Abstract
Biofilm infections are a common complication of prosthetic devices in humans. Previous in vitro research has determined that low-frequency ultrasound combined with aminoglycoside antibiotics is an effective method of killing biofilms. We report the development of an in vivo model to determine if ultrasound enhances antibiotic action. Two 24-h-old Escherichia coli (ATCC 10798) biofilms grown on polyethylene disks were implanted subcutaneously on the backs of New Zealand White female rabbits, one on each side of the spine. Low-frequency (28.48-kHz) and low-power-density (100- and 300-mW/cm2) continuous ultrasound treatment was applied for 24 h with and without systemic administration of gentamicin. The disks were then removed, and the number of viable bacteria on each disk was determined. At the low ultrasonic power used in this study, exposure to ultrasound only (no gentamicin) caused no significant difference in bacterial viability. In the presence of antibiotic, there was a significant reduction due to 300-mW/cm2 ultrasound (P = 0.0485) but no significant reduction due to 100-mW/cm2 ultrasound. Tissue damage to the skin was noted at the 300-mW/cm2 treatment level. Further development of this technique has promise in treatment of clinical implant infections.
Prosthetic implants are commonly used in modern health care. In 1996, it was estimated that 4.4 million people in the United States had at least one fixation device, and 1.3 million of these individuals had artificial joints (5). Even though there is some initial inflammatory response to an implanted device (1, 6), the body becomes accustomed to the device and the quality of life of the individual usually improves. However, some devices occasionally become colonized with bacteria. There are many routes of infection, from surgical procedures to migration of the bacteria from the skin along a catheter surface into the body (10, 11, 21, 27). Whatever the route, these types of infections pose a serious problem to the patient. Both gram-positive and gram-negative organisms have been found to infect prosthetic devices (27).
Bacterial infections of prosthetic devices generally develop into biofilm infections in which the bacteria are sequestered in a layer of exopolymers. The exopolymer or biofilm appears to protect the bacteria from immune responses such as opsonization, complement-mediated lysis, and phagocytosis, as well as from normal levels of antimicrobial therapy (17). It has been noted that vancomycin levels in Staphylococcus epidermidis biofilms far exceeded MIC and MBC levels and did not appear to be a significant penetration barrier to the antibiotic (3). It has been suggested that the biofilm may increase the concentration of an antibiotic by trapping and concentrating it in a manner similar to that used to trap and concentrate nutrients (2). On the other hand, it has also been postulated that antibiotics are not fully able to penetrate the layer of exopolymers and some bacteria are never exposed to the antibiotic at full concentrations in serum (25, 26). It has been estimated that 50 to 500 times the normal therapeutic dose of antibiotic is required to eradicate young biofilms (≤24 h old) and 5,000 times the normal dose is required to eliminate older biofilms (≥48 h old) (8). This poses a serious health threat to the patient because not only does the biofilm cause a potentially life-threatening infection but the levels of antibiotics estimated to be effective at eliminating the biofilm are not achievable with the recommended dosage. Usually, an infection is treated by removing the infected device, treating the patient with safe levels of antibiotic, and replacing the device at a later time. This can be a costly, time-consuming, and even life-threatening procedure. Clearly, a more effective method for dealing with biofilm infections of implanted devices is necessary.
Ultrasound has been used in many applications in medicine and research. Our laboratory has shown that low-frequency ultrasound when combined with antibiotics can significantly enhance the bactericidal action of antibiotics in vitro in both planktonic and biofilm forms (7, 15, 16, 18–20, 22, 23). The mechanism by which ultrasound enhances antibiotic action is unclear but may be due to perturbation of the cell membrane or to stress responses by the bacteria (18).
In this paper we report that low-frequency ultrasound also enhances the effectiveness of gentamicin against Escherichia coli biofilms in vivo in a rabbit model.
MATERIALS AND METHODS
Experimental animals.
Forty-one 2-kg New Zealand White female rabbits were used in this study. Animals were treated and maintained under the regulations of the Institutional Animal Care and Use Committee of Brigham Young University and those established by the U.S. Department of Agriculture.
Measurement of subcutaneous heating.
Preliminary studies were conducted on one rabbit to establish parameters and procedures for the study. Temperature increases in skin tissue due to ultrasonic treatment were measured with a thermocouple in a stainless-steel sheath (Phystek MT-23/5; Physitemp Instruments, Newark, N.J.) inserted subcutaneously in the back of the rabbit. The purpose of these measurements was to determine if ultrasound treatment caused sufficient temperature increase in the tissue to cause tissue damage or discomfort to the animal during the procedure. The rabbit was preanesthetized with ketamine (35 mg/kg of body weight) and xylazine (5 mg/kg) intramuscularly and prepared for surgery as described below. The thermocouple probe was inserted subcutaneously, and the ultrasonic transducer (Edo Acoustics, Salt Lake City, Utah) operating at 28.48 kHz and 100 or 300 mW/cm2 was placed on the skin above the end of the probe. To protect the transducer and wiring, a tubular silicone rubber flange was placed around the transducer and held in place by a harness worn by the rabbit. The wiring was fed up and out through the top of the cage so that after recovery from surgery the rabbit was free to move about the cage. Temperature was measured for 24 h.
Preparation of the biofilm.
E. coli ATCC 10798 was selected for this study because it has been previously shown to produce a thick biofilm in only 14 h of growth (7, 15). The MIC of gentamicin for planktonic E. coli was determined to be 12 μg/ml by methods described previously (16). Twenty-four hours prior to surgery, an overnight culture grown in 10 ml of tryptic soy broth (TSB) was centrifuged at 4,800 rpm for 10 min (GS-15R; Beckman, Fullerton, Calif.). The supernatant was removed, and the bacteria were resuspended in 10 ml of sterile phosphate-buffered saline. Sterile polyethylene disks (diameter, 1.5 cm; thickness, 0.01 mm; 0.5-cm square tab) were used to grow the biofilm in this study. The disks were placed in separate sterile glass petri dishes each containing 20 ml of TSB. Twenty microliters of the suspended cells was inoculated into each of the petri dishes. The dishes were shaken at 100 rpm while incubating at 37°C for 8 h. After 8 h, the disks were rinsed with 1 ml of physiological saline solution to remove planktonic bacteria and were transferred to petri dishes containing sterile TSB. This procedure was again repeated 16 h after the original inoculation to form a biofilm on the polyethylene disks. Presence of the bacteria in the biofilm was confirmed by confocal laser microscopy. Mean thickness of the biofilm was determined to be 285 μm. Mean log10 counts per square centimeter of biofilms, including both sides, prior to insertion were 7.29 ± 0.04 (n = 47). Two control biofilms were grown at the same time and under the same conditions as the biofilms implanted into the rabbit. After the biofilms were implanted, the controls were immediately plated by the procedure described below to determine the approximate number of viable CFU on the disks implanted into the rabbit.
Implantation of biofilm-infected disks.
Rabbits were preanesthetized with a combination of xylazine (5 mg/kg) and ketamine HCl (35 mg/kg) administered intramuscularly into the caudal thigh muscles as a single dose. Anesthesia was maintained during the surgical procedure with isofluorane and oxygen delivered via face mask.
The entire area from the dorsal midline to the abdomen was denuded of all fur. An aseptic prep of the site was performed with three surgical scrubs using undiluted Betadine scrub, followed by an alcohol rinse, and finally the site was painted with Betadine solution. With the animal in sternal recumbency, the surgical site was draped after the final application of Betadine solution. Under sterile conditions, two 2-cm incisions were made perpendicular to and bilateral to the vertebral column near the midline of the rabbit. Blunt dissection with a Metzenbaum scissor was used to create implant sites by tunneling subcutaneously just beneath the cutaneous trunci in the lateral position parallel to the vertebral column. In each procedure, a sterile disk was first inserted to ensure that the subcutaneous tunnel would position the implant correctly. The sterile disk was then removed, and the disk colonized with E. coli biofilm described above was sutured into place to the underside of the skin through two small holes in the tab of the disk. The incision was closed with a simple interrupted pattern using nonabsorbable 2-0 multifilament polyamide nylon suture.
In experiments using antibiotic, injectable gentamicin (Gentocin; Schering-Plough, Kenilworth, N.J.) (8 mg/kg) was administered subcutaneously in a pulsed dose (24) every 24 h commencing immediately after rabbits had recovered from surgery. Rabbits received a total of two doses of gentamicin throughout the experiment. The final dose was received 24 h prior to the recovery of the implant. Rabbits received ibuprofen (70 mg/kg) orally every 8 to 12 h postoperatively to alleviate discomfort from the procedure. Rectal temperature was measured every 8 h after the surgical procedure coincident with the ibuprofen and antibiotic treatments. Blood was sampled from the marginal ear vein at regular intervals pre-, mid-, and posttreatment to determine if bacteria had escaped the biofilm into the bloodstream. The initial blood sample was taken before surgery and gentamicin therapy, the second sample was taken just prior to gentamicin treatment and the commencement of ultrasound therapy, and the third sample was taken prior to recovery of the disks and 24 h after the final gentamicin dose. Blood was diluted and plated onto nutrient agar (NA) plates by the membrane filtration procedure.
In some experiments, a 49-g ultrasound transducer was fixed in place over one of the biofilm-infected implant sites 24 h postsurgery. This was done by fitting the rabbits with a silicone rubber flange and a canvas jacket designed to secure the transducer for ultrasound administration. The flange was glued to the skin with a mixture of Nexaband (Tri-Point, Raleigh, N.C.) glue and 25% hexane. The transducer was fixed in place with an acoustically conductive gel adhesive (Tensive; Parker Laboratories). Continuous 28.48-kHz, 100- or 300-mW/cm2 ultrasound was administered to one implant site for 24 h. Air from a compressed air tank was delivered to the inside of the flange at about 62 cm3/min to convect heat away from the transducer.
Ultrasound setup.
Nonfocused ultrasonic transducers were used in this experiment to deliver the ultrasound. A function generator (Hewlett Packard 3312A) created a continuous sinusoidal wave at 28.48 kHz that was amplified by an RF amplifier (model 240L; ENI, Rochester, N.Y.). A 10:1 voltage transformer (Edo Acoustics) was then used to boost the voltage going to the transducer. Prior to the experiments, the transducers were calibrated by correlating acoustic intensity output with voltage input. Acoustic intensity was measured with a calibrated hydrophone (Bruel and Kjaer, Naerum, Denmark).
Removal of biofilm-infected disks.
After 24 h of ultrasound, animals were humanely euthanized with an intravenous 26% sodium pentobarbital–7.8% isopropyl alcohol (Sleepaway, Ft. Dodge, Iowa) euthanasia solution (1 ml/2.5 kg). Skin, subcutaneous tissue, and muscle from around the implant site were taken for histopathology from all animals. For two of the animals, heart, liver, and kidney tissues were also studied to determine if septicemia or other pathology was present. Histopathology was performed by ARUP Laboratories, Salt Lake City, Utah.
After the rabbits were euthanized, the implanted disks were removed with sterile forceps from the subcutaneous fascia and fibrous capsule, and the small tab was cut from the disk with sterile scissors, dropping the remaining circular disk (3.53 cm2) into a large test tube of 0.5% trypsin in phosphate-buffered saline. The disks were subjected to 1-W/cm2 ultrasound at 70 kHz in a Sonicor SC-100 sonicating bath (Sonicor Instrument Co., Copiaque, N.Y.) for 30 min at 37°C to remove all adherent bacteria. It was previously determined that this ultrasound treatment would not kill bacteria remaining on the biofilm (15). Bacteria removed from the disk were serially diluted in physiological saline solution and plated on NA plates by the membrane filtration method. The first tube in the dilution series contained 0.1% polysorbate 80 (Tween 80) to reduce clumping. The 45-μm-pore-size membrane filter (Gelman Sciences, Ann Arbor, Mich.) used in the plating procedure was placed onto an NA plate and incubated for 48 h at 37°C. The colonies on the membranes were then counted.
Experimental controls.
Several experiments with appropriate control groups were conducted to determine the role of the study variables: antibiotic, ultrasound, and weight of the transducer. The sequence of performing control experiments was randomized to remove statistical bias throughout the procedure. The side of the rabbit (left or right) over which a weight simulating the transducer or the transducer itself was placed was also randomized. The first control group (n = 1) received two sterile polyethylene disks with no biofilm and a weight over one disk. The second group (n = 4) received two biofilm-coated disks only. The third group (n = 4) received two biofilm-coated polyethylene disks with a weight over one of the disks. The fourth group (n = 4) received two biofilm-coated polyethylene disks and injectable antibiotic (8 mg/kg every 24 h). The fifth group (n = 4) received two biofilm-coated polyethylene disks with 28.48-kHz, 100-mW/cm2 ultrasound over one of the disks. These experiments were necessary to establish the response of the rabbit to each of the parameters of the test: the polyethylene implant, the reaction to bacterial biofilm, the effect (if any) of the weight of the ultrasonic transducer, the effect of antibiotic therapy, and the effect of 100-mW/cm2 ultrasound.
Experimental groups.
There were eight rabbits in the first experimental group and three rabbits in the second experimental group. The first group received 28.48-kHz, 100-mW/cm2 continuous ultrasound for 24 h, and the second group received 28.48-kHz, 300-mW/cm2 continuous ultrasound for 24 h. The side upon which the transducer was placed (right or left) was randomized.
Statistical analysis.
Data were analyzed by the MIXED procedure of the SAS statistical software (SAS Institute, Cary, N.C.) to evaluate differences between groups relative to variation between and within animals. Data in the 100-mW/cm2 experimental group were analyzed by using a two-way additive model with rabbits and treatments as factors. Data in the 300-mW/cm2 experimental group were analyzed by a one-tailed Student’s t test of the mean difference in paired observations between treated and nontreated sides. This procedure is a special case of the more general procedure used for the analysis of the 100-mW/cm2 group. Based on variances estimated for the 100-mW/cm2 group, with a practical significant difference between treated and nontreated sides of 1 log10, it was determined that only three replicate experiments would be necessary for the 300-mW/cm2 group.
RESULTS
During the first temperature experiment at 100 mW/cm2, the temperature of the skin never exceeded 40°C, and therefore the ultrasound at 100 mW/cm2 and 28.48 kHz was considered safe to use throughout the 24-h treatment. There was some damage to the skin during treatment after the temperature probe experiment at 300 mW/cm2. The skin in the area of ultrasound treatment turned red and a lesion formed 5 days after ultrasound treatment. No damage to the skin was noted before this time. The lesion healed after 2 weeks but left a visible scar on the skin.
There were no viable CFU in the first control rabbit, which received polyethylene implants without biofilm, thus indicating that the surgical procedure was aseptic. There was no statistically significant side-to-side variation in the rabbits in the second control group, which received only biofilm (P = 0.93; data not shown). In the third control group, there was no evidence that the weight simulating the weight of the transducer had an effect on viable counts in the biofilm (P = 0.26; data not shown). In the fourth control group, there was a significant reduction (P = 0.055; data not shown) in viable counts due to antibiotic treatment from approximately 6.22 to 5.24 log10 CFU/cm2, but it was small (mean difference, 0.89 ± 0.42). In the fifth control group, there was no statistically significant evidence that ultrasound without antibiotic had an effect on the number of viable bacteria in the biofilm (P = 0.80; data not shown).
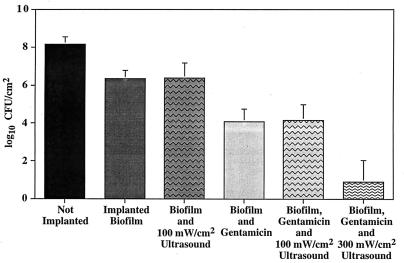
A similar statistical analysis was performed for the 100-mW/cm2 experimental group receiving both ultrasound and antibiotic. Mean viable counts on the ultrasound-treated side (log10 4.22 ± 0.29) were similar to those on the untreated side (log10 4.11 ± 0.23), and there was no significant difference between the ultrasound-treated and nontreated sides (P = 0.611; data not shown). There was little variation from side to side, but there was significant variation in viable CFU from rabbit to rabbit (P = 0.0034), indicating that the matched-pairs design was appropriate to account for variation between animals. A one-tailed Student’s t test of the mean difference between ultrasound-treated and nontreated sides in the 300-mW/cm2 group receiving both ultrasound and antibiotic showed that there was significant evidence of a reduction in viability (P = 0.0485) (Fig. 1). The average counts were reduced from 3.49 to 0.89 log10 CFU/cm2 by application of ultrasound. In one of the animals, ultrasound reduced viable counts to undetectable levels.
FIG. 1.
Results for biofilms implanted in rabbits treated with antibiotics and 100- or 300-mW/cm2 ultrasound. The data are log10 viable CFU from biofilm-coated disks. Not Implanted, control disks grown under the same conditions and at the same time as those implanted into the rabbit but plated immediately to determine average CFU in the original biofilm; Implanted Biofilm, disks recovered from the rabbit which received no treatment, removed 48 h postplacement. Error bars indicate one standard deviation from the mean.
Histopathology indicated necrosis, edema, a fibrinous capsule, and inflammation in the skin and skeletal muscle tissues around the implant sites. In addition, there was ulceration in skin tissue on the treated side. Visual observation showed a darkening of the skin under the transducer, indicating evidence of a burn or other type of skin damage. Large numbers of bacteria were present in the tissues of the 100-mW/cm2 group but not the 300-mW/cm2 group.
None of the blood samples, before, during, or after treatment from any of the rabbits showed any signs of E. coli bacteremia (data not shown). The tissue around the implant site had large numbers of bacteria, but none of the other organs and tissues sampled showed any colonization by E. coli.
DISCUSSION
The purpose of these experiments was to determine if low-frequency (28.48-kHz), low-power (100- and 300-mW/cm2) ultrasound in combination with antibiotic therapy would cause significant reduction in the number of viable bacteria in an implanted bacterial biofilm.
Our model showed up to a 4.0 log10 reduction in viable bacteria in the biofilm with antibiotics alone but never complete eradication of CFU on the implant. These results are consistent with other research involving implantation of a bacterial biofilm and treatment with antibiotics in a rabbit model (6, 10, 13, 14).
We showed that there was no significant difference in viable counts between ultrasound-treated and nontreated sides in the 100-mW/cm2 group. In the 300-mW/cm2 group, viable counts were reduced a total of 6.0 log10, with a 2.39-log10 (P = 0.048) further reduction of biofilms treated with antibiotics and ultrasound versus treatment with antibiotic alone. This indicates that ultrasound at this power density enhanced antibiotic action against biofilm bacteria and viable counts can be significantly reduced with a combination of ultrasound and aminoglycoside antibiotic.
Another significant observation is that the ultrasonic procedure, even at higher intensity, did not cause bacteremia. No bacteria were detected in the blood, heart, liver, or kidney. Apparently, this low power density did not cause the biofilm to break up in a way to disseminate bacteria into the blood. This is consistent with our in vitro observations (20).
There was no damage to the rabbit skin at 100 mW/cm2, but there was damage at the 300-mW/cm2 power level, which we presume to be due to cavitation induced in the skin tissue by ultrasound. It was noted that the damage to skin in the temperature probe experiment was not observed until several days after the experiment, whereas skin damage was immediately apparent in experiments with implanted disks. We attribute this difference to the stress and inflammation caused by the surgery and septic disk, which may have compromised blood flow to the adjacent skin.
Ibuprofen was administered to alleviate discomfort to the animal. Ibuprofen at high concentrations (1 mg/ml) has been shown to inhibit adherence of Staphylococcus epidermidis to medical polymers (4) and inhibit polymorphonuclear leukocyte (PMN) function (9, 12). However, we do not think that the use of ibuprofen compromised the significance of these data, because the biofilms were formed before they were implanted and the ibuprofen levels (70 mg/kg) were much lower than those used for in vitro tests of PMN function. Even if there were some reduction of PMN function, it would occur on both implants, and thus the side-to-side comparison shows enhancement of the action of the antibiotic working with the host immune system.
This research has demonstrated significant evidence that low-frequency and low-power-density ultrasound, when combined with aminoglycoside antibiotics, reduces the number of viable bacteria in an in vivo biofilm. Although the skin damage at higher powers was disappointing, we are very encouraged by the low bacterial counts and the absence of dissemination of bacteria to the blood and other organs. Obviously, there are many more refinements to be made to improve this procedure before it can be used clinically. Our next aim is to study intermediate intensities and pulsed ultrasound to determine if bacteria can be killed without tissue damage. We also plan to extend this technology to other bacteria, antibiotics, and implant models.
ACKNOWLEDGMENTS
Support was provided by NIH grant R01HL59923 and a grant from the Whitaker Foundation.
The transducers and technical expertise were donated by Gordon Snow of Edo Acoustics.
REFERENCES
- 1.Anderson J M. Inflammatory response to implants. Trans Am Soc Artif Intern Organs. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Costerton J W, Cheng K J, Geesy G G, et al. Biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche R O, Dhir A, Miller A J, Landon G C, Raad I I, Musher D M. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170:720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 4.Farber B F, Wolff A G. The use of nonsteroidal antiinflammatory drugs to prevent adherence of Staphylococcus epidermidis to medical polymers. J Infect Dis. 1992;166:861–865. doi: 10.1093/infdis/166.4.861. [DOI] [PubMed] [Google Scholar]
- 5.Isiklar Z U, Darouiche R O, Landon G C, Beck T. Efficacy of antibiotics alone for orthopaedic device related infections. Clin Orthop Relat Res. 1996;332:184–189. doi: 10.1097/00003086-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Jayabalan M, Kumar N S, Rathinham K, Kumari T V. In vivo biocompatibility of an aliphatic crosslinked polyurethane in rabbit. J Biomed Mater Res. 1991;25:1431–1442. doi: 10.1002/jbm.820251203. [DOI] [PubMed] [Google Scholar]
- 7.Johnson L L, Peterson R V, Pitt W G. Treatment of bacterial biofilms on polymeric biomaterials using antibiotics and ultrasound. J Biomater Sci Polym Ed. 1998;9:1177–1185. doi: 10.1163/156856298x00712. [DOI] [PubMed] [Google Scholar]
- 8.Khoury A E, Lam K, Ellis B, Costerton J W. Prevention and control of bacterial infections associated with medical devices. Am Soc Artif Intern Organs Trans. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Maderazo E G, Breaux S P, Woronick C L. Inhibition of human polymorphonuclear leukocyte cell responses by ibuprofen. J Pharm Sci. 1984;73:1403–1406. doi: 10.1002/jps.2600731020. [DOI] [PubMed] [Google Scholar]
- 10.Morck D W, Olson M E, McKay S G, Lam K, Prosser B, Cleeland R, Costerton J W. Therapeutic efficacy of fleroxacin for eliminating catheter-associated urinary tract infection in a rabbit model. Am J Med. 1993;94(Suppl. 3A):23S–30S. [PubMed] [Google Scholar]
- 11.Nickel J C, Grant S K, Lam K, Olson M E, Costerton J W. Bacteriologically stressed animal model of new closed catheter drainage system with microbicidal outlet tube. Urology. 1991;38:280–289. doi: 10.1016/s0090-4295(91)80363-c. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen V G, Webster R O. Inhibition of human polymorphonuclear leukocyte functions by ibuprofen. Immunopharmacology. 1987;13:61–71. doi: 10.1016/0162-3109(87)90027-0. [DOI] [PubMed] [Google Scholar]
- 13.Olson M E, Nickel J C, Khoury A E, Morck D W, Cleeland R, Costerton J W. Amindocillin treatment of catheter-associated bacteriuria in rabbits. J Infect Dis. 1989;159:1065–1072. doi: 10.1093/infdis/159.6.1065. [DOI] [PubMed] [Google Scholar]
- 14.Owusu-Ababio G, Rogers J A, Morck D W, Olson M E. Efficacy of sustained release ciprofloxacin microspheres against device-associated biofilm infection in a rabbit peritoneal model. J Med Microbiol. 1995;43:368–376. doi: 10.1099/00222615-43-5-368. [DOI] [PubMed] [Google Scholar]
- 15.Peterson R V, Pitt W G. Mechanisms of the ultrasonically-enhanced killing of E. coli biofilms on polyethylene surfaces. Master’s thesis. Provo, Utah: Brigham Young University; 1997. [Google Scholar]
- 16.Pitt W G, McBride M O, Lunceford J K, Roper R J, Sagers R D. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob Agents Chemother. 1994;38:2577–2582. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potera C. Biofilms invade microbiology. Science. 1996;273:1795–1797. doi: 10.1126/science.273.5283.1795. [DOI] [PubMed] [Google Scholar]
- 18.Qian Z, Sagers R D, Pitt W G. Investigation of the mechanism of the bioacoustic effect. J Biomed Mater Res. 1999;44:198–205. doi: 10.1002/(sici)1097-4636(199902)44:2<198::aid-jbm10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Qian Z, Sagers R D, Pitt W G. The effect of ultrasonic frequency upon enhanced killing of P. aeruginosa biofilms. Ann Biomed Eng. 1997;25:69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 20.Qian Z, Stoodley P, Pitt W G. Effect of low-intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials. 1996;17:1975–1980. doi: 10.1016/0142-9612(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 21.Read R R, Eberwein P, Dasgupta M K, Grant S K, Lam K, Nickel C, Costerton J W. Peritonitis in peritoneal dialysis: bacterial colonization by biofilm spread along the catheter surface. Kidney Int. 1989;35:614–621. doi: 10.1038/ki.1989.30. [DOI] [PubMed] [Google Scholar]
- 22.Rediske A M, Hymas W C, Wilkinson R, Pitt W G. Ultrasonic enhancement of antibiotic action on several species of bacteria. J Gen Appl Microbiol. 1998;44:283–388. doi: 10.2323/jgam.44.283. [DOI] [PubMed] [Google Scholar]
- 23.Rediske A M, Rapoport N, Pitt W G. Reversing bacterial resistance to antibiotics with ultrasound. Lett Appl Microbiol. 1998;28:81–84. doi: 10.1046/j.1365-2672.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal K L. Bacterial infections and antibiotic therapy in small mammals. Suppl Compend Contin Educ Pract Vet. 1998;20:13–22. [Google Scholar]
- 25.Stewart P S, Griebe T, Srinivasan R, Chen C-I, Yu F P, DeBeer D, McFeters G A. Comparison of respiratory activity and culturability during monochloramine disinfection of binary population biofilms. Appl Environ Microbiol. 1994;60:1690–1692. doi: 10.1128/aem.60.5.1690-1692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suci P A, Mittelman M W, Yu F P, Geesey G G. Investigation of ciprofloxacin penetration into Pseudomonas aerugionsa biofilms. Antimicrob Agents Chemother. 1994;38:2125–2133. doi: 10.1128/aac.38.9.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward K H, Olson M E, Lam K, Costerton J W. Mechanism of persistent infection associated with peritoneal implants. J Med Microbiol. 1992;36:406–413. doi: 10.1099/00222615-36-6-406. [DOI] [PubMed] [Google Scholar]