Abstract
Drought stress adversely affects plant health and productivity. Recently, drought-resistant bacterial isolates are used to combat drought resistance in crops. In this in vitro study, 20 bacterial isolates were isolated from harsh soil; their drought tolerance was evaluated using four concentrations of polyethylene glycol (PEG) 6000. The two most efficient isolates (DS4 and DS9) were selected and identified using 16S rRNA genetic sequencing. They were registered in the NCBI database and deposited under accession numbers MW916285 and MW916307 for Bacillus cereus (DS4) and Bacillus albus (DS9), respectively. These isolates were screened for plant growth-promoting properties compared to non-stressed conditions. Biochemical parameters; Proline, salicylic acid, gibberellic acid (GA), indole acetic acid (IAA), antioxidant activity, and antioxidant enzymes were measured under the same conditions, and in vitro seed germination was tested under stress conditions and inoculation with selected isolates. The results showed that under the harsh conditions of PEG6000, DS4 produced the highest amount of IAA of 1.61 µg/ml, followed by DS9 with 0.9 µg/ml. The highest amount of GA (49.95 µg/ml) was produced by DS9. On the other hand, the highest amount of siderophore was produced from DS4 isolate followed by DS9. Additionally, DS4 isolate recorded the highest exopolysaccharide (EPS) content of 3.4 mg/ml under PEG (-1.2 MPa) followed by DS9. The antioxidant activity increased in PEG concentrations depending manner, and the activity of the antioxidant enzymes increased, as catalase (CAT) recorded the highest activity in DS4 with an amount of 1.095 mg/ml. additionally, an increase in biofilm formation was observed under drought conditions. The isolated mixture protected the plant from the harmful effects of drought and showed an increase in the measured variables. Under unstressed conditions, the highest rates of emulsification index (EI 24%) were obtained for DS4 and DS9, at 14.92 and 11.54, respectively, and decreased under stress. The highest values of germination, total seedling length, and vigor index were obtained upon inoculation with the combination of two strains, and were 100%, 4.10 cm, and 410, respectively. Therefore, two strains combination is an effective vaccine capable of developing and improving drought tolerance in dryland plants.
Keywords: Drought tolerance, PGPR, 16s rRNA, Antioxidant activity, Antioxidant enzymes
1. Introduction
Abiotic stresses, such as drought, salinity, and extreme temperatures, as well as pollutants, such as pesticides and heavy metals, are the main factors affecting plant growth and yield and cause significant economic losses. Most abiotic stresses lead to oxidative stress, hence an increase in reactive oxygen species (ROS) at the cellular level (Kerchev et al., 2020). ROS are not only toxic molecules but also signals that regulate various developmental processes and responses to the environment (Tsukagoshi et al., 2010, Mhamdi and Van Breusegem, 2018).
Drought is a major environmental problem currently presenting a challenge to most countries worldwide, as it leads to decreased plant growth and yield, which thus affect the agricultural and food industry. There is thus a need to seek ways of reducing this risk by improving plant growth under drought stress conditions (Chukwuneme et al., 2020). Owing to certain limitations in genetic techniques (Coleman-Derr and Tringe, 2014, Philippot et al., 2013) and the cost-intensive agricultural practices such as soil improvement (Jongdee et al., 2006), searches have been undertaken to identify better and more efficient ways of tackling drought stress. Recently, an increasing number of environmentally friendly approaches have been used to support agricultural sustainability, including bio-stimulants (Chiaiese et al., 2018, du Jardin, 2015). Bio-stimulants enhance the plant defenses, increase yield, improve fruit quality, and reduce plant stress (Shukla et al., 2019). The application of biostimulants affect metabolic processes, improve ion transport, and modify plant hormones. Stress tolerance is perhaps the most significant benefit of bio-stimulants (Backer et al., 2018, Paul et al., 2019, Polo and Mata, 2018).
Microorganisms have considerable applications in agriculture, with the central target of supplanting nutrients and chemicals. The incorporation of microorganisms as an active and vital component in the agricultural system to stimulate drought tolerance in plants needs to be recommended to increase sustainable crop production. Viruses, bacteria, algae, and fungi or their products can be applied as biofertilizers, biopesticides, bioherbicides, and in bioremediation. Amongst them, bacteria possibly boost plant growth through the production of plant-essential phytohormones, mineral solubilization, and in addition, it might enhance an antagonistic effect against the pathogens. In plants growing under extreme stress conditions, Plant Growth-Promoting Bacteria (PGPB) inoculation was shown to enhance stress tolerance, at least in part, by enhancing the root length, allowing better access to water (Cohen et al., 2015, Kang et al., 2014). Beneficial bacterial species with the ability to promote plant growth have been studied and applied to mitigate the harmful effects of drought (Enebe and Babalola, 2018).
Bacteria avoid drought damage across several mechanisms; the production of phytohormones, such as indole acetic acid (IAA), gibberellins (GA), biosurfactant (SINGH et al., 2018), and siderophores (Wang et al., 2014). In addition, bacteria stimulate oxygen radical scavenging (Coleman-Derr and Tringe, 2014, Ndeddy Aka and Babalola, 2017) by proline production (Hayat et al., 2012) and antioxidant enzymes (Rezayian et al., 2018). Furthermore, previous studies demonstrated that Salicylic acid (SA) relieves drought stress by inducing antioxidant enzymes (Habibi, 2012). Moreover, exopolysaccharide (EPS) is produced to protect bacterial cells from drought, by regulating nutrients and water flow across plant roots through biofilm formation (Ali et al., 2014), which can increase cell survival under stress (Ozturk and Aslim, 2010). Additionally, bacteria must be able to survive on the seed surface and then colonize the root system for optimal interaction with the plant (Bloemberg and Lugtenberg, 2001). Owing to these outstanding characteristics of PGPB, plants can grow well under drought conditions.
Like plants, in the case of water scarcity, bacteria may also be affected by hydric stress. Under conditions of water deficiency, bacteria need to adapt to osmotic stress. Therefore, selecting bacteria that are the most tolerant of drought is very useful to produce new inoculants for use in arid areas (García et al., 2017). Most studies have focused on certain bacterial species, mostly Pseudomonas and Bacillus (Chukwuneme et al., 2020), so the use of other types of bacteria has not been sufficiently studied. As such, this study was conducted to isolate and characterize other bacterial types and determine their ability to produce some plant growth-promoting (PGP) substances under different drought conditions.
2. Materials and methods
2.1. Isolation and screening of drought-tolerant bacteria
Bacteria were isolated from soil samples collected from different regions of Egypt using a serial dilution method. The morphologically distinct bacterial colonies were isolated, purified, and maintained on slants at 4 °C, and 60% glycerol stock was preserved at −80 °C for future use.
Subsequently, the ability of the purified isolates to grow under drought stress conditions was examined by adding different concentrations of polyethylene glycol (PEG 6000) (-0.15, −0.49, −0.73, and −1.2 Mega Pascal (MPa) in tryptic soy broth (TSB) (Vardharajula and Sk Z, 2014), and they were inoculated with 1% bacterial cultures overnight. After incubation at 28 °C for 24 h under shaking conditions (150 rpm), the growth was measured using a spectrophotometer at 600 nm (SCO-Tech, SPUV19, Germany) while using a sterile medium as a blank. Three replicates of each isolate at the same concentrations as mentioned previously were measured (Sandhya et al., 2009).
Optical density (OD) values of drought-tolerant isolates were used for categorization as highly sensitive OD < 0.3; sensitive OD 0.3 to 0.39; tolerant OD 0.4 to 0.5, and completely tolerant OD > 0.5 (Susilowati et al., 2018).
2.2. Assessment of features of drought-tolerant bacteria (PGP)
2.2.1. Cell-free extract preparation
Tubes of TSB medium were prepared under unstressed conditions with different water potentials using the above-mentioned PEG 6000 concentrations and amended with 1.0 mM L-tryptophan. Each tube was inoculated with 1% of the overnight grown culture of each tolerant and highly tolerant isolate and incubated at 28 °C for 24 h on a shaking incubator at 150 rpm. By centrifugation at 10,000 × g for 5 min, a cell-free extract supernatant was obtained, collected, and used for the following experiments.
2.2.2. Indole acetic acid (IAA) production
A total of 1.5 ml of the bacterial-free extract and 4 ml of Salkowski reagent (12 g of FeCl3 per liter in 7.9 M H2SO4) were mixed. Then, the solution mixture was incubated in the dark for 24 h. The intensity of the resulting pink color was measured at a wavelength of 520 nm using a spectrophotometer. The IAA concentration was determined using a standard curve of known concentrations of IAA (Gusmiaty et al., 2019).
2.2.3. Gibberellins (GA) production
One milliliter of Folin Ciocalteu reagent (HIMEDIA Co., Germany) and 1.0 ml of HCl conc. were added to 1.0 ml of the bacterial-free extract in a test tube, followed by the addition of 3 ml of distilled water (DW). After that, the mixture was allowed to boil in a water bath for 5 min, and then left to cool. The produced bluish-green color was measured using a spectrophotometer (SCO-Tech, SPUV19, Germany) at 760 nm. Likewise, the color was also measured in gibberellic acid standard solution (GA3) (Abou-Aly et al., 2019).
2.2.4. Siderophore production
In accordance with a previous study (Carson et al., 1992), two types of siderophore were measured (Hydroxamate and catecholate). Bacterial-free extract (1.0 ml) was mixed with 3.0 ml of 2% aqueous FeCl3 solution. The previous mixture was measured at two different wavelengths in the UV–visible spectrum range; 430 nm for hydroxamate and 495 nm for catecholate. A blank tube contained 1 ml of DW and 3 ml of FeCl3.
2.2.5. Exopolysaccharide (EPS) estimation
Bacterial isolates were cultured in 50 ml of optimized mineral salt medium (12.6% K2HPO4, 18.2% KH2PO4, 10% NH4NO3, 1% MgSO4·7H2O, 0.6% MnSO4, 1% CaCl2·2H2O, 0.06% FeSO4·2H2O, 1% sodium molybdate, 1.5% NaCl, and 0.2% glucose), in accordance with a slightly modified version of a previously reported procedure (Naseem and Bano, 2014) and incubated for 10 days under stressed and unstressed conditions. After incubation at 28 °C in a shaker incubator (150 rpm), 500 μl of 1 mM EDTA was added to harvest the cells, followed by intense shaking, and then centrifuged at 15,000 rpm for 10 min. The supernatant was separated and mixed with two volumes of ice ethanol (95%). Then, centrifugation was performed twice at 15,000 rpm for 30 min. The sediments were collected, washed, and dried until the weight was stable and then the dry weight was taken.
2.3. Oxidative stress features of drought-tolerant bacteria
2.3.1. Proline
Proline production was estimated as described previously (Abou-Aly et al., 2019); 2.0 ml of bacterial supernatant and 2.0 ml of glacial acetic acid were added to 2.0 ml of acid ninhydrin (2.5 g of ninhydrin in 60 ml of glacial acetic acid and 40 ml of 6 M phosphoric acid with warming until it melted) in a glass tube, and put in a boiling bath for 1.0 h, moved to an ice bath. After that, 4.0 ml of toluene was mixed vigorously for 15–20 s. The absorbance was read at 520 nm and toluene was used as a blank.
2.3.2. Salicylic acid
SA production by bacterial isolates was estimated in accordance with a previously reported method (Abou-Aly et al., 2019) as follows: 4.0 ml of bacterial supernatant was acidified with 1 N HCl until the pH reached 2. Salicylic acid was extracted in chloroform (CHCl3) 1:1 (v/v), followed by the addition of 4.0 ml of DW and 5.0 ml of 2 M FeCl3. The absorbance was read spectrophotometrically at 527 nm, with chloroform used as a blank.
2.3.3. Antioxidant enzymes
2.3.3.1. Polyphenol oxidase activity (PPO)
The PPO was measured as described previously (Oktay et al., 1995). A total of 100 µl (100 mM sodium phosphate buffer, pH 7.0), 500 µl (5 mM 4-methylcatechol), and 500 µl of crude extract and then 3000 µl of DW were added. The absorbance was read spectrophotometrically at 420 nm.
2.3.3.2. Peroxidase activity (PO)
The PO was observed using 4-methyl catechol as a substrate, in accordance with a previous study (Onsa et al., 2004). A mixture of 100 µl of 100 mM potassium phosphate buffer (pH 7.0), 500 µl of 5 mM H2O2, 500 µl of 5 mM 4-methyl catechol, and 500 µl of crude extract was established, which was made up to a total volume of 4000 µl by DW. Its absorbance was measured at 420 nm using a spectrophotometer. One unit of enzyme activity under assay conditions was defined as the amount of enzyme that caused a change of 0.001 in absorbance per min.
2.3.3.3. Catalase (CAT)
The CAT was determined by observing the decrease in absorbance at 240 nm resulting from the decomposition of H2O2, as described previously (Desoky et al., 2020, Vílchez et al., 2016). The mixture included 500 µl of 75 mM H2O2, 1500 µl of 100 mM potassium phosphate buffer (pH 7.0), 200 µl of enzyme extract, and 800 µl of DW.
2.4. Molecular identification of isolates
2.4.1. DNA extraction and PCR amplification
Genomic DNA of the two isolates was extracted using ZR Soil Microbe DNA MiniPrep™ (Zymo Research, USA) extraction kit, in accordance with the manufacturer’s instructions. The DNA was amplified using universal 16S rRNA primers 27F: 5′-AGAGTTTGGATCMTGGCTCAG-3′ and 1492R: 5′-CGGTTACCTTGTTACGACTT-3′ (Abdelatty et al., 2021). PCR was carried out in thermal cycle PCR machine (SensoQuest, Cat # 049974) with a reaction volume of 50 µl. Each reaction contained 0.4 μM of each primer with a concentration of 10 pM, 400 μM of dNTP mix, 5 µl of 10 µl PCR reaction buffer, 2 μM MgCl2, 2.5 units of TAKARA Taq DNA polymerase (Cat. #: R001AM), 1 μl of template DNA, and the final volume was adjusted with sterilized double DW. The PCR program was as follows: initial denaturation at 95 °C for 3 min; then 35 cycles of denaturation at 95 °C for 50 s, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min; followed by final extension at 72 °C for 10 min. Amplified PCR products were subjected to 2% agarose gel electrophoresis and stained with ethidium bromide using a GeneRulerTM 1 kb DNA ladder (Cat. #: SM0313), followed by visualization using a gel Doc™ EZ Imaging System with Image Lab™ Software (Bio-Rad, Cat# No. 7,569,130.).
2.4.2. DNA purification and sequencing analysis
The PCR products were purified using QIAquick Gel Extraction Kit (Cat. #: 28706), in accordance with the manufacturer’s instructions, and were sequenced by Macrogen Company, South Korea. The obtained 16S rRNA sequences were aligned and compared with the known sequences in the NCBI nucleotide database by the BLAST algorithm to find closely related bacteria. Jalview software (Waterhouse et al., 2009) was applied to show single-nucleotide polymorphisms (SNPs) and the consensus resulting from the alignment of our obtained sequences with the nearest strains in the NCBI database (http://www.jalview.org/). Analysis of the evolutionary relationship using MEGA X was performed following the neighbor-joining method, as previously reported (Kumar et al., 2018).
2.5. Quantitative determination of biofilm under stress conditions
The ability of Bacillus cereus and Bacillus albus to generate biofilms under PEG6000 stress condition (at concentration of −0.71 MPa) was assessed using the colorimetric method (Auger et al., 2006). Fresh cultures of Bacillus cereus and Bacillus albus (final concentration 4 log10 CFU/ml) were loaded individually into wells of the 96-well microtiter plate and used to inoculate tryptone soya broth (TSB) this is used to represent unstressed conditions. While for the stressed conditions, the TSB was supplemented with PEG6000 at a final concentration of −0.71 MPa, then inoculated with bacterial isolates. The plates were incubated statically at 37 °C for 24 h. Quantitative estimation of the generated biofilms were detected by measuring the absorbance (OD) at 570 nm as described previously by Esmael et al., 2021.
2.6. Antioxidant activities and biosurfactant production
2.6.1. Antioxidant activities
One milliliter from the cell-free supernatant of the two isolates was mixed with 3 ml of 0.1 mM 2-diphenyl-2-picryl hydrazyl hydrate (DPPH) and incubated for 30 min in the dark. Absorbance was measured in triplicate at 515 nm using a spectrophotometer (SCO-Tech, SPUV19, Germany), and using 1 ml of ethanol added to 3 ml of DPPH solution as a control. DPPH scavenging activity was calculated as described previously (Saad et al., 2021, Sahitya et al., 2018) using the following formula:
2.6.2. Biosurfactant production
The emulsification index (% EI 24) was determined in accordance with a previous study (Adnan et al., 2018). Equal volumes of culture supernatant and toluene were mixed in a clear test tube with vortexing for 2 min and left to stand for 24 h. % EI 24 was calculated using the following equation:
2.7. In vitro evaluation of seed viability in Petri dishes
In the absence and presence of −0.73 MPa PEG 6000, seed germination tests were performed to evaluate the effects on seed germination of isolate inoculation individually and in combination (Rincón et al., 2008). Before the test, drought-sensitive maize seeds obtained from the Agricultural Botany Department, Egypt, were washed and sterilized using 70% ethanol for 5 min, followed by 2% sodium hypochlorite (NaClO2) solution for 15 min, and washed several times with sterile DW to clear away the remnants of the disinfectant. Thereafter, two filter papers were added in the Petri plates (replicated three times), after which 10 ml of each bacterial strain suspension with −0.73 MPa or 10 ml of sterile water as a control was placed in each Petri dish. Sterile seeds were soaked in 10 ml of bacterial strain suspension with and without PEG for 5 h in a rotary shaker at 150 rpm; after that, six seeds were placed in each Petri dish and incubated at 25 °C for 10 days (Chukwuneme et al., 2020). Germinated seeds in each Petri dish were counted and two seedlings per plate were randomly selected to measure total seedling length (shoot and root length). Percentage germination and vigor index were calculated in accordance with a previously reported method (Chukwuneme et al., 2020) as follows:
Here, n is the number of germinated seeds after 7 days and N is the total number of seeds.
3. Results
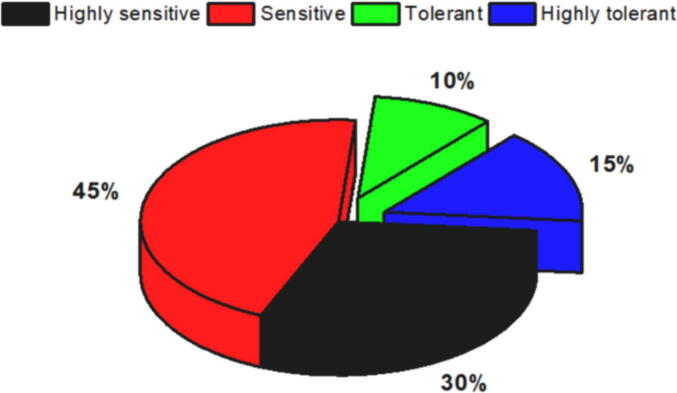
In total, 20 different bacterial isolates were isolated from various arid soils using TSB. The isolates were named “DS,” followed by their isolation number. The results in Fig. 1 show that with osmotic stress concentrations applied using PEG 6000, six (30%) of the isolates were categorized as highly sensitive, nine (45%) as sensitive, two (10%) as tolerant, and three (15%) as highly tolerant. The five bacterial isolates (named DS2, DS4, DS5, DS8, and DS9) that showed tolerant and highly tolerant activities against PEG 6000 were selected for further experiments in this work.
Fig. 1.
Percentages of tolerance levels of bacterial isolates on TSB medium fortified with various PEG 6000 concentrations.
3.1. PGP characteristics of drought-tolerant bacterial isolates
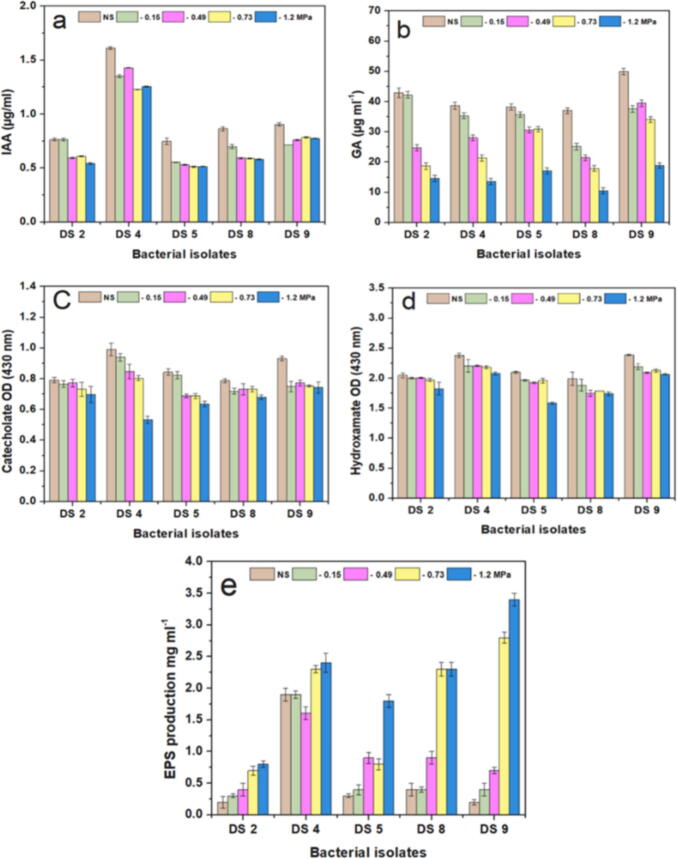
The five most tolerant isolates were screened for the PGP properties of IAA, GA, siderophores, and EPS with and without PEG 6000. Drought stress had variable effects on all isolates in the tested experiments, as shown in Fig. 2. From the results in Fig. 2a, all five tested isolates gave the highest IAA amounts under unstressed conditions and produced various amounts at different drought stresses. Maximum IAA production was recorded for isolate DS4, ranging from 1.23 to 1.61 μg/ml, followed by isolate DS9 (0.72–0.90 μg/ml), while isolate DS5 showed the lowest amounts (0.51–0.75 μg/ml).
Fig. 2.
Bar graphs illustrate the PGP characteristics of drought-tolerant bacterial isolates under unstressed conditions (NS) and PEG 6000 stressed concentrations (-0.15, −0.49, −0.73, and −1.2 MPa). (a) IAA, (b) GA, (c) siderophore (catecholate and hydroxamate), and (d) EPS production.
Regarding the trend of GA production, it decreased gradually upon exposure to increasing concentrations of PEG 6000 (Fig. 2b). Isolate DS9 produced the highest amount of GA, within the range of 18.87–49.95 μg/ml, followed by isolate DS2, while isolate DS8 was found to produce the lowest amount of GA (10.50–37.00 μg/ml). To determine whether the drought-resistant bacterial isolates were producing siderophores, the production of two siderophores types, hydroxamate, and catecholate, were examined (Fig. 2c-d). Our results indicated that all tested bacterial isolates were able to produce siderophores under unstressed and stressed conditions while the amount of both the hydroxamate and catecholate varies inversely with the concentration (MPa) of PEG 6000. Bacterial isolate DS4 was the most potent isolate in terms of producing siderophore, followed by isolates DS9 and DS2 respectively. Regarding the production of exopolysaccharides (EPS), a significant increase in this variable was observed under drought stress conditions compared with that under unstressed conditions (Fig. 2d). The highest EPS production was achieved by isolate DS4 and then isolate DS9 under unstressed conditions, namely, at −0.15 and −0.49 MPa, which were 1.90 and 1.61 mg ml−1, while at −0.73 and −1.2 PEG 6000 concentrations they were 2.80 and 3.40 mg ml−1, respectively. The quantity of EPS produced by the isolate DS8 was close to that of the isolate DS4, at only −1.2 MPa, and the lowest EPS production was obtained by DS2.
3.2. Traits associated with oxidative stress of drought-tolerant bacteria.
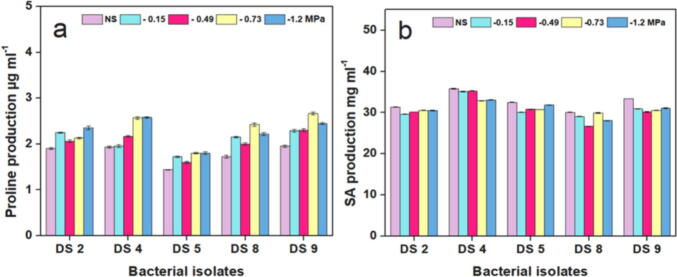
The production of proline, salicylic acid, and oxidative enzymes by drought-tolerant bacterial isolates was assessed, as shown in Fig. 3. Regarding the production of proline (Fig. 3a), compared with that under unstressed conditions, proline production increased under the different PEG concentrations. Except at −1.2 MPa, the maximum proline quantities in unstressed conditions and media supplemented with different concentrations of PEG were produced by isolate DS9, ranging from 1.95 to 2.66 µg/ml. In this respect, the proline production by DS4 was higher than those of the other isolates. Meanwhile, the minimum proline quantities were recorded by DS5, ranging from 1.44 to 1.80 µg ml−1.
Fig. 3.
Bar graphs represent the production of (a) proline and (b) Salicylic acid (SA) by drought-tolerant bacterial isolates under unstressed conditions (NS) and various PEG 6000 stressed concentrations (-0.15, −0.49, −0.73, and −1.2 MPa).
Regarding Salicylic acid (SA) production, all of the examined isolates were able to produce various amounts of SA with and without stress, as shown in Fig. 3b. The highest and lowest levels of SA with and without stress were produced by isolates DS4 and DS8, respectively. Meanwhile, higher SA amounts were produced by DS9 (30.15–33.38 mg ml−1) than by DS5 (30.08–32.50 mg ml−1) and DS2 (29.60–31.33 mg ml−1).
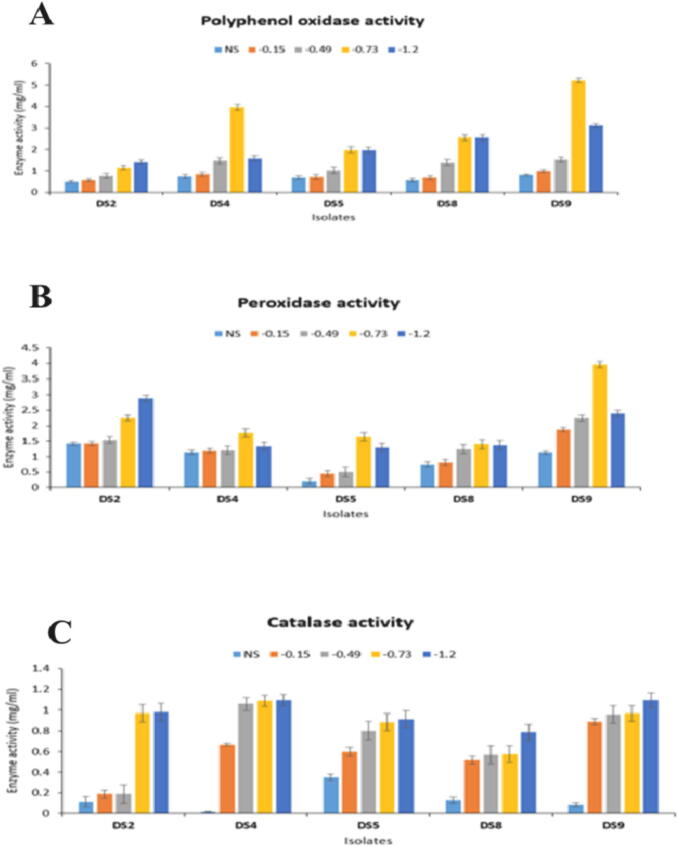
The activities of those antioxidant enzymes for the selected isolates were strongly influenced by the intensity of drought stress (Fig. 4) and declined in the control without stress compared with the levels at other PEG concentrations.
Fig. 4.
Effect of different PEG 6000 concentrations (MPa) on the activities of: (a) polyphenol oxidase enzyme, (b) peroxidase enzyme, and (c) catalase enzyme. Different colors of the columns representing different PEG6000 concentrations (unstressed, −0.15, −0.49, −0.73 and −1.2 MPa). The data displayed are the mean of three replicates and error bars indicates the deviations in the values.
Our results showed that PPO and PO exhibited variable activities with increasing PEG concentrations for five tested isolates. DS9 recorded the highest PPO and PO activity, followed by DS4 for PPO, but followed by DS2 for PO. Additionally, CAT activity increased dramatically with increasing PEG concentrations until peaking at −1.2 MPa. DS4 had the maximum activity (0.015, 0.668, 1.058, 1.088, and 1.095 mg/ml) while the minimum activity was observed for DS8 at 0.128, 0.520, 0.570, 0.578, and 0.788 mg/ml at control conditions, −0.15, −0.49, −0.73, and −1.2 MPa, respectively. Depending on the results of the preceding experiments, isolates DS4 and DS9 were selected for further study because they exhibited the highest PGPB activities.
3.3. Molecular characterization and phylogenetic analysis
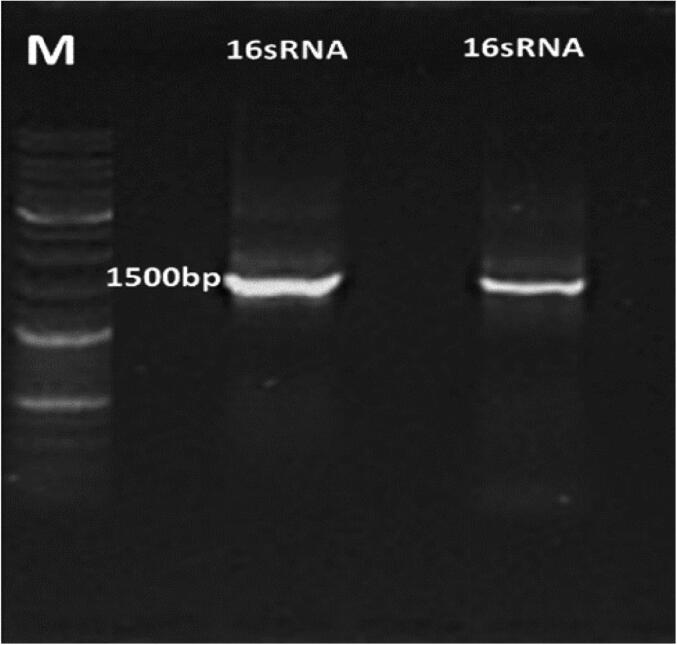
The amplified PCR products of the 16S rRNA gene (1.5 kb in length) from the two isolates were sequenced and the assembled sequences were submitted to the NCBI database under accession numbers MW916285.1 for Bacillus cereus and MW916307.1 for Bacillus albus. The query 16S rRNA gene sequences were aligned with the nearest sequences, exhibiting identity rates ranging from 96% to 98%, with an E-value of zero.
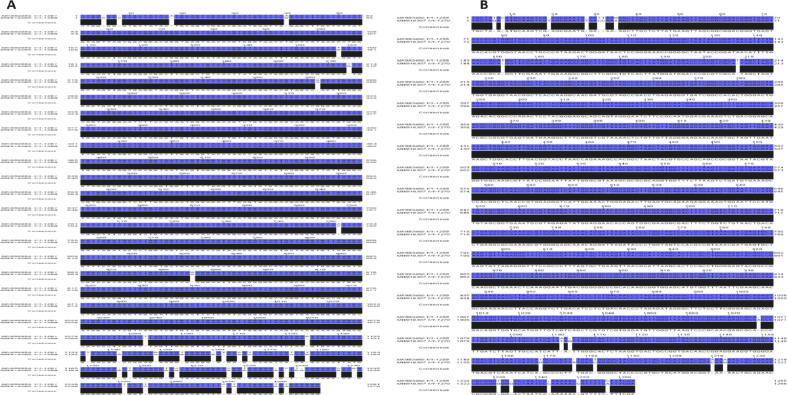
BlastN and Jalview alignment results for these two isolates revealed that the nearest deposited sequences in the database are Bacillus cereus (MK956956.1) and Bacillus albus (MK993460.1), with identity rates of 96.97% and 98.26%, respectively. The results on SNPs indicated that there were 22 SNPs and 16 GAPs between the obtained sequence Bacillus cereus (MK956956.1) and the nearest one of Bacillus cereus MK956956.1. Meanwhile, there were 9 SNPs and 11 GAPs between Bacillus albus (MK993460.1) and the nearest one of Bacillus albus MK993460.1 (Fig. 5, Fig. 6).
Fig. 5.
PCR products of 16S rRNA gene of the two selected PGP isolates.
Fig. 6.
Image shows SNPs and GAPs between (a) Bacillus cereus (MW916285.1) and the nearest sequence (MK956956.1), and (b) Bacillus albus (MW916307.1) and the nearest sequence (MK993460.1) deposited in GenBank.
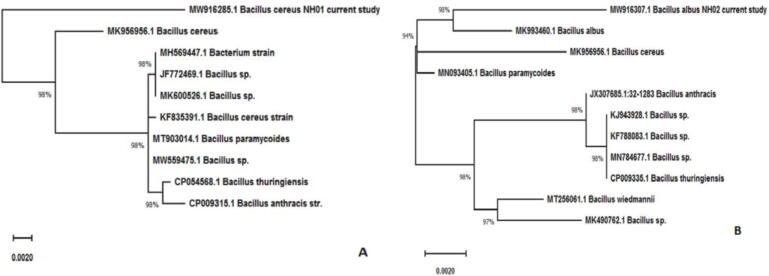
The phylogenetic tree indicated the genetic relationship of the bacterial isolates with the closest reference bacterial species. The obtained isolates Bacillus cereus (MW916285) and Bacillus albus (MW916307) were in the same clades as the nearest sequences Bacillus cereus (96%, MK956956.1) and Bacillus albus (98%, MK993460.1), respectively, in the database (Fig. 7).
Fig. 7.
The phylogenetic tree of A) the obtained sequence of Bacillus cereus (MW916285.1) with the nearest one of Bacillus cereus (MK956956.1), and B) the obtained sequence of Bacillus albus (MW916307.1) with the nearest sequence of Bacillus albus (MK993460.1) deposited in GenBank. This tree was made using the maximum likelihood method with MEGA X. Each bar represents 0.002 changes per nucleotide.
3.4. Evaluation of biofilm formation by the selected PGP bacteria under stress condition
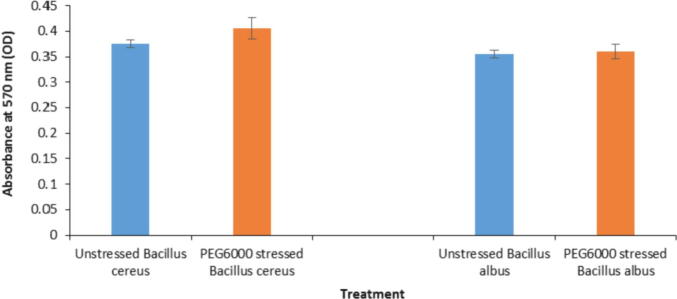
Bacteria adapt to different stress conditions by the generation of biofilm, which is a noteworthy strategy to survive in plant rhizosphere. Fig. 8 shows the absorbance values at 570 nm (OD) which indicates the activity of biofilm formation for the two selected PGPB (Bacillus cereus and Bacillus albus) under normal and PEG6000-stress conditions. The data showed that the bacterial activity of biofilm formation was slightly increased under the stress condition as compared to the normal conditions.
Fig. 8.
Quantitative evaluation of biofilm formation by Bacillus cereus and Bacillus albus in NB cultures (unstressed controls) and NB cultures supplemented with PEG6000 (-0.71 MPa).
3.5. Antioxidant activities and biosurfactant production
The preceding experiments showed that isolated DS4 and DS9 (here after Bacillus cereus and Bacillus albus) were the two most potent strains in terms of drought tolerance. These two isolates were selected and used as candidates for DPPH scavenging activity and biosurfactant production under PEG stressed and normal unstressed conditions.
Concerning antioxidant activity (Fig. 9a), the two isolates were used to estimate the ability to inhibit 2,2-diphenyl-2-becquerel hydrazyl hydrate (DPPH). DPPH radical scavenging activity (%) decreased with increasing PEG concentrations until −1.2 MPa, meaning that the inhibition of DPPH increases with increasing PEG concentration. Notably, isolate DS9 exhibited lower scavenging activity (%) than DS4, which implies a higher ability of DS9 to inhibit DPPH.
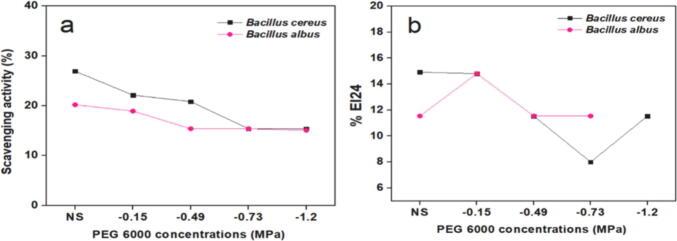
Fig. 9.
Percentage of DPPH scavenging activity (a) and emulsification index (b) for both DS4 and DS9 (strain names) under different concentrations of PEG 6000, compared with unstressed conditions (NS).
To determine biosurfactant production, the emulsification capacity assay was used (Fig. 9b). Under unstressed conditions, the highest rates of EI 24% were obtained for DS4 and DS9, at 14.92 and 11.54, respectively. EI 24% decreased with increasing drought stress. Additionally, DS4 gave higher EI 24% than DS9. Notably, DS9 was not able to produce biosurfactant at −1.2 MPa.
3.6. Seed germination test
Seed germination was assessed with DS4 and DS9 (Bacillus cereus and Bacillus albus) under unstressed conditions compared with that under-drought stress at −0.73 MPa, as presented in Table 1. Under unstressed conditions, germination rate, total seedling length (cm), and vigor index gave the highest values when inoculating seeds with either strain individually or them in combination, compared with the findings for uninoculated seeds (T1). Under drought stress, the highest values of germination %, total seedling length (cm), and vigor index were obtained upon inoculation with the combination of two strains, and were 100%, 4.10 cm, and 410, respectively.
Table 1.
Germination (%), total seedling length (cm), and vigor index of maize seeds inoculated with both DS4 and DS9 either alone or by co-inoculation under drought stress (-0.73 MPa) and without stress.
| Treatments | Germination % | Total seedling length (cm) | Vigor index |
|---|---|---|---|
| T1 (un-inoculated seeds) | 83.33 | 3.50 | 291.66 |
| T2 (un-inoculated seeds + − 0.73 MPa) | 16.67 | 0.50 | 8.340 |
| T3 (seeds + DS4 strain) | 100.00 | 4.00 | 400.00 |
| T4 (seeds + DS4 strain+ − 0.73 MPa) | 66.67 | 3.10 | 206.677 |
| T5 (seeds + DS9 strain) | 100.00 | 3.60 | 360.00 |
| T6 (seeds + DS9 strain+ − 0.73 MPa) | 50.00 | 2.90 | 145.00 |
| T7 (seeds + DS4 strain + DS4 strain) | 100.00 | 4.10 | 410.00 |
| T8 (seeds + DS4 strain + DS9 strain + − 0.73 MPa) | 83.33 | 4.10 | 341.65 |
4. Discussion
Drought is a major environmental problem that reduce plant growth and yields, additionally, affect the agricultural and food industry. various ecofriendly methods have been used to support agricultural sustainability (Chiaiese et al., 2018, du Jardin, 2015), including biostimulants. Biostimulants enhance plant defense, increase yield, improve fruit quality, and reduce plant stress (Shukla et al., 2019). Plant Growth-Promoting Bacteria (PGPB) inoculation was shown to enhance stress tolerance, promote plant growth and mitigate the harmful effects of drought (Enebe and Babalola, 2018). PEG 6000 reduces water availability and thus leads to drought stress (Arun K. et al., 2020).Under the drought stress conditions, IAA production decreased (Sandhya et al., 2009). Additionally, phytohormones play a major role in plant development (Sathya et al., 2017) through stimulating plant cell growth and division (Jayakumar et al., 2020). They also enhance lateral root formation and absorptive surface area, thereby improving the acquisition of water by plants (Rajkumar et al., 2017). The difference between the strains in the amount of phytohormones produced can be influenced by the culture conditions, growth stage, and substrate availability (Susilowati et al., 2018).
Siderophores are used as one of the most important survival strategies for microbes because they form complexes with Fe and improve its solubility and uptake under conditions with a lack of iron availability (Rajkumar et al., 2017). Previous study of Arzanesh et al., (2011) analyzed siderophores and their relationship to drought resistance, which found that the strain that produces a higher level of siderophores is associated with excellent drought resistance of the host plants. Under stress conditions, Pseudomonas sp., produced higher EPS levels than under unstressed conditions, suggesting that the formation of EPS in bacteria occurs as a reaction to stress (Ali et al., 2014).
Microbial EPS production under stressed conditions is a form of physiological adaptation enabling microbes to survive. Therefore, the ability to produce EPS by bacterial cells is used as a criterion of drought tolerance in bacteria (Sandhya et al., 2009). Depending on the genus and species of bacteria, the quantity and composition of EPS differ greatly, which also often depends on the environmental conditions.
Proline accumulation is one of the mechanisms involved as a stress response and drought tolerance has been shown to be enhanced in plants with high proline content. Membrane integrity must be maintained under drought stress to avoid protein denaturation; therefore, proline can interact with enzymes to maintain protein structure and activity (Kavi Kishor et al., 2005). In addition to being an excellent osmolyte, proline plays three major roles during stress, namely, as a metal chelator, a signaling molecule, and an antioxidative defense molecule (Hayat et al., 2012). The accumulation of greater amounts of proline upon bacterial inoculation may be due to maintenance of the water potential (Kumari et al., 2016).
SA is a phenolic compound that regulates plant growth, development, and response to biotic and abiotic stresses (Miura and Tada, 2014). Under stressed conditions, SA is involved in the control of important plant physiological processes such as photosynthesis, plant–water relationships, nitrogen metabolism, antioxidant defense system, and glycine betaine development, thus protecting plants against abiotic stresses (Khan et al., 2014).
The large amounts of ROS are produced upon exposure to drought stress, especially hydroxyl radical and single oxygen (1O2) (both of which are highly reactive), causing oxidative damage to many cell components (Cruz De Carvalho, 2008). Antioxidant enzymes such as Polyphenol oxidase (PPO), peroxidase (PO), and catalase (CAT) play important roles in the scavenging of ROS, and it has been found that, as a result of drought, these enzymes are activated in response to stress, indicating that high antioxidant capacity is associated with stress tolerance (Zandalinas et al., 2018). An increase in antioxidant enzyme activity under drought stress plays an important role in fighting stress in plants. Meanwhile, CAT induces the dismutation of H2O2 into water and molecular O2 (Singh et al., 2020). In addition, PO degrades H2O2 by oxidizing co-substrates such as phenolic compounds and/or antioxidants, whereas PPO oxidizes some phenols to quinones (Apel and Hirt, 2004, Demir and Kocaçalişkan, 2001).
The activity of antioxidants is not only critical during acute drought stress, but also interferes with recovery from water limitation and dehydration resuscitation (Laxa et al., 2019).
Previous studies have shown that Bacillus species can be used as a plant growth-promoting bacteria, with the advantages of rapid reproduction, simple nutrition and strong environmental compatibility (Rehman et al., 2019). Also Hung et al., (2020) reported that Bacillus spp., exhibited many plants growth-promoting properties. In addition, Kasim et al., (2016) detected an increase in the biofilm generation of twenty PGPRs under increasing salt concentrations. Under different environmental stress conditions bacterial competition is being increased for nutrients and subsequently, bacteria revert from the planktonic life style to the aggregated or biofilm form to protect the bacterial cells in the rhizosphere from the elevated stress (Qurashi and Sabri, 2012). Additionally, increasing the formation of exopolysaccharides (ES) under stress conditions also supports the generation of biofilms in PGPB.
Biosurfactant is a multifunctional microbial metabolite (chelating/complexion/solubilizing agent), which is environmentally friendly due to its low toxicity and biodegradability (Akladious et al., 2019). Biosurfactant efficiently operates to solubilize and increase the supply of micronutrients and trace metals (Sheng et al., 2008). The biosurfactant production by microbes depends on nutritional and physical conditions (Rodrigues et al., 2006).
The beneficial effects of the tested strains on the growth characteristics could be attributed to the production of PGP substances, as previously confirmed in this study. These results are in harmony with those obtained in previous studies (Chukwuneme et al., 2020, Zahid et al., 2015). Besides inoculating plants with individual strains of bacteria, co-inoculation of two or more strains leads to greater tolerance of drought stress in plants (Wang et al., 2012). Additionally, seeds inoculated with either single strain showed higher values than uninoculated seeds (T1). Generally, the ability of bacteria to improve plant growth under drought stress conditions is due to their osmoregulation, antioxidant activities, and biofilm formation, as mentioned previously in this study, to adapt and protect them from stress damage by avoiding oxidative damage (Bashan and de-Bashan, 2010, Dimkpa et al., 2009).
5. Conclusion
Climate change has already and will increasingly lead to undesirable environmental conditions and greater drought stress in different locations. This can reduce agricultural productivity and result in the loss of arable land. Various approaches have been developed to reduce the effects of drought on crop plants, but these have some potential negative effects in addition to being expensive, so it is necessary to use environmentally friendly and low-cost microbial inoculations that further enhance the growth of drought-tolerant plants through a variety of mechanisms, such as the production of growth-promoting substances, besides the production of certain substances and enzymes to overcome oxidative stress during drought. This study showed that inoculating maize seeds with strain DS4 or DS9 increased the germination rate and seedling length and reduced the harmful effects of drought. Moreover, good results were obtained when the maize seeds were inoculated with a mixture of these two strains, compared with the findings for each strain alone. Hence, there is a need for further evaluation and screening of the efficacy of these drought-tolerant strains and the ability to produce PGP traits in soil–plant systems. Finally, additional studies are needed to understand the interaction between plants and microbes genetically, in order to mitigate drought tolerance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Noha M. Ashry, Email: noha.ashry@fagr.bu.edu.eg.
Mohamed T. El-Saadony, Email: m.talaatelsadony@gmail.com.
References
- Abdelatty A.M., Mandouh M.I., Mohamed S.A., Busato S., Badr O.A.M., Bionaz M., Elolimy A.A., Moustafa M.M.A., Farid O.A.A., Al-Mokaddem A.K. Azolla leaf meal at 5% of the diet improves growth performance, intestinal morphology and p70S6K1 activation, and affects cecal microbiota in broiler chicken. Animal. 2021;15(10):100362. doi: 10.1016/j.animal.2021.100362. [DOI] [PubMed] [Google Scholar]
- Abou-Aly H.E., Youssef A.M., El-Meihy R.M., Tawfik T.A., El-Akshar E.A. Evaluation of heavy metals tolerant bacterial strains as antioxidant agents and plant growth promoters. Biocatal. Agric. Biotechnol. 2019;19:101110. doi: 10.1016/j.bcab.2019.101110. [DOI] [Google Scholar]
- Adnan M., Alshammari E., Ashraf S.A., Patel K., Lad K., Patel M. Physiological and molecular characterization of biosurfactant producing endophytic fungi Xylaria regalis from the cones of Thuja plicata as a potent plant growth promoter with Its potential application. Biomed Res. Int. 2018;2018:1–11. doi: 10.1155/2018/7362148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akladious S.A., Gomaa E.Z., El-Mahdy O.M. Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG - 4) causing root rot in faba bean (Vicia faba) plants. Eur. J. Plant Pathol. 2019;153(4):1237–1257. doi: 10.1007/s10658-018-01639-1. [DOI] [Google Scholar]
- Ali S.Z., Sandhya V., Venkateswar Rao L. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014;64(2):493–502. doi: 10.1007/s13213-013-0680-3. [DOI] [Google Scholar]
- Apel, K., Hirt, H., 2004. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed]
- Arun K., D., Sabarinathan, K.G., Gomathy, M., Kannan, R., Balachandar, D., 2020. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. J. Basic Microbiol. 60, 768–786. https://doi.org/10.1002/jobm.202000011. [DOI] [PubMed]
- Arzanesh M.H., Alikhani H.A., Khavazi K., Rahimian H.A., Miransari M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011;27(2):197–205. doi: 10.1007/s11274-010-0444-1. [DOI] [Google Scholar]
- Auger S., Krin E., Aymerich S., Gohar M. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl. Environ. Microbiol. 2006;72(1):937–941. doi: 10.1128/AEM.72.1.937-941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer R., Rokem J.S., Ilangumaran G., Lamont J., Praslickova D., Ricci E., Subramanian S., Smith D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018;871:1–17. doi: 10.3389/fpls.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan, Y., de-Bashan, L.E., 2010. How the plant growth-promoting bacterium azospirillum promotes plant growth-a critical assessment, 1st ed, Advances in Agronomy. Elsevier Inc. https://doi.org/10.1016/S0065-2113(10)08002-8
- Bloemberg, G. V., Lugtenberg, B.J.J., 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4, 343–350. https://doi.org/10.1016/S1369-5266(00)00183-7. [DOI] [PubMed]
- Carson K.C., Dilworth M.J., Glenn A.R. Siderophore production and iron transport in rhizobium leguminosarvm bv. viciae MNF710. J. Plant Nutr. 1992;15:2203–2220. doi: 10.1080/01904169209364469. [DOI] [Google Scholar]
- Chiaiese P., Corrado G., Colla G., Kyriacou M.C., Rouphael Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018;871:1–6. doi: 10.3389/fpls.2018.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukwuneme C.F., Babalola O.O., Kutu F.R., Ojuederie O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020;15(1):93–105. doi: 10.1080/17429145.2020.1752833. [DOI] [Google Scholar]
- Cohen A.C., Bottini R., Pontin M., Berli F.J., Moreno D., Boccanlandro H., Travaglia C.N., Piccoli P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015;153(1):79–90. doi: 10.1111/ppl.2015.153.issue-110.1111/ppl.12221. [DOI] [PubMed] [Google Scholar]
- Coleman-Derr D., Tringe S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014;5:1–6. doi: 10.3389/fmicb.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008;3(3):156–165. doi: 10.4161/psb:3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir, Y., Kocaçalişkan, I., 2001. Effects of NaCl and proline on polyphenol oxidase activity in bean seedlings. Biol. Plant. https://doi.org/10.1023/A:1013715425310.
- Desoky E.S.M., Saad A.M., El-Saadony M.T., Merwad A.R.M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatalysis and Agricultural Biotechnology. 2020;30:101878. [Google Scholar]
- Dimkpa C., Weinand T., Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- du Jardin P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam) 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- Enebe M.C., Babalola O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl. Microbiol. Biotechnol. 2018;102(18):7821–7835. doi: 10.1007/s00253-018-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmael A., Azab E., Gobouri A.A., Nasr-eldin M.A., Moustafa M.M.A., Mohamed S.A., Badr O.A.M., Abdelatty A.M. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant salmonella typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms. 2021;9:1–19. doi: 10.3390/microorganisms9020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J.E., Maroniche G., Creus C., Suárez-Rodríguez R., Ramirez-Trujillo J.A., Groppa M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017;202:21–29. doi: 10.1016/j.micres.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Gusmiaty, Restu M., Bachtiar B., Larekeng SH. Gibberellin and IAA Production by Rhizobacteria from Various Private Forest. IOP Conf. Ser. Earth Environ. Sci. 2019;270(1):012018. doi: 10.1088/1755-1315/270/1/012018. [DOI] [Google Scholar]
- Habibi G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012;56:57–63. [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012;7(11):1456–1466. doi: 10.4161/psb:21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Zhao Y., Fan L., Jin Q., Yang G., Xu Z. Improvement of manganese phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere. 2020;260:127614. doi: 10.1016/j.chemosphere.2020.127614. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Padmakumar P., Nair I.C., Radhakrishnan E.K. Drought tolerant bacterial endophytes with potential plant probiotic effects from Ananas comosus. Biologia (Bratisl). 2020;75(10):1769–1778. doi: 10.2478/s11756-020-00483-1. [DOI] [Google Scholar]
- Jongdee B., Pantuwan G., Fukai S., Fischer K. Improving drought tolerance in rainfed lowland rice: An example from Thailand. Agric. Water Manag. 2006;80(1-3):225–240. doi: 10.1016/j.agwat.2005.07.015. [DOI] [Google Scholar]
- Kang S.-M., Khan A.L., Waqas M., You Y.-H., Kim J.-H., Kim J.-G., Hamayun M., Lee I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014;9(1):673–682. doi: 10.1080/17429145.2014.894587. [DOI] [Google Scholar]
- Kasim W.A., Gaafar R.M., Abou-Ali R.M., Omar M.N., Hewait H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016;61(2):217–227. doi: 10.1016/j.aoas.2016.07.003. [DOI] [Google Scholar]
- Kavi Kishor P.B., Sangam S., Amrutha R.N., Sri Laxmi P., Naidu K.R., Rao K.R.S.S., Rao S., Reddy K.J., Theriappan P., Sreenivasulu N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005;88:424–438. [Google Scholar]
- Kerchev P., van der Meer T., Sujeeth N., Verlee A., Stevens C.V., Van Breusegem F., Gechev T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020;40:107503. doi: 10.1016/j.biotechadv.2019.107503. [DOI] [PubMed] [Google Scholar]
- Khan M.I.R., Asgher M., Khan N.A. Plant Physiology and Biochemistry Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol. Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K., 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Kumari, S., Vaishnav, A., Jain, S., Varma, A., Choudhary, D.K., 2016. Induced drought tolerance through wild and mutant bacterial strain Pseudomonas simiae in mung bean (Vigna radiata L.). World J. Microbiol. Biotechnol. 32, 1–10. https://doi.org/10.1007/s11274-015-1974-3. [DOI] [PubMed]
- Lakshmi Sahitya, U., Krishna, M.S.R., Sri Deepthi, R., Shiva Prasad, G., Peda Kasim, D., 2018. Seed Antioxidants Interplay with Drought Stress Tolerance Indices in Chilli (Capsicum annuum L.) Seedlings. Biomed Res. Int. 2018. https://doi.org/10.1155/2018/1605096. [DOI] [PMC free article] [PubMed]
- Laxa M., Liebthal M., Telman W., Chibani K., Dietz K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants. 2019;8(4):94. doi: 10.3390/antiox8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A., Van Breusegem F. Reactive oxygen species in plant development. Dev. 2018;145 doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014;5:4–9. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem H., Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014;9(1):689–701. doi: 10.1080/17429145.2014.902125. [DOI] [Google Scholar]
- Ndeddy Aka R.J., Babalola O.O. Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Bioremediat. J. 2017;21(1):1–19. doi: 10.1080/10889868.2017.1282933. [DOI] [Google Scholar]
- Oktay K., Schenken R.S., Nelson J.F. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol. Reprod. 1995;53:295–301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- Onsa G.H., bin Saari N., Selamat J., Bakar J. Purification and characterization of membrane-bound peroxidases from Metroxylon sagu. Food Chem. 2004;85(3):365–376. doi: 10.1016/j.foodchem.2003.07.013. [DOI] [Google Scholar]
- Ozturk S., Aslim B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2010;17(3):595–602. doi: 10.1007/s11356-009-0233-2. [DOI] [PubMed] [Google Scholar]
- Paul, K., Sorrentino, M., Lucini, L., Rouphael, Y., Cardarelli, M., Bonini, P., Reynaud, H., Canaguier, R., Trtílek, M., Panzarová, K., Colla, G., 2019. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 10, 1–17. https://doi.org/10.3389/fpls.2019.00047. [DOI] [PMC free article] [PubMed]
- Philippot L., Raaijmakers J.M., Lemanceau P., van der Putten W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013;11(11):789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- Polo J., Mata P. Evaluation of a biostimulant (Pepton) based in enzymatic hydrolyzed animal protein in comparison to seaweed extracts on root development, vegetative growth, flowering, and yield of gold cherry tomatoes grown under low stress ambient field conditions. Front. Plant Sci. 2018;8:1–8. doi: 10.3389/fpls.2017.02261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurashi A.W., Sabri A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Brazilian J. Microbiol. 2012;43:1183–1191. doi: 10.1590/S1517-83822012000300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar M., Bruno L.B., Banu J.R. Alleviation of environmental stress in plants: The role of beneficial Pseudomonas spp. Crit. Rev. Environ. Sci. Technol. 2017;47(6):372–407. doi: 10.1080/10643389.2017.1318619. [DOI] [Google Scholar]
- Rehman B., Hassan T.U., Bano A. Potential of indole-3-acetic acid-producing rhizobacteria to resist Pb toxicity in polluted soil. Soil Sediment Contam. 2019;28(1):101–121. doi: 10.1080/15320383.2018.1539947. [DOI] [Google Scholar]
- Rezayian M., Niknam V., Ebrahimzadeh H. Effects of drought stress on the seedling growth, development, and metabolic activity in different cultivars of canola. Soil Sci. Plant Nutr. 2018;64(3):360–369. doi: 10.1080/00380768.2018.1436407. [DOI] [Google Scholar]
- Rincon A., Valladares F., Gimeno T.E., Pueyo J.J. Water stress responses of two Mediterranean tree species influenced by native soil microorganisms and inoculation with a plant growth promoting rhizobacterium. Tree Physiol. 2008;28(11):1693–1701. doi: 10.1093/treephys/28.11.1693. [DOI] [PubMed] [Google Scholar]
- Rodrigues L., Banat I.M., Teixeira J., Oliveira R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006;57:609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- Sandhya, V., Z., A.S., Grover, M., Reddy, G., Venkateswarlu, B., 2009. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol. Fertil. Soils 46, 17–26. https://doi.org/10.1007/s00374-009-0401-z.
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT. 2021;148:11668. [Google Scholar]
- Sathya A., Vijayabharathi R., Gopalakrishnan S. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3. Biotech. 2017;7:1–10. doi: 10.1007/s13205-017-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X., He L., Wang Q., Ye H., Jiang C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008;155(1-2):17–22. doi: 10.1016/j.jhazmat.2007.10.107. [DOI] [PubMed] [Google Scholar]
- Shukla P.S., Mantin E.G., Adil M., Bajpai S., Critchley A.T., Prithiviraj B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019;10:1–22. doi: 10.3389/fpls.2019.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Kaur S., Kumar A. In vitro drought tolerance in selected elite clones of Eucalyptus tereticornis Sm. Acta Physiol. Plant. 2020;42:1–9. doi: 10.1007/s11738-019-3009-4. [DOI] [Google Scholar]
- SINGH R., GLICK B.R., RATHORE D. Biosurfactants as a biological tool to increase micronutrient availability in soil: A Review. Pedosphere. 2018;28(2):170–189. doi: 10.1016/S1002-0160(18)60018-9. [DOI] [Google Scholar]
- Susilowati A., Puspita A.A., Yunus A. Drought resistant of bacteria producing exopolysaccharide and IAA in rhizosphere of soybean plant (Glycine max) in Wonogiri Regency Central Java Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018;142:012058. doi: 10.1088/1755-1315/142/1/012058. [DOI] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143(4):606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Vardharajula, S., Sk Z, A., 2014. Exopolysaccharide production by drought tolerant Bacillus spp. and effect on soil aggregation under drought stress. J. Microbiol. Biotechnol. Food Sci. 4, 51–57. https://doi.org/10.15414/jmbfs.2014.4.1.51-57.
- Vílchez J.I., García-Fontana C., Román-Naranjo D., González-López J., Manzanera M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016;7:1–11. doi: 10.3389/fmicb.2016.01577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C.J., Yang, W., Wang, C., Gu, C., Niu, D.D., Liu, H.X., Wang, Y.P., Guo, J.H., 2012. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS One 7, 1–10. https://doi.org/10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed]
- Wang S., Ouyang L., Ju X., Zhang L., Zhang Q., Li Y. Survey of Plant Drought-Resistance Promoting Bacteria from Populus euphratica Tree Living in Arid Area. Indian J. Microbiol. 2014;54(4):419–426. doi: 10.1007/s12088-014-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid M., Kaleem Abbasi M., Hameed S., Rahim N. Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.) Front. Microbiol. 2015;6:1–11. doi: 10.3389/fmicb.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas S.I., Mittler R., Balfagón D., Arbona V., Gómez‐Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018;162(1):2–12. doi: 10.1111/ppl.2018.162.issue-110.1111/ppl.12540. [DOI] [PubMed] [Google Scholar]