Abstract
Intravenous ampicillin-sulbactam is effective in the treatment of various infections in adults, but little is known about the pharmacokinetics (PK) of ampicillin-sulbactam in children. The objective of this study was to determine the PK of ampicillin and sulbactam in pediatric patients with intra-abdominal infection, skin and/or skin structure infection, or periorbital-preseptal and facial cellulitis. Intravenous ampicillin and sulbactam (2:1), 40 to 80 mg/kg of body weight, were given every 6 h for 2 to 6 days to 28 pediatric patients. The ages ranged from 1 to 6 years for 10 patients, 6.1 to 10 years for 9 patients, and 10.1 to 12 years for 9 patients. Multiple blood samples were obtained and analyzed for ampicillin and sulbactam in plasma and serum by high-performance liquid chromatography. The mean maximum concentration of drug in serum ranged from 177 to 200 μg/ml for ampicillin and 82 to 102 μg/ml for sulbactam in the three age groups. The mean total clearance, steady-state distribution volume, and half-life were 4.76 ml/min/kg, 0.32 liter/kg, and 0.77 h, respectively, for ampicillin and 4.95 ml/min/kg, 0.34 liter/kg, and 0.81 h, respectively, for sulbactam. Dose or gender did not affect the PK of ampicillin or sulbactam. The PK of ampicillin and sulbactam in these patients were comparable to those reported in adults. The combination was well tolerated in pediatric patients.
An ampicillin and sulbactam combination (Unasyn) has been approved by the U.S. Food and Drug Administration (FDA) for intravenous administration. It is indicated for the treatment of intra-abdominal, skin and skin structure, and gynecological infections in adults. This combination is effective against various microorganisms, including β-lactamase-producing Escherichia coli, Klebsiella species, Staphylococcus aureus, and Bacteroides species (2, 18).
Ampicillin-sulbactam was recently approved by the FDA for children with skin and/or skin structure infections (18). However, pediatric patients also receive this drug to treat infections for which it is approved in adults. This is true for many drugs, including albuterol, captopril, cisapride, digoxin, morphine, and ranitidine, which are routinely used in pediatric practice (13, 14).
New FDA regulation for labeling of prescription drugs has been implemented to facilitate approval of more drugs for pediatric patients. This regulation would provide a pediatric approval for a drug already indicated in a similar disease in adults, based only on pharmacokinetic, pharmacodynamic, and safety studies in the pediatric population (3).
The pharmacokinetics of ampicillin-sulbactam has been determined in 12 studies, involving a total of 51 Japanese pediatric patients. These studies included various ages (2.4 months to 17 years), doses (15 to 60 mg/kg of body weight/dose), administration times (bolus to 60-min infusion), and analytical methods for measurement of these drugs in serum (5–8, 10, 11, 15–17, 20, 21, 24). Because no studies had been conducted in the United States, we determined the pharmacokinetics and safety of ampicillin-sulbactam in children with intra-abdominal infection, skin and/or skin structure infection, or periorbital-preseptal or facial cellulitis.
MATERIALS AND METHODS
Hospitalized pediatric patients (3 months to 12 years old, as specified by the protocol) requiring parenteral antibiotic therapy for an intra-abdominal infection, skin and/or skin structure infection, or periorbital-preseptal and facial cellulitis were eligible to be considered for this study. The exclusion criteria included known or suspected hypersensitivity to penicillin or cephalosporins and clinically significant renal dysfunction as evidenced by a serum creatinine level of >2.5 mg/dl, a blood urea nitrogen level of >50 mg/dl, or an estimated creatinine clearance of <50 ml/min/1.73 m2 (approximate body surface area). Patients were also excluded if they were terminally ill; had an underlying disease that might interfere with evaluation of any adverse experiences; had immunological or neutrophil function disorders (e.g., chronic granulomatous disease); had a leukocyte count of <3,000/mm3, platelet count of <100,000/mm3, and hemoglobin level of <9.0 g/dl; had a history of seizures; had poorly controlled diabetes mellitus; had glycogen storage disease or a strong family history (parents or siblings) of glycogen storage disease; were in a clinical study of another investigational drug or had received an investigational drug within the preceding four weeks; were pregnant; or had signs or symptoms of meningitis.
The study protocol was approved by the Human Subjects Research Committees. A written informed consent was obtained by a parent or guardian of each patient prior to enrollment into the study.
Ampicillin sodium-sulbactam sodium (2:1) (provided by Pfizer, Inc.) was to be given at a dose of 37.5 to 75 mg/kg every 6 h by an intravenous infusion over 15 to 40 min by an infusion device. In these open label pharmacokinetic studies, selection of dose was based on the severity of the infection as determined by the investigator. The highest dose to be prescribed was 75 mg/kg every 6 h. A minimum of four doses were administered to assure steady-state conditions.
Blood samples were obtained just prior to a dose (0 h) and at 0.25, 0.50, 1.0, 1.5, 2.0, 4.0, and 6.0 h after initiation of infusion. For some patients, additional blood samples were collected at 0, 0.25, 2.0, and 6.0 h during a second dosing interval, at least 1 day after the first pharmacokinetic study.
Ampicillin and sulbactam concentrations in serum or plasma were measured by high-performance liquid chromatography with UV detection. Some sites provided only serum samples, while others provided only plasma samples for the entire study. The method was validated with both serum and plasma and was found comparable for the two biological fluids. Based on the quality control serum and plasma samples that were included along with the calibration and patient samples, both assays were comparable, and hence patient data from serum samples could be pooled with patient data obtained from the plasma samples.
The method consisted of extracting ampicillin and sulbactam simultaneously with added cefazolin as an internal standard from serum or plasma after protein precipitation by acetonitrile. The mobile phase was aqueous tetrabutyl ammonium dihydrogen phosphate-acetonitrile at pH 7.5. The postcolumn derivatization was done with 2 N sodium hydroxide and an aqueous methanolic solution of mercuric chloride. A reversed-phase C8 column and a UV wavelength of 290 nm were used.
Good interday precision was demonstrated for analysis of plasma and serum quality control samples spiked with sulbactam concentrations of 0.75, 7.5, 35, and 150 μg/ml and ampicillin at 1.5, 15, 70, and 400 μg/ml. These analyses exhibited coefficients of variation ranging from 0.5 to 7.9% for sulbactam and from 1.3 to 10.1% for ampicillin for the four quality control samples. Within-day precision of the back-calculated calibration standards was within a coefficient of variation of 8.1% for sulbactam and within 11.4% for ampicillin. Accuracy of the method was determined by comparing the means of the measured concentrations of the controls with their nominal concentrations. Across all concentrations, the mean values were within 11.3% of their expected values for sulbactam and ampicillin.
Linearity of the method was demonstrated for sulbactam in the range of 0.5 to 50 μg/ml and for ampicillin in the range of 1 to 100 μg/ml. For instances in which concentrations exceeded these values, samples were diluted with human plasma or serum as appropriate and reanalyzed. The correlation coefficient was 0.9978 or greater for all standard curves. The limit of quantitation of the method was 0.5 μg/ml for sulbactam and 1.0 μg/ml for ampicillin. At these concentrations, the coefficient of variation of the standard did not exceed 9.0% for either compound. Stability of the compounds at −70°C was demonstrated up to 16 months in serum and 14 months in plasma.
Data analysis.
Pharmacokinetic parameters—maximum concentration of drug in serum (Cmax), area under the concentration-time curve over the dosing interval τ (6 h) (AUCτ), clearance (CL), half-life (t1/2), and volume of distribution at steady state (Vss)—were determined for each drug (ampicillin and sulbactam) as (units are indicated in parentheses): Cmax (micrograms per milliliter), determined from visual inspection of the data; AUCτ (microgram · hour per milliliter), determined by the linear trapezoidal method; CL (milliliters per minute per kilogram), determined by the equation CL = dose/AUCτ; t1/2 (hours), determined by the equation t1/2 = ln(2)/β, where the slope of the natural logarithms of the concentrations versus time is denoted by β, the elimination rate constant calculated from the linear least-squares regression of the data; Vss (liters per kilogram), determined by the equation Vss = MRT × CL, where MRT (mean residence time) is calculated as AUMCτ/AUCτ + [τ × Cmin]/[β × AUCτ] (AUMCτ is the first moment of the concentration-time curve over the dosing interval τ, determined analogously to AUCτ, and Cmin is the concentration at 6 h [23]).
The mean concentration-time data of ampicillin and sulbactam in serum were fit to a single-dose, two-compartment open model (23). The simulation of multiple-dose levels was obtained by generating the single-dose curve from the fitted model and applying the superposition method.
Statistical comparisons were made by analysis of variance, with a P value set at <0.05. The relationship between total clearance and age was analyzed by linear regression.
RESULTS
A total of 28 pediatric patients (19 males and 9 females) completed this study. The diagnosis was skin and/or skin structure infection in 15 patients, intra-abdominal infection in 12 children, and periorbital-preseptal and facial cellulitis in 1 patient. The ages ranged from 1 to 6 years for 10 patients, 6.1 to 10 years for 9 patients, and 10.1 to 12 years for 9 patients.
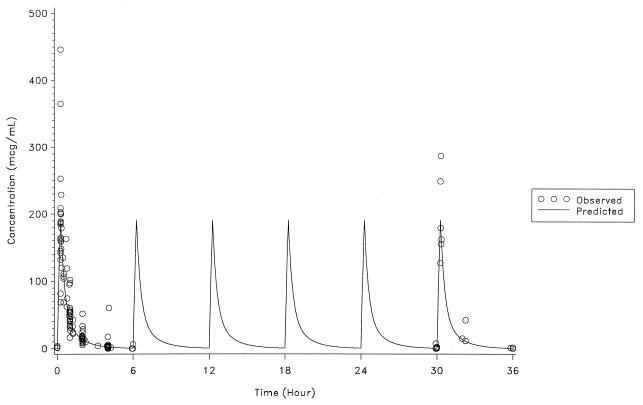
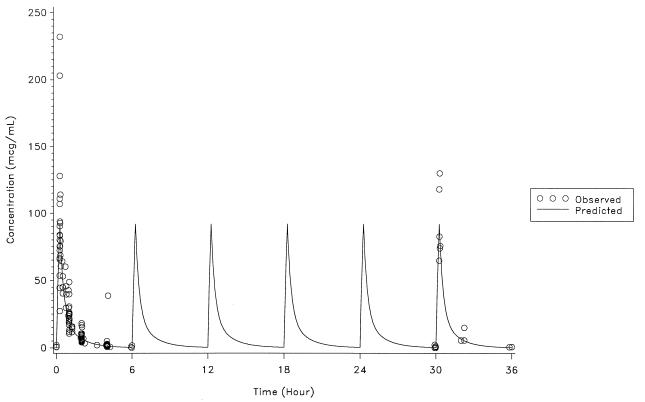
The actual intravenous dose of ampicillin-sulbactam (2:1) ranged from 40 to 80 mg/kg every 6 h during 2 to 6 days of therapy. The infusion duration was 15 min in 23 patients, 17 to 25 min in 4 patients, and 40 min in one patient. The mean Cmax ranged from 177 to 200 μg/ml for ampicillin and 82 to 102 μg/ml for sulbactam in three age groups (Table 1). The Cmax occurred at the end of the infusion period. The individual observed and predicted serum concentration-time data for ampicillin and sulbactam are shown in Fig. 1 and 2, respectively.
TABLE 1.
Pharmacokinetic parameters of ampicillin and sulbactam during the first dosing interval
| Drug | Parameter | Age group
|
|||||
|---|---|---|---|---|---|---|---|
| 1–6 yr
|
6.1–10 yr
|
10.1–12 yr
|
|||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| Ampicillin-sulbactam | Dose (mg/kg) | 10 | 69.5 (10.2) | 9 | 63.7 (13.2) | 9 | 65.9 (11.2) |
| Ampicillin | Cmax (μg/ml) | 10 | 200 (118) | 9 | 183 (36.5) | 9 | 177 (31.9) |
| AUCτ (μg · h/ml) | 9 | 179 (79.2) | 8 | 159 (42.9) | 9 | 175 (45.5) | |
| CL (ml/min/kg) | 9 | 5.11 (2.36) | 8 | 4.88 (1.49) | 9 | 4.31 (0.92) | |
| t1/2 (h) | 9 | 0.74 (0.07) | 8 | 0.72 (0.11) | 9 | 0.85 (0.18) | |
| Vss (liter/kg) | 9 | 0.34 (0.17) | 8 | 0.30 (0.08) | 9 | 0.32 (0.07) | |
| Sulbactam | Cmax (μg/ml) | 10 | 102 (64.2) | 9 | 85.8 (21.2) | 9 | 81.9 (20.7) |
| AUCτ (μg · h/ml) | 9 | 90.5 (42.8) | 8 | 76.8 (25.2) | 9 | 81.8 (15.7) | |
| CL (ml/min/kg) | 9 | 5.10 (2.24) | 8 | 5.17 (1.73) | 9 | 4.60 (1.09) | |
| t1/2 (h) | 9 | 0.77 (0.08) | 8 | 0.76 (0.10) | 9 | 0.89 (0.13) | |
| Vss (liter/kg) | 9 | 0.34 (0.16) | 8 | 0.34 (0.10) | 9 | 0.35 (0.10) | |
FIG. 1.
Observed and predicted ampicillin concentrations.
FIG. 2.
Observed and predicted sulbactam concentrations.
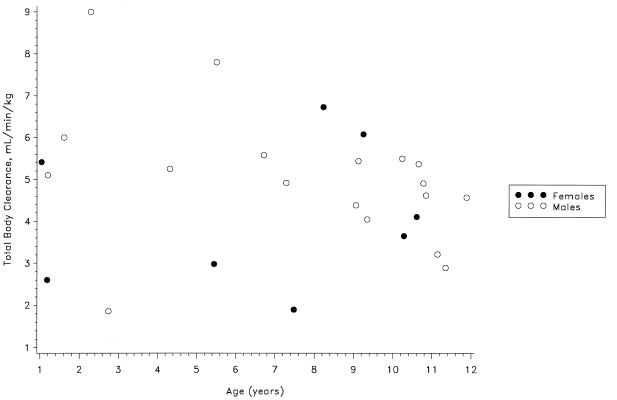
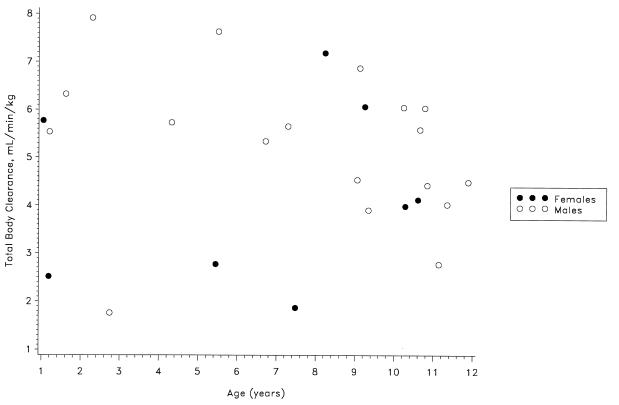
The dose or gender did not influence the pharmacokinetics of ampicillin or sulbactam. The data were analyzed in three age groups of similar size to determine the influence of age on the pharmacokinetics of ampicillin and sulbactam. As shown in Table 1, the mean CL, Vss, and t1/2 were similar in the three age groups (P > 0.05). Although the Cmax for both ampicillin and sulbactam appeared to decline with age, the correlations were not statistically significant. For all patients as a group, the CL, Vss, and t1/2 for ampicillin (means ± standard deviations) were 4.76 ± 1.67 ml/min/kg, 0.32 ± 0.11 liter/kg, and 0.77 ± 0.14 h, respectively; the respective values for sulbactam were 4.95 ± 1.70 ml/min/kg, 0.34 ± 0.12 liter/kg, and 0.81 ± 0.12 h. The interpatient variability in the pharmacokinetic parameters could not be explained by any patient factors. Figures 3 and 4 show no apparent trend for a relationship between clearance of ampicillin or sulbactam and patient age. No significant adverse effects were associated with the use of ampicillin-sulbactam in these pediatric patients.
FIG. 3.
Ampicillin clearance versus age.
FIG. 4.
Sulbactam clearance versus age.
DISCUSSION
Many microorganisms initially susceptible to penicillin have become resistant due to the formation of β-lactamases. Sulbactam is a beta-lactam with little intrinsic antibiotic activity. It acts primarily by irreversible inactivation of β-lactamases produced by many bacteria. Since sulbactam is poorly absorbed after oral administration, it is combined with ampicillin for intravenous administration for the treatment of various infections.
The results from this study indicate that the pharmacokinetics of ampicillin and sulbactam are similar in pediatric patients. The Cmax and AUC for ampicillin were nearly twice those of sulbactam, as expected from their 2:1 ratio in the intravenous dose.
The pharmacokinetics of ampicillin and sulbactam in these patients appeared to be comparable to those in adults (19). In healthy adults, the mean Vss and t1/2 have been reported to be about 0.25 liter/kg and 1 h, respectively, for both ampicillin and sulbactam (4). Another study of healthy subjects found a mean t1/2 of 1.1 h for ampicillin and 1.14 h for sulbactam (23).
These drugs are eliminated primarily by the kidney, and their elimination may be decreased in neonates as a consequence of underdeveloped renal function (1, 9, 22). The t1/2 has ranged from 2 to 21 h for ampicillin and 3 to 72 h for sulbactam in infants less than 1 week old (1). The t1/2 in children, however, has ranged from 0.8 to 1.0 h for both ampicillin and sulbactam (6, 10, 11, 17, 24) and is comparable to the t1/2 observed in our study.
The patients in this study ranged from 1 to 12 years old, and the age did not appear to affect the pharmacokinetics of ampicillin or sulbactam. This can be explained by the fact that the renal function is fully developed for excretion of penicillins by the end of 1 year after birth (12).
The results of this study with children between 1 and 12 years old showed that the pharmacokinetics of ampicillin-sulbactam was independent of dose and gender. The drug was well tolerated in these patients. These data may be useful in treating pediatric patients with ampicillin and sulbactam.
Based on the observed Cmax values and published MIC data (2, 18), ampicillin-sulbactam may be given intravenously at a dose of 75 mg/kg every 6 h to children 1 year old or older. Additional studies are needed to determine the dosage regimens for pediatric patients less than 1 year old.
ACKNOWLEDGMENTS
We thank the following investigators for patient enrollment in this study: William J. Barson and Denis R. King, Children’s Hospital, Columbus, Ohio; Adnan S. Dajani, Children’s Hospital of Michigan, Detroit, Michigan; Gerald M. Haase, Children’s Hospital, Denver, Colo.; Christopher J. Harrison, Creighton University, Omaha, Nebr.; Gordon E. Schutze and Thomas G. Wells, Arkansas Children’s Hospital, Little Rock; and Maria Stephan, Children’s Hospital Medical Center, Cincinnati, Ohio.
REFERENCES
- 1.Axline S G, Yaffe S J, Simon H J. Clinical pharmacology of antimicrobials in premature infants. II. Ampicillin, methicillin, oxacillin, neomycin and colistin. Pediatrics. 1967;39:97–103. [PubMed] [Google Scholar]
- 2.Benson J, Nahata M C. Sulbactam/ampicillin, a new beta-lactamase inhibitor/beta-lactam antibiotic combination. Drug Intell Clin Pharm. 1988;22:534–541. doi: 10.1177/106002808802200702. [DOI] [PubMed] [Google Scholar]
- 3.Federal Register. FDA, 21CFR. Part 201 (docket no. 92N0165). Specific requirements on the content and format of labeling for human prescription drugs: revision of “Pediatric Use” subsection in the laboratory. Fed Regist. 1994;59:64240–64250. [Google Scholar]
- 4.Foulds G. Pharmacokinetics of sulbactam/ampicillin in humans: a review. J Infect Dis. 1991;8(Suppl.):S503–S511. doi: 10.1093/clinids/8.supplement_5.503. [DOI] [PubMed] [Google Scholar]
- 5.Haruta T, Kuroki S, Okura K, Yoshioka N, Yamaoka K, Hashimoto H. Bacteriological, pharmacokinetic and clinical studies of sulbactam/ampicillin in the pediatric field. Jpn J Antibiot. 1989;42:719–724. [PubMed] [Google Scholar]
- 6.Hattori K, Higashino H, Kobayashi T, Yamamoto C, Nobori U, Nakamura Y, Satou Y, Kurokawa H, Nogi S, Kobayashi Y. Pharmacokinetic and clinical studies on sulbactam/ampicillin in children. Jpn J Antibiot. 1989;42:701–717. [PubMed] [Google Scholar]
- 7.Hayashi M, Kida K, Matsuda H. Pharmacokinetics, bacteriological and clinical evaluation of sulbactam/ampicillin in pediatrics. Jpn J Antibiot. 1989;42:743–753. [PubMed] [Google Scholar]
- 8.Ito S, Mayumi M, Ito M, Milawa H. Clinical evaluation of sulbactam/ampicillin in the pediatric field. Jpn J Antibiot. 1989;42:675–685. [Google Scholar]
- 9.Kaplan J M, McCracken G H, Horton L J. Pharmacologic studies in neonates given large dosages of ampicillin. J Pediatr. 1974;84:571–578. doi: 10.1016/s0022-3476(74)80684-0. [DOI] [PubMed] [Google Scholar]
- 10.Meguro H, Arimasu O, Shiraishi H, Sugamata K, Hiruma F, Abe T, Fujii R, Mashiko J, Nagao Y, Okamoto Y. Clinical evaluation of sulbactam/ampicillin in pediatric infections. Jpn J Antibiot. 1989;42:612–622. [PubMed] [Google Scholar]
- 11.Motohiro T, Sakata Y, Oda K, Aramaki M, Tanaka K, Koga T, Shimada Y, Fujimoto T, Yamashita F, Sakamoto H, et al. Pharmacokinetic, bacteriological and clinical studies of sulbactam/ampicillin in pediatric patients. Jpn J Antibiot. 1989;42:773–790. [PubMed] [Google Scholar]
- 12.Nahata M C. Pediatrics. In: DiPiro J, Talbert R, Yu G, Matzke G, Wells B, Posey M, editors. Pharmacotherapy: a pathophysiologic approach. 3rd ed. Norwalk, Conn: Appleton & Lange; 1997. pp. 77–86. [Google Scholar]
- 13.Nahata M C. Need for conducting research on medications unlabeled for use in pediatric patients. Ann Pharmacother. 1994;28:1103–1104. doi: 10.1177/106002809402800917. [DOI] [PubMed] [Google Scholar]
- 14.Nahata M C. New regulations for pediatric labeling of prescription drugs. Ann Pharmacother. 1996;30:1032–1033. doi: 10.1177/106002809603000921. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Miyazu M, Kasai K, Iwai N, Taneda Y. Studies of sulbactam/ampicillin in the field of pediatrics. Jpn J Antibiot. 1989;42:662–674. [PubMed] [Google Scholar]
- 16.Nakao Y, Kimura H, Miura K, Miyajima Y, Ishikawa H, Hayakawa F, Kuno D. Pharmacokinetic, bacteriological and clinical studies on sulbactam/ampicillin in pediatrics. Jpn J Antibiot. 1989;42:639–650. [PubMed] [Google Scholar]
- 17.Nishimura T, Tabuki K. Laboratory and clinical studies of sulbactam/ampicillin in the pediatric field. Jpn J Antibiot. 1989;42:687–700. [PubMed] [Google Scholar]
- 18.Physicians’ desk reference, 53rd ed. 1999. Package information: ampicillin/sulbactam (Unasyn), p. 2421–2424. Medical Economics Co., Montvale, N.J.
- 19.Ripa S, Ferrante L, Prenna M. Pharmacokinetics of sulbactam/ampicillin in humans after intravenous and intramuscular injection. Chemotherapy. 1990;36:185–192. doi: 10.1159/000238765. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Narita A, Suzuki H, Nakazawa S, Matsumoto K, Nakanishi Y, Niino K, Nakazawa S. Clinical and pharmacokinetic studies on intravenous administration of sulbactam/ampicillin in the field of pediatrics. Jpn J Antibiot. 1989;42:623–638. [PubMed] [Google Scholar]
- 21.Sato Y, Ishikawa K, Iwata S, Akita H, Okikawa T, Sunakawa K. Pharmacokinetic and bacteriological studies on sulbactam/ampicillin in the field of pediatrics. Jpn J Antibiot. 1989;42:579–593. [PubMed] [Google Scholar]
- 22.Sutton A M, Turner T L, Cockburn F, McAllister T A. Pharmacokinetic study of sulbactam and ampicillin administered concomitantly by intraarterial or intravenous infusion in the newborn. Rev Infect Dis. 1986;8(Suppl. 5):S518–S522. doi: 10.1093/clinids/8.supplement_5.s518. [DOI] [PubMed] [Google Scholar]
- 23.Wagner J G. Fundamentals of clinical pharmacokinetics. Hamilton, Ill: Drug Intelligence Publications, Inc.; 1975. pp. 90–100. [Google Scholar]
- 24.Yanagashima M, Yanai M, Yanagi T, Tsuji Y. Pharmacokinetic and clinical studies of sulbactam/ampicillin in the pediatric field. Jpn J Antibiot. 1989;42:754–756. [PubMed] [Google Scholar]