Abstract
Carcinogenic and health hazard causing heavy metals have been increasing in our dietary stuffs due to large amount of industrial effluents being dumped in water bodies that are ultimately used for irrigation purposes. The study was aimed to assess and compare the mean concentrations of heavy metals (Cd, As and Pb) in soil and vegetables irrigated with four different sources (Ground water, river water, domestic sewage water and industrial untreated effluents and domestic waste water receiving drains) for the estimation of carcinogenic and non-carcinogenic health risk associated with them. Prepared samples were analyzed by through ICP-OES. Statistical analysis revealed that domestic sewage water and drains water usage for irrigation purposes leads to high values of Estimated Daily Intake (EDI) of metals through vegetation. To assess the carcinogenic effects values daily intakes, Total hazard quotients (THQs) and Health indexes (HI), while for carcinogenic effects, Total cancer risks (TCR) were determined. The results of present study revealed that the daily intakes of these metals are far less than that of permissible levels but their bio-accumulating behavior produce high risks to human health. The HI values revealed that waste water usage is producing the vegetables of high health risks. In adults, the HI of Phaseolus vulgaris, Spinacia oleracea, Brassica compestris, Raphnus sativus, Daucus carota and Solanum tuberosum assessed as 0.81, 1.52, 1.26, 0.12, 0.22, and 0.15 (ground water irrigation), 0.046, 0.75, 0.51, 0.68, 0.90 0.064 (River Ravi water irrigation), 1.23, 3.34, 4.81, 4.23, 1.41 and 3.43 (domestic sewage irrigation) and 3.04, 5.50, 6.08, 2.50, 5.34 and 5.13 (Drain waste water irrigation), respectively. It was observed that cancer risks of As exceeded the threshold (1 × 10−4) in all i.e. ground river, domestic sewage and drain water grown vegetables, while, Cd and Pb were in permissible range.
Keywords: Health risk, Heavy metal, Toxicity, Daily intake, Hazard quotients, Hazard indexes, Cancer risks, Sustainability, Bioaccumulation
Abbreviations: GW, Ground water; RW, River water; DomWW, domestic waste water; DRWW, Drain wastewaster
1. Introduction
Human health always remained a major issue in highley populated countaries. Heavy industrialization leads to abundant consumption of ground water resources. In turn, wastewater is the cheaper source for irrigation of lands. Under deveolping countaries also facing the problems of electric power generation, so like oher under deveolping countarie Pakistan also face problem of high electricity prices which leads to enforce the agriculture farmers to utlized the wastewaters for irrigation. There are some common practices for irrigation are used individually and in some areas working together to cover the need of water for irrigation. The use of waste water for irrigation purpose, troubling the human health (Alturiqi et al., 2020). because waste water not only increase the load of bacteria, fungi, parasitic protozoans and essential trace elements (Ohoro et al., 2019), but it may also enhance the toxic non-essential trace elements to soils where the plants are going to be grown. As, Cd and Pb are non-essential heavy metals that are entering in our food chains through soils (Margenat et al., 2019, Khalid et al., 2017). Among all cotaminants heavy metals have gained more interest due to their persistant, bioaccumulating and biomagnifying nature (Zakaria et al., 2021, Pandiyan et al., 2021). Heavy metals increases the oxidative stress by enhancing the reactive osygen species (ROS) in aerobic cells (Orun et al., 2008).
Heavy metals cause serious carcinogenic and noncarcinogeneic effects in body i.e. at low level ingestion of arsenic may cause noncarcinogenic effects like sore throat and irritated lungs, nausea, vomaiting, darkening of skin lesions and warts (Signes-Pastor et al., 216)). Furthermore, its chronic intake may lead to neurobehavioral and neuropathic alterations, loss of memory, lowering of intellectual level, infertility disorder, fetal damage, premature delivery, cardiovasclar dieases, chronic cough and chronic bronchitis (Milton and Rahman, 2002). While, its carcnogenic effecs may be shown in the form of lung and bladder cancer usually (Cohen et al., 2015; Sanyal et al., 2020). In turn, Cadmium (Cd) cause chronic diseases to human. Mainly, it cause disorders in liver, kidneys testicles, pancreas and bones (Fu and Xi, 2020). CD2+ may also lowers the activities of catalase, glutathione peroxidase, superoxide dismutase in liver (Talas et al., 2008). Moreover, Cd not anly damage skeltal system by distrubing the metabolism of Ca, Zn, Cu, and Fe and Mg (Kim et al., 2015) but it may also can play a role initial role in cancer forming in breasts, pancreas, lungs, prostate, kidneys, nasophyrnx and testicles (Genchi et al. 2020). Similarly, Lead(Pb) cause the neuro toxicity in adult and childrens. Mainly, Pb affect the brain, kidney, liver and reproductive system (Brewer et al., 2020). It may cause the lowering of IQ level, perception power. It may also made the hyperactivity of nervous system (Fu and Xi, 2020). In addition, Pb burden in blood cause bladder cancer (Awadalla et al., 2020).
In current situations of hilghy populated Lahore it is very necessary to assess the contaminantion levels of food and its associated health risks. This research is mainly, aimed to study the the toxicity level of heavy metals in soil and vegetables. estimated daily intakes heavy metals, total hazard quotients, hazard indexs and TCRs in vegetables irrigated with four different irrigation systems. In addition health risk factors are measure individually both for adult and child.
2. Materials and methods:
2.1. Study area description
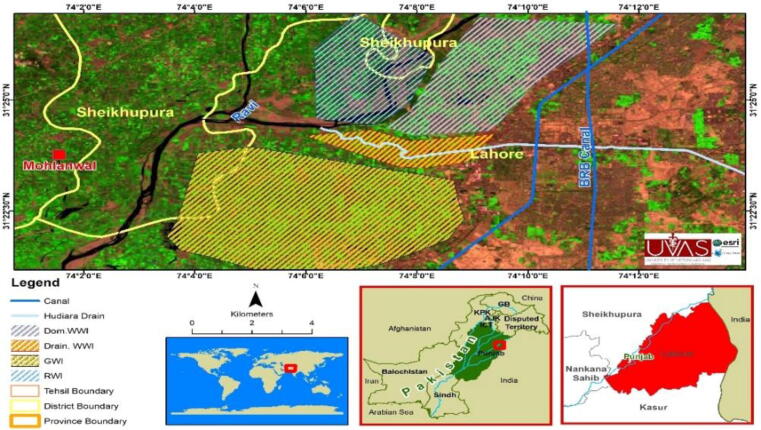
This study was carried out in the vicinity of Lahore District Pakistan The area lies between the Lat 31.2233″ N. 74.20″ E and Lat. 31.250″ N Long 74.120″ E. the detail of study area can be viewed in Fig. 1. Lahore is 2nd largest, thickly populated and industrial city, having almost 3007 industrial units (Khan et al., 2015). It receives its all organic food from its surrounding villages and several towns which are occupied by of large numbers of industries.
Fig. 1.
ESRI GIS based map of study area of Lahore, Pakistan.
The organic foods that it receives had grown under four different types of source for irrigation i.e. ground water (30 m to 125 m depth), river Ravi water, domestic sewage water and Hudiara drain. Hudiara drain is the main effluents receiving drain. There are almost 85 large industries have been working around the Hudiara drain only along 54.4 Km in Pakistani side (Khan et al., 2003). The food stuffs grown with these irrigation sources after consumption have threaten the health of humans living in this city and its surrounding areas.
2.2. Approach for sampling
Samples of human edible parts of vegetables along with their related and soil samples were collected from each type of land classified on the basis of water source for irrigation. Samples of survey based highly consumed vegetables Spinacia oleracea, Brassica compestris, Raphanus sativus, Phaseolus vulgaris, Daucus carota and Solanum tuberosum were collected from each source of irrigation by following the APHA, (2012).
2.3. Sampling and preparation
2.3.1. Soil
Soil samples were mechanically ground, sieved properly and stored for further processing. 1 g of each soil samples were mixed with 10 ml HNO3, heated on hot plate to 95 °C for 10 min and then cool down. After cooling, added 5 ml HNO3 and refluxed for 30 min till brown fumes aroused from digesting samples. We added 5 ml HNO3 in repetition untill brown fumes ended after this we added 3 ml of H2O2 to make all possible oxidation process completed. In the last added 10 ml HCl and again heated for 30 min to accomplish the digestion as described by Kacholi and Sahu, (2018).
2.3.2. Vegetables samples preparation
Plant’s edible parts samples were collected separately. All the stuck dust particles were removed by washing with de-ionized distilled water. After oven drying at 70–80 °C for 24–48 h all the samples were crumbled to make fine powder. Digestions of all the vegetables and blank samples were done by following the optimized method as described Gebeyehu and Bayissa, (2020) Added 9 ml HNO3 and 3 ml HCl at digestion time of 45 min on hot plates at 180 °C in 1 g dried samples. Blank samples were also prepared by following optimum conditions
2.4. Heavy metal analysis
After digestion all the, soil and plants digested samples were diluted up to 50 ml in volumetric flask with 2% HNO3 and then filtered by using whatman filter paper No. 42 into 50 ml vacuumed volumetric flask. All the samples were analyzed for heavy metals by using inductively coupled plasma optical plasma emission spectrometry (ICP-OES). After every three-sample analysis, blank samples were run to ensure the correct measurement by the machine. The correctness each metal's analysis was checked using standard reference solutions of each metal.
2.5. Bioaccumulation factors (BAFs)
This factor determined the bio-availability of heavy metals to plants from soil. BAFs were calculated by using the following equation.
| (1) |
where Cp and Cs stand for the heavy metal concentration in plants and soil respectively.
2.6. Health risk assessment
2.6.1. Estimated daily intake (EDI)
The daily intake of metals depends on rate of daily food consumption. By using the following equation (2), the estimated daily Intake of metals are calculated.
| (2) |
where Ef represented the exposure frequency (365 days of whole year), ED denoted thee exposure duration (70 years), Cm represented the concentration of metals (mg/g), Cf stands for conversion factor from wet to dry weight of vegetable, which is 0.085 as proposed by (Rattan et al., 2005), FIR is ingestion rate of vegetable which is 0.345Kg for adult and 0.232Kg for children (Yaacob et al., 2018), Bw is average body weight (70Kg) and AT is average time (365 day × 70 years) as reported by Antoinie et al., (2017).
2.6.2. Target hazard quotient (THQ)
Different biomagnifying contaminants had caused carcinogenic and non-carcinogenic effects in our body. Target Health Quotients is the parameter which is considered to assess the non-carcinogenic effects as described by Ezemonye et al., 2019, Agoro et al., 2020. THQs can be calculated by the following equation (3).
| (3) |
where EDI is estimated daily intake of metal (mg/day/Kg) and RfD is Oral Reference dose of heavy metal. Values of RfD of As, Cd and Pb are presented in Table 1. It is presumed that if THQ value is greater than 1 then body definitely show non-carcinogenic effects. But if it remained less than the limit (1) then it is considered to be negligible chances of non-carcinogenic effects in human body.
Table 1.
Constant parameters used in calculation of THQ and Cancer risk.
| Parameters | Metals | Reference dose | Reference |
|---|---|---|---|
| Oral Reference dose | As | 0.0003 | (Antoine et al., 2017) |
| Cd | 0.001 | (Antoine et al., 2017) | |
| Pb | 0.0035 | (Rai et al., (2019) | |
| Oral Cancer Slope factors | As | 1.5 | (Antoine et al., 2017) |
| Cd | 0.38 | (Yang et al., 2018) | |
| Pb | 0.0085 | (Yang et al., 2018) |
2.6.3. Hazard Index:
The cummulative noncarcinogenic effects of all heavy metals coming throgh a particular vegetable is evaluated by the Hazard index equation as described by Gebeyehu and Bayissa (2020)
| (4) |
2.6.4. Cancer risk
Cancer risk is a unit less probability of getting cancer. It may be measured by the following equation (4) as described by Liang et al., (2017).
| (5) |
where EDI is estimated daily intake of metal and CPSo is the oral slope factor.
The oral cancer slope factor is the tool which converts the average daily intake of contaminant into gradual risk of developing cancer with life time exposure. All the details of CPSo of As, Cd and Pb are presented in Table 1.
2.7. Statistical analysis
To calculate the means and Standard deviation of replicates of soil and vegetable sample’s data were calculated with the help of statistical analysis system (SAS).
3. Results
The demand of organic food has also been increased many times due to over population in recent few decades. Because of very high demands of pure and organic food, some farmers are using waste water without any discrimination to irrigate their fields. Overall, effect of such contaminations may cause many risks human health. So it is very necessary to measure and compare the level of contamination done by each irrigating sources used in Lahore.
3.1. Mean concntration of heavy metals in soils
Mean concentrations of heavy metals in soils of relevant fields are given in Table 2. In ground water irrigated field soils the minimum and maximum values of As, Cd and Pb were recorded as 2.36 mg Kg−1 and 6.39 mg Kg−1, 1.27 mg Kg−1 and 3.46 mg Kg−1, and 2.41 mg Kg−1 and 4.35 mg Kg−1 respectively. The lowest and highest mean concentrations of As, Cd and Pb in soils irrigated with river water were observed as 3.87 mg Kg−1 and 9.21 mg Kg−1, 2.57 mg Kg−1 and 3.99 mg Kg−1 and 3.75 mgKg−1 and 7.84 mgKg−1respectively. The results of the present study also found ranges of As, Cd and Pb in soils grown with domestic waste water as 6.40–17.51 mg Kg−1, 3.574–5.57 mg Kg−1 and 9.59–11.88 mg Kg−1 respectively. While, under drain water irrigations soils contamination level of As, Cd and Pb varied from 12.55 mg Kg−1, 3.81 mg Kg−1 and 13.32 mg Kg−1 to 19.19 mg Kg−1, 12.61 mg Kg−1 and 15.45 mg Kg−1 respectively. It has been observed that Ground water irrigating soils have lowest level of contamination while, DrWW irrigating soils have highest level of contamination.
Table 2.
Comparisons of mean concentrations (mg Kg−1) ± S.D of selected heavy metals in soil of some vegetable fields treated four different irrigation systems.
|
GWI |
RWI |
Dom.WWI |
Dr.WWI |
||
|---|---|---|---|---|---|
| Fields of vegetables | HMs | Means ± SDs | Means ± SDs | Means ± SDs | Means ± SDs |
|
Phaseolus vulgaris (Green bean) |
As | 4.86 ± 0.17 | 5.58 ± 0.46 | 6.78 ± 0.06 | 14.73 ± 0.21 |
| Cd | 3.29 ± 0.24 | 3.61 ± 0.03 | 5.10 ± 0.88 | 8.61 ± 0.07 | |
| Pb | 3.56 ± 0.11 | 4.78 ± 0.12 | 4.78 ± 0.12 | 12.58 ± 0.16 | |
| Spinacia oleracea (Spinach) | As | 6.39 ± 0.21 | 9,21 ± 1.59 | 10.12 ± 0.01 | 13.82 ± 0.58 |
| Cd | 1.33 ± 0.05 | 3.06 ± 0.02 | 3.24 ± 0.33 | 3.81 ± 0.04 | |
| Pb | 4.35 ± 0.02 | 7.84 ± 0.05 | 9.60 ± 0.07 | 10.32 ± 0.29 | |
| Brassica compestris (Mustard) | As | 3.17 ± 0.43 | 3.87 ± 0.05 | 14.38 ± 2.57 | 12.55 ± 0.17 |
| Cd | 3.46 ± 0.21 | 3.44 ± 0.33 | 5.44 ± 0.33 | 8.56 ± 0.28 | |
| Pb | 4.23 ± 0.11 | 6.66 ± 0.17 | 10.33 ± 0.26 | 13.36 ± 0.40 | |
|
Raphanus sativus (Radish) |
As | 3.34 ± 0.51 | 5.86 ± 0.11 | 17.51 ± 3.09 | 19.19 ± 0.27 |
| Cd | 3.36 ± 0.03 | 2.57 ± 0.05 | 5.57 ± 0.04 | 9.11 ± 0.03 | |
| Pb | 4.30 ± 0.10 | 6.10 ± 0.44 | 11.90 ± 0.99 | 12.58 ± 0.23 | |
|
Daucus carota (Carrot) |
As | 2.69 ± 0.61 | 4.91 ± 0.07 | 6.41 ± 0.16 | 14.26 ± 0.04 |
| Cd | 2.53 ± 0.02 | 3.99 ± 0.02 | 3.99 ± 0.02 | 7.32 ± 0.06 | |
| Pb | 2.41 ± 0.03 | 3.75 ± 0.08 | 10.89 ± 0.07 | 15.45 ± 0.11 | |
|
Solanum tubersom (Potato) |
As | 2.36 ± 0.10 | 4.19 ± 0.15 | 10.48 ± 0.08 | 114.28 ± 0.12 |
| Cd | 1.27 ± 0.12 | 3.85 ± 0.06 | 3.57 ± 0.19 | 2.61 ± 0.35 | |
| Pb | 3.22 ± 0.01 | 5.09 ± 0.01 | 11.88 ± 0.33 | 10.40 ± 0.01 |
Permissible limit of standard os India, 2000, Ashwathi (As = NA, Cd = 3–6 mg Kg−1, Pb = 250–500 mg Kg−1).
Permissible limit of EU, 2006 (European Commission, 2006;Joint FAO/WHO Expert Committee on Food Additives, 2014) (As = NA, Cd = 5 mg Kg−1, Pb = 100 mg Kg−1).
Permissible limit of FAO/WHO 2001 (As = 20 mg Kg−1, Cd = 0.3 mg Kg−1, Pb = 100 mg Kg−1).
3.2. Mean concntration of heavy metals in vegetables
The mean concentrations of As ranged from 0.073 mg Kg−1 (S. tuberosum), 0.194 mg Kg−1 (B. compestris), 0.477 mg Kg−1 (P.vulgaris) and 1.5 mg Kg−1 (R. sativus) to 1.07 mg Kg−1 (S. oleracea), 0.59 mg Kg−1 (D. carota), 3.038 mg Kg−1 (B. compestris) and 3.50 mg Kg−1 (S. oleracea) irrigated with ground water, river Ravi water, domestic sewage water and effluent receiving drains water, respectively. Similarly, the mean concentrtions of Cd were recorded lowest as 0.008 mg Kg−1 (S. tuberosum), 0.014 mg Kg−1 (S. tuberosum), 0.149 mg Kg−1 (R. sativus) and), 0.149 mg Kg−1 (R. sativus) and highest as 0.07 mg Kg−1 (B. compestris), 0.143 mg Kg−1 (B. compestris), 0.828 mg Kg−1 (P.vulgaris) and 0.944 (B. compestris)) grown with ground water, river Ravi water, domestic sewage water and drains water, respectively.
While, range of Pb were observed as 0.0.003 (D. carota)-0.75 mg Kg−1 (S. oleracea), 0.15 (R. sativus)-1.51 mg Kg−1 (D. carota), 1.06 (R. sativus)-3,39 (B. compestris) and 2.86 (R. sativus)-7.83 (B. compestris) irrigated with ground water, river water, domestic sewage water and drains water respectively. All the details of mean concntration of heavy metals in vegetables were presented in Table 3.
3.3. Biolaccumulation factors (BAFs)
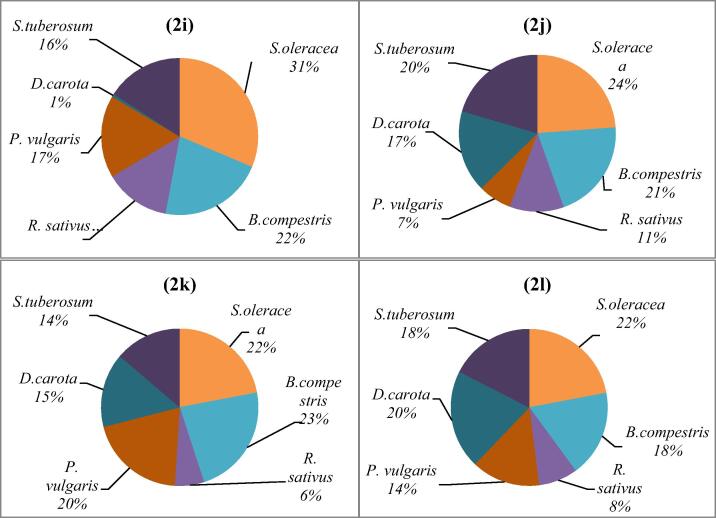
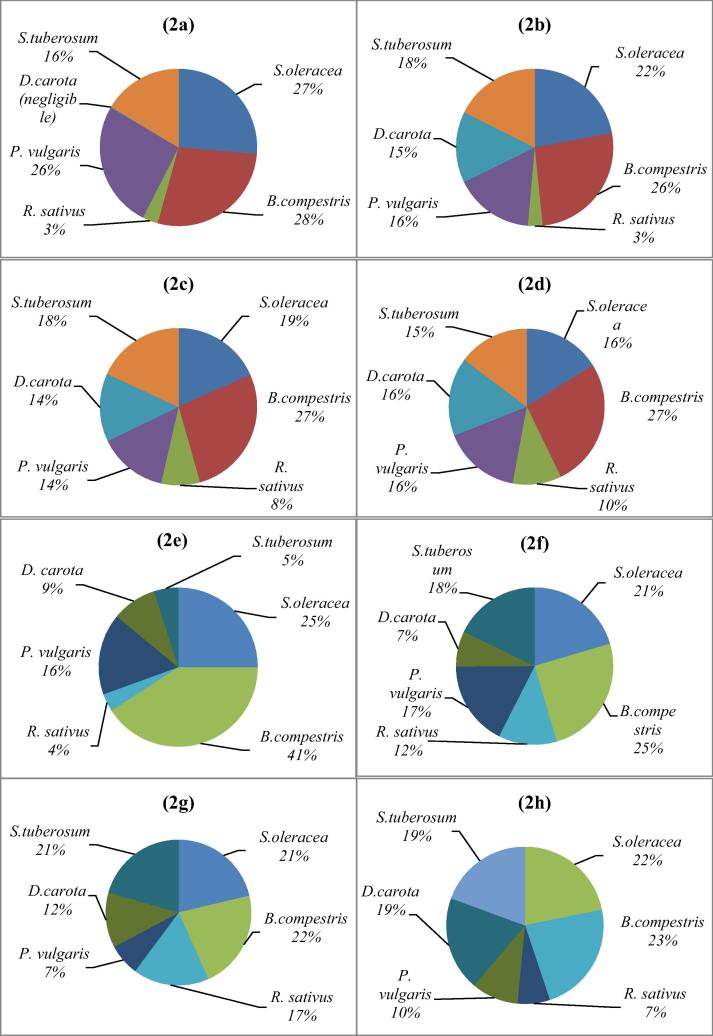
BAfs investigation is helpful tool to compare the accumulation tendencies of the different plant. Fig. 2 represented that percentage BAF of heavy metal in some vegetables. The result of this study indicated that ranking of accumulation factor of Pb under GWI B. Compestris > S. oleracea > P. vulgaris > S. tubersum > R. sativus > D. carota. Whereas, under RWI Pb accumulation ranked as B. compestris > S. oleracea > S. tubersum > P. vulgaris D. carota > R. sativus. While, with domestic WWI Pb BAFs were ranked as B. compestris > S. oleracea > S. tubersum P. vulgaris = D. carota > R. sativus. However, BAF of Pb under drain WWI ranked as B. compestris > S. oleracea > P. vulgaris = D. carota > S. tubersum > R. sativus.
Fig. 2.
Bioaccumulation Factors of selected heavy metals in some vegetables raised with different irrigation systems.
BAfs for As in vegetables treated with GWI, RWI, domestic WWI and drain WWI were observed in decreasing order as B. compestris > S. oleracea > P. vulgaris > D. carota > S. tubersum R. sativus, B. compestris > S. oleracea > S. tubersum > P. vulgaris > R. sativus > D. carota, B. compestris > S. oleracea = S. tubersum > R. sativus > D. carota > P. vulgaris and B. compestris > S. oleracea > D. carota = S. tubersum > P. vulgaris > R. sativus respectively.
BAFs of Cd were rancked as B. compestris > S. oleracea > P. vulgaris > D. carota > S. tubersum R. sativus, B. compestris > S. oleracea > S. tubersum > P. vulgaris > R. sativus > D. carota, S. oleracea > B. compestris = S. tubersum = D. carota > R. sativus > P. vulgaris and B. compestris > S. oleracea > D. carota = S. tubersum > P. vulgaris > R. sativus under irrigation with GWI, RWI, domestic WWI and drain WWI, respectively.
Fig. 2. (2a) BAFs of Pb in GWI vegetables, (2b) BAFs of Pb in RWI vegetables, (2c) BAFs of Pb in DomWWI vegetables, (2d) BAFs of Pb in DrWWI vegetables, (2e) BAFs of As in GWI vegetables, (2f) BAFs of As in RWI vegetables, (2g) BAFs of As in DomWWI vegetables, (2h) BAFs of As in DrWWI vegetables, (3i) BAFs of Cd in GWI vegetables, (2j) BAFs of Cd in RWI vegetables, (2k) BAFs of Cd in DomWWI vegetables, (2l) BAFs of Cd in DrWWI vegetables,
3.4. Health risk assessment
3.4.1. Estimated daily intakes (EDI) of heavy metals
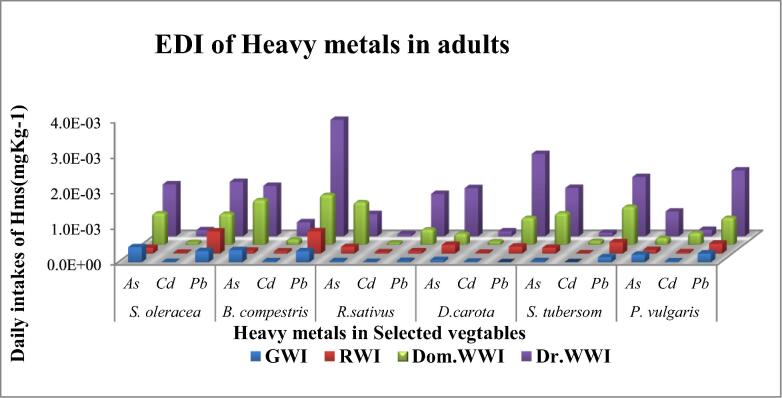
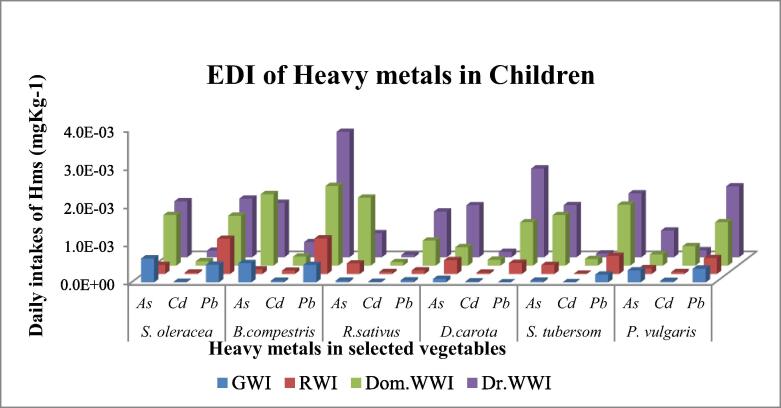
Estimated daily intakes (EDI) in terms of mg/Kg/day were calculated for the child and adult seperately by using equation (2) and computations were shown in Fig. 3a, Fig. 3b respectively.
Fig. 3a.
Estimated daily intake of selected heavy metals to human children from some vegetables raised with different irrigation systems.
Fig. 3b.
Estimated daily intake of selected heavy metals to human adults from some vegetables raised with different irrigation systems.
In adults, the consumption of S. oleracea gives the lowest and highest EDI of As, Cd and Pb were observed as 1.63 × 10−4 (RWI) and 1.47 × 10−3 (DrWWI), 9.6 × 10−6 (GWI) and 1.71 × 10−4 (DrWWI), 3.16 × 10−4 (GWI) and 1.53 × 10−3 (DrWWI) under four different irrigation sources respectively. While, through S.oleracea consumption, EDI for As, Cd and Pb to children ranged as 2.39 × 10−4 (RWI)-1.47 × 10−3 (DrWWI), 1.42 × 10−5 (GWI)-1.71 × 10−3 (DrWWI) and 4.65 × 10−4 (GWI)-1.5 × 10−3 (DrWI) respectively. In adults, B. compestris consumption EDI of As, Cd and Pb varied from 8.13 × 10−5(RWI), 2.93 × 10−5(GWI) and 3.12 × 10−4 (GWI) to 1.42 × 10−3(DrWWI), 3.95 × 10−4(DrWWI) and 3.28 × 10−3(DrWWI), among four different sources of irrigation respectively. While, the utilization of B. compestris by child, EDI of As ranged from 1.20 × 10−4(RWI) to 1.87 × 10−3(DomWWI), Cd varied from 4.31 × 10−5(GWI) to 3.95 × 10−4(DrWWI) and Pb ranged from 4.58 (GWI) × 10−4 to 3.28 × 10−3(Dr.WWI).
Lowest EDI of As, Cd and Pb were observed in ground water irrigated R. sativus as 3.06 × 10−5, 9.60 × 10−6 and 3.60 × 10−5, respectively. While highest EDI of As, Cd and Pb were recoded in waste water of drain water irrigated R. sativus 1.2 × 10−3, 6.24 × 10−5 and 1.20 × 10−3 mg/Kg/Day respectively. Similar trends as R. sativus were also shown by the D. carota S. tubersom and P. vulgaris in adults and child.
In adults and childrens the EDI of heavy metals due to consumption of S. oleracea, have followed the decreasing order as As > Pb > Cd, Pb > As > Cd, As > Pb > Cd, and Pb > As > Cd under GW, RW, DomWW and Dr.WW irrigation, respectively. The EDI of corrosponding heavy metals through ingestion of B. compestris ranked in the decreasing order as As > Pb > Cd, Pb > As > Cd, Pb > As > Cd, and Pb > As > Cd under GW, RW, DomWW and DrWW irrigation, respectively. Similarly, ingestion of R. sativus may incorporated these metals in the decreasing order as Pb > As > Cd, As > Pb > Cd, As > Pb > Cd, and Pb > As > Cd under GW, RW, DomWW and DrWW irrigation, respectively. The EDI of As, Cd and Pb due to ingestion of D. carota showed trend in the decreasing order as As > Cd > Pb, As > Pb > Cd, Pb > As > Cd, and Pb > As > Cd under GW, RW, DomWW and DrWW irrigation, respectively. However, the consumption of S. tubersom and P. vulgaris both showed similar trends in the decreasing order as Pb > As > Cd, Pb > As > Cd, Pb > As > Cd and Pb > As > Cd under GW, RW DomWW, and DrWW irrigateon, respectively, in adult and child. All the comparisons are presented in Fig. 3a, Fig. 3b.
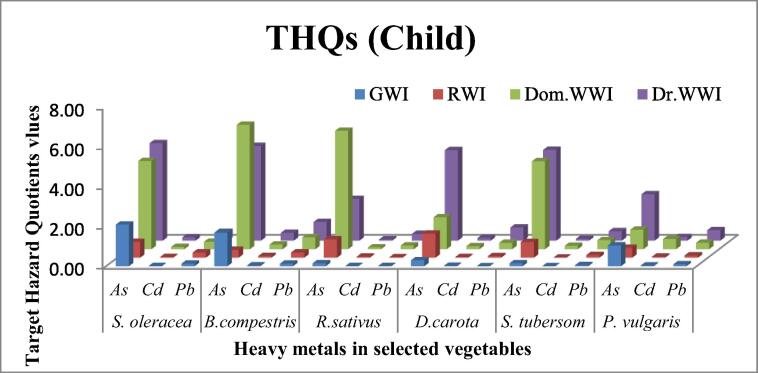
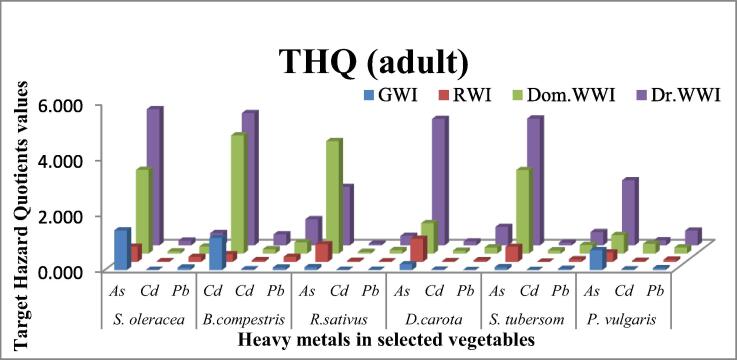
3.4.2. Target hazard quotient (THQ)
To study the carcinogenic effects, total hazard quotients are investigated. The data of THQs for child and adult obtained by using Equation (3) is presented in Fig. 4a, Fig. 4b respectively. THQ of As under GWI has been found in the range of 0.10 (R. sativus = S. tubersom) to 1.42 (S. oleracea). The corrosponding value of THQ of under GWI varied from 0.003 (S. tubersom) to 0.026 (P. vulgaris). while, the range of Pb in the same treatment assessed as 0.00 to 0.09 in S. tubersom and S. oleracea, respectively. THQ of As, Cd and Pb in vegetables grown on RWI showed the variation from 0.27(B. compestris), 0.01 (S. tubersom), 0.02 (R. sativus) to 0.82 (D. carota), 0.05 (B. compestris) and 0.18 (S. oleracea and B. compestris), respectively.
Fig. 4a.
Target hazard quotient of selected heavy metals to human children from some vegetables raised with different irrigation systems.
Fig. 4b.
Target hazard quotient of selected heavy metals to human adults from some vegetables raised with different irrigation systems.
The results indicated that vegetables cultivated under Dom.WWI has higher THQs than GWI and RWI vegetables, but less than Dr.WWI vegetavles. THQ of As, Cd and Pb in Dom.WWI vegetables varied from 0.67 (P.vulgaris), 0.06 (R. sativus) and 0.13 (R. sativus) to 4.24 (B. compestris), 0.35 (P. vulgaris) and 0.41 (B.compestris), respectively. However, THQ of AS, Cd and Pb got highest ranges of 2.09 (R. sativus)-4.89 (S. oleracea), 0.06 (R. sativus)-0.40 (B. compestris) and 0.34 (R. sativus) if they were grown under Dr.WWI.
In case of child the values of THQs of As, noticed greater than threshold (1) and are ranked in decreasing order as 4.89 (S. oleracea) > 4.75(B. compestris) > 4.55(S. tubersom) > 4.54(D. carota) > 2.33 (P. vulgaris) and 2.09 (R. sativus) under Dr.WWI vegetables. While, As also crossed the limit (1) for THQs in DomWWI vegetables. Their decreasing order was remained as 6.24 (B. compestris) > 5.94 (R. sativus) > 4.42 (S. oleracea) > 4.41 (S. tubersom) > 1.60 (D. carota). Whereas, P. vulgaris showed very near to cross the limit value as 0.98.
However, THQs assessment indicated that Ground water and river water is also not safe to childrens. It was noticed that consumption of S.oleracea(2.09) and B. compestris(1.69) under GWI and D. carota (1.21) under RWI exceeded the threshold level. The results indicated that vegetables cultivated under drain waste water irrigation have very high THQs for As followed by Pb nad Cd. As in all vegetables under Dr.WWI and exceeded than the limited value (1). Corrosponding THQs values for Arsenic under Dom.WWI all vegetables also showed exceeded vlaue than the limited value except P. vulgaris. The same pattern of THQs is also recorded in childrens with more alarming values. It has been observed that vlues of As of THQs transcend far away than limited value, which is very alarming condition to consume such vegetables especially, grown with Dr.WWI.
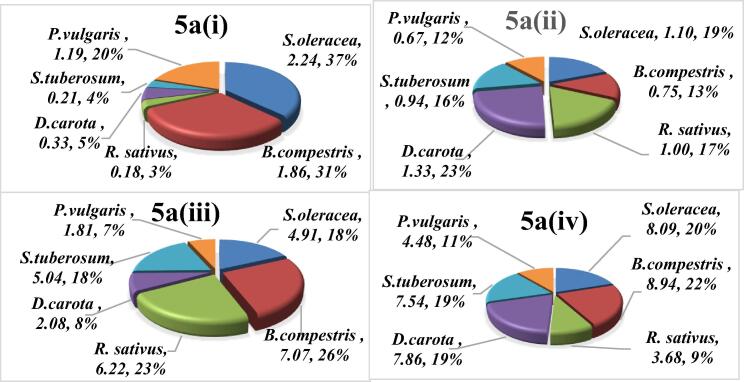
3.4.3. Hazard index (HI)
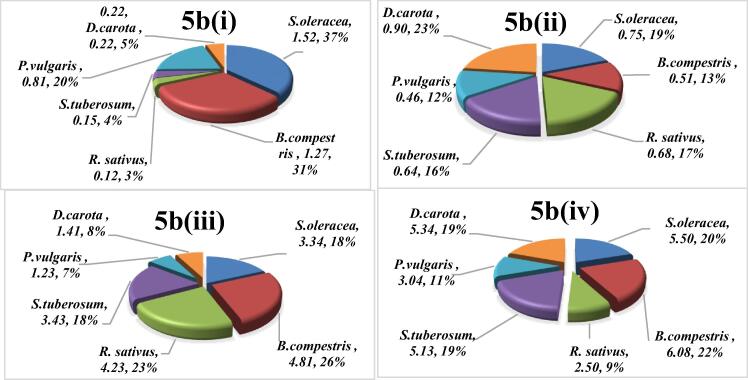
The collective effect of all heavy metals is evaluated by the Hazard index. HI for each vegetable is computed by using Equation (4) and the results are indicated in Fig. 5a, Fig. 5b for child and adult respectively. HI investigtion revealed that under GWI individual contribution of of S.oleracea (37%) followed by B. compestris (31%), P. vulgaris (20%), D. carota (5%), S. tubersom (4%) and R. sativus (3%) was recorded. It has been further noticed that under this irrigation S. olerace and B. compestris exceeded the threshold level. Similarly, RW irrigated vegetables also make their part to overall cummulative effect in decreasinng order as D. carota (23%) > S.oleracea (19%) > R. sativus (17%) > S. tubersom (16%) > B. compestris (13%) > P. vulgaris (12%). While, under domestic wastewater irrigation B.compestris (26%), R. sativus (23), S. tubersom (18%), S. oleracea (18%), D. carota (8%) and P. vulgaris (7%) ranked according to percentage participation. However, contribution of vegetables grown on DRWW were measured as in the decreasing order as R. sativus (9%) < P. vulgaris (11%) < S. tubersom (19%) = D. carota (19%) < S. oleracea (20%) < B. compestris (22%). Due to DomWW and DrWW irrigation, all selected vegetables were exceeded the threshold.
Fig. 5a.
Hazard index of heavy metal contaminated vegetables to human children from some vegetables raised with different irrigation systems.
Fig. 5b.
Hazard index of heavy metal contaminated vegetables to human adults from some vegetables raised with different irrigation systems.
Where, 5a(i), 5a(ii), 5a(iii), and 5a(iv) showed the HI of some vegetables to children grown with ground GWI, RWI, DomWWI and DrWWI respectively.
Where, 5b(i), 5b(ii), 5b(iii), and 5b(iv) showed the HI of some vegetables to adults grown with ground GWI, RWI, DomWWI and DrWWI respectively.
3.4.4. Carcinogenic risk assessement (CR)
The consumption of heavy metal contaminated dietary stuff for long term exposure throughout the life on daily basis, considered to be carcinogenic effects (Cancer in different parts of body). United States Environmental Protection Agency, 2011 stated that if cancer risk values lie between 10−6 to 10−4, it causes tolerable risk but if it is less than 10−6 it can be ignored while, CR values more than 10−4 is unacceptable. It has been determined that almost all vegetables under all irrigation system only Arsenic’s CR had surpassed the limit (1.0x10−4). The results of CR for children and adults have been calculated by using equation5 and were presented in Table 4.
Table 4.
Carcinogenic risk to adult and children (CR) values from selected heavy metals in some vegetables.
| Vegetables | Heavy metals |
CR to Children |
CR to adult |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GWI | RWI | DomWWI | DrWWI | GWI | RWI | DomWWI | DrWWI | ||
| S. oleracea | As | 9.4 × 10−4 | 3.6 × 10−4 | 2.0 × 10−3 | 3.2 × 10−3 | 6.4 × 10−4 | 2.4 × 10−4 | 1.4 × 10−3 | 2.2 × 10−3 |
| Cd | 5.4 × 10−6 | 1.4 × 10−5 | 4.4 × 10−5 | 9.6 × 10−5 | 3.7 × 10−6 | 9.2 × 10−6 | 3.0 × 10−5 | 6.5 × 10−5 | |
| Pb | 3.9 × 10−6 | 7.8 × 10−6 | 1.1 × 10−5 | 1.9 × 10−5 | 2.7 × 10−6 | 5.3 × 10−6 | 7.6 × 10−6 | 1.3 × 10−5 | |
| B. compestris | As | 7.6 × 10−4 | 1.8 × 10−4 | 2.8 × 10−3 | 3.1 × 10−3 | 5.2 × 10−4 | 1.2 × 10−4 | 1.9 × 10−3 | 2.1 × 10−3 |
| Cd | 1.6 × 10−5 | 3.3 × 10−5 | 8.9 × 10−5 | 2.2 × 10−4 | 1.1 × 10−5 | 2.3 × 10−5 | 6.1 × 10−5 | 1.5 × 10−4 | |
| Pb | 3.9 × 10−6 | 7.9 × 10−6 | 1.8 × 10−5 | 4.1 × 10−5 | 2.6 × 10−6 | 5.4 × 10−6 | 1.2 × 10−5 | 2.8 × 10−5 | |
| R. sativus | As | 6.7 × 10−5 | 4.2 × 10−4 | 2.7 × 10−3 | 1.4 × 10−3 | 4.6 × 10−5 | 2.8 × 10−4 | 1.8 × 10−3 | 9.4 × 10−4 |
| Cd | 5.4 × 10−6 | 2.0 × 10−5 | 3.5 × 10−5 | 3.5 × 10−5 | 3.7 × 10−6 | 1.4 × 10−5 | 2.4 × 10−5 | 2.4 × 10−5 | |
| Pb | 4.5 × 10−7 | 8.1 × 10−7 | 5.6 × 10−6 | 1.5 × 10−5 | 3.1 × 10−7 | 5.5 × 10−7 | 3.8 × 10−6 | 1.0 × 10−5 | |
| D. carota | As | 1.4 × 10−4 | 5.5 × 10−4 | 7.2 × 10−4 | 3.0 × 10−3 | 9.3 × 10−5 | 3.7 × 10−4 | 4.9 × 10−4 | 2.0 × 10−3 |
| Cd | 9.6 × 10−6 | 1.3 × 10−5 | 5.8 × 10−5 | 7.9 × 10−5 | 6.5 × 10−6 | 8.9 × 10−6 | 3.9 × 10−5 | 5.3 × 10−5 | |
| Pb | 1.6 × 10−8 | 2.5 × 10−6 | 9.6 × 10−6 | 2.9 × 10−5 | 1.1 × 10−8 | 1.7 × 10−6 | 6.6 × 10−6 | 2.0 × 10−5 | |
| S. tubersom | As | 6.7 × 10−5 | 3.6 × 10−4 | 2.0 × 10−3 | 3.0 × 10−3 | 4.6 × 10−5 | 2.4 × 10−4 | 1.3 × 10−3 | 2.0 × 10−3 |
| Cd | 1.9 × 10−6 | 3.3 × 10−6 | 6.6 × 10−5 | 5.5 × 10−5 | 1.3 × 10−6 | 2.2 × 10−6 | 4.5 × 10−5 | 3.7 × 10−5 | |
| Pb | 1.7 × 10−6 | 4.0 × 10−6 | 1.4 × 10−5 | 2.1 × 10−5 | 1.2 × 10−6 | 2.8 × 10−6 | 9.2 × 10−6 | 1.4 × 10−5 | |
| P. vulgaris | As | 4.7 × 10−4 | 2.2 × 10−4 | 4.4 × 10−4 | 1.5 × 10−3 | 3.2 × 10−4 | 1.5 × 10−4 | 3.0 × 10−4 | 1.0 × 10−3 |
| Cd | 1.5 × 10−5 | 2.0 × 10−5 | 1.9 × 10−4 | 1.0 × 10−4 | 1.0 × 10−5 | 1.4 × 10−5 | 1.3 × 10−4 | 6.9 × 10−5 | |
| Pb | 3.1 × 10−6 | 3.5 × 10−6 | 9.7 × 10−6 | 2.3 × 10−5 | 2.1 × 10−6 | 2.4 × 10−6 | 6.6 × 10−6 | 1.6 × 10−5 | |
4. Discussion
Source of irrigation had a great impact on bioavailability of heavy metals to plants. In the present study effects of irrigation system on bioavailabailty of heavy metal were assessed to compare the levels of risks associated with irrigation system.
4.1. Levels of heavy metals in soil
To assess the health risk it is necessary to determine the levels of heavy metals in soil where the vegetables are to be grown. Because solid and liquid waste management and waste dumping had increase the levels of heavy metals in soil (Zhuang et al., 2009). Soils polluted with heavy metals augement the more metalic ions uptake in plants (Wei et al., 2021, Guerra Sierra et al., 2021).
The result of current study showed that level of As and Pb remained under the permisible limit of indian standards Awashthi, (2000), FAO/WHO (2001) and Union standards, (2006) as reported by Mahmood and Malik (2014). But Cd exceeded in soils where vegetable were grown with drain WWI. Similar findings of Mahfooz et al., (2020) also confirm that bioavailability of metals had increased by irrigating the vegetable and crops with wastewater.
4.2. Levels of heavy metals (mg Kg−1) in vegetables
The results of the present investigation were compared with maximum permisible levels (MPL) given by authorities as presented in Table 3. It has been assessed that all vegetaables have been contaminated by As,Cd and Pb under RWI, DomWWI and DrWWI, except GW irrigated vegetables where they showed partial contamination. The mean concentration of heavy metals in GWI vegetables remained less than maximum permisible level (MPL) as recommended by FAO/WHO (2014). The reasons of lower amounts of these metals under ground water irrigation may be that heavy metals leached down to deeper layer and distributed in few meters depth soil (singh et al 2010). The comparisons of results of this study with background values indicated that ground water has less effect on bioaccumulationin in plants than wastewater usage for irrigation (Mahmood and Malik 2014). Likuku and Obuseng, (2015) reported that Pb in Capsicum annum (green pepper) and solanum lycopersicum (tomatoes) grown with ground water bioaccumulated in lesser concentrations than in corrosponding vegetable grwon with waste water. It has also been assessed that uptake of metal concentraion in vegetables grown with DrWW remained highest followed by DomWW, RW water and GW. The highest uptake in drain irrigated vegetables may be due to dischargeing the traffic emmision, discharge from Pb storage battaries, lots of textile mils, paint industries and tanneries in Hudiara drain in Lahore(Mahmood and Malik, 2014, Parveen et al., 2012). Domestic sewage water is also used for irrigation in the vicinity of Lahore.
Table 3.
Comparisons of mean concentrations (mg Kg−1) ± s.d of selected heavy metals in some vegetable’s consumable parts grown under four different irrigation systems.
|
GWI |
RWI |
Dom.WWI |
Dr.WWI |
Standards | ||
|---|---|---|---|---|---|---|
| Plants names | HMs | Means ± SDs | Means ± SDs | Means ± SDs | Means ± SDs | |
| Grain | ||||||
|
Phaseolus vulgaris (Green bean) |
As | 0.51 ± 0.118 | 0.243 ± 0.108 | 0.477 ± 0.016 | 1.67 ± 0.170 | 0.2a |
| Cd | 0.063 ± 0.034 | 0.086 ± 0.011 | 0.828 ± 0.059 | 0.435 ± 0.174 | 0.05b | |
| Pb | 0.585 ± 0.097 | 0.676 ± 0.112 | 1.845 ± 0.140 | 4.433 ± 0.515 | 0.1b | |
| Leafy | ||||||
|
Spinacia oleracea (Spinach) |
As | 1.017 ± 0.004 | 0.388 ± 0.155 | 2.151 ± 0.240 | 3.50 ± 1.210 | 0.2a |
| Cd | 0.023 ± 0.012 | 0.058 ± 0.021 | 0.187 ± 0.139 | 0.409 ± 0.236 | 0.05b | |
| Pb | 0.754 ± 0.071 | 1.50 ± 0.405 | 2.121 ± 0.455 | 3.664 ± 0.988 | 0.3b | |
|
Brassica compestris (Mustard) |
As | 0.821 ± 0.126 | 0.194 ± 0.08 | 3.038 ± 0.534 | 3.40.01 | 0.2a |
| Cd | 0.07 ± 0.020 | 0.143 ± 0.157 | 0.381 ± 0.206 | 0.944 ± 0.124 | 0.05b | |
| Pb | 0.744 ± 0.169 | 1.51 ± 0.435 | 3.389 ± 0.214 | 7.83 ± 0.623 | 0.3b | |
| Roots | ||||||
|
Raphanus sativus (Radish) |
As | 0.073 ± 0.035 | 0.45 ± 0.380 | 2.891 ± 0.297 | 1.501 ± 0.440 | 0.2a |
| Cd | 0.023 ± 0.005 | 0.086 ± 0.065 | 0.149 ± 0.173 | 0.149 ± 0.172 | 0.05b | |
| Pb | 0.086 ± 0.006 | 0.155 ± 0.125 | 1.065 ± 0.051 | 2.858 ± 0.382 | 0.1b | |
|
Daucus carota (Carrot) |
As | 0.148 ± 0.043 | 0.59 ± 0.136 | 0.781 ± 0.067 | 3.25 ± 0.170 | 0.2a |
| Cd | 0.041 ± 0.022 | 0.056 ± 0.039 | 0.246 ± 0.382 | 0.336 ± 0.165 | 0.05b | |
| Pb | 0.003 ± 0.00 | 0.472 ± 0.049 | 1.841 ± 0.061 | 5.543 ± 0.288 | 0.1b | |
| Stem tuber | ||||||
|
Solanum tubersom (Potato) |
As | 0.073 ± 0.013 | 0.385 ± 0.036 | 2.147 ± 0.056 | 3.26 ± 1.270 | 0.2a |
| Cd | 0.008 ± 0.000 | 0.014 ± 0.000 | 0.28 ± 0.081 | 0.233 ± 0.089 | 0.05b | |
| Pb | 0.332 ± 0.013 | 0.773 ± 0.089 | 2.591 ± 0.216 | 3.998 ± 0.096 | 0.1b | |
(a) Codex Alimentarius Commission. Joint FAO/WHO food standards program, 2014.
(b) (FAO/WHO, 2014).
The household sewage untreated wastewater coming from these villiages and towns and is used for irrigation purposes dirceclty. This source has lower contamination level due to no or small home industrial effluents dumping as compared to waste water drains that receives waste water from large industries. The river Ravi is the only river which run along the Lahore. Its water is also used for irrigation. It also recieves wastewater from domestic and industrial drains, but due to large water body and dilution due to water coming from heavy rain water and melting of glaciers makes lesser effects to plants than domestic wastewaters and industrial drain wastewaters.
The results of this research also determined that leafy vegetables i.e. S. oleracea and B. compestris gained more heavy metals than roots, leaf and stem tubers. the similar results were obtained by Hu et al., 2017a, Hu et al., 2017b. Gebeyehu and Bayissa (2020) who also found that leafy vegetables significantly accumulate more As, Cd and Pb than fruity vegetable. The results of present study also indicated that As, Cd and Pb had contaminated the 37.5% ground water irrigated vegetables. While, other sources of irrigation made the vegetables 100% contaminated.
4.3. Bioaccumulation factors (BAFs)
The main root of entry of persistant contaminants in food chain is the uptake of these contaminants from soil to plants. The ratio of uptaking of heavy metals to plants from soil is known as bioaccumulation facor. there are various factors which can play their role in variation of bioaccumulation factors e.g. source of irrigatiing water, physico-chemical condition of water and soil, type of plant, type and concentration of heavy metal in water and soil (Sharma et. al., 2018; Gao et al., 2018). It has been assessed that leafy vegetables have higher BAFs than fruit and root vegeables. The similar finding was also reported by Hu et al., 2017a, Gebeyehu and Bayissa, 2020, Meng et al., 2021. Furthermore, they also indicate that leafy vegetables had higher potential health risk than root, stem tuber and fruity vegetable. The values of BAFs of present study indicated that use of wastewater leads to higher bioavailability to plants than groundwater irrigation. Because vegetables grown with wastewater had higher heavy metal concentrations as compred to groundwater irrigated vegetables (Mahmood and malik, 2014).
4.4. Health risk assessment
4.4.1. Estimated daily intake (EDI) of heavy metals from dietary stuffs
The vlues of EDI of heavy metals recorded during this investigation has been found far lower than Maximum Tolerable Daily Intake doses (MTDI). MTDI of As, Pb, and Cd were reported by Shaheen et al., 2016, Gebeyehu and Bayissa, 2020 as 0.13 mg/day, 0.21 mg/day and 0.07 mg/day respectively. These values of MTDI like this study were also compared with their data by Haque et al., 2021, Gebeyehu and Bayissa, 2020, and they observed the similar results to present study. Previous investigations as this study revealed that EDI of heavy metals was lower than the MTDI but their accumulating nature in living organisms can cause various carcinogenic and non carcinogenic effects in body. So calculations of EDI is very useful tool for the assessment target hazard quotients, health index and cancer risks.
4.4.2. Target hazard quotient
It is considered that if THQ > 1 then carcinogenic effects will be observed in body, but if it remianed less than 1 then there will be no noncarcinogenic effects in human body. In present study, THQ of As for adult, 100% and 83.3% vegetables under DrWWI and DomWWI exceeded the threshold, while, the Pb and Cd under the same irrigation system remained lower than threshold in all vegetables. Howeever, 33.33% GWI vegetables surpassed the limit(1) of THQ only for As. While, under RWI no heavy metal showed higher THQ than threshold level. Finally, it was concluded that As is the metal which has higher risks for non-carcinogenic effects than Pb and Cd. It has also been concluded that DomWWI and DrWWI systems are threatening the human health. Higher THQs enhance the danger for the childeren because values of THQs become double for childrens. Many studies had also similar finding to this study. Gebeyehu and Bayissa, (2020) indicated that As and Hg found to be greater than unity in tomato and As, Hg and Co also found to be > 1 in cabbage. Ametepey et al., (2018) find that THQs for Cd and Cr supassed the threshold level but others remained in safer limits. However, Meng et al., 2021 observed that Cd and Pb showed THQs lower than unity as the THQs for Cd and Pb of this study showed less than 1. Hu et al., (2017a) obsereved that excessive use of leafy vegetables leads to higher THQs for heavy metals under any irrigation system. As this investigation determined that S. oleracea and B. compestris has always exceeded under all irrigation system. This is may be due to higher transpiration rate higher uptake of metals along with water by wide leaf of these vegetables.
4.4.3. Hazard indexes (HI)
This study indicated the consumption of vegetables grown under DomWWI and Dr WWI systems with respect to heavy metals is not fit for childeren health. All the vegetables under these irrigation system showed surpassed HI than threshold 1. While, RW irrigated S. oleracea, R. sativus and D. carota also crossed the limited value. However, GW irrigated S. oleracea and B. compestris and P. vulgaris showed higher HI than unity. In contrast, for same vegetables consumpttion showed very alarminng HI to children but with few degree lesser effects. The result of present study revealed that Leafy vegeetables participated more than other vegetables under irrigation system. Many other studies like this investigation summarized the consumption of wastewater irrigated vegetables leads to higher HI (Alturiqi et al., 2020, Haque et al., 2021, Onyele and Anyanwu, 2018, Kumar and Thakur, 2018). Based on the previous studies it is concluded that higher contents of heavy metals posed carcinogeneic and non carcinogenic effects to human especially to childen.
4.4.4. Cancer risk (CR)
In the present investigation it was observed that arsenic posed a serious threat to human health. In almost all irrigation systems, all vegetables accumulate As to such extent that it can serious cancerous effects. While, Cd under drain and domestic showed cancer causing range. The consumption of heavy metals contaminated vegetables leads to higher CR values than threshold (1.0x10−4) as determined by several studies (Antoine et al., 2017, Li et al., 2018, Liang et al., 2019).
5. Conclusion
The main pupose of this study was to estimate the food quality provided to the humans. To find the required results it is necessary to assess the quality of irrigaton system. It has been seeen that all the vegetables of Lahore were brought to local markets from nearyby towns and villiages. The irrigation system for these vegetables has some variation, e.g. some vegetables were irrigated with ground water by means of tubewells, A large area of Lahore and sheikhupura is irrgated with river Ravi water similarly, fields attached to villages and other housing societies are irrigated with domestic waste water. However, Hudiara drain wastewater is also a source of irrigation which receives ample amount of industrial effluents. As, Cd and Pb in these vegetables sample measured and compared to assess the health risk associated with each source of water. Contents of As, Cd and Pb were obsereved highest in drain water irrigated soil and in their respective vegetables as compared to ground and river water irrigated vegetables. Similar trends of Bioaccumulation factor were assessed under these irrigation system. Leafy vegetables were found to be more dangerous for health than other types of vegetables. However, Estimated daily intkes of each metal in each system of irrigation was observed far less than the maximum tolerable daily intake of these metals. But their incremental accumulating behaviour showed higher target hazard quotient than threshold(1). Consequently, it has been determined that HI of wastewater irrigated vegetables were also observed higher than threshold. In some cases As content in ground and river water irrigation had also contaminated the foods. HI of heavy metals contamnated vegetables for adult and childern were also compared and it was found that far higher HI values in childeren than adult made our concern more stronger. Present study also assessed that As content is reached to highly alarming level that it can cancer in different parts of body because its CR values were recorded highest and more than threshold (1.0x10−4). While other metals were almost in safe range in terms of carcinogenic factors. So more investigation is needed in future to assess the health risk associated with heavy metals contaminated vegetables.
Authors contribution
NH, A, and MSA have contributed in the research idea and design, data
gaining, and experimental analysis and explanation of data. While, K.S.A and A. J. contributed in the sampling and statistical analaysis of data.
Ethical approval
There is no ethical conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are genuinely thankful to Dr. Muhammad Shafiq and Dr. Asmatullah. Both persons paid vital intelectual contribution for this study. Moreover, Dr. Arshad Assistant Professor at wildlife department for providing me lab ficilities for Acid digestion of samples.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agoro M.A., Adeniji A.O., Adefisoye M.A., Okoh O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in eastern cape province, South Africa. Water. 2020;12(10):2746. [Google Scholar]
- Alturiqi A.S., Albedair L.A., Ali M.H. Health risk assessment of heavy metals in irrigation water, soil and vegetables from different farms in Riyadh district, Saudi Arabia. J. Elementol. 2020;25(4):1269–1279. [Google Scholar]
- Ametepey S.T., Cobbina S.J., Akpabey F.J., Duwiejuah A.B., Abuntori Z.N. Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis, Ghana. Int. J. Food Contaminat. 2018;5(1):1–8. [Google Scholar]
- Antoine J.M., Fung L.A.H., Grant C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017;4:181–187. doi: 10.1016/j.toxrep.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHA. 2012. Standard methods for the examination of water and waste water, 21st Edn. American Public Health Association, Washington 2462.
- Awadalla A., Mortada W.I., Abol-Enein H., Shokeir A.A. Correlation between blood levels of cadmium and lead and the expression of microRNA-21 in Egyptian bladder cancer patients. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awashthi, S.K., 2000. Prevention of food adulteration Act no.37 of 1954, central and state rules as amended for 1999, 3rd Edition, Ashoka Land House New Delhi.
- Brewer, G.J., Prasad, A.S. eds., 2020. Essential and Toxic Trace Elements and Vitamins in Human Health. Academic Press.
- European Commission, 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union, 364(324-365).
- Ezemonye L.I., Adebayo P.O., Enuneku A.A., Tongo I., Ogbomida E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019;6:1–9. doi: 10.1016/j.toxrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2014) General standards for contaminants and toxins in food and feed (CODEX STAN 193-1995).
- Fu Z., Xi S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods. 2020;30(3):167–176. doi: 10.1080/15376516.2019.1701594. [DOI] [PubMed] [Google Scholar]
- Gebeyehu H.R., Bayissa L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PloS one. 2020;15(1) doi: 10.1371/journal.pone.0227883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health. 2020;17(11):3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra Sierra B.E., Muñoz Guerrero J., Sokolski S. Phytoremediation of heavy metals in tropical soils an overview. Sustainability. 2021;13(5):2574. [Google Scholar]
- Haque M.M., Niloy N.M., Khirul M.A., Alam M.F., Tareq S.M. Appraisal of probabilistic human health risks of heavy metals in vegetables from industrial, non-industrial and arsenic contaminated areas of Bangladesh. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Huang B., Tian K., Holm P.E., Zhang Y. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere. 2017;167:82–90. doi: 10.1016/j.chemosphere.2016.09.122. [DOI] [PubMed] [Google Scholar]
- Hu Y., Cheng H., Tao S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017;107:111–130. doi: 10.1016/j.envint.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Joint Fao/Who Food Standards Programme Codex Alimentarius Commission 37th Session: Report of the Eighth Session of the Codex Committe on Contaminants in Foods; World Health Organization: Geneva, Switzerland, 2014. http://www.fao.org/.
- Kacholi, D.S., Sahu, M., 2018. Levels and health risk assessment of heavy metals in soil, water, and vegetables of Dar es Salaam, Tanzania. Journal of Chemistry, 2018.
- Khalid S., Shahid M., Niazi N.K., Murtaza B., Bibi I., Dumat C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017;182:247–268. [Google Scholar]
- Khan M., Khan H.N., Aslam H. Hudiara drain–a case of trans-boundary Water. Pak. J. Biol. Sci. 2003;6(2):167–175. [Google Scholar]
- Khan M.U., Shahbaz N., Waheed S., Mahmood A., Shinwari Z.K., Malik R.N. Comparative health risk surveillance of heavy metals via dietary foodstuff consumption in different land-use types of Pakistan. Human Ecol. Risk Assess.: Int. J. 2015;22(1):168–186. [Google Scholar]
- Kim H.S., Kim Y.J., Seo Y.R. An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J. Can. Prevent. 2015;20(4):232. doi: 10.15430/JCP.2015.20.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Thakur R.K. Health risk assessment of heavy metals via dietary intake of vegetables grown in wastewater irrigated areas of Jagjeetpur, Haridwar India. Arch. Agricult. Environ. Sci. 2018;3(1):73–80. [Google Scholar]
- Li X., Li Z., Lin C.J., Bi X., Liu J., Feng X., Zhang H., Chen J., Wu T. Health risks of heavy metal exposure through vegetable consumption near a large-scale Pb/Zn smelter in central China. Ecotoxicol. Environ. Saf. 2018;161:99–110. doi: 10.1016/j.ecoenv.2018.05.080. [DOI] [PubMed] [Google Scholar]
- Liang G., Gong W., Li B., Zuo J., Pan L., Liu X. Analysis of heavy metals in foodstuffs and an assessment of the health risks to the general public via consumption in Beijing, China. Int. J. Environ. Res. Public Health. 2019;16(6):909. doi: 10.3390/ijerph16060909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yi X., Dang Z., Wang Q., Luo H., Tang J. Heavy metal contamination and health risk assessment in the vicinity of a tailing pond in Guangdong, China. Int. J. Environ. Res. Public Health. 2017;14(12):1557. doi: 10.3390/ijerph14121557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likuku A.S., Obuseng G. International Conference on Plant, Marine and Environmental Sciences. 2015. Health risk assessment of heavy metals via dietary intake of vegetables irrigated with treated wastewater around Gaborone, Botswana. [Google Scholar]
- Mahfooz Y., Yasar A., Guijian L., Islam Q.U., Akhtar A.B.T., Rasheed R., Irshad S., Naeem U. Critical risk analysis of metals toxicity in wastewater irrigated soil and crops: a study of a semi-arid developing region. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-69815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A., Malik R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore Pakistan. Arab. J. Chem. 2014;7(1):91–99. [Google Scholar]
- Margenat A., Matamoros V., Díez S., Cañameras N., Comas J., Bayona J.M. Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environ. Int. 2019;124:49–57. doi: 10.1016/j.envint.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Meng M., Yang L., Wei B., Cao Z., Yu J., Liao X. Plastic shed production systems: the migration of heavy metals from soil to vegetables and human health risk assessment. Ecotoxicol. Environ. Saf. 2021;215 doi: 10.1016/j.ecoenv.2021.112106. [DOI] [PubMed] [Google Scholar]
- Milton A.H., Rahman M. Respiratory effects and arsenic contaminated well water in Bangladesh. Int. J. Environ. Health Res. 2002;12(2):175–179. doi: 10.1080/09603120220129346. [DOI] [PubMed] [Google Scholar]
- Ohoro C.R., Adeniji A.O., Okoh A.I., Okoh O.O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: a review. Int. J. Environ. Res. Public Health. 2019;16(17):3026. doi: 10.3390/ijerph16173026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyele O.G., Anyanwu E.D. Human health risk assessment of some heavy metals in a rural spring, southeastern Nigeria. Afr. J. Environ. Nat. Sci. Res. 2018;1(1):15–23. [Google Scholar]
- Orun I., Talas Z.S., Ozdemir I., Alkan A., Erdogan K. Antioxidative role of selenium on some tissues of (Cd2+), Cr3+)-induced rainbow trout. Ecotoxicol. Environ. Saf. 2008;71(1):71–75. doi: 10.1016/j.ecoenv.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pandiyan J., Mahboob S., Govindarajan M., Al-Ghanim K.A., Ahmed Z., Al-Mulhm N., Jagadheesan R., Krishnappa K. An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: a perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 2021;28(2):1218–1225. doi: 10.1016/j.sjbs.2020.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen N., Ghaffar A., Shirazi S.A., Bhalli M.N. Spatial distribution of heavy metal contamination in road side soils of Faisalabad-Pakistan. Pak. J. Sci. 2012;64(4):309. [Google Scholar]
- Rai P.K., Lee S.S., Zhang M., Tsang Y.F., Kim K.H. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Rattan R.K., Datta S.P., Chhonkar P.K., Suribabu K., Singh A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—a case study. Agric. Ecosyst. Environ. 2005;109(3–4):310–322. [Google Scholar]
- Sanyal, T., Bhattacharjee, P., Paul, S., Bhattacharjee, P., 2020. Recent advances in arsenic research: significance of differential susceptibility and sustainable strategies for mitigation. Frontiers in public health, 8. [DOI] [PMC free article] [PubMed]
- Shaheen N., Irfan N.M., Khan I.N., Islam S., Islam M.S., Ahmed M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere. 2016;152:431–438. doi: 10.1016/j.chemosphere.2016.02.060. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor A.J., Carey M., Meharg A.A. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016;191:128–134. doi: 10.1016/j.foodchem.2014.11.078. [DOI] [PubMed] [Google Scholar]
- Singh A., Sharma R.K., Agrawal M., Marshall F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010;51(2):375–387. [Google Scholar]
- Talas Z.S., Orun I., Ozdemir I., et al. Antioxidative role of selenium against the toxic effect of heavy metals (Cd+2, Cr+3) on liver of rainbow trout (Oncorhynchus mykiss Walbaum 1792) Fish Physiol Biochem. 2008;34:217–222. doi: 10.1007/s10695-007-9179-9. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA), 2011. Exposure Factors Handbook Edition (Final).
- Wei Z., Van Le Q., Peng W., Yang Y., Yang H., Gu H., Lam S.S., Sonne C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123658. [DOI] [PubMed] [Google Scholar]
- Yaacob A., Yap C.K., Nulit R., Omar H., Al-Shami S.A., Bakhtiari A.R. Assessment of health risks of the toxic Cd and Pb between leafy and fruit vegetables collected from selected farming areas of Peninsular Malaysia. Integr. Food Nutr. Metab. 2018;5(3):1–9. [Google Scholar]
- Yang J., Ma S., Zhou J., Song Y., Li F. Heavy metal contamination in soils and vegetables and health risk assessment of inhabitants in Daye, China. J. Int. Med. Res. 2018;46(8):3374–3387. doi: 10.1177/0300060518758585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria Z., Zulkafflee N.S., Mohd Redzuan N.A., Selamat J., Ismail M.R., Praveena S.M., Tóth G., Abdull Razis A.F. Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants. 2021;10(6):1070. doi: 10.3390/plants10061070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang P., McBride M.B., Xia H., Li N., Li Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009;407(5):1551–1561. doi: 10.1016/j.scitotenv.2008.10.061. [DOI] [PubMed] [Google Scholar]