Abstract
This study was aimed to determine the neuroprotective influence of Stellaria media in terms of restoring normal state of the rat’s hippocampus and cortex after oxidative insult caused by in vitro ischemia and reperfusion. Cell viability and membrane integrity were assessed using MTT and lactate dehydrogenase (LDH) assay, respectively. Ischemic insult was introduced in the rat brain’s hippocampal and cortical slivers by exposing oxygen and glucose deficiency (OGD) for 2 h, followed by 1 h of re-perfusion. Cellular oxidative stress levels were quantified by incorporating 2ʹ,7ʹ-dichlorofluorescein diacetate fluorescent probes. Additionally, the lipid peroxidation was assessed using TBARS assay. Findings revealed significant neuroprotection against OGD-induced mitochondrial impairment at 40 µg/mL of S. media in rat’s hippocampal and cortical slices. The LDH levels were decreased significantly (P < 0.001) during pre-incubation and reoxygenation periods using varied concentrations of S. media extract. Cellular oxidative stress levels results showed significant (P < 0.001) reduction in dichlorofluorescein fluorescence in slices homogenate of hippocampus and cortex using S. media extract. The lipid peroxidation assay results showed decreased (P < 0.01) levels of malondialdehyde in liver tissues of treated rats treated (200 mg/kg body weight) when compared to the ischemic animal. In summary, findings clearly indicated the neuroprotective effects of extract against in vitro ischemia in brain hippocampal and cortex slivers. S. media could undoubtedly be utilized as a healing agent in preventing neuronal cells’ loss during is chemic-reperfusion process.
Keywords: Ischemic stroke, LDH, Lipid peroxidation, S. media, Neuroprotection, OGD
1. Introduction
Ischemic stroke is the second most common cause of deaths in the world which often occurs due to a blockage in the artery that supplies blood to the brain. Sometimes, it may cause permanent disability in the affected individuals (Chugh, 2019). The ischemic events are supposed to be lethal because they are generally associated with the massive depletion of oxygen and glucose that leads to the neuronal death (Donkor, 2018). In cerebral ischemia, severe cellular degeneration occurs since the brain is highly reliant on enhanced supply of both oxygen and glucose. Cerebral ischemic conditions occur when a significant amount of blood supply is reduced in the particular regions of the brain. As a result of “transient global ischemia”, selective neuronal damage occurs (Belov Kirdajova et al., 2020). On the other hand, a cascade of events takes place as a result of ischemic reperfusion mechanism that includes secretion of excitatory amino acids, reduction in cellular energy resources, generation of reactive oxygen species (ROS), and mitochondrial dysfunction. All these events play a vital role in the progression of stress oxidative (Nita and Grzybowski, 2016). The oxidative stress results in cellular damage due to the formation of oxygen-derived free radicals and hydrogen peroxide. It can happen through alteration in membrane fluidity and chain reaction of membrane lipid peroxidation (Phaniendra et al., 2015). Thus, identifying new therapeutic agents from natural resources against ischemic stroke is a colossal demand of this hour which can act on numerous sites of neurotoxic cascade.
In recent years, the researcher’s interest in identifying and characterizing new medicinal flora to treat and cure neurodegenerative diseases and brain impairment has increased. Approximately 120 traditional medicinal plants are being used to treat the varied disorders associated with the central nervous system (Kumar, 2006). In India, many medicinal plants viz. Bacopa monnierae, Allium sativum, Celastrus paniculatus, Centella asiatica, Withania somnifera, Nicotiana tabaccum, Ricinus communis, Ginkgo biloba, Salvia officinalis, Huperiza serrata, Angelica sinensis, Hypericum perforatum, Uncaria tomentosa, Physostigma venosum, Curcuma longa, Terminalia chebula, Acorus calmus, Crocus sativus, Enhydra fluctuans, Valeriana wallichii, and Glycyrrhiza glabra are explored for their neuroprotective effects (Kumar and Khanum, 2012). In Chinese traditional medicinal system, various plants that are documented for the treatment of stroke are: Scutellaria baicalensis, Ledebouriella divaricata, Angelica pubescens, Morus alba, Uncaria rhynchophylla, Salvia miltiorrhiza, and Ligusticum chuanxiong (Gong and Sucher, 1999).
Stellaria media belongs to the family Caryophyllaceae which is a family of dicot angiosperms consisting of 85 genera and 2630 species. It is a cool-seasoned annual plant which is found throughout the Himalayas up to a height of 4300 m. It is also known as ‘Star chickweed’, ‘Mouse ear chickweed’, and ‘Common chickweed’. The plant is abundantly found all around and does not require cultivation. Fresh edible plants can be harvested upon appearance of flowers between May and July. It can be used fresh or in dried form. Vanillic acid, p-hydroxybenzoic acid, ferulic acid, caffeic acid, and chlorogenic acid are the major phenolic components of this plant (Kitanov, 1992). On the other hand, apigenin, genistein, vicenin-2, and gypsogenin are the prime flavonoids and saponin constituents: (Hodisan and Sancraian, 1989). The plant contains a total of 16 free amino acids, of which, 9 were essential amino acids, including valine, threonine, isoleucine, leucine, methionine, phenylalanine, histidine, lysine, and arginine (Shan et al., 2010).
In order to mimic the ischemic conditions, animal models are extensively being used for exploring neuroprotection and mechanism of cell demise in forebrain slices that are subjected to oxygen and glucose deficiency (OGD) and reoxygenation (Cárdenas et al., 2000). In view of this, the present study was designed to evaluate the lipid peroxidation and neuroprotective effects of S. media against OGD-induced injury in rat brain’s hippocampal and cortical slices.
2. Materials and methods
2.1. Ethical issue
This study was performed at Department of Biotechnology, University of Science and Technology Bannu-KP, Pakistan, after approval by the Ethical Committee of the department (Trial number: Biotech/USTB/0121; dated 13/07/2018). Protocols adopted in this investigation were in accordance with the guidelines of the Brazilian College of Animal Experimentation, affiliated with the International Council for Laboratory Animal Science.
2.2. Animals used
Male Wister rats (age – 8–10 weeks; weight – 200–240 g) were obtained from National Institute of Health, Islamabad, Pakistan. They were retained in steel enclosures with unrestricted access to food and water. The cages were kept in a well-wired room and maintained at controlled temperature with 12 h light–dark cycle.
2.3. Plant collection and extraction
S. media was purchased from cantonment board nursery of district Bannu-KP and it was recognized and validated at Department of Botany, UST Bannu-KP, Pakistan. The voucher was deposited at the herbarium (voucher number: USTB/Bot/0141). Following a gentle wash, the collected leaves were dried under shade. After 30 days, these leaves were powdered by means of a local blending machine. About 200 g of the powder were steeped in 1 L of ethanol for 7 days and kept in an orbital shaker. After a week, the product was filtrated and concentrated using a rotary evaporator. The shady green product obtained was exposed to freeze drying and a fine powder was obtained which was stored at 4 °C for further use.””
2.4. Slices preparation
Rats were decapitated for in vivo observations. Experimental container and animal’s brain immediately after incision are shown in Fig. 1. The hippocampus and cortex were immediately dissected out into slices of 400 µm in size on a block of ice with the help of a Mcllwain tissue chopper. The hippocampal and cortical slices were equally divided into two sets i.e. control and OGD.”The slices were pre-incubated in a pre-incubation solution (in mM: sodium chloride 120, potassium chloride 0.5, sodium bicarbonate 3.5, calcium chloride 1.5, magnesium chloride 1.3, sodium phosphate monobasic 1.25, and D-glucose 10; pH 7.4) (Cárdenas et al., 2000).
Fig. 1.
(a) Experimental container and (b) animal’s brain immediately after incision.
2.5. OGD and reoxygenation
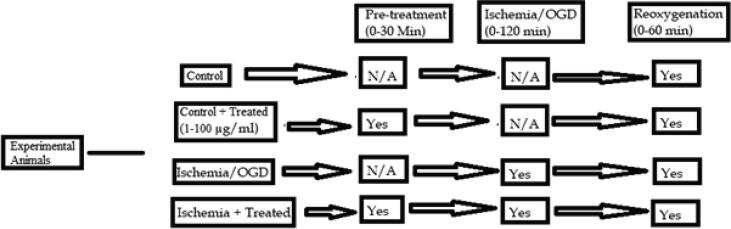
Both the control and experimental groups were pre-treated for 30 min in the presence (1–100 μg/mL) or absence of S. media in a CSF. The OGD and control experiments were done correspondingly using three slices of the same rat in each tube. After pre-treatment, the medium in the OGD-groups was changed with another glucose free artificial cerebrospinal fluid. In order to mimic ischemia like conditions, incubation of the OGD slices was done at 37 °C with an anaerobic gas mixture supply (5% CO2, 95% argon) for 2 h, and termed as OGD period. The control tubes were incubated for 2 h at 37 °C with 95% O2 in combination with 5% CO2. Subsequently, the medium was changed from both control and OGD-groups. Reperfusion was done by incubating the tubes for 1 h in the presence (1–100 μg/mL) or absence of S. media (Kamdem et al., 2012a). The entire experimental plan is represented in Fig. 2.
Fig. 2.
Schematic representation of OGD and reoxygenation in rats.
2.6. Cellular viability and membrane integrity assay
The cellular viability analysis was performed using MTT [3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay (Mosmann, 1983). After 2 h of OGD and 1 h of reperfusion, 10 μL of MTT solution (50 μg/mL) were added and the samples were incubated for further 30 min at 37 °C. The optical density was recorded at 630 nm and the net absorbance (A630) was considered as an index of cell viability. After reperfusion period, neuronal damage persuaded by OGD was enumerated by the quantity of lactate dehydrogenase (LDH) released in the solution. The LDH values were recorded spectrophotometrically at 340 nm using a commercial kit LDH FS DGKC (DiaSys Diagnostic System GmbH, Germany). The LDH activity was calculated using the following formula:
where, X = sample absorbance value at 340 nm.
2.7. Non protein thiols (NPSH) assay
The detection and measurement of thiols are major tasks to investigate certain processes involved in the biological systems. Thiols are significant antioxidants that provide shield to nucleic acid, proteins and cellular lipids against peroxidative stress due to their ability to react with free radicals. The change in content of sulfhydryl groups indicates the effect of certain drug on tissues. Hippocampal and cortex slivers from all plates were homogenized in 20 μL of 10 mM Tris-HCl (pH 7.4). Ten μL of this solution was used for the test. The NPSH was determined by the modified assay of Ellman (1959). Briefly, 50 μL of tissue homogenates were precipitated with 50 μL of 10% TCA (trichloroacetic acid) and then the samples were centrifuged at 3000 × g for 10 min at 4 °C. The protein pellet was excluded and free -SH groups were measured in the supernatant.”
2.8. Quantification of ROS
Cellular oxidative stress levels were quantified by incorporating DCFH-DA (2ʹ,7ʹ-dichlorofluorescein diacetate) fluorescent probes. After 2 h of OGD, followed by 1 h of reoxygenation,”5 μM of DCFH-DA were added into the supernatant and incubated for 1 h in dark. The formation of fluorescence product due to DCFH-DA oxidation was measured and values were recorded. Slivers of each taster were mixed homogenously, and a portion of this homogenous solution was utilized to enumerate intracellular ROS production in the slivers homogenate (Kamdem et al., 2012a). Dichlorofluorescein (DCF) fluorescence was observed using spectrofluorophotometer (Parkin Elmar L45) with an emission and excitation wavelengths of 525 and 488 nm, respectively.”
2.9. Lipid peroxidation
The lipid peroxidation was depicted using thiobarbituric acid reactive substance (TBARS) assay as per the protocol of Okhawa et al. (1979). The homogenate of liver was centrifuged at 4000 × g for 10 min and the collected supernatant was utilized for assessing malondialdehyde (MDA) content. About 100 µL of the low speed supernatant were incubated for 60 min at 37 °C in the presence (200 mg/kg) or absence of S. media extract. After incubation, TBA, sodium dodecyl sulphate (8.1%), and acetate buffer (pH 7.4) were added and incubated for 60 min at 100 °C. The formation of light pink color indicated the reaction of MDA with thiobarbituric acid. The content was allowed to cool and the absorbance was recorded at 532 nm using a spectrophotometer.”
2.10. Data statistics
Mean ± standard error of mean (mean ± SEM) was used to express the data. One way analysis of variance, followed by Kruskall-Wallis test was done to study the ROS produced in the medium. Data exhibiting a P value of < 0.01 or < 0.001 were considered statistically significant. Cohen’s d value was calculated for standardized effect size. The value obtained was 0.091.
3. Results
3.1. Cellular viability
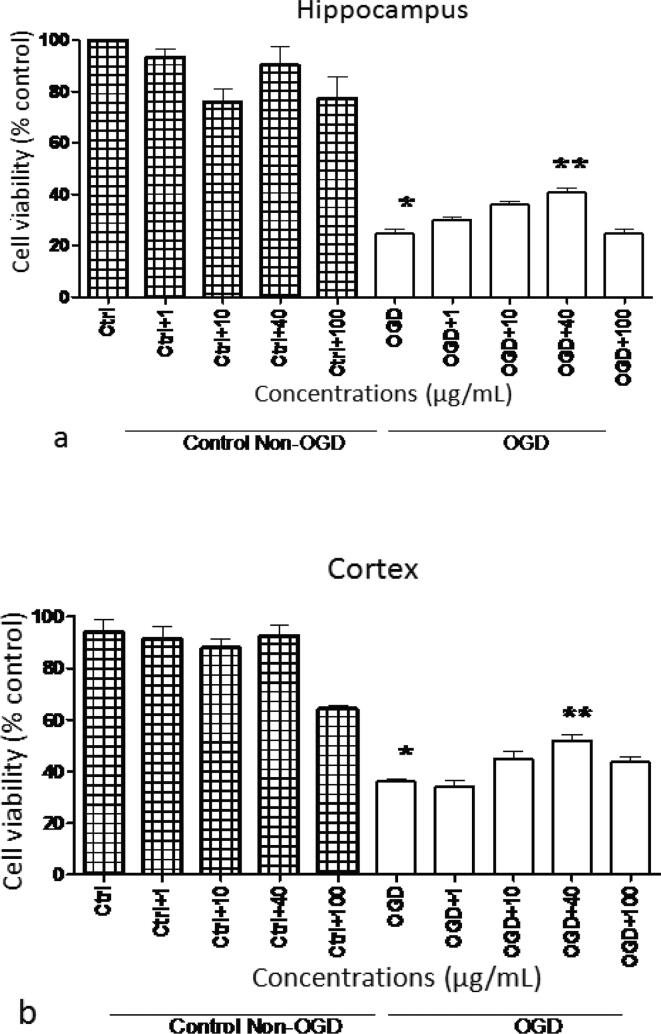
The cellular viability assay displayed significant neuroprotection against OGD-induced mitochondrial impairment in rat hippocampal (Fig. 3a) and cortex slices (Fig. 3b) by S. media (40 µg/mL). On the other hand, S. media did not show any prominent effects in control slices of hippocampus and cortex.
Fig. 3.
Effect of various concentrations of S. media extract on cellular viability in (a) hippocampus and (b) cortex. Data were mentioned as percentage of control. All the tests were carried out in triplicate and values were expressed as mean ± SEM. *represents remarked change from control slivers at P < 0.001. **represents momentous variance from slivers exposed to ischemic episode at P < 0.001.
3.2. Membrane integrity using LDH‘ assay
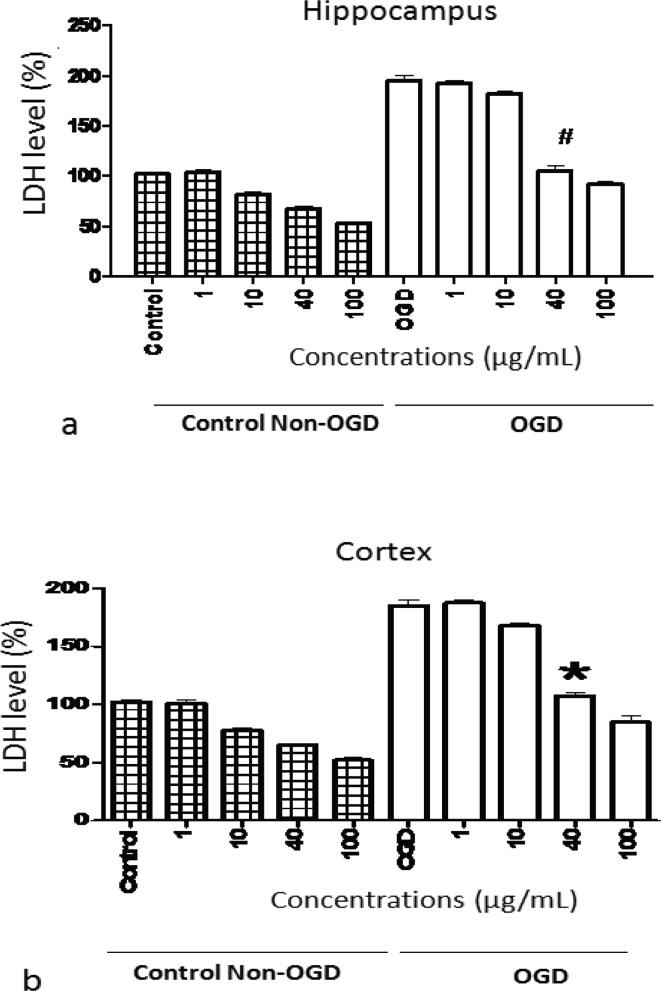
The impact of OGD and S. media on the secretion of LDH from hippocampus and cortical slivers is presented in Fig. 4. The LDH levels were decreased significantly (P < 0.001) during pre-incubation and reoxygenation periods using S. media extract. The attenuation of the LDH levels by the extract was reported in a concentration dependent manner.
Fig. 4.
Effect of various concentrations of S. media extract on OGD-induced LDH release from (a) hippocampus and (b) cortex. All the tests were carried out in triplicate and values were represented as mean ± SEM. #, *represents remarked variance from control slivers at P < 0.001.
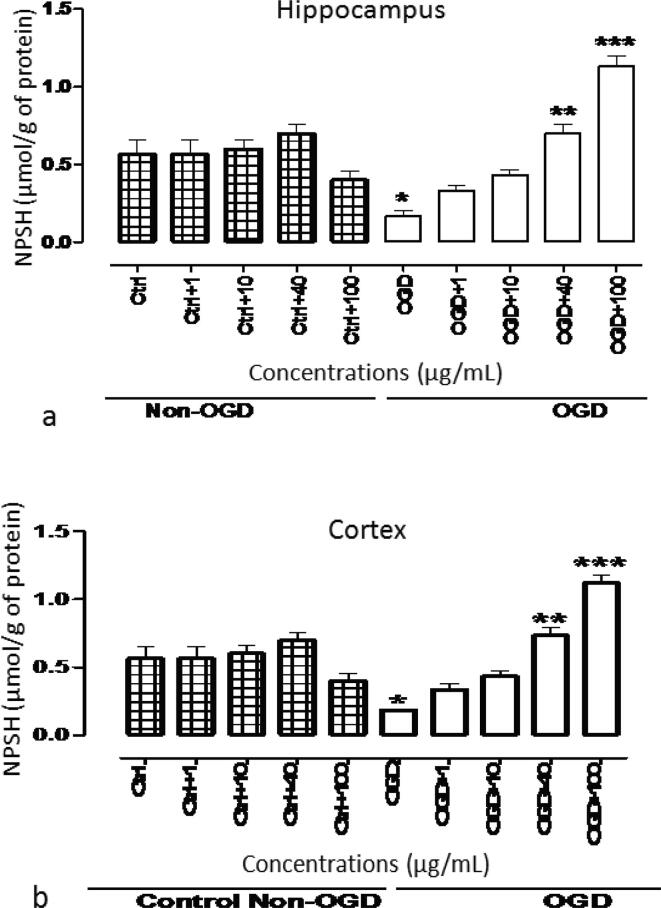
3.3. NPSH content and effect of extract
After 2 h of OGD followed by 1 h of reperfusion period, NPSH was markedly decreased when compared with the levels obtained in control slivers. The levels were significantly (P < 0.001) increased when S. mdeia was present during pre-incubation before ischemic insult and during incubation after reoxygenation time interval. The increase in NPSH in both hippocampus and cortex was found to be in a concentration dependent manner (Fig. 5).
Fig. 5.
NPSH content in rat’s (a) hippocampus and (b) cortex after 2 h of OGD, followed by 1 h of reperfusion. Values are represented as mean ± SEM. *show remarkable variance from untreated control slivers (P < 0.05). ** and *** present remarkable variance from slivers exposed to ischemic event at P < 0.01 and P < 0.001, respectively.
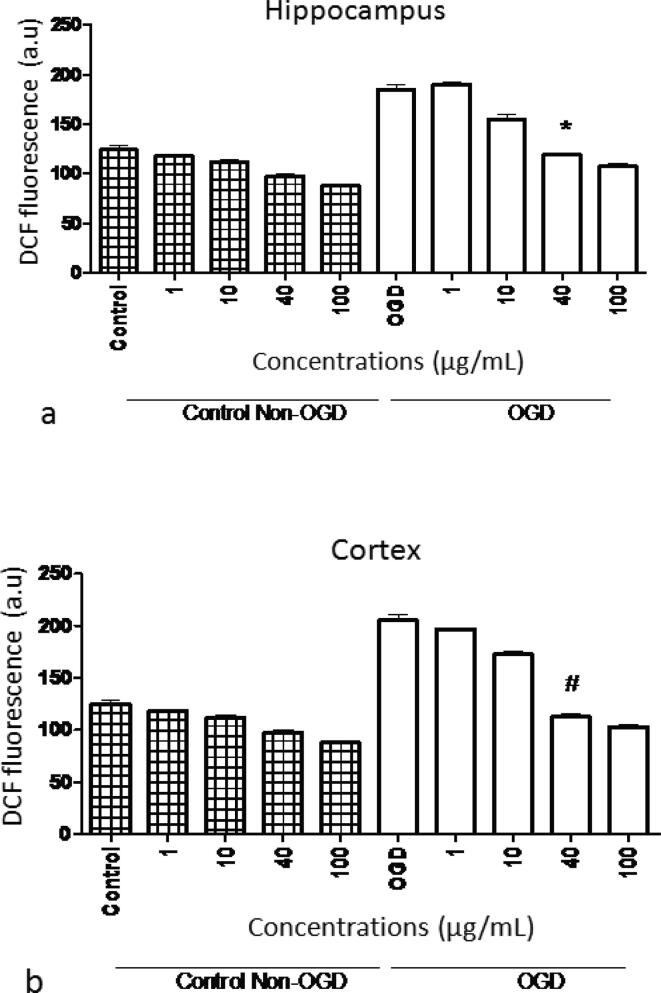
3.4. Production of ROS
OGD resulted in an increased fluorescence of DCF in the medium as compared to the non-OGD group. The presence of S. media extract reduced DCF fluorescence significantly (P < 0.001) in a concentration dependent manner (1–100 µg/mL). Results showed that S. media significantly (P < 0.001) prevented ROS secretion in the medium gained from slices kept under basal conditions at 40 µg/mL. Furthermore, the same type of protection was observed in slices homogenate of hippocampus and cortex when the extract was added before OGD and during reoxygenation (P < 0.001). However, the extract did not show any considerable effect on DCFH-DA oxidation in the control group (Fig. 6).
Fig. 6.
Effect of various concentrations of S. media extract on OGD-induced ROS production in slices homogenates in (a) hippocampus and (b) cortex. All the tests were carried out in triplicate and values were represented as mean ± SEM. *,#represent remarked variance from control slivers at P < 0.001.
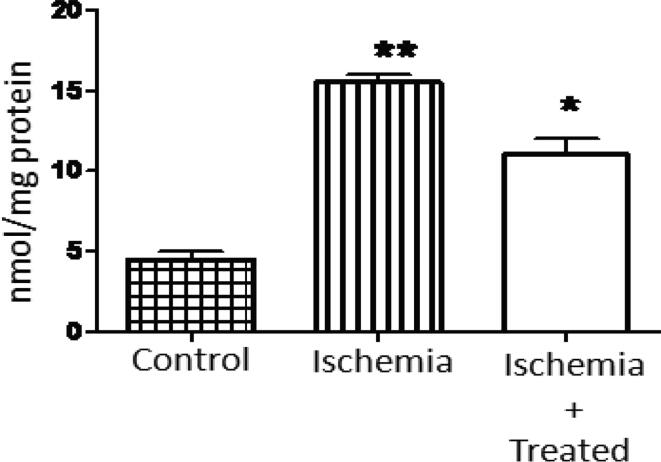
3.5. Lipid peroxidation assay
Assessing TBARS is an important measurement of determining the extent to which lipid peroxidation occurs. Results of this investigation showed that the MDA levels were enhanced in ischemic rat’s liver tissues. On the other hand, the MDA levels were reduced in liver tissues of rats treated with S. media extract (200 mg/kg body weight) when compared to the ischemic animal (P < 0.01) (Fig. 7).
Fig. 7.
Level of MDA in the liver of the control, ischemic, and S. media extract (200 mg/kg) treated ischemic animals. *,**represent remarked changes from control at P < 0.001.
4. Discussion
In order to treat cerebral ischemia and other related neurodegenerative disorders, the development of neuroprotective molecules from traditionally available herbal medicines is considered a promising strategy (Sharifi-Rad et al., 2020). In this context, neuroprotective effects of S. media were investigated using in vitro ischemic model in rat’s hippocampus and cortex slivers which is extensively mentioned in the literature in order to study the neuronal impairment as a result of ischemic reperfusion (Sharifi-Rad et al., 2020, Alzobaidi et al., 2021). Some of the researchers used the drugs to reduce the consequences of ischemic reperfusion injury before ischemic event or after reoxygenation period but some other researchers used the drug for both ischemic event and reoxygenation (Konrath et al., 2008, Simão et al., 2011). In this investigation, different concentrations of S. media were used prior to inducing ischemic conditions and throughout the reoxygenation period. Our objective was to assess a probable prophylactic effect before ischemia and its protective effects on injury after reoxygenation. Findings of this study showed that S. media extract provided significant protection to slices against the adverse effects of OGD.
In comparison with the previously conducted studies, our findings displayed that the exposure of hippocampus and cortical slices to ischemic conditions and reoxygenation resulted in an enhanced release of LDH, both in slices homogenates and incubation medium with respect to the control. In general, the secretion of LDH is observed upon cell death (Kamdem et al., 2012a, Kamdem et al., 2012b). This cellular damage is associated with the higher susceptibility of both hippocampus and cortex to the oxidative stress produced due to OGD and reoxygenation (Venkateshappa et al., 2012).
NPSH is a significant antioxidant molecule which controls free radicals that are produced endogenously. It was measured in slivers homogenates after I/R injury. It was observed that NPSH content was meaningfully reduced in slivers exposed to ischemia alone in comparison to control sliver (non-OGD, without treatment) (Kamdem et al., 2012b). Conversely, S. media extract remarkably prohibited I/R-induced reduction in NPSH when present before ischemic event and during the reoxygenation time.
Our results demonstrated the ROS lowering ability of the extract during reperfusion and before OGD period. Findings suggested that S. media might be used as a preventive agent against neurotoxic effects of ischemic damage, but not as a remedial agent for the treatment of brain ischemia. The ROS production is generally related to the normal metabolic mechanism; however, their over-production occurs in certain pathological conditions, including ischemic reperfusion that can lead to stress oxidative (Zorov et al., 2014). Results obtained in this context suggested that free radicals caused neurotoxicity in both hippocampus and cortex, and S. media possessed the ability to counterfeit the ROS cytotoxicity. Lipid peroxidation is an important marker to assess the oxidative damage (Cherian et al., 2019). The ischemic rats treated with S. media significantly (P < 0.01) decreased the MDA levels showing its promising ability to minimize the oxidative stress.
5. Conclusions
In a nutshell, S. media offered a significant neuroprotection attribute against cerebral ischemic reperfusion damage. The observed effect was credited to the decrease in ROS level, reduction in LDH secretion, protection of slivers dipping capacity of MTT, and MDA lowering ability of the extract. S. media can certainly be used as an ideal healing agent towards the prevention of neuronal cells’ loss during ischemic-reperfusion process. Further investigation is required to isolate active component from the extract that are responsible for neuroprotective and pharmacological effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors extend their sincere acknowledgement to Higher Education Commission of Pakistan for financial support for this project “20-5082/NRPU/RND/HEC 2014”. The authors express their appreciation to the Researchers Supporting Project (RSP-2021/193), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Umar Khayam Sahibzada, Email: umar.sahibzada@gmail.com.
Ameer Khusro, Email: armankhan0301@gmail.com.
References
- Alzobaidi N., Quasimi H., Emad N.A., Alhalmi A., Naqvi M. Bioactive compounds and traditional herbal medicine: Promising approaches for the treatment of dementia. Degener. Neurol. Neuromuscul. Dis. 2021;11:1–14. doi: 10.2147/DNND.S299589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov Kirdajova D., Kriska J., Tureckova J., Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell Neurosci. 2020;14:51. doi: 10.3389/fncel.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas, A., Moro, M.A., Hurtado, O., Leza, J.C., Lorenzo, P., Castrillo, A., et al., 2000. Implication of glutamate in the expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. J. Neurochem. 74, 2041–2048. [DOI] [PubMed]
- Cherian D.A., Peter T., Narayanan A., Madhavan S.S., Achammada S., Vynat G.P. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019;11(Suppl 2):S297–S300. doi: 10.4103/JPBS.JPBS_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh C. Acute ischemic stroke: Management approach. Ind. J. Crit. Care Med. 2019;23(Suppl 2):S140–S146. doi: 10.5005/jp-journals-10071-23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor E.S. Stroke in the 21st Century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res. Treat. 2018;2018:3238165. doi: 10.1155/2018/3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gong X., Sucher N.J. Stroke therapy in traditional Chinese medicine (TCM): Prospects for drug discovery and development. Trends Pharmacol. Sci. 1999;20:191–196. doi: 10.1016/s0165-6147(98)01276-0. [DOI] [PubMed] [Google Scholar]
- Hodisan V., Sancraian A. Triterpenoid saponins from Stellaria media (L.) Cyr. Farmacia. 1989;37:105. [Google Scholar]
- Kamdem, J.P., Stefanello, S.T., Boligon, A.A., Wagner, C., Kade, I.J., Pereira, R.P., et al., 2012a In vitro antioxidant activity of stem bark of Trichilia catigua Adr. Juss (Meliaceae). Acta Pharm. 62, 371-382. [DOI] [PubMed]
- Kamdem J.P., Waczuk E.P., Kade I.J., Wagner C., Boligon A.A., Athayde M.L., et al. Catuaba (Trichilia catigua) prevents against oxidative damage induced by in vitro ischemia-reperfusion in rat hippocampal slices. Neurochem. Res. 2012;37:2826–2835. doi: 10.1007/s11064-012-0876-0. [DOI] [PubMed] [Google Scholar]
- Kitanov G.M. Phenolic acids and flavanoids from Stellaria media (L.) Vill. (caryophyllaceae) Pharmazie. 1992;47:470–471. [Google Scholar]
- Konrath E.L., Santin K., Nassif M., Latini A., Henriques A., Salbego C. Antioxidant and pro-oxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro. Neurotoxicology. 2008;29(6):1136–1140. doi: 10.1016/j.neuro.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacog. Rev. 2012;6(12):81. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. 2006;20(12):1023–1035. doi: 10.1002/ptr.1970. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular grow and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016;2016:1–23. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y.u., Zhou J., Guang Zhao H., Feng X.u., Dong Y., Xia B. Amino-acid and mineral composition of Stellaria media. Chem. Nat. Compd. 2010;46(4):667–668. [Google Scholar]
- Sharifi-Rad M., Lankatillake C., Dias D.A., Docea A.O., Mahomoodally M.F., Lobine D., Chazot P.L., Kurt B., Boyunegmez Tumer T., Catarina Moreira A., Sharopov F., Martorell M., Martins N., Cho W.C., Calina D., Sharifi-Rad J. Impact of natural compounds on neurodegenerative disorders: from preclinical to pharmacotherapeutics. J. Clin. Med. 2020;9(4):1061. doi: 10.3390/jcm9041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F., Matté A., Matté C., Soares F.M.S., Wyse A.T.S., Netto C.A., Salbego C.G. Resveratrol prevents oxidative stress and inhibition of Na+K+-ATPase activity induced by transient global cerebral ischemia in rats. J. Nutr. Biochem. 2011;22(10):921–928. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Venkateshappa C., Harish G., Mahadevan A., Srinivas Bharath M.M., Shankar S.K. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem. Res. 2012;37(8):1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]