Abstract
Loss-of-function mutations in parkin is associated with onset of juvenile Parkinson’s disease (PD). Resveratrol is a polyphenolic stilbene with neuroprotective activity. Here, we evaluated the rescue action of resveratrol in parkin mutant D. melanogaster. The control flies (w1118) received diet-containing 2% ethanol (vehicle), while the PD flies received diets-containing resveratrol (15, 30 and 60 mg/kg diet) for 21 days to assess survival rate. Consequently, similar treatments were carried out for 10 days to evaluate locomotor activity, oxidative stress and antioxidant markers. We also determined mRNA levels of Superoxide dismutase 1 (Sod1, an antioxidant gene) and ple, which encodes tyrosine hydroxylase, the rate-limiting step in dopamine synthesis. Our data showed that resveratrol improved survival rate and climbing activity of PD flies compared to untreated PD flies. Additionally, resveratrol protected against decreased activities of acetylcholinesterase and catalase and levels of non-protein thiols and total thiols displayed by PD flies. Moreover, resveratrol mitigated against parkin mutant-induced accumulations of hydrogen peroxide, nitric oxide and malondialdehyde. Resveratrol attenuated downregulation of ple and Sod1 and reduction in mitochondrial fluorescence intensity displayed by PD flies. Overall, resveratrol alleviated oxidative stress and locomotor deficit associated with parkin loss-of-function mutation and therefore might be useful for the management of PD.
Subject terms: Biochemistry, Neuroscience, Stress and resilience
Introduction
Parkinson's disease (PD) is an idiopathic and neurodegenerative disease. The hallmark of PD is depletion in dopamine level, due to dopaminergic neuronal loss in the substantia nigra pars compacta and striatum, leading to movement disorder1,2. Apart from the environmentally-induced sporadic form, mutations in several genes such as SNCA, PRKN, ATP13A2, DJ-1, LRRK2 and Pink1 have been linked with the familial form of PD3,4. Therefore, most PD incidences are sporadic, with only about 10–15% being familial cases5. In addition, 50% of all autosomal recessive familial or juvenile cases and nearly 15% of sporadic cases exhibit mutations in Parkin gene6. Indeed, mutations in Parkin gene are the second most frequent genetic causes of PD7. They occur in both early-onset familiar and sporadic forms of PD, with patients displaying clinical features of early and late parkinsonism8. The Parkin gene is composed of twelve exons that encode a 465 amino acid length protein (Parkin) with N-terminal ubiquitin-like motif, in-between ring-finger (IBR) domain, and C-terminal two-RING finger motif. The C-terminal motif possesses the ubiquitin ligase activity, whereas the loss of ubiquitin ligase activity in Parkin has been implicated in the pathogenicity of PD7.
Parkin is an E3 ubiquitin ligase and a downstream substrate of PTEN-induced kinase 1 (Pink1). Together with Pink1 and ubiquitin, Parkin maintains mitochondrial integrity during mitochondrial stress by ubiquitinating several outer mitochondrial membrane protein targets. Consequently, this leads to sequestration and mitophagy of depolarized or damaged mitochondria4. Apart from mitophagy, Parkin is also involved in mitochondrial biogenesis and dynamics leading to mitochondrial quality control system. Therefore, the homeostatic roles of mitochondria in energy-dependent tissue function and health, are through the quality control of proteins, partly involving Parkin9. The loss-of-function mutation of Parkin causes accumulation of depolarized mitochondria in energy-dependent tissues, such as muscles and brains10. This eventually induces mitochondrial dysfunctions, oxidative stress and inflammation11. Indeed, muscular and neurodegenerations, which are phenotypic features of PD, have been shown through Parkin knockout in different animal models8.
The loss-of-function mutations in Drosophila PARK2 (parkin) ortholog, causes PD-like phenotypes such as shortened life span, locomotor deficit12,13 and dopaminergic neuronal loss14 due to dysfunctional mitochondrial-mediated oxidative stress15,16. Drosophila has made it possible to carry out genetic manipulations to generate numerous PD models such as the expression of human genes with pathogenic mutations or targeted mutation of conserved orthologs17. Thus, D. melanogaster does not only provide a unique opportunity for understanding the mechanism of pathogenesis of PD, but also help in identifying targets for therapies.
Currently, pharmacological drugs employed in the management of PD, such as levodopa, dopamine agonist or monoamine oxidase B inhibitors, only treat the symptoms. Of note, the beneficial effects of levodopa, the most commonly used conventional drug, wears out over time and its clinical value declines as the disease advances. There are also major side-effects such as dyskinesia, swelling and impulse control disorders noticed in PD patients18. Owing to the lack of effective therapy in the management of PD, there may be a dramatic increase in the incidence of PD patients in the coming decades.
Oxidative stress has been implicated in the pathogenesis of PD. In healthy state, the body maintains a balance between oxidative stress and antioxidant status in order to ensure survival19. Thus, in order to reduce oxidative stress associated with PD, compounds possessing anti-oxidative property are currently being promoted as potential co-adjuvant molecules in the treatment of PD20,21. Truly, there is a growing line of evidence indicating that parkin mutant flies have been rescued with antioxidant-rich supplements and natural compounds22–24.
Natural polyphenols are promising medicinal and pharmaceutical compounds for the management of PD due to their high antioxidant content and low toxic properties. Resveratrol is a stilbene found in several plants such as grapes, berries and peanuts25. It has both anti-inflammatory and antioxidant26 properties. We have previously reported the protective role of resveratrol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model of PD27. Here, we hypothesized that resveratrol could serve as a therapeutic agent in Drosophila parkin mutant model of PD. In this regard, we sought to validate the potential therapeutic treatment of oxidative stress symptoms associated with parkin loss-of-function mutation in D. melanogaster.
Results
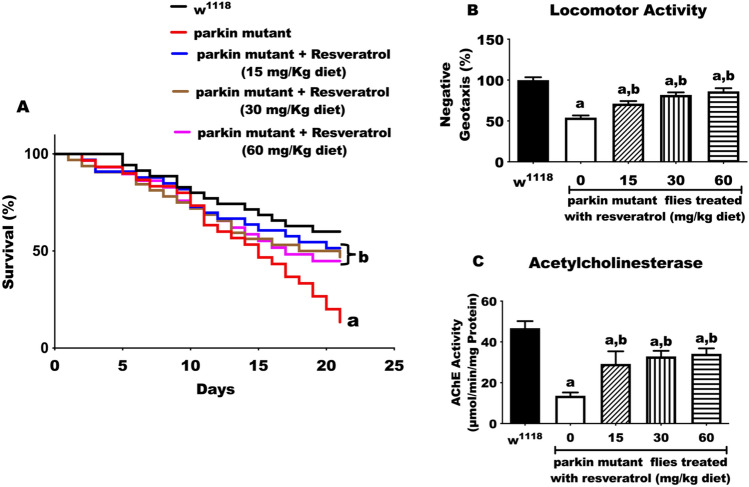
Resveratrol improves survival rate of parkin mutant D. melanogaster
After 21 days, untreated parkin mutant (PD) flies had a 75% decrease in survival rate compared with the wild type w1118 flies (Control, Fig. 1). However, the PD flies fed with diet-containing resveratrol (15, 30 and 60 mg/kg diet) had significant increases in survival rates compared with the untreated PD flies.
Figure 1.
Effects of resveratrol on survival rate, locomotor (climbing) activity and acetylcholinesterase concentration of PD Drosophila melanogaster. (A) 21 days survival rate, (B) locomotor (climbing) activity and (C) acetylcholinesterase concentration of control and PD flies fed with diet-containing resveratrol (0, 15, 30 and 60 mg/kg diets) for 10 days. Values are expressed as mean ± standard error of mean (SEM) of 30 flies/vial, 5 replicates per treatment group. (a) Significant difference from control; (b) significant difference from PD flies.
Resveratrol improves climbing activity of parkin mutant D. melanogaster
A significant reduction in climbing rate was observed in PD flies compared with PD flies treated with resveratrol. The PD flies exposed to resveratrol showed a significant increase in climbing activity when compared with untreated PD flies (p < 0.05; Fig. 1B). Also, a significant reduction in the activity of acetylcholinesterase (AChE) by 3.4-fold was observed in untreated PD flies compared with control. However, the treatment of PD flies with resveratrol improved AChE activity compared with untreated PD flies (approx. 2.1, 2.4 and 2.5 folds for 15, 30 and 60 mg/kg diet of resveratrol, respectively p < 0.05), but not up to the level of the control flies (Fig. 1C).
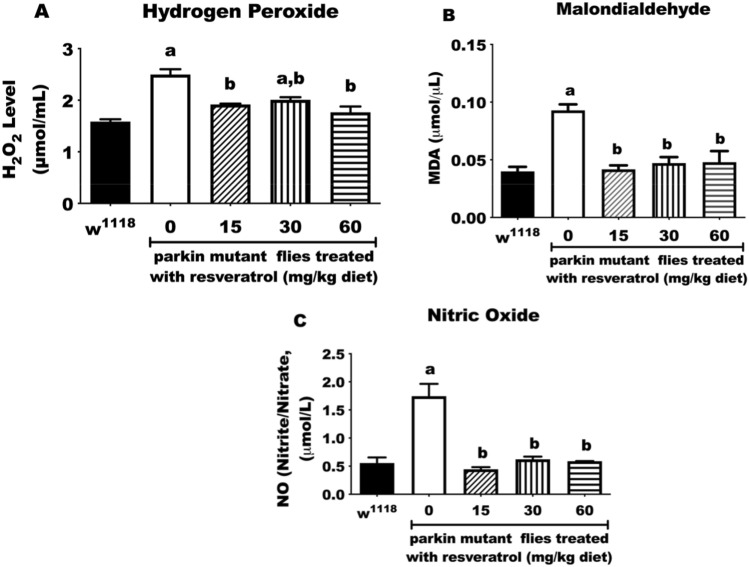
Resveratrol alleviates oxidative stress and inflammatory markers in parkin mutant flies after 10 days of treatment
Oxidative stress makers (hydrogen peroxide (H2O2) and malondialdehyde (MDA)) as well as inflammatory marker (nitric oxide (NO)) may be detrimental to tissues when produced in excessive amounts27. We found that PD flies displayed significantly elevated levels of H2O2, MDA and NO (measured as nitrates/nitrites) (p < 0.05, Fig. 2A–C, respectively) compared with the control group. However, treatment of PD flies with diet-containing resveratrol significantly reduced H2O2, MDA and NO levels compared with untreated PD flies (p < 0.05; Fig. 2).
Figure 2.
Resveratrol alleviates oxidative stress and inflammatory markers in PD flies after 10 days of treatment. (A) Hydrogen peroxide, (B) lipid peroxidation and (C) nitric oxide (nitrites/nitrates) of control and PD flies fed with diets-containing resveratrol (0, 15, 30 and 60 mg/kg diets). Values are expressed as mean ± SEM of 30 flies/vial, 5 replicates per group. (a) Significant difference from control; (b) significant difference from untreated PD flies.
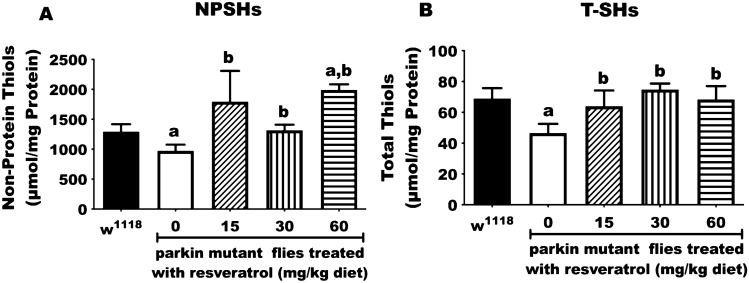
Resveratrol ameliorates reduced levels of non-protein thiols and total thiols displayed in parkin mutant D. melanogaster
The thiol system consists of glutathione and other cysteine-containing molecules28 that function to maintain the redox balance in biological system29. The PD flies displayed significantly reduced levels of non-protein thiols (Fig. 3A) and total thiols (Fig. 3B) by 1.32 and 1.48 folds, respectively, compared with the control group. However, resveratrol significantly increased the levels of non-protein thiols and total thiol compared with untreated PD flies (p < 0.05; Fig. 3).
Figure 3.
Resveratrol ameliorates reduced levels of non-protein thiols and total thiols of parkin mutant D. melanogaster. (A) Non-protein thiols and (B) total thiols of control and parkin mutant D. melanogaster treated with resveratrol (0, 15, 30 and 60 mg/kg diets) for 10 days. Values are expressed as mean ± SEM of 30 flies/vial, 5 replicates per treatment group. (a) Significant difference from control; (b) significant difference from untreated PD flies.
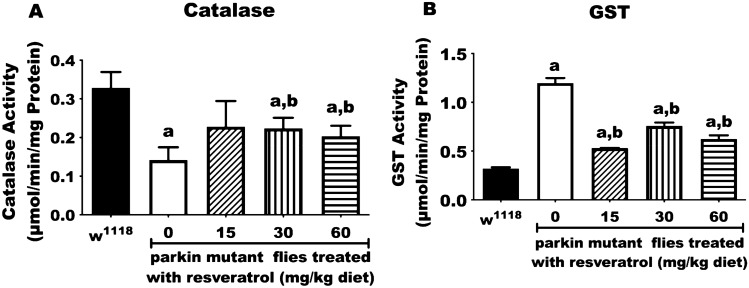
Resveratrol mitigates alterations of catalase and glutathione-S-transferase (GST) activities in parkin mutant D. melanogaster
In healthy state, there is a balance between oxidative stress and antioxidant markers30. Thus, we determined the activities of two antioxidant enzymes, catalase and GST in control and PD flies. We observed that PD flies displayed significantly reduced catalase activity (Fig. 4A) and elevated activity of GST (Fig. 4B) compared with the control flies. The treatment of PD flies with resveratrol improved catalase and GST activities compared with untreated PD flies, but not up to the level of the control flies (Fig. 4).
Figure 4.
Resveratrol mitigates alterations of catalase and Glutathione-S-Transferase activities in parkin mutant D. melanogaster. (A) Catalase activity and (B) glutathione S-transferase activity of control and PD flies exposed to resveratrol (0, 15, 30 and 60 mg/kg diets) for 10 days. Values are expressed as mean ± SEM of 30 flies/vial, 5 replicates per treatment group. (a) Significant difference from control; (b) significant difference from untreated PD flies.
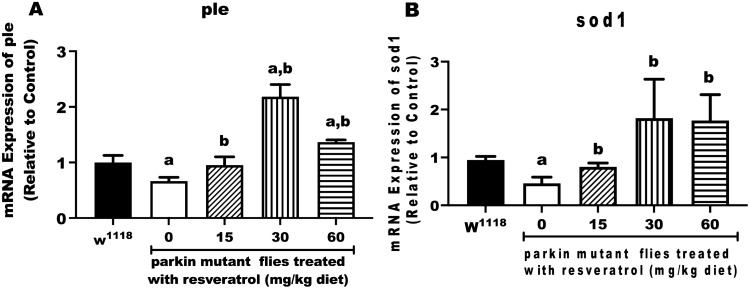
Resveratrol prevents down-regulation of ple and Sod1 genes in parkin mutant D. melanogaster
In order to understand the action of resveratrol at the molecular level, we evaluated its effects on ple and Sod1. The ple encodes tyrosine hydroxylase, the enzyme that catalyzes the first-rate limiting step in dopamine biosynthesis31. The Sod1 encodes superoxide dismutase1 (SOD1)32, the enzyme that catalyzes the dismutation of superoxide radical to hydrogen peroxide. The effects of resveratrol (15, 30 and 60 mg/kg diet) on mRNA expression of ple and Sod1 in PD flies are shown in Fig. 5. The PD flies displayed significant reduction in mRNA levels of ple and Sod1 compared with the control. However, resveratrol enhanced mRNA levels of ple and Sod1 in PD flies, with 15 mg/kg diet of resveratrol showing the highest ameliorative effects on ple and Sod1 expressions.
Figure 5.
Resveratrol prevents down-regulation of ple and Sod1 in parkin mutant D. melanogaster. (A) mRNA level of ple and (B) mRNA level of Sod1 of control and PD flies treated with resveratrol (0, 15, 30 and 60 mg/kg diets) for 10 days. Values are expressed as mean ± SEM. (a) Significant difference from control; (b) significant difference from untreated PD flies.
Histology of control and parkin mutant fly brains
The histology data in Fig. 6 indicated no detectable lesion in the brains of control as well as PD flies treated with diets containing resveratrol after 10 days of treatment (Fig. 6).
Figure 6.
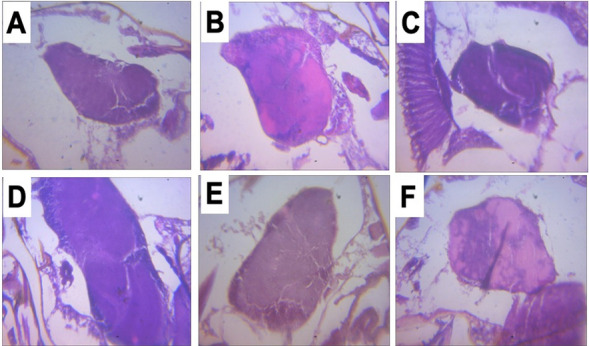
Histology of control and PD fly brains after exposure to resveratrol. (A) Control, (B) untreated PD fly, (C) PD fly treated with resveratrol (15 mg/kg diet), (D) PD fly treated with resveratrol (30 mg/kg diet) and (E) PD fly treated with resveratrol (60 mg/kg/diet) for 10 days.
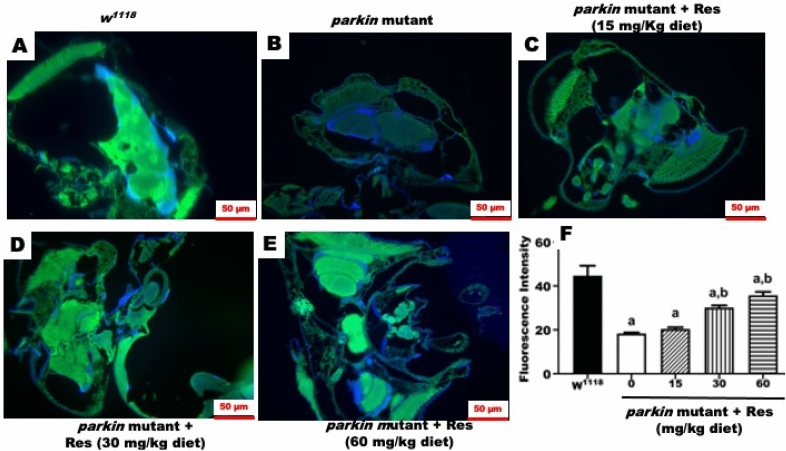
Action of resveratrol on mitochondrial fluorescence intensity in parkin mutant D. melanogaster
To understand the action of resveratrol on mitochondrial abundance, the brains were stained with MitoTracker Green (Invitrogen™) as shown in Fig. 7. MitoTracker is a mitochondrial selective probe that covalently binds to mitochondrial proteins via reaction with cysteine residues. This assay is independent of mitochondrial membrane potential and mainly used to represent mitochondrial mass33. We found that mitochondrial mass, indicated by fluorescence intensity of MitoTracker Green, was markedly reduced in the brain of PD flies (Fig. 7). However, the treatment of PD flies with resveratrol (30 and 60 mg/kg diet) showed significant increase in mitochondrial fluorescence intensity compared with untreated PD flies (Fig. 7).
Figure 7.
MitoTracker staining of brains of control and PD flies. (A) Control brain, (B) untreated PD fly brain, (C) PD fly brain treated with resveratrol (15 mg/kg diet), (D) PD fly brain treated with resveratrol (30 mg/kg diet), (E) PD fly brain treated with resveratrol (60 mg/kg/diet) for 10 days and (F) mitochondrial fluorescence intensity. (a) Significant difference from control; (b) significant difference from untreated PD flies. DAPI: blue; MitoTracker: green.
Discussion
Mutations in parkin have been established as one of the causes of PD34. Parkin is an E3 ubiquitin-protein ligase and a member of the quality control protein system. Available PD treatments, only provide symptomatic reliefs, but could not reduce, reverse or halt the neurodegenerative process35. Since oxidative stress is linked with the pathogenesis of PD36, any agent that can maintain the redox and oxidative stress-antioxidant balance might be a promising therapeutic drug for PD37. Resveratrol (3,5,4’-trihydroxystilbene) is a naturally existing polyphenolic compound found in nuts and fruits such grapes38,39. It has been reported to possess both anti-inflammatory and antioxidative properties40. It also possesses neuroprotective capacity as previously reported27. Here, we carried out different experiments to ascertain if resveratrol could alleviate oxidative stress associated with parkin loss-of-function in D. melanogaster.
In this study, resveratrol improved the survival rate of parkin mutant flies. Different authors have reported the life span-prolonging effect of resveratrol in animal models, including vertebrates (rodents), yeast, and invertebrates (nematodes and insects)41. Indeed, resveratrol has been shown to act via different targets such as mediation of autophagocytic death, decreased oxidative stress41, activation of sirtuins42, reduced inflammation43 and lipofuscin formation44. The observation that resveratrol increased the survival rate of parkin mutant flies aligns with our previous study where resveratrol extended the lifespan of D. melanogaste27, and that of Valenzano et al.45.
Acetylcholine plays important roles in movement and balance46. Thus, reduction in its activity may impair acetylcholine function which includes movement. The PD flies displayed reduced locomotor (climbing) activity and acetylcholinesterase activity, which imply dysfunctional cholinergic transmission system. Further, the fact that resveratrol improved locomotor activity and acetylcholinesterase activity in PD flies further indicated its neuroprotective property.
Phase II drug metabolizing enzymes are the major detoxification enzymes that perform important roles in cellular defense. Glutathione-S-transferase (GST), a phase II drug metabolizing enzyme, participates in the detoxification of xenobiotics via conjugation with glutathione47. Indeed, overexpression of GST activity has been shown to be protective in parkin mutant fly model48. In this study, parkin mutant flies showed increased GST activity, which might be an adaptive response to parkin mutation. In addition, the levels of non-protein thiols and total thiols were reduced in parkin mutant flies. Interestingly, these markers were improved when PD flies were fed with diet-containing resveratrol. The thiol system consists of glutathione and other cysteine-containing molecules28 that function to maintain the redox balance in biological system29. Glutathione acts as an antioxidant and it also acts as a vital factor in the detoxification of xenobiotics together with GST. Thus, the reduction in the levels of these thiols suggest redox imbalance elicited by parkin mutation in D. melanogaster. The observed increase in the level of non-protein thiols in PD flies treated with resveratrol (15 and 30 mg/kg diet) might be an adaptive protective response due to parkin mutation.
Catalase is a first-line antioxidant defence enzyme that catalyzes the conversion of H2O2 to water and molecular oxygen. Since catalase is required for the breakdown of H2O2, an inhibition of its activity would be expected to result in increased accumulation of H2O2. The loss of function of Parkin protein caused an overload of reactive oxygen species, including H2O2, as a result of defective mitophagy. In PD, oxidative stress is deleterious due to accumulation of H2O249. Our results showed an increased level of H2O2 in the parkin mutant flies, indicating oxidative stress. The observation that resveratrol reduced H2O2 level, implies that oxidative stress in the flies was minimised.
Nitric oxide is a gaseous and easily diffusible molecule that acts as neurotransmitter in synapses50. Above physiological level, it can act as a reactive nitrogen species and induce oxidative stress in tissues. It forms secondary intermediates called peroxynitrites, which react with macromolecules, causing nitrosative damage. Since the brain has the highest amounts of saturated fats, it is a target for oxidation, nitration and peroxidation51. S-nitrosylation of Parkin produces a loss of function of the protein contributing to PD52. An elevation of nitrates/nitrites with increased lipid peroxidation as observed in our study, suggests oxidative damage. The observation that resveratrol ameliorated increased nitric oxide level displayed in PD flies further confirms its antioxidative activity.
In D. melanogaster, ple encodes tyrosine hydroxylase, the rate-limiting step in Dopamine (DA) synthesis31. In mammals, dopamine is mainly associated with motor control and reward. In flies, dopamine modulates a range of behaviours such as locomotion, learning, sleep and courtship53. As found in mammals, flies synthesize DA from the aromatic amino acid, tyrosine, via the coordinated action of tyrosine hydroxylase and DOPA decarboxylase54. Our data revealed the downregulation of ple in PD flies compared with the control flies. This might imply a consequent reduction in the synthesis of DA, which is the hallmark of PD. Resveratrol offered protective action by upregulating ple with maximum rescue effect at 15 mg/kg diet concentration. This result thus revealed the neuroprotective action of resveratrol (15 mg/kg diet) with respect to ple expression, which might consequently lead to increase in DA synthesis.
Superoxide dismutase 1 (SOD1), encoded by Sod1 in flies, plays important function in the antioxidant system32. It offers protection against ROS by breaking down superoxide radicals to oxygen and H2O2, thereby making the latter susceptible to catalase degradation. This consequently prevents oxidative stress that can predispose to different diseases such as PD55. Thus, a reduction in the mRNA level of Sod1 noted in the PD flies further implies that oxidative oxidative stress accompanied parkin mutation. This further explains the reason for the accumulation of H2O2 observed in this study. Conversely, treatment of PD flies with resveratrol (15 mg/kg diet) restored mRNA level of Sod1, which might imply increase in the activity of SOD1 compared with PD flies, leading to the breakdown of excess superoxide radicals in the flies.
There was no detectable histological lesion in the brains of PD flies treated with resveratrol. Thus, we evaluated mitochondrial abundance in the brains of flies using MitoTracker Green, which is commonly used to stain mitochondria56. It binds covalently to sulfhydryl groups of mitochondrial proteins33. There was reduction in mitochondrial intensity in the brains of PD flies compared with control flies. Interestingly, resveratrol significantly increased mitochondria intensity in the brains of PD flies.
Taken together, resveratrol improved survival rate and locomotor performance of PD flies. It maintains oxidant-antioxidant homeostasis and prevented down regulation of ple and Sod1 genes in PD flies (Fig. 8). We did not investigate the reason(s) for the apparent lack of concentration-dependent correlations of resveratrol in the levels of thiols, activities of catalase and GST as well as mRNA levels of ple and Sod1 genes in this study. Nevertheless, this study indicated that appropriate concentrations of resveratrol can reduce oxidative stress associated with parkin loss-of-function mutation and therefore might be harnessed for the management of PD. It would be interesting to understand if resveratrol could rescue other forms of genetic-induced models of PD.
Figure 8.
Rescue mechanisms of resveratrol in parkin mutant Drosophila melanogaster model of Parkinson’s disease. Resveratrol alleviates oxidative stress, improved survival rate and climbing activity in PD flies. File: Drosophila-drawing.svg—Wikimedia Commons, (n.d.). https://commons.wikimedia.org/wiki/File:Drosophila-drawing.svg (accessed February 21, 2022).
Materials and methods
Chemicals
All chemicals used in this study were commercial analytical grade products. Reveratrol 98% (HPLC) was purchased from AK Scientific, 30,023 Ahern Ave, Union City, CA 94587, USA.
Fly stocks
The D. melanogaster strain w; parkin[25]/TM6B.GFP12 and wildtype w1118 were the flies previously used in Dr. Alexander Whitworth’s laboratory, MRC Mitochondrial Biology Unit, University of Cambridge, UK. The flies were maintained at temperature 25 ± 2 °C and 60% relative humidity, under 12 h dark/light cycle in the Drosophila laboratory, Department of Biochemistry, Faculty of Basic Medical Science, College of Medicine, University of Ibadan, Ibadan, Nigeria. The Drosophila medium was composed of cornmeal medium, brewer's yeast, agar–agar and nipagin (preservative).
Generation of parkin mutant (PD) D. melanogaster
Male and female, w; parkin25/TM6B.GFP, flies were allowed to mate in vials containing diet for 3 days and removed. Then, larvae and pupae were followed up to the adult stage, and the pupae without tubby body were noted. Following eclosion, the w; parkin25/parkin25 (PD) flies were separated, reared under controlled environmental conditions and monitored for 72 h before further studies. Thereafter, they were carefully selected, under brief CO2 anesthesia, 3 days after eclosion. We used the w1118 flies with white eyes (the strain from which the parkin25/parkin25 flies were generated) as control.
Treatment of PD flies with resveratrol
The control flies were treated with diet-containing vehicle (ethanol, 2% final concentration), while the PD flies were treated with resveratrol: 15 mg/kg diet (0.15 mg/10 g diet, approximately 6.57 mM); 30 mg/kg diet (0.30 mg/10 g diet, approximately, 13.14 mM) and 60 mg/kg diet (0.60 mg/10 g diet, approximately, 26.28 mM), for 21 days to evaluate survival rate. These concentrations were selected based on our previous study involving treatment of wild-type D. melanogaster with different concentrations of resveratrol (7.5, 15.0, 30.0, 60.0 and 120.0 mg/kg diet), in which 15, 30 and 60 mg/kg diets of resveratrol extended lifespan by 20.9, 39.5 and 41.86%, respectively27.
Survival rate analysis
Survival rate was carried out to determine the appropriate concentrations and duration of exposure of flies to resveratrol. Thus, 1-to 3-days old control and PD flies were divided into different groups of 30 flies each in 5 replicates. The survival study was carried out by recording the number of dead flies daily for the entire exposure period (21 days). The diet mixed with resveratrol was changed every 5 days. At the end of the treatment, data was analyzed and plotted as percent of live flies57. Kaplan–Meier curves were plotted and log-rank tests performed. Based on the data obtained, 10 days duration of treatment and concentrations of resveratrol (15.0, 30.0 and 60.0 mg/kg diet) were chosen for subsequent treatments to evaluate the rescue role of resveratrol in PD flies by carrying out different behavioural, oxidative stress and antioxidant markers as well as mRNA expression of ple and Sod1 genes.
Preparation of sample for biochemical assays
Control and PD flies of age 1-to 3- days old were divided into different groups of 30 flies each in 5 replicates and treated with resveratrol (15.0, 30.0 and 60.0 mg/kg diet) for 10 days. Thereafter, they were anaesthetized under CO2, weighed and homogenized in 0.1 M phosphate buffer, pH 7.0 (ratio of 1 mg: 10 μL), and centrifuged at 4000 × g for 10 min at 4 °C. The supernatants were then separated from pellets into labelled clean microfuge tubes and used for the analyses of oxidative stress, antioxidant and inflammatory markers.
Behavioural assay
This was carried out manually using negative geotaxis/locomotor assay58. Briefly, ten flies from control and PD groups were briefly anaesthetized using ice and placed in glass column (15 cm length and 1.5 cm in diameter). After recovery from anaesthesia, they were gently tapped to the bottom of the column. Then, the number of flies that climbed to the 6 cm mark were recorded. The data were then expressed as percentage of flies that crossed up to and beyond the 6 cm mark of the column.
Determination of total thiols and non-protein thiol content
Total thiol content was assayed by the method of Ellman59. Briefly, the reaction mixture contained 170 μL of 0.1 M potassium phosphate buffer (pH 7.4), 20 μL of sample, and 10 μL of 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB). After incubation for 30 min at room temperature, the absorbance was measured at 412 nm using SpectraMax microplate reader (Molecular Devices). For non-protein thiol, the sample was precipitated with 4% sulphosalicyclic acid (4%) in the ratio of 1:1. The samples were kept at 4 °C for 1 h and then subjected to centrifugation at 5000 rpm for 10 min at 4 °C. The assay mixture consisted of 170 µl of 0.1 M phosphate buffer, 20 µl of supernatant and 10 µl of DTNB. The reaction was allowed to incubate for 30 min at room temperature, and the absorbance was read at 412 nm using SpectraMax microplate reader. For both total thiols and non-protein thiols, reduced Glutathione (GSH) was used as standard, and the data were expressed as in μmol/mg of protein.
Determination of glutathione-S-transferase activity
Glutathione-S-transferase activity was determined using the method of Habig and Jacoby60 using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The assay mixture was made up of 270 μL of solution A (20 mL of 0.25 M potassium phosphate buffer, pH 7.0, with 2.5 mM EDTA, 10.5 mL of distilled water and 500 μL of 0.1 M GSH at 25 °C), 20 μL of the sample (1:5 dilution), and 10 μL of 25 mM CDNB. The reaction mixture was monitored at 340 nm for 5 min at 10 s intervals in a SpectraMax microplate reader (Molecular Devices). The results were expressed as μmol/mins/mg protein.
Determination of catalase activity
Catalase activity was determined based on the method of Aebi61. Briefly, the H2O2 clearance was monitored at 240 nm, for 2 min (10 s intervals) and at 25 °C with a UV/Visible spectrophotometer. The reaction assay contained 1800 μL of 50 mM phosphate buffer (pH 7.0), 180 μL of 300 mM H2O2, and 20 μL of sample (1:50 dilution). The loss in absorbance of H2O2 was monitored for 2 min at 240 nm and thereafter used to calculate catalase activity expressed as μmol of H2O2 consumed/mins/mgprotein.
Determination of acetylcholinesterase (AChE) activity
The method of Ellman et al.62 was used to determine the activity of AChE. The assay mixture contained 135 μL of distilled water, 20 μL of 100 mM potassium phosphate buffer (pH 7.4), 10 mM DTNB, 5 μL of sample and 20 μL of 8 mM acetylthiocholine as initiator. The reaction was then monitored for 5 min at 15 s intervals at 412 nm using a SpectraMax microplate reader (Molecular devices). The enzyme activity was estimated as μmol of acetylthiocholine hydrolyzed/min/mg protein.
Hydrogen peroxide generation
Hydrogen peroxide level was determined according to the method of Wolff63. The assay mixture contained 590 μl of FOX1 (Ferrous Oxidation-Xylenol orange) reagent and 10 μl of sample. This was followed by 30 min incubation at room temperature, and the absorbance measured at 560 nm. The concentration of the hydrogen peroxide generated was determined using extinction coefficient of H2O2 and expressed as μmol/ml.
Protein determination
The method of Lowry et al.64 was used to determine total protein content using Bovine Serum Albumin (BSA) as a standard.
Determination of nitric oxide level
The nitric oxide (nitrate/nitrite) level was determined using Griess reaction method65. The fly homogenate was incubated with Griess reagent at room temperature for 20 min, and the absorbance was read at 550 nm. The concentration of NO in the sample was calculated using the standard calibration curve of NaNO265 and expressed as μmol/L.
Lipid peroxidation assay
The assay was performed as described by Ohkawa et al.66. The reaction mixture was made by adding 5 µL of 10 mM butylhydroxytoluene, 200 µL of 0.67% thiobarbituric acid, 600 µL of 1% O-phosphoric acid, 105 µL distilled water, and 90 µL whole fly homogenate. The resultant mixture was incubated at 90 °C for 45 min, and the OD was measured at 535 nm. The results were expressed as μmol of malondialdehyde (MDA) formed/μL.
The isolation of RNA and quantitative real- time RT-PCR
Total RNA was isolated from 25 mg whole flies using Trizol (TRI Reagent®, Zymol Research) using manufacturer’s protocol as previously described67. The RNA isolated was resuspended in 50 µl RNase-free water, quantified spectrophotometrically using MaestroNanoDrop Pro (Maestro Gen) and visualized in 2% agarose gel after gel electrophoresis (BioRad). Total RNA (0.3 µg) was used for cDNA synthesis following the manufacturer’s protocol for ProtoScript II kit (New Egland BioLabs) and carried out in BioRad T100 thermal cycler. The primer sequences used in this study (ple and Sod1, Table 1) were obtained from the GeneBank overview (GenBank Overview (nih.gov)) and designed using the primer BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) which were custom synthesized by Invitrogen. Subsequently, qPCR was carried out and the mRNA expression levels were standardized to two genes (Beta Tubulin 86D and RPL32). Luna Universal qPCR Master mix kit was used for qPCR. A total reaction volume of 20 µl was used with 9 ng of cDNA, Luna Universal qPCR Mix, 10 µM forward and reverse primers, and nuclease-free water. The cycling condition was as follows: an initial denaturation at 95 °C for 60 s followed by 40 cycles of 95 °C for 15 s, 60 °C for 60 s followed by a dissociation curve analysis. The SYBR fluorescence was analysed by the SDS 2.1 software (Applied Biosystems, ABI Prisms 7900HT). The reaction was carried out in triplicates of each independent group. Dissociation curves at 60–95 °C was carried out to ascertain the amplification of a single specific product for each reaction. The 2−ΔΔCT method was used to determine the expression values of each gene.
Table 1.
Sequence of primers.
| Genes | Forward sequence | Reverse sequence |
|---|---|---|
| Β-Tubulin | TGGGCCCGTCTGGACCACAA | TCGCCGTCACCGGAGTCCAT |
| RPL32 | CCCAAGATCGTGAAGAAGCG | TGGGCTTGCGCCATTTGTG |
| ple | CAGCAAGGCAAATGATTACGGT | AATCCGGGGTGGTTCATGTC |
| Sod1 | GGAGTCGGTGATGTTGACCT | GTTCGGTGACAACACCAATG |
Histology of fly brains
Adult fly brains were fixed in 10% neutral buffered formalin, deparaffinized and processed for Hematoxylin and Eosin (H/E) histological staining68. The slides were viewed using light microscopy and interpreted by Veterinary Pathologists who were blinded to the control and PD fly brains.
Mitochondrial staining in fly brains
Apart from H/E histological study, we carried out additional experiment to evaluate mitochondrial mass/abundance (based on fluorescence intensity of MitoTracker™ Green stain, Invitrogen™)56 in the brains of control and parkin mutant flies treated with resveratrol for 10 days. Briefly, the fly brains (15/group) were excised, fixed in 4% buffered formalin for 40 min and transferred to 30% sucrose solution in PBS. Thereafter, the whole brains in a group were dehydrated and embedded in paraffin. Then, 5 µm sections of fly brains were prepared from a block and stained on charged slides. The slides were deparaffinised in xylene solutions (10 min each) and rehydrated in decreasing concentrations of ethanol solution in PBS. The slides were then stained with 0.1% MitoTracker™ Green (Invitrogen™) in PBS for 20 min. Nuclei were stained with DAPI (0.1 µg/mL) and slides were mounted in PBS-Glycerol (1:1) solution containing N-propyl gallate. Images were acquired using Zeiss Axioscop fluorescent microscope and signal intensity quantitation as well as background correction were carried out using ImageJ.
Statistical analysis
For statistical analysis, GraphPad Prism 9 was used. The Kaplan–Meier’s method was used to analyze the survival rate and comparisons were made with the log-rank tests. For biochemical analyses, statistical significance was evaluated using one way analysis of variance (ANOVA), followed by Dunnett’s post hoc test. Data points correspond to the mean of independent experiments and error bars (S.E.M); the level of significance was set at p < 0.05 and indicated in the charts.
Acknowledgements
We thank the International Centre for Genetic Engineering and Biotechnology (ICGEB), Italy for the award of the grant (CRP/NGA18-02) used to purchase equipment, reagents, materials and resources used in this project. AW was supported by Medical Research Council Core Funding (MC_UU_00015/6).
Author contributions
A.A., A.D.B and A.O.A. performed experiments, and analysed data, with assistance from I.O.A., J.B.T.R., F.S. and O.O.J. In addition, A.O.A. conceived the study, while A.J.W. and A.O.A. designed experiments and supervised the work. A.A. and A.O.A. wrote the manuscript with input from all authors.
Data availability
All data that support the findings of this study are available on reasonable request to the corresponding author. The contributing authors declare that all relevant data are included in the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexander J. Whitworth, Email: a.whitworth@mrc-mbu.cam.ac.uk
Amos O. Abolaji, Email: ao.abolaji@ui.edu.ng
References
- 1.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Lang AE. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:1–21. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Lara DL, Gonzalez-Enriquez GV, Torres-Mendoza BM, Gonzalez-Usigli H, Cardenas-Bedoya J, Macias-Islas MA, Ortiz GG. Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson’s disease. Biomed. Pharmacother. 2020;129:1–7. doi: 10.1016/j.biopha.2020.110485. [DOI] [PubMed] [Google Scholar]
- 3.Bekris LM, Mata IF, Zabetian CP. The genetics of Parkinson disease. J. Geriatr. Psychiatry Neurol. 2010;23:228–242. doi: 10.1177/0891988710383572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn PM, Moreira PI, Ambrósio AF, Alves CH. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020;8:1–20. doi: 10.1186/s40478-020-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papapetropoulos S, Adi N, Ellul J, Argyriou AA, Chroni E. A prospective study of familial versus sporadic Parkinson’s disease. Neurodegener. Dis. 2007;4:424–427. doi: 10.1159/000107702. [DOI] [PubMed] [Google Scholar]
- 6.Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Brice A. Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 7.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson's disease. Movement. Disord. 2010;25:S32–S39. doi: 10.1002/mds.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattori N, Mizuno Y. Pathogenetic mechanisms of parkin in Parkinson's disease. Lancet. 2004;364:722–724. doi: 10.1002/mds.22798. [DOI] [PubMed] [Google Scholar]
- 9.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Xu E, Musich PR, Lin F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019;25:816–824. doi: 10.1111/cns.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsche M, König IR, Delcambre S, Petrucci S, Balck A, Brüggemann N, Klein C. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain. 2020;143:3041–3051. doi: 10.1093/brain/awaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. PNAS. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Dev. Dis. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 14.Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Jackson GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J. Neurosci. 2007;27:981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini N, Oelhafen S, Hua H, Georgiev O, Schaffner W, Büeler H. Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol. Dis. 2010;40:82–92. doi: 10.1016/j.nbd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog. Neurobiol. 2013;106:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt VL, Whitworth AJ. Mechanisms of Parkinson's disease: Lessons from Drosophila. Curr. Top. Dev. Biol. 2017;121:173–200. doi: 10.1016/bs.ctdb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jiménez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson's disease. Mov. Disord. 2016;31:861–881. doi: 10.1002/mds.26662. [DOI] [PubMed] [Google Scholar]
- 19.Casani S, Gómez-Pastor R, Matallana E, Paricio N. Antioxidant compound supplementation prevents oxidative damage in a Drosophila model of Parkinson's disease. Free Radic. Biol. Med. 2013;61:151–160. doi: 10.1016/j.freeradbiomed.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Biosa A, Sanchez-Martinez A, Filograna R, Terriente-Felix A, Alam SM, Beltramini M, Bubacco L, Bisaglia M, Whitworth AJ. Superoxide dismutating molecules rescue the toxic effects of PINK1 and parkin loss. Hum. Mol. Genet. 2018;27:1618–1629. doi: 10.1093/hmg/ddy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C. Low doses of paraquat and polyphenols prolong life span and locomotor activity in knock-down parkin Drosophila melanogaster exposed to oxidative stress stimuli: Implication in autosomal recessive juvenile parkinsonism. Gene. 2013;512:355–363. doi: 10.1016/j.gene.2012.09.120. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan P, Majhi O, Biswas A, Joshi VK, Sinha D. Enhanced accumulation of reduced glutathione by Scopoletin improves survivability of dopaminergic neurons in Parkinson’s model. Cell Death Dis. 2020;11:1–11. doi: 10.1038/s41419-020-02942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta-Mol. Basis Dis. 1842;1282–1294:2014. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannu N, Bhatnagar A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019;109:2237–2251. doi: 10.1016/j.biopha.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 25.Meng X, Zhou J, Zhao CN, Gan RY, Li HB. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods. 2020;9:1–27. doi: 10.3390/foods-9030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwager J, Richard N, Widmer F, Raederstorff D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 2017;17:1–12. doi: 10.1186/s12906-017-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative damage and behavioral deficits in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2018;503:1042–1048. doi: 10.1016/j.bbrc.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 28.Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DP, Sies H. The redox code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan R, Chen Y. Management of oxidative stress: Crosstalk between brown/beige adipose tissues and skeletal muscles. Front. Physiol. 2021;12:712372. doi: 10.3389/fphys.2021.712372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Yang Y, Liang W, Wang T, Wang S, Wang X, Wang Y, Jiang H, Feng H. Neuroprotection by urate on the mutant hSOD1-related cellular and Drosophila models of amyotrophic lateral sclerosis: Implication for GSH synthesis via activating Akt/GSK3β/Nrf2/GCLC pathways. Brain Res. Bull. 2019;146:87–301. doi: 10.1016/j.brainresbull.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Holmila RJ, Vance SA, Chen X, et al. Mitochondria-targeted probes for imaging protein sulfenylation. Sci. Rep. 2018;8:6635. doi: 10.1038/s41598-018-24493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu YT, Tai CH, Lin CH, Wu RM. Updates on the genetics of Parkinson's disease: Clinical implications and future treatment. Acta Neurol. Taiwan. 2021;30(3):83–93. [PubMed] [Google Scholar]
- 35.Smith GA, Heuer A, Dunnett SB, Lane EL. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice II: Predicting L-DOPA-induced dyskinesia. Behav. Brain Res. 2012;226:281–292. doi: 10.1016/j.bbr.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Talebi M, Vadoud SA, Haratian A, Talebi M, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S. The interplay between oxidative stress and autophagy: Focus on the development of neurological diseases. Behav. Brain Funct. 2022;18(1):3. doi: 10.1186/s12993-022-00187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ounthaisong U, Tangyuenyongwatana P. Cross-docking study of flavonoids against tyrosinase enzymes using PyRx 0.8 virtual screening tool. TJPS. 2017;8:41. [Google Scholar]
- 38.Feng C, Chen J, Ye W, Liao K, Wang Z, Song X, Qiao M. Synthetic biology-driven Microbial production of resveratrol: Advances and perspectives. Front. Bioeng. Biotechnol. 2022;10:833920. doi: 10.3389/fbioe.2022.833920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King RE, Bomser JA, Min DB. Bioactivity of resveratrol. Compr. Rev. Food Sci. Food Saf. 2006;5:65–70. doi: 10.1111/j.1541-4337.2006.00001.x. [DOI] [Google Scholar]
- 40.Chupradit S, Bokov D, Zamanian MY, Heidari M, Hakimizadeh E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2021 doi: 10.1111/fcp.12746. [DOI] [PubMed] [Google Scholar]
- 41.Hector KL, Lagisz M, Nakagawa S. The effect of resveratrol on longevity across species: A meta-analysis. Biol. Lett. 2012;8:790–793. doi: 10.1098/rsbl.2012.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, Li HB. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021 doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, Li G. Effects of resveratrol on longevity, cognitive ability and aging-related histological markers in the annual fish Nothobranchius guentheri. Exp. Gerontol. 2012;47:940–949. doi: 10.1016/j.exger.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 46.Abdalla FH, Cardoso AM, Pereira LB, Schmatz R, Gonçalves JF, Stefanello N, Mazzanti CM. Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol. Cell Biochem. 2013;381:1–8. doi: 10.1007/s11010-013-1659-x. [DOI] [PubMed] [Google Scholar]
- 47.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 48.Whitworth AJ, Theodore DA, Greene JC, Beneš H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. PNAS. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiasalari Z, Khalili M, Baluchnejadmojarad T, Roghani M. Protective effect of oral hesperetin against unilateral striatal 6-hydroxydopamine damage in the rat. Neurochem. Res. 2016;41:1065–1072. doi: 10.1007/s11064-015-1796-6. [DOI] [PubMed] [Google Scholar]
- 50.Guerra DD, Bok R, Vyas V, Orlicky DJ, Lorca RA, Hurt KJ. Akt phosphorylation of neuronal nitric oxide synthase regulates gastrointestinal motility in mouse ileum. PNAS. 2019;116:17541–17546. doi: 10.1073/pnas.1905902116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dreyfus H, Harth S, Giuliani-Debernardi A, Roos M, Mack G, Mandel P. Gangliosides in various brain areas of three inbred strains of mice. Neurochem. Res. 1982;7:477–488. doi: 10.1007/BF00965499. [DOI] [PubMed] [Google Scholar]
- 52.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-Nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 53.Van Swinderen B, Andretic R. Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. Biol. Sci. 2011;278(1707):906–913. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Figueira FH, de Quadros Oliveira N, de Aguiar LM, Escarrone AL, Primel EG, Barros DM, da Rosa CE. Exposure to atrazine alters behaviour and disrupts the dopaminergic system in Drosophila melanogaster. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;202:94–102. doi: 10.1016/j.cbpc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Otaki Y, Watanabe T, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Konta T, Shibata Y, Sato H, Kawasaki R, Daimon M, Ueno Y, Kato T, Kayama T, Kubota I. The impact of superoxide dismutase-1 genetic variation on cardiovascular and all-cause mortality in a prospective cohort study: The yamagata (Takahata) study. PLoS ONE. 2016;11:1–12. doi: 10.1371/journal.pone.0164732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baltzer C, Tiefenböck SK, Marti M, Frei C. Nutrition controls mitochondrial biogenesis in the Drosophila adipose tissue through Delg and Cyclin D/Cdk4. PLoS ONE. 2009;4(9):e6935. doi: 10.1371/journal.pone.0006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abolaji AO, Kamdem JP, Lugokenski TH, Nascimento TK, Waczuk EP, Farombi EO, Rocha JBT. Involvement of oxidative stress in 4-vinylcyclohexene- induced toxicity in Drosophila melanogaster. Free Rad. Biol. Med. 2014;71:99–108. doi: 10.1016/j.freeradbiomed.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 59.Elmann GL. Tissue sulphydril grups/Elmann GL. Arch. Biochem. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 60.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 61.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 62.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 63.Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182. doi: 10.1016/S0076-6879(94)33021-2. [DOI] [Google Scholar]
- 64.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/S00219258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 65.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaubaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 66.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 67.Abolaji AO, Kamdem JP, Lugokenski TH, Farombi EO, Souza DO, da Silva Loreto ÉL, Rocha JB. Ovotoxicants 4-vinylcyclohexene 1, 2-monoepoxide and 4- vinylcyclohexene diepoxide disrupt redox status and modify different electrophile sensitive target enzymes and genes in Drosophila melanogaster. Redox. Biol. 2015;5:328–339. doi: 10.1016/j.redox.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bancroft JD, Gamble M. Theory and Practice of Histology Techniques. 6. Churchill; 2008. p. 83e134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available on reasonable request to the corresponding author. The contributing authors declare that all relevant data are included in the paper.