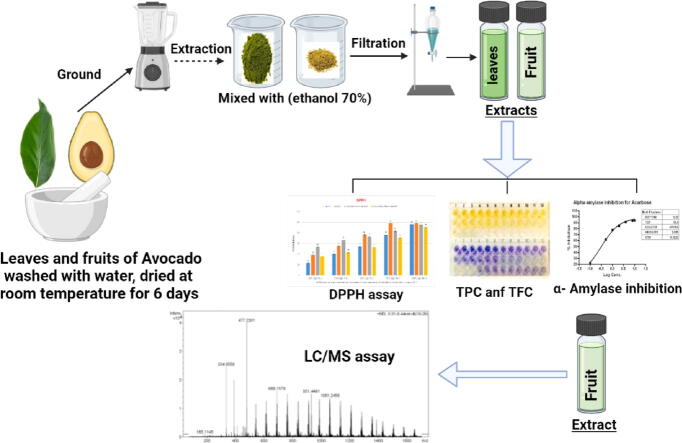
Graphical abstract
Keywords: Avocado fruit, Leaves, Extraction, Antioxidant activity, Amylase inhibition, Phenolic compounds, LC/MS
Abstract
Diabetes is a worldwide public health disease. Currently, the most effective way to treat diabetes is to mitigate postprandial hyperglycemia by inhibiting carbohydrate hydrolysis enzymes in the digestive system. Plant extracts are rich in bioactive compounds, which can be used in diabetes treatment. This study aims to evaluate the polyphenols content in ethanolic extracts of avocado fruit and leaves (Persea americana Mill.). Additionally, their antioxidant activity using DPPH, while the inhibition ability of α-amylase was examined by reacting different amounts of the extracts with α-amylase compared to acarbose as standard inhibitor. The active compounds were detected in the extracts by LC/MS. The obtained results showed that the leaf extract recorded a significant content of total phenolic compounds compared to the fruit extract (178.95 and 145.7 mg GAE /g dry weight, respectively). The total flavonoid values ranged from 32.5 to 70.08 mg QE/g dry weight of fruit and leaves extracts, respectively. Twenty-six phytogenic compounds were detected in leaf and fruit extract by LC/MS. These compounds belong to fatty acids, sterols, triterpenes, phenolic acids, and flavonoids. The antioxidant activity of the extracts is due to the exist of phytogenic compounds, i.e., polyphenols and flavonoids. The antioxidant activity increased in a concentration dependant manner. Avocado fruit extract (1000 µg/mL) scavenged 95% of DPPḢ while leaf extract rummaged 91.03% of free radicals compared with Vit C and BHT. Additionally, higher α-amylase inhibitory activity was observed in fruit extract than the leaf extract, where the fruit and leaf extract (1000 μg/ml) inhibited the enzyme by 92.13% and 88.95%, respectively. The obtained results showed that the ethanolic extracts of avocado could have a significant impact on human health due to their high content of polyphenols.
1. Introduction
The use of natural extracts with pharmacological activity has gain great interest because they have therapeutic potential in treatment of different diseases with low side effects. Hyperglycemia increases the production of different active free radicals, which results in diabetic complications, such as oxidative damage of tissue and nephropathy, neuropathy, retinopathy, and memory disorder (Maritim et al., 2003). Abnormal blood sugar levels are a symptom of diabetes, This is due to the immune system inability to create enough insulin (Petersmann et al., 2018). Hyperglycemia occurs after feeding because of starch hydrolyzes by α-amylase and α-glucosidase to large amounts of glucose. The intestinal enzymes must be inhibited to treat type 2 diabetes (Wang et al., 2013). Glucosidase is a one of digestive enzymes that degrade dietetic carbohydrates in simple monosaccharides. Glucosidase inhibitors, such as acarbose, reduce the speed of carbohydrate digestion and delays the absorption of carbohydrates from the digestive tract. Therefore, they can prevent the start of type 2 diabetes by reducing the level of postprandial glucose (Liu et al., 2011). Avocado (Persea americana Mill.) is subtropical/tropical fruit, a member of Lauraceae and Persea family. It is a Mexican origin. It is extensively cultivated in the Central Region of the United State and consumed worldwide (Melgar et al., 2018). It has become very popular recently and is often sold as a “superfood” due to its unique nutritional content, phytochemical composition, and health benefits. (Segovia et al., 2018). In addition to being used as food, is traditionally used for a variety of medicinal purposes, including lowering blood pressure, lowering blood sugar, antivirals, antidiarrheals, and cardiovascular disease (Cortés-Rojo et al., 2019). Therefore, these fruits also have analgesic, anti-inflammatory, and antifungal properties (Jesus et al., 2015). In general, it has been hypothesized that biomolecular oxidation can cause several health problems, including diabetes, pancreatitis, and ageing. (Finkel and Holbrook, 2000). There is evidence that free radicals are to responsible for these potential threats. (Halliwell and Chirico, 1993). Free radicals are unstable species because they have unpaired electron pairings with biological macromolecules. Free radicals are well recognized to play a crucial role in extraversion, mainly in organs as brain and pancreas. (Shulman et al., 2004). The uncontrolled activity of ROS is related to health disorders, such as diabetes, hypertension, cancer, neurological illnesses, gastric ulcers, reperfusion therapy, arthritis, and inflammatory diseases. (Vajragupta et al., 2000). Secondary metabolites seem to be the most analysed phytochemical group in fruits and vegetables (Domínguez-Avila et al., 2017). The most abundant phenolic compounds in avocado were phenolic acids, flavonoids and tannins (Tremocoldi et al., 2018). Avocado wastes have recently rekindled researchers' interest due to their high content of carbohydrates, lipids, protein, fiber, minerals, and other physiologically active components (Lara-Flores et al., 2018). Organic acids, phenolic alcohol derivatives, flavonoids, procyanidins, terpenes, alkaloids, saponins, acetin, phytosterols, and other polar and non-polar chemicals are illustrations. LC-MS, GC–MS, and FTIR are the most devices which used to detect and quantify these physiologically active compounds (Alkhalaf et al., 2019). Avocado leaves are a medicinal herb that are commonly utilised in extract formulations for therapeutic purposes as well as in folk medicine as a tea (Isaac, 2020), This could be because of its diuretic characteristics. (Wright et al., 2007). Rhamnetin, luteolin, rutin, quercetin, and apigenin are phytochemicals extracted from avocado leaves that can inhibit the progression of oxidative stress-related disorders (Owolabi et al., 2010). Avocado wastes are a rich source of several bioactive chemicals with significant nutritional, health, and pharmacological implications, the potential of avocado by-products were discussed in a recent review of (Coman et al., 2020). Avocado bioactive compounds have been shown to reduce oxidative stress (Melgar et al., 2018) and inflammatory processes (Tremocoldi et al., 2018), as well as modulate lipids, according to Pahua-Ramos et al., (2012). This prospective study was designed to investigate the bioactive compounds included phenolic and flavonoids in avocado extracts by LC/MS, detecting their activities; antioxidant activity, and α-amylase. Aiming to provide more results about biochemical structure of avocado extracts and study of the effect of these extracts as hypoglycemic agent.
2. Material and methods
2.1. Chemicals
Merck Company supplied the ethanol. Sigma-Aldrich Company provided the Folin-Ciocalteau-phenol reagent (FC), 1,1 diphenyl-2-picryl-hydrazyl (DPPH), ascorbic acid, and butylated hydroxyl toluene (BHT). Sigma-Aldrich Company supplied α-amylase enzyme and acarbose inhibitor. All the other chemicals were of the highest commercial purity.
2.2. Preparation of plant extracts
Avocado leaves and fruits (500 g) were dried in a vacuum oven (45 °C) for 5 h. 100 g of the powder was homogenized in 1000 mL ethanol 70% (1:10, w/v) and stirred for 3 h at room temperature, then filtrated through (Whatman No.1) paper (Saad et al, 2021a). A rotary evaporator was used to recover the solvent in the filtrate and the obtained free solvent extracts were adjusted to a concentration of 100 mg/mL and stored at 4 °C for further analyses.
2.3. Phytochemical analyses
2.3.1. Determination of total phenolics
The total polyphenols (TP) were evaluated according to Saad et al, (2021b). In brief, 50 µL of fruit and leaves avocado extracts (500 µg/mL) was mixed with 50 µL of diluted Folin-Ciocalteu reagent and 50 µL Na2CO3 (7.5%) in microtiter plate wells, and incubated at room temperature for 60 min, then the absorbance was measured at 760 nm using microtiter plate reader (BioTek Elx808, USA). The total polyphenols content was presented as mg gallic acid equivalent/mL of solutions.
2.3.2. Determination of total flavonoids
Total flavonoids content (TF) was estimated as per Saad et al, (2021c). 20 µL of of fruit and leaves avocado extracts (500 µg/mL) was mixed with 20 µL of sodium nitrite (5%) in microtiter plate wells and 20 µL of ethanolic AlCl3 (10%) was added. The plate was incubated at room temperature for 10 min. 50 µL of NaOH was added to stop the reaction. the absorbance was measured at 450 nm, and TF was expressed as mg quercetin equivalent per mL of each solution.
2.4. DPPH radical scavenging activity
The free radical scavenging activity of polyphenolic compounds was measured using 1, 1-diphenyl-2-picryl-hydrazil (DPPH•) according to the method of (WANG et al., 2008, El-Saadony et al., 2020) DPPH• (0.1 mM) was prepared in methanol. Successive concentration from avocado fruit and leaves extracts and standard materials; BHT and Ascorbic Acid were prepared as 100, 250, 500, 750, and 1000 µg/ml in methanol. 1 mL of DPPH• Solution of 3 mL of each concentration was added, then vigorously stirred up the mixture and permitted to stand 30 min at room temperature. The same procedure was used to test checks, except for samples of methanol. A spectrophotometer read the absorption at 517 nm (Jasco, serial No. C317961148, Japan). According to following equation DPPH• radical scavenging activity was calculated:
| (1) |
Where Abs control was the absorbance of the control and Abs sample was the absorbance in the presence of the sample of Avocado extracts (fruits and leaves).
2.5. Determination of α-amylase inhibitory activity
The hydrolysis of 1,4 glycosidic bonds in starch and other similar carbohydrates is catalysed by -amylase (EC 3.2.1.1). In particular, α-amylase participates in glucose digestion and is considered a key enzyme that can control postprandial hyperglycemia. The assay's principle is based on the fact that reducing sugars have the ability to reduce a variety of reagents; chemically, they form an aldehyde or ketone in basic solution, and the aldehyde group of glucose converts DNS to its reduced form (3-amino-5-nitrosalicylic acid), which results in a change in the amount of light absorbed at 540 nm. The proportion of each extract's -amylase inhibitory activity values was obtained using this method, as follows:
| (2) |
the extracts inhibitory values were estimated by (El Souda et al., 2017).
2.6. Detection bioactive compounds by LC/MS
Electrospray Ionisation Mass Spectrometry (ESI-MS, XEVO TQD, Waters Corporation, Milford, MA01757 U.S.A) was used to detect the bioactive compounds in avocado extracts. The device occupied with C18 column (ACQUITY UPLC – BEH); 1.7–2.1 µm × 50 mm with flow rate of 0.2 mL\min. The solvent system consisted of (A) Water containing 0.1 % formic acid. (B) Methanol containing 0.1 % formic acid.
The sample (100 μg/mL) solution was prepared using methanol (HPLC analytical grade), filtered using a membrane disc filter (0.2 μm) then subjected to LC-ESI-MS analysis. 10 μL of sample was injected into the UPLC instrument equipped with reverse phase C-18 column. The sample was eluted using gradient elution. The parameters for analysis were carried out using negative ion mode as follows: source temperature 150 °C, cone voltage 30 eV, capillary voltage 3 kV, desolvation temperature 440 °C, cone gas flow 50 L/h, and desolvation gas flow 900 L/h. The data were detected in 100–1000 m/z mass spectra range and processed using the Maslynx 4.1 software, then identified by comparing its retention time (Rt) and mass spectrum with reported data (Bakr et al., 2021).
2.7. Statistical analysis
One way ANOVA in SPSS 16 was used to conduct statistical analysis. The results were obtained as a mean standard deviation (SD). The significance of differences between means was assessed at p ‹ 0.05 using post hoc Duncan's test.
3. Results
The higher polyphenol and flavonoids contents were detected in fruit and leaves ethanolic extracts. It can be seen from the data that the leaves’ extract reported significantly more than Fruit extract it was 178.95 and 145.7 mg GAE acid/g DW, respectively. The values of flavonoids contents ranged from 32.5 to 70.08 mg QE /g DW for fruit and leaves ethanolic extracts, respectively.
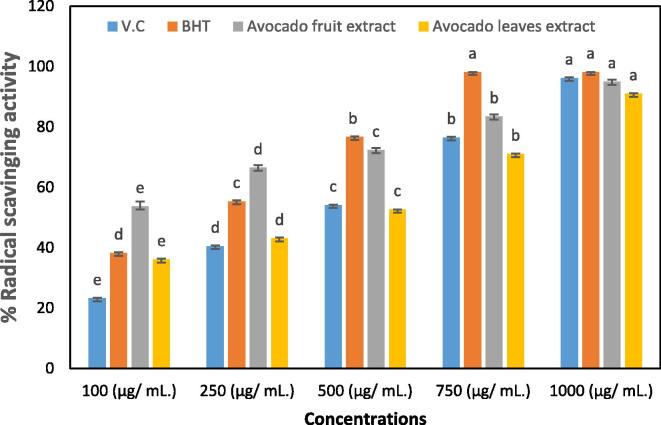
Table 1 and Fig. 1 illustrate the free radical scavenging activities of Persea americana fruits and leaves. DPPH value of concentration (1000 µg / mL) of avocado fruit extract was 95.00 ± 0.68 %, while bring down at lower concentrations 100, 250, 500 and 750 µg/mL causes 63.70 ± 1.6, 66.47 ± 0.82, 72.32 ± 0.74 and 83.43 ± 0.76 %. The results of avocado leaves extract were 91.03 ± 0.19 %, At 1000 µg / mL, which also reduces at concentrations 100, 250, 500 and 750 µg/mL the inhibition percent were 36.11 ± 0.27, 43.07 ± 0.30, 52.66 ± 0.05, and 71.03 ± 0.19 %, respectively. These results are compared with V.C and BHT as standard the outcome at high concentrate 1000 µg/mL was 99.84 ± 0.15 and 98.18 ± 0.17 %, respectively although the reduction of the different concentrations 100, 250, 500 and 750 µg/mL caused 73.00 ± 1.15, 90.09 ± 0.51, 94.42 ± 0.54 and 96.62 ± 0.23 %, respectively for vitamin C and it’s were 80.33 ± 0.29, 95.99 ± 0.07, 96.16 ± 0.18 and 98.05 ± 0.08 % for BHT butylated hydroxytoluene, respectively.
Table 1.
DPPH scavenging activity of avocado fruit and leaves crude extracts compared with two-reference standard Vit. C and BHT.
| Conc. (μg/ mL.) | AFE | ALE | V.C | BHT |
|---|---|---|---|---|
| 100 | 63.70 ± 1.6c | 36.11 ± 0.27e | 73.00 ± 1.15e | 80.33 ± 0.29c |
| 250 | 66.47 ± 0.82c | 43.07 ± 0.30d | 90.09 ± 0.51d | 95.99 ± 0.07b |
| 500 | 72.32 ± 0.74b | 52.66 ± 0.05c | 94.42 ± 0.54c | 96.16 ± 0.18b |
| 750 | 83.43 ± 0.76b | 71.03 ± 0.19b | 96.62 ± 0.23b | 98.05 ± 0.08a |
| 1000 | 95.00 ± 0.68a | 91.03 ± 0.19b | 99.84 ± 0.15a | 98.18 ± 0.17a |
| IC50 | 55.03c | 421.6a | 86.10b | 49.19d |
Avocado fruit extract (AFE); Avocado leaves extract (ALE). The values are presented as a mean ± standard Error (SEM). Significant differences (p ≤ 0.05) are indicated by different superscripts within the same column.
Fig. 1.
DPPH radical scavenging of Avocado fruit and leaves crude extract in comparison with V.C and BHTas an antioxidants standard.
Table 2 shows the values of the α-amylase inhibitory activity affected by avocado fruit and leaves ethanolic extracts compared with acarbose inhibitor, both fruit and leaves extracts recorded lower values as 21.00 ± 0.30, 37.98 ± 0.52, and 51.38 ± 0.21%, and 18.67 ± 0.36, 30.69 ± 0.20 and 42.51 ± 0.51 %, respectively at concentrations 100, 250, and 500 μg/mL. while the avocado fruit extract had more inhibitory effects on α-amylase at high concentrations of (750 and 1000 μg/mL) than the avocado leaves and acarbose, where values were 82.60 ± 0.36 and 92.13 ± 0.03 % and 75.52 ± 0.24 and 88.95 ± 0.34 % and 78.50 ± 0.22 and 88.34 ± 0.23 %, respectively.
Table 2.
α- Amylase inhibition activity of the Avocado fruit and leaves ethanolic extracts compared with Acarbose inhibitor.
| Conc. (μg/ mL.) | AFE | ALE | Acarbose inhibitor |
|---|---|---|---|
| 100 | 21.00 ± 0.30e | 18.67 ± 0.36e | 23.35 ± 0.28e |
| 250 | 37.98 ± 0.52d | 30.69 ± 0.20d | 57.09 ± 0.15d |
| 500 | 51.38 ± 0.21c | 42.51 ± 0.51c | 69.54 ± 0.19c |
| 750 | 82.60 ± 0.36b | 75.52 ± 0.24b | 78.50 ± 0.22b |
| 1000 | 92.13 ± 0.03a | 88.95 ± 0.34a | 88.34 ± 0.23a |
| IC50 | 418 | 682 | 226 |
The results are presented as a mean ± standard Error (SEM). Significant differences (p ≤ 0.05) are indicated by different superscripts within the same column.
3.1. LC/MS analysis of Persea americana fruit extract
Table 3 shows that LC/MS analysis of fruit extract identified 26 chemical peaks for several classes such as fatty acids, sterols, triterpenes, phenolic acids, and flavonoids. In the MS spectrum of the tested extract, strong peaks of flavonoids fractions; M−162 and M−146, M−132, indicating the extense of flavonoids O-glycosides; hexoside or O-rhamnoside (deoxyhexose), O-pentoside. Isorhamentin derivatives allocated in peak no (6) showed molecular ion at (M−H) + at m/z 461.3723 with a main fragment ion at m/z 315, 300, 176 corresponding to (M−H- glucuronide group 194 which undergo decarboxylation to give characteristic fragment of uronic acid 176 So peak No 6 assigned for isorhamentin-O- glucuronide. Peak No (9) & (10) showed molecular ion at (M−H) + at m/z 447.3365 & 460.4063 with a main fragment ion at m/z 315 corresponding to isorhamentin nucleus derived from loss of [M−H−pentose]; and [M−H- -rhamnose- methoxy]; So, peaks No 9& 10 are assigned for isorhamnetin-O-pentoside & isorhamentin-O-rhamnoside, respectively. Peak No 7 with molecular ion at 431.2636 and main fragments 311 correspond to vitexin previously reported in the plant (Castro-López et al., 2019). Peak 11 with molecular ion peak at 595.5849 and fragment peak at 271, 433, correspond to (M + H − 2hexose) and is assigned to apigenin-O-dihexoside. Peak No 14 with molecular ion peak at 269.3015 assigned to aglycone apigenin. Tricin: Peaks 8 with molecular ion m/z 607.2245 and the base peak of aglycone appears at m/z 329. Due to the loss of rhamnosyle, pentoside unit so peak No 8 [M−H −146–132]- is assigned to Tricin-O-rhamnosyl pentoside. Peak No. 12 showed a molecular ion peak at m/z 329.55 [M−H]-, assigned to aglycone tricin; compounds 8 & 9 were first detected in the LC/MS of Persea americana Mill fruit extract. Anthocyanins related to Peak 3, 4 showed a molecular ion peak at m/z 577.5127(M−H]-; 433.3954 [M−H]-, and fragment ions at 425, 289 & 433,300, peak No 3 & 4 assigned to Procyanidin dimer and Peonidin 3-O-pentoside respectively. Peak 5 showed a molecular ion peak at m/z 289.6310 [M−H]-, correspond to catechin. Phenolic acids ionize in negative ESI and appear early in LC spectrum (Rt) due to their high polarity. Qunic acid was detected at 191.1228 and coumaric acid at 163.0880(peak 1& 2). The late Rt (26 to 30 min) showed several unsaturated, and saturated fatty acids starting with peak no 19, 21, 22, 23, 24, 25 and 26 respectively; 391.3430 Hexacosatrienoic acid, 277.3062 Linolenic acid; 253.3136 Palmitoleic acid; 279.3407 linoleic acid; 255.3120 Palmitic acid; 281.3320 Oleic acid ;283.3519; Stearic acid. Cycloartenol (Peak 15) 425.3806 (M + H) + ; Avenasterol (16) 413.3064 (M + H) + ; Campasterol (18)399.3789 (M−H)+ ; ɣ- Tocopherol (17) 415.3818; (M−H)+ ; α- Tocopherol (20) 430.3855 (M + H)+.
Table 3.
Provisional identification of chemical compounds in Persea Americana fruit total ethanolic extract by LC-MS technique.
| No. | Rt | Mol. ion(+) | Mol. ion(-) | formula | fragment | Compounds |
|---|---|---|---|---|---|---|
| 1 | 0.87 | 191.1228 | C9H20O4 | 191 | Qunic acid | |
| 2 | 7.74 | 163.0880 | C9H8O3 | 163 | p.Coumaric acid | |
| 3 | 15.00 | 577.5127(-) | C30H25O12 | 425, | Procyanidin dimer B | |
| 4 | 15.05 | 433.3954 | C21H21O11 | Peonidin 3-O-pentoside | ||
| 5 | 15.19 | 289.6310 | C15H14O6 | 289, 245 | Catechin | |
| 6 | 15.30 | 461.3723 | 315, 300, 176 | Isorhament-O-glycouride | ||
| 7 | 16.10 | 431.2636 | C21H20O10 | 311, | Vitexin | |
| 8 | 16.21 | 607.2245(-) | 329, 475, 607 | Tricin-O-rhamnosylpentoside | ||
| 9 | 20.97 | 447.3365 | 300,315,447 | Isorhamentin -O- pentoside | ||
| 10 | 21.16 | 460.4063 | 300, 460 | Isorhamentin- O - rhamnoside | ||
| 11 | 22.14 | 595.5849 | 269, 433, 595 | Apigenin-O-dihexoside | ||
| 12 | 22.55 | 329.55(-) | C17H14O7 | 329 | Tricin aglycone | |
| 13 | 25.37 | 281.3471 | C15H10O6 | 281 | Kaempferol aglycone | |
| 14 | 25.49 | 269.3015 | C15H10O5 | 269 | Apigenin aglycone | |
| 15 | 26.15 | 425.3806 | C30H50O | Cycloartenol | ||
| 16 | 26.22 | 413.3064 | C29H48O | Avenasterol | ||
| 17 | 26.31 | 415.3818 | C28H48O2 | γ - Tocopherol | ||
| 18 | 26.42 | 399.3789 | C28H48O | Campasterol | ||
| 19 | 26.48 | 391.3430 | C16H26O2 | Hexacosatrienoic acid | ||
| 20 | 26.90 | 430.3855 | C29H50O2 | α- Tocopherol | ||
| 21 | 27.53 | 277.3062 | C18H30O2 | Linolenic acid | ||
| 22 | 27.89 | 253.3136 | C16H30O2 | Palmitoleic acid | ||
| 23 | 28.64 | 279.3407 | C18H32O2 | linoleic acid | ||
| 24 | 28.77 | 255.3120 | C16H32O2 | Palmitic acid | ||
| 25 | 28.85 | 281.3320 | C18H34O2 | Oleic acid | ||
| 26 | 29.92 | 283.3519 | C18H36O2 | Stearic acid |
4. Discussion
The natural products of plant origin are mainly quarries to find promising reading candidates and play an ordering role in the next drug development program (Sharifi-Rad et al., 2018). Availability, low cost, and minimal side effects are especially the main key player of all treatments available in rural areas (Mansouri et al., 2015). In addition, many plants are released from undesirable side effects and provide a rich biological source of biologically active chemical products having a powerful pharmacological behavior (Sharifi-Rad et al., 2018). Many plants are considered a basic source of powerful antidiabetic medications. In developing countries, particularly medicinal plants deal with diabetes and exceed the burden of traditional drugs to the population (Arumugam et al., 2013). Recently, it is recommended to treat diseases such as diabetic containing diabetes using medicinal plants (Mansouri et al., 2015), which including bioactive compounds, flavonoids, terpenoids, saponins, carotenoids, alkaloids, glycolic, etc. act as anti-diabetic agents (Durazzo et al., 2018). Natural products of plant origin are rich in bioactive compounds with antioxidant (Saad et al., 2020a; El-Saadony et al., 2020; Abdel-Moneim et al, 2021) and antimicrobial activity (El-Tarabily et al, 2021). They are used as food additives for preserving juices (El-Saadony et al, 2021a), meat (Saad et al., 2020b, El-Saadony et al., 2021b), bakery (Saad et al., 2015; Saad et al, 2021d), and dairy (El-Saadony et al, 2021c). These activities are attributed to biologically active compounds (i.e, polyphenols, carotenoids, lignans, coumarin, glycosinological, etc.), resulting in possible beneficial properties of each plant matrix, which can be represented a first step for biological understanding. The antigram effects resulting from behavioral and beneficial activity plants generally occur by increasing insulin secretion, or due to the ability to improve pancreatic tissue performance carried out by reducing the intestinal absorption of glucose. The adverse effects of these episodes are significant and include subsequent Diabetes mellitus (DM) problems. Because ROS has been linked to the disease's aetiology, it is possible to use antioxidants for the logical technique for managing and expanding its potential burden (Swelum et al., 2020). It was therefore proven that antioxidants prevent the demise of the pancreatic beta cells through inhibition of automated oxidation reactions and therefore regulate diabetes progression towards complications (Liu et al., 2007). Synthetic antioxidants, on the other hand, are restricted in their usage due to their carcinogenicity, while plant antioxidants degrade at a much lower level of toxicity. The plants containing a natural antioxidant retain the function of the beta cell and prevent combined Diabetes mellitus (DM) (Aslan et al., 2010). This study demonstrates the antioxidant and α-amylase inhibitory activity of avocado fruit and leaves extracts. Alpha-amylase is a key enzyme for starch digestion and plays an important role in determining the amount of glucose released. Inhibition of enzyme activity is considered a potential method to control starch digestion and regulate postprandial hyperglycemia in diabetic patients. It is crucial in the creation of nutritional health products as well as the treatment of diabetes. The pathogenesis of diabetes is linked to oxidative free radicals (Baynes and Thorpe, 1999).
LC/MS analysis of the fruit extract of the avocado Persea americana Mill was conducted because of its highest inhibitory effect as antioxidants and its ability to inhibit the activity of the enzyme alpha-amylase the results revealed presence of unsaturated and saturated fatty acids, sterol, triterpenes, flavonoids, and phenolic compounds. Flavonoids have dominated in Lauraceae which Persea americana is a member. Kaempferol, apigenin and isorhamentin glycosides were the main flavaniods in this family (Owolabi et al., 2010) and (Tremocoldi et al., 2018) reported that Persea americana Millis contains Anthocyanins and catechin. (Wang et al., 2020) reported saturated and unsaturated fatty acids i.e., linolenic acid, Palmitoleic acid , linoleic acid, Palmitic acid, Oleic acid, Stearic acid in avocado plants at Rts (26–30 min). Cycloartenol (Peak 15); Avenasterol (16) ,Campasterol (18); ɣ- Tocopherol (17); α- Tocopherol (20) all these compounds were previously reported in the plant (Ramos-Aguilar et al., 2019). The presence of these compounds may be a reasonable cause of the hypoglycemic potential observed in this study. It has been shown to have anti-diabetic characteristics by regenerating pancreatic cells and suppressing carbohydrate metabolism enzyme activity (Kwon et al., 2008); (Chika and Bello, 2010). C. Droserifolia aqueous extract is traditionally used to treat hyperglycemia, according to (Abdel Motaal et al., 2020), Multiple in vitro and in vivo investigations have revealed anti-diabetic potential. The flavonol glycosides accounted for 78 % of the water extract, and there were five main peaks. These 5 flavonol glycosides have been demonstrated to inhibit intestinal enzymes involved in the hydrolysis of polysaccharides to glucose in vitro tests. Furthermore, by inhibiting aldose reductase and reducing oxidative stress, which is linked to the aetiology of diabetes complications, these flavonol glycosides can prevent insulin resistance. Generally, the attributes exhibited of avocado fruit extract are related to its phenols and flavonoids, which are recognized for having antioxidant capabilities. Flavonoids can be a natural phenolic compound of the highest diversity and widely distributed nature compounds (Ward, 1990). Its wide spectrum chemical and biological activities have been reported by (Prasad et al., 2009), including free radical scavenging. Flavanol has similar characteristics (Miliauskas et al., 2004), and it has been found to be an effective hypoglycemic agent (Kim et al., 2004) and had high alpha-glucosidase and alpha-amylase inhibitory effects (Li et al., 2018).
5. Conclusion
The general clarification of this study was LC/MS, antioxidants, total phenols, and total ethanol extract flavonoids, from avocado extracts, which made its application as an antidiabetic agent credible. In the treatment of diabetes, it is very important to highly use affinity plant inhibitors to regulate the activity of α-amylase. It promotes the use of a therapeutically significant natural plant because of its availability and relative safety (Persea americana Millis).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Taha F. Taha, Email: Taha_farhytaha@yahoo.com.
Ahmed Saad, Email: ahmedm4187@gmail.com.
References
- Abdel Motaal A., Salem H.H., Almaghaslah D., Alsayari A., Bin Muhsinah A., Alfaifi M.Y., Elbehairi S.E.I., Shati A.A., El-Askary H. Flavonol Glycosides. In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules. 2020;25(24):5864. doi: 10.3390/molecules25245864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalaf M.I., Alansari W.S., Ibrahim E.A., ELhalwagy M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. - Sci. 2019;31(4):1358–1362. [Google Scholar]
- Arumugam G., Manjula P., Paari N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013;2(3):196–200. [Google Scholar]
- Aslan M., Orhan N., Orhan D.D., Ergun F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010;128(2):384–389. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Bakr R.O., Shahat E.A., Elissawy A.E., Fayez A.M., Eldahshan O.A. Evaluation of the hepatoprotective activity of Pulicaria incisa subspecies candolleana and in silico screening of its isolated phenolics. J. Ethnopharmacol. 2021;271:113767. doi: 10.1016/j.jep.2020.113767. [DOI] [PubMed] [Google Scholar]
- Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Castro-López C., Bautista-Hernández I., González-Hernández M.D., Martínez-Ávila G.C.G., Rojas R., Gutiérrez-Díez A., Medina-Herrera N., Aguirre-Arzola V.E. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules. 2019;24(1):173. doi: 10.3390/molecules24010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chika A., Bello S.O. Antihyperglycaemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2010;129(1):34–37. doi: 10.1016/j.jep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Coman V., Teleky B.-E., Mitrea L., Martău G.A., Szabo K., Călinoiu L.-F., Vodnar D.C. Bioactive potential of fruit and vegetable wastes, Advances in food and nutrition research. Elsevier. 2020:157–225. doi: 10.1016/bs.afnr.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Cortés-Rojo C., Montoya-Pérez R., Rodríguez-Orozco A.R., Saavedra-Molina A., Calderón-Cortés E. Avocado Oil and Diabetic Complications Related to Mitochondrial Dysfunction, Bioactive Food as Dietary Interventions for Diabetes. Elsevier. 2019:89–101. [Google Scholar]
- Domínguez-Avila J.A., Wall-Medrano A., Velderrain-Rodríguez G.R., Chen C.-Y., Salazar-López N.J., Robles-Sánchez M., González-Aguilar G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Func. 2017;8(1):15–38. doi: 10.1039/c6fo01475e. [DOI] [PubMed] [Google Scholar]
- Durazzo A., D’Addezio L., Camilli E., Piccinelli R., Turrini A., Marletta L., Marconi S., Lucarini M., Lisciani S., Gabrielli P., Gambelli L., Aguzzi A., Sette S. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules (Basel, Switzerland) 2018;23(8):1844. doi: 10.3390/molecules23081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Souda S.M., Mohammed RedaS, Ibrahim FatenM, Matloub AzzaA. Harpullia pendula Planch leaves: phenolics, in vitro antioxidant and α-amylase inhibitory activity. Egypt. Pharm. J. 2017;16(2):103. doi: 10.4103/epj.epj_10_17. [DOI] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., E. Abd El-Hack M. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9(5):639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., S. F. Khalil O., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: Biological activity, shelf life and quality properties during cold preservation. Saudi Journal of Biological Sciences. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Italian Journal of Animal Science. 2021;20(1):762–776. [Google Scholar]
- El-Saadony M.T., Saad A.M., Elakkad H.A., El-Tahan A.M., Alshahrani O.A., Alshilawi M.S., Ahmed A.I. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4° C through controlling chemical and microbial fluctuations. Saudi Journal of Biological Sciences. 2021 doi: 10.1016/j.sjbs.2021.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Halliwell, B., Chirico, S., 1993. Lipid peroxidation: its mechanism, measurement, and significance. The American journal of clinical nutrition 57, 715S-725S [DOI] [PubMed]
- Isaac U.E. Ameliorative Effect of Administering Avocado (Persea americana) Leaf Extract on Lead Acetate Toxicity in the Brain-cerebellum of Albino Rats. J Altern Complement Med. 2020:29–37. [Google Scholar]
- Jesus D., Oliveira J.R., Oliveira F.E., Higa K.C., Junqueira J.C., Jorge A.O.C., Back-Brito G.N., Oliveira L.D. Persea americana Glycolic Extract. In Vitro Study of Antimicrobial Activity against Candida albicans Biofilm and Cytotoxicity Evaluation. Sci. World J. 2015;2015:1–5. doi: 10.1155/2015/531972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Moon B.H., Lee H.J., Choi D.H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 2004;93(2–3):227–230. doi: 10.1016/j.jep.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Kwon Y.-I., Apostolidis E., Shetty K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresource Technol. 2008;99(8):2981–2988. doi: 10.1016/j.biortech.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Lara-Flores A.A., Araújo R.G., Rodríguez-Jasso R.M., Aguedo M., Aguilar C.N., Trajano H.L., Ruiz H.A. Bioeconomy and biorefinery: valorization of hemicellulose from lignocellulosic biomass and potential use of avocado residues as a promising resource of bioproducts. Waste to Wealth. Springer. 2018:141–170. [Google Scholar]
- Li, K., Yao, F., Xue, Q., Fan, H., Yang, L., Li, X., Sun, L., Liu, Y., 2018. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chemistry Central journal 12, 82-82. [DOI] [PMC free article] [PubMed]
- Liu C.-T., Sheen L.-Y., Lii C.-K. Does garlic have a role as an antidiabetic agent? Molecular Nutrition & Food Research. 2007;51(11):1353–1364. doi: 10.1002/mnfr.200700082. [DOI] [PubMed] [Google Scholar]
- Liu L., Deseo M.A., Morris C., Winter K.M., Leach D.N. Investigation of α-glucosidase inhibitory activity of wheat bran and germ. Food Chem. 2011;126(2):553–561. [Google Scholar]
- Mansouri E., Kooti W., Bazvand M., Ghasemi Boroon M., Amirzargar A., Afrisham R., Afzalzadeh M.R., Ashtary-Larky D., Jalali N. The Effect of Hydro-Alcoholic Extract of Foeniculum vulgare Mill on Leukocytes and Hematological Tests in Male Rats. Jundishapur J. Nat. Pharm. Prod. 2015;10(1) doi: 10.5812/jjnpp.10.17795/jjnpp-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Melgar B., Dias M.I., Ciric A., Sokovic M., Garcia-Castello E.M., Rodriguez-Lopez A.D., Barros L., Ferreira I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind Crops Prod. 2018;111:212–218. [Google Scholar]
- Miliauskas G., Venskutonis P.R., van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85(2):231–237. [Google Scholar]
- Owolabi Mbang A., Abass Moyosola M., Emeka Promise M., Jaja Smith I., Nnoli M., Dosa B.S. Biochemical and histologic changes in rats after prolonged administration of the crude aqueous extract of the leaves of Vitex grandifolia. Pharmacognosy Research. 2010;2(5):273. doi: 10.4103/0974-8490.72322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahua-Ramos M.E., Ortiz-Moreno A., Chamorro-Cevallos G., Hernández-Navarro M.D., Garduño-Siciliano L., Necoechea-Mondragón H., Hernández-Ortega M. Hypolipidemic Effect of Avocado (Persea americana Mill) Seed in a Hypercholesterolemic Mouse Model. Plant Foods for Human Nutrition. 2012;67(1):10–16. doi: 10.1007/s11130-012-0280-6. [DOI] [PubMed] [Google Scholar]
- Petersmann A., Nauck M., Müller-Wieland D., Kerner W., Müller U., Landgraf R., Freckmann G., Heinemann L. Definition, Classification and Diagnosis of Diabetes Mellitus. Experimental and Clinical Endocrinology & Diabetes. 2018;126(07):406–410. doi: 10.1055/a-0584-6223. [DOI] [PubMed] [Google Scholar]
- Prasad K.N., Yang B., Dong X., Jiang G., Zhang H., Xie H., Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov. Food Sci. Emerg. Technol. 2009;10(4):627–632. [Google Scholar]
- Ramos-Aguilar A.L., Ornelas-Paz J., Tapia-Vargas L.M., Ruiz-Cruz S., Gardea-Béjar A.A., Yahia E.M., Ornelas-Paz J.d.J., Pérez-Martínez J.D., Rios-Velasco C., Ibarra-Junquera V. The importance of the bioactive compounds of avocado fruit (Persea americana Mill) on human health. Biotecnia. 2019;21(3):154–162. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. In: Int. Veg J., editor. Sci; 2020. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts; pp. 1–11. [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: Characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26(1):567–577. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26(15):4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad, A.M., El-Saadony, M.T., El-Tahan, A.M., Sayed, S., Moustafa, M.A., Taha, A.E., Taha, T.F., Ramadan, M.M., 2021c. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. in press [DOI] [PMC free article] [PubMed]
- Saad A.M., Elmassry R.A., Wahdan K.M., Ramadan F.M. Chickpea (Cicer arietinum) steep liquor as leavening agent: effect on dough rheology and sensory properties of bread. . Acta Periodica Technologica. 2015;46:91–102. [Google Scholar]
- Saad A.M., El‐Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. 2021;56(7):3255–3268. [Google Scholar]
- Segovia F., Hidalgo G., Villasante J., Ramis X., Almajano M. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules. 2018;23(10):2421. doi: 10.3390/molecules23102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M., Roberts T.H., Matthews K.R., Bezerra C.F., Morais-Braga M.F.B., Coutinho H.D.M., Sharopov F., Salehi B., Yousaf Z., Sharifi-Rad M., del Mar Contreras M., Varoni E.M., Verma D.R., Iriti M., Sharifi-Rad J. Ethnobotany of the genus Taraxacum-Phytochemicals and antimicrobial activity. Phytotherapy Research. 2018;32(11):2131–2145. doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- Shulman R.G., Rothman D.L., Behar K.L., Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends in Neurosciences. 2004;27(8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Tremocoldi, M.A., Rosalen, P.L., Franchin, M., Massarioli, A.P., Denny, C., Daiuto, É.R., Paschoal, J.A.R., Melo, P.S., Alencar, S.M.d., 2018. Exploration of avocado by-products as natural sources of bioactive compounds. PloS one 13, e0192577-e0192577. [DOI] [PMC free article] [PubMed]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Mohamed E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajragupta O., Boonchoong P., Wongkrajang Y. Comparative quantitative structure–activity study of radical scavengers. Bioorganic & Medicinal Chemistry. 2000;8(11):2617–2628. doi: 10.1016/s0968-0896(00)00195-4. [DOI] [PubMed] [Google Scholar]
- WANG B., LI B., ZENG Q., LIU H. Antioxidant and free radical scavenging activities of pigments extracted from molasses alcohol wastewater. Food Chemistry. 2008;107(3):1198–1204. [Google Scholar]
- Wang H., Liu T., Huang D. Starch Hydrolase Inhibitors from Edible Plants, Advances in Food and Nutrition Research. Elsevier. 2013:103–136. doi: 10.1016/B978-0-12-416555-7.00003-5. [DOI] [PubMed] [Google Scholar]
- Wang, M., Yu, P., Chittiboyina, A.G., Chen, D., Zhao, J., Avula, B., Wang, Y.-H., Khan, I.A., 2020. Characterization, Quantification and Quality Assessment of Avocado (Persea americana Mill.) Oils. Molecules (Basel, Switzerland) 25, 1453. [DOI] [PMC free article] [PubMed]
- Ward, R.S., 1990. Carbon-13 NMR of flavonoids (studies in organic chemistry series, no. 39). P. K. Agrawal (Ed.). Elsevier, Amsterdam, 1989. Magnetic Resonance in Chemistry 28, 562-563.
- Wright C.I., Van-Buren L., Kroner C.I., Koning M.M.G. Herbal medicines as diuretics: A review of the scientific evidence. J. Ethnopharmacol. 2007;114(1):1–31. doi: 10.1016/j.jep.2007.07.023. [DOI] [PubMed] [Google Scholar]