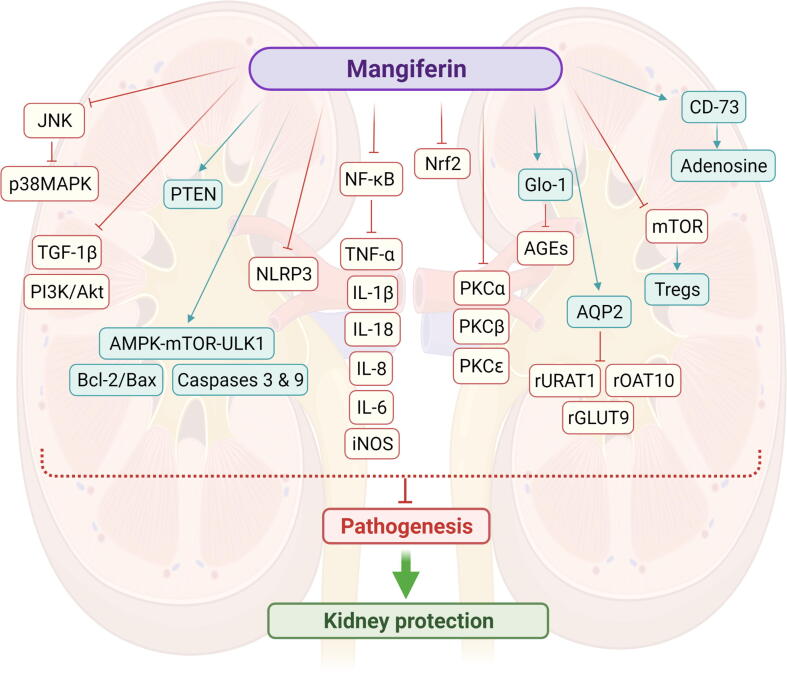
A comprehensive account of the reno-protective activity of mangiferin against kidney disorders based on experimental models is presented. The plausible mechanism of action has been extensively studied whereby the reno-protection may occur mainly through the antioxidant, anti-inflammatory, anti-apoptotic, autophagy activation, immunomodulation, regulation of renal urate transporters and modulation of specific signalling pathways (Fig. 7). Besides, combined mangiferin with other hypoglycaemic drugs would be greatly suggested to induce synergistic effects. Studies on the efficacy and safety of mangiferin in clinical trials are further warranted to confirm its medicinal potential as therapeutic agent for kidney disorders in humans. Despite high-cost clinical studies and the risk of few individuals trying drugs with low absorption, a vast amount of research is dedicated solely to improving mangiferin bioavailability. Therefore, it is very likely that a better-absorbed mangiferin modulating intestinal permeability may find use in treating kidney disorders in the future.
Fig. 7.
Overview of reno-protective actions of mangiferin against kidney disorders through different signaling pathways. Mangiferin significantly reduced kidney morphological damage as well as inflammation, fibrosis, and apoptotic histological markers. The inflammatory cytokines IL-1, IL-6, IL-8, IL-18, and TNF- α were also decreased by mangiferin. The anti-inflammatory and antioxidant actions of mangiferin were mediated by Nrf2 and NF-κB. Abbreviations: AGEs, Advanced glycation end products; AMPK, AMP-activated protein kinase; AQP2, Aquaporin 2; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Glo-1, glyoxalase; IL-1β, Interleukin-1β; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-18, Interleukin-18; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; MAPKs, mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; OAT10, renal organic anion transporter 10; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; NLRP3, Nod-like receptor protein 3; Nrf2, Nuclear factor erythroid 2-related factor 2; PKC, Protein kinase C; ULK1, Unc51-like kinase 1; rGLUT9, renal glucose transporter 9; rURAT1, renal urate-anion transporter 1; TGF-1β, Transforming growth factor beta 1; TNF-α, T necrosis factor α; Tregs, Regulatory T cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the Ministry of Higher Education (MOHE) Malaysia for the financial support provided via the Fundamental Research Grant Scheme [Ref: FRGS/1/2020/SKK06/UNIKL/02/4]. The authors also would like to acknowledge Universiti Kuala Lumpur Royal College of Medicine Perak, Malaysia for providing the necessary facilities and resources to complete the study. The figures were created with the support of BioRender.com under a paid subscription. SRB grateful to the Prof. Jagadeesh Bayry (INSERM, Centre de Recherche des Cordeliers, Sorbonne Universités, Université de Paris, France and IIT Palakkad, Palakkad, Kerala, India) for providing post-doctoral position.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adeola H.A., Bano A., Vats R., Vashishtha A., Verma D., Kaushik D., Mittal V., Rahman M.H., Najda A., Albadrani G.M., Sayed A.A., Farouk S.M., Hassanein E.H.M., Akhtar M.F., Saleem A., Abdel-Daim M.M., Bhardwaj R. Bioactive compounds and their libraries: An insight into prospective phytotherapeutics approach for oral mucocutaneous cancers. Biomed. Pharmacother. 2021;141:111809. doi: 10.1016/j.biopha.2021.111809. [DOI] [PubMed] [Google Scholar]
- Alelign T., Petros B. Kidney stone disease: An update on current concepts. Adv. Urol. 2018:1–12. doi: 10.1155/2018/3068365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaani S., Meara A., Rovin B.H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 2017;12(5):825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilkanthawar V., Daswadkar S., patil V., Joshi L. Review on molecular docking: Novel drug discovery and drug designing tool. Int. J. Eng. Res. 2020;11(4):647–665. [Google Scholar]
- Bastos A., Onuchic L. Molecular and cellular pathogenesis of autosomal dominant polycystic kidney disease. Braz. J. Med. Biol. Res. 2011;44:606–617. doi: 10.1590/s0100-879x2011007500068. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Ahmmed S.M., Saha B.P., Mukherjee P.K. Soya phospholipid complex of mangiferin enhances its hepatoprotectivity by improving its bioavailability and pharmacokinetics. J. Sci. Food Agric. 2014;94(7):1380–1388. doi: 10.1002/jsfa.6422. [DOI] [PubMed] [Google Scholar]
- Bonam S.R., Bayry J., Tschan M.P., Muller S. Progress and challenges in the use of MAP1LC3 as a legitimate marker for measuring dynamic autophagy in vivo. Cells. 2020;9(5):1321. doi: 10.3390/cells9051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam S.R., Wang F., Muller S. Autophagy: A new concept in autoimmunity regulation and a novel therapeutic option. J. Autoimmun. 2018;94:16–32. doi: 10.1016/j.jaut.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Ciarla S., Struglia M., Giorgini P., Striuli R., Necozione S., Properzi G., Ferri C. Serum uric acid levels and metabolic syndrome. Arch. Physiol. Biochem. 2014;120(3):119–122. doi: 10.3109/13813455.2014.924145. [DOI] [PubMed] [Google Scholar]
- Dagar N., Das P., Bisht P., Taraphdar A.K., Velayutham R., Arumugam S. Diabetic nephropathy: A twisted thread to unravel. Life Sci. 2021;278:119635-1–119635-10. doi: 10.1016/j.lfs.2021.119635. [DOI] [PubMed] [Google Scholar]
- Ghosh G., Duyne G.V., Ghosh S., Sigler P.B. Structure of NF-κB p50 homodimer bound to a κB site. Nature. 1995;373(6512):303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Das J., Sil P.C. D (+) galactosamine induced oxidative and nitrosative stress-mediated renal damage in rats via NF-κB and inducible nitric oxide synthase (iNOS) pathways is ameliorated by a polyphenol xanthone, mangiferin. Free Radic. Res. 2012;46(2):116–132. doi: 10.3109/10715762.2011.644240. [DOI] [PubMed] [Google Scholar]
- Hajiaghaalipour F., Khalilpourfarshbafi M., Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 2015;11(5):508–524. doi: 10.7150/ijbs.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Peng X., Zhu J., Chen X., Liu H., Tang C., Dong Z., Liu F., Peng Y. Mangiferin attenuate sepsis-induced acute kidney injury via antioxidant and anti-inflammatory effects. Am. J. Nephrol. 2014;40(5):441–450. doi: 10.1159/000369220. [DOI] [PubMed] [Google Scholar]
- Hoover P.J., Costenbader K.H. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist's perspective. Kidney Int. 2016;90:487–492. doi: 10.1016/j.kint.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultström M., Becirovic-Agic M., Jönsson S. Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiol. Genom. 2018;50(3):127–141. doi: 10.1152/physiolgenomics.00037.2017. [DOI] [PubMed] [Google Scholar]
- Imran M., Arshad M.S., Butt M.S., Kwon J.-H., Arshad M.U., Sultan M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Heal. Dis. 2017;16:1–17. doi: 10.1186/s12944-017-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal D.I., Chonchol M., Chen W., Targher G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 2013;61(1):134–146. doi: 10.1053/j.ajkd.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra A., Arora M.K., Kisku A., Sharma S. The multifaceted role of mangiferin in health and diseases: A review. Adv. Tradit. Med. 2021;21(4):619–643. [Google Scholar]
- Jiamboonsri P., Pithayanukul P., Bavovada R., Leanpolchareanchai J., Yin T., Gao S., Hu M. Factors influencing oral bioavailability of Thai mango seed kernel extract and its key phenolic principles. Molecules. 2015;20(12):21254–21273. doi: 10.3390/molecules201219759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotshna, Khare, P., Shanker, K., 2016. Mangiferin: A review of sources and interventions for biological activities. Biofactors. 42, 504-514. [DOI] [PubMed]
- Kabir M.T., Tabassum N., Uddin M.S., Aziz F., Behl T., Mathew B., Rahman M.H., Akter R., Rauf A., Aleya L., Singla R.K. Therapeutic potential of polyphenols in the management of diabetic neuropathy. Evid. Based Complement. Alternat. Med. 2021;2021:1–20. doi: 10.1155/2021/9940169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S., Zargar A.H., Jain S.M., Sethi B., Chowdhury S., Singh A.K., Thomas N., Unnikrishnan A., Thakkar P.B., Malve H. Diabetes insipidus: The other diabetes. Indian J. Endocrinol. Metab. 2016;20:9–21. doi: 10.4103/2230-8210.172273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A.H., Liu J., Kumar G., Skapek S.X., Falck J.R., Imig J.D. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27(8):2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J., Jawad A.S. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology. 2017;56:i67–i77. doi: 10.1093/rheumatology/kew399. [DOI] [PubMed] [Google Scholar]
- Li X., Yan Z., Carlström M., Tian J., Zhang X., Zhang W., Wu S., Ye F. Mangiferin ameliorates hyperuricemic nephropathy which is associated with downregulation of AQP2 and increased urinary uric acid excretion. Front. Pharmacol. 2020;11:49–61. doi: 10.3389/fphar.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.-L., Lu W., Zhou J.-Y., Chen Y., Zhang Q., Liu H., Qiu F., Dai Z. Mangiferin attenuates murine lupus nephritis by inducing CD4+ Foxp3+ regulatory T cells via suppression of mTOR signaling. Cell. Physiol. Biochem. 2018;50(4):1560–1573. doi: 10.1159/000494654. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;1:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang K., Tang Y., Sun Z., Jian L., Li Z., Wu B., Huang C. Structure elucidation of in vivo and in vitro metabolites of mangiferin. J. Pharm. Biomed. Anal. 2011;55(5):1075–1082. doi: 10.1016/j.jpba.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Liu J.-Y., Morisseau C., Huang H., Hammock B.D. Screening of soluble epoxide hydrolase inhibitory ingredients from traditional Chinese medicines for anti-inflammatory use. J. Ethnopharmacol. 2016;194:475–482. doi: 10.1016/j.jep.2016.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.-W., Zhu, X., Zhang, L., Lu, Q., Wang, J.-Y., Zhang, F., Guo, H., Yin, J.-L., Yin, X.-X., 2013. Up-regulation of glyoxalase 1 by mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 721, 355-364. [DOI] [PubMed]
- Lum P.T., Sekar M., Gan S.H., Pandy V., Bonam S.R. Protective effect of mangiferin on memory impairment: A systematic review. Saudi J. Biol. Sci. 2021;28(1):917–927. doi: 10.1016/j.sjbs.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowski A., Kus P., Goralska E., Wozniak D. Mangiferin–a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013;13:439–455. [PubMed] [Google Scholar]
- Mei S., Ma H., Chen X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021;149:111997-1–111997-13. doi: 10.1016/j.fct.2021.111997. [DOI] [PubMed] [Google Scholar]
- Nogueira A., Pires M.J., Oliveira P.A. Pathophysiological mechanisms of renal fibrosis: A review of animal models and therapeutic strategies. In Vivo. 2017;31:1–22. doi: 10.21873/invivo.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P.B., Ghosh S., Sil P.C. Bioactive Natural Products; Wiley Online Library: 2015. Beneficial effect of naturally occurring antioxidants against oxidative stress–mediated organ dysfunctions. [Google Scholar]
- Pal, P.B., Sinha, K., Sil, P.C., 2014. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PloS one. 9, e107220-1-e107220-20. [DOI] [PMC free article] [PubMed]
- Perez-Ruiz F., Dalbeth N., Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv. Ther. 2015;32:31–41. doi: 10.1007/s12325-014-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petejova N., Martinek A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc. 2016;160:183–194. doi: 10.5507/bp.2015.050. [DOI] [PubMed] [Google Scholar]
- Pulipati S., Babu P.S., Narasu M.L., Anusha N. An overview on urinary tract infections and effective natural remedies. J. Med. Plants. 2017;5:50–56. [Google Scholar]
- Rahman M.A., Hannan M.A., Dash R., Rahman M.H., Islam R., Uddin M.J., Sohag A.A.M., Rahman M.H., Rhim H. Phytochemicals as a complement to cancer chemotherapy: Pharmacological modulation of the autophagy-apoptosis pathway. Front. Pharmacol. 2021;12:1–20. doi: 10.3389/fphar.2021.639628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., Rengarajan T., Nishigaki Y., Palaniswami R., Nishigaki I. In vitro studies on mangiferin protection against cadmium-induced human renal endothelial damage and cell death via the MAP kinase and NF-κ B pathways. J. Recept. Signal Transduct. Res. 2016;36:57–66. doi: 10.3109/10799893.2015.1019137. [DOI] [PubMed] [Google Scholar]
- Rivero A., Mora C., Muros M., García J., Herrera H., Navarro-González J.F. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci. 2009;116:479–492. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- Rock K.L., Kataoka H., Lai J.-J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossing P. Diabetic nephropathy: Worldwide epidemic and effects of current treatment on natural history. Curr. Diab. Rep. 2006;6:479–483. doi: 10.1007/s11892-006-0083-y. [DOI] [PubMed] [Google Scholar]
- Sadhukhan P., Saha S., Dutta S., Sil P.C. Mangiferin ameliorates cisplatin induced acute kidney injury by upregulating Nrf-2 via the activation of PI3K and exhibits synergistic anticancer activity with cisplatin. Front. Pharmacol. 2018;9:638–650. doi: 10.3389/fphar.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Mahalanobish S., Dutta S., Sil P.C. Mangiferin ameliorates collateral neuropathy in tBHP induced apoptotic nephropathy by inflammation mediated kidney to brain crosstalk. Food Funct. 2019;10:5981–5999. doi: 10.1039/c9fo00329k. [DOI] [PubMed] [Google Scholar]
- Saha S., Sadhukhan P., Sil P.C. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. Biofactors. 2016;42:459–474. doi: 10.1002/biof.1292. [DOI] [PubMed] [Google Scholar]
- Saha S., Sadhukhan P., Sinha K., Agarwal N., Sil P.C. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem. Biophys. Rep. 2016;5:313–327. doi: 10.1016/j.bbrep.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A.K., Verma V.K., Mutneja E., Malik S., Nag T.C., Dinda A.K., Arya D.S., Bhatia J. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol. Cell. Biochem. 2019;452:141–152. doi: 10.1007/s11010-018-3420-y. [DOI] [PubMed] [Google Scholar]
- Samadarsi R., Dutta D. Anti-oxidative effect of mangiferin-chitosan nanoparticles on oxidative stress-induced renal cells. Int. J. Biol. Macromol. 2020;151:36–46. doi: 10.1016/j.ijbiomac.2020.02.112. [DOI] [PubMed] [Google Scholar]
- Sanz A.B., Sanchez-Niño M.D., Ramos A.M., Moreno J.A., Santamaria B., Ruiz-Ortega M., Egido J., Ortiz A. NF-κB in renal inflammation. J. Am. Soc. Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- Sarko D., Georges R. Kidney-specific drug delivery: Review of opportunities, achievements, and challenges. J. Anal. Pharm. Res. 2016;2:33–38. [Google Scholar]
- Sekar M. Molecules of Interest–Mangiferin–A Review. Annu. Res. Rev. Biol. 2015;5:307–320. [Google Scholar]
- Sekar V., Mani S., Malarvizhi R., Barathidasan R., Vasanthi H.R. Positive interaction of mangiferin with selected oral hypoglycemic drugs: A therapeutic strategy to alleviate diabetic nephropathy in experimental rats. Mol. Biol. Rep. 2020;47:4465–4475. doi: 10.1007/s11033-020-05517-0. [DOI] [PubMed] [Google Scholar]
- Sellamuthu P.S., Arulselvan P., Muniappan B.P., Kandasamy M. Effect of mangiferin isolated from Salacia chinensis regulates the kidney carbohydrate metabolism in streptozotocin–induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012;2:S1583–S1587. [Google Scholar]
- Soetikno V., Watanabe K., Sari F.R., Harima M., Thandavarayan R.A., Veeraveedu P.T., Arozal W., Sukumaran V., Lakshmanan A.P., Arumugam S. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011;55:1655–1665. doi: 10.1002/mnfr.201100080. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu W., Tang K., Zang J., Li D., Gao H. Mangiferin alleviates renal interstitial fibrosis in streptozotocin-induced diabetic mice through regulating the PTEN/PI3K/Akt signaling pathway. Int. J. Diabetes Res. 2020;2020:1–12. doi: 10.1155/2020/9481720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succar L., Pianta T.J., Davidson T., Pickering J.W., Endre Z.H. Subclinical chronic kidney disease modifies the diagnosis of experimental acute kidney injury. Kidney Int. 2017;92:680–692. doi: 10.1016/j.kint.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Tanaka D., Tsuda Y., Shiyama T., Nishimura T., Chiyo N., Tominaga Y., Sawada N., Mimoto T., Kusunose N. A practical use of ligand efficiency indices out of the fragment-based approach: Ligand efficiency-guided lead identification of soluble epoxide hydrolase inhibitors. J. Med. Chem. 2011;54:851–857. doi: 10.1021/jm101273e. [DOI] [PubMed] [Google Scholar]
- Tanner R.M., Shimbo D., Irvin M.R., Spruill T.M., Bromfield S.G., Seals S.R., Young B.A., Muntner P. Chronic kidney disease and incident apparent treatment-resistant hypertension among blacks: Data from the Jackson Heart Study. J. Clin. Hypertens. 2017;19:1117–1124. doi: 10.1111/jch.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H., Hogan J.J., Herlitz L.C., Lerma E.V. Springer International Publishing; 2019. Glomerulonephritis. [Google Scholar]
- Vazquez-Olivo G., Antunes-Ricardo M., Gutiérrez-Uribe J.A., Osuna-Enciso T., León-Félix J., Heredia J.B. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Mangifera indica L.) J. Sci. Food Agric. 2019;99:3481–3489. doi: 10.1002/jsfa.9567. [DOI] [PubMed] [Google Scholar]
- Walia V., Chaudhary S.K., Sethiya N.K. Neurochem; Int.: 2020. Therapeutic Potential of Mangiferin in the Treatment of Various Neuropsychiatric and Neurodegenerative Disorders; p. 104939. [DOI] [PubMed] [Google Scholar]
- Wang B., Wan J., Gong X., Kuang G., Cheng X., Min S. Mangiferin attenuates renal ischemia-reperfusion injury by inhibiting inflammation and inducing adenosine production. Int. Immunopharmacol. 2015;25:148–154. doi: 10.1016/j.intimp.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Wang, X., Gao, L., Lin, H., Song, J., Wang, J., Yin, Y., Zhao, J., Xu, X., Li, Z., Li, L., 2018. Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. Eur. J. Pharmacol. 824, 170-178. [DOI] [PubMed]
- Wei W., Ma N., Fan X., Yu Q., Ci X. The role of Nrf2 in acute kidney injury: Novel molecular mechanisms and therapeutic approaches. Free Radic. Biol. Med. 2020;158:1–12. doi: 10.1016/j.freeradbiomed.2020.06.025. [DOI] [PubMed] [Google Scholar]
- Xu G.-K., Sun C.-Y., Qin X.-Y., Han Y., Li Y., Xie G.-Y., Qin M.-J. Effects of ethanol extract of Bombax ceiba leaves and its main constituent mangiferin on diabetic nephropathy in mice. Chin. J. Nat. Med. 2017;15:597–605. doi: 10.1016/S1875-5364(17)30087-0. [DOI] [PubMed] [Google Scholar]
- Yang H., Gao L., Niu Y., Zhou Y., Lin H., Jiang J., Kong X., Liu X., Li L. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol. Pharm. Bull. 2015;38:1591–1598. doi: 10.1248/bpb.b15-00402. [DOI] [PubMed] [Google Scholar]
- Zhang B., Kandhi S., Yang Y.-M., Le Y., Deng W., Qin J., Jiang H., Froogh G., Sun D., Huang A. A novel mechanism of ascorbate direct modulation of soluble epoxide hydrolase. Prostaglandins Other Lipid Mediat. 2017;131:59–66. doi: 10.1016/j.prostaglandins.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang D., Han S., Zhou Y., Qi B., Wang X. Therapeutic effects of mangiferin on sepsis-associated acute lung and kidney injuries via the downregulation of vascular permeability and protection of inflammatory and oxidative damages. Eur. J. Pharm. Sci. 2020;152:105400-1–105400-11. doi: 10.1016/j.ejps.2020.105400. [DOI] [PubMed] [Google Scholar]
- Zhang H., Sun S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015;5:1–12. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Sun X., Zhang Z. Kidney–targeted drug delivery systems. Acta Pharm. Sin. B. 2014;4:37–42. doi: 10.1016/j.apsb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Cheng Y.Q., Du L., Li Y., Zhang F., Guo H., Liu Y.W., Yin X.X. Mangiferin attenuates renal fibrosis through down-regulation of osteopontin in diabetic rats. Phytother. Res. 2015;29:295–302. doi: 10.1002/ptr.5254. [DOI] [PubMed] [Google Scholar]
- Zoccali C., Mallamaci F. Uric acid, hypertension, and cardiovascular and renal complications. Curr. Hypertens. Rep. 2013;15:531–537. doi: 10.1007/s11906-013-0391-y. [DOI] [PubMed] [Google Scholar]