Abstract
The Hepatitis B virus (HBV) infection is one of the most widespread viral infections of humans. HBV causes acute and chronic hepatitis. Chronic hepatitis leads to hepatocellular carcinoma, which is a significant cause of death. DNA-based immunization programs to control the spread of Hepatitis B in developing countries are costly and require special storage and transportation. The alternative way is to express Hepatitis B surface antigen (HBsAg) in plants to develop oral vaccines. In this study, HBsAg gene was isolated, cloned, and then transformed in tomato plants. The transgenic tomato plants were confirmed through RT-qPCR. HBsAg expression was analysed in mature green and red stages of tomato fruit through quantitative real-time PCR. It was observed that expression of HBsAg was high in matured red tomato as compared to mature green. The present study is the first step to developing Solanum lycopersicum as an edible vaccine production system in this world region.
Keywords: Hepatitis B surface antigen, Tomato, Agrobacterium-mediated transformation, Edible vaccines
1. Introduction
Hepatitis B virus (HBV) is a double-stranded DNA virus that belongs to Hepadnaviridae family (Rehermann and Nascimbeni, 2005). The genome of HBV virus is 3.2 kb in length, and it is the smallest among DNA viruses (Kim et al., 2016). There are ten genotypes, and these genotypes have different geographical distributions (Sunbul, 2014). The present HBV epidemic results from introducing and disseminating new subgenotypes from main A, D, and F genotypes over a long period. Genotypes A and D are mostly found in Africa, Europe, and India; B and C in Asia; genotype E is restricted to West Africa, and genotype F prevalence is in Central and South America. The lipid envelope of infection virion contains hepatitis B surface antigen surrounded by an inner nucleocapsid composed of hepatitis B core antigen. (Gerlich and Robinson 1980).
Hepatitis B virus is the leading cause of acute and chronic liver disorders, and its infection is a serious global health issue (Madihi et al., 2020). About 40% of chronic hepatitis cases lead to hepatocellular carcinoma, the second major cause of death. Deaths due to liver cirrhosis and hepatocellular carcinoma are 1.34 million per year (WHO, 2018).
Although effective vaccines are available against hepatitis B infection, it is still a major health issue in the developing world (Kumar et al., 2007). The current immunization programs of HBV are based on recombinant vaccines derived from the costly microbial fermentation process and mammalian cell cultures. Furthermore, these vaccines require well-developed infrastructure to keep them in cold-chain throughout their manufacturing process, transportation, and storage which is not affordable for developing countries. In contrast, plant-based vaccines can be produced at a much lower cost and are independent of cold storage requirements (Penney et al., 2011). Molecular farming in plants has many advantages over existing expression systems as it does not require skilled labor and expensive equipment (Fischer and Emans, 2000, Giddings et al., 2000, Julian et al., 2003, Twyman et al., 2003). The production cost of recombinant proteins in a plant expression system is 2–10% of the cost of microbial fermentation and 0.1% of the cost of mammalian cells or transgenic animals (Molowa et al., 2002). Plants-based vaccines prefer mammalian expression systems as no external carbon source is needed for plants because they are fueled by photosynthesis. Plants-based vaccines are free of contamination by a mammalian pathogen, another critical advantage of a plant vaccine. Because of these advantages, the production of antigens, vaccines, and other eukaryotic proteins in plants is more attractive (Gunasekaran and Gothandam 2020).
The production of edible vaccines based on transgenic plants is one of the positive directions in new types of vaccines. HBsAg gene has already been expressed in tobacco (Mason et al., 1992, Thanavala et al., 1995, Sunil Kumar et al., 2003), potato (Richter et al., 2000), banana (Elkholy et al., 2009), tomato (Salyaev et al., 2007, Srinivas et al., 2008) and other fruits and vegetables (Marcondes and Hansen, 2008, Pniewski et al., 2006).
The above research works identified the potential use of HbsAg gene in plant systems to develop edible vaccines. In the present project, the same technology employed in the local registered variety of tomatoes will not pose any future intellectual property rights issues. At present, tomato is an important crop plant cultivated worldwide, and its production and consumption continue to increase. Tomato is also a major source of essential nutrients. Most importantly, the tomato's successful molecular farming model has been established (Srinivas et al., 2008, Fisher and Schillberg, ,2004). In Pakistan, tomato cultivation and consumption have increased in fresh and processed forms, hence selected for the present study.
2. Materials and methods
2.1. Isolation and cloning of HBsAg gene
HBsAg gene, complete cds having gene bank accession number AY738913.1 was retrieved from nucleotide database and was analyzed for restriction sites through BioEdit software. Unique BamH1 and Sac1 enzymes were selected to clone the HBsAg gene under 35S promoter in pBI121. Full-length primers with the above unique restriction sites were designed. The forward primer was added with BamH1 site beside 5′-CG-3′ protective bases, while the reverse primer was added with Sac1 restriction site beside 5′-C-3′ protective base. The sequence of forwarding primer was 5′-CGGGATCCATGGAGAACATCACATCAGGA-3′, and the reverse primer was 5′-CGAGCTCTTAAATGTATACCCAAAGACA-3′. DNA from an infected person was isolated using DNA purification kit (Thermo Scientific, #K0721). HBsAg gene was amplified from 100 ng of DNA using phusion high fidelity DNA polymerase (Thermo Scientific, #F530L). The amplified product was purified using GeneJET PCR Purification Kit (Thermo Scientific, #K0701). A 1000 ng of the purified product was digested with BamH1 (Thermo Scientific, #ER0051) and Sac1 (Thermo Scientific, #ER1131) enzymes. Similarly, pBI121 was also digested with BamH1 and Sac1 enzymes. The restricted backbone of pBI121 was gel-eluted using GeneJET Gel Extraction Kit (Thermo Scientific, #K0691). A ligation reaction was performed using T4 DNA Ligase (New England Biolabs, #M0202). The ligated product was transformed into DH5α through electroporation. PCR was carried out from plasmids extracted from transformed colonies for confirmation of clone. Further confirmation of clone was carried out through sequencing. The resultant pBI121:HBsAg was transformed and confirmed in Agrobacterium tumefaciens strain GV3101pMP90 (2nd Lab China #AC1001).
2.2. Genetic transformation of tomato
The Rio Grande tomato variety seeds were surface-sterilized initially with 70% ethanol for 1 min and then with Clorox (5.25% sodium hypochlorite) for 10 min. The seeds were then rinsed three times with distilled water to remove the traces of clorox and then sterilized. For seven days, the seeds were germinated on MS0 medium at 25 ± 2 °C under a 16/8 light and dark. Cotyledonary leaves were cut from seven days old seedlings and were used for infection by transformed Agrobacterium tumefaciens. Agrobacterium suspension was prepared by inoculating a single colony in 25 ml of liquid LB media containing 50 mg/l kanamycin and 25 mg/l rifampicin and incubated for 36 h in the dark at 28 °C while shaking at 170 rpm. The 36 h grown culture was centrifuged at 4000 rpm for 5 min, and the supernatant was discarded. The pellet of the cell was suspended in inoculation media, and OD660 was adjusted to 0.2. All media compositions are given in Table 1. This Agrobacterium suspension was added to cotyledonary leaves and placed on a shaker at 50 rpm for 20 min. The cotyledonary leaves were filter dried and placed on co-cultivation media for two days. The cotyledonary leaves were shifted to pre-selection media after rinsing three times with autoclaved distilled water and a final wash with inoculation media containing 500 mg/l cefotaxime for 15 min. After 07 days, cotyledonary leaves were shifted to selection media, allowing explants to regenerate shoots for 45 days in 16/8 light/dark at 25 °C ± 2 °C. Passaging was regularly done after 15 days. Shoots having a length of 5 cm were placed on rooting media. Well-established roots were developed after 21 days. The plantlets with well-established roots were acclimatized with ambient temperature and humidity conditions in the growth room and then transplanted in pots containing soil.
2.3. Molecular analysis of transgenic plants
DNA was extracted from T0 transformed and non-transformed plants using GeneJET Plant Genomic DNA Purification Kit (Thermo Scientific #K0791). T0 transformed plants were analyzed for transgene using PCR. The sequences of full-length primers used in PCR were 5′-ATGGAGAACATCACATCAGGA-3′ and 5′-TTAAATGTATACCCAAAGACA-3′.
For transcript analysis, total RNA was extracted from mature red tomato fruits of T1 plants using GeneJET RNA Purification Kit (Thermo Scientific, #K0731) as described previously (Ibrahim et al. 2021). Nanodrop was used to measure the quality and quantity of RNA. A260/A280 value was kept above 1.8 while A260/A230 value was maintained above 2. About 500 ng high quality of RNA was transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, #K1622). 2 µl of cDNA was used as a template to amplify the HBsAg gene using gene-specific 5′-GAACCTGCACGACTCCTGCT-3′ and 5′-GAGCCAGGAGAAACGGGCTGA-3′ primers. The amplified products were analyzed by a gel documentation system (Bio-Rad, USA).
Quantitative Real-Time PCR was also carried out to check the relative expression of HBsAg gene in mature green and mature red stages of tomato fruits using FastStart Universal SYBR Green Master (Rox) (Roche, USA). Tomato LeEF-1 having gene bank accession numberX14449.1was used as an endogenous reference for normalization in the experiment. The sequence of forwarding primer of endogenous control was 5′-CTCTCAGGCTGACTGTGCTG-3′, and of reverse, primer was 5′-GTTCACGGGTCTGACCATCT-3′.
Gene-specific primers were designed using primer3 free online software to be used in quantitative PCR. A free edition of Beacon Designer software evaluated the integrity, specificity, and quality of primers. Cross-dimers, self-dimers, and hairpin structures were avoided. Matches of 3 bp at three ends of primers were also avoided. The quality of the amplicon structure was also evaluated by mFold software. Following a previous protocol (Thornton and Basu, 2011), Real-Time PCR primers were designed. PCR master mix was prepared by mixing 12.5 µl FastStart Universal SYBR Green Master (ROX), 0.5 µl each of forward and reverse primer (15 µM), 400 ng of template cDNA to the final volume of 25 µl. The PCR profile was set at 95 °C for 10 min for initial activation of FastStart Taq DNA polymerase and then 40 cycles of 95 °C for15 seconds, 60 °C for 1 min, and a final melt curve analysis of 60 to 95 °C for 1 min. The data was recorded as relative quantity.
3. Results
3.1. Isolation and cloning of HBsAg gene
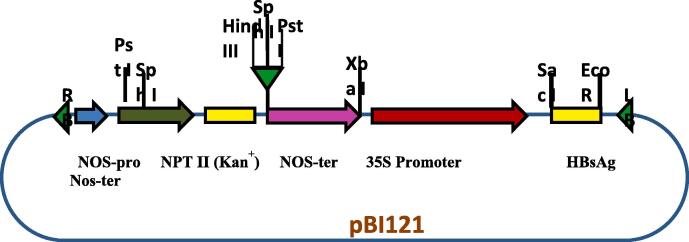
The 696 bp fragment containing 681 bp HBsAg gene was amplified with primers containing BamH1 and Sac1 restriction sites (Fig. 1a). The amplified product was purified, and 1 µg of the purified product was digested with BamH1 and Sac1 simultaneously. Similarly, pBI121 was also digested with BamH1 and Sac1 enzymes, releasing a larger fragment containing the backbone of pBI121 (Fig. 1b). A ligation reaction was performed between pBI121 backbone and inserted containing HBsAg gene. The clone was confirmed through PCR using full-length primers, which produced 681 bp fragments, indicating that HBsAg gene has been successfully cloned into pBI121 under 35S promoter. The clone is named as pBI121:HBsAg. The physical map of pBI121:HBsAg was shown in Fig. 2.
Fig. 1.
Different steps in isolation and cloning of HBsAg gene. M, 1 kb DNA ladder; lane 1 and 2, 696 bp fragments amplified from DNA extracted from the blood of HBV patient. An Agarose gel electrophoresis of amplified HBsAg gene from DNA. b, restriction of pBI121 with BamH1 and Sac1 restriction enzymes. M, 1 kb DNA ladder; lane 1, pBI121 digestion produced two fragments, upper band is the backbone of pBI121, which was gel eluted. c, confirmation of pBI121:HBsAg through PCR. M, 1 kb DNA ladder; Lane 1–6, 681 bp fragment amplified from plasmids extracted from transformed colonies; lane 7, water control; lane 8, plasmid control.
Fig. 2.
Physical map of resultant pBI121:HBsAg .
3.2. Genetic transformation of tomato
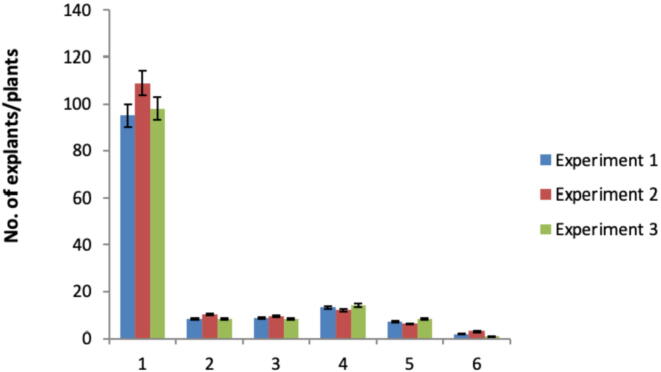
About 302 cotyledonary leaves of Solanum lycopersicum L.cv. Rio Grande were transformed with A. tumefaciens having pBI121:HBsAg in three different experiments. Of 302 cotyledonary leaves, only 26 cotyledonary leaves could survive on selection media and regenerated plants with an average transformation efficiency of 8.58%. The 39 well-rooted plants were produced on rooting media. Ou of it twenty-one plants acclimatized to ambient temperature and humidity conditions (Fig. 3). Six plants went through all stages of growth and development and produced enough seeds for characterization (Table 2). The different steps involved in the transformation of tomato through Agrobacterium are shown in Fig. 4.
Fig. 3.
Summary of transformation experiments. 1: Total explants used, 2: kanamycin-resistant explants, 3: Transformation efficiency (%), 4: Rooted plantlets, 5: Acclimatized 6: Plants produced seeds.
Fig 4.
Regeneration and shoot induction from transformed cotyledonary leaves. a, Transformed cotyledonary leaves; b, Shoot induction from transformed cotyledonary leaves on selection media; c, shoot elongation on selection media.
3.3. Molecular analysis of transgenic plants
The DNA template from transgenic plants gave amplification of 681 bp fragment while no such amplification was observed from DNA of control plant (Fig. 5a). Transgene expression was detected by Reverse transcriptase PCR (RT-PCR) analysis which amplified 155 bp fragments from transgenic plants (Fig. 5b).
Fig 5.
Confirmation and expression of the transgene in transgenic plants. a. PCR confirmation of transgene. M, 1 Kb DNA ladder; lane 1–6, amplification of 681 bp fragment from transgenic plants; lane 7, control plant; lane 8, water control; lane 9, plasmid control. b. expression of the transgene. M, 1 Kb DNA ladder; lane 1–6, amplification of 155 bp fragment from transgenic plants; lane 7, control plant; lane 8, water control; lane 9, plasmid control.
3.4. Expression analysis of HBsAgin tomato fruits
The quantitative expression of the HBsAg gene was measured in mature green and mature red stages of tomato fruits. Mature red tomato showed higher HBsAg gene expression in all events than mature green (Fig. 6). Expression of HBsAg gene was 2.47 to 3.6 fold higher in mature red tomatoes compared to mature green. Maximum fold difference was observed in event 6, and minimum fold difference was observed in event 1 (Table 3).
Fig. 6.
Expression analysis of HBsAg gene in fruits of different tomato events. In every event, the expression is higher in the mature red stage.
4. Discussion
Hepatitis B virus (HBV) is becoming a major threat to the whole world. Current immunization programs are based on recombinant vaccines derived from microbial fermentation and mammalian cell cultures. Using mammalian and yeast expression systems for the production of HBV vaccines requires expert personnel and excellent infrastructure. These vaccines are expensive, require a specific purification system, cold storage, and a unique transportation facility. Developing countries have limited facilities and resources to develop and sustain such recombinant vaccines to immunize its population. An attractive alternative to these is plant-based edible vaccines which are cost-effective and most suitable for developing countries (Twyman et al., 2003). Plant-based vaccine production seems to be effective and less laborious (Baesi et al., 2011).
The surface protein of the hepatitis B (HBsAg) virus is immunogenic and varies from 22 nm to 42 nm in diameter. These surface antigens have been used to develop vaccines against the hepatitis B virus. Hepatitis B surface antigen proved itself as a candidate region of the virus to be efficiently used in vaccine development (Arakawa et al., 1998). HBsAg is the first viral antigen that has been expressed in potatoes and bananas (Mason et al., 1992). The HBsAg protein assembles in plant tissues similar to those found in the commercial vaccine and blood of infected humans (Mason et al., 1992).
In the present investigation, a gene encoding the same hepatitis B surface antigen was isolated and transformed in the tomato plant. Its expression level was analyzed in the mature green and mature red stages of tomato fruit. Ripe red tomato showed higher expression of HBsAg gene in all events compared to mature green. Transcript expression of the HBsAg gene was 2.47 to 3.6 fold higher in mature red tomatoes compared to mature green. Ming Lou et al., 2007 also found enhanced expression of the HBsAg gene in mature red tomato fruits compared to unripe fruits and leaf tissues. In the present study, the expression of HbsAg was carried out by CaMV35S promoter, and this promoter is considered suitable for most dicot plants (Rajabi-Memari et al., 2006) while Lou et al., 2007 used fruit specific promoter to drive the expression of HbsAg.
Thanavala et al. (2005) expressed hepatitis B surface antigen (HBsAg) in potatoes, and they recommended that plant-based edible vaccine should be considered an essential part of global immunization. Elkholy et al. 2009 expressed HBsAg in bananas, and its expression was measured by western blot. Their study also concluded the feasibility of the expression of HBsAg in plants. Transgenic lettuce (Lactuca sativa L.) expressing hepatitis B virus surface antigen was found immunogenic in mice and human volunteers (Kapusta et al., 1999).
Different proteins, including immunogenic, have been expressed in the plant system and orally administrated to humans. Transgenic lettuce and potato expressing HBsAg have been found to elicit an immune response in humans (Chikwamba et al., 2002, Mason et al., 1992). Plant selection should be made according to the eating habits of the targeted population and the agricultural infrastructure of that region. Keeping in view the production and consumption of tomatoes as fresh and processed food in Pakistan, it has been selected to express HBsAg. Six transgenic events showed successful expression of the HBsAg gene. It is proposed that in future studies, the immunogenicity and biosafety of the different transformed events will be compared using mice as an experimental organism. The present study was the first step in Pakistan to develop tomatoes as an edible vaccine production system in this world region.
5. Conclusions
An efficient transformation system was developed in tomatoes to produce edible vaccines against the hepatitis B virus. The results of this work recommended that plants can be used to produce immunogenic proteins against different diseases. This research concluded that tomato is a suitable candidate for the production of vaccines as it can be easily transformed, produced rapidly, and eaten raw. Transgenic plants expressing different antigens have been done successfully, but still, many questions remain unsolved. So further work needs to be done in the future, such as dose optimization and checking the antigen's immunogenicity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors (SM and FAM) express their sincere appreciation to the Researchers Supporting Project Number (RSP 2021/24) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.11.012.
Contributor Information
Shahid Mahboob, Email: mushahid@ksu.edu.sa.
Ghulam Muhammad Ali, Email: drgmali@yahoo.ca.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Arakawa T., Chong D.K., Langridge W.H. Effificacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 1998;16:292–297. doi: 10.1038/nbt0398-292. [DOI] [PubMed] [Google Scholar]
- Baesi M., Nabati A.D., Rajabi-Memari H., Siahpoosh M.R., Abdollahi M.R., Jaberolansar N. Cloning and transformation of hepatitis B surface antigen(HBsAg) gene to tomato (Lycopersicon esculentum Mill.) Jundishapur J. Nat. Pharm. Prod. 2011;6:32–41. [Google Scholar]
- Chikwamba R., Cunnick J., Hathaway D., McMurry J., Mason H., Wang K. A functional antigen in a practical crop: LT-B producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT) Transgenic Res. 2002;11:479–493. doi: 10.1023/a:1020393426750. [DOI] [PubMed] [Google Scholar]
- Elkholy F.S., Ismail R.M., Bahieldin A., Sadik A.S., Madkour M.A. Expression of Hepatitis B surface Antigen (HBsAg) gene in transgenic banana (Musa Sp.) Arab J. Biotech. 2009;12:291–302. [Google Scholar]
- Fischer R., Emans N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000;9:279–299. doi: 10.1023/a:1008975123362. [DOI] [PubMed] [Google Scholar]
- Fisher, R., Schillberg, S.,2004. Molecular Farming: Plant-made Pharmaceuticals and Technical Proteins,Fisher, R.; Schillberg, S.;Eds.; Weinheim: Wiley-VCH Verlag GmbH & Co: Weinheim Germany.
- Gerlich W., Robinson W.S. Hepatitis B virus contains protein attached to the 5′ end of its complete strand. Cell. 1980;21:801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Giddings G., Allison G., Brooks D., Carter A. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 2000;18(11):1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- Gunasekaran B., Gothandam K.M. A review on edible vaccines and their prospects. Braz. J. of Med. Biol. Res. 2020;53:1–2. doi: 10.1590/1414-431X20198749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S., Saleem B., Rehman N., Zafar S.A., Naeem M.K., Khan M.R. CRISPR/Cas9 mediated disruption of Inositol Pentakisphosphate 2-Kinase 1 (TaIPK1) reduces phytic acid and improves iron and zinc accumulation in wheat grains. J. Adv. Res. 2021 doi: 10.1016/j.jare.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.-C., Drake P.M.W., Christou P. The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003;4(10):794–805. doi: 10.1038/nrg1177. [DOI] [PubMed] [Google Scholar]
- Kapusta, J.,Modelska, A.,Figlerowicz,M., Pniewski,T., Letellier, M.,Lisowa, O.,Yusibov,V.,Koprowski,H.,Plucienniczak, A., Legocki, A. B.A.,1999. Plant-Derived Edible Vaccine Against Hepatitis b Virus. F.A.S.E.B.13,1796-1798. [DOI] [PubMed]
- Kim H., Lee S.A., Kim B.J.X. region mutations of hepatitis B virus related to clinical severity. World J. Gastroenterol. 2016;22:5467–5478. doi: 10.3748/wjg.v22.i24.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G.B.S., Ganapathi T.R., Bapat V.A. Production of hepatitis B surface antigen in recombinant plant systems: an update. Biotechnol. Prog. 2007;23(3):532–539. doi: 10.1021/bp0602754. [DOI] [PubMed] [Google Scholar]
- Sunil Kumar G.B., Ganapathi T.R., Revathi C.J., Prasad K.S.N., Bapat V.A. Expression of hepatitis B surface antigen in tobacco cell suspension cultures. Protein Expres. Purif. 2003;32(1):10–17. doi: 10.1016/j.pep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Lou X.M., Yao Q.H., Zhang Z., Peng R.H., Xiong A.S., Wang H.K. Expression of the Human Hepatitis B Virus Large Surface Antigen Gene in Transgenic Tomato. Clin. Vaccine Immunol. 2007;14:464–469. doi: 10.1128/CVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madihi S., Syed H., Lazar F., Zyad A., Benani A. A Systematic Review of the Current Hepatitis B Viral Infection and Hepatocellular Carcinoma Situation in Mediterranean Countries. Bio. Med. Res. Int. 2020;2020:1–16. doi: 10.1155/2020/7027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes J., Hansen E. Transgenic lettuce seedlings carrying hepatitis B virus antigen HBsAg. Braz. J. Infect. Dis. 2008;12(6):469–471. doi: 10.1590/s1413-86702008000600004. [DOI] [PubMed] [Google Scholar]
- Mason H.S., Lam D.M.K., Arntzen C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Nat. Acad. Sci. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molowa,D.,Shenouda, M., Meyers, A.,Tublin, P., Fein,A.,2002. The state of biologics manufacturing: part 2. JP Morgan, editor. Market analysis of the state of manufacturing of biologics including mAbs. 1-16.
- Penney C.A., Thomas D.R., Deen S.S., Walmsley A.M. Plant-made vaccines in support of the millennium development goals. Plant Cell Rep. 2011;30(5):789–798. doi: 10.1007/s00299-010-0995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pniewski T., Kapusta J., Płucienniczak A. Agrobacterium-mediated transformation of yellow lupin to generate callus tissue producing HBV surface antigen in a long-term culture. J. Appl. Genet. 2006;47(4):309–318. doi: 10.1007/BF03194640. [DOI] [PubMed] [Google Scholar]
- Rajabi-Memari H., Jalali-Javaran M., Rasaee M.J., Rahbarizadeh F., Forouzandeh-Moghadam M., Esmaili A. Expression and Characterization of a Recombinant Single-Domain Monoclonal Antibody Against MUC1 Mucin in Tobacco Plants. Hybridoma. 2006;25(4):209–215. doi: 10.1089/hyb.2006.25.209. [DOI] [PubMed] [Google Scholar]
- Rehermann B., Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Richter L.J., Thanavala Y., Arntzen C.J., Mason H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000;18(11):1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- Salyaev R.K., Rekoslavskaya N.I., Stolbikov A.S., Hammond R.W., Shchelkunov S.N. Synthesis of Hepatitis B Virus Surface Antigen in tomato plants transgenic for the preS2-S gene. Dokl. Biochem. Biophys. 2007;416(1):290–293. doi: 10.1134/s1607672907050171. [DOI] [PubMed] [Google Scholar]
- Srinivas L., Sunil Kumar G.B., Ganapathi T.R., Revathi C.J., Bapat V.A. Transient and stable expression of hepatitis B surface antigen in tomato (Lycopersicon esculentum L.) Plant Biotechnol. 2008;2(1):1–6. [Google Scholar]
- Sunbul Mustafa. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014;20(18):5427. doi: 10.3748/wjg.v20.i18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N., Goodwin P., Arntzen C.J., Mason H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. 2005;102(9):3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanavala Y., Yang Y.F., Lyons P., Mason H.S., Arntzen C. Immunogenicity of transgenic plant derived hepatitis B surface antigen. Proc. Natl. Acad. Sci. 1995;92(8):3358–3361. doi: 10.1073/pnas.92.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton B., Basu C. Real-Time PCR (qPCR) Primer Design Using Free Online Software. Biochem. Mol. Biol. Educ. 2011;39(2):145–154. doi: 10.1002/bmb.20461. [DOI] [PubMed] [Google Scholar]
- Twyman R.M., Stoger E., Schillberg S., Christou P., Fischer R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol. 2003;21(12):570–578. doi: 10.1016/j.tibtech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- WHO,World Hepatitis Day . World HealthOrganization,2018, https://www.who.int/campaigns/world-hepatitis-day
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.