Abstract
Simple objective modalities are required for evaluating suspected autoimmune gastritis (AIG). This cross-sectional study aimed to examine whether pepsinogen, gastrin, and endoscopic findings can predict AIG. The diagnostic performance of endoscopic findings and serology in distinguishing AIG was evaluated. AIG was diagnosed in patients (N = 31) with anti-parietal cell antibody and/or intrinsic factor antibody positivity and histological findings consistent with AIG. Non-AIG patients (N = 301) were seronegative for anti-parietal cell antibodies. Receiver operating characteristic curve analysis of the entire cohort (N = 332) identified an endoscopic atrophic grade cutoff point of O3 on the Kimura–Takemoto classification (area under the curve [AUC]: 0.909), while those of pepsinogen-I, I/II ratio, and gastrin were 20.1 ng/mL (AUC: 0.932), 1.8 (AUC: 0.913), and 355 pg/mL (AUC: 0.912), respectively. In severe atrophy cases (≥ O3, N = 58, AIG/control; 27/31), the cutoff values of pepsinogen-I, I/II ratio, and gastrin were 9.8 ng/mL (AUC: 0.895), 1.8 (AUC: 0.86), and 355 pg/mL (AUC: 0.897), respectively. In conclusion, endoscopic atrophy is a predictor of AIG. High serum gastrin and low pepsinogen-I and I/II ratio are predictors even in the case of severe atrophy, suggesting their usefulness when the diagnosis of AIG is difficult or as serological screening tests.
Subject terms: Gastritis, Diagnostic markers, Autoimmunity, Gastrointestinal hormones, Oesophagogastroscopy
Introduction
Autoimmune gastritis (AIG) is an uncommon chronic gastritis characterized by immune-mediated destruction of parietal cells1,2. AIG develops by the following two mechanisms: (1) a decrease in parietal cells resulting in hypochlorhydria or achlorhydria, and a lack of intrinsic factor; and (2) corpus-restricted inflammation progressing to severe oxyntic gland atrophy2,3. Hypergastrinemia, neuroendocrine tumor, and iron deficiency anemia induced by hypochlorhydria; macrocytic-megaloblastic anemia induced by intrinsic factor-dependent vitamin B-12 deficiency; and low serum pepsinogen (PG) I secondary to advanced oxyntic gastric atrophy are the major clinical findings. Although it is clinically important to detect AIG considering the high coincidence rates of gastric cancer, neuroendocrine tumors, macrocytic-megaloblastic anemia, and other autoimmune disorders4, diagnosis is usually difficult, and most cases are overlooked because of their nonspecific and subtle clinical manifestations5,6.
The two diagnostic autoantibodies of this disease are the anti-parietal cell antibody (APCA) and anti-intrinsic factor antibody (AIFA)3; however, AIG cannot be diagnosed merely based on these autoantibodies, and they are not recommended for screening owing to lack of cost-effectiveness, the high false positive rate of APCA, and low sensitivity of AIFA7,8. Histological examination is another established diagnostic tool for AIG2,3; however, despite its high sensitivity, negative results cannot always rule out AIG because of the inherent limitations of biopsy in only a limited area of the gastric mucosa. Since distinct diagnostic criteria for AIG have not yet been established, the method of diagnosis differs considerably among investigators and includes histology alone9,10; histology and autoantibodies11–13; histology, autoantibodies, endoscopy, and gastrin14; histology and serology15; autoantibodies and endoscopy16; autoantibodies or pernicious anemia; and endoscopy and serology without any histological information17. However, the most reliable and objective finding would be a combination of autoantibodies and histology, which is considered the gold standard by several investigators18. Thus, we aimed to examine other potential predictors of AIG, focusing on endoscopic findings, PG, and gastrin.
PG and gastrin are useful in establishing a diagnosis of AIG when autoantibody and histological findings are inconclusive, for example, when false positive or false negative results are observed in strongly suspected cases. Furthermore, these markers are included in the serological screening tests such as the ABC method (PG)19 and Gastropanel® (PG and gastrin)20. Using these tests, clinicians can effectively evaluate the presence of suspected AIG without any histological evidence.
Macroscopic endoscopic findings are also clinically significant as the potential first diagnostic clues of AIG. Although the most common initial findings of AIG are hematological disorders12,21, several investigators suggested that gastrointestinal symptoms are another important clue. Carabotti et al. reported that 56.7% of AIG cases are associated with gastrointestinal symptoms, 69.8% of which are dyspepsia22. Soykan et al. reported that an unexpectedly high rate of 50.4% of patients with AIG complained of abdominal symptoms23. Miceli et al. reported that 34% of AIG cases were diagnosed according to histological findings of gastritis after endoscopy12, and a multicenter study in Japan demonstrated that the most common modality for diagnosing AIG was endoscopy17. These studies suggest the importance of endoscopy as an initial detection tool in some patients with AIG who have abdominal symptoms or those who are negative for anemia or other autoimmune diseases.
In this cross-sectional study, we assessed the performance of endoscopic findings and serology (PG and gastrin) in diagnosing AIG in (1) patients with AIG strictly defined based on autoantibody positivity and characteristic histological findings, and (2) non-AIG patients seronegative for autoantibodies.
Results
Baseline characteristics of the whole study population
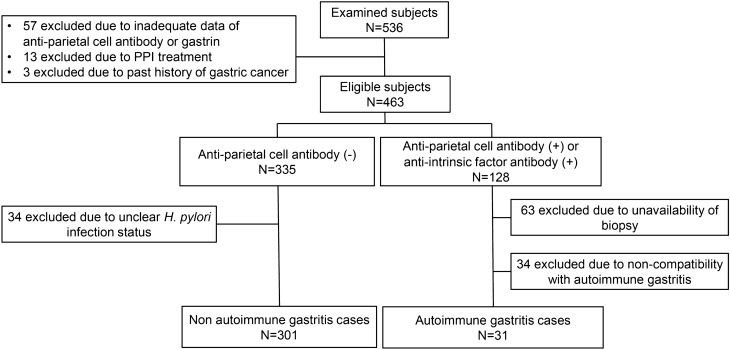
As shown in Fig. 1, 301 non-AIG patients (male, 53.8%; median age [interquartile range (IQR)], 70 [63.5–75] years) and 31 patients with AIG (male, 58.1%; median age [IQR], 73 [66–76] years) were finally enrolled for analysis in this study. Among patients with AIG, 13 initially presented to our hospital, and the remaining 18 were already being followed up at our hospital. The reasons for performing endoscopy were as follows: positive results in the ABC method (n = 14, 45.2%), abnormalities on barium X-ray (n = 5, 16.1%), annual endoscopic follow-up (n = 3, 9.7%), screening in asymptomatic individuals (n = 6, 19.3%), and others (n = 3, 9.7%).
Figure 1.
Study flowchart of patient selection.PPI, proton pump inhibitor.
Age, sex, serum biomarker levels (PG and gastrin), Helicobacter pylori (H. pylori) serology, and autoantibodies were evaluated for all patients, as shown in Table 1. Supplementary Table S1 shows the detailed histological findings in patients with AIG (according to our definition). Oxyntic mucosal atrophy and lymphocyte and plasma cell infiltration were observed in all cases. Supplementary Table S2 presents the observed Kappa values among the three endoscopists. As a Kappa value of ≥ 0.60 is usually considered reliable and applicable for evaluation, the “endoscopic atrophic border” (mean, 0.654) and disappearance of the gastric fold (mean, 0.622) were adapted as parameters to be analyzed in this study. However, three other endoscopic findings, including corpus-predominant advanced atrophy compared to the antral part (mean, 0.335), sticky mucosa (mean, 0.352), and hyperplastic polyp (mean, 0.516), were excluded from the analysis as endoscopic findings generally are not highly reliable.
Table 1.
Baseline characteristics of all participants.
| Baseline characteristics | Autoimmune gastritis (N = 31) | Non-autoimmune gastritis (N = 301) | P-value* |
|---|---|---|---|
| Male, N (%) | 18 (58.1) | 162 (53.8) | 0.652 |
| Age, years, median (IQR) | 73 (66–76) | 70 (63–75) | 0.132 |
| Gastric cancer, N (%) | 2/31 (6.5%) | 8/301 (2.7%) | 0.237 |
| Pepsinogen I (ng/mL), median (IQR) | 6.4 (3.8–15.9) | 43.3 (31.2–59.5) | < 0.001* |
| Pepsinogen II (ng/mL), median (IQR) | 7.7 (4.9–10.3) | 8.3 (5.6–13.0) | 0.273 |
| Pepsinogen I/II ratio, median (IQR) | 1.0 (0.6–1.5) | 5.5 (3.6–7.2) | < 0.001* |
| H. pylori antibody titer > 10 U/mL, N (%) | 4/31 (12.9%) | 105/301 (34.9%) | < 0.05* |
|
H. pylori infection status Uninfected/present infection/eradicated/past infection, N (%) |
17/6/8/0 (54.9%/19.4%/25.8%/0%) | 77/106/89/29 (25.6%/35.2%/29.6%/9.6%) | < 0.01* |
| Gastrin (pg/mL), median (IQR) | 1,310 (448–2,490) | 102.0 (82–144) | < 0.001* |
| < 100/100–350/350–1,000/ > 1,000 pg/mL, N (%) | 2/3/7/19 (6.5%/9.7%/22.6%/61.3%) | 141/140/15/5 (46.8%/46.5%/5.0%/1.7%) | < 0.001* |
| Anti-parietal cell antibody positive, N (%) | 29/31 (93.5%) | 0/301 (0%) | < 0.001* |
| Anti-intrinsic factor antibody positive, N (%) | 15/27 (55.6%) | NA | NA |
|
Endoscopic atrophic border†, N (%) C0/C1–3/O1–2/O3/O4, N |
0/2/2/7/20 (0%/6.5%/6.5%/22.6%/64.5%) | 71/67/132/22/9 (23.5%/22.3%/43.9%/7.3%/3.0%) | < 0.001* |
| Endoscopic atrophic border† > O3, N (%) | 27/31 (87.1%) | 31/301 (10.2%) | < 0.001* |
| Disappearance of the gastric fold, N (%) | 19/31 (61.2%) | 20/301 (6.6%) | < 0.001* |
Data are provided as numbers (%) or median (interquartile range [IQR]).
*P-value: Fisher’s exact test or Man–Whitney U test; autoimmune gastritis vs. non-autoimmune gastritis.
†Endoscopic atrophic border was based on the Kimura–Takemoto classification. We defined O4 as marked vascular visibility observed in the greater curvature of the corpus, which is defined as O3 in the Kimura–Takemoto classification.
The clinical characteristics of patients with AIG and non-AIG gastritis were compared (Table 1). The median PG I levels (6.4 [3.8–15.9] vs. 43.3 [31.2–59.5] ng/mL, P < 0.001; normal value, < 70 ng/mL) and PG I/II ratios (1.0 [0.6–1.5] vs. 5.5 [3.6–7.2], P < 0.001; normal value, < 3) were significantly lower, and gastrin levels (1,310 [448–2,490] vs. 102.0 [82–144] pg/mL, P < 0.001; normal value, < 140 pg/mL) were significantly higher in patients with AIG than in those without. Severe endoscopic atrophy (atrophic border of O3 and O4) (87.1% vs. 10.2%, P < 0.001) and the disappearance of the gastric fold (61.2% vs. 6.6%, P < 0.001) were significantly more common in patients with AIG than in those without. Age, sex, PG II level, and the prevalence of gastric cancer were similar between the two groups. The detailed clinical findings of AIG are shown in Table 2. Briefly, urea breath tests (≥ 5%) and H. pylori serology (≥ 10 U/mL) were positive in 8/20 (40%) and 4/31 (12.9%) patients, respectively. Two-thirds of patients with AIG (20/31, 64.6%) showed a moderate to high titer (≥ 20) of APCA. Anemia, pernicious anemia, and Hashimoto’s thyroiditis were diagnosed in 8/31 (25.8%), 6/23 (26.1%), and 11/27 patients (40.7%), respectively. One case each of primary biliary cholangitis and Hunter’s glossitis was diagnosed before AIG diagnosis.
Table 2.
Detailed clinical and laboratory findings of autoimmune gastritis.
| Baseline characteristics | Autoimmune gastrits (N-31) |
|---|---|
| Diagnosis of H. pylori infection | |
| Urea breath test* | |
| > 5‰/2.5–5‰/ < 2.5‰, N (%) | 8/6/6 (40%/30%/30%) |
| H. pylori antibody titer | |
| ≥ 10 U/mL /3–9.9 U/mL / < 3 U/mL, N (%) | 4/7/20 (12.9%/22.6%/64.5%) |
| H. pylori culture† | |
| positive, N (%) | 2/12 (16.7%) |
| Anti-parietal cell antibody titer | |
| Serum dilution (negative/1:10/1:20–40/1: ≥ 80), N (%) | 2/9/14/6 (6.5%/29%/45.2%/19.4%) |
| Associated disorders | |
| Anemia, N (%)‡ | 8/31 (25.8%) |
| Low Vitamin B 12, N (%)§ | 12/23 (52.2%) |
| Pernicious anemia, N (%)§ | 6/23 (26.1%) |
| Hashimoto's disease, N (%)|| | 11/27 (40.7%) |
| Gastric cancer, N (%) | 2/31 (6.5%) |
| intestinal/diffuse, N | 1/1 |
| early/advanced, N | 2/0 |
*The urea breath test was performed in 20 participants.
†H. pylori culture was performed in 12 participants.
‡Anemia was defined as hemoglobin < 13.0 g/dL in men or < 11.4 g/dL in women.
§Vitamin B12 and pernicious anemia were evaluated in 23 participants. Low vitamin B12 was defined as vitamin B12 level < 233 pg/mL. Pernicious anemia was defined as vitamin B12 < 233 pg/mL, mean corpuscular volume > 80 fl, and hemoglobin < 13 g/dL in men or 11.4 g/dL in women.
||Hashimoto’s disease was evaluated in 27 participants.
Diagnostic ability of each clinical parameter in predicting AIG
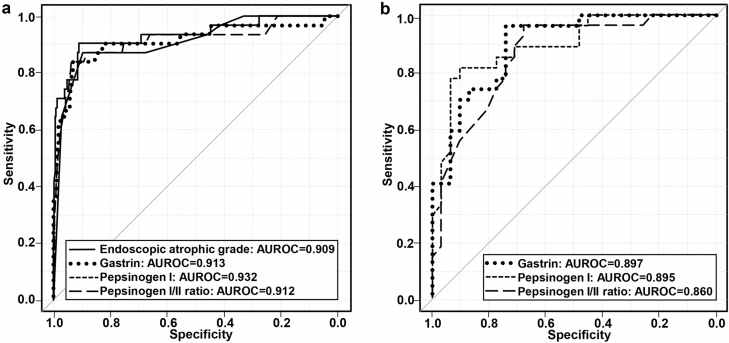
The diagnostic ability of endoscopic findings, gastrin, and PG for predicting AIG was evaluated by ROC analysis of the entire study cohort (N = 332, AIG = 31, control = 301) (Table 3). The AUROC for an endoscopic atrophic border was 0.909 (95% CI 0.848–0.970), and the threshold point was O3 on the Kimura–Takemoto classification, with a sensitivity of 87.1%, specificity of 89.7%, and overall accuracy of 89.5%. The AUROC for the disappearance of the gastric fold was 0.773 (95% CI 0.66–0.879) with a sensitivity of 61.3%, specificity of 93.4%, and overall accuracy of 90.4%. The optimal cutoff value of gastrin was 355 pg/mL (AUROC, 0.912; 95% CI 0.844–0.983), with a sensitivity of 83.9%, specificity of 93.4%, and overall accuracy of 92.5%, while that of PG I was 20.1 ng/mL (AUROC, 0.932; 95% CI 0.874–0.990), with a sensitivity of 90.3%, specificity of 91.0%, and overall accuracy of 91.0%. The optimal cutoff value of the PG I/II ratio was 1.8 (AUROC, 0.913; 95% CI 0.840–0.981), with a sensitivity of 83.9%, specificity of 93.7%, and overall accuracy of 92.8%. The difference in the AUROCs of the endoscopic atrophic border and disappearance of the gastric fold was significant (P < 0.01); however, those between the endoscopic atrophic border and gastrin, PG I, and I/II ratio were not significant, according to the Delong test. Figure 2A illustrates the comparison of AUROCs for the endoscopic atrophic grade, PG I, PG I/II ratio, and gastrin, showing that all AUROCs were ≥ 0.80.
Table 3.
Diagnostic performance of clinical parameters in predicting autoimmune gastritis in the overall cohort.
| Clinical parameters | AUROC | SE | 95% CI | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Endoscopic atrophic grade* | 0.909 | 0.031 | 0.848–0.970 | O3 | 87.1 | 89.7 | 46.6 | 98.5 | 89.5 |
| Disappearance of the gastric fold† | 0.773 | 0.054 | 0.66–0.879 | Disappearance of the gastric fold | 61.3 | 93.4 | 48.7 | 95.9 | 90.4 |
| Serum gastrin concentration (pg/mL) | 0.913 | 0.035 | 0.844–0.981 | 355 | 83.9 | 93.4 | 56.5 | 98.3 | 92.5 |
| Pepsinogen I (ng/mL) | 0.932 | 0.03 | 0.874–0.990 | 20.1 | 90.3 | 91 | 50 | 98.5 | 91 |
| Pepsinogen I/II ratio | 0.912 | 0.03 | 0.840–0.983 | 1.8 | 83.9 | 93.7 | 58.1 | 97.9 | 92.8 |
AUROC area under the receiver operating characteristic curve, 95% CI 95% confidence interval, NPV negative predictive value, PPV positive predictive value, SE standard error.
*Endoscopic atrophic grade was based on the Kimura–Takemoto classification (C0–O3), and O4 is defined as marked vascular visibility observed in the greater curvature of the corpus.
†P < 0.05 in comparison with the AUROC for the disappearance of the gastric fold, by the Delong test.
Figure 2.
(A) Receiver operating characteristic curves showing the ability of each variable to distinguish autoimmune gastritis in the overall cohort. Receiver operating characteristic curves for the performance of endoscopic atrophy, disappearance of the gastric fold, pepsinogen I level, pepsinogen I/II ratio, and gastrin in distinguishing patients with autoimmune gastritis (N = 31) from those with non-autoimmune gastritis (N = 301). Area under the receiver operating characteristic curve (AUROC) of endoscopic atrophic grade, the disappearance of the gastric fold, gastrin, pepsinogen I, and pepsinogen I/II ratio was 0.909, 0.773, 0.913, 0.932 and 0.912, respectively; additionally, the optimal cutoff points for endoscopic atrophic grade, gastrin, pepsinogen I, and pepsinogen I/II ratio were, O3, 355 pg/mL, 20.1 ng/mL, and 1.8, respectively. (B) Receiver operating characteristic curves showing each variable’s ability to distinguish autoimmune gastritis in patients with severe endoscopic atrophy (≥ O3 on the Kimura–Takemoto classification). Receiver operating characteristic curves for the performance of pepsinogen I level, pepsinogen I/II ratio, and gastrin in distinguishing patients with autoimmune gastritis (N = 27) from those with non-autoimmune gastritis (N = 31). Area under the receiver operating characteristic curve (AUROC) of gastrin, pepsinogen I, and pepsinogen I/II ratio was 0.897, 0.895 and 0.86, respectively, and their optimal cutoff values were 355 pg/mL, 9.8 ng/mL, and 1.8, respectively.
Diagnostic ability of each serological parameter in predicting AIG in patients with advanced atrophy
The subgroup of patients with severe endoscopic atrophy ≥ O3 (N = 58 [AIG = 27, control = 31]) was extracted (Supplementary Table S3), and the diagnostic ability of gastrin and PG in predicting AIG in this subgroup of patients was also evaluated by ROC analysis. The median values of PG I (5.9 ng/mL vs. 18.9 ng/mL, P < 0.001; normal value, < 70 ng/mL), PG I/II ratio (0.9 vs. 3.3, P < 0.001; normal value, < 3), and prevalence of H. pylori seropositive cases (7.4% vs. 54.8%, P < 0.001; normal value, < 140 pg/mL) were significantly lower and the median gastrin level (1310 pg/mL vs. 102 pg/mL, P < 0.001) was significantly higher in patients with AIG than in those without. The prevalence of severe endoscopic atrophy extending to the entire corpus (O4) was significantly higher in patients with AIG than in those without (80.6% vs. 10.7%, P < 0.001). ROC analysis revealed that the optimal cutoff value of gastrin was 355 pg/mL (AUROC, 0.897; 95% CI 0.819–0.975), with a sensitivity of 96.3%, specificity of 74.2%, and overall accuracy of 84.5%, while that of PG I was 9.8 ng/mL (AUROC, 0.895; 95% CI 0.813–0.977), with a sensitivity of 81.5%, specificity of 90.3%, and overall accuracy of 86.2%. The optimal cutoff value of the PG I/II ratio was 1.8 (AUROC, 0.86; 95% CI 0.765–0.956), with a sensitivity of 96.3%, specificity of 67.7%, and overall accuracy of 81.0%. The AUROCs of PG I and I/II ratio were not significantly different from that of serum gastrin according to the Delong test (Table 4). Figure 2B shows a comparison of AUROCs between PG I, PG I/II ratio, and gastrin.
Table 4.
Diagnostic performance of serum gastrin and pepsinogen in predicting autoimmune gastritis in cases with severe endoscopic atrophy (≥ O3 on the Kimura–Takemoto classification).
| Clinical parameters | AUROC | SE | 95% CI | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Serum gastrin concentration (pg/mL) | 0.897 | 0.04 | 0.819–0.975 | 355 | 96.3 | 74.2 | 76.5 | 95.8 | 84.5 |
| Pepsinogen I (ng/mL) | 0.895 | 0.049 | 0.813–0.977 | 9.8 | 81.5 | 90.3 | 87.5 | 82.4 | 86.2 |
| Pepsinogen I/II ratio | 0.86 | 0.078 | 0.765–0.956 | 1.8 | 96.3 | 67.7 | 71.4 | 91.3 | 81.0 |
AUROC area under the receiver operating characteristic curve, 95% CI 95% confidence interval, NPV negative predictive value, PPV positive predictive value, SE standard error.
Discussion
In this study, we evaluated the relevance of several clinical findings in AIG diagnosis among (1) patients diagnosed by autoantibody positivity and characteristic histology and (2) non-AIG patients diagnosed based on autoantibody negativity whose H. pylori infection status was precisely evaluated. The ROC curve analysis results of the entire study population (N = 332) indicated that the diagnostic performance of advanced endoscopic atrophy (≥ O3 in the Kimura–Takemoto classification) and serology, including PG (PG I, ≤ 20.1 ng/mL; PG I/II ratio, ≤ 1.8) and gastrin (≥ 355 pg/mL), was high; therefore, they have sufficient diagnostic performance for use in daily clinical practice.
As our results showed that advanced endoscopic atrophy itself may be a marker of AIG, it may be useful if highly suspected cases of AIG among patients with advanced atrophy can be further evaluated by serology (i.e., two-stage identification). Based on this idea, an ROC curve analysis of patients with severe gastric atrophy only (≥ O3, N = 58) was performed. Our results indicated that the diagnostic performance of PG (PG I, ≤ 9.8 ng/mL; PG I/II ratio, ≤ 1.8) and gastrin (≥ 355 pg/mL) was also high (AUROC, > 0.85) and that they may be valid predictors. These findings are especially useful when severe atrophy is found during endoscopy, which may be the commonest way of identifying AIG in clinical practice.
In our study, gastrin (355 pg/mL) and PG I/II ratio (1.8) exhibited the same cutoff values for the prediction of AIG in both the overall study cohort and the subset of patients with advanced atrophy. These results highlight how they may be especially useful as serum markers in clinical practice. Considering the high AUROC of these serological markers, we advocate that PG and gastrin should be additionally checked in cases where the diagnosis is challenging, for example, when pathological examination of biopsy specimens does not show findings typical of AIG despite a positive APCA result. Re-biopsy and histological evaluation may be recommended if PG or gastrin levels are strongly suggestive of AIG. Moreover, in serological screening tests, these cutoff values are useful in detecting AIG; in this study; endoscopy was indicated in approximately half (45.2%) of the AIG cases based on a positive serology test using the ABC method.
The endoscopic atrophic grade (Kimura–Takemoto classification) is considered highly objective by several investigators24,25, and this atrophy grade was imposed for all upper gastrointestinal endoscopic cases in the Japan Endoscopy Database Project26. Although O4 is useful in detecting AIG of high prevalence compared with non-AIG (64.5% vs. 3.0%), new endoscopic criteria are not necessary for AIG diagnosis as it is included as part of grade O3 in the Kimura–Takemoto classification. In AIG diagnosis, endoscopy is often performed merely to take biopsies for histopathological diagnosis with low specificity and objectivity3,10,27. However, herein, we demonstrated that the “endoscopic atrophic border” had a Kappa value of 0.654. This is consistent with the report by Terao et al.17, wherein high interobserver variability (0.705) was shown in the “degree of atrophy;” however, in the case of “sticky mucosa,” our Kappa value seems to be lower than their value (0.352 vs. 0.604). Although corpus-predominant advanced atrophy compared with the antral region, which we referred to as the “corpus-predominant atrophic pattern,” has been used as a diagnostic criterion15,16, it did not have a reliable Kappa value in our study. Regarding the disappearance of the gastric fold in the gastric corpus, it cannot be used as a diagnostic modality because it has a low AUROC value (0.773), even though its Kappa value was > 0.6. Hence, other objective endoscopic findings should be evaluated.
In this study, the cutoff values of gastrin for the diagnosis of AIG obtained from the ROC analysis (355 pg/mL) were higher than those previously reported by Checchi et al.28. The cutoff value of gastrin for diagnosing AIG with severe atrophy was reported as 395 pg/mL11, and Terao et al.17 set hypergastrinemia > 350 pg/mL as one of the criteria for AIG in a multicenter study, which is similar to the 355 pg/mL cutoff value for gastrin in this study. In Checchi et al.’s study, the cutoff value of gastrin (43 pg/mL) was close to normal (< 39.3 pg/mL), suggesting the possible diagnosis of individuals without AIG as having AIG28. Simple comparisons with other reports are difficult because of differences in the gastrin unit of measurement (pg/mL and pmol/L)10,29.
Regarding PGs, various cutoff values have been reported by several investigators; however, few reports involved an ROC analysis using a histology-based definition of AIG10,28,30. The cutoff values for PG I (20.1 ng/mL) and PG I/II ratio (1.8) in our study were similar to those reported by Koc et al.30, but significantly lower than those in the studies by Venerito et al.10 (PG I, 50 ng/mL; PG I/II ratio, 5) and Checchi et al. (PG I/II ratio, 14)28. However, considering that the definition of AIG employed by Venerito et al. was confined to the histology of enterochromaffin-like hyperplasia and the almost normal cutoff value of gastrin reported by Checchi et al., their cutoff values may be set higher than the actual physiological values. The evaluations of the cutoff value of PGs to predict AIG in other studies were inherently limited by various issues. A report evaluated the ROC curve of PGs in appropriately defined patients with AIG, but did not calculate the cutoff value31. Three more studies did not analyze the cutoff value in a strictly defined AIG cohort, including participants with multifocal atrophy32, pangastritis29, or severe atrophy of Operative Link on Gastric Intestinal Metaplasia Assessment > stage 2 and autoantibody positivity15. Because Koc et al. only analyzed a limited number of patients (N = 16) with AIG, this is the first report to demonstrate the practical cutoff value of PG in the diagnosis of AIG using a sufficient cohort of strictly defined patients with AIG.
In this study, individuals on acid inhibitors were strictly excluded; however, in clinical practice, the possible effects of acid inhibitory drugs should be considered when using these cutoff values. Hypergastrinemia characterized by gastrin levels over 500 pg/mL, which is induced by PPIs or potassium competitive acid blockers (PCABs)33,34, can be a major problem in serological screening for AIG. However, it is unclear whether eradication influences gastrin in patients with AIG because severe hypochlorhydria is induced by AIG in many cases, and eradication may not affect it. As for PG and gastrin, these would theoretically normalize after eradication; however, because of the extremely low PG level and extremely high gastrin level in AIG, eradication treatment does not seem to affect the results of serological screening.
In our study, the median value (IQR) of gastrin was 1,310 (448–2,490) pg/mL, and the PG I level and PG I/II ratio were 6.4 (3.8–15.9) ng/mL and 1.0 (0.6–1.5), respectively, suggesting that the clinical characteristics of AIG in our study were generally comparable to those in previous studies10,17,27,35. However, 45.2% of patients with AIG in our study had H. pylori infection (19.4% and 25.8% of present and previous infection cases, respectively), an incidence higher than those in previous reports of seropositivity rates (Terao et al.17, 7.8%; Venerito et al.10, 18.2% [4/22]). In our study, 29/31 patients with AIG (94%) were accurately evaluated by more than two modalities to establish H. pylori infection, and eradication was diagnosed by normalization of the urea breath test, which may explain the high infection rate in our study.
The prevalence of AIG (5.8%, 31/536) in this study is higher than the reported overall prevalence in Japan (0.49%)16. Eighteen patients with AIG, who were included in the analysis, were already being followed-up at our hospital with regular endoscopy. Thus, the prevalence of first-visit cases was 13/536 (2.4%), which is still higher than the national prevalence rate. However, we believe that this is reasonable given how rare cases tend to cluster at major referral hospitals, such as ours.
This study has some limitations. First, the identification of patients with AIG was based only on autoantibodies and histological findings. In this study, 2/31 histologically defined AIG cases (6.4%) had normal gastrin levels, suggesting that false positive cases were inevitably included (although there is a possibility that these were very early stage AIG cases), and that additional criteria using serological markers, such as gastrin and/or PG, may be required to increase the accuracy of the diagnosis of AIG, as described earlier. Second, other endoscopic findings, including remnant oxyntic mucosa, scattered minute whitish protrusions, and intestinal metaplasia were not evaluated. They may be potential predictive factors of AIG and should, therefore, be evaluated. Furthermore, the small sample size of patients from a single institution may limit the generalizability of the obtained results.
In conclusion, in this study, we demonstrated that endoscopic findings of severe atrophy (≥ O3 in the Kimura–Takemoto classification) are useful predictors of AIG. High serum gastrin levels and low PG I and I/II ratio are also predictors of AIG. The cutoff values of serum gastrin levels (≥ 355 pg/mL) and low PG I/II ratio (≤ 1.8) can be used in both the general population and those with endoscopic atrophy (≥ O3). Thus, patients with advanced atrophy with a strong suspicion of AIG can be further evaluated using these cutoff values. Clinicians should also note that AIG can be suspected by serology, especially when false negative or positive results are suspected or during serological screening for gastritis involving PG and/or gastrin.
Methods
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study design was approved by the Ethics Committee of Tokyo Dental College Ichikawa General Hospital (approval numbers: I-283 RII/2016, I-283 RIII/2017, I-283 RIV/2018, I-283 RV/2019, and I-283 RVI/2021). Written informed consent was obtained from all participants.
Study design and participants
In this cross-sectional study, 536 consecutive patients who underwent upper gastrointestinal endoscopy at Tokyo Dental College Ichikawa General Hospital between January 2017 and March 2020 were enrolled after obtaining written informed consent. The exclusion criteria for this study were as follows: (1) use of histamine-2 receptor antagonists, PPIs, or PCABs within the preceding 2 months; (2) presence of viral diseases; (3) pregnancy or lactation; (4) presence of renal and/or liver dysfunction; or (5) a past history of gastric cancer or any type of esophageal or gastric surgery36. Most of the excluded individuals were excluded by interview before enrollment.
A flowchart of the target group selection is shown in Fig. 1. “AIG” was defined based on both (1) seropositivity for APCA and/or AIFA, and (2) histological findings consistent with AIG. Patients positive for either APCA or AIFA were classified into the following two subgroups: patients in whom biopsy of the gastric body was performed, and those without biopsy. The latter were excluded from the analysis. For AIG diagnosis, in the case of APCA positivity only, at least three of the five histological findings compatible with AIG, as described in the “Histology” section, were necessary. In the case of AIFA positivity, irrespective of APCA, at least two of the five histological findings were necessary because the sensitivity of AIFA in diagnosing AIG is considerably higher than that of APCA8. Non-AIG controls were defined as individuals who tested negative for APCA, and 57 were excluded due to inadequate APCA or gastrin data, 13 due to PPI treatment, and 3 due to history of gastric cancer.
H. pylori infection status in the control participants was defined as follows: Patients who were seronegative for APCA and with a clear history of successful eradication, confirmed by a negative result on either a urea breath or stool antigen test, were defined as “H. pylori-eradicated patients.” In contrast, those with a result < 2.5% in the urea breath test were classified as negative according to the manufacturer’s recommended cutoff value. Patients negative for APCA and with a history of eradication were further classified into two subgroups: patients positive (≥ 10 U/mL) and negative (< 10 U/ml) for H. pylori antibody titer. The former was defined as “present H. pylori infection cases.” The latter individuals with an atrophy grade from C0 to C1, which is regarded as an atrophy score of 0 (negative) in the Kyoto classification37, were defined as “H. pylori-uninfected cases.” The patient group negative for H. pylori antibody (< 10 U/mL) and endoscopic atrophy (≥ C-2) may include those who are H. pylori-infected and those with previous infection-induced atrophic gastritis36,38,39. Among these patients, those with at least one positive urea breath, stool antigen, or culture test were classified as “present H. pylori infection cases,” whereas previous infection-induced atrophic gastritis cases were defined when negative results were obtained in urea breath or stool antigen tests, as we reported previously40. If a urea breath or stool antigen test was not performed, individuals with an eradication history and those with endoscopic atrophy (≥ C2) and seronegative results of H. pylori were excluded.
H. pylori infection status was defined differently in patients with AIG because of the extreme case bias. Those with at least one positive urea breath, stool antigen, H. pylori antibody titer, or culture test were classified as “present H. pylori infection cases”. The cutoff value for a positive diagnosis in the urea breath test was set at 5% instead of 2.5%41 because false-positive results tend to be obtained in AIG. Patients with an apparent history of successful eradication, confirmed by a negative result in either a urea breath or stool antigen test, were defined as “H. pylori-eradicated patients.” The cutoff value for a negative result in these urea breath tests was strictly 2.5%. It is difficult to differentiate between H. pylori-uninfected individuals and those with a previous infection among patients with AIG; thus, for simplicity, patients other than those with a present infection or history of eradication were defined as H. pylori-uninfected.
To evaluate the diagnostic validity and determine the threshold values of the serological tests and endoscopic findings for predicting AIG, an ROC curve analysis was performed.
Measurement of serum biomarkers
Fasting serum samples (after an overnight fast) were collected before endoscopy. PG, gastrin, H. pylori antibody titers, and APCA were examined in the enrolled patients. Briefly, serum PG I and PG II levels were measured using a commercial chemiluminescence enzyme immunoassay kit (Architect Pepsinogen I, Pepsinogen II, Abbott Japan Co. Ltd., Tokyo, Japan); H. pylori IgG antibodies were measured using an enzyme immunoassay kit (E Plate “Eiken” H. pylori antibody, Eiken Chemical Co. Ltd., Tokyo, Japan); and gastrin level was determined with radioimmunoassay (Gastrin RIA Kit II, Fujirebio Diagnostics Co., Ltd., Tokyo, Japan), as previously reported36. In patients clinically suspected of having AIG (e.g., severe atrophy of ≥ O3 according to the Kimura–Takemoto classification or APCA positivity), thyroglobulin antibodies, thyroid microsomal antibodies, serum vitamin B12, and AIFA were additionally measured. APCA and AIFA measurements were outsourced to SRL Co. Ltd. (Tokyo, Japan), and thyroglobulin and thyroid microsomal antibody measurements were performed by LSI Medience Co., Ltd. (Tokyo, Japan). Anemia was defined as hemoglobin < 13 g/dL (men) or 11.4 g/dL (women); vitamin B12 level < 233 pg/mL was considered low; and pernicious anemia was defined as vitamin B12 < 233 pg/mL, mean corpuscular volume (MCV) > 80 fL, and hemoglobin < 13 g/dL (men) or 11.4 g/dL (women)17. Hashimoto’s disease was defined as positivity for thyroglobulin antibodies or thyroid microsomal antibodies. APCA and AIFA were analyzed using a fluorescent antibody test and chemiluminescence enzyme immunoassay, respectively. APCA was considered positive when fluorescence was produced at a dilution of tenfold or more according to the manufacturer’s instruction. Although there is limited information on the sensitivity of APCA, a study reported that 33.59% of APCA-positive cases were diagnosed as AIG7.
Histology
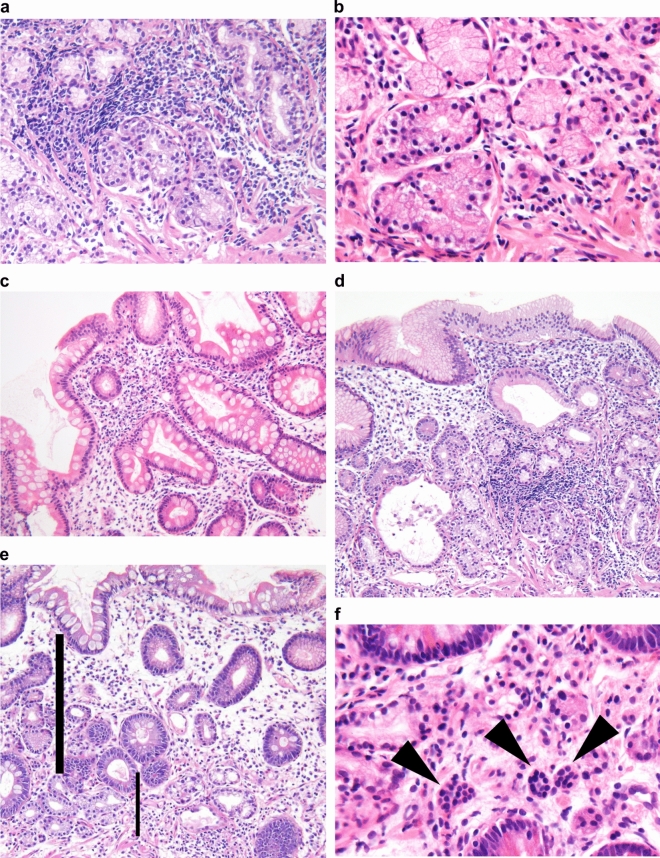
Patients positive for either APCA or AIFA with available gastric corpus biopsies were histologically evaluated by an experienced pathologist (AS) who was blinded to the patients’ endoscopic and serological information and medical charts. Biopsy specimens were fixed with buffered formalin and stained using hematoxylin and eosin. The following five findings were evaluated to diagnose AIG based on previous reports3,9–14: (1) oxyntic mucosal atrophy, (2) pseudo-pyloric and intestinal metaplasia, (3) diffuse lymphocytic cell infiltration, (4) proportion of gastric pits/duct in the gastric mucosa (> 1.0 or none), and (5) enterochromaffin-like cell hyperplasia. Typical histological findings are shown in Fig. 3a–f.
Figure 3.
Targeted histological findings of autoimmune gastritis. Oxyntic mucosal atrophy with lymphocytic infiltrates (a). Epithelium of pseudo-pyloric (b) and intestinal metaplasia (c). Diffuse lymphocyte cell infiltration, which is heavier in the deep portion than in the lamina propria (d). Increased proportion of gastric pits (bold line) induced by severe atrophy, which was defined as the proportion of gastric pits (bold line)/gastric duct (thin line) (e). Enterochromaffin-like cell hyperplasia (arrowhead) (f).
Endoscopic examination
Upper gastrointestinal endoscopy was performed using an Olympus Elite system and Olympus electronic panendoscopes, including the GIF-HQ290 or GIF-HQ290Z (Olympus, Optical Co. Ltd, Tokyo, Japan). Based on previous reports13,14,16,17, the following five endoscopic findings considered specific for AIG were evaluated individually: (1) endoscopic atrophic grade, (2) characteristic corpus-predominant atrophic pattern (corpus-predominant advanced atrophy compared with the antral part), (3) disappearance of the fold in the greater curvature of the gastric body in the sufflation state, (4) presence of sticky adherent dense mucous, and (5) hyperplastic polyps. Sticky adherent dense mucous has a denser, creamy white-yellowish color and firmly adheres to the mucosa, as defined by Terao et al.17. The endoscopic atrophic grade was defined according to the Kimura–Takemoto classification42, which categorized gastric mucosal atrophy into closed (C1–3) and open types (O1–3). We added “O4” for severe endoscopic atrophic corpus gastritis, which was defined as marked vascular visibility observed not only on the lesser curvature but also on the whole greater curvature of the corpus. This is included in the O3 category of the Kimura–Takemoto classification and is similar to the definition provided by Terao et al.17; however, it was not associated with the disappearance of the fold according to our definition. Figures 4a–d depict typical endoscopic findings of O4 (Fig. 4a), O3 (Fig. 4b), O2 (Fig. 4c), and O1 (Fig. 4d). Figure 4e shows the endoscopic findings of the corpus-predominant atrophic and non-atrophic patterns in the antrum (Fig. 4e-1), despite severe corpus atrophy (Fig. 4e-2). Figures 4a, 4b, and 4e-2 show the disappearance of the fold. Figure 4f shows sticky adherent dense mucous, and Fig. 4g shows multiple hyperplastic polyps.
Figure 4.
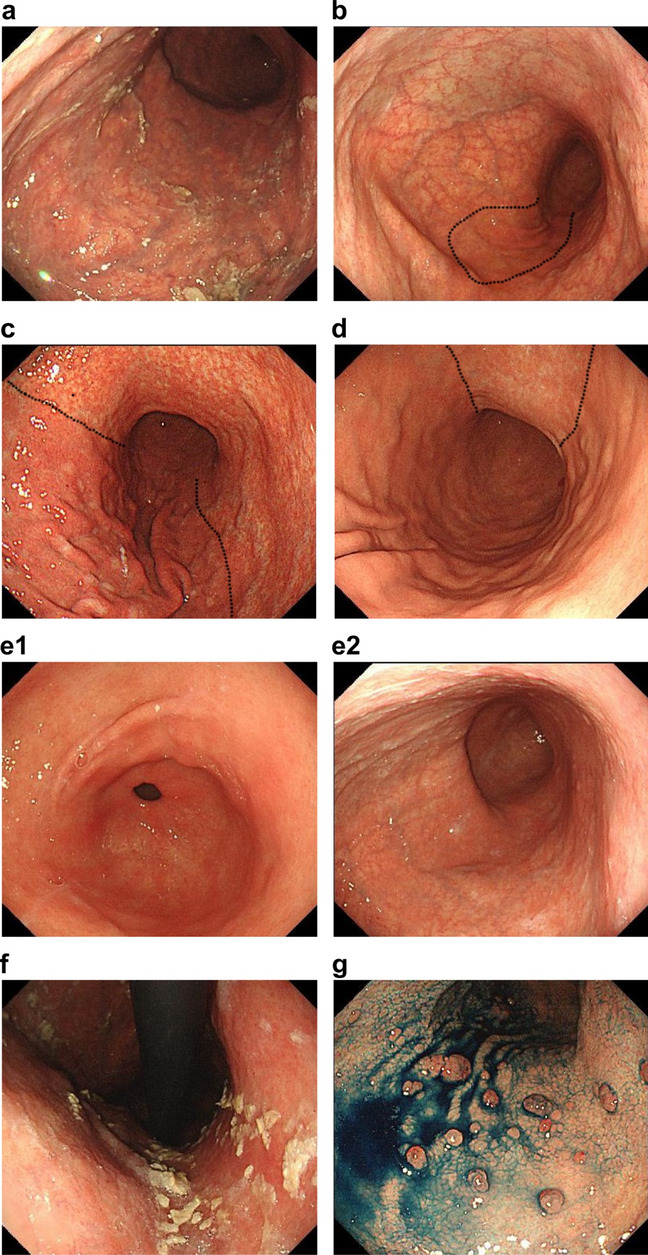
Representative endoscopic findings associated with autoimmune gastritis. Endoscopic findings of O4 with vascular visibility in the entire area of the greater curvature of the corpus (a). O3 with spared vascular visibility in a part of the greater curvature of the corpus (b). O2 with spared vascular visibility in the entire area of the greater curvature of the corpus (c). O1 with vascular visibility in the lesser curvature of the corpus (d). The endoscopic atrophic border is indicated by a dotted line (b–d). Endoscopic findings of corpus-predominant advanced atrophy (O4) with a non-atrophic antral part (antrum [e1] and corpus [e2]). Endoscopic findigs of the disappearance of the fold in the greater curvature of the gastric body (a, b, e2). Typical endoscopic findings of sticky adherent dense mucous (f) and multiple hyperplastic polyps in the proximal stomach (g).
Three experienced endoscopists (KN, YH, and HH), blinded to the clinical, serological, and histological status of the patients, independently evaluated the five endoscopic images and determined the final endoscopic findings by consensus.
Statistical analyses
Serum gastrin and PG values are presented as medians (IQR), and statistical analyses were performed using nonparametric tests. The Mann–Whitney U test was used to determine the significance of the differences between patients with and without AIG. The χ2 test or Fisher’s exact test was performed to analyze categorical variables. Interobserver agreement between the diagnosis of endoscopic findings was analyzed using Kappa values. The strength of agreement was determined based on the guidelines by Landis and Koch43, and ≥ 0.6 was considered suitable. AUROC values were used to identify the optimal cutoff value. Comparison of AUROC values was performed using the Delong test. Sensitivities, specificities, positive predictive values, negative predictive values and overall accuracy were also calculated. All statistical analyses were performed using SPSS (version 25; SPSS, Chicago, IL, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)44, and a two-sided P < 0.05 was considered statistically significant.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.jp) for their writing support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- AIG
Autoimmune gastritis
- AIF
Anti-intrinsic factor
- APCA
Anti-parietal cell antibody
- AUROC
Areas under the receiver-operating characteristic curve
- CIs
Confidence intervals
- IQR
Interquartile range
- NPV
Negative predictive value
- OR
Odds ratio
- PCAB
Potassium competitive acid blocker
- PG
Pepsinogen
- PPI
Proton pump inhibitor
- PPV
Positive predictive value
- ROC
Receiver-operating characteristic
- SE
Standard error
Author contributions
Conception and design: H.K. Analysis and interpretation of the data: H.K., K.N., A.S., Y.H., and H.H. Drafting of the article: H.K. Critical revision of the article for important intellectual content: K.N., K.O., T.K., K.A., S.T., S.M., T.K., and J.N. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07947-1.
References
- 1.Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Am. J. Dig. Dis. 1973;18:426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- 2.Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis—pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013;10:529–541. doi: 10.1038/nrgastro.2013.101. [DOI] [PubMed] [Google Scholar]
- 3.Coati I, et al. Autoimmune gastritis: Pathologist’s viewpoint. World J. Gastroenterol. 2015;21:12179–12189. doi: 10.3748/wjg.v21.i42.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmud N, et al. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2019;32:67–72. doi: 10.20524/aog.2018.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miceli E, et al. Natural history of autoimmune atrophic gastritis: A prospective, single centre, long-term experience. Aliment. Pharmacol. Ther. 2019;50:1172–1180. doi: 10.1111/apt.15540. [DOI] [PubMed] [Google Scholar]
- 6.Nishizawa T, et al. Clue of diagnosis for autoimmune gastritis. Digestion. 2021;102:903–910. doi: 10.1159/000516624. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, et al. Analysis of clinical characteristics of 2243 with positive anti-gastric parietal cell antibody. J. Clin. Lab. Anal. 2020;34:e23264. doi: 10.1002/jcla.23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahner E, et al. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am. J. Gastroenterol. 2009;104:2071–2079. doi: 10.1038/ajg.2009.231. [DOI] [PubMed] [Google Scholar]
- 9.Villanacci V, et al. Autoimmune gastritis: Relationships with anemia and Helicobacter pylori status. Scand. J. Gastroenterol. 2017;52:674–677. doi: 10.1080/00365521.2017.1288758. [DOI] [PubMed] [Google Scholar]
- 10.Venerito M, et al. Oxyntic gastric atrophy in Helicobacter pylori gastritis is distinct from autoimmune gastritis. J. Clin. Pathol. 2016;69:677–685. doi: 10.1136/jclinpath-2015-203405. [DOI] [PubMed] [Google Scholar]
- 11.Alexandraki KI, et al. Are patients with autoimmune thyroid disease and autoimmune gastritis at risk of gastric neuroendocrine neoplasms type 1? Clin. Endocrinol. (Oxf.) 2014;80:685–690. doi: 10.1111/cen.12346. [DOI] [PubMed] [Google Scholar]
- 12.Miceli E, et al. Common features of patients with autoimmune atrophic gastritis. Clin. Gastroenterol. Hepatol. 2012;10:812–814. doi: 10.1016/j.cgh.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Demir AM, et al. Autoimmune atrophic gastritis: The role of Helicobacter pylori infection in children. Helicobacter. 2020;25:e12716. doi: 10.1111/hel.12716. [DOI] [PubMed] [Google Scholar]
- 14.Kawanaka M, et al. High prevalence of autoimmune gastritis in patients with nonalcoholic steatohepatitis. Intern. Med. 2019;58:2907–2913. doi: 10.2169/internalmedicine.2693-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Re V, et al. Pepsinogens to distinguish patients with gastric intestinal metaplasia and Helicobacter pylori infection among populations at risk for gastric cancer. Clin. Transl. Gastroenterol. 2016;7:e183. doi: 10.1038/ctg.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notsu T, et al. Prevalence of autoimmune gastritis in individuals undergoing medical checkups in Japan. Intern. Med. 2019;58:1817–1823. doi: 10.2169/internalmedicine.2292-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terao S, et al. Multicenter study of autoimmune gastritis in Japan: Clinical and endoscopic characteristics. Dig. Endosc. 2020;32:364–372. doi: 10.1111/den.13500. [DOI] [PubMed] [Google Scholar]
- 18.Kamada T, Maruyama Y, Monobe Y, Haruma K. Endoscopic features and clinical importance of autoimmune gastritis. Dig. Endosc. 2021 doi: 10.1111/den.14175. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, et al. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels–The ABC method. Digestion. 2016;93:13–18. doi: 10.1159/000441742. [DOI] [PubMed] [Google Scholar]
- 20.Syrjänen K. A panel of serum biomarkers (GastroPanel®) in non-invasive diagnosis of atrophic gastritis. Systematic review and meta-analysis. Anticancer Res. 2016;36:5133–5144. doi: 10.21873/anticanres.11083. [DOI] [PubMed] [Google Scholar]
- 21.Lenti MV, et al. Cell blood count alterations and patterns of anaemia in autoimmune atrophic gastritis at diagnosis: A multicentre study. J. Clin. Med. 2019;8:1992. doi: 10.3390/jcm8111992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carabotti M, et al. Upper gastrointestinal symptoms in autoimmune gastritis: A cross-sectional study. Medicine. 2017;96:e5784. doi: 10.1097/MD.0000000000005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soykan I, Yakut M, Keskin O, Bektaş M. Clinical profiles, endoscopic and laboratory features and associated factors in patients with autoimmune gastritis. Digestion. 2012;86:20–26. doi: 10.1159/000338295. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J. Gastroenterol. 2005;40:123–127. doi: 10.1007/s00535-004-1511-x. [DOI] [PubMed] [Google Scholar]
- 25.Quach DT, Hiyama T. Assessment of endoscopic gastric atrophy according to the Kimura-Takemoto classification and its potential application in daily practice. Clin. Endosc. 2019;52:321–327. doi: 10.5946/ce.2019.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda K, et al. Design paper: Japan Endoscopy Database (JED): A prospective, large database project related to gastroenterological endoscopy in Japan. Dig. Endosc. 2018;30:5–19. doi: 10.1111/den.12964. [DOI] [PubMed] [Google Scholar]
- 27.Massironi S, Zilli A, Elvevi A, Invernizzi P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019;18:215–222. doi: 10.1016/j.autrev.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Checchi S, et al. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J. Clin. Endocrinol. Metab. 2007;92:4346–4351. doi: 10.1210/jc.2007-0988. [DOI] [PubMed] [Google Scholar]
- 29.Varis K, Kekki M, Härkönen M, Sipponen P, Samloff IM. Serum pepsinogen I and serum gastrin in the screening of atrophic pangastritis with high risk of gastric cancer. Scand. J. Gastroenterol. Suppl. 1991;186:117–123. doi: 10.3109/00365529109103998. [DOI] [PubMed] [Google Scholar]
- 30.Koc DO, Bektas S. Serum pepsinogen levels and OLGA/OLGIM staging in the assessment of atrophic gastritis types. Postgrad. Med J. 2020 doi: 10.1136/postgradmedj-2020-139183. [DOI] [PubMed] [Google Scholar]
- 31.Miceli E, et al. A laboratory score in the diagnosis of autoimmune atrophic gastritis: A prospective study. J. Clin. Gastroenterol. 2015;49:e1–e5. doi: 10.1097/MCG.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 32.Lahner E, et al. Autoantibodies toward ATP4A and ATP4B subunits of gastric proton pump H+, K+ ATPase are reliable serological pre-endoscopic markers of corpus atrophic gastritis. Clin. Transl. Gastroenterol. 2020;11:e00240. doi: 10.14309/ctg.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima Y, et al. Does the novel potassium-competitive acid blocker vonoprazan cause more hypergastrinemia than conventional proton pump inhibitors? A multicenter prospective cross-sectional study. Digestion. 2018;97:70–75. doi: 10.1159/000484217. [DOI] [PubMed] [Google Scholar]
- 34.Shiotani A, et al. Hypergastrinemia in long-term use of proton pump inhibitors. Digestion. 2018;97:154–162. doi: 10.1159/000484688. [DOI] [PubMed] [Google Scholar]
- 35.Yakut M, et al. The association between precancerous gastric lesions and serum pepsinogens, serum gastrin, vascular endothelial growth factor, serum interleukin-1 beta, serum toll-like receptor-4 levels and Helicobacter pylori Cag A status. Clin. Res. Hepatol. Gastroenterol. 2013;37:302–311. doi: 10.1016/j.clinre.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Kishikawa H, et al. Cutoff pepsinogen level for predicting unintendedly eradicated cases of Helicobacter pylori infection in subjects with seemingly normal pepsinogen levels. Digestion. 2017;95:229–236. doi: 10.1159/000469705. [DOI] [PubMed] [Google Scholar]
- 37.Sumi N, et al. Diagnosis of histological gastritis based on the Kyoto classification of gastritis in Japanese subjects–including evaluation of aging and sex difference of histological gastritis. Scand. J. Gastroenterol. 2022;57:260–265. doi: 10.1080/00365521.2021.2002927. [DOI] [PubMed] [Google Scholar]
- 38.Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori antibody titer and gastric cancer screening. Dis. Markers. 2015;2015:156719. doi: 10.1155/2015/156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue M, et al. High-negative anti-Helicobacter pylori IgG antibody titers and long-term risk of gastric cancer: Results from a large-scale population-based cohort study in Japan. Cancer Epidemiol. Biomark. Prev. 2020;29:420–426. doi: 10.1158/1055-9965.EPI-19-0993. [DOI] [PubMed] [Google Scholar]
- 40.Kishikawa H, et al. Previous Helicobacter pylori infection–induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter. 2020;25:e12669. doi: 10.1111/hel.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham DY, Klein PD. Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterol. Clin. North Am. 2000;29:885–893. doi: 10.1016/s0889-8553(05)70156-4. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 43.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 44.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.